Abstract
OBJECTIVE
This study describes the normal variations in serum and cervicovaginal (CVF) cytokine levels throughout pregnancy.
STUDY DESIGN
This multicenter, prospective study examined trimester-specific maternal serum and CVF cytokines (IL-1α, IL-1β, IL-6, IL-8, IL-10, TNFα and CRP). A two-factor linear mixed modelling approach compared cytokine distribution, while pair-wise comparisons evaluated differences over time.
RESULTS
Trimester-specific serum cytokine data were available for 288, 243 and 221 patients; whereas CVF cytokine data was available for 273, 229 and 198 patients. CVF had significantly higher concentrations of IL-1α, IL-1β, IL-6, IL-8 and MMP-8 (P < 0.001), irrespective of the trimester. At all time-points, IL-10 and CRP concentrations were higher in serum than CVF (P < 0.001). Serum IL-10 increased significantly throughout pregnancy (P < 0.001).
CONCLUSION
Differences in cytokine distribution across different biological fluids are evident throughout pregnancy. These findings provide a framework for examining patterns of changes in cytokines throughout pregnancy.
Keywords: cytokines, inflammatory markers, pregnancy, serum, cervicovaginal fluid
INTRODUCTION
Pregnancy is an unique immunological state with an ever changing balance in immune responses.1 This process of changing immune responses lasts throughout gestation and requires an appropriate balance between pro-inflammatory and anti-inflammatory responses.2 Early in gestation, the implanting blastocyst incites a predominantly pro-inflammatory state followed by an anti-inflammatory state, which allows for fetal growth and development. Near term, a renewed pro-inflammatory process may promote maternal adaption in preparation for the process of delivery.3–6 Therefore, the gestational process is characterized by selective pro-inflammatory and anti-inflammatory conditions, depending upon the stage of gestation.7,8
Modest elevations in both serum pro- and anti-inflammatory cytokine levels have been observed in normal pregnancies as compared to the non-pregnancy condition.9–14 Cytokines reflecting Th1 cell responses mediate inflammation and have been associated with fetal rejection. The production of cytokines by Th2 cells influences an anti-inflammatory milieu and a Th1/Th2 ratio is associated with a successful pregnancy.15–18 Dysregulation in this immune network has been associated with pregnancy complications19,20, with increased serum pro-inflammatory cytokines and decreased anti-inflammatory cytokine levels raising the risk for preterm labor and preeclampsia.13–21 Elevated maternal IL-6 in cervicovaginal fluid and serum, and CRP levels in serum have been identified as risk factors for preterm birth (PTB) <32 weeks.22–25 Additionally, infants identified as small for gestational age (SGA) have been associated with low pro-inflammatory and anti-inflammatory maternal serum cytokines.26 Research by Heng et al. (2014) explored biomarkers in CVF as potential predictors for term and preterm labor, and concluded the medium to be an excellent source to study cytokine changes throughout pregnancy, noting the importance of multiple biomarker modelling to achieve predictive efficacy.27,28 In addition to cytokines, there is evidence of increased expression of chemokines, as well as increased activity of select matrix metalloproteinases (MMP 8, 9) in spontaneous preterm birth and rupture of membranes.29 Further, cytokine-induced MMP expression can be inhibited due to the effect of progesterone on the decidua; thus contributing to a potential dysregulation in the host response.30 Given the complexity of the immune responses and the importance in normal and adverse pregnancy outcomes, describing the status of cytokines throughout pregnancy in both CVF and serum is a necessary precursor for elucidating which biomarker(s) may serve best to predict adverse events.
MATERIALS AND METHODS
Human Samples
This study reports the analysis of a multicenter, prospective, longitudinal study of women with a singleton gestation. Patients were screened at the University of Kentucky and University of Virginia prenatal clinics to exclude any pre-existing diabetes, heart disease, a medical history of HIV, bacterial vaginosis, sexually transmitted infections, chronic conditions with implications for immune function, any autoimmune disease or illicit drug use. The Institutional Review Boards at the affiliated sites approved the study protocol. Informed written consent for the study was obtained, and all of the women volunteers received modest compensation for participation. Maternal serum (288, 243 and 221 patients) and CVF (273, 229 and 198 patients) were collected during each trimester to measure the following cytokine levels: IL-1α, IL-1β, IL-6, IL-8, IL-10, TNFα and CRP. For MMP-8 cytokine data, serum was collected from 205, 170 and 152, and CVF from 185, 156 and 128 patients during the three trimesters, respectively. Participant age, number of prior pregnancies (parity), race, education and income were collected by self-report. Body mass index (BMI) was calculated from the patient’s height and weight recordings.
Sample collection and cytokine estimation
Clotted blood samples were centrifuged at 2000 rpm for 10 minutes, and the serum was divided into three aliquots and stored at −80°C. Samples were analyzed undiluted. For CVF specimens, samples were obtained after the speculum was placed and the cervix visualized. An ectocervical sample was collected using an Aware Messenger (Calypte)™ swab and by sweeping the cervix 360 degrees and kept in place for 30 seconds to maximize saturation. When removing the swab, a vaginal sample was obtained by sweeping 360 degrees in the vaginal vault/posterior fornix. The swab was then placed in the proprietary container, firmly pressed against the inner cryovial wall to ensure maximum seepage of fluid into the buffer container; and the vial cap secured. All samples were immediately refrigerated, transported to the lab within 6 hours where they were stored at −20°C for a minimum of 24 hours. To further process, samples were thawed 4°C, and then centrifuged at 3750rpm for 15 minutes. For long-term storage, the samples were split into three aliquots and stored at −80°C. Cytokines IL-1α, IL-1β, IL-6, IL-8, IL-10 and TNFα, were measured using multiplex beadlyte assay (MPXHCYTO-60K-06) on a Luminex IS-100 (Austin, Tx) according to the manufacturer’s recommendations. High sensitivity testing was used for samples below minimum detectable concentrations. Singleplex assays were used for CRP (Millipore, Billerica, MA) and MMP-8 (R&D Systems Minneapolis, MN). The dynamic range of the CRP assay was 50–0.016 ng/ml with a minimum detection concentration of 0.0012 ng/ml; and MMP-8 range was 66,700–91.5 pg/ml with a MinDC of 16.6 pg/ml (samples were diluted 1:10 from MMP-8 analysis). All cytokine data were generated using Milliplex Analyst Software.
Statistical analysis
The non-normally distributed raw cytokine levels were log-transformed prior to all analysis. Pearson’s product moment correlation was used to determine associations among cytokines by medium (serum vs. CVF) and trimester, Two-factor linear mixed modeling evaluated differences in cytokine levels between sources and over time. The first factor was cytokine source (serum/cervicovaginal) and the second factor was trimester (first/second/third). Each model contained the main effects for cytokine source and trimester as well as their interaction. If the interaction term was not significant in the model it was removed and the model with only main effects was subsequently fit. In all models, women were included as a random factor to account for repeated measures on individuals over time. All data analysis was conducted using SAS, version 9.4, with an alpha level of 0.05.
RESULTS
Patient demographic and clinical characteristics are summarized in Table 1. The majority were White/non-Hispanic (74%) and had at least a high-school diploma/GED (87%). Median parity among this sample was one (interquartile range= 1–3) and average BMI was 26 ± 6.4 kg/m2. At enrollment, 66 women (20%) had a history of prior preterm birth and 25 women (4.8%) had a history of preeclampsia. The overall rate of preterm birth was 13.9% for the cohort, with nearly 10% experiencing spontaneous PTB.
Table 1.
Demographic and clinical characteristics of patients
| Variable | Mean (SD), Median (IQR) or n (%) |
|---|---|
| Age (years) | 26 (5.4) |
| BMI (kg/m2) | 26.6 (6.4) |
| Parity | 1 (1–3) |
| Race | |
| Caucasian | 190 (74.1%) |
| African American | 41 (16.2%) |
| Hispanic | 11 (4.3%) |
| Other | 11 (4.3) |
| Education | |
| Less than high school/GED | 32 (12.6%) |
| At least high school | 222 (87.4%) |
| Income | |
| Less than $20,000 | 92 (39.2%) |
| $20,000 - $39,999 | 52 (22.1%) |
| $40,000 and over | 91 (38.7%) |
Baseline characteristics of women with available serum or cervicovaginal fluid data at least one trimester during pregnancy. Note: Numbers vary due to missing data.
Correlations among cytokines at each trimester
Pearson’s correlations were conducted to determine the patterns of association among cytokines in serum and CVF separately. Most of the serum cytokines, with the exception of interleukins and CRP, and almost all of the CVF cytokines were positively and significantly correlated with each other, for each trimester (see Tables 2 and 3).
Table 2.
Pearson product moment correlations for serum cytokines across pregnancy
| Systemic Cytokines | First Trimester | |||||||
| N | 288 | 288 | 288 | 288 | 288 | 288 | 288 | 205 |
| IL-1α | IL-1β | IL-6 | IL-8 | IL-10 | TNFα | CRP | MMP-8 | |
| IL-1α | 1 | .70** | .57** | .47** | .43** | .43** | −.02 | .25** |
| IL-1β | 1 | .57** | .45** | .47** | .50** | −.003 | .28** | |
| IL-6 | 1 | .59** | .51** | .49** | −.02 | .07 | ||
| IL-8 | 1 | .33** | .34** | −.02 | .19* | |||
| IL-10 | 1 | .29** | .12* | −.03 | ||||
| TNFα | 1 | −.01 | .15* | |||||
| CRP | 1 | .18* | ||||||
| MMP-8 | 1 | |||||||
| Second Trimester | ||||||||
| N | 243 | 243 | 243 | 243 | 243 | 243 | 243 | 170 |
| IL-1α | 1 | .63** | .58** | .46** | .45** | .38** | −.04 | .27** |
| IL-1β | 1 | .56** | .48** | .43** | .43** | −.13* | .32** | |
| IL-6 | 1 | .67** | .49** | .45** | −.11 | .15* | ||
| IL-8 | 1 | .36** | .48** | −.17* | .29** | |||
| IL-10 | 1 | .33** | .04 | .08 | ||||
| TNFα | 1 | −.08 | .18* | |||||
| CRP | 1 | −.003 | ||||||
| MMP-8 | 1 | |||||||
| Third Trimester | ||||||||
| N | 221 | 221 | 221 | 221 | 221 | 221 | 221 | 152 |
| IL-1α | 1 | .63** | .58** | .35** | .49** | .40** | −.11 | .07 |
| IL-1β | 1 | .59** | .51** | .49** | .39** | −.13 | .10 | |
| IL-6 | 1 | .62** | .53** | .46** | −.07 | .08 | ||
| IL-8 | 1 | .32** | .36** | −.12 | .26* | |||
| IL-10 | 1 | .34** | .02 | .13 | ||||
| TNFα | 1 | −.04 | .15 | |||||
| CRP | 1 | −.02 | ||||||
| MMP-8 | 1 | |||||||
Note:
P < 0.001
P < 0.05
Table 3.
Pearson product moment correlations for CVF cytokines across pregnancy
| CVF Cytokines | First Trimester | |||||||
| N | 273 | 273 | 273 | 273 | 273 | 273 | 273 | 185 |
| IL-1α | IL-1β | IL-6 | IL-8 | IL-10 | TNFα | CRP | MMP-8 | |
| IL-1α | 1 | .61** | .13* | .63** | .20** | .35** | .39** | .45** |
| IL-1β | 1 | .29** | .73** | .37** | .64** | .55** | .54** | |
| IL-6 | 1 | .24** | .42** | .41** | .18* | .20* | ||
| IL-8 | 1 | .33** | .46** | .42** | .71** | |||
| IL-10 | 1 | .45** | .20** | .31** | ||||
| TNFα | 1 | .46** | .28** | |||||
| CRP | 1 | .33** | ||||||
| MMP-8 | 1 | |||||||
| Second Trimester | ||||||||
| N | 229 | 229 | 229 | 229 | 229 | 229 | 229 | 156 |
| IL-1α | 1 | .63** | .16* | .67** | .45** | .28** | .43** | .61** |
| IL-1β | 1 | .41** | .74** | .52** | .58** | .63** | .64** | |
| IL-6 | 1 | .46** | .51** | .54** | .36** | .34** | ||
| IL-8 | 1 | .51** | .48** | .51** | .78** | |||
| IL-10 | 1 | .48** | .42** | .29** | ||||
| TNFα | 1 | .46** | .29** | |||||
| CRP | 1 | .54** | ||||||
| MMP-8 | 1 | |||||||
| Third Trimester | ||||||||
| N | 198 | 198 | 198 | 198 | 198 | 198 | 198 | 128 |
| IL-1α | 1 | .56** | .18* | .64** | .37** | .21* | .30** | .40** |
| IL-1β | 1 | .48** | .78** | .53** | .51** | .61** | .55** | |
| IL-6 | 1 | .45** | .42** | .52** | .38** | .31** | ||
| IL-8 | 1 | .42** | .45** | .42** | .58** | |||
| IL-10 | 1 | .39** | .30** | .30** | ||||
| TNFα | 1 | .48** | .27* | |||||
| CRP | 1 | .40** | ||||||
| MMP-8 | 1 | |||||||
Note:
P < 0.001
P < 0.05
Overall changes in cytokines levels over time
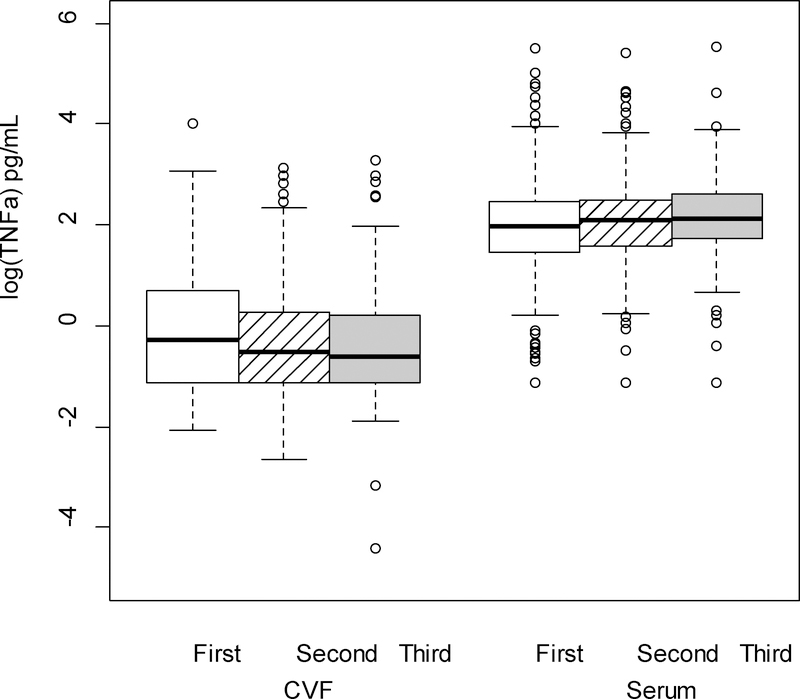
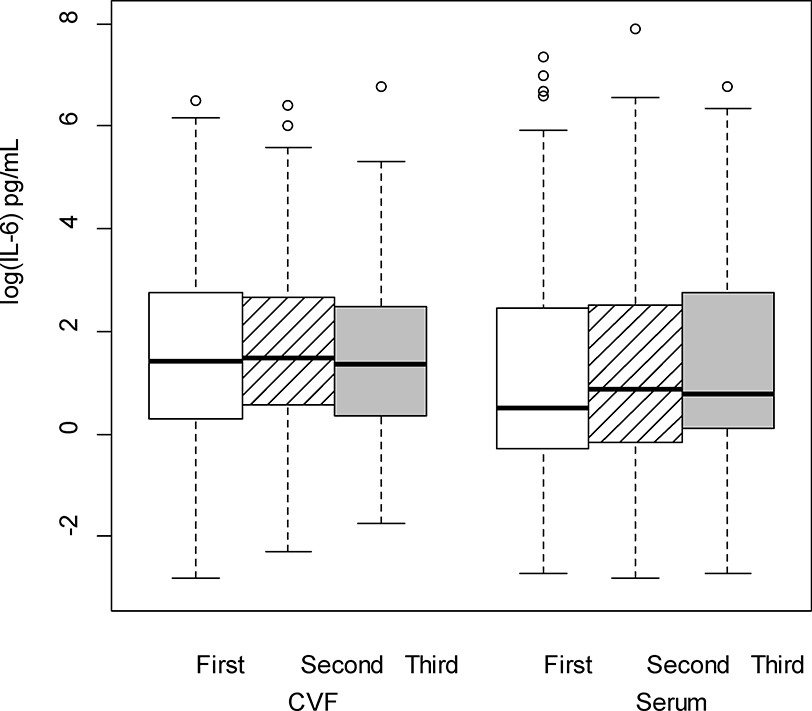
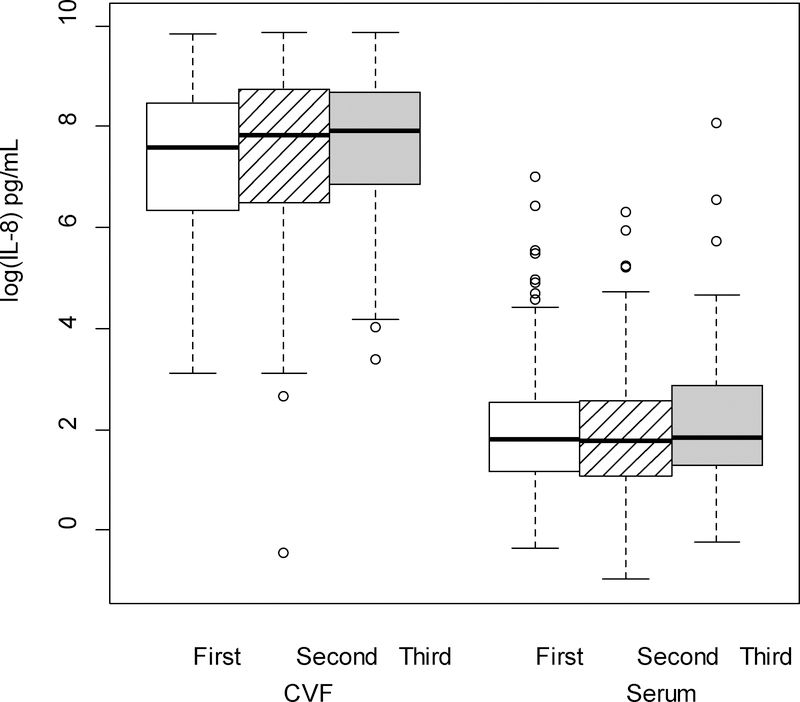
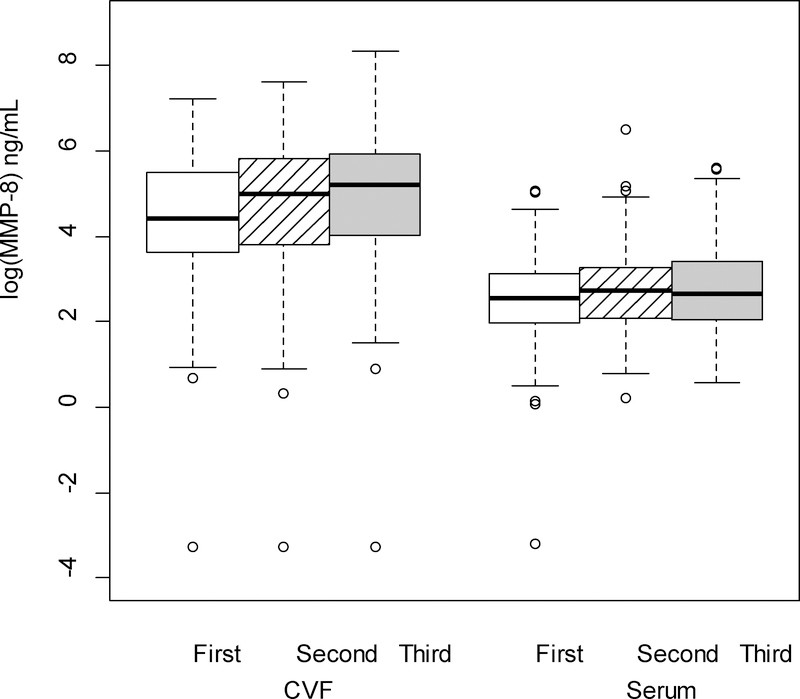
The cytokine levels, both from serum and CVF, demonstrated significant variations across pregnancy. There were no differences in the rate of change between serum and CVF cytokines across trimesters (i.e., each of the interactions between trimester and source were nonsignificant) for any cytokine except TNFα. Therefore, averaging across trimester, concentrations of CVF IL-1β, IL-6, IL-8, IL-1α and MMP-8 (P < 0.001 for all comparisons; Figures 1–5 respectively) were significantly greater than serum levels, while concentrations of serum IL-10 and CRP (P < 0.001 for all comparisons; Figures 6–7 respectively) were significantly greater than CVF levels.
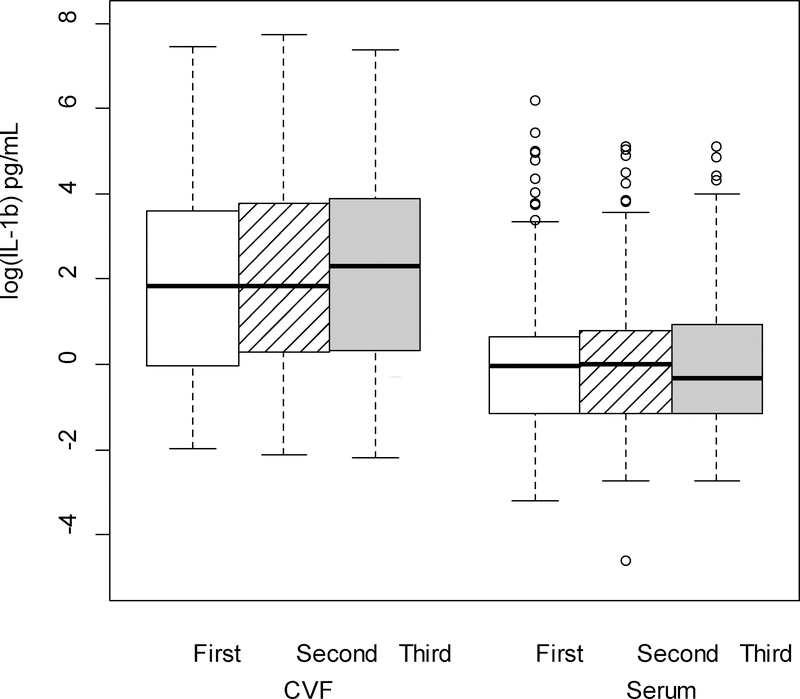
Figure 1.
IL-1β (log-transformed) levels in the cervicovaginal fluid (CVF) were significantly higher than serum levels across trimester of pregnancy (p < .001; linear mixed model F test of fixed effects).
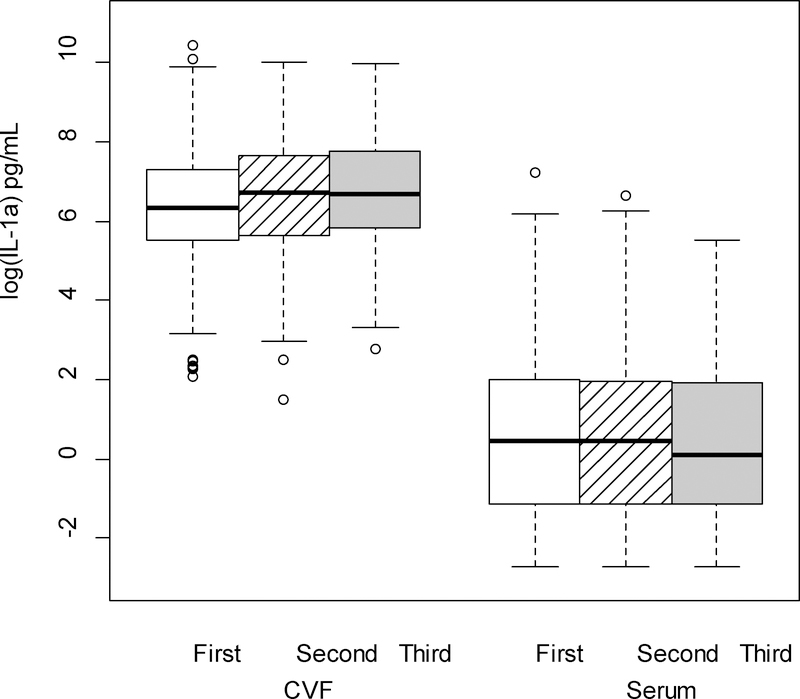
Figure 5.
IL-1α (log-transformed) levels in cervicovaginal fluid (CVF) were significantly higher than serum levels across trimester of pregnancy (p < .001; linear mixed model F test of fixed effects).
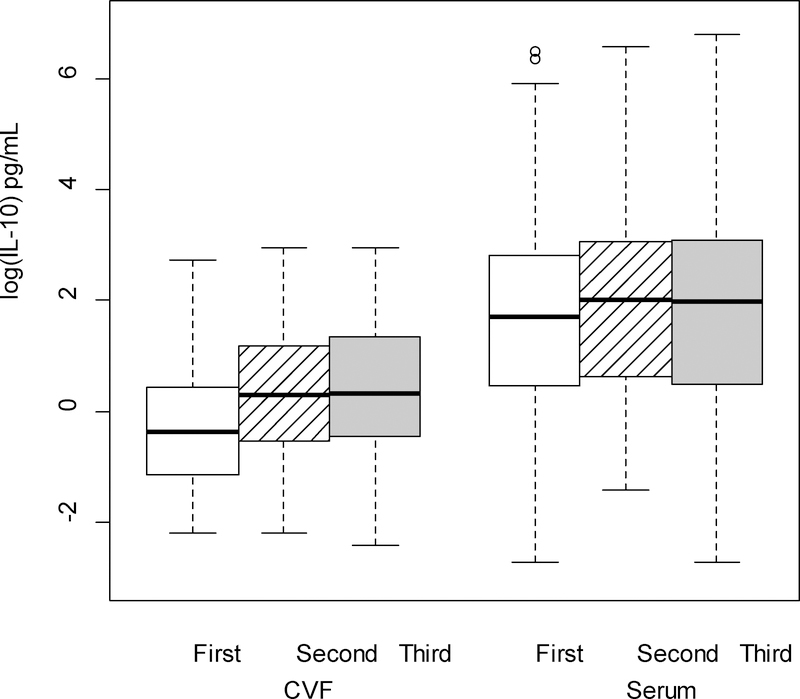
Figure 6.
IL-10 (log-transformed) levels in the serum were significantly higher than levels in the cervicovaginal fluid (CVF; p < .001 from linear mixed model F test of fixed effects), across trimester of pregnancy. Regardless source of cytokine (CVF/serum), levels were significantly lower in the first trimester compared to the second and third trimesters (p < .001 for both comparisons).
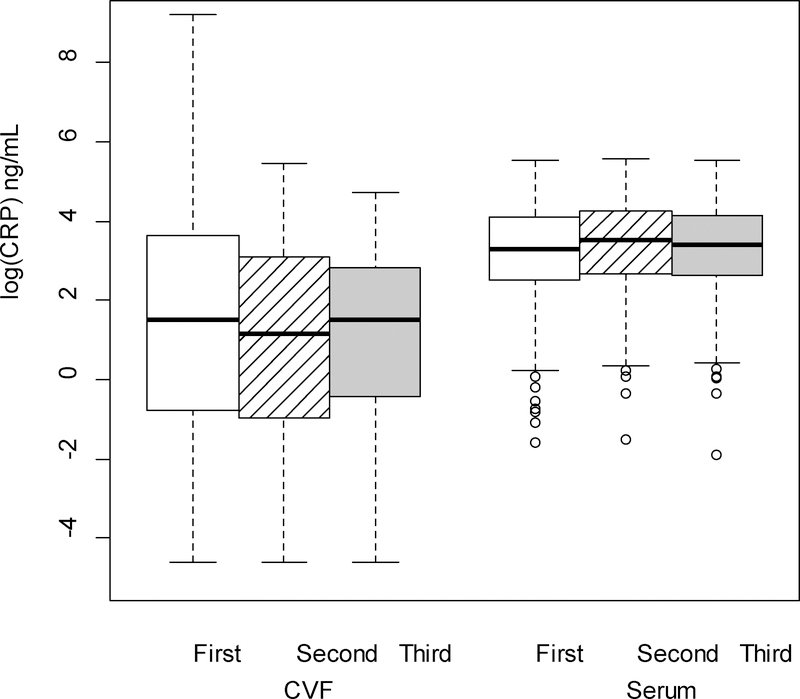
Figure 7.
CRP (log-transformed) levels in the serum were significantly higher than levels in the cervicovaginal fluid (CVF; p < .001 from linear mixed model F test of fixed effects), across trimester of pregnancy.
Regardless of source (CVF or serum), concentrations significantly varied across trimester for IL-8, MMP-8 and IL-10. There was no change in IL-8 from first to second trimester, but there were significant increases in concentrations from first to third (P = 0.006) and second to third (P = 0.03) trimesters. MMP-8 levels significantly increased from first to third (P = 0.013) trimester; however the changes from first to second and second to third trimesters were not significant. Concentrations for IL-10 increased significantly from first to second (P < 0.001) and first to third trimesters (P < 0.001); the change from second to third trimester was not significant. Concentrations did not significantly vary across trimester for IL-6, IL-1α, IL-1β or CRP.
The interaction between cytokine and trimester was significant for TNFα (P < 0.001; Figure 8). CVF concentrations were significantly lower at each trimester compared to serum (P <0.001 for all three comparisons). In comparing changes in CVF TNFα concentrations over time, there was a significant decrease from first to second (P = 0.048) and first to third (P = 0.002) trimesters, although, levels did not differ between second and third trimester. Among serum TNFα concentrations, there was a significant increase from first to third (P = 0.004) trimester, but the changes from first to second and second to third trimesters were not significant.
Figure 8.
TNF-α (log-transformed) levels varied significantly across pregnancy between serum and cervicovaginal fluid (CVF) specimens (p < .001 from F test of fixed effects for the interaction). In the post-hoc analysis, within the CVF, first trimester levels were significantly higher than second and third trimester levels (p =.048 and p = .002, respectively). Within the serum, first trimester levels were significantly lower than third trimester levels (p = .004). At each trimester, concentrations of TNF-α were significantly lower in the CVF compared to serum specimens (p <.001 for all comparisons).
DISCUSSION
Our data confirm pro-inflammatory and anti-inflammatory cytokine concentrations vary in distribution across pregnancy, although, changes in cytokine levels across gestation have not been well delineated previously. In this study, we analyzed cytokine concentrations in serum and CVF. IL-1β, IL-6, IL-8, and MMP-8 concentrations were significantly higher in CVF than serum, while IL-1α, IL-10, and CRP were significantly higher in serum. The differences between serum and CVF concentrations of TNFα varied by trimester. Of note, IL-10, an important anti-inflammatory cytokine for immune modulation increased significantly from early to late pregnancy, which is consistent with a report by Coussons-Read et al.23 In our data, TNFα also significantly increased in serum from the first to the third trimester of pregnancy consistent with previous reports.31,32 Finally, our data are supported by a report by Larsson et al who noted that serum CRP concentrations increase consistently throughout pregnancy.33
Cervicovaginal specimens may provide additional and more selective alterations in the immune response within the reproductive tract. Hence, we also aimed to describe variation in cytokine distribution at this mucosal surface. Interestingly, in contrast to serum, CVF CRP concentration decreased in middle as compared to early and late pregnancy samples. Potentially, the increasing levels of MMP-8 in CVF from early to late pregnancy may reflect its biological function as a collagen-cleaving enzyme in preparation for labor, though does not appear to be involved during the labor process.34 IL-6 has been identified as a main cytokine associated with adverse perinatal health outcomes.35–38 In this study, significantly higher levels of IL-6 in CVF versus serum suggest cervicovaginal specimens may allow better discrimination between normal and pathologic conditions. IL-6 concentrations did not vary significantly across the pregnancy timeline in this study. Similar to Heng et al. (2014), there were no significant changes in CVF IL-1α in second and third trimester samples.28
This research is one of the first projects to compare trimester-specific immune markers in two biological fluids. One strength of this study is the longitudinal design, which assessed women with singleton gestation at each trimester. A weakness is the attrition observed throughout the trimesters and lack of control for exposures such as antibiotics. In addition, varied collection times within the trimesters (limited to a 6-week window per protocol) may have influenced the variation for determination of the interpersonal gestational immunological changes.
Despite these concerns, our findings provide a framework for examining patterns of changes in cytokines over the time course of pregnancy. Further research to measure the range of maternal and fetal immune responsiveness during gestation is warranted, particularly in response to pathophysiologies occurring early in pregnancy.
Figure 2.
IL-6 (log-transformed) levels in cervicovaginal fluid (CVF) were significantly higher than serum levels across trimester of pregnancy (p < .001; linear mixed model F test of fixed effects).
Figure 3.
IL-8 (log-transformed) levels in the cervicovaginal fluid (CVF) were significantly higher than serum levels across trimester of pregnancy (p < .001; linear mixed model F test of fixed effects). Regardless source of cytokine (CVF/serum), levels were significantly lower in the first trimester (p=.006) and second trimester (p=.03) compared to the third trimester.
Figure 4.
MMP-8 (log-transformed) levels in the cervicovaginal fluid (CVF) were significantly higher than levels in the serum (p < .001 from linear mixed model F test of fixed effects) across trimester of pregnancy. Regardless source of cytokine (CVF/serum), levels were significantly higher in the third trimester compared to the first trimester (p = .013 from F test of fixed effects).
ACKNOWLEDGEMENTS
Financial support for the research
Financial support for this research was provided in part by National Institutes for Health Building Interdisciplinary Research Careers in Women’s Health (BIRCWH: K12DA14040) to K.B.A; Center for Biomedical Research Excellence (COBRE: 5P20GM103538) to J.L.E. and University of Kentucky Clinical and Translational Research Center KL2RR033171 to J.E. The project described was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant number UL1TR000117. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Findings were presented at the 36th Annual Meeting of the Society for Maternal Fetal Medicine: The pregnancy Meeting, Atlanta, Georgia, February 1st - 6th, 2016.
We acknowledge the expert support of Wendy F Hansen MD, and Jason Stevens (Research Analyst). Dr. Hansen is a Professor and John W Greene Chair in the Department of Obstetrics and Gynecology of the University of Kentucky College of Medicine. Mr. Stevens is the principal research analyst for the University of Kentucky (UK) Center for Oral Health Research. We are also grateful to the medical and nursing faculty and staff at the UK prenatal clinics, and all of the women who participated in study for making this research possible.
Contributor Information
Kristin B Ashford, College of Nursing, 417 Nursing Building University of Kentucky, 751 Rose Street, Lexington; KY 40536-0200.
Niraj Chavan, Obstetrics & Gynecology, Division of Maternal-Fetal Medicine, University of Kentucky.
Jeffrey L. Ebersole, Center for Oral Health Research, University of Kentucky.
Amanda T. Wiggins, College of Nursing, University of Kentucky.
Savita Sharma, College of Nursing, University of Kentucky.
Ms. Andrea McCubbin, College of Nursing, University of Kentucky.
Ms. Janine Barnett, College of Nursing, University of Kentucky.
John O’Brien, Obstetrics & Gynecology, Division of Maternal-Fetal Medicine, University of Kentucky.
REFERENCES
- 1.Sykes, A.MacIntyre D, Yap XJ, et al. Changes in the Th1 : Th2 Cytokine Bias in Pregnancy and the Effects of the Anti-Inflammatory Cyclopentenone Prostaglandin 15-Deoxy-Δ12,14-Prostaglandin J2. Mediators of Inflammation 2012;2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mor G, Cardenas I. The immune system in pregnancy: a unique complexity. American journal of reproductive immunology 2010;63(6):425–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Romero R, Espinoza J, Gonçalves LF, et al. The role of inflammation and infection in preterm birth In, Seminars in reproductive medicine: Copyright© 2007 by Thieme Publishers, Inc., 333 Seventh Avenue, New York, NY 10001, USA; 2007:021–039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Romero R, Espinoza J, Gonçalves LF, et al. Inflammation in preterm and term labour and delivery In, Seminars in Fetal and Neonatal Medicine: Elsevier; 2006:317–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Romero R, Espinoza J, Kusanovic JP, et al. The preterm parturition syndrome. BJOG: An International Journal of Obstetrics & Gynaecology 2006;113(s3):17–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paulesu L, Bhattacharjee J, Bechi N, et al. Pro-inflammatory cytokines in animal and human gestation. Current pharmaceutical design 2010;16(32):3601–3615 [DOI] [PubMed] [Google Scholar]
- 7.Moorman NJ, Cristea IM, Terhune SS, et al. Human cytomegalovirus protein UL38 inhibits host cell stress responses by antagonizing the tuberous sclerosis protein complex. Cell host & microbe 2008;3(4):253–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Graham C, Chooniedass R, Stefura WP, et al. In vivo immune signatures of healthy human pregnancy: Inherently inflammatory or anti-inflammatory? PloS one 2017;12(6):e0177813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belo L, Santos-Silva A, Rocha S, et al. Fluctuations in C-reactive protein concentration and neutrophil activation during normal human pregnancy. European journal of obstetrics, gynecology, and reproductive biology 2005;123(1):46–51 [DOI] [PubMed] [Google Scholar]
- 10.Curry AE, Vogel I, Skogstrand K, et al. Maternal plasma cytokines in early- and mid-gestation of normal human pregnancy and their association with maternal factors. Journal of reproductive immunology 2008;77(2):152–160 [DOI] [PubMed] [Google Scholar]
- 11.Sharma A, Satyam A, Sharma JB. Leptin, IL-10 and inflammatory markers (TNF-alpha, IL-6 and IL-8) in pre-eclamptic, normotensive pregnant and healthy non-pregnant women. American journal of reproductive immunology 2007;58(1):21–30 [DOI] [PubMed] [Google Scholar]
- 12.Kraus TA, Sperling RS, Engel SM, et al. Peripheral blood cytokine profiling during pregnancy and post-partum periods. American journal of reproductive immunology 2010;64(6):411–426 [DOI] [PubMed] [Google Scholar]
- 13.Chatterjee P, Chiasson VL, Bounds KR, Mitchell BM. Regulation of the anti-inflammatory cytokines interleukin-4 and interleukin-10 during pregnancy. Frontiers in immunology 2014;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barrera D, Díaz L, Noyola-Martínez N, Halhali A. Vitamin D and inflammatory cytokines in healthy and preeclamptic pregnancies. Nutrients 2015;7(8):6465–6490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mosmann TR, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunology today 1996;17(3):138–146 [DOI] [PubMed] [Google Scholar]
- 16.O’Garra A, Arai N. The molecular basis of T helper 1 and T helper 2 cell differentiation. Trends in cell biology 2000;10(12):542–550 [DOI] [PubMed] [Google Scholar]
- 17.Wegmann TG, Lin H, Guilbert L, Mosmann TR. Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a TH2 phenomenon? Immunology today 1993;14(7):353–356 [DOI] [PubMed] [Google Scholar]
- 18.Sykes L, MacIntyre DA, Yap XJ, et al. Changes in the Th1: Th2 Cytokine Bias in Pregnancy and the Effects of the Anti-Inflammatory Cyclopentenone Prostaglandin 15-Deoxy--Prostaglandin. Mediators of inflammation 2012;2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Romero R, Gotsch F, Pineles B, Kusanovic JP. Inflammation in pregnancy: its roles in reproductive physiology, obstetrical complications, and fetal injury. Nutr Rev 2007;65(12 Pt 2):S194–202 [DOI] [PubMed] [Google Scholar]
- 20.Robinson DP, Klein SL. Pregnancy and pregnancy-associated hormones alter immune responses and disease pathogenesis. Hormones and behavior 2012;62(3):263–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.RJ Ruiz NJ, Murphey C, Marti CN, Godbold E, and Pickler RH. Second trimester maternal plasma levels of cytokines IL-1Ra, Il-6 and IL-10 and preterm birth. J Perinatol 2012;32(7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoram Sorokin MD, Romero Roberto, Mele Lisa, Wapner Ronald J., Iams Jay, D., Dudley Donald J., Spong Catherine Y., Peaceman Alan M.,, Leveno Kenneth J., Harper Margaret, Caritis Steve N., Menachem, et al. Maternal serum interleukin-6, c-reactive protein, and matrix metalloproteinase-9 concentrations as risk factors for preterm birth < 32 weeks and adverse neonatal outcomes. Am J Perinatol 2010;27(8):631–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paternoster DM, Stella A, Gerace P, et al. Biochemical markers for the prediction of spontaneous pre-term birth. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics 2002;79(2):123–129 [DOI] [PubMed] [Google Scholar]
- 24.Chan RL. Biochemical markers of spontaneous preterm birth in asymptomatic women. BioMed research international 2014;2014:164081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abdelazim IA. Relation between interleukin-6 in the cervicovaginal fluid and subclinical chorioamnionitis in patients with preterm premature rupture of membranes. Asian Pacific Journal of Reproduction 2013;2(1):38–41 [Google Scholar]
- 26.Georgiou HM, Thio YS, Russell C, et al. Association between maternal serum cytokine profiles at 7–10 weeks’ gestation and birthweight in small for gestational age infants. American journal of obstetrics and gynecology 2011;204(5):415. e411–415. e412 [DOI] [PubMed] [Google Scholar]
- 27.Heng YJ, Liong S, Permezel M, et al. Human cervicovaginal fluid biomarkers to predict term and preterm labor. Frontiers in physiology 2015;6:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heng YJ, Liong S, Permezel M, et al. The interplay of the interleukin 1 system in pregnancy and labor. Reproductive Sciences 2014;21(1):122–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Menon R, Fortunato SJ. Infection and the role of inflammation in preterm premature rupture of the membranes. Best practice & research Clinical obstetrics & gynaecology 2007;21(3):467–478 [DOI] [PubMed] [Google Scholar]
- 30.Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science 2014;345(6198):760–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coussons-Read ME, Okun ML, Nettles CD. Psychosocial stress increases inflammatory markers and alters cytokine production across pregnancy. Brain, behavior, and immunity 2007;21(3):343–350 [DOI] [PubMed] [Google Scholar]
- 32.Winkler G, Cseh K, Baranyi E, et al. Tumor necrosis factor system in insulin resistance in gestational diabetes. Diabetes research and clinical practice 2002;56(2):93–99 [DOI] [PubMed] [Google Scholar]
- 33.Larsson A, Palm M, Hansson LO, Basu S, Axelsson O. Reference values for alpha1-acid glycoprotein, alpha1-antitrypsin, albumin, haptoglobin, C-reactive protein, IgA, IgG and IgM during pregnancy. Acta obstetricia et gynecologica Scandinavica 2008;87(10):1084–1088 [DOI] [PubMed] [Google Scholar]
- 34.Heng YJ, Quinzio MKD, Liong S, et al. Temporal investigation of matrix metalloproteinases and their inhibitors in human cervicovaginal fluid in late pregnancy and labor. Reproductive Sciences 2012;19(1):55–63 [DOI] [PubMed] [Google Scholar]
- 35.Coussons-Read MELM, Carey JC, Kreither MO, D’Anna K, Argys L, et al. The occurrence of preterm delivery is linked to pregnancy-specific distress and elevated inflammatory markers across gestation. Brain Behavior and Immunity 2012;26:650–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lockwood CJ, Ghidini A, Wein R, et al. Increased interleukin-6 concentrations in cervical secretions are associated with preterm delivery. American journal of obstetrics and gynecology 1994;171(4):1097–1102 [DOI] [PubMed] [Google Scholar]
- 37.Holst RM, Mattsby-Baltzer I, Wennerholm UB, Hagberg H, Jacobsson B. Interleukin-6 and interleukin-8 in cervical fluid in a population of Swedish women in preterm labor: relationship to microbial invasion of the amniotic fluid, intra-amniotic inflammation, and preterm delivery. Acta obstetricia et gynecologica Scandinavica 2005;84(6):551–557 [DOI] [PubMed] [Google Scholar]
- 38.Hatzidaki E, Gourgiotis D, Manoura A, et al. Interleukin-6 in preterm premature rupture of membranes as an indicator of neonatal outcome. Acta obstetricia et gynecologica Scandinavica 2005;84(7):632–638 [DOI] [PubMed] [Google Scholar]