Abstract
Background:
Because of the decreasing susceptibility of Neisseria gonorrhoeae to cephalosporin therapy, the Centers for Disease Control and Prevention recommends test of cure (TOC) 1 week after gonorrhea (GC) treatment if therapies other than ceftriaxone are used. In addition, the Centers for Disease Control and Prevention asks clinicians, particularly those caring for men who have sex with men (MSM) on the west coast, to consider retesting all MSM at 1 week. However, it is unclear if this is acceptable to providers and patients or if nucleic acid amplification tests (NAATs) are useful for TOC at 7 days.
Methods:
Between January and July 2012, MSM with GC were advised to return 1 week after treatment for TOC using NAAT. A multi-variate logistic regression model was used to determine demographic and behavioral differences between MSM who returned for follow-up and MSM who did not.
Results:
Of 737 men with GC, 194 (26.3%) returned between 3 and 21 days of treatment. Individuals who returned were more likely to have no GC history (P = 0.0001) and to report no initial symptoms (P = 0.02) when compared with individuals who did not return for TOC. Of those who returned, 0% of urethral samples, 7.4% of rectal samples, and 5.3% of pharyngeal samples were NAAT positive at TOC.
Conclusions:
Although TOC may be an important strategy in reducing complications and the spread of GC, low return rates may make implementation challenging. If implemented, extra efforts should be considered to enhance return rates among individuals with a history of GC. If TOCs are recommended at 1 week and NAATs are used, the interpretation of positive results, particularly those from extragenital sites, may be difficult.
Gonorrhea (GC), caused by Neisseria gonorrhoeae, is the second most commonly reported notifiable disease in the United States with 321,849 cases reported in 2011.1 Data from the US Centers for Disease Control and Prevention’s (CDC) STD Surveillance Network have shown that the proportion of sexually transmitted disease (STD) clinic attendees reporting a history of GC infection within the past year was 6.8% for women, 9.7% for men who have sex with women, and 12.8% for men who have sex with men (MSM).2
Although symptoms are common with urethral GC infections in MSM, infections of the pharynx and rectum (collectively known as extragenital GC infections) are predominantly asymptomatic. A study by Kent et al.3 found that 84% of MSM who were diagnosed at 2 San Francisco clinics with a rectal GC infection were asymptomatic at time of diagnosis, and a study by Morris et al.4 found that 92% of MSM diagnosed as having pharyngeal infections were asymptomatic in the Project EXPLORE study. Furthermore, Kent et al. found that among all MSM diagnosed as having GC in their study, 64% had extragenital infections. The high prevalence of GC in MSM, the asymptomatic nature of extragenital infections, and the high proportion of extragenital GC infections underscore the importance ofscreening to detect infection.
Nucleic acid amplification tests (NAATs) have largely replaced culture for GC detection because they are more sensitive.5 They are automated and approved by the Food and Drug Administration for testing urine and self-collected vaginal swabs. Although GC NAATs are not approved by the Food and Drug Administration for extragenital testing, many laboratories have validated them for this purpose.6–8 Prompt detection and treatment of GC in its early stages is important to prevent complications and continued transmission.
GC differs from most other bacterial STDs because of its formidable ability to develop antibiotic resistance, which limits the options for effective treatment and control of the disease.9–13 N. gonorrhoeae has readily acquired resistance to numerous first-line antimicrobial agents used for the treatment of gonococcal infections. In a July 2011 Morbidity and Mortality Weekly Report, the CDC published worrisome trends of decreasing cephalosporin sensitivity among N. gonorrhoeae isolates collected as part of the Gonococcocal Isolate Surveillance Project. Of particular concern was the decreasing susceptibility to oral cephalosporins with the percentage of isolates with cefixime minimum inhibitory concentrations of at least 0.25 μg/mL increasing from 0% in 2000 to 4.0% in 2010. Regionally, these increases were more pronounced among MSM in the western United States. Based on this emerging trend, the CDC asked clinicians caring for patients with GC, particularly MSM in the western United States, to consider having patients return for a test of cure (TOC) at 1 week, with culture as the preferred method or NAAT if culture was not available.14 In August 2012, the CDC published an update to the 2010 STD treatment guidelines recommending dual therapy with an intra-muscular injection of ceftriaxone plus azithromycin or doxycycline as first-line therapy for uncomplicated GC at all anatomic sites.11 If a therapy other than ceftriaxone is used, a TOC is recommended at 7 days posttreatment.
Given these revised CDC recommendations for GC TOC and given that STD clinics have largely replaced culture with NAAT, there is concern that nucleic acid from nonviable organisms may remain and give a falsely positive result if patients are tested too soon after treatment.15
Two studies have evaluated the use of NAATs for GC TOC, one using ligase chain reaction and the other using an in-house porA pseudogene polymerase chain reaction (PCR) test.15,16 Bachmann et al. concluded that TOC should take place via PCR at least 10 days after treatment, whereas the study by Hjelmevoll et al. concluded that the appropriate time for GC TOC with their in-house PCR seemed to be 14 days after treatment. However, both of these studies had relatively small sample sizes of 130 and 30, respectively. To date, there have been no published studies of the time to negative NAAT for either rectal or pharyngeal GC infection using current, commercially available NAATs such as the transcription-mediated amplification test which detects RNA.
The objectives of the present study were 4-fold: (1) to evaluate the feasibility of TOC at 7 days after treatment for MSM with GC in a large lesbian, gay, bisexual, and transgender (LGBT) community-based clinic, (2) to assess the demographic and behavioral differences between individuals who present for TOC and individuals who do not return for TOC, (3) to determine if TOC results differ across various anatomical sites of infection at the individual and aggregate levels, and (4) to determine the proportion of MSM with GC who continue to have a positive GC test result at TOC.
MATERIALS AND METHODS
The Los Angeles LGBT Center (the Center) operates a clinic in Los Angeles, California, that provides STD testing and treatment to more than 12,000 unique, primarily MSM, patients per year. Each patient undergoes a counselor-administered assessment with questions on demographics, sexual risk behaviors, and symptoms. Men who have sex with men who present for STD testing and/or treatment provide self-collected rectal and urine samples for GC and chlamydia NAAT testing and then are seen by a laboratory technician for pharyngeal GC NAAT testing. Gonorrhea NAATs are performed using APTIMA Combo 2 Assay (Hologic Gen-Probe, San Diego, CA), a transcription-mediated amplification test that detects RNA.
Patients who present with urethral discharge, dysuria, or rectal symptoms at the time of the initial visit are presumptively treated with single doses of ceftriaxone (250 mg, intramuscular) and azithromycin (1 g, oral), or alternative therapy if indicated. Asymptomatic patients who test positive and do not receive treatment at the time of initial screening are called to schedule a return appointment for treatment. Before implementation of the modified TOC protocol, patients at the Center with GC were advised to return in 3 weeks for TOC after treatment. Patients returning with persistent symptoms after treatment are queried about potential reexposure, to assess the likelihood of potential treatment failure, and treated as appropriate.
From January 23, 2012, to July 20, 2012, the modified TOC protocol was implemented, which changed the recommended return time from 3 weeks to 7 days for patients who met the criteria for classification as MSM: (1) male and (2) either identified as gay or bisexual or had sex with another man in the past year. Each patient was informed by treatment staff about emerging antibiotic resistance in GC and the need to return for TOC.
When the patient returned for TOC, NAAT and culture samples were taken from the anatomical sites that were previously positive, and the patient was instructed to return in 3 months for routine screening. Pharyngeal and rectal NAATs have been internally validated by the County of Los Angeles, Department of Public Health Laboratory, allowing results to be used for clinical management. Specimens for culture were collected and directly plated onto modified Thayer-Martin media by a provider. Specimens were immediately sealed in a plastic zipper storage bag with a carbon dioxide releasing tablet and placed in an incubator at 35°C to 37°C.
Statistical Methods
Per the protocol, the treatment staff recommended that patients return 7 days after treatment to receive TOC. However, patients were included in the analysis if they came back between 3 and 21 days after treatment. To determine the TOC return rate, and thus feasibility of the modified TOC protocol, the number of MSM who returned within the 3 to 21-day window for a TOC visit was divided by the total number of MSM who tested positive for GC and were treated during the study period. Although TOC visits were not tracked in 2011, the number of MSM who returned within 21 days of treatment of GC retesting from January to July 2011 was divided by the total number of MSM who tested positive for GC and were treated to obtain a TOC return rate comparison before the study.
A multivariate logistic regression model with backward elimination was used to determine if TOC return differed by previous receipt of post-exposure HIV prophylaxis at the clinic, self-reported history of GC infection, site of infection (urethral or extragenital only), or symptoms during the initial visit, controlling for demographic characteristics such as age group, race/ethnicity, and education level. All analyses were performed in SAS version 9.3 (Cary, NC).
Ethics
The project was exempted as non-research by the County of Los Angeles, Department of Public Health Institutional Review Board (FWA00000071; Project Numbers: 2013-05-434 and 2011-12-361). In addition, the project underwent review at CDC and was determined not to be research involving human subjects.17,18
RESULTS
Of the 737 MSM patients who tested positive for GC and were treated after program implementation, 194 returned between 3 and 21 days after treatment, for a TOC return rate of approximately 26.3%. Of these 194 patients, 4.6% returned between 3 and 6 days after treatment, 41.8% returned at 7 days, 35.1% returned between 8 and 14 days, and 18.6% returned between 15 and 21 days (Fig. 1). The proportion of patients who returned for TOC substantially decreased from a return rate of 40.7% in January to 23.6% in July (Fig. 2). From January to July in 2011, 7.3% of 536 MSM with laboratory confirmed GC were retested within 21 days of their initial treatment.
Figure 1.
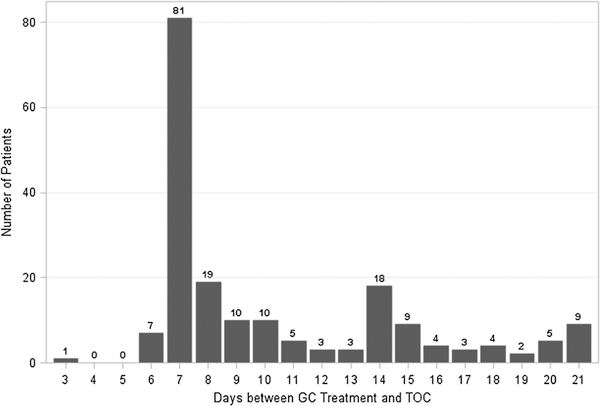
Distribution of times from treatment to TOC for all patients returning for TOC (n = 194), January 2012 to July 2012.
Figure 2.
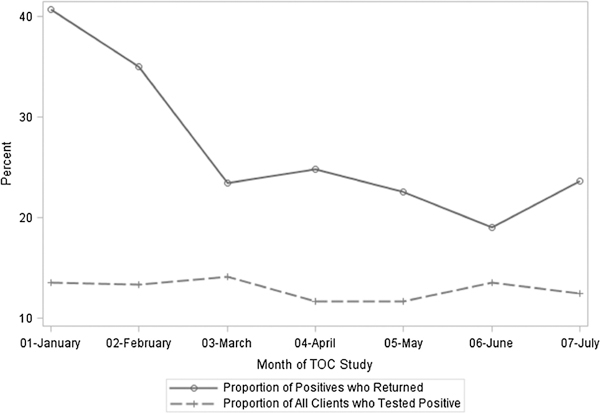
Proportion of GC cases returning for a TOC by month, January 2012 to July 2012.
There were no differences in the rate of return based on demographics such as age group, race/ethnicity, or education level (Table 1). Patients were more likely to return for TOC if they previously received a 28-day regimen of postexposure HIV prophylaxis from the clinic (P = 0.03), did not have a self-reported history of GC (P = 0.0001), and were asymptomatic at intake (P = 0.02; Table 2). However, the site of infection (urethral vs. extragenital only) was not significant and was removed in backward elimination from the multivariate model (P = 0.37).
TABLE 1.
Demographics of MSM by Return Status (n = 737), January 2012 to July 2012
| Total |
MSM Who Returned between 3 and 21 d After Treatment |
|||
|---|---|---|---|---|
| Demographic Category | n | Column % | n | Row % |
| Age group, y | ||||
| <20 | 18 | 2.4 | 2 | 11.1 |
| 20–24 | 183 | 24.8 | 52 | 28.4 |
| 25–29 | 215 | 29.2 | 62 | 28.8 |
| 30–39 | 204 | 27.7 | 42 | 20.6 |
| 40–49 | 86 | 11.7 | 27 | 31.4 |
| 50+ | 31 | 4.2 | 9 | 29.0 |
| Race/Ethnicity | ||||
| White | 337 | 45.7 | 93 | 27.6 |
| Black/African American | 68 | 9.2 | 14 | 20.6 |
| Hispanic | 267 | 36.2 | 67 | 25.1 |
| Asian/PI | 47 | 6.4 | 17 | 36.2 |
| Other | 18 | 2.4 | 3 | 16.7 |
| Education | ||||
| High school or below | 143 | 19.4 | 28 | 19.6 |
| Some college | 190 | 25.8 | 49 | 25.8 |
| College graduate | 354 | 48.0 | 109 | 30.8 |
| Postgraduate study/degree | 46 | 6.2 | 8 | 17.4 |
| Unknown | 4 | 0.5 | 0 | 0.0 |
| Previous HIV post-exposure prophylaxis patient | ||||
| Yes | 52 | 7.1 | 21 | 40.4 |
| No | 685 | 92.9 | 173 | 25.3 |
| History of GC infection | ||||
| No history of GC infection | 403 | 54.7 | 131 | 32.5 |
| No recent history of GC infection | 244 | 33.1 | 40 | 16.4 |
| Infection in last 12 mo | 86 | 11.7 | 23 | 26.7 |
| Unknown | 4 | 0.5 | 0 | 0.0 |
| Symptoms at initial positive test result | ||||
| Symptomatic | 351 | 47.6 | 72 | 20.5 |
| Asymptomatic | 386 | 52.4 | 122 | 31.6 |
| Initial anatomical infection site(s) | ||||
| Genital infection | 214 | 29.0 | 42 | 19.6 |
| Extragenital infection | 523 | 71.0 | 152 | 29.1 |
| Totals | 737 | 100 | 194 | 100 |
TABLE 2.
Multivariate Logistic Regression of TOC Return Regressed on Demographic and STI History Predictors, January 2012 to July 2012
| Demographic Category | Estimate | SE | P | OR (95% CI) |
|---|---|---|---|---|
| Age group, y | 0.13 | |||
| <20 | −0.93 | 0.79 | 0.24 | 0.39 (0.08–1.84) |
| 20–24 | 0.34 | 0.25 | 0.18 | 1.41 (0.85–2.32) |
| 25–29 | 0.36 | 0.24 | 0.14 | 1.43 (0.89–2.29) |
| 30–39 | — | — | Reference | — |
| 40–49 | 0.72 | 0.30 | 0.02 | 2.05 (1.13–3.72) |
| 50+ | 0.45 | 0.45 | 0.32 | 1.57 (0.65–3.82) |
| Race/Ethnicity | 0.64 | |||
| White | — | — | Reference | — |
| Black/African American | −0.27 | 0.34 | 0.43 | 0.76 (0.39–1.49) |
| Asian/PI | 0.28 | 0.34 | 0.41 | 1.32 (0.68–2.56) |
| Hispanic | −0.11 | 0.20 | 0.58 | 0.89 (0.60–1.33) |
| Other | −0.59 | 0.66 | 0.38 | 0.56 (0.15–2.04) |
| Education | 0.08 | |||
| High school or below | −0.49 | 0.26 | 0.06 | 0.61 (0.37–1.03) |
| Some college | −0.24 | 0.22 | 0.27 | 0.79 (0.51–1.21) |
| College degree | — | — | Reference | — |
| Postgraduate study/degree | −0.82 | 0.42 | 0.05 | 0.44 (0.19Y1.01) |
| Previous HIV postexposure prophylaxis patient | ||||
| No | — | — | Reference | — |
| Yes | 0.68 | 0.32 | 0.03 | 1.97 (1.06Y3.68) |
| Symptoms at initial positive test result | ||||
| Symptomatic | — | — | Reference | — |
| Asymptomatic | 0.41 | 0.18 | 0.02 | 1.51 (1.06–2.15) |
| History of GC infection | 0.0001 | |||
| No history of GC (reference = GC in past year) | 0.44 | 0.28 | 0.12 | 1.55 (0.89–2.70) |
| Ever had GC (reference = GC in past year) | −0.46 | 0.32 | 0.15 | 0.63 (0.34–1.18) |
| No history of GC (reference = ever had GC) | 0.89 | 0.52 | −0.0001 | 2.44 (1.61–3.71) |
To determine NAAT clearance time, the interval from treatment to TOC was plotted by site of infection and TOC result. Of the 95 patients retested for GC of the urethra, no patients tested positive at the TOC visit (Fig. 3).
Figure 3.
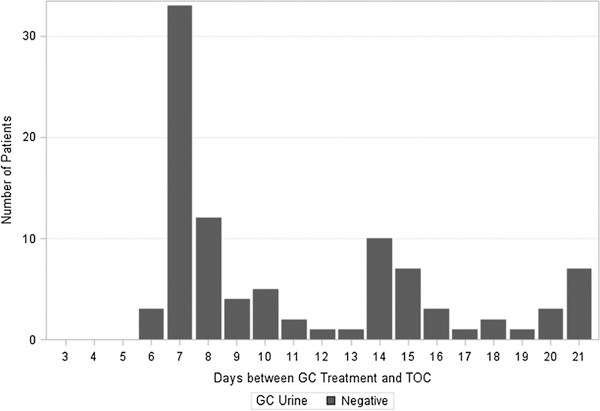
Distribution of days between GC treatment and TOC by NAAT result for rectal specimens (n = 135), January 2012 to July 2012.
Of the 135 MSM retested for rectal GC, 10 were NAAT positive and 4 were indeterminate. Six of the NAAT positives returned 7 days after treatment, 2 returned 8 days after treatment, and 2 returned 14 days after treatment (Fig. 4). Cultures were taken at the same time as the NAATs for 90 of the 135 patients retested for rectal GC. Of these 90 samples, 9 were NAAT positive and all were culture negative.
Figure 4.
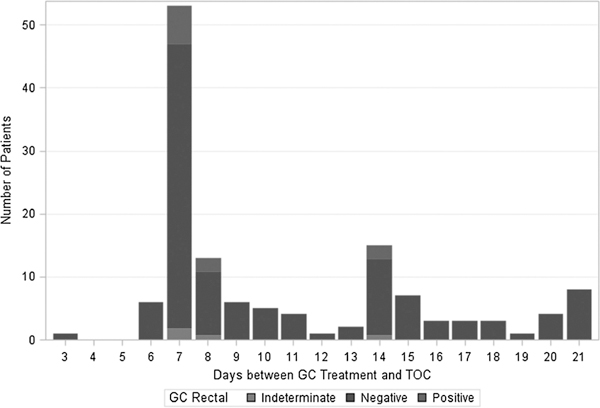
Distribution of days between GC treatment and TOC by NAAT result of rectal Specimens (n = 135), January 2012 to July 2012.
Of the 134 patients retested for GC of the throat, 7 were NAAT positive and 2 were indeterminate. Patients with positive NAATs returned on days 3, 6, 7 (n = 3), 8, and 10 (Fig. 5). Similarly, 85 of the 134 patients retested for pharyngeal GC had concurrent NAAT and culture testing. Of these 85 samples, 5 were NAAT positive and all were culture negative.
Figure 5.
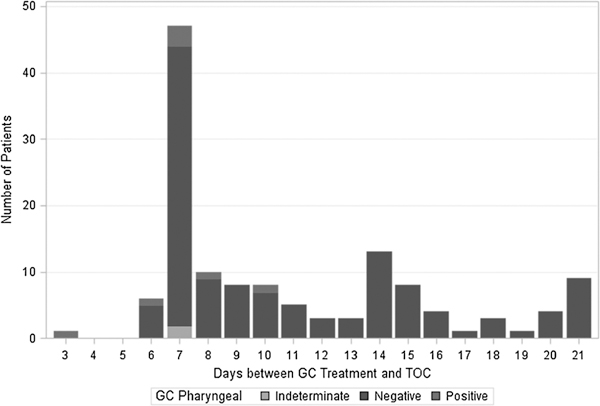
Distribution of days between GC treatment and TOC by NAAT result of rectal Specimens (n = 134), January 2012 to July 2012.
There were 17 unique patients who tested NAAT positive at follow-up. Only 3 of these patients reported reexposure between treatment and TOC, and their TOCs were at 8 and 14 days (rectal) and 10 days (pharyngeal) after treatment. Of the remaining 14 patients, 12 eventually returned outside the study window and all were NAAT negative on retest. Two patients were lost to follow-up. Of the 6 unique patients with indeterminate rectal or throat NAATs, 4 eventually returned outside the study window and were NAAT negative on retest; 2 were lost to follow-up.
DISCUSSION
Because of the threat of cephalosporin-resistant GC and limited remaining treatment options, the management of patients with GC can be difficult. Confirming eradication of infection with a TOC after non-ceftriaxone-based therapies, or in appropriately treated populations at highest risk for resistant N. gonorrhoeae, can be an important strategy to reduce complications and the spread of cephalosporin-resistant GC. However, TOC in practice may be challenging for many reasons.
One impediment to implementing routine TOC for MSM may be patient compliance. We found that the overall TOC uptake between 3 and 21 days after treatment was suboptimal at 26.3%. Compliance with TOC visits has been a common problem with data from similar studies indicating rates of return ranging from 46% to 60%.16,19,20 Our results showed even lower TOC uptake, and this might have been attributed to the fact that the other studies had a longer active window period for TOC. However, the 26.3% rate of TOC return during our study compared favorably to 7.3% retesting within 21 days in January to July of 2011, before the TOC protocol was modified, showing that a substantially higher proportion of MSM responded to the modified recommendation. If TOC is to become recommended in MSM or other populations, there is a need to explore strategies to increase rates of return.
There are other potential patient-related reasons affecting return rate for TOC. Our evaluation found that the strongest predictors of TOC uptake were asymptomatic status and prior history of GC. Patients who were asymptomatic might be motivated to return for TOC to learn if their infection had cleared, whereas symptomatic patients may be likely to assume successful treatment if their symptoms resolved. Patients with their first episode of GC may be more likely to follow their provider’s recommendation and less likely to be complacent. For individuals with a history of GC, providers electing to use TOC may emphasize to the patient that prior outcome experience for GC infections may no longer be reliable. If TOC is to become an important part of resistant GC control, then specific counseling about returning for testing despite GC history may be important.
There were 2 potential clinic-related reasons for the low TOC rate. There appeared to be a waning interest in clinic staff advocating the program over time as evidenced by the proportion of GC positive patients who returned for TOC substantially decreasing from a return rate of 40.7% in January to 23.6% in July. Another potential barrier was the high clinic volume during this time. Over the 26 weeks of the study, the clinic’s laboratory reached capacity at numerous points and turned away walk-in patients who were seeking services. Therefore, patients who returned for TOC may have been inadvertently turned away.
Despite the low return rate, we found that 91% of MSM returning between 3 and 21 days had a negative TOC NAAT. All 17 positive TOC NAATs were extragenital specimens; all urine specimens were negative. In addition, no rectal TOC specimens were positive after 14 days and no pharyngeal TOC specimens were positive after 10 days, although the number of patients returning after 14 days was relatively low. Our evaluation supports other published studies showing that 1 week may be an appropriate time for TOC NAAT for urethral samples. It seems, however, that the clearance time for extragenital sites may be longer. Further study is needed to more definitively determine the time to negative result for all TOC NAATs, especially those from extragenital sites.
This protocol was initially designed to obtain cultures for comparison to NAAT tests; however, difficulties culturing the organism prevented accurate assessment of NAAT concordance. The Center has successfully collected specimens for urethral cultures for the CDC’s Gonococcocal Isolate Surveillance Project program for several years but had relied solely on NAAT for extragenital testing for approximately the past 6 years and had only reimplemented throat and rectal cultures for an extragenital surveillance program just before the beginning of this TOC evaluation. There were initial difficulties in isolating the organism from extragenital specimens, and this was only slightly improved with changes in specimen collection, equipment, and transport. In previous studies, GC culture was positive for only approximately 40% of persons with positive NAATs.21,22 The difficulty in detecting extragenital GC by culture, even in a high-volume clinic with fresh culture plates, an incubator, and good experience with urethral cultures, raises concerns about the feasibility of culture in smaller and less equipped settings. This underscores the importance of understanding the time to clearance of extragenital NAATs after treatment.
Our evaluation has other notable limitations. First, despite clear protocol recommendations to provide the TOC messages, we do not know if all clinical staff provided the TOC messaging for all GC-positive patients with the same fidelity. Future efforts will need to incorporate other means, such as active outreach after diagnosis and treatment, to improve TOC rates. Second, although these results may be generalizable to MSM who test at LGBT-identified medical centers in the United States, it is unclear if these results are generalizable to all MSM or other populations. Third, given the design of the evaluation and the problems with the extragenital cultures, we have no way to assess whether the positive TOC NAAT represent false positives or true infections. Because the sensitivity of extragenital cultures at the Center was presumably low, the validity of these results should be approached with caution. Lastly, the patients were encouraged to make an appointment for TOC, but because the clinic functions largely on a walk-in basis, many patients retained the option to walk in for TOC and may have been turned away due to capacity issues.
Compliance with TOC may be challenging, and routine TOC visits can impose a significant burden on clinic resources. These obstacles can be partially ameliorated by streamlining such visits to reduce unnecessary clinician and counselor time. Self-collected pharyngeal and rectal swabs, validated by several studies, would further help reduce staff involvement, reduce patient time in clinic, and may also allow a greater clinic patient volume.23–25 A brief, computerized check-in procedure, which could include questions about symptoms and sexual exposure since treatment, could be used to triage those patients for whom clinical evaluation and retreatment would be advisable rather than waiting for TOC results. Although these aforementioned audio computer-assisted self-interview techniques have shown promise in other settings, future studies should validate this technique for TOC purposes.26
In conclusion, although TOC may be important for the control and surveillance of emerging gonococcal antibiotic resistance, the optimal timing of NAAT based TOCs needs to be more clearly defined, especially for extragenital infections. This is important because MSM are at the highest risk for cephalosporin resistance, and GC infections at extragenital sites are frequently asymptomatic, difficult to culture, and represent an important reservoir of GC infection in MSM.27,28 Furthermore, if clinics wish to enhance TOC rates, special efforts may be needed to encourage MSM with a history of GC infection to return. Finally, if it is determined that TOC should be routinely recommended for all clinics serving MSM, then clinic resources will need to focus on consistent messaging, active follow-up to reinforce the need for TOC, and an efficient process for TOC and retreatment when necessary.
Acknowledgments
Source of funding: The authors acknowledge no support pertinent to this manuscript.
Footnotes
Conflicts of interest: None declared for any authors.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention/the Agency for Toxic Substances and Disease Registry.
REFERENCES
- 1.Centers for Disease Control and Prevention. 2011 Sexually transmitted diseases surveillance—Gonorrhea [CDC Web site]. December 13, 2012. Available at: http://www.cdc.gov/std/stats11/gonorrhea.htm. Accessed September 1, 2013.
- 2.Newman LM, Dowell D, Bernstein K, et al. A tale of two gonorrhea epidemics: Results from the STD Surveillance Network. Public Health Rep 2012; 127:282–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kent CK, Chaw JK, Wong W, et al. Prevalence of rectal, urethral, and pharyngeal chlamydia and gonorrhea detected in 2 clinical settings among men who have sex with men: San Francisco, California, 2003. Clin Infect Dis 2005; 41:67–74. [DOI] [PubMed] [Google Scholar]
- 4.Morris SR, Klausner JD, Buchbinder SP, et al. Prevalence and incidence of pharyngeal gonorrhea in a longitudinal sample of men who have sex with men: The EXPLORE study. Clin Infect Dis 2006; 43:1284–1289. [DOI] [PubMed] [Google Scholar]
- 5.Boyadzhyan B, Yashina T, Patnaik M, et al. Comparison of the APTIMA CT and GC assays with the APTIMA combo 2 assay, the Abbott LCx assay, and direct fluorescent-antibody and culture assays for detection of Chlamydia trachomatis and Neisseria gonorrhoeae. J Clin Microbiol 2004; 42:3089–3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bachmann LH, Johnson RE, Cheng H, et al. Nucleic acid amplification tests for diagnosis of Neisseria gonorrhoeae oropharyngeal infections. J Clin Microbiol 2009; 47:902–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bachmann LH, Johnson RE, Cheng H, et al. Nucleic acid amplification tests for diagnosis of Neisseria gonorrhoeae and Chlamydia trachomatis rectal infections. J Clin Microbiol 2010; 48:1827–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klausner JD, Bernstein KT, Pandori M, et al. Clinic-based testing for rectal and pharyngeal Neisseria gonorrhoeae and Chlamydia trachomatis infections by community-based organizations—Five cities, United States, 2007. MMWR Morb Mortal Wkly Rep 2009; 58:716–719. [PubMed] [Google Scholar]
- 9.Allen VG, Mitterni L, Seah C, et al. Neisseria gonorrhoeae treatment failure and susceptibility to cefixime in Toronto, Canada. JAMA 2013; 309:163–170. [DOI] [PubMed] [Google Scholar]
- 10.Del Rio C, Hall G, Hook EW, et al. Update to CDC’s sexually transmitted diseases treatment guidelines, 2006: Fluoroquinolones no longer recommended for treatment of gonococcal infections. MMWR Morb Mortal Wkly Rep 2007; 56:332–336. [PubMed] [Google Scholar]
- 11.Del Rio C, Hall G, Holmes K, et al. Update to CDC’s sexually transmitted diseases treatment guidelines, 2010: Oral cephalosporins no longer a recommended treatment for gonococcal infections. MMWR Morb Mortal Wkly Rep 2012; 61:590–594. [PubMed] [Google Scholar]
- 12.Lewis DA, Lukehart SA. Antimicrobial resistance in Neisseria gonorrhoeae and Treponema pallidum: Evolution, therapeutic challenges and the need to strengthen global surveillance. Sex Transm Infect. 2011; 87:ii39–ii43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohnishi M, Saika T, Hoshina S, et al. Ceftriaxone-resistant Neisseria gonorrhoeae, Japan. Emerg Infect Dis 2011; 17:148–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Del Rio C, Hall G, Hook EW, et al. Cephalosporin susceptibility among Neisseria gonorrhoeae isolates—United States, 2000–2010. MMWR Morb Mortal Wkly Rep 2011; 60:873–877. [PubMed] [Google Scholar]
- 15.Bachmann LH, Desmond RA, Stephens J, et al. Duration of persistence of gonococcal DNA detected by ligase chain reaction in men and women following recommended therapy for uncomplicated gonorrhea. J Clin Microbiol 2002; 40:3596–3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hjelmevoll SO, Olsen ME, Sollid JU, et al. Appropriate time for test-of-cure when diagnosing gonorrhoea with a nucleic acid amplification test. Acta Derm Venereol 2012; 92:316–319. [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. Defining Public Health research and Public Health Non-research [CDC Web site]. July 29, 2010. Available at: http://www.cdc.gov/od/science/integrity/docs/cdc-policy-distinguishing-public-health-research-nonresearch.pdf. Accessed March 31, 2014.
- 18.U.S. Department of Health & Human Services (HHS). Protection of Human Subjects, 45 CFR § 46 [HHS Web site]. July 14, 2009. Available at: http://www.hhs.gov/ohrp/humansubjects/guidance/45cfr46.html. Accessed March 31, 2014.
- 19.Gratrix J, Bergman J, Egan C, et al. Retrospective review of pharyngeal gonorrhea treatment failures in Alberta, Canada. Sex Trans Dis 2013; 40:877–879. [DOI] [PubMed] [Google Scholar]
- 20.Rodgers S, Murgatroyd M, Perez K, et al. Challenges in implementing the new BASHH guidelines for the management of gonorrhea. Int J STD AIDS 2014; 25:145–147. [DOI] [PubMed] [Google Scholar]
- 21.Schachter J, Moncada J, Liska S, et al. Nucleic acid amplification tests in the diagnosis of chlamydial and gonococcal infections of the oropharynx and rectum in men who have sex with men. Sex Transm Dis 2008; 35:637–642. [DOI] [PubMed] [Google Scholar]
- 22.Page-Shafer KG, Graves A, Kent C, et al. Increased sensitivity of DNA amplification testing for the detection of pharyngeal gonorrhea in men who have sex with men. Clin Infect Dis 2002; 34:173–176. [DOI] [PubMed] [Google Scholar]
- 23.Alexander S, Ison C, Parry J, et al. Self-taken pharyngeal and rectal swabs are appropriate for the detection of Chlamydia trachomatis and Neisseria gonorrhoeae in asymptomatic men who have sex with men. Sex Transm Dis 2008; 84:488–492. [DOI] [PubMed] [Google Scholar]
- 24.Rotblatt H, Montoya JA, Plant A, et al. There is no place like home—First-year use of the I Know home testing program for chlamydia and gonorrhea. Am J Public Health 2013; 103:1376–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barbee L, Dombrowski J, Kerani R, et al. Introduction of extragenital self-obtained nucleic acid amplification tests (NAAT) for gonorrhea and chlamydial infection in MSM in an STD clinic. Presented at: 8th National STD Prevention Conference [D7.4]; 2012; Minneapolis. [Google Scholar]
- 26.Metzger DS, Koblin B, Turner C, et al. Randomized controlled trial of audio computer-assisted self-interviewing: utility and acceptability in longitudinal studies. HIVNET Vaccine Preparedness Study Protocol Team. Am J Epidemiol 2000; 152:99–106. [DOI] [PubMed] [Google Scholar]
- 27.Klein EJ, Fisher LS, Chow AW, et al. Anorectal gonococcal infection. Ann Intern Med 1977; 86:340–346. [DOI] [PubMed] [Google Scholar]
- 28.Bro Jorgensen A, Jensen T. Gonococcal pharyngeal infections. Report of 110 cases. Br J Vener Dis. 1973; 49:491–499. [DOI] [PMC free article] [PubMed] [Google Scholar]


