Abstract
Purpose
Clinical studies suggest that 25-hydroxyvitamin D (25[OH]D) deficiency plays a pivotal role in both type 2 diabetes mellitus (T2DM) and cognitive impairment. However, it is unclear if 25(OH)D deficiency could be a possible cause of cognitive impairment in T2DM. Vitamin-D binding protein (VDBP) acts as a major 25(OH)D transporter. Preclinical study has demonstrated improvement in cognitive function by VDBP via inhibiting synaptic degeneration. The aim of the study was to assess the association between serum 25(OH)D, VDBP and cognitive impairment in T2DM patients.
Patients and methods
In this case-control study, cognitive function was assessed using the Mini-Mental State Examination (MMSE) and serum 25(OH)D and VDBP levels were estimated using ELISA kits.
Results
A total of 88 subjects were included in the study. T2DM patients had lower serum 25(OH)D (p=0.02), VDBP levels (p=0.04) and MMSE scores (p<0.0001) than controls. T2DM patients had higher prevalence of 25(OH)D deficiency and insufficiency, aOR 0.322 (0.128–0.809), p=0.016 and cognitive impairment, aOR 4.405 (1.617–12.002); p=0.004. Cognitive impairment was associated with serum 25(OH)D, aOR 0.131 (0.027–0.638); p=0.014 and VDBP, aOR 1.008 (1.001–1.015), p=0.029. A general linear model showed a significant association of MMSE with serum 25(OH)D (p=0.022).
Conclusion
Serum 25(OH)D deficiency and cognitive impairment was higher in T2DM patients. Routine assessment of cognitive function is suggested to prevent further behavioral complications. The association of VDBP and cognitive impairment in T2DM needs further exploration.
Keywords: hypovitaminosis D, Mini-Mental State Examination, vitamin-D insufficiency, cognitive dysfunction
Introduction
Diabetes is a common and complex metabolic disease that can lead to end-organ damage in almost all vital organs, including the brain.1 As shown by the International Diabetes Federation, 451 million adults had diabetes in 2017, which is estimated to increase to 693 million by 2045.2 A growing group of evidence suggests that type 2 diabetes mellitus (T2DM) is associated with lower levels of cognitive function and may be a risk factor for the development of mild cognitive impairment (MCI).3,4
Vitamin-D is an endogenously produced hormone which regulates calcium levels in the body and maintains bone mineral density.5 Recent reports suggest the association of vitamin-D with cardiovascular diseases, cancer,6,7 multiple sclerosis, hypertension7 and diabetes.6,7 Additionally, vitamin-D has also been reported to affect glucose homeostasis6 and metabolism.8 Vitamin-D levels are most commonly measured by serum 25-hydroxyvitamin D (25[OH]D) concentration.5 Evidence suggests that 25(OH)D deficiency may play a role in cognitive impairment in adults.3 Cross-sectional studies conducted amongst the geriatric population have revealed significant association between 25(OH)D deficiency and cognitive dysfunction.5,9,10 In current studies 25(OH)D deficiency has been found to be associated with cognitive impairment. However, the involvement of 25(OH)D deficiency in T2DM and cognitive impairment needs to be explored.3
Vitamin-D binding protein (VDBP), also known as group-specific component acts as a major 25(OH)D transporter.11,12 Even after ligand binding, 98–99% VDBP binding sites remain unoccupied, which suggests a function beyond 25(OH)D transport.12 Reports have suggested that VDBP has been shown to scavenge actin.11,12 A retrospective, cross-sectional study revealed that serum VDBP levels are decreased in those with type 1 diabetes.12 Additionally, it has been reported in a preclinical study that VDBP improves cognitive function by inhibiting synaptic degeneration. Although, VDBP has been found to be associated with AD and MCI,13–16 association of VDBP and cognitive function in T2DM patients has not yet been reported.
In view of the above, the present case-control study was conducted to assess cognitive function in T2DM patients. Additionally, another purpose of the present study was to compare 25(OH)D and VDBP levels of T2DM patients with that of healthy controls. Further, the association between serum 25(OH)D, VDBP levels, and cognitive function was also assessed.
Materials and methods
Subjects
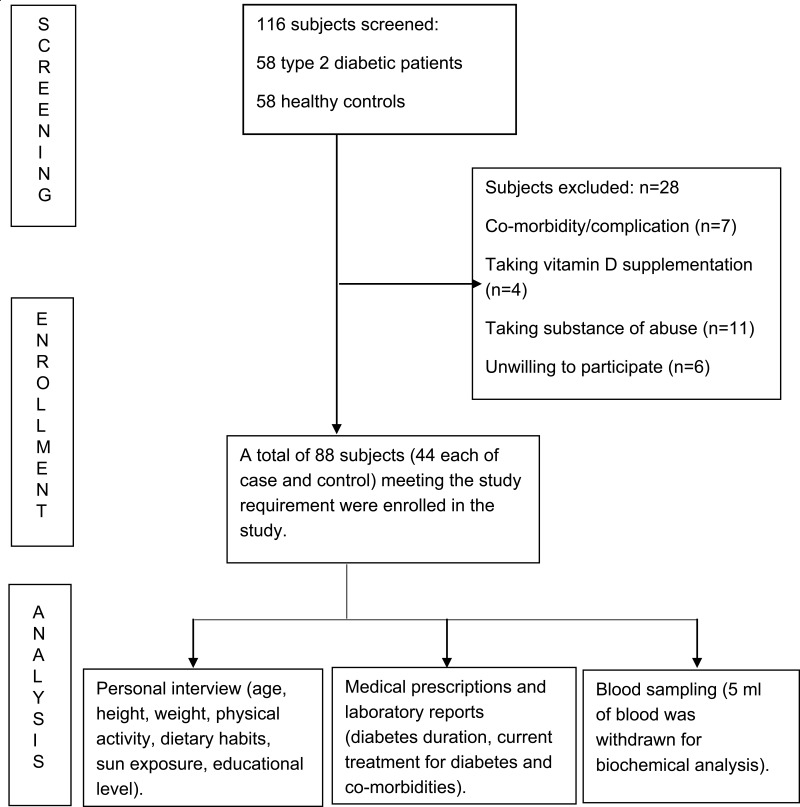
This was a case-control study that recruited T2DM patients and healthy controls. Men and women aged ≥19–≤65 years, willing to give written informed consent were included. Patients diagnosed with T2DM were included as cases. Healthy subjects were included as controls. Patients with T1DM, history of severe psychiatric disorders, taking any substance of abuse, on psychotropic drug, complications of diabetes (hypertension, amputation, blindness, renal insufficiency and dialysis), liver disease, renal disease, primary hyperparathyroidism, cancer, HIV and obesity, taking vitamin-D supplement, women who were pregnant or taking oral contraceptive pills, and unwillingness to give written informed consent were excluded. Healthy subjects taking substance of abuse, vitamin-D supplements, obese and unwilling to give written informed consent were excluded. Overall, 116 consecutive subjects visiting Diabetic clinics and Medicine OPDs were approached for participation. Cases and controls were matched by their demographic characteristics. A total of 44 T2DM patients and 44 controls aged ≥19–≤65 years, age- and sex- matched were included in the study. The study was approved by the Jamia Hamdard Institutional Ethics Committee. Written informed consent was obtained from the participants.
Clinical data
A standard format was used for the documentation of demographic and clinical data of the subjects. The recorded data included age, height, weight, history of alcohol or tobacco consumption, physical activity, dietary habits, sun exposure, educational level, diabetes duration, current treatment for diabetes and associated co-morbidities. Body mass index (BMI) was calculated using the measured weight and height for each subject. Fasting plasma glucose and HbA1c were also recorded for cases. Available medical prescriptions and laboratory reports of the healthy subjects obtained through their health checkup were accessed to confirm their eligibility for enrollment (Figure 1).
Figure 1.
Flow diagram of patient flow in the study.
Serum 25(OH)D and VDBP analysis
A fasting 5-mL blood sample was collected from the subjects. Samples were then centrifuged at 3000 rpm for 20 minutes to separate serum. Supernatant serum was divided into aliquots and stored aseptically at −80°C until analysis. Serum 25(OH)D and VDBP levels were quantified for each 40 μL of serum sample using a highly sensitive ELISA kit (SHANGHAI YEHUA Biological Technology Co., Ltd., Shanghai, People's Republic of China), which measures free 25(OH)D. Serum 25(OH)D deficiency and 25(OH)D insufficiency have been defined as serum 25(OH)D level <20 ng/mL and <30 ng/mL, respectively.17
Cognitive assessment
Cognitive function was assessed using the Mini-Mental State Exam (MMSE) or Hindi Mental State Examination. Cognitive assessment was performed on the day of sample collection of the patient. The MMSE is a fully structured scale that consists of 30 points grouped into seven categories: orientation to place, orientation to time, registration, attention and concentration, recall, language, and visual construction. The MMSE score ranges from 0 to 30, with higher scores indicating better performance.18 Scores from 24–30 indicate no cognitive impairment, 18–23 indicate mild cognitive impairment, and 0–17 indicate severe cognitive impairment.19
Statistical analysis
Data contained both continuous and categorical variables. Therefore, quantitative variables are expressed as mean ± standard deviation (SD). Normality of the continuous variables were tested by the Kolmogorov-Smirnov and Shapiro-Wilk tests. Association between two continuous variables were assessed by the Student’s t-test or Mann–Whitney U test. χ2 and Fisher exact tests were used to compare differences in the frequencies of categorical variables. The variables which were found to have a p-value<0.2 were then included in a binary logistic regression model to check for further association. The odds ratio for insignificant results is not shown in the results. A Generalised Logistic Model was used to represent the relation between exposure and outcome variables. For all statistical tests a two-sided p-value<0.05 was considered as the level of significance. All statistical analyses were performed using IBM SPSS (version 22.0, IBM Corporation, Armonk, NY, USA) software.
Based on previous literature in the Indian population,4 we calculated sample size using the formula, n=(Zα/2+Zβ)2 * 2*σ2/d2, with 90% power and 5% type one error-rate. Where, σ2=population variance; d=difference between the means; Zβ=power of the study=90%; and Zα/2=type one error=5%. An estimated 56 individuals were required in the study. We further assumed maximum 25% non-response rate. So, we increased our sample size by 25%. Thus, a minimum of 35 subjects were estimated to need recruiting into each group.
Ethics
The study was conducted in agreement with the Declaration of Helsinki and approved by Jamia Hamdard Institutional Ethics Committee.
Results
Baseline characteristics
A total of 88 subjects were included. The study comprised two groups: cases (patients diagnosed with T2DM) and controls (healthy individuals). Thus, 44 subjects in each group were included, of which 39 (44.32%) were females and 49 (55.68%) were males. The socio-demographic characteristics of study participants are shown in Table 1.
Table 1.
Demographic data of cases and controls
| Characteristic | Cases (n=44) | Control (n=44) |
|---|---|---|
| Age (years) | 45.70±7.53 | 44.91±6.05 |
| Sex | ||
| Female | 21 (47.7) | 18 (40.9) |
| Male | 23 (52.3) | 26 (59.1) |
| BMI (kg/m2) | 26.1±2.51 | 25.33±2.91 |
| Education | ||
| Educated | 33 (75) | 38 (86.4) |
| Uneducated | 11 (25) | 6 (13.6) |
| Employment | ||
| Employed | 26 (59.1) | 30 (68.2) |
| Unemployed | 18 (40.9) | 14 (31.8) |
| Exercise (hours/day) | 0.4±0.65 | 0.28±0.41 |
| Diet | ||
| Vegetarian | 9 (20.5) | 6 (13.6) |
| Non-vegetarian | 35 (79.5) | 38 (86.4) |
| Sun exposure (hours/day) | 0.97±1.65 | 0.57±0.759 |
| FPG (mg/dL) | 193.48±84.04 | 97.53±8.68** |
| Duration of diabetes (years) | 5.42±5.36 | |
| HbA1c (%) | 9.05±2.47 | |
| Diabetes treatment | ||
| Oral hypoglycemic | 36 (81.81) | |
| Insulin | 8 (18.18) |
Notes: **p<0.0001. Data presented as mean ± SD or n (%)
Abbreviations: n, number; BMI, body mass index; FPG, fasting plasma glucose; HbA1c, glycated hemoglobin.
Assessment of cognitive function
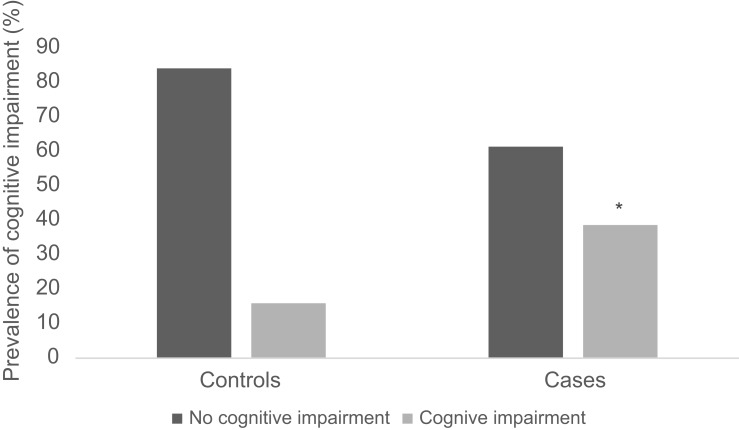
The mean MMSE score for cases (23.73±3.32) was significantly lower as compared to controls (28.32±2.64), p<0.0001. Additionally, the prevalence of cognitive impairment was significantly higher in cases, 17 (38.64%) than in controls, 7 (15.91%) (Figure 2), aOR 4.405 (1.617–12.002); p=0.004 (Table 2). The risk associated with the prevalence of cognitive impairment was 3.328 (1.121–9.141) among cases than in controls. Cognitive function was found to be associated with education (aOR 0.123, 95% CI 0.023–0.667; p<0.015). No association was found with duration of disease, FPG and HbA1c, in cases. Association of various parameters with MMSE scores is represented in Table 3.
Figure 2.
Prevalence of cognitive impairment in controls and cases.
Note: *p<0.05.
Table 2.
Association of variables among cases and controls
| Variable | Cases (n=44) | Controls (n=44) | Crude OR | p-value | Adjusted OR* | p-value |
|---|---|---|---|---|---|---|
| Vitamin D deficiency | 34 (77.3) | 23 (52.3) | 0.400 [0.144–1.112] | 0.079 | 0.322 [0.128–0.809] | 0.016 |
| Vitamin D sufficiency | 10 (22.7) | 21 (47.7) | 1 | 1 | ||
| Cognitive impairment | 17 (38.6) | 7 (15.9) | 5.810 [1.408–23.904] | 0.015 | 4.405 [1.617–12.002] | 0.004 |
| No cognitive impairment | 27 (61.4) | 37 (84.1) | 1 | 1 | ||
| VDBP | 1.003 [0.999–1.007] | 0.350 | 1.003 [0.999–1.007] | 0.051 |
Note: Data presented as n (%). *Adjusted for age, gender, employment status, education, HbA1c, sun-exposure, exercise, diet.
Abbreviations: n, number; VDBP, vitamin-D binding protein.
Table 3.
Association of various parameters with MMSE scores
| Parameter | Cognitive impairment (n=27) | No cognitive impairment (n=61) | Crude OR | p-value | Adjusted OR | p-value |
|---|---|---|---|---|---|---|
| Age | NA | NA | 1.021 [0.925–1.126] | 0.685 | NA | NA |
| Male | 12 (24.5) | 37 (75.5) | 1 | NA | NA | |
| Female | 15 (38.5) | 24 (31.5) | 0.501 [0.017–14.350] | 0.686 | ||
| BMI | NA | NA | 0.843 [0.619–1.150] | 0.281 | NA | NA |
| Educated | 18 (25.4) | 53 (74.6) | 1 | 1 | ||
| Uneducated | 9 (52.9) | 8 (47.1) | 0.113 [0.015–0.832] | 0.032 | 0.123 [0.023–0.667] | 0.015 |
| Employed | 14 (25) | 42 (75) | 1 | NA | NA | |
| Unemployed | 13 (40.6) | 19 (59.4) | 1.344 [0.455–3.968] | 0.593 | ||
| Exercise | NA | NA | 1.201 [0.255–5.669] | 0.817 | NA | NA |
| Sun exposure | NA | NA | 0.996 [0.566–1.752] | 0.988 | NA | NA |
| T2DM cases | 17 (38.6) | 27 (61.4) | 3.625 [0.710–18.499] | 0.121 | 1.008 [1.000–1.016] | 0.044 |
| Controls | 7 (15.9) | 37 (84.1) | 1 | 1 | ||
| Vitamin D deficiency | 22 (91.7) | 35 (54.7) | 0.135[0.027–0.669] | 0.014 | 0.131 [0.027–0.638] | 0.014 |
| Vitamin D sufficiency | 2 (8.3) | 29 (45.3) | 1 | 1 | ||
| VDBP | NA | NA | 1.021 [0.925–1.126] | 0.035 | 1.008 [1.001–1.015] | 0.029 |
Note: Data presented as n (%).
Abbreviations: n, number; NA, not applicable; BMI, body mass index; T2DM, type 2 diabetes mellitus; VDBP, vitamin-D binding protein.
Assessment of serum 25(OH)D levels
Serum 25(OH)D levels were significantly lower in cases (23.09±13.06 ng/mL) compared to the healthy controls (32.12±21.18 ng/mL), p=0.02. The prevalence of 25(OH)D insufficiency was significantly higher in cases (34 [77.3%]) than in controls (23 [52.3%]), aOR 0.322 (0.128–0.809), p=0.016 (Table 2). Serum 25(OH)D levels were lower in women (21.49±11.28) than in men (24.56±14.60), although the difference did not reach statistical significance (p=0.27). Serum 25(OH)D inversely correlated with age (OR 0.871, 95% CI 0.77–0.99; p=0.04).
Assessment of VDBP levels
Serum VDBP level was significantly lower in cases (236.35±117.12) as compared to controls (282.30±129.85), p=0.04. Serum VDBP levels were found to be significantly associated with BMI (r=0.409; p=0.006), in cases. No association was found between VDBP levels and other clinical parameters.
Association of serum 25(OH)D level and cognitive function
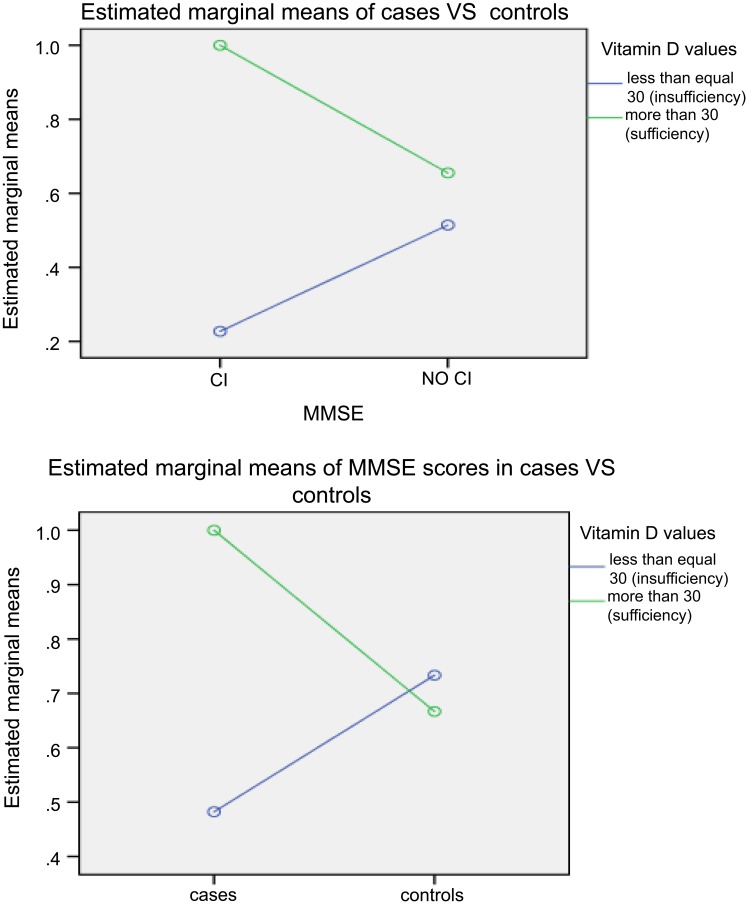
A positive association was found between serum 25(OH)D levels and MMSE scores in cases, p=0.022 (Figure 3). Serum 25(OH)D was significantly associated with cognitive impairment, aOR 0.131(0.027–0.638); p=0.014 (Table 3).
Figure 3.
Generalised linear model of mini-mental state examination scores and vitamin D.
Association of VDBP levels and cognitive function
VDBP levels were significantly associated with MMSE scores in cases, aOR 1.012 (1.003–1.022), p=0.01. Additionally, VDBP levels were significantly lower in cases with cognitive impairment (176.38±64.34) than in cases without cognitive impairment (274.10±127.70), p=0.01.
Discussion
The diverse effects of vitamin-D on calcium homeostasis and glucose levels have evoked an interest in investigating its role in T2DM patients.20 Several clinical reports have demonstrated the association of 25(OH)D deficiency and T2DM, paradoxically, some reports contradict the same. Interestingly, supplementation with vitamin-D has been reported to improve homeostasis, insulin resistance, and glucose levels. Thus, we investigated serum 25(OH)D levels in our study in order to unveil the exact association with T2DM.6 In the present study, we observed that serum 25(OH)D levels were significantly lower in cases as compared to controls. Additionally, patients with diabetes had higher prevalence of 25(OH)D insufficiency as compared to healthy subjects. Our results are in consensus with similar case-control studies demonstrating lower serum 25(OH)D concentrations in cases patients compared with the controls.7,21,22 Further, a cross-sectional clinical study suggested an inverse association between the serum concentration of 25(OH)D3 and the odds of newly diagnosed T2DM, thereby showing a protective role of vitamin-D against the development of diabetes.23 Thus, the results of the present study are in agreement with previous findings and postulate that 25(OH)D deficiency may have an impact on T2DM.
Several mechanisms have been suggested regarding the association of low 25(OH)D concentrations with T2DM. The effect of 25(OH)D on diabetes may be mediated through effects on glucose homeostasis, b-cell function, and insulin secretion.7,8,23 Serum 25(OH)D may affect the pancreatic beta-cell function via binding of circulating 1,25-dihydroxyvitamin-D to the beta-cell vitamin-D receptor. Activation of 25(OH)D by 1-alpha-hydroxylase, expressed in beta cells may also be involved. Enhancement of insulin sensitivity may be mediated by stimulation of expression of insulin receptors and/or by activating peroxisome proliferator-activated receptor, a transcription factor associated with the fatty acid metabolism in skeletal muscle and adipose tissue. 25(OH)D may also affect insulin secretion and sensitivity indirectly via its role in regulating extracellular calcium concentration and flux through cell membranes in the beta cell and peripheral.4 Since 25(OH)D has anti-inflammatory7,22,24 and immunoregulatory effects, it can ameliorate low-grade chronic inflammation by modulating the generation of cytokines, associated with systemic inflammation in T2DM.7
In the present study, subjects with T2DM had lower MMSE scores than those without T2DM. Low MMSE scores were found to be associated with lower education. The MMSE scores were not associated with HbA1c levels and duration of disease. These findings are supported by similar case-control studies demonstrating lower MMSE score in patients with diabetes than in controls.4,25 Previous studies have suggested that hypovitaminosis D is related to cognitive impairment.3 Cross-sectional studies on older adults have shown a positive association between 25(OH)D deficiency and cognitive impairment.9,26,27 Another cross-sectional study on adults demonstrated that low 25(OH)D level is associated with greater risk of cognitive impairment.5 However, little is known about the association of 25(OH)D and cognitive function in T2DM patients. The present study demonstrated a significant inverse association of serum 25(OH)D levels and cognitive impairment. Similar to our results, a cross-sectional study reported an inverse association of 25(OH)D and cognitive impairment in T2DM patients.3 A community-based cohort study revealed an association of low plasma 25(OH)D levels with greater odds of cognitive impairment in older adults.9 Moreover, a retrospective study including patients with mild stage of Alzheimer’s disease (AD) revealed slower progression to severe stages of AD, in subjects treated with vitamin-D compared with those without treatment.28 Hydroxylases for activation of vitamin-D and VDR have been detected in brain regions responsible for cognition and memory, thus, suggesting the role of vitamin-D in cognition.10 The binding of 1,25-(OH)2D3 on VDR triggers protective mechanisms against degenerative processes implicated in AD.3 There is a robust evidence that vitamin-D contributes to neuroprotection7,10,17 by modulating the production of nerve growth, decreasing L-type calcium channel expression,7,17 regulating the toxicity of reactive oxygen species,3,17 and neurotrophic factors such as nerve growth factor, glial cell-derived neurotrophic factor,17 nitric oxide synthase and increasing glutathione levels.3 In in vitro models, vitamin-D reduces the accumulation of amyloid β-42 (Aβ) by stimulating Aβ phagocytosis and clearance while protecting against apoptosis.17 The present study did not find any association between 25(OH)D levels and MMSE scores healthy controls. As in our study, many studies performed in younger adults found no association between 25(OH)D levels and cognition. A prospective cohort study including late middle age adults did not find significant associations between 25(OH)D and cognitive test scores.29 Another cross-sectional study found that none of the psychometric measures were associated with 25(OH)D levels in the adult groups. However, the elderly group had a significant difference between 25(OH)D quintiles performance on a learning and memory task.30 Moreover, reports from The Tromso Study indicate that the levels of 25(OH)D seem to be predictive of cognitive outcome in older individuals only.31
Vitamin-D binding protein (VDBP) acts as a major protein carrier for serum 25(OH)D and activated vitamin-D.7,32 It has been considered to be the main determinant of 25(OH)D levels. Thus, measuring VDBP is likely to unveil the causal pathway between 25(OH)D and T2DM.32 Therefore, the present study assessed VDBP levels in order to correlate its association with cognitive functions and T2DM. The present study demonstrated significantly lower levels of VDBP in patients with diabetes as compared to healthy controls. This finding is in line with previous literature. A similar case control study found significantly lower levels of VDBP in patients with diabetes as compared to controls.33 A retrospective, cross-sectional, cases-control study on T1DM patients revealed significantly lower serum VDBP levels in T1DM patients as compared to the controls.12 In addition, another case-control study demonstrated decreased serum VDBP levels in T2DM patients with normal albuminuria than in controls, however the difference was insignificant.34 Elevated urinary loss of VDBP12,33 and decreased reabsorption in proximal tubule by megalin/Dab233 have been suggested to cause lower blood levels of VDBP and vitamin-D in patients with diabetes. Furthermore, high glucose has been demonstrated to downregulate VDBP, signifying that uncontrolled glycemia may contribute to lower VDBP and vitamin-D levels in diabetes.33 Additionally, VDBP concentrations have been demonstrated to be inversely associated with hyperinsulinemia and insulin resistance.35
In the present study, a significant correlation was found between the levels of VDBP and cognitive functions among the T2DM patients. Our results indicated significantly lower levels of VDBP in cases with cognitive impairment. The role of VDBP on cognition is both scarce and inconsistent. VDBP has been shown to reduce Aβ aggregation in vitro, using thioflavin T fluorescence assay. In addition, DBP has also been demonstrated to prevent Aβ-mediated death in cultured mouse hippocampal HT22 cell line. Furthermore, DBP has been found to decrease Aβ-induced synaptic loss in the hippocampus and rescued memory deficits in mice after injection of Aβ into the lateral ventricle.11 Moreover, in a case-control study, VDBP levels were found to be significantly lower in MCI subjects than in AD subjects and controls.13 These results suggest protective effect of DBP against Aβ by direct interaction, indicating that DBP might be a promising therapeutic agent for the treatment of AD.11
Our study had several limitations that need to be mentioned. Some of the data such as, duration of T2DM, education status and family history were self-reported or were obtained from patient’s medical records. This could have led to recall bias. Next, as this was a case-control, cross sectional study, no causal relationship can be inferred. Lastly, the sample size was small which might not represent all T2DM patients.
Our study indicates T2DM patients are at higher risk of having hypovitaminosis D as compared to their healthy counterparts. Additionally, cognitive function of T2DM patients is poorer than in healthy people. Moreover, 25(OH)D deficiency is associated with poorer cognitive function. Therefore, frequent assessment of cognitive function is recommended to prevent and restrict any further decline in cognitive function. Additionally, assessment of vitamin-D is advised in T2DM patients. The present study also suggests that lower VDBP levels have an adverse effect on cognitive function in T2DM patients. However, further studies are warranted to conclude its mechanism in diabetes and its associated comorbidities. Besides, prospective studies with large sample sizes need to be conducted to further elucidate the association of vitamin-D, VDBP and cognitive function in T2DM patients.
Acknowledgments
We are thankful to University Grants Commission for providing fellowship to Dr Rizwana Parveen.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Herath PM, Cherbuin N, Eramudugolla R, et al. The effect of diabetes medication on cognitive function: evidence from the PATH through life study. Biomed Res Int. 2016;7208429:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.International Diabetes Federation. IDF Diabetes Atlas, 8th ed. Brussels: International Diabetes Federation; 2017. [Google Scholar]
- 3.Chen RH, Zhao XH, Gu Z, et al. Serum levels of 25-hydroxyvitamin D are associated with cognitive impairment in type 2 diabetic adults. Endocrine. 2014;45:319–324. doi: 10.1007/s12020-013-0041-9 [DOI] [PubMed] [Google Scholar]
- 4.Shuba N, Karan. Assessment of the cognitive status in diabetes mellitus. J Clin Diagn Res. 2012;10:1658–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Darwish H, Zeinoun P, Ghusn H, Khoury B, Tamim H, Khoury S. Serum 25-hydroxyvitamin D predicts cognitive performance in adults. Neuropsychiatr Dis Treat. 2015;2217–2223. doi: 10.2147/NDT [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fondjo LA, Owiredu W, Sakyi SA, et al. Vitamin D status and its association with insulin resistance among type 2 diabetics: a case-control study in Ghana. PLoS One. 2017;4:e0175388. doi: 10.1371/journal.pone.0175388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Celikbilek A, Gocmen AY, Tanik N, et al. Decreased serum vitamin D levels are associated with diabetic peripheral neuropathy in a rural area of Turkey. Acta Neurol Belg. 2015;1:47–52. doi: 10.1007/s13760-014-0304-0 [DOI] [PubMed] [Google Scholar]
- 8.Pittas AG, Dawson-Hughes B. Vitamin D and diabetes. J Steroid Biochem Mol Biol. 2010;1-2:425–429. doi: 10.1016/j.jsbmb.2010.03.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chei CL, Raman P, Yin ZX, et al. Vitamin D levels and cognition in elderly adults in China. J Am Geriatr Soc. 2014;11:2125–2129. doi: 10.1111/jgs.13082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Granic A, Hill TR, Kirkwood TB, et al. Serum 25-hydroxyvitamin D and cognitive decline in the very old: the newcastle 85+ study. Eur J Neurol. 2015;1(106–15):e6–e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moon M, Song H, Hong HJ, et al. Vitamin D-binding protein interacts with Abeta and suppresses Abeta-mediated pathology. Cell Death Differ. 2013;4:630–638. doi: 10.1038/cdd.2012.161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blanton D, Han Z, Bierschenk L, et al. Reduced serum vitamin D-binding protein levels are associated with type 1 diabetes. Diabetes. 2011;10:2566–2570. doi: 10.2337/db11-0576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muenchhoff J, Poljak A, Song F, et al. Plasma protein profiling of mild cognitive impairment and Alzheimer’s disease across two independent cohorts. J Alzheimers Dis. 2015;4:1355–1373. [DOI] [PubMed] [Google Scholar]
- 14.Bishnoi RJ, Palmer RF, Royall DR. Vitamin D binding protein as a serum biomarker of Alzheimer’s disease. J Alzheimers Dis. 2015;1:37–45. [DOI] [PubMed] [Google Scholar]
- 15.Song F, Poljak A, Kochan NA, et al. Plasma protein profiling of mild cognitive impairment and Alzheimer’s disease using iTRAQ quantitative proteomics. Proteome Sci. 2014;12(1):5. doi: 10.1186/1477-5956-12-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rozek W, Ricardo-Dukelow M, Holloway S, et al. Cerebrospinal fluid proteomic profiling of HIV-1-infected patients with cognitive impairment. J Proteome Res. 2007;6(11):4189–4199. doi: 10.1021/pr070220c [DOI] [PubMed] [Google Scholar]
- 17.Schlögl M, Holick MF. Vitamin D and neurocognitive function. Clin Interv Aging. 2014;559–568. doi: 10.2147/CIA.S51785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alencar RC, Cobas RA, Gomes MB. Assessment of cognitive status in patients with type 2 diabetes through the mini-mental status examination: a cross-sectional study. Diabetol Metab Syndr. 2010;10. doi: 10.1186/1758-5996-2-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. [DOI] [PubMed] [Google Scholar]
- 20.Sheth JJ, Shah A, Sheth FJ. et al. Does vitamin D play a significant role in type 2 diabetes? BMC Endocr Disord;2015. 5. doi: 10.1186/s12902-015-0003-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laway BA, Kotwal SK, Shah ZA. Pattern of 25 hydroxy vitamin D status in North Indian people with newly detected type 2 diabetes: a prospective case control study. Indian J Endocrinol Metab. 2014;5:726–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Payne JF, Ray R, Watson DG, et al. Vitamin D insufficiency in diabetic retinopathy. Endocr Pract. 2012;2:185–193. doi: 10.4158/EP11147.OR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahmadieh H, Azar ST, Lakkis N, et al. Hypovitaminosis d in patients with type 2 diabetes mellitus: a relation to disease control and complications. ISRN Endocrinol. 2013; 2013:641098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dalgard C, Petersen MS, Weihe P, Grandjean P. Vitamin D status in relation to glucose metabolism and type 2 diabetes in septuagenarians. Diabetes Care. 2011;34:1284–1288. doi: 10.2337/dc10-2084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rajeshkanna NR, Valli S, Thuvaragah P. Relation between diabetes mellitus-type 2 and cognitive impairment: a predictor of Alzheimer’s disease. Ijmrhs. 2014;3(4):903–910. [Google Scholar]
- 26.Annweiler C, Schott AM, Allali G, et al. Association of vitamin D deficiency with cognitive impairment in older women: cross-sectional study. Neurology. 2010;74(1):27–32. doi: 10.1212/WNL.0b013e3181beecd3 [DOI] [PubMed] [Google Scholar]
- 27.Brouwer-Brolsma EM, Dhonukshe-Rutten RA, van Wijngaarden JP, et al. Cognitive performance: a cross-sectional study on serum vitamin D and its interplay with glucose homeostasis in dutch older adults. J Am Med Dir Assoc. 2015;16(7):621–627. doi: 10.1016/j.jamda.2015.02.013 [DOI] [PubMed] [Google Scholar]
- 28.Chaves M, Toral A, Bisonni A, et al. [Treatment with vitamin D and slowing of progression to severe stage of Alzheimer’s disease]. Vertex B Aires Argent. 2014;25(114):85–91. [PubMed] [Google Scholar]
- 29.Schneider AL, Lutsey PL, Alonso A, et al. Vitamin D and cognitive function and dementia risk in a biracial cohort: the ARIC brain MRI study. Eur J Neurol. 2014;21(9):1211–1218. doi: 10.1111/ene.12460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGrath J, Scragg R, Chant D, Eyles D, Burne T, Obradovic D. No association between serum 25-hydroxyvitamin D3 level and performance on psychometric tests in NHANES III. Neuroepidemiology. 2007;29(1–2):49–54. doi: 10.1159/000108918 [DOI] [PubMed] [Google Scholar]
- 31.Landel V, Annweiler C, Millet P, Morello M, Féron F. Vitamin D, cognition and Alzheimer’s disease: the therapeutic benefit is in the D-tails. J Alzheimers Dis. 2016;53(2):419–444. doi: 10.3233/JAD-150943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leong A, Rehman W, Dastani Z, et al. The causal effect of vitamin D binding protein (DBP) levels on calcemic and cardiometabolic diseases: a Mendelian randomization study. PLoS Med. 2014;10:e1001751. doi: 10.1371/journal.pmed.1001751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jain SK, Kahlon G, Bass P, Levine SN, Warden C. Can L -cysteine and vitamin D rescue vitamin D and vitamin D binding protein levels in blood plasma of African American type 2 diabetic patients? Antioxid. Redox Signal. 2015;23(8):688–693. doi: 10.1089/ars.2015.6320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fawzy MS, Abu AlSel BT. Assessment of vitamin D-binding protein and early prediction of nephropathy in type 2 saudi diabetic patients. J Diabetes Res. 2018;2018:1–13. doi: 10.1155/2018/8517929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ashraf AP, Huisingh C, Alvarez JA, Wang X, Gower BA. Insulin resistance indices are inversely associated with vitamin D binding protein concentrations. J Clin Endocrinol Metab. 2014;99(1):178–183. doi: 10.1210/jc.2013-2452 [DOI] [PMC free article] [PubMed] [Google Scholar]