Abstract
Toxoplasma gondii infection induces a robust CD8 T cell immunity in the infected host, which is critical for keeping chronic infection under control. IFNγ production and cytolytic activity exhibited by CD8 T cells are critical functions needed to prevent the reactivation of latent infection. Paradoxically, the susceptible mice infected with the parasite develop encephalitis irrespective of the presence of vigorous CD8 T cell immunity. Recent studies from our laboratory have demonstrated that these animals have defect in the memory CD8 T cell population, which become dysfunctional due to exhibition of inhibitory receptors like PD-1. Although the blockade of PD-1-PDL-1 pathway rescues the CD8 response, PD-1hi expressing cells are refractory to the treatment. In this review, we discuss the development of CD8 memory response during chronic infection, mechanism responsible for their dysfunctionality, and possible therapeutic measures that can be taken to reverse the process.
Keywords: Toxoplasma gondii, CD8+ T cells, Cytokines, PD-1, Cytotoxicity
Toxoplasma gondii and protective immunity
Toxoplasma gondii, an obligate intracellular pathogen, is one of the most common parasite infecting humans worldwide [1, 2]. According to CDC estimates, up to one third of the global population is chronically infected with Toxoplasma gondii. In the USA, approximately 22 % of the population 12 years and over is afflicted with the parasite, whereas up to 95 % population in the other regions of the world has been shown to be infected (http://www.cdc.gov/parasites/toxoplasmosis/).
Similar to many other intracellular pathogens including viruses, bacteria, and protozoa, the protective immunity against T. gondii is highly dependent on the development of robust cell-mediated immunity [3, 4]. In the case of T. gondii, although innate immune mechanisms mediated by macrophages and NK cells can restrict acute infection during early stages, the presence of optimal adaptive immunity is essential for keeping the pathogen under control [5, 6]. Among the T cells, CD4 population due to their ability to produce cytokines like IFNγ are important for later stages of acute infection [7, 8], while long-term immunity which is essential for the maintenance of chronicity is primarily dependent on CD8 T cells [9-11]. The first report suggesting the importance of CD8 T cells in response to T. gondii infection was reported by studies conducted by Khan et.al who demonstrated that mice immunized with the major membrane protein (SAG-1) developed a strong response. Interestingly, immune CD8 T cells from these animals caused lysis of extracellular parasites [12]. Subsequently, it was reported that antigen-specific CD8 cloned T cells raised against the same antigen, upon adoptive transfer protect naive animals against lethal infection [10]. In between this period, there were number of reports by other laboratories which demonstrated the importance of CD8 T cells during Toxoplasma infection [13, 14]. In one of the studies, it was reported that CD8 CTLs (cytotoxic T lymphocytes) generated by the vaccine strain are critical for the protection against a virulent strain of parasite [15]. The importance of CD8 T cells in chronic toxoplasmosis was reported by Brown and McLeod, who demonstrated the role of these cells in determining the cyst burden [16]. Similarly, a number of other studies have further confirmed that CD8 T cells are essential in keeping chronic toxoplasma infection under control, thus establishing them as a dominant component of long-term immunity needed to keep the reactivation process in check [9, 17-19]. In addition to their role in chronic infection, CD8 T cells due to their ability to produce IFNγ may also contribute to protection during acute toxoplasmosis. Nevertheless, in addition to cytokine production, cytotoxic activity of these cells mediated by perforin is critical for preventing encephalitis due to reactivation of latent infection [17, 20]. It is important to state that the importance of CD8 T cells in controlling TE (toxoplasmic encephalitis) can be extended to humans, as the disease in HIV infected population occurred during advanced stages of infection, when CD8 T cell immunity in these patients was weakened [21].
In recent years, multifactorial steps in CD8 T cell activation, effector function acquisition, and memory cell differentiation have unfolded. However, many questions still remain unaddressed and the process may vary with the pathogen involved. Moreover, the process in T. gondii may be more complex in mice susceptible to the parasite (which develop TE) where, in spite of a very vigorous CD8 T cell effector immunity, the memory response is severely compromised [22]. In this article, we will discuss available knowledge about the multi-step process involved in CD8 T cell response to T. gondii and the factor(s) which inhibit the development of robust long-term immunity in a TE model.
Factors responsible for elicitation of CD8 T cell response against T. gondii
-
Antigen presentation and MHC class I molecules
The first step for the induction of CD8 T cell response is initiated by interaction of CD8 with antigen presenting cells and subsequent presentation of antigenic peptide [23]. Studies conducted with various mouse strains demonstrated that differences in MHC class I haplotype could influence the outcome of infection [16, 24, 25], thus further emphasizing the importance of CD8 T cells in the immunoprotection against the parasite. Importantly, studies conducted with mice expressing human MHC class I transgenes have demonstrated the allelic dependence for the control of T. gondii infection [26]. Thus, identifying the class I restricted T. gondii-specific epitope would be helpful in determining the antigens involved in eliciting CD8 T cell immune response against the parasite. Although several studies identified the antigens and secreted proteins like rhoptry, dense granule and microneme as T cell antigens, whether epitopes contained in these antigens correspond to dominant T. gondii-specific CD8 T cell response in the infected mice was not determined till recently [27-31]. While most of the CD8 epitopes are recognized by cells from BALB/C (H-2 L) mice, Wilson et.al identified a H-2 K restricted CD8 T cell epitope derived from Tgd057, a protein of unknown function [32]. However, based on the complex nature of the pathogen studies related to identification of multiple CD8 T cell epitopes responsible for the elicitation of protective immunity against T. gondii, needs to be intensified and their specificity in terms of stage-specific recognition determined. This information will be important in designing CD8-based vaccine for acute and chronic stage of infection.
-
Role of co-stimulatory molecules in the development of CD8 T cell immunity
Apart from T cell receptor–peptide interaction, a second cell-cell interplay involving co-stimulatory molecules is important for optimal CD8 T cell activation [33, 34]. Co-stimulation occurs mainly via interaction of two families of proteins, the immunoglobulin (Ig) superfamily members B7/CD28, and the TNF receptor/TNF superfamily [35]. Although the blockade of co-stimulatory molecules like CD80 and CD86 have been shown to dampen T cell activation in human PBMC [36], the mouse model demonstrates that animals lacking CD28 (a receptor for both CD80 and CD86) were not susceptible to T. gondii infection, in spite of lower IFNγ production by T cells [37]. However, when infected mice were rechallenged with the virulent (RH) parasite strain, the animals succumbed to infection. Similar observations have been made with CD40-CD40L pathway, and limited data available suggests that although these molecules may play a role in T cell activation, their absence does not profoundly affect the protective immunity against the parasite [38]. Along the same lines studies conducted with CD28 homolog, inducible T cell co-stimulator (ICOS) molecule demonstrated that CD8 T cell immunity in knockout mice was not significantly affected [39]. As the great majority of brain resident CD8 T cells express ICOS during chronic infection, it remains to be seen if the ICOS-ICOSL interaction has differential role in acute versus chronic infection [40]. Overall, a clear picture about the importance of co-stimulatory molecules like CD28-CD80/CD86, CD40-CD40L, ICOS-ICOS-L interaction in the development of CD8 T cell immunity during acute versus chronic infection needs to be investigated. Based on available reports, it may be fair to postulate that during acute infection, the stimulus from the parasite is so strong that requirement for these interactions is bypassed. However, positive signals from these interactions may be needed for the generation/maintenance of adequate CD8 T cell numbers and function. Recent studies performed in our laboratory have demonstrated that reinvigoration of CD8 T cell functionality during chronic toxoplasmosis remains ineffective if CD40-CD40L interaction is blocked [41]. Thus, in depth studies need to be conducted to determine the role of co-stimulatory molecules in the maintenance of CD8 T cell functionality, which is essential for keeping chronic infection under control.
The development of effective CD8 T cell immunity during infection without any immune-pathological consequences relies on a delicate balance on positive signals from co-stimulatory molecules and negative signal from inhibitory receptors like CTLA-4 and PD-1 [42, 43]. Although the role of CTLA-4 on CD8 T cell responses during T. gondii infection has not been studied in detail, the importance of PD-1 molecule on these cells has been recently evaluated and will be addressed separately.
-
Cytokines responsible for the development of CD8 T cell immunity against T. gondii
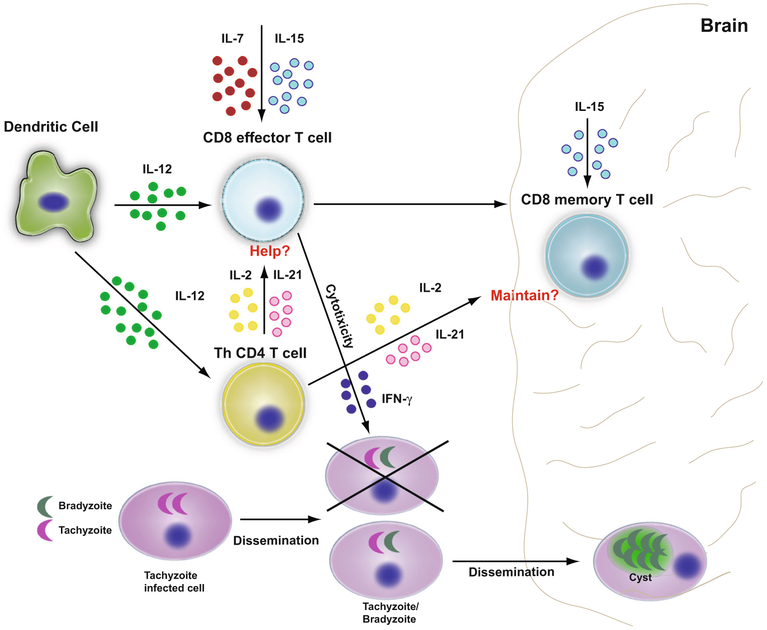
Once antigen-specific CD8 T cells are generated, they need to expand and differentiate which is modulated by cytokine milieu [44]. During infection, a number of inflammatory cell types like neutrophils, macrophages, and dendritic cells traffic to the site of infection and release cytokines and chemokines which decides the fate of CD8 T cell response [32, 45-50]. One of the pivotal cytokine which is responsible for the generation of CD8 T cell effectors or short lived effector cells (SLEC) is IL-12 [47, 51, 52]. The biologically active form of IL-12, IL-12p70 (composed of two subunits) [53] produced during T. gondii infection can induce CD8 T cells effector response [32]. The production of cytokine is strain dependent which affects CD8 effectors, underlying the importance of the cytokine in their generation [48]. Apart from IL-12, other related cytokines that are known to be important for CD8 T cell immunity are γ chain cytokines like IL-7 and IL-15 [54]. Unlike other pathogens, T. gondii does not induce a potent IL-2 response [55-57], which explains the fact that mice lacking CD4 T cells (which is a primary source of IL-2) generate a normal CD8 T cell effector response [58]. However, the studies related to IL-2 need to be investigated further as it has been reported that IL-2−/− mice exhibit poor CD8 T cell immunity [59]. Based on the data obtained from CD4 deficient mice [58], the role of IL-2 during toxoplasma infection may be restricted to maintenance of CD8 T memory and cytokine may be important in programming these cells for long-term survival (Fig. 1).
A possible role of IL-7 in the development of CD8 T cell response was reported several years ago by our laboratory in which it was demonstrated that exogenous treatment of infected animals with cytokine led to augmentation of cytotoxic CD8 T cell responses [55]. In the study conducted few years ago, we observed that in the absence of IL-15, endogenous IL-7 is critical for the development of CD8 T cell memory precursors via expression of anti-apoptotic protein Bcl-2 [54]. In contrast, the role of related γ chain cytokine IL-15, in the maintenance of CD8 T cell memory response has been established by our laboratory [54, 60-63]. Moreover, a dominant role of IL-15 independent of IL-7 in CD8 T cell recall response during secondary infection has been reported [64]. The importance of IL-15 has also been demonstrated during acute infection, where it regulates CD8 T cell burst size but not per cell function [54]. Interestingly, IL-15−/− mice survive T. gondii infection [65], which may be attributed to IL-7, as treatment with anti IL-7 antibody abrogates the protection in knock out animals [54]. These conclusions drawn from these studies is that although the role of both IL-7 and IL-15 is needed for the development of CD8 memory precursors, the later is primarily involved in the maintenance of this response, thus presumably playing an important role during chronic infection (Fig. 1). However, the role of other cytokines involved in the priming and maintenance of CD8 T cells needs to be further studied. In this regard, IL-21, another γc cytokine has been shown to affect the potency of CD8 T cell response in viral infections [66, 67]. Recent unpublished studies from our laboratory have observed the role of this cytokine in the maintenance of CD8 T cell immunity against T. gondii and will be discussed later in this review.
-
Helper role of CD4 T cells in the development of CD8 T cell response
The helper role of CD4 T cells in the induction and maintenance of CD8 T cell response has been debated for quite some time [68]. As regards to T. gondii, it was believed that along with CD8, CD4 T cells play a synergistic role in the protection against latent infection [69]. As for the involvement of CD4 T cells specifically in the induction of CD8 T cell response, we reported that robust CD8 T cell immunity in the absence of CD4 T cells can be induced [58]. In subsequent studies, it was demonstrated that in the absence of CD4 T cells, NK cells played an important role in the elicitation of CD8 T cell immunity [70]. Furthermore, depletion of NK cells in the CD4 −/− mice severely compromised the development of CD8 T cell response against the infection. The role of NK cells in helping the generation of CD8 T cell response was demonstrated to be mediated by their interaction with DC via NKG2D molecule [71]. The crosstalk enhanced the IL-12 production by DC leading to generation of strong CD8 T cell response. The role of NK cells in the development of CD8 T cell immunity has potential therapeutic implications in immunocompromised situations like HIV patients who can have severely depleted CD4 T cell numbers. Interestingly, although CD8 T cell response against T. gondii in the absence of CD4 T cells could be developed, it was not maintained [58]. These studies imply the importance of CD4 help in the maintenance of CD8 T cell response against the parasite and raise the question about the nature of help provided by these cells for the persistence of protective immunity. The important role of CD4 T cells in the long-term CD8 T cell immunity may be attributed to the programming of these cells at the time of induction. It is possible that in the absence of conventional CD4 T cells, CD8 T cells are not well programmed to maintain their numbers or even functionality, thus resulting in the inability of the susceptible host to prevent encephalitis. However, this cannot be extended to normal wild-type animals that develop encephalitis even when CD4 T cells are present. The loss of functionality, especially in memory CD8 T cells during chronic toxoplasmosis has been reported and will be discussed in a later part of this review [72]. Although the factors which trigger CD8 T cell dysfunction have not been worked out, loss of CD4 T cells during acute infection, which was reported several years ago, could be an important factor [73] and needs to be studied in detail. The nature of CD4 T cell help needed for development and maintenance of long-term CD8 functionality needs to be characterized and is currently under active investigation in our laboratory. In this regard, as stated above, it is important to note that during primary T. gondii infection, the parasite apparently does not induce a potent IL-2 response, which has been recently reported to be important for CD8 T cell functionality during secondary infection [74]. It may be postulated that a lack of IL-2 response results from CD4 T cell loss during acute infection, which ultimately effects the long-term survival of memory CD8 T cell population.
Fig. 1.
CD8 T cells immunity during T. gondii infection. During acute toxoplasma infection, strong IL-12 release by dendritic cells generates robust CD8 T cell effector response. The cytokine is also responsible for polarized Th1 response, which most likely helps in the expansion of effector CD8+ T cell immunity. While IL-7 and IL-15 (both γ chain cytokines) are required for the CD8 T cell effector development, maintenance of long-term memory response is dependent on IL-15. CD8 T cells secret IFNγ, which can inhibit the parasites replication and also eliminate the infected cells by their cytolytic ability. Under immunological pressure the disseminated tachyzoites convert to bradyzoites and remain apparently in a quiescent state in the tissues especially brain. Possible role of cytokines like IL-2 and IL-21 produced primarily by CD4 in this scenario needs further elucidation
CD8 T cells response during chronic Toxoplasmosis
As stated above, role of CD8 T cells in keeping chronic toxoplasmosis under control is well established. Paradoxically, despite a robust CD8 T cell response during acute phase of infection, long-term immunity against this pathogen is compromised in certain susceptible strains leading to host mortality [75]. Differential susceptibility to T. gondii reactivation in AIDS patients was also noted in a study conducted during pre-HAART era, which reported that only 30 % of AIDS patients with low CD4 count and Toxoplasma seropositivity, who were not on effective prophylaxis, developed reactivated toxoplasmosis [76]. Considering that memory CD8 T cells can persist for a lifetime and can mediate a robust recall response, it remains questionable whether the susceptibility to toxoplasmic reactivation is due to potential attrition of memory CD8 T cell response. As mentioned earlier, recent studies from our laboratory have reported that in C57BL/6 (mice susceptible to toxoplasmic encephalitis), CD8 T cells during later phase chronic encephalitis exhibit progressive attrition of functionality, increased apoptosis and poor recall response along with elevated expression of PD-1, an inhibitory receptor, a phenomenon referred to as CD8 exhaustion [22, 75]. Unlike chronic viral models, where this phenomenon has been extensively reported, T. gondii model represents an interesting or rather unique situation where despite initial control of parasitemia, the cells eventually get exhausted and lose their functionality.
With regards to T. gondii infection, the ability of CD8 T cells to produce IFNγ and exhibit cytotoxicity against the infected targets is critical for maintaining the chronicity of toxoplasma infection [10, 17, 77]. Loss of these functions, presumably even one of them may compromise the host ability to maintain the chronicity of infection. Thus, CD8 T cells possessing polyfunctional properties (like producing IFNγ and exhibiting cytotoxic activity against infected targets) most likely are best equipped to keep infection under control. In recent years, studies with virus models have shown that polyfunctional activity of virus-specific CD8 T cells, rather than the absolute numbers, correlates with the protection against the pathogen [78]. Along the same lines, we observed that concomitant with Toxoplasma reactivation and elevated CD8 mediated PD-1 expression, the frequency of polyfunctional CD8 T cells declined sharply in spleen and brain [22]. Interestingly, inhibitory receptor(s) like PD-1 are preferentially expressed on polyfunctional memory CD8 T cells, which become apoptotic leading to attrition of this population during chronic toxoplasmosis [22]. Although anti PDL-1 therapy revived CD8 T cell response, some of the functions remained refractory to this treatment [75]. Moreover, continuous anti PDL-1 treatment was needed to prevent the mortality of the chronically infected mice (Khan et.al unpublished observations).
Possible mechanism of CD8 T cell dysfunction during chronic infection
Though the mechanism(s) for CD8 T cell dysfunction during viral infections especially LCMV have started to unravel [79], very little information related to this phenomenon during T. gondii infection is available. As stated earlier, unlike viral infections where CD8 T cell exhaustion occurs apparently due to persistently high viral load, during toxoplasma infection, the dysfunction is manifested long after the initial control of parasitemia. Recent studies from our laboratory suggest a key role of γ chain cytokine IL-21 in controlling CD8 T cell dysfunction in the mouse model of encephalitis. (Khan et.al, manuscript in preparation). In these animals, unlike those who do not develop encephalitis, a significant dip in cytokine levels was observed. Moreover, significantly enhanced CD8 T cell dysfunction in mice lacking IL-21R was noted. Interestingly, another γ chain cytokine IL-15, which is critical for maintenance of CD8 T cell memory [80], apparently had minimal effect on CD8 T cell exhaustion as treatment with exogenous cytokine did not rescue the cells expressing high levels of PD-1 (Khan et al. manuscript in progress). The question which demands attention is as follows: the kind of effect IL-21 has on memory CD8 T cells that are responsible for the maintenance of their functionality and long-term survival (Fig. 1). The studies conducted in our laboratory have shown that in the absence of functional IL-21, the expression of positive costimulatory molecules on CD8 T cells from infected C57BL/6 is strongly downregulated which may be the cause of their functional impairment during chronic infection (Khan et al. manuscript in progress). The importance of some of the costimulatory molecules in the survival of memory CD8 T cells has been reported [81]. It should be noted that we have demonstrated that reversal of CD8 exhaustion by anti PDL-1 administration is dependent on CD40-CD40L interaction as CD8 T cells lacking CD40 are unresponsive to the treatment [41]. Thus, a balance between positive and negative costimulatory molecules for the development and maintenance of robust CD8 T cell immunity is needed to be studied in further detail.
Defective memory cell development during T. gondii infection
Memory CD8 T cells are critical for long-term protection against intracellular pathogens. Although during chronic toxoplasmosis, the majority of T. gondii-specific polyfunctional CD8 T cells exhibit cardinal markers of memory phenotype (CD44 and CD127) [22]. They show high PD-1 expression and on subset-specific analysis; they also express high levels of CD43, a marker of effector cell lineage. Memory cells expressing this marker have been reported to exhibit poor recall response [82] which is a hallmark of conventional population. It is important to note that in a recent study using a Listeria monocytogenes model, it has been demonstrated that the infection induces a strong central memory (CD62Lhi) CD8 T cell response and the cells have stem cell-like properties. These central memory cells are highly potent in term of recall response and clearance of infection [83]. Thus, it appears that during chronic infection like T. gondii, there is a skewed memory CD8 development which results in the inefficient control of chronic infection leading to encephalitis in the host. Rather than subscribing to the view that the chronic stage of infection is represented by bradyzoites containing cysts which are quiescent till any severe immune defect occurs in the host [84], we believe that in the presence of inadequate memory response, mild reactivation of infection may be an ongoing process in the hosts suffering from chronic toxoplasmosis. The dissemination of parasites, although controlled by the non-conventional memory CD8 T cells, is not sufficient to clear the infection. Thus, lack of development of conventional central memory population is needed to restrict the chronic infection and has strong therapeutic implications.
Conclusions and perspectives
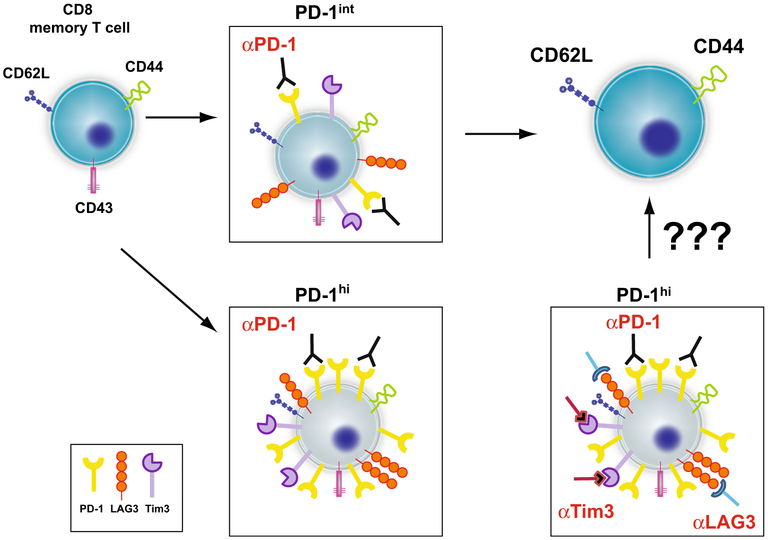
There is little doubt that CD8 T cells are critical for long-term protection against T. gondii infection, especially during the chronic phase of the disease. Although the infection generates a robust CD8 T cell effector response during acute infection, long-term immunity is compromised due to exhaustion of memory cells. For vaccine-based studies, CD8 T cell population needs to be targeted and the measures that will allow to maintain the functionality of memory cells need to be investigated. It is also very important to identify dominant class I restricted epitopes for T. gondii recognized during acute and chronic toxoplasmosis. Some of the work in this direction is being conducted in McLeod laboratory using human transgenic mice approach [85, 86]. However, the biggest challenge in the encephalitis model remains the dysfunctionality of memory CD8 T cell population. Without reversing the phenomenon of exhaustion, robust long-term CD8 T cell immunity against chronic infection will not be achievable and host will remain susceptible to encephalitis. Although blockade PD-1 receptor (a major inhibitor molecule) ameliorates the dysfunctionality of CD8 T cells and ensures the survival of infected animals, long-term treatment is needed, which can have severe consequences like autoimmune reactions in the host. However, recent studies in our laboratory have shown that combinatorial treatment with anti PDL-1 and antibody to other inhibitory receptor LAG-3 is more effective (Hwang et al. manuscript in progress). Thus, it is very essential to evaluate the pattern of inhibitory receptors expressed by highly exhausted CD8 (PD-1hi) expressing memory T cell population during chronic toxoplasmosis. Most likely, cocktail of antibodies against multiple inhibitory receptors like PD-1, LAG-3, and Tim3 may be needed to rescue this population (Fig. 2). Thus, short and effect treatment with antibody cocktail against these multiple inhibitory receptors, which restore memory CD8 T cell population in chronically, infected hosts’ needs to be worked out.
Fig. 2.
Restoration of CD8 T cell memory against chronic toxoplasmosis by inhibitory receptor blockade. CD8 memory Tcells (CD62Lhi CD44hi CD43hi) generated during chronic toxoplasma infection exhibit increased expression of inhibitory molecule, especially PD-1. The blockade of PD-1-PDL-1 interaction by antibody treatment is able to restore the functionality of PD-1inter memory cells but fail to reverse the exhaustion in PD-1hi expressing population. Treatment with cocktail, comprising of antibodies against multiple receptors TIM-3 and LAG-3 in addition to anti PDL-1 may be needed for rescue of highly exhausted CD8 T cells (PD-1hi) population
References
- 1.Dubey JP (1998) Advances in the life cycle of Toxoplasma gondii. Int J Parasitol 28:1019–1024 [DOI] [PubMed] [Google Scholar]
- 2.Hill DE, Dubey JP (2013) Toxoplasma gondii prevalence in farm animals in the United States. Int J Parasitol 43:107–113 [DOI] [PubMed] [Google Scholar]
- 3.Kaufmann SH (1999) Cell-mediated immunity: dealing a direct blow to pathogens. Curr Biol 9:R97–R99 [DOI] [PubMed] [Google Scholar]
- 4.Thorne KJ, Blackwell JM (1983) Cell-mediated killing of protozoa. Adv Parasitol 22:43–151 [DOI] [PubMed] [Google Scholar]
- 5.Yap GS, Sher A (1999) Cell-mediated immunity to Toxoplasma gondii: initiation, regulation and effector function. Immunobiology 201:240–247 [DOI] [PubMed] [Google Scholar]
- 6.Denkers EY (1999) T lymphocyte-dependent effector mechanisms of immunity to Toxoplasma gondii. Microbes Infect 1:699–708 [DOI] [PubMed] [Google Scholar]
- 7.Liesenfeld O, Kosek J, Remington JS, Suzuki Y (1996) Association of CD4+ T cell-dependent, interferon-gamma-mediated necrosis of the small intestine with genetic susceptibility of mice to peroral infection with Toxoplasma gondii. J Exp Med 184:597–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen SB, Maurer KJ, Egan CE, Oghumu S, Satoskar AR, Denkers EY (2013) CXCR3-dependent CD4(+) T cells are required to activate inflammatory monocytes for defense against intestinal infection. PLoS Pathog 9:e1003706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gigley JP, Fox BA, Bzik DJ (2009) Long-term immunity to lethal acute or chronic type II Toxoplasma gondii infection is effectively induced in genetically susceptible C57BL/6 mice by immunization with an attenuated type I vaccine strain. Infect Immun 77:5380–5388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khan IA, Ely KH, Kasper LH (1994) Antigen-specific CD8+ Tcell clone protects against acute Toxoplasma gondii infection in mice. J Immunol 152:1856–1860 [PubMed] [Google Scholar]
- 11.Khan IA, Green WR, Kasper LH, Green KA, Schwartzman JD (1999) Immune CD8(+) T cells prevent reactivation of Toxoplasma gondii infection in the immunocompromised host. Infect Immun 67:5869–5876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khan IA, Smith KA, Kasper LH (1988) Induction of antigen-specific parasiticidal cytotoxic T cell splenocytes by a major membrane protein (P30) of Toxoplasma gondii. J Immunol 141:3600–3605 [PubMed] [Google Scholar]
- 13.Suzuki Y, Remington JS (1990) The effect of anti-IFN-gamma antibody on the protective effect of Lyt-2+ immune T cells against toxoplasmosis in mice. J Immunol 144:1954–1956 [PubMed] [Google Scholar]
- 14.Parker SJ, Roberts CW, Alexander J (1991) CD8+ T cells are the major lymphocyte subpopulation involved in the protective immune response to Toxoplasma gondii in mice. Clin Exp Immunol 84:207–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hakim FT, Gazzinelli RT, Denkers E, Hieny S, Shearer GM, Sher A (1991) CD8+ T cells from mice vaccinated against Toxoplasma gondii are cytotoxic for parasite-infected or antigen-pulsed host cells. J Immunol 147:2310–2316 [PubMed] [Google Scholar]
- 16.Brown CR, McLeod R (1990) Class I MHC genes and CD8+ T cells determine cyst number in Toxoplasma gondii infection. J Immunol 145:3438–3441 [PubMed] [Google Scholar]
- 17.Suzuki Y, Wang X, Jortner BS, Payne L, Ni Y, Michie SA, Xu B, Kudo T, Perkins S (2010) Removal of Toxoplasma gondii cysts from the brain by perforin-mediated activity of CD8+ T cells. Am J Pathol 176:1607–1613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feliu V, Vasseur V, Grover HS, Chu HH, Brown MJ, Wang J, Boyle JP, Robey EA, Shastri N, Blanchard N (2013) Location of the CD8 T cell epitope within the antigenic precursor determines immunogenicity and protection against the Toxoplasma gondii parasite. PLoS Pathog 9:e1003449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhadra R, Cobb DA, Khan IA (2013) Donor CD8+ T cells prevent Toxoplasma gondii de-encystation but fail to rescue the exhausted endogenous CD8+ T cell population. Infect Immun 81:3414–3425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Denkers EY, Yap G, Scharton-Kersten T, Charest H, Butcher BA, Caspar P, Heiny S, Sher A (1997) Perforin-mediated cytolysis plays a limited role in host resistance to Toxoplasma gondii. J Immunol 159:1903–1908 [PubMed] [Google Scholar]
- 21.Shearer GM, Bernstein DC, Tung KS, Via CS, Redfield R, Salahuddin SZ, Gallo RC (1986) A model for the selective loss of major histocompatibility complex self-restricted T cell immune responses during the development of acquired immune deficiency syndrome (AIDS). J Immunol 137:2514–2521 [PubMed] [Google Scholar]
- 22.Bhadra R, Gigley JP, Khan IA (2012) PD-1-mediated attrition of polyfunctional memory CD8+ T cells in chronic toxoplasma infection. J Infect Dis 206:125–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xia D, Hao S, Xiang J (2006) CD8+ cytotoxic T-APC stimulate central memory CD8+ T cell responses via acquired peptide-MHC class I complexes and CD80 costimulation, and IL-2 secretion. J Immunol 177:2976–2984 [DOI] [PubMed] [Google Scholar]
- 24.McLeod R, Eisenhauer P, Mack D, Brown C, Filice G, Spitalny G (1989) Immune responses associated with early survival after peroral infection with Toxoplasma gondii. J Immunol 142:3247–3255 [PubMed] [Google Scholar]
- 25.Deckert-Schluter M, Schluter D, Schmidt D, Schwendemann G, Wiestler OD, Hof H (1994) Toxoplasma encephalitis in congenic B10 and BALB mice: impact of genetic factors on the immune response. Infect Immun 62:221–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown CR, David CS, Khare SJ, McLeod R (1994) Effects of human class I transgenes on Toxoplasma gondii cyst formation. J Immunol 152:4537–4541 [PubMed] [Google Scholar]
- 27.Hiszczynska-Sawicka E, Li H, Xu JB, Oledzka G, Kur J, Bickerstaffe R, Stankiewicz M (2010) Comparison of immune response in sheep immunized with DNA vaccine encoding Toxoplasma gondii GRA7 antigen in different adjuvant formulations. Exp Parasitol 124:365–372 [DOI] [PubMed] [Google Scholar]
- 28.Beghetto E, Nielsen HV, Del Porto P, Buffolano W, Guglietta S, Felici F, Petersen E, Gargano N (2005) A combination of antigenic regions of Toxoplasma gondii microneme proteins induces protective immunity against oral infection with parasite cysts. J Infect Dis 191:637–645 [DOI] [PubMed] [Google Scholar]
- 29.Reichmann G, Dlugonska H, Fischer HG (2002) Characterization of TgROP9 (p36), a novel rhoptry protein of Toxoplasma gondii tachyzoites identified by T cell clone. Mol Biochem Parasitol 119:43–54 [DOI] [PubMed] [Google Scholar]
- 30.Scorza T, D’Souza S, Laloup M, Dewit J, De Braekeleer J, Verschueren H, Vercammen M, Huygen K, Jongert E (2003) A GRA1 DNA vaccine primes cytolytic CD8(+) T cells to control acute Toxoplasma gondii infection. Infect Immun 71:309–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blanchard N, Gonzalez F, Schaeffer M, Joncker NT, Cheng T, Shastri AJ, Robey EA, Shastri N (2008) Immunodominant, protective response to the parasite Toxoplasma gondii requires antigen processing in the endoplasmic reticulum. Nat Immunol 9:937–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilson DC, Grotenbreg GM, Liu K, Zhao Y, Frickel EM, Gubbels MJ, Ploegh HL, Yap GS (2010) Differential regulation of effector- and central-memory responses to Toxoplasma gondii Infection by IL-12 revealed by tracking of Tgd057-specific CD8+ T cells. PLoS Pathog 6:e1000815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mescher MF, Curtsinger JM, Agarwal P, Casey KA, Gerner M, Hammerbeck CD, Popescu F, Xiao Z (2006) Signals required for programming effector and memory development by CD8+ T cells. Immunol Rev 211:81–92 [DOI] [PubMed] [Google Scholar]
- 34.Williams MA, Bevan MJ (2007) Effector and memory CTL differentiation. Annu Rev Immunol 25:171–192 [DOI] [PubMed] [Google Scholar]
- 35.Sharpe AH (2009) Mechanisms of costimulation. Immunol Rev 229:5–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Subauste CS, de Waal MR, Fuh F (1998) Role of CD80 (B7.1) and CD86 (B7.2) in the immune response to an intracellular pathogen. J Immunol 160:1831–1840 [PubMed] [Google Scholar]
- 37.Reichmann G, Villegas EN, Craig L, Peach R, Hunter CA (1999) The CD28/B7 interaction is not required for resistance to Toxoplasma gondii in the brain but contributes to the development of immunopathology. J Immunol 163:3354–3362 [PubMed] [Google Scholar]
- 38.Reichmann G, Walker W, Villegas EN, Craig L, Cai G, Alexander J, Hunter CA (2000) The CD40/CD40 ligand interaction is required for resistance to toxoplasmic encephalitis. Infect Immun 68:1312–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilson EH, Zaph C, Mohrs M, Welcher A, Siu J, Artis D, Hunter CA (2006) B7RP-1-ICOS interactions are required for optimal infection-induced expansion of CD4+ Th1 and Th2 responses. J Immunol 177:2365–2372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Villegas EN, Lieberman LA, Mason N, Blass SL, Zediak VP, Peach R, Horan T, Yoshinaga S, Hunter CA (2002) A role for inducible costimulator protein in the CD28- independent mechanism of resistance to Toxoplasma gondii. J Immunol 169:937–943 [DOI] [PubMed] [Google Scholar]
- 41.Bhadra R, Gigley JP, Khan IA (2011) Cutting edge: CD40-CD40 ligand pathway plays a critical CD8-intrinsic and -extrinsic role during rescue of exhausted CD8 Tcells. J Immunol 187:4421–4425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alegre ML, Frauwirth KA, Thompson CB (2001) T-cell regulation by CD28 and CTLA-4. Nat Rev Immunol 1:220–228 [DOI] [PubMed] [Google Scholar]
- 43.Jin HT, Ahmed R, Okazaki T (2011) Role of PD-1 in regulating T-cell immunity. Curr Top Microbiol Immunol 350:17–37 [DOI] [PubMed] [Google Scholar]
- 44.Kim MT, Harty JT (2014) Impact of inflammatory cytokines on effector and memory CD8+ T cells. Front Immunol 5:295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bliss SK, Butcher BA, Denkers EY (2000) Rapid recruitment of neutrophils containing prestored IL-12 during microbial infection. J Immunol 165:4515–4521 [DOI] [PubMed] [Google Scholar]
- 46.Bliss SK, Gavrilescu LC, Alcaraz A, Denkers EY (2001) Neutrophil depletion during Toxoplasma gondii infection leads to impaired immunity and lethal systemic pathology. Infect Immun 69:4898–4905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yap G, Pesin M, Sher A (2000) Cutting edge: IL-12 is required for the maintenance of IFN-gamma production in T cells mediating chronic resistance to the intracellular pathogen, Toxoplasma gondii. J Immunol 165:628–631 [DOI] [PubMed] [Google Scholar]
- 48.Robben PM, Mordue DG, Truscott SM, Takeda K, Akira S, Sibley LD (2004) Production of IL-12 by macrophages infected with Toxoplasma gondii depends on the parasite genotype. J Immunol 172:3686–3694 [DOI] [PubMed] [Google Scholar]
- 49.Aliberti J, Reis e Sousa C, Schito M, Hieny S, Wells T, Huffnagle GB, Sher A (2000) CCR5 provides a signal for microbial induced production of IL-12 by CD8 alpha+ dendritic cells. Nat Immunol 1:83–87 [DOI] [PubMed] [Google Scholar]
- 50.Del Rio L, Bennouna S, Salinas J, Denkers EY (2001) CXCR2 deficiency confers impaired neutrophil recruitment and increased susceptibility during Toxoplasma gondii infection. J Immunol 167:6503–6509 [DOI] [PubMed] [Google Scholar]
- 51.Khan IA, Matsuura T, Kasper LH (1994) Interleukin-12 enhances murine survival against acute toxoplasmosis. Infect Immun 62:1639–1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gazzinelli RT, Hieny S, Wynn TA, Wolf S, Sher A (1993) Interleukin 12 is required for the T-lymphocyte-independent induction of interferon gamma by an intracellular parasite and induces resistance in T-cell-deficient hosts. Proc Natl Acad Sci U S A 90:6115–6119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Trinchieri G, Pflanz S, Kastelein RA (2003) The IL-12 family of heterodimeric cytokines: new players in the regulation of T cell responses. Immunity 19:641–644 [DOI] [PubMed] [Google Scholar]
- 54.Bhadra R, Guan H, Khan IA (2010) Absence of both IL-7 and IL-15 severely impairs the development of CD8 T cell response against Toxoplasma gondii. PLoS One 5:e10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kasper LH, Matsuura T, Khan IA (1995) IL-7 stimulates protective immunity in mice against the intracellular pathogen, Toxoplasma gondii. J Immunol 155:4798–4804 [PubMed] [Google Scholar]
- 56.Haque S, Khan I, Haque A, Kasper L (1994) Impairment of the cellular immune response in acute murine toxoplasmosis: regulation of interleukin 2 production and macrophage-mediated inhibitory effects. Infect Immun 62:2908–2916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Candolfi E, Hunter CA, Remington JS (1994) Mitogen- and antigen-specific proliferation of T cells in murine toxoplasmosis is inhibited by reactive nitrogen intermediates. Infect Immun 62:1995–2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Casciotti L, Ely KH, Williams ME, Khan IA (2002) CD8(+)-T-cell immunity against Toxoplasma gondii can be induced but not maintained in mice lacking conventional CD4(+) T cells. Infect Immun 70:434–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Villegas EN, Lieberman LA, Carding SR, Hunter CA (2002) Susceptibility of interleukin-2-deficient mice to Toxoplasma gondii is associated with a defect in the production of gamma interferon. Infect Immun 70:4757–4761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Khan IA, Kasper LH (1996) IL-15 augments CD8+ T cell-mediated immunity against Toxoplasma gondii infection in mice. J Immunol 157:2103–2108 [PubMed] [Google Scholar]
- 61.Khan IA, Casciotti L (1999) IL-15 prolongs the duration of CD8+ T cell-mediated immunity in mice infected with a vaccine strain of Toxoplasma gondii. J Immunol 163:4503–4509 [PubMed] [Google Scholar]
- 62.Combe CL, Moretto MM, Schwartzman JD, Gigley JP, Bzik DJ, Khan IA (2006) Lack of IL-15 results in the suboptimal priming of CD4+ T cell response against an intracellular parasite. Proc Natl Acad Sci U S A 103:6635–6640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Khan IA, Moretto M, Wei XQ, Williams M, Schwartzman JD, Liew FY (2002) Treatment with soluble interleukin-15Ralpha exacerbates intracellular parasitic infection by blocking the development of memory CD8+ T cell response. J Exp Med 195:1463–1470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bhadra R, Khan IA (2012) IL-7 and IL-15 do not synergize during CD8 T cell recall response against an obligate intracellular parasite. Microbes Infect 14:1160–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lieberman LA, Villegas EN, Hunter CA (2004) Interleukin-15-deficient mice develop protective immunity to Toxoplasma gondii. Infect Immun 72:6729–6732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yi JS, Ingram JT, Zajac AJ (2010) IL-21 deficiency influences CD8 T cell quality and recall responses following an acute viral infection. J Immunol 185:4835–4845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Elsaesser H, Sauer K, Brooks DG (2009) IL-21 is required to control chronic viral infection. Science 324:1569–1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wiesel M, Oxenius A (2012) From crucial to negligible: functional CD8(+) T-cell responses and their dependence on CD4(+) T-cell help. Eur J Immunol 42:1080–1088 [DOI] [PubMed] [Google Scholar]
- 69.Gazzinelli R, Xu Y, Hieny S, Cheever A, Sher A (1992) Simultaneous depletion of CD4+ and CD8+ T lymphocytes is required to reactivate chronic infection with Toxoplasma gondii. J Immunol 149:175–180 [PubMed] [Google Scholar]
- 70.Combe CL, Curiel TJ, Moretto MM, Khan IA (2005) NK cells help to induce CD8(+)-T-cell immunity against Toxoplasma gondii in the absence of CD4(+) T cells. Infect Immun 73:4913–4921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Guan H, Moretto M, Bzik DJ, Gigley J, Khan IA (2007) NK cells enhance dendritic cell response against parasite antigens via NKG2D pathway. J Immunol 179:590–596 [DOI] [PubMed] [Google Scholar]
- 72.Gigley JP, Bhadra R, Khan IA (2011) CD8 T cells and Toxoplasma gondii: a new paradigm. J Parasitol Res 2011:243796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Khan IA, Matsuura T, Kasper LH (1996) Activation-mediated CD4+ T cell unresponsiveness during acute Toxoplasma gondii infection in mice. Int Immunol 8:887–896 [DOI] [PubMed] [Google Scholar]
- 74.Sa Q, Woodward J, Suzuki Y (2013) IL-2 produced by CD8+ immune T cells can augment their IFN-gamma production independently from their proliferation in the secondary response to an intracellular pathogen. J Immunol 190:2199–2207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bhadra R, Gigley JP, Weiss LM, Khan IA (2011) Control of Toxoplasma reactivation by rescue of dysfunctional CD8+ T-cell response via PD-1-PDL-1 blockade. Proc Natl Acad Sci U S A 108:9196–9201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Porter SB, Sande MA (1992) Toxoplasmosis of the central nervous system in the acquired immunodeficiency syndrome. N Engl J Med 327:1643–1648 [DOI] [PubMed] [Google Scholar]
- 77.Jordan KA, Hunter CA (2010) Regulation of CD8+ T cell responses to infection with parasitic protozoa. Exp Parasitol 126:318–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, Abraham J, Lederman MM, Benito JM, Goepfert PA, Connors M, Roederer M, Koup RA (2006) HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood 107:4781–4789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DA, Wherry EJ (2009) Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol 10:29–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sprent J, Surh CD (2001) Generation and maintenance of memory T cells. Curr Opin Immunol 13:248–254 [DOI] [PubMed] [Google Scholar]
- 81.Duttagupta PA, Boesteanu AC, Katsikis PD (2009) Costimulation signals for memory CD8+ T cells during viral infections. Crit Rev Immunol 29:469–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hikono H, Kohlmeier JE, Takamura S, Wittmer ST, Roberts AD, Woodland DL (2007) Activation phenotype, rather than central- or effector-memory phenotype, predicts the recall efficacy of memory CD8+ T cells. J Exp Med 204:1625–1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Graef P, Buchholz VR, Stemberger C, Flossdorf M, Henkel L, Schiemann M, Drexler I, Hofer T, Riddell SR, Busch DH (2014) Serial transfer of single-cell-derived immunocompetence reveals stemness of CD8(+) central memory T cells. Immunity 41:116–126 [DOI] [PubMed] [Google Scholar]
- 84.Melzer TC, Cranston HJ, Weiss LM, Halonen SK (2010) Host cell preference of Toxoplasma gondii cysts in murine brain: a confocal study. J Neuroparasitology 1, N100505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cong H, Mui EJ, Witola WH, Sidney J, Alexander J, Sette A, Maewal A, El Bissati K, Zhou Y, Suzuki Y, Lee D, Woods S, Sommerville C, Henriquez FL, Roberts CW, McLeod R (2012) Toxoplasma gondii HLA-B*0702-restricted GRA7(20–28) peptide with adjuvants and a universal helper T cell epitope elicits CD8(+) T cells producing interferon-gamma and reduces parasite burden in HLA-B*0702 mice. Hum Immunol 73:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.El Bissati K, Zhou Y, Dasgupta D, Cobb D, Dubey JP, Burkhard P, Lanar DE, McLeod R (2014) Effectiveness of a novel immunogenic nanoparticle platform for Toxoplasma peptide vaccine in HLA transgenic mice. Vaccine 32:3243–3248 [DOI] [PMC free article] [PubMed] [Google Scholar]