ABSTRACT
Cell migration is central to many developmental, physiologic and pathological processes, including cancer progression. The Ral GTPases (RalA and RalB) which act down-stream the Ras oncogenes, are key players in the coordination between membrane trafficking and actin polymerization. A major direct effector of Ral, the exocyst complex, works in polarized exocytosis and is at the center of multiple protein-protein interactions that support cell migration by promoting protrusion formation, front-rear polarization, and extra-cellular matrix degradation. In this review we describe the recent advancements in deciphering the molecular mechanisms underlying this role of Ral via exocyst on cell migration. Among others, we will discuss the recently identified cross-talk between Ral and Rac1 pathways: exocyst binds to a negative regulator (the RacGAP SH3BP1) and to the major effector (the Wave Regulatory Complex, WRC) of Rac1, the master regulator of protrusions. Next challenge will be to better characterize the dynamics in space and in time of these molecular interplays, to better understand the pleiotropic functions of Ral in both normal and cancer cells.
KEYWORDS: exocyst, migration, Rac, Ral, Rho, Ras, Wave
The Ral GTPases in cell migration, invasion and metastasis
The activation of Ras GTPases (K-Ras, H-Ras, N-Ras) by constitutive mutations is one of the most frequent oncogenic event in human cancers (roughly 30% of all tumors, 90% of pancreas tumors, 35% of colon tumors, 20% of lung tumors) (www.cbioportal.org). Three major down-stream pathways are stimulated by Ras proteins: the MAP kinases, the PI3 kinases and the Ral GTPases. Despite the fact that the selective targeting of the downstream kinase effectors of Ras (MAPK and PI3K) has seen substantial research investments, no effective targeted therapies currently exist for patients with tumors carrying mutant Ras. However, the targeting of Ral GTPases, downstream Ras, has not been extensively exploited.
RalA and RalB are monomeric GTPases belonging to the Ras family. By cycling between active GTP-bound and inactive GDP-bound states, they regulate a variety of cellular process, including survival, vesicle trafficking, autophagy and migration, which is the focus of this review. Ral proteins are key transducers of the oncogenic Ras signaling. Molecularly, some direct activators of Ral (the Ras-dependent RalGEFs: RalGDS, Rgl1, Rgl2, Rgl3) are direct effectors of Ras, supporting the existence of a direct Ral-Ras signaling axis. Indeed, the Ral pathway is a key player in human cancer progression, particularly in invasion and metastasis.1-3
A role for Ral in cell migration was first described in Drosophila melanogaster: the expression of the dominant-negative Ral gene delayed the onset of migration of “border cells” (a group of specific follicle cells that migrate in the egg chamber during the fly oogenesis), without affecting their migratory speed.4 In addition, by using a FRET-based biosensor approach in mammalian cells, Ral activity was locally detected at lamellipodia, strongly suggesting a crucial function for Ral in promoting protrusion formation.5 Finally, by using gene silencing approaches, RalB, but not RalA, was found to be required for the migration of both normal6 and cancer cells,7 pointing out non-overlapping and possibly even antagonist functions for the 2 Ral proteins with regard to the regulation of cell motility.
Concerning the role of Ral in in vivo invasion and metastasis, initial data came from the studies in mice: 3T3 cells could be transformed by the RasV12G37 mutant (a Ras effector mutant that activates RalGEFs but not Raf or PI3kinase8), and the RasV12G37-cells formed aggressive lung metastasis following lateral tail-vein injection; moreover, expression of a dominant negative RalB inhibited metastatic activity of RasV12G37-cells.9 Later, by silencing RalA or RalB in human pancreatic cancer cell lines, it was shown that both RalA and RalB are required for tumor metastasis when assessed by tail-vein injection but that RalB may play a more important role than RalA in this process.10 Consistently, in in vivo experimental metastasis models, using hamster cells injected in hamster, both constitutively active RalAV23 and RalBV23 increased the metastatic potential of transformed cells, with RalB being much more effective.11,12
Given this well-established role of Ral proteins, particularly of RalB, in cancer metastasis, it is a compelling priority of fundamental research to better characterize the underlying molecular mechanisms, i.e. to define in details the protein-protein physical and functional interaction networks and pathways cross-talks that link Ral to the migration/invasion machinery. We describe here the recent efforts to untangle the molecular complexity of these interactions.
Exocyst complex, a critical Ral effector for driving cell migration
Exocyst is a major Ral effector and a crucial mediator of the Ral functions in migration.6,13,14 It is an evolutionary conserved complex, originally identified for its role in polarized exocytosis and composed of 8 subunits (EXOC1–8 in humans).15 Active GTP-bound Ral proteins directly interact with the EXOC2/Sec 5 and EXOC8/Exo84 subunits (since the yeast nomenclature is still widely used, in this review it is added after the human nomenclature). Binding to Ral-GTP has been reported to regulate both localization and assembly of exocyst, but it remains unclear how exactly Ral controls exocyst functions.
A striking accumulation of findings identified exocyst as a hub at the center of multiple interactions with partners that are closely involved in cell migration. A concept emerging from this exocyst-centered network is that the exocyst complex is a key player in the spatio-temporal coordination between membrane trafficking and actin cytoskeleton plasticity during cell motility. Indeed, on one side exocyst shuttles between trafficking intracellular vesicles and the plasmamembrane, and on the other side it physically interacts with many regulators of the actin cytoskeleton, including several components of Rho-family pathways (Rac, Rho, Cdc42). Here below, we present the description of these interactions, classified according to their functional role in the 3 processes critical for migration: protrusion formation, front-rear polarization, and extra-cellular matrix degradation or deformation (Table 1, Fig. 1).
Table 1.
Direct partners of exocyst for migration/invasion functions.
| Exocyst subunits | Direct interactors | ||||
|---|---|---|---|---|---|
| Yeast names | |||||
| EXOC1 | Sec 3 | IQGAP27 | Kibra25 | WASH29 | |
| EXOC2 | Sec 5 | RalA, RalB | GEF-H132 | Paxillin13 | |
| EXOC3 | Sec 6 | Cyfip (Wave complex)20 | |||
| EXOC4 | Sec 8 | IQGAP27 | SH3BP117 | ||
| EXOC5 | Sec 10 | ||||
| EXOC6 | Sec 15 | ||||
| EXOC7 | Exo70 | Abi (Wave complex)20 | ARPC1 (Arp2/3 complex)21 | ||
| EXOC8 | Exo84 | RalA, RalB | SH3BP117 | WASH29 | Par626 |
Figure 1.
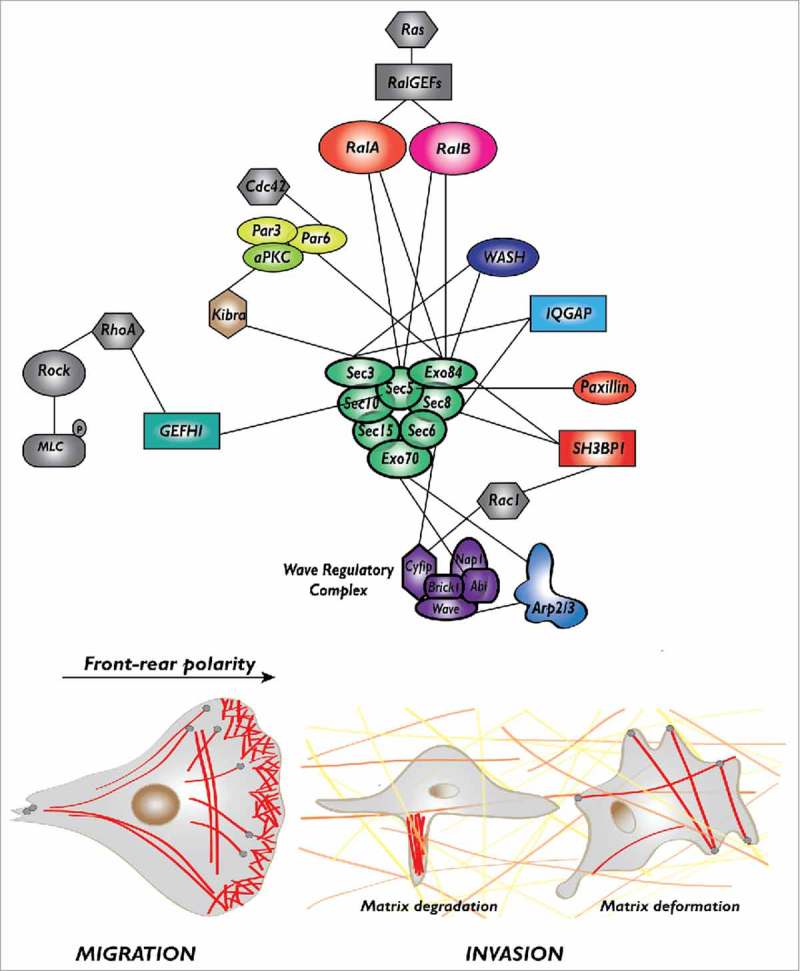
The protein-protein interaction network of Ral-exocyst controlling migration and invasion. The exocyst, a major effector of Ral proteins, directly interact with several key regulators of protrusion formation (Wave Regulatory Complex, Arp2/3 complex, SH3BP1, paxillin), front-rear polarity (Par complex) and extra-cellular matrix degradation (WASH, IQGAP) or deformation (GEFH1), to drive cell migration and invasion.
Protrusion formation. Cell protrusions are driven by the localized polymerization of actin which is regulated by a well-characterized molecular machinery: at the cell leading edge the active GTP-bound Rac1 GTPase binds and activates its target, the Wave Regulatory Complex (WRC), that in turn activates the Arp2/3 complex which promotes the branching of new actin filaments.16 Several molecular links connect exocyst to this Rac-WRC-Arp2/3 pathway.
First, EXOC4/Sec 8 and EXOC8/Exo84 bind to a negative regulator of Rac, the RacGAP SH3BP1;17 this interaction is essential to inactivate Rac1-GTP at the front, promoting together with several RacGEFS (including βPIX, DOCK3, Asef, and Tiam1) the continuous oscillation between the GDP-bound and GTP-bound conformations (namely the GTPase flux).18,19 The importance of Rac1 inactivation by SH3BP1 at the front for cell migration is demonstrated by the disorganization of the protrusions and the impaired cell velocity in cells depleted of SH3BP1 or expressing a constitutively active Rac1V12.17
Second, EXOC7/Exo70 and EXOC3/Sec 6 bind to the Abi and Cyfip subunits of the WRC complex, respectively, presumably contributing to the trafficking of WRC toward the edge of nascent protrusions20 (see below for an integrated model of WRC activation).
Third, EXOC7/Exo70 binds to the ARPC1 subunit of the Arp2/3 complex and directly stimulates Arp2/3 activity promoting actin branching.21,22 In addition, EXOC7/Exo70 interacts with PI(4,5)P2 phospholipid and induces plasma-membrane curvatures which might contribute to protrusion formation and migration.23 Finally, another molecular link at protrusions involves the interaction between EXOC2/Sec 5 and paxillin; this interaction targets exocyst to focal complexes, possibly contributing to the turnover of cell adhesions to substrate, a process essential for cell migration.13
Front-rear polarization. The Par complex, composed of Par3, Par6, aPKC, is a major molecular determinant of apico-basal cell polarity down-stream the Cdc42 GTPase in epithelial cells, but also of front-rear polarity axis during cell migration.24 An aPKC-exocyst (EXOC1/Sec 3) interaction, mediated by Kibra, an aPKC partner, is necessary for the localized activation of JNK at the leading edge, leading to localized paxillin phosphorylation, which controls the turnover of focal adhesion complexes.25 A Par6-exocyst (EXOC8/Exo84) interaction, promoted by RalA, regulates polarity in rat neuroblasts.26 These findings indicate that a direct molecular link between exocyst and Par complex contributes to the establishment of front-rear polarity in migrating cells.
Extra-cellular matrix degradation or deformation. Migration through an extra-cellular matrix (ECM) implies degradation and/or deformation of the ECM.
Exocyst is required for metalloproteinase secretion, ECM proteolysis, invadopodia formation and invasion of cancer cells.27,28 The molecular interactions underlying this role in ECM degradation involve the binding of EXOC1/Sec 3 and EXOC4/Sec 8 to the scaffold protein IQGAP1,27 and of EXOC1/Sec 3 and EXOC8/Exo84 to WASH,29 the endosomal Arp2/3 activator. Interestingly, ERK1/2-dependent phosphorylation of EXOC7/Exo70 promotes exocyst assembly, metalloproteinase secretion and invadopodia, possibly coordinating growth factor signaling and exocytosis.30
Moreover, exocyst, via association of EXOC2/Sec 5 with the RhoGEF GEF-H1,31 an activator of the RhoA GTPase, was also shown to contribute to cancer dissemination driven by ECM deformation independently of proteolysis, by activating the RhoA-ROCK pathway which generates actomyosin-dependent contractility forces that actively remodel the extracellular matrix.32
It is intriguing the fact that 2 different exocyst subunits were often found to bind to the same partner (EXOC2/Sec 5 and EXOC8/Exo84 to Ral proteins, EXOC7/Exo70 and EXOC3/Sec 6 to WRC, EXOC4/Sec 8 and EXOC8/Exo84 to SH3BP1, EXOC1/Sec 3 and EXOC4/Sec 8 to IQGAP1, EXOC1/Sec 3 and EXOC8/Exo84 to WASH). One might speculate that isolated subunit provides binding sites with weak affinities and that efficient binding is achieved only when a complete exocyst complex is well formed. Indeed, as recently observed by negative-stain electron microscopy33 and by reconstruction of the in vivo 3D architecture via fluorescence microscopy mapping,34 in the whole complex the exocyst subunits are intricately packed together to form an elongated architecture exposing composite surfaces available for binders.
Other players down-stream Ral for cell migration: RalBP1 and exosomes
Besides exocyst, another well-established effector of Ral proteins is RalBP1, also known as RLIP76.35,36 RalBP1 contains a RhoGAP domain with GTPase activating activity for Cdc42 and Rac1, 2 key Rho GTPases which control actin dynamics. Despite this apparently straightforward link between RalBP1 and these 2 Rho-family GTPases, the contribution of Ral-RalBP1 signaling to cell migration is controversial. In normal rat NRK cells RalBP1 was reported to be dispensable for motility,6 while it appears to be required in mouse 3T3 cells.37 The situation is rather complex since RalBP1 is an effector of Ral-GTP but also of R-Ras-GTP.37 It has been proposed that RalBP1 associates at recycling endosomes with activated R-Ras and with ARNO, a guanine nucleotide exchange factor for Arf6, to regulate Rac signaling, cell spreading and migration.37-39 Even more intriguingly, it has been reported that in pancreatic cancer cells RalBP1 functions down-stream RalB to regulate invadopodia formation, but surprisingly through a GAP-independent mechanism, since GAP deficient mutant rescued the invadopodium defect induced by loss of RalBP1; to the contrary, the supposed ATPase function of RalBP1, and perhaps its plasma membrane transport role, is required for invadopodium formation.40
Very recently Ral was found to regulate the secretion of extracellular vesicles (EVs), also called exosomes, which are secreted vesicles arising from the fusion of multivesicular bodies (MVBs) with the plasma-membrane and contributing to exchange information with distant cells by carrying proteins, lipids and nucleic acids.41 This Ral function, which does not involve the exocyst complex, was discovered and described in the C. elegans nematode and it appears conserved in mammals,42,43 but the precise mechanism of action of RalA and RalB in exosome secretion remains to be determined. Interestingly, accumulating evidences support a role of exosomes in tumor invasion,44,45 suggesting the appealing possibility that Ral pathway might contribute to cell migration via regulation of exosomes.
The cross-talk between Ral and Rac pathways
Here we will discuss more in details the cross-talk between Ral and Rac1 that our group recently identified17,19,20 (Fig. 2). As mentioned above, the Ral effector exocyst directly bind to a negative Rac regulator, the RacGAP SH3BP1, and to a major Rac effector, the Wave Regulatory Complex (WRC). Both SH3BP1 and WRC localize at the cell front in migrating cells, together with the exocyst. The fact that depletion of exocyst subunits decrease SH3BP1 and WRC recruitment suggests that its function in polarized exocytosis might contribute to the local delivery of regulatory molecules to migration front. It is tempting to speculate that exocyst works as “molecular taxi” physically transporting signaling molecules, including players of Rac pathway, from cytoplasm to leading edge. To experimentally challenge this hypothesis, we are currently performing advanced time-lapse imaging coupled to acute activation of Ral pathway.
Figure 2.
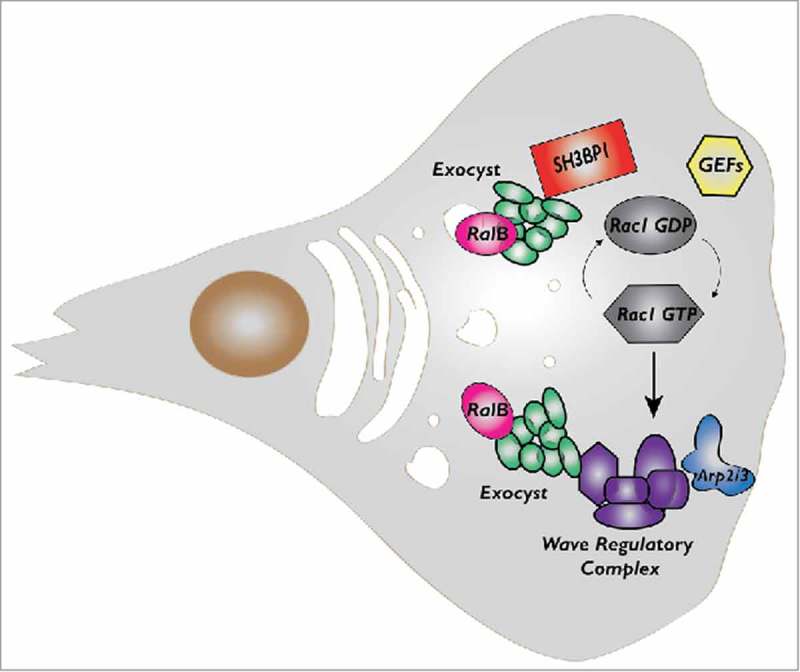
The cross-talk between RalB and Rac1 pathways during cell migration. While both RalA and RalB may participate to control cell migration, here we indicate RalB because its role is more robust and universal. RalB regulates assembly and localization of exocyst complex which in turn directly binds to a negative Rac1 regulator, the RacGAP SH3BP1, and to a major Rac1 effector, the Wave Regulatory Complex (WRC). RalB, exocyst, SH3BP1 and WRC, they all localize at the cell front in migrating cells even though the features and dynamics of their translocations appear different and need more investigation.
By integrating our findings with previous works we propose a dynamic model for WRC activation (Fig. 3). Exocyst, possibly by working as molecular taxi, would recruit WRC from cytosol to leading edge. Here WRC may be activated by the basal presence of low level of Rac1-GTP and of membrane PIP3 (phosphatidylinositol-trisphosphate). The positive feedback loop between actin cytoskeleton and Rac146,47 would lead to sustained Rac activity and stronger WRC stimulation. We speculate that Rac1-GTP might be responsible for retaining WRC at the edge, but this appealing hypothesis remains to be tested.
Figure 3.
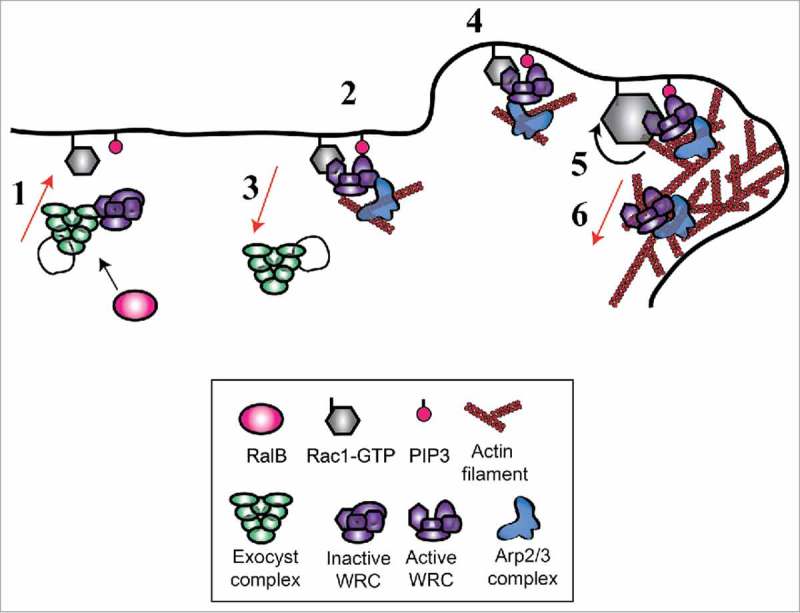
Model for Wave Regulatory Complex (WRC) recruitment and activation. Via interaction with exocyst, WRC is recruited to the front plasma-membrane (1) where it is moderately activated by the existing Rac1-GTP and PIP3 molecules (2). Rac1-GTP retains WRC at the edge, exocyst dissociates from WRC and recycles in the cytosol (3). This initial WRC activation is sufficient to start protrusion (4). The positive feedback loop between actin filaments and Rac146,47 leads to sustained Rac1 activation and stronger WRC stimulation (5). During the protrusion progression, a fraction of the WRC complex is incorporated in the actin network to be recycled (6).
This model provides a possible explanation for a paradoxical observation: the activation of Rac1 does not occur at the moment of forward movements of the cell edge, but with a non-negligible delay estimated, depending on the study, of 40 seconds48 or 2.2 minutes.47 The response function that quantitatively describes the conversion from the Rac1 molecular activity to the morphological changes was identified by a computational approach.49 Since Rac1 is generally considered the master regulator of protrusion, a puzzling question raised: how the cause (Rac1 activation) could occur after the effect (protrusion)? In our model, global Rac1 activation temporally occurs only after the effect of the positive feedback loop from actin cytoskeleton (step 5). The initiation of the protrusion (step 4) precedes the activation of the majority of Rac1 molecules, because it relies on the exocyst-dependent WRC translocation. This temporal event order would convincingly explain why the peak of Rac1 activity happens only after membrane forward movement. Consistently, while massive Rac1 activation is delayed, we observed that WRC recruitment is synchronous with edge movement.20 In other words, the temporal trigger of protrusion would be WRC recruitment rather than Rac1 activation.
Ral and migration plasticity
Cells move by using a variety of mechanisms, which result from the activation of different motility programs. Schematically, at least 3 distinct migration/invasion modes have been described: mesenchymal, amoeboid and collective.50 Both in embryogenesis and in cancer the cells show an impressive capacity to switch among these various migration/invasion programs.
Each program relies on specific molecular machineries whose activity must be tightly coordinated in space and time to generate productive movement. For instance, mesenchymal migration is driven by Rac1 that, via the Wave Regulatory Complex (WRC), activates Arp2/3-dependent actin polymerization to generate membrane protrusions; mesenchymal moving cells are elongated and attach to substrates via integrin-mediated adhesions. On the contrary, amoeboid migration relies more on RhoA-ROCK signaling to generate actomyosin contractility and mechanical forces exploited by cells to move forward; amoeboid moving cells squeeze through the interstitial spaces without need of matrix degradation or strong adhesion, in a manner largely independent from proteases and integrins.
Very interestingly, the RalB-Exocyst pathway is a common regulator of both Rac-driven and Rho-driven migration. As discussed in the previous chapter, in mesenchymal moving cells there is a cross-talk between RalB via exocyst and the Rac1 pathway (Fig. 2). On the other side, as mentioned above, there is also a cross-talk between RalB-exocyst and RhoA pathway via the interaction of EXOC2/Sec 5 with the RhoGEF GEF-H1,31,51,52 which stimulates the RhoA-ROCK signaling, cell contractility, mechanical forces, and cell dissemination by matrix deformation.32 This role of RalB in contractility-driven migration has been formally shown in a specific model of lung A549 cancer cells treated with TGF-β to promote epithelial-mesenchymal transition (EMT).32 It will be important to test whether it is a general mechanism conserved in amoeboid moving cells.
During migration/invasion, vesicles trafficking, actin polymerization and actomyosin contractility must be tightly coordinated. The switch among the various migration/invasion programs requires a continuous adjustment of the balance between these 3 processes, which are all regulated by Ral via the exocyst. These observations together support a potential role for Ral pathway in migration plasticity that warrants further investigations.
Conclusions
We start to have a good understanding on the molecular actors and on the protein-protein interactions underlying the role of Ral pathway, in particular of RalB, in cell migration and cancer invasion. However, we still lack a spatiotemporal dimension where to place these components and interactions. For example, it is very reasonable to assume that the interaction between exocyst and WRC that drives cell protrusion occur at distinct location and/or at different moment than the interaction between exocyst and GEFH1 that drives cell contractility. One future challenge is to better characterize these dynamics by advanced live imaging with high resolution, in space and in time.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by Fondation ARC pour la Recherche sur le Cancer (PJA 20151203371 to MCP), Ligue National contre le Cancer (RS14/75–54 to MCP), by Institut national de la Santé et de la Recherche médicale (Inserm, PC201530 to MCP), and Association Christelle Bouillot (to JC).
References
- [1].Camonis JH, White MA. Ral GTPases: corrupting the exocyst in cancer cells. Trends Cell Biol 2005; 15:327-32; PMID:15953551; https://doi.org/ 10.1016/j.tcb.2005.04.002 [DOI] [PubMed] [Google Scholar]
- [2].Shirakawa R, Horiuchi H. Ral GTPases: crucial mediators of exocytosis and tumourigenesis. J Biochem (Tokyo) 2015; 157:285-99; https://doi.org/ 10.1093/jb/mvv029 [DOI] [PubMed] [Google Scholar]
- [3].Gentry LR, Martin TD, Reiner DJ, Der CJ. Ral small GTPase signaling and oncogenesis: More than just 15 minutes of fame. Biochim Biophys Acta 2014; 1843:2976-88; PMID:25219551; https://doi.org/ 10.1016/j.bbamcr.2014.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Lee T, Feig L, Montell DJ. Two distinct roles for Ras in a developmentally regulated cell migration. Dev Camb Engl 1996; 122:409-18 [DOI] [PubMed] [Google Scholar]
- [5].Takaya A, Ohba Y, Kurokawa K, Matsuda M. RalA activation at nascent lamellipodia of epidermal growth factor-stimulated Cos7 cells and migrating Madin-Darby canine kidney cells. Mol Biol Cell 2004; 15:2549-57; PMID:15034142; https://doi.org/ 10.1091/mbc.E03-11-0857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Rossé C, Hatzoglou A, Parrini M-C, White MA, Chavrier P, Camonis J. RalB mobilizes the exocyst to drive cell migration. Mol Cell Biol 2006; 26:727-34; PMID:16382162; https://doi.org/ 10.1128/MCB.26.2.727-734.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Oxford G, Owens CR, Titus BJ, Foreman TL, Herlevsen MC, Smith SC, Theodorescu D. RalA and RalB: antagonistic relatives in cancer cell migration. Cancer Res 2005; 65:7111-20; PMID:16103060; https://doi.org/ 10.1158/0008-5472.CAN-04-1957 [DOI] [PubMed] [Google Scholar]
- [8].White MA, Nicolette C, Minden A, Polverino A, Van Aelst L, Karin M, Wigler MH. Multiple Ras functions can contribute to mammalian cell transformation. Cell 1995; 80:533-41; PMID:7867061; https://doi.org/ 10.1016/0092-8674(95)90507-3 [DOI] [PubMed] [Google Scholar]
- [9].Ward Y, Wang W, Woodhouse E, Linnoila I, Liotta L, Kelly K. Signal pathways which promote invasion and metastasis: critical and distinct contributions of extracellular signal-regulated kinase and Ral-specific guanine exchange factor pathways. Mol Cell Biol 2001; 21:5958-69; PMID:11486034; https://doi.org/ 10.1128/MCB.21.17.5958-5969.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lim K-H, O'Hayer K, Adam SJ, Kendall SD, Campbell PM, Der CJ, Counter CM. Divergent roles for RalA and RalB in malignant growth of human pancreatic carcinoma cells. Curr Biol CB 2006; 16:2385-94; PMID:17174914; https://doi.org/ 10.1016/j.cub.2006.10.023 [DOI] [PubMed] [Google Scholar]
- [11].Tchevkina E, Agapova L, Dyakova N, Martinjuk A, Komelkov A, Tatosyan A. The small G-protein RalA stimulates metastasis of transformed cells. Oncogene 2005; 24:329-35; PMID:15467745; https://doi.org/ 10.1038/sj.onc.1208094 [DOI] [PubMed] [Google Scholar]
- [12].Rybko VA, Knizhnik AV, Komelkov AV, Aushev VN, Trukhanova LS, Tchevkina EM. Different metastasis promotive potency of small G-proteins RalA and RalB in in vivo hamster tumor model. Cancer Cell Int 2011; 11:22; PMID:21714887; https://doi.org/ 10.1186/1475-2867-11-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Spiczka KS, Yeaman C. Ral-regulated interaction between Sec 5 and paxillin targets Exocyst to focal complexes during cell migration. J Cell Sci 2008; 121:2880-91; PMID:18697830; https://doi.org/ 10.1242/jcs.031641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hazelett CC, Yeaman C. Sec 5 and Exo84 mediate distinct aspects of RalA-dependent cell polarization. PloS One 2012; 7:e39602; PMID:22761837; https://doi.org/ 10.1371/journal.pone.0039602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wu B, Guo W. The Exocyst at a Glance. J Cell Sci 2015; 128:2957-64; PMID:26240175; https://doi.org/ 10.1242/jcs.156398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Derivery E, Gautreau A. Generation of branched actin networks: assembly and regulation of the N-WASP and WAVE molecular machines. BioEssays News Rev Mol Cell Dev Biol 2010; 32:119-31; https://doi.org/ 10.1002/bies.200900123 [DOI] [PubMed] [Google Scholar]
- [17].Parrini MC, Sadou-Dubourgnoux A, Aoki K, Kunida K, Biondini M, Hatzoglou A, Poullet P, Formstecher E, Yeaman C, Matsuda M, et al.. SH3BP1, an exocyst-associated RhoGAP, inactivates Rac1 at the front to drive cell motility. Mol Cell 2011; 42:650-61; PMID:21658605; https://doi.org/ 10.1016/j.molcel.2011.03.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Miller AL, Bement WM. Regulation of cytokinesis by Rho GTPase flux. Nat Cell Biol 2009; 11:71-7; PMID:19060892; https://doi.org/ 10.1038/ncb1814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Parrini MC, Camonis J. Cell motility: The necessity of Rac1 GDP/GTP flux. Commun Integr Biol 2011; 4:772-4; PMID:22446552; https://doi.org/ 10.4161/cib.17772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Biondini M, Sadou-Dubourgnoux A, Paul-Gilloteaux P, Zago G, Arslanhan MD, Waharte F, Formstecher E, Hertzog M, Yu J, Guerois R, et al.. Direct interaction between exocyst and Wave complexes promotes cell protrusions and motility. J Cell Sci 2016; 129:3756-69; PMID:27591259; https://doi.org/ 10.1242/jcs.187336 [DOI] [PubMed] [Google Scholar]
- [21].Zuo X, Zhang J, Zhang Y, Hsu S-C, Zhou D, Guo W. Exo70 interacts with the Arp2/3 complex and regulates cell migration. Nat Cell Biol 2006; 8:1383-8; PMID:17086175; https://doi.org/ 10.1038/ncb1505 [DOI] [PubMed] [Google Scholar]
- [22].Liu J, Zhao Y, Sun Y, He B, Yang C, Svitkina T, Goldman YE, Guo W. Exo70 stimulates the Arp2/3 complex for lamellipodia formation and directional cell migration. Curr Biol CB 2012; 22:1510-5; PMID:22748316; https://doi.org/ 10.1016/j.cub.2012.05.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Zhao Y, Liu J, Yang C, Capraro BR, Baumgart T, Bradley RP, Ramakrishnan N, Xu X, Radhakrishnan R, Svitkina T, et al.. Exo70 generates membrane curvature for morphogenesis and cell migration. Dev Cell 2013; 26:266-78; PMID:23948253; https://doi.org/ 10.1016/j.devcel.2013.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Khursheed M, Bashyam MD. Apico-basal polarity complex and cancer. J Biosci 2014; 39:145-55; PMID:24499799; https://doi.org/ 10.1007/s12038-013-9410-z [DOI] [PubMed] [Google Scholar]
- [25].Rosse C, Formstecher E, Boeckeler K, Zhao Y, Kremerskothen J, White MD, Camonis JH, Parker PJ. An aPKC-exocyst complex controls paxillin phosphorylation and migration through localised JNK1 activation. PLoS Biol 2009; 7:e1000235; PMID:19885391; https://doi.org/ 10.1371/journal.pbio.1000235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Das A, Gajendra S, Falenta K, Oudin MJ, Peschard P, Feng S, Wu B, Marshall CJ, Doherty P, Guo W, et al.. RalA promotes a direct exocyst-Par6 interaction to regulate polarity in neuronal development. J Cell Sci 2014; 127:686-99; PMID:24284074; https://doi.org/ 10.1242/jcs.145037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Sakurai-Yageta M, Recchi C, Le Dez G, Sibarita J-B, Daviet L, Camonis J, D'Souza-Schorey C, Chavrier P. The interaction of IQGAP1 with the exocyst complex is required for tumor cell invasion downstream of Cdc42 and RhoA. J Cell Biol 2008; 181:985-98; PMID:18541705; https://doi.org/ 10.1083/jcb.200709076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Liu J, Yue P, Artym VV, Mueller SC, Guo W. The role of the exocyst in matrix metalloproteinase secretion and actin dynamics during tumor cell invadopodia formation. Mol Biol Cell 2009; 20:3763-71; PMID:19535457; https://doi.org/ 10.1091/mbc.E08-09-0967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Monteiro P, Rossé C, Castro-Castro A, Irondelle M, Lagoutte E, Paul-Gilloteaux P, Desnos C, Formstecher E, Darchen F, Perrais D, et al.. Endosomal WASH and exocyst complexes control exocytosis of MT1-MMP at invadopodia. J Cell Biol 2013; 203:1063-79; PMID:24344185; https://doi.org/ 10.1083/jcb.201306162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ren J, Guo W. ERK1/2 regulate exocytosis through direct phosphorylation of the exocyst component Exo70. Dev Cell 2012; 22:967-78; PMID:22595671; https://doi.org/ 10.1016/j.devcel.2012.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Pathak R, Delorme-Walker VD, Howell MC, Anselmo AN, White MA, Bokoch GM, Dermardirossian C. The microtubule-associated Rho activating factor GEF-H1 interacts with exocyst complex to regulate vesicle traffic. Dev Cell 2012; 23:397-411; PMID:22898781; https://doi.org/ 10.1016/j.devcel.2012.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Biondini M, Duclos G, Meyer-Schaller N, Silberzan P, Camonis J, Parrini MC. RalB regulates contractility-driven cancer dissemination upon TGFβ stimulation via the RhoGEF GEF-H1. Sci Rep 2015; 5:11759; https://doi.org/ 10.1038/srep11759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Heider MR, Gu M, Duffy CM, Mirza AM, Marcotte LL, Walls AC, Farrall N, Hakhverdyan Z, Field MC, Rout MP, et al.. Subunit connectivity, assembly determinants and architecture of the yeast exocyst complex. Nat Struct Mol Biol 2016; 23:59-66; PMID:26656853; https://doi.org/ 10.1038/nsmb.3146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Picco A, Irastorza-Azcarate I, Specht T, Böke D, Pazos I, Rivier-Cordey A-S, Devos DP, Kaksonen M, Gallego O. The In Vivo Architecture of the Exocyst Provides Structural Basis for Exocytosis. Cell 2017; 168:400-12.e18; PMID:28129539; https://doi.org/ 10.1016/j.cell.2017.01.004 [DOI] [PubMed] [Google Scholar]
- [35].Jullien-Flores V, Dorseuil O, Romero F, Letourneur F, Saragosti S, Berger R, Tavitian A, Gacon G, Camonis JH. Bridging Ral GTPase to Rho pathways. RLIP76, a Ral effector with CDC42/Rac GTPase-activating protein activity. J Biol Chem 1995; 270:22473-7; PMID:7673236; https://doi.org/ 10.1074/jbc.270.38.22473 [DOI] [PubMed] [Google Scholar]
- [36].Cantor SB, Urano T, Feig LA. Identification and characterization of Ral-binding protein 1, a potential downstream target of Ral GTPases. Mol Cell Biol 1995; 15:4578-84; PMID:7623849; https://doi.org/ 10.1128/MCB.15.8.4578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Goldfinger LE, Ptak C, Jeffery ED, Shabanowitz J, Hunt DF, Ginsberg MH. RLIP76 (RalBP1) is an R-Ras effector that mediates adhesion-dependent Rac activation and cell migration. J Cell Biol 2006; 174:877-88; PMID:16966426; https://doi.org/ 10.1083/jcb.200603111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Lee S, Wurtzel JGT, Goldfinger LE. The RLIP76 N-terminus binds ARNO to regulate PI 3-kinase, Arf6 and Rac signaling, cell spreading and migration. Biochem Biophys Res Commun 2014; 454:560-5; PMID:25450693; https://doi.org/ 10.1016/j.bbrc.2014.10.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Wurtzel JGT, Lee S, Singhal SS, Awasthi S, Ginsberg MH, Goldfinger LE. RLIP76 regulates Arf6-dependent cell spreading and migration by linking ARNO with activated R-Ras at recycling endosomes. Biochem Biophys Res Commun 2015; 467:785-91; PMID:26498519; https://doi.org/ 10.1016/j.bbrc.2015.10.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Neel NF, Rossman KL, Martin TD, Hayes TK, Yeh JJ, Der CJ. The RalB small GTPase mediates formation of invadopodia through a GTPase-activating protein-independent function of the RalBP1/RLIP76 effector. Mol Cell Biol 2012; 32:1374-86; PMID:22331470; https://doi.org/ 10.1128/MCB.06291-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Tkach M, Théry C. Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell 2016; 164:1226-32; PMID:26967288; https://doi.org/ 10.1016/j.cell.2016.01.043 [DOI] [PubMed] [Google Scholar]
- [42].Hyenne V, Apaydin A, Rodriguez D, Spiegelhalter C, Hoff-Yoessle S, Diem M, Tak S, Lefebvre O, Schwab Y, Goetz JG, et al.. RAL-1 controls multivesicular body biogenesis and exosome secretion. J Cell Biol 2015; 211:27-37; PMID:26459596; https://doi.org/ 10.1083/jcb.201504136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Hyenne V, Labouesse M, Goetz JG. The Small GTPase Ral orchestrates MVB biogenesis and exosome secretion. Small GTPases 2016; Epub ahead of print; 1-7; PMID:27875100; https://doi.org/ 10.1080/21541248.2016.1251378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Hoshino D, Kirkbride KC, Costello K, Clark ES, Sinha S, Grega-Larson N, Tyska MJ, Weaver AM. Exosome secretion is enhanced by invadopodia and drives invasive behavior. Cell Rep 2013; 5:1159-68; PMID:24290760; https://doi.org/ 10.1016/j.celrep.2013.10.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Sung BH, Ketova T, Hoshino D, Zijlstra A, Weaver AM. Directional cell movement through tissues is controlled by exosome secretion. Nat Commun 2015; 6:7164; PMID:25968605; https://doi.org/ 10.1038/ncomms8164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Castro-Castro A, Ojeda V, Barreira M, Sauzeau V, Navarro-Lérida I, Muriel O, Couceiro JR, Pimentel-Muíños FX, Del Pozo MA, Bustelo XR. Coronin 1A promotes a cytoskeletal-based feedback loop that facilitates Rac1 translocation and activation. EMBO J 2011; 30:3913-27; PMID:21873980; https://doi.org/ 10.1038/emboj.2011.310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Kunida K, Matsuda M, Aoki K. FRET imaging and statistical signal processing reveal positive and negative feedback loops regulating the morphology of randomly migrating HT-1080 cells. J Cell Sci 2012; 125:2381-92; PMID:22344265; https://doi.org/ 10.1242/jcs.096859 [DOI] [PubMed] [Google Scholar]
- [48].Machacek M, Hodgson L, Welch C, Elliott H, Pertz O, Nalbant P, Abell A, Johnson GL, Hahn KM, Danuser G. Coordination of Rho GTPase activities during cell protrusion. Nature 2009; 461:99-103; PMID:19693013; https://doi.org/ 10.1038/nature08242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Yamao M, Naoki H, Kunida K, Aoki K, Matsuda M, Ishii S. Distinct predictive performance of Rac1 and Cdc42 in cell migration. Sci Rep 2015; 5:17527; PMID:26634649; https://doi.org/ 10.1038/srep17527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Friedl P, Alexander S. Cancer invasion and the microenvironment: plasticity and reciprocity. Cell 2011; 147:992-1009; PMID:22118458; https://doi.org/ 10.1016/j.cell.2011.11.016 [DOI] [PubMed] [Google Scholar]
- [51].Kent OA, Sandí M-J, Burston HE, Brown KR, Rottapel R. An oncogenic KRAS transcription program activates the RHOGEF ARHGEF2 to mediate transformed phenotypes in pancreatic cancer. Oncotarget 2016; 8(3):4484-4500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Meiri D, Marshall CB, Mokady D, LaRose J, Mullin M, Gingras A-C, Ikura M, Rottapel R. Mechanistic insight into GPCR-mediated activation of the microtubule-associated RhoA exchange factor GEF-H1. Nat Commun 2014; 5:4857; PMID:25209408; https://doi.org/ 10.1038/ncomms5857 [DOI] [PubMed] [Google Scholar]


