Abstract
Introduction
The benefit of transanal total mesorectal excision (TaTME) for mid and low rectal cancer is conflicting.
Aim
To assess and compare the short-term outcomes of TaTME with conventional laparoscopic total mesorectal excision (LaTME) for middle and low rectal cancer.
Material and methods
We searched PubMed, Embase and Cochrane Library databases for studies addressing TaTME versus conventional LaTME for rectal cancer between 2008 and December 2018. Randomized controlled trials (RCTs) and retrospective studies which compared TaTME with LaTME were included.
Results
Twelve retrospective case-control studies were identified, including a total of 899 patients. We did not find significant differences in overall intraoperative complications, blood loss, conversion rate, operative time, overall postoperative complication, anastomotic leakage, ileus, or urinary morbidity. Also no significant differences in oncological outcomes including circumferential resection margin (CRM), positive CRM, distal margin distance (DRM), positive DRM, quality of mesorectum, number of harvested lymph nodes, temporary stoma or local recurrence were found. Although the TaTME group had better postoperative outcomes (readmission, reoperation, length of hospital stay) on average, the difference did not reach statistical significance.
Conclusions
Transanal total mesorectal excision offers a safe and feasible alternative to LaTME although the clinicopathological features were not superior to LaTME in this study. Currently, with the lack of evidence on benefits of TaTME, further evaluation of TaTME requires large randomized control trials to be conducted.
Keywords: rectal cancer, transanal total mesorectal excision, laparoscopic total mesorectal excision, meta-analysis
Introduction
Colorectal cancer is one of the most common cancers worldwide [1]. Since laparoscopic surgery was first applied in colorectal cancer in 1991, the technique has spread worldwide [2]. Compared to open surgery, laparoscopic surgery for rectal cancer is safe and feasible with comparable short-term outcomes and long-term outcomes [3–6].
Since the principles of total mesorectal excision (TME) were first described by Heald et al. in 1982 [7], it has become a standard procedure for rectal cancer, and reduced the local recurrence to less than 5% [8–10]. However, there remained some difficulties in middle or low rectal cancer, especially in a low location, obese patients, or males with a deep, narrow pelvis. In 2010, the down-to-up approach, transanal total mesorectal excision (TaTME), was introduced to solve these problems [11–13]. And then, there were several randomized controlled trials focusing on middle and low rectal cancer compared TaTME with laparoscopic TME (LaTME) [14, 15].
Previous meta-analyses had demonstrated a relative merit of TaTME over LaTME [16–20]. However, these studies had a relatively small sample size. What is more, some previous meta-analyses included data from abdominoperineal resections, which may generate bias, affecting outcomes [18]. Hence, we conducted this meta-analysis to assess and compare the short-term outcomes of TaTME with LaTME for middle and low rectal cancer. Intraoperative outcomes, postoperative outcomes, oncological outcomes and local recurrence were measured with meta-analytical methods.
Aim
The aim of the study was to assess and compare the short-term outcomes of TaTME with conventional LaTME for middle and low rectal cancer.
Material and methods
This meta-analysis adheres to the Preferred Reporting Items for Systematic Reviews and Meta-analysis and Meta-analysis guidelines [21, 22].
Literature-search strategy
Literature searches of PubMed, Embase and Cochrane Library databases for studies addressing TaTME versus conventional LaTME for rectal cancer between 2008 and December 2018 were performed. Only English-language publications were involved. The search terms were “Transanal or transanal total mesorectal excision or TaTME or transanal minimally invasive surgery or TAMIS or transanal endoscopic microsurgery or TEM or natural orifice transluminal endoscopic surgery or NOTES or natural orifice specimen extraction or NOSE or transanal specimen extraction” and “rectal cancer or proctectomy”.
Inclusion and exclusion criteria
Randomized controlled trials (RCTs) and retrospective studies that comparing TaTME with LaTME were included. All the included studies had to have at least one of the relevant outcomes mentioned below. The exclusion criteria were as follows: (a) lack of the sufficient data or outcomes of interest; (b) duplicate publication; (c) non-comparative studies, editorials, letters, conference abstracts, review articles, case reports and animal experimental studies; (d) studies included high rectal cancer (tumor distance from anal verge more than 10 cm) and abdomino-perineal resection (APR).
Data extraction and outcomes of interest
Two independent authors extracted and summarized the data from the included studies independently.
The intraoperative outcomes were estimated blood loss, operative time, conversion rate, and intraoperative complications. The postoperative outcomes were overall postoperative complications, anastomotic leakage, ileus, urinary morbidity, reoperation, readmission rate, and length of hospital stay. The oncological outcomes were quality of mesorectum, circumferential resection margin (CRM), positive CRM, distal margin distance (DRM), positive DRM, harvested lymph nodes and local recurrence.
Quality assessment
For continuous variables weighted mean differences (WMDs) were calculated. For dichotomous variables odds ratios (ORs) were calculated. For continuous data as median and range values, the means and standard deviations were calculated by the formula described by Liberati et al. [22].
Statistical analysis
Statistical heterogeneity between studies was assessed using the χ2 test with significance set at p < 0.10 [23]. A random effects model was used and funnel plots were used to evaluate publication bias. The Newcastle-Ottawa scale was used to evaluate the methodological quality of all the retrospective studies.
Statistical analyses were done using RevMan 5.3 software (Cochrane Collaboration, Oxford, UK).
Results
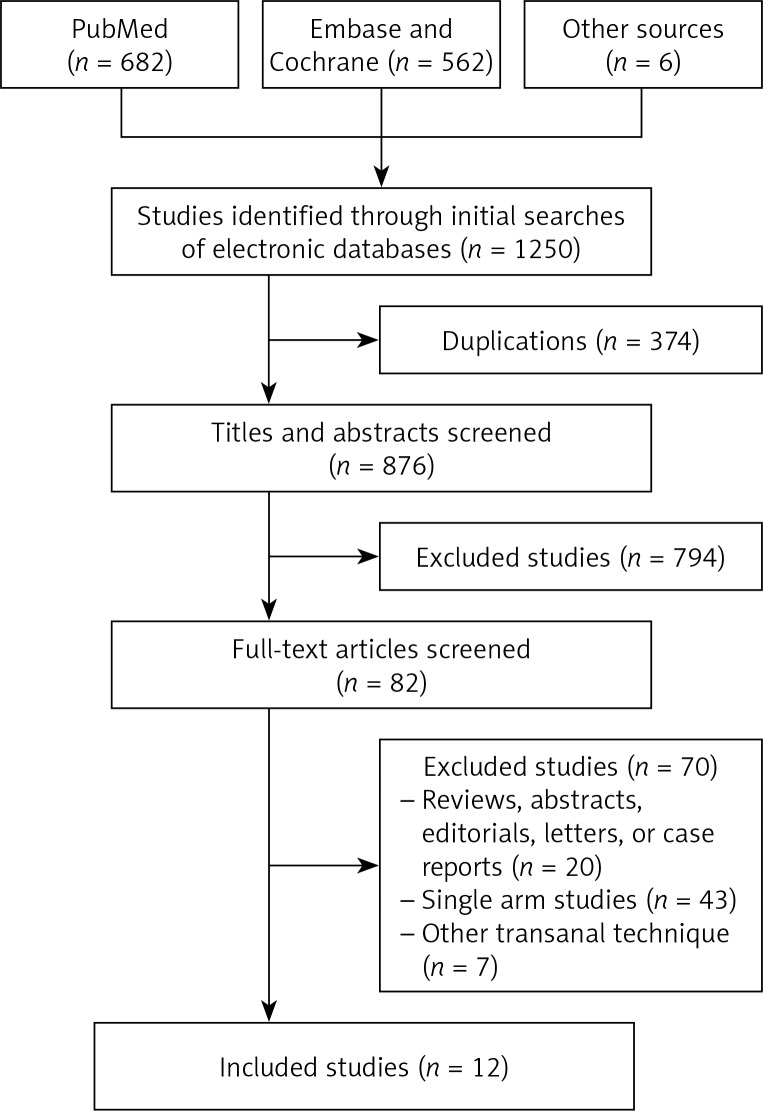
One thousand two hundred and forty-seven citations were retrieved from the search strategy. Finally, twelve studies [24–35] were included in the analysis, with a total of 899 patients (411 patients in TaTME group, 488 patients in LaTME group) (Figure 1). The characteristics of eligible studies are shown in Table I.
Figure 1.
Flow diagram of trial identification, screening, inclusion and exclusion
Table I.
Characteristics of studies included in this meta-analysis
| Study | Design | Country | Patients TaTME/LaTME | BMI | T stage | Tumor location | Neoadjuvant therapy TaTME/LaTME | Quality score | |
|---|---|---|---|---|---|---|---|---|---|
| TaTME | LaTME | ||||||||
| Fernandez-Hevia 2014 [24] | MCC | Spain | 37/37 | 23.7 ±3.6 | 25.1 ±4.0 | T2–T4 | L + M | 28/23 | 7 |
| De’Angelis 2015 [25] | MCC | France | 32/32 | 25.2 ±3.5 | 24.5 ±3.2 | T2–T4 | L | 27/23 | 8 |
| Chen 2016 [26] | MCC | Taiwan, China | 50/100 | 24.2 ±3.7 | 24.6 ±3.1 | T2–T3 | L + M | 50/100 | 7 |
| Chouillard 2016 [27] | Prospective cohort study | France | 18/15 | 27.1 ±4.5 | 29.0 ±4.2 | T1–T3 | L + M | 14/12 | 7 |
| Lelong 2016 [28] | MCC | France | 34/38 | 24 (18.6–45.0) | 24.2 (17.7–32.7) | T1–T4 | L | 30/35 | 8 |
| Rasulov 2016 [29] | Prospective cohort study | Russia | 22/23 | 26.0 (19.7–32.3) | 26.0 (18.3–37.2) | T1–T4 | L + M | 19/11 | 8 |
| Chang 2017 [30] | MCC | Taiwan, China | 23/23 | 25.8 ±4.3 | 25.0 ±3.0 | T1–T3 | L | 8/14 | 7 |
| Mege 2018 [31] | MCC | France | 34/34 | 25 ±4 | 25 ±3 | T1–T4 | L | 29/29 | 8 |
| Persiani 2018 [32] | MCC | Italy | 46/46 | 25 (19.1–32.8) | 25.6 (18.8–33.4) | T1–T3 | L + M | 26/32 | 7 |
| Chen YT 2018 [33] | MCC | Taiwan, China | 39/64 | 25.4 ±4.0 | 24.6 ±3.3 | T1–T3 | L + M | 115/31 | 8 |
| Roodbeen 2018 [34] | MCC | Netherlands | 41/41 | 26.7 ±1.9 | 26.1 ±4.0 | T1–T4 | L | 18/18 | 7 |
| Rubinkiewicz 2018 [35] | MCC | Poland | 35/35 | 26.1 ±4.09 | 27.1 ±4.71 | T1–T3 | L | 31/31 | 7 |
TaTME – transanal total mesorectal excision, LaTME – laparoscopic total mesorectal excision, BMI – body mass index, MCC – matched case control. Tumor location: L – low, M – middle.
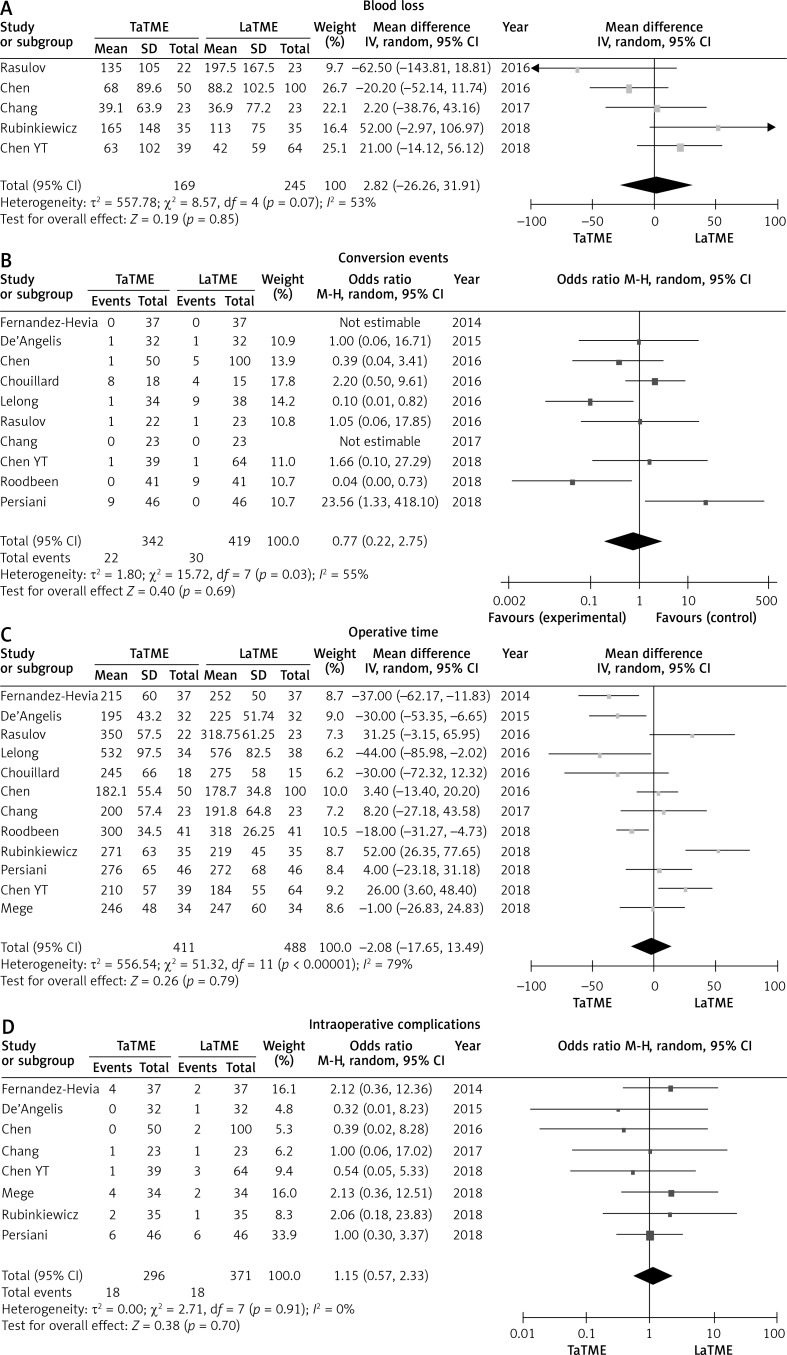
Meta-analysis revealed no statistically significant difference in intraoperative outcomes: There were no statistically significant differences in blood loss (p = 0.85), operative time (p = 0.79), conversion rate (p = 0.69) or intraoperative complications (p = 0.70) between the two groups (Figure 2). There was no heterogeneity among studies, I 2 = 0%.
Figure 2.
Forest plots describing estimated blood loss (A), conversion events (B), operative time (C) and intraoperative complications (D) between TaTME and LaTME
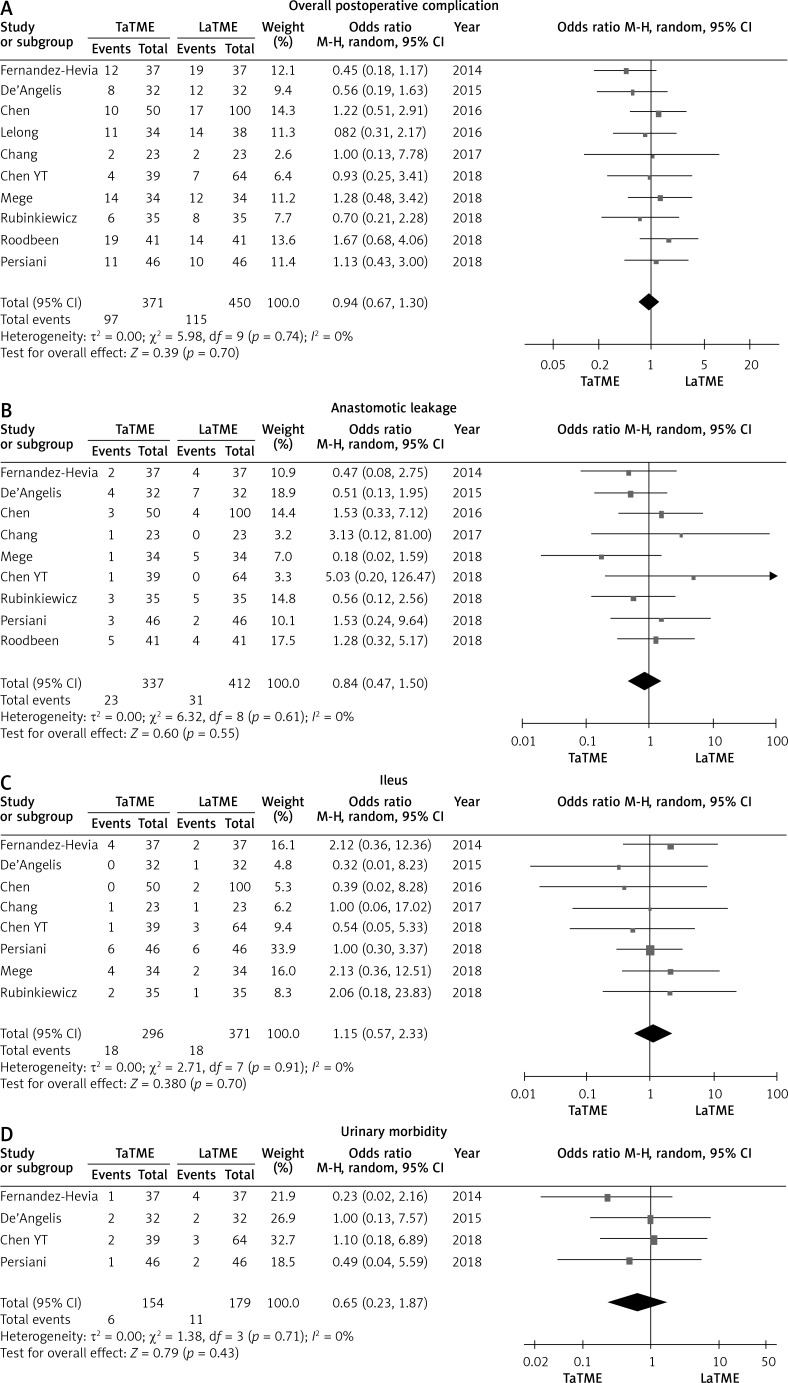
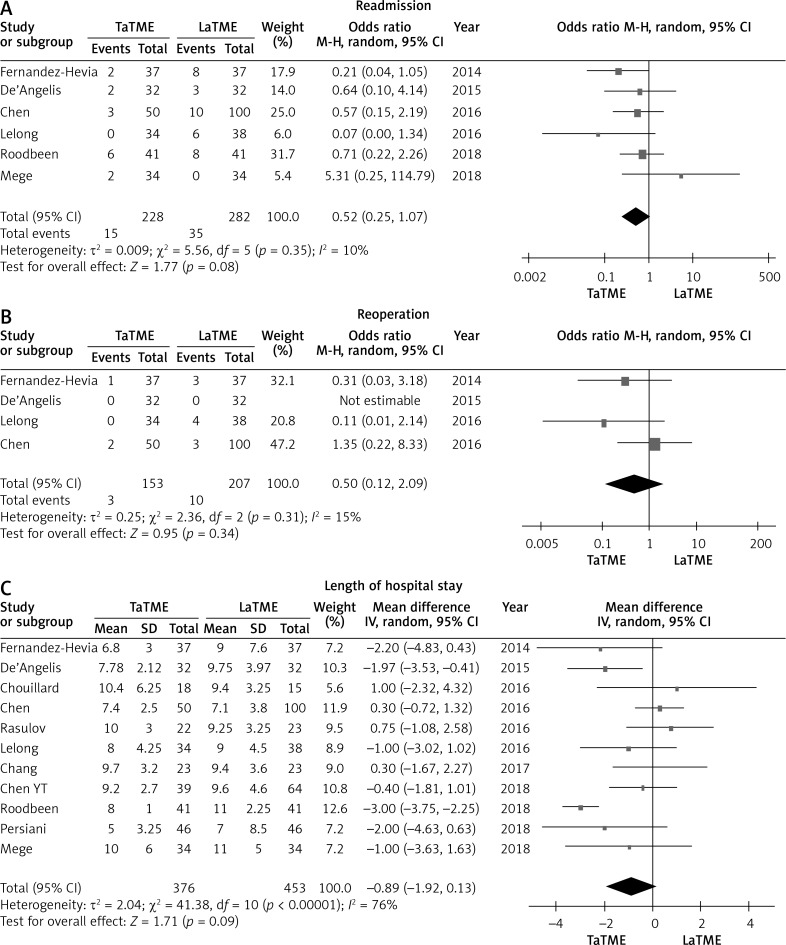
Ten studies [24–26, 28, 30–35] that assessed 821 patients reported on overall postoperative complication rate. Meta-analysis showed no statistically significant differences in overall postoperative complication (p = 0.39), anastomotic leakage (p = 0.60), ileus (p = 0.38) or urinary morbidity (p = 0.79) between the two groups (Figure 3). The TaTME group had non-significantly better postoperative outcomes in readmission (p = 0.08), reoperation (p = 0.34) and length of hospital stay (p = 0.09) (Figure 4).
Figure 3.
Forest plots describing postoperative outcomes: overall postoperative complication (A), anastomotic leakage (B), ileus (C), urinary morbidity (D) between TaTME and LaTME
Figure 4.
Forest plots describing postoperative outcomes: readmission (A), reoperation (B), length of hospital stay (C) between TaTME and LaTME
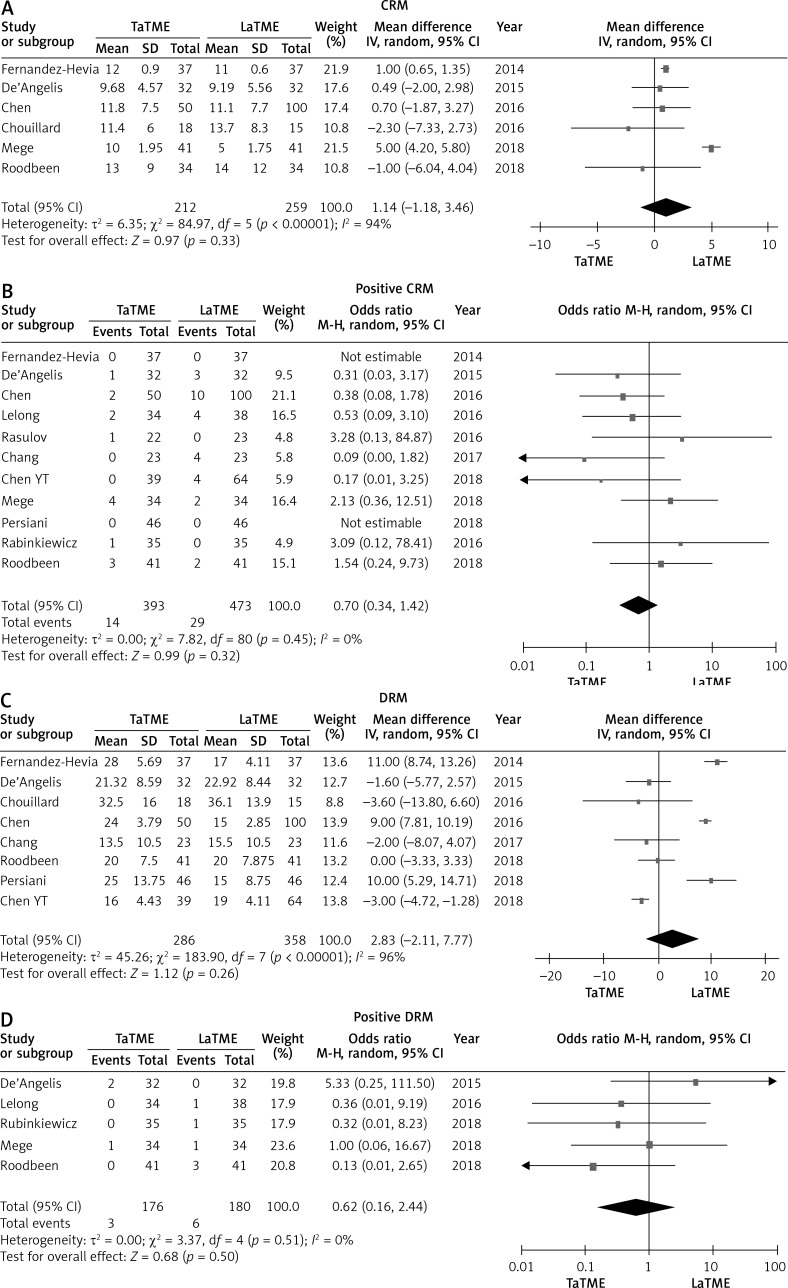
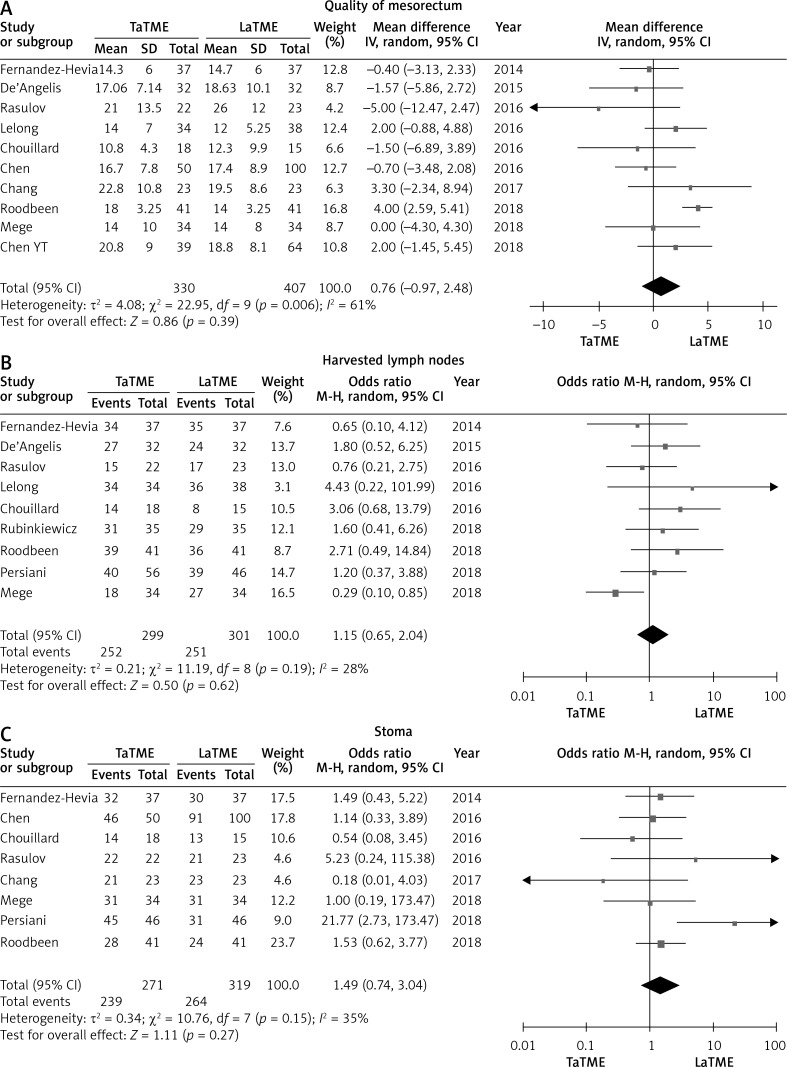
There were six studies [24–27, 31, 34] that reported CRM, eleven studies [24–26, 28–35] that reported positive CRM, eight studies [24–27, 30, 32–34] that reported DRM and five studies [25, 28, 31, 34, 35] that reported positive DRM. No differences were found in these pathological outcomes (Figure 5). Meanwhile, we did not find statistically significant differences in quality of mesorectum (p = 0.39), harvested lymph nodes (p = 0.62) or temporary stoma (p = 0.27) (Figure 6).
Figure 5.
Forest plots describing oncological outcomes: CRM (A), positive CRM (B), DRM (C), positive DRM (D) between TaTME and LaTME
Figure 6.
Forest plots describing oncological outcomes: quality of mesorectum (A), number of harvested lymph nodes (B) and temporary stoma (C) between TaTME and LaTME
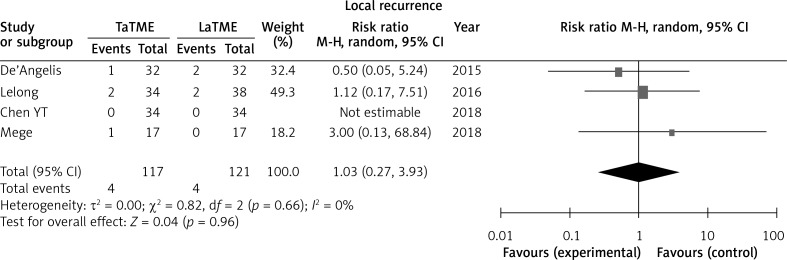
Four studies [25, 28, 31, 33] reported local recurrence; no difference was found in this outcome (Figure 7).
Figure 7.
Forest plot describing oncological outcome of local recurrence
Publication bias
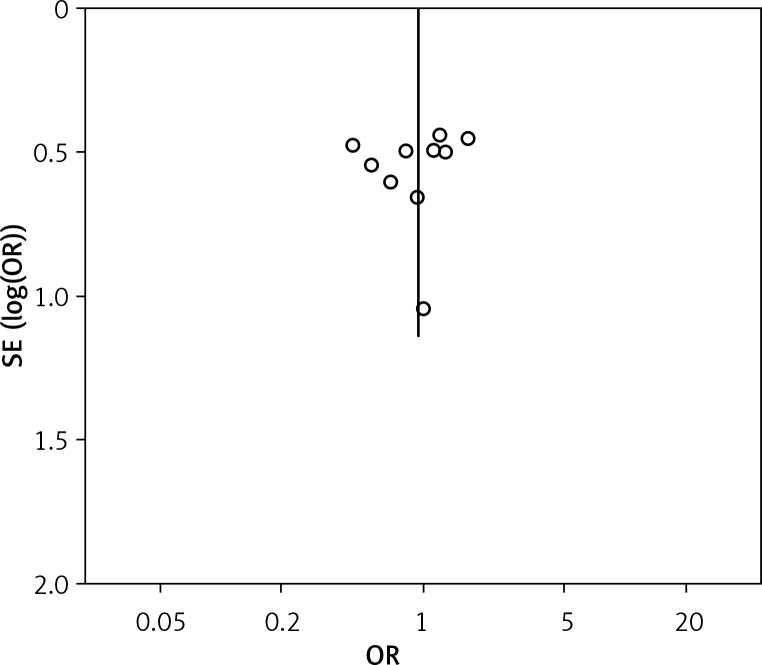
The funnel plot based on overall complication rate indicated no obvious publication bias (Figure 8).
Figure 8.
Funnel plot showing publication bias based on overall complication rate
Discussion
This study was the largest meta-analysis including 899 patients (411 patients in TaTME group, 488 patients in LaTME group). Our results showed no significant difference between TaTME and LaTME in overall intraoperative complications, postoperative outcomes, oncological outcomes or local recurrence. We hope that our findings can illustrate the safety and feasibility of TaTME, and promote its application in middle and low rectal cancer.
The TaTME is a novel technique which is expected to have better oncological outcomes. Lots of previous studies had shown that TaTME is superior to LaTME and may benefit in some surgical and pathological outcomes, but no RCT results prove these findings. There have been many meta-analyses [16–20] about TaTME in the last 3 years, but most of them contained substantial bias, and the results of Rubinkiewicz et al. [18] showed no significant differences in clinical outcomes between TaTME and LaTME recently. But we found this negative result based on overall complications and some surgical outcomes without systematically analyzing intraoperative outcomes, postoperative outcomes, oncological outcomes. What is more, TaTME is more suitable for middle and low rectal cancer, and it is inappropriate to include high rectal cancer, which was included in Rubinkiewicz’s study [35].
In this study, we included several of the most recent papers which were not included in previous analyses, and systematically analyzed surgical outcomes aiming to find out new proof of differences of clinical outcomes between TaTME and LaTME. Previous meta-analyses [16, 17] had conflicting results in conversion rate and postoperative complications. In this meta-analysis which included 899 patients, we were able to show evidence of decrease of the overall postoperative complication rate, urinary morbidity and readmission rate in the TaTME group. However, we found no significant difference in conversion rate in our result. As previous reports, temporary stoma may affect recovery after surgery [36–38], but there was no difference in the rate of temporary stoma between two groups.
In our study, the quality of mesorectum did not reach statistical significance between the two groups. A previous meta-analysis [16] including six studies found a significant difference in the complete rate of complete mesorectum. But after adding more studies in this study, no significant result was found in the complete rate of complete mesorectum. Interestingly, the TaTME group had better postoperative outcomes (readmission, reoperation, length of hospital stay) on average, although the difference did not reach statistical significance. The heterogeneities in these parameters were 10%, 15%, 76% respectively, which may be an important factor affecting these results.
Fewer studies have assessed long-term observation. Only Zhang’s meta-analysis [19] reported 2-year survival and 2-years disease-free survival between TaTME and LaTME, which included only two studies, and found no significant result. It is impossible to prove the superiority of any technique due to lack of new data. One of the most different procedures between TaTME and LaTME is separating the rectum during the small pelvis. Therefore, we think the rate of local recurrence is an important long-term outcome. In this study we first compared local recurrence between TaTME and LaTME; the result showed no difference between TaTME and LaTME. It means that changing this key operative approach may not affect the surgical outcome of TME. It still requires more time for long-term follow-up in RCT studies, or more any other long-term outcome data from non-RCT results.
This meta-analysis has several limitations that must be taken into account. Firstly, all the included studies were observational studies but without RCTs. Without adequate random sequence generation and blinding, the risk of bias might increase. Therefore, the quality of the evidence pooled from these retrospective trials must be judged as low. Secondly, there may be publication bias due to all the included studies being in English, and these data were not from a high-volume center, which may also affect the results. Finally, no long-term outcome, such as overall survival and disease free survival, was measured in the analysis.
Conclusions
TaTME offers a safe and feasible alternative to LaTME although the clinicopathological features were not superior to LaTME in this study. Currently, in view of the lack of evidence on benefits of TaTME, further evaluation of TaTME necessitates large randomized control trials.
Acknowledgments
Dezheng Lin, Zhaoliang Yu and Wenpei Chen contributed equally to this study and should be considered as co-first authors.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Ries LA, Wingo PA, Miller DS, et al. The annual report to the nation on the status of cancer, 1973-1997, with a special section on colorectal cancer. Cancer. 2000;88:2398–424. doi: 10.1002/(sici)1097-0142(20000515)88:10<2398::aid-cncr26>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 2.Jacobs M, Verdeja JC, Goldstein HS. Minimally invasive colon resection (laparoscopic colectomy) Surg Laparosc Endosc. 1991;1:144–50. [PubMed] [Google Scholar]
- 3.Kearney DE, Coffey JC. A Randomized trial of laparoscopic versus open surgery for rectal cancer. N Engl J Med. 2015;373:194. doi: 10.1056/NEJMc1505367. [DOI] [PubMed] [Google Scholar]
- 4.Jeong SY, Park JW, Nam BH, et al. Open versus laparoscopic surgery for mid-rectal or low-rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): survival outcomes of an open-label, non-inferiority, randomised controlled trial. Lancet Oncol. 2014;15:767–74. doi: 10.1016/S1470-2045(14)70205-0. [DOI] [PubMed] [Google Scholar]
- 5.Pedziwiatr M, Malczak P, Mizera M, et al. There is no difference in outcome between laparoscopic and open surgery for rectal cancer: a systematic review and meta-analysis on short- and long-term oncologic outcomes. Tech Coloproctol. 2017;21:595–604. doi: 10.1007/s10151-017-1662-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malczak P, Mizera M, Torbicz G, et al. Is the laparoscopic approach for rectal cancer superior to open surgery? A systematic review and meta-analysis on short-term surgical outcomes. Videosurgery Miniinv. 2018;13:129–40. doi: 10.5114/wiitm.2018.75845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heald RJ, Husband EM, Ryall RD. The mesorectum in rectal cancer surgery: the clue to pelvic recurrence? Br J Surg. 1982;69:613–6. doi: 10.1002/bjs.1800691019. [DOI] [PubMed] [Google Scholar]
- 8.Enker WE, Thaler HT, Cranor ML, Polyak T. Total mesorectal excision in the operative treatment of carcinoma of the rectum. J Am Coll Surg. 1995;181:335–46. [PubMed] [Google Scholar]
- 9.Bach SP, Hill J, Monson JR, et al. A predictive model for local recurrence after transanal endoscopic microsurgery for rectal cancer. Br J Surg. 2009;96:280–90. doi: 10.1002/bjs.6456. [DOI] [PubMed] [Google Scholar]
- 10.Enker WE. Potency, cure, and local control in the operative treatment of rectal cancer. Arch Surg. 1992;127:1396–401. doi: 10.1001/archsurg.1992.01420120030005. discussion 1402. [DOI] [PubMed] [Google Scholar]
- 11.Sylla P, Rattner DW, Delgado S, Lacy AM. NOTES transanal rectal cancer resection using transanal endoscopic microsurgery and laparoscopic assistance. Surg Endosc. 2010;24:1205–10. doi: 10.1007/s00464-010-0965-6. [DOI] [PubMed] [Google Scholar]
- 12.Zhang H, Zhang YS, Jin XW, et al. Transanal single-port laparoscopic total mesorectal excision in the treatment of rectal cancer. Tech Coloproctol. 2013;17:117–23. doi: 10.1007/s10151-012-0882-x. [DOI] [PubMed] [Google Scholar]
- 13.Heald RJ. A new solution to some old problems: transanal TME. Tech Coloproctol. 2013;17:257–8. doi: 10.1007/s10151-013-0984-0. [DOI] [PubMed] [Google Scholar]
- 14.Deijen CL, Velthuis S, Tsai A, et al. COLOR III: a multicentre randomised clinical trial comparing transanal TME versus laparoscopic TME for mid and low rectal cancer. Surg Endosc. 2016;30:3210–5. doi: 10.1007/s00464-015-4615-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lelong B, de Chaisemartin C, Meillat H, et al. A multicentre randomised controlled trial to evaluate the efficacy, morbidity and functional outcome of endoscopic transanal proctectomy versus laparoscopic proctectomy for low-lying rectal cancer (ETAP-GRECCAR 11 TRIAL): rationale and design. BMC Cancer. 2017;17:253. doi: 10.1186/s12885-017-3200-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu W, Xu Z, Cheng H, et al. Comparison of short-term clinical outcomes between transanal and laparoscopic total mesorectal excision for the treatment of mid and low rectal cancer: a meta-analysis. Eur J Surg Oncol. 2016;42:1841–50. doi: 10.1016/j.ejso.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 17.Ma B, Gao P, Song Y, et al. Transanal total mesorectal excision (taTME) for rectal cancer: a systematic review and meta-analysis of oncological and perioperative outcomes compared with laparoscopic total mesorectal excision. BMC Cancer. 2016;16:380. doi: 10.1186/s12885-016-2428-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rubinkiewicz M, Czerwinska A, Zarzycki P, et al. Comparison of short-term clinical and pathological outcomes after transanal versus laparoscopic total mesorectal excision for low anterior rectal resection due to rectal cancer: a systematic review with meta-analysis. J Clin Med. 2018;7:448. doi: 10.3390/jcm7110448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang X, Gao Y, Dai X, et al. Short- and long-term outcomes of transanal versus laparoscopic total mesorectal excision for mid-to-low rectal cancer: a meta-analysis. Surg Endosc. 2018 doi: 10.1007/s00464-018-6527-z. [DOI] [PubMed] [Google Scholar]
- 20.Jiang HP, Li YS, Wang B, et al. Pathological outcomes of transanal versus laparoscopic total mesorectal excision for rectal cancer: a systematic review with meta-analysis. Surg Endosc. 2018;32:2632–42. doi: 10.1007/s00464-018-6103-6. [DOI] [PubMed] [Google Scholar]
- 21.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 22.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fernandez-Hevia M, Delgado S, Castells A, et al. Transanal total mesorectal excision in rectal cancer: short-term outcomes in comparison with laparoscopic surgery. Ann Surg. 2015;261:221–7. doi: 10.1097/SLA.0000000000000865. [DOI] [PubMed] [Google Scholar]
- 25.De’Angelis N, Portigliotti L, Azoulay D, Brunetti F. Transanal total mesorectal excision for rectal cancer: a single center experience and systematic review of the literature. Langenbecks Arch Surg. 2015;400:945–59. doi: 10.1007/s00423-015-1350-7. [DOI] [PubMed] [Google Scholar]
- 26.Chen CC, Lai YL, Jiang JK, et al. Transanal total mesorectal excision versus laparoscopic surgery for rectal cancer receiving neoadjuvant chemoradiation: a matched case-control study. Ann Surg Oncol. 2016;23:1169–76. doi: 10.1245/s10434-015-4997-y. [DOI] [PubMed] [Google Scholar]
- 27.Chouillard E, Regnier A, Vitte RL, et al. Transanal NOTES total mesorectal excision (TME) in patients with rectal cancer: is anatomy better preserved? Tech Coloproctol. 2016;20:537–44. doi: 10.1007/s10151-016-1449-z. [DOI] [PubMed] [Google Scholar]
- 28.Lelong B, Meillat H, Zemmour C, et al. Short- and mid-term outcomes after endoscopic transanal or laparoscopic transabdominal total mesorectal excision for low rectal cancer: a single institutional case-control study. J Am Coll Surg. 2017;224:917–25. doi: 10.1016/j.jamcollsurg.2016.12.019. [DOI] [PubMed] [Google Scholar]
- 29.Rasulov AO, Mamedli ZZ, Gordeyev SS, et al. Short-term outcomes after transanal and laparoscopic total mesorectal excision for rectal cancer. Tech Coloproctol. 2016;20:227–34. doi: 10.1007/s10151-015-1421-3. [DOI] [PubMed] [Google Scholar]
- 30.Chang TC, Kiu KT. Transanal total mesorectal excision in lower rectal cancer: comparison of short-term outcomes with conventional laparoscopic total mesorectal excision. J Laparoendosc Adv Surg Tech A. 2018;28:365–9. doi: 10.1089/lap.2017.0520. [DOI] [PubMed] [Google Scholar]
- 31.Mege D, Hain E, Lakkis Z, et al. Is trans-anal total mesorectal excision really safe and better than laparoscopic total mesorectal excision with a perineal approach first in patients with low rectal cancer? A learning curve with case-matched study in 68 patients. Colorectal Dis. 2018;20:O143–51. doi: 10.1111/codi.14238. [DOI] [PubMed] [Google Scholar]
- 32.Persiani R, Biondi A, Pennestri F, et al. Transanal total mesorectal excision vs laparoscopic total mesorectal excision in the treatment of low and middle rectal cancer: a propensity score matching analysis. Dis Colon Rectum. 2018;61:809–16. doi: 10.1097/DCR.0000000000001063. [DOI] [PubMed] [Google Scholar]
- 33.Chen YT, Kiu KT, Yen MH, Chang TC. Comparison of the short-term outcomes in lower rectal cancer using three different surgical techniques: transanal total mesorectal excision (TME), laparoscopic TME, and open TME. Asian J Surg. 2018 doi: 10.1016/j.asjsur.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 34.Roodbeen SX, Penna M, Mackenzie H, et al. Transanal total mesorectal excision (TaTME) versus laparoscopic TME for MRI-defined low rectal cancer: a propensity score-matched analysis of oncological outcomes. Surg Endosc. 2018 doi: 10.1007/s00464-018-6530-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rubinkiewicz M, Nowakowski M, Wierdak M, et al. Transanal total mesorectal excision for low rectal cancer: a case-matched study comparing TaTME versus standard laparoscopic TME. Cancer Manag Res. 2018;10:5239–45. doi: 10.2147/CMAR.S181214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Veltcamp HM, Koedam T, Knol JJ, et al. Quality of life after rectal cancer surgery: differences between laparoscopic and transanal total mesorectal excision. Surg Endosc. 2019;33:79–87. doi: 10.1007/s00464-018-6276-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klek S, Pisarska M, Milian-Ciesielska K, et al. Early closure of the protective ileostomy after rectal resection should become part of the Enhanced Recovery After Surgery (ERAS) protocol: a randomized, prospective, two-center clinical trial. Videosurgery Miniinv. 2018;13:435–41. doi: 10.5114/wiitm.2018.79574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nowakowski MM, Rubinkiewicz M, Gajewska N, et al. Defunctioning ileostomy and mechanical bowel preparation may contribute to development of low anterior resection syndrome. Videosurgery Miniinv. 2018;13:306–14. doi: 10.5114/wiitm.2018.76913. [DOI] [PMC free article] [PubMed] [Google Scholar]