ABSTRACT
The exocyst complex mediates the tethering of secretory vesicles to the plasma membrane before SNARE-mediated membrane fusion. Recent studies have implicated the exocyst in a wide range of cellular processes. Particularly, research on the Exo70 subunit of the complex has linked the function of the exocyst in exocytosis to cell adhesion, migration and invasion. In this review, we will discuss the recent work on how Exo70 regulates these cellular processes, and how small GTPases and kinases interact with Exo70 to orchestrate its function in exocytosis and cytoskeleton organization. The study of Exo70 contributes to the understanding of many pathophysiological processes from organogenesis to cancer metastasis.
KEYWORDS: cell adhesion, cell invasion, cell migration, Exo70, exocyst, invadopodia, lamellipodia
The exocyst is an octameric protein complex consisting of Sec3, Sec5, Sec6, Sec8, Sec10, Sec15, Exo70 and Exo84. It primarily functions in the tethering of secretory vesicles to the plasma membrane before the soluble N-ethylmaleimide–sensitive factor attachment protein receptors (SNARE)-mediated membrane fusion.1,2 Recent studies from different fields have implicated the exocyst in various pathophysiological processes, such as cell cycle progression, tumor invasion and primary ciliogenesis.1,3 Particularly, study of the Exo70 subunit of the complex has unveiled some new mechanisms that link exocytosis to cell adhesion, migration and tumor invasion. Exo70, together with Sec3, mediates the association of the exocyst complex to the plasma membrane, which is a critical step for vesicle tethering.4,5 Exo70 directly interacts with PI(4,5)P2 in the plasma membrane through several negatively charged residues at its C-terminus. Disrupting the association of Exo70 and Sec3 with the plasma membrane results in defects in exocytosis. While the role of Exo70 in exocytosis has been well-established, we highlight the other cellular processes that implicate this interesting protein.
Exo70 in cell adhesion
Cells are connected to their neighboring cells or attached to the extracellular matrix (ECM). These adhesive interactions are important for maintaining cellular functions including proliferation and survival. Recent evidence has shown that Exo70 contributes to cell-ECM interaction. Exo70 is implicated in the targeting of Caveolin-1-positive vesicles to the plasma membrane during cell re-attachment to ECM (Fig. 1).6 Caveolin-1 is a major component of caveolae, which are believed to modulate the cell interaction with ECM through the association with integrin-mediated adhesion.7 The depletion of Exo70 impaired the delivery of Caveolin-1-positive vesicles to plasma membrane and consequently inhibited cell spreading.6 Similar phenotype was observed by silencing Sec5, another component of exocyst, in MEFs.8 In addition, Sec5 has also been implicated in focal complex formation.9 These studies suggest that Exo70 coordinates with other exocyst components in this function.
Figure 1.
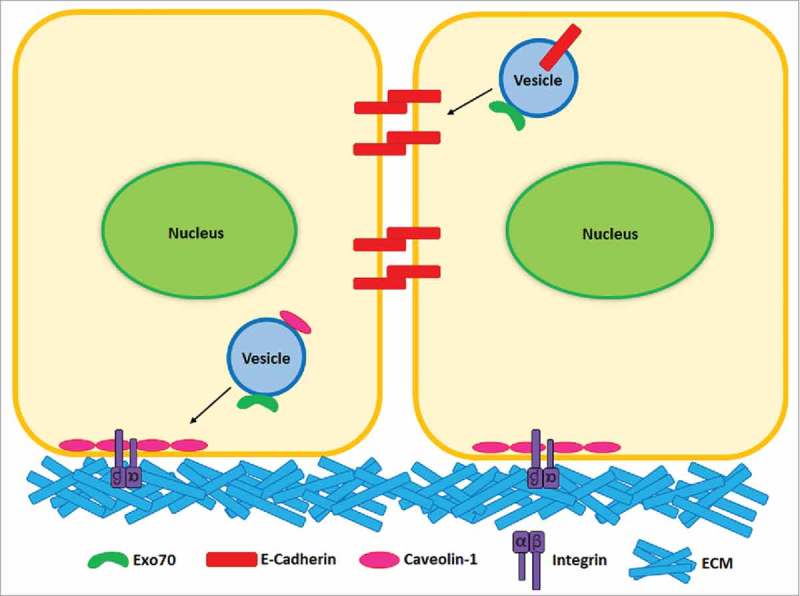
Exo70 in cell-ECM and cell-cell interaction. Exo70 targets integrin- and Caveolin-1-positive vesicles to the plasma membrane during cell attachment to ECM. Exo70 also directs the transport of E-Cadherin-positive vesicles to the lateral membrane to promote the formation of adherens junction.
In epithelial cells, the exocyst has been implicated in the formation of junctions.10-14 It was shown that Exo70 mediates the directional transport of E-Cadherin to lateral membrane and promotes the clustering of E-Cadherin on plasma membrane during the formation of adherens junctions (Fig. 1).15 Immunofluorescence microscopy studies indicated that lack of Exo70 led to an abnormal distribution of E-Cadherin on the lateral membrane and impaired maturation of adherens junction. Interestingly, Exo70 has several splicing isoforms that are differentially expressed in epithelial cells (“Exo70-E”) and mesenchymal cells (“Exo70-M”), respectively. Expression of Exo70-E contributes to epithelia formation and mesenchymal-epithelial transition.16
Exo70 in cell migration and invasion
Cell migration involves actin network reorganization, membrane remodeling, and trafficking of signaling and adhesion proteins to the leading edge. Actin polymerization and branching is controlled by the Arp2/3 complex.17 Exo70 directly binds to the Arp2/3 complex, and is localized to the leading edge of migrating cell.18,19 Exo70 enhances the interaction of the Arp2/3 complex with WAVE2 and accelerates actin branching (Fig. 2). This process is likely to be independent of the holo-exocyst complex as the stimulatory effect can be detected in vitro with recombinant Exo70.19 It was recently reported that Exo70 is also involved in the interaction of the exocyst complex and the WAVE regulatory complex (WRC).20 The WRC regulates the dynamics of actin cytoskeleton by stimulating the activity of the Arp2/3 complex at the plasma membrane to control cell motility. The interaction between Exo70 and WRC is responsible for the recruitment of WRC to sites of the plasma membrane where protrusions are formed in migrating cells. WRC recruitment coincides with leading edge movement, demonstrating that the association of Exo70 with WRC contributes to cell motility. It is very likely that Exo70 plays dual roles in migrating cells through mediating actin cytoskeleton: a kinetic activator that directly controls actin branching and a molecular carrier that transports regulatory molecules of Arp2/3 complex. In addition to remodeling actin, Exo70 can form oligomers via its N-terminus to generate negative curvature on the plasma membrane, a process that could also be independent of the whole exocyst complex.21 This membrane-deforming ability contributes to protrusion formation (Fig. 2). As elaborated above, the exocyst also mediates the delivery of focal adhesion molecules such as integrins to the plasma membrane. In addition, Exo70 was shown to interact with PIPKIγi2 to direct the polarized integrin trafficking during directional cell migration.22
Figure 2.
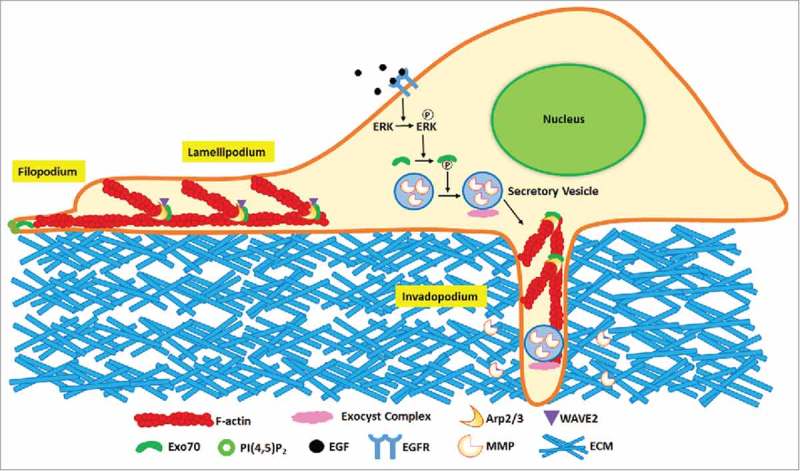
Exo70 in cell migration and invasion. During cell migration, Exo70 generates membrane curvature, which contributes to membrane protrusion at the leading edge. Exo70 also promotes the interaction of the Arp2/3 complex with its activators such as WAVE2, stimulating actin filament nucleation and branching. During cell invasion, Exo70, together with other exocyst components, participates in the secretion of matrix metalloproteinases (MMPs), which mediate ECM degradation by invadopodia. Phosphorylation of Exo70 by ERK promotes the assembly of Exo70 with other exocyst components, thereby enhancing MMP exocytosis.
Cell invasion often involves the generation of matrix-degrading structures that are termed as invadopodia. Mueller and colleagues proposed a sequential model of invadopodia formation, which starts with actin-based structure precursor and then mature into ECM degradation.23 Inhibition of invadopodial structure assembly blocks the ECM degradation. With RNAi knockdown of the Exo70, the number of invadopodial sites, as represented by actin puncta, diminishes, and ECM degradation is reduced significantly.24 In addition to modulating the Arp2/3 complex-mediated actin remodeling, Exo70 regulates the invadopodial activity by promoting MMPs secretion (Fig. 2),24 Gelatin zymography detected a significant reduction in the levels of MMP-2 and MMP-9 in Exo70 knockdown cells, whereas re-expression of RNAi-resistant Exo70 recovers the secretion of the two proteins.
Regulation of Exo70
The Rho family of small GTPases regulates many cellular processes. In yeast, Exo70 was identified as downstream effector of Rho3 and Cdc42, both of which were implicated in regulating polarized exocytosis.25-27 In mammalian cells, Exo70 is associated with TC10, another Rho family protein. In response to insulin, GTP-TC10 recruits Exo70 and other members of the exocyst, which tethers Glut4-containing vesicles to the plasma membrane.28 In developing neurons, NGF induces TC10-Exo70 complex assembly and this complex locally prevents Cdc42-dependent activation of N-WASP at the plasma membrane.29 Additionally, TC10-Exo70 complex stimulated by IGF functions in axonal membrane expansion and polarized delivery of IGF-1 receptor.30
Recent studies underlie the involvement of MAPK signaling in the secretory pathway. Exo70 was identified as a direct substrate of ERK1/2.31 Upon EGF stimulation, ERK1/2 phosphorylates Exo70 at serine 250. The phosphorylation of Exo70 promotes the assembly of the exocyst complex, which regulates MMP exocytosis and invadopodia activity (Fig. 2). It was also found that Exo70 is highly phosphorylated in metastatic melanoma cells from patients with BRAFV600E mutation; inhibition of the RAF-MEK-ERK signaling pathway decreases the phosphorylation of Exo70 and inhibited invadopodia formation in melanoma cells.32
Conclusions and perspectives
In this mini-review, we summarize the recent studies regarding the function of Exo70 in cells. Through the targeting of cargos to specific membrane domains, and through its involvement in membrane curvature induction and actin remodeling, Exo70 plays important roles in many cellular processes.
Despite these progresses, many questions remain unanswered. For example, as Exo70 was shown to interact with many molecules, how are these interactions spatially and temporally orchestrated for certain cellular functions? Also, since there are so many isoforms for Exo70,32,33 what controls the expression of these isoforms in cells, in different tissues, and at different developmental stages? These questions are much better addressed in plant biology,33 but still need to be studied in depth in mammalian cells. Answering these questions will not only elucidate the function of Exo70 and the exocyst complex, but also contribute to the understanding of many pathophysiological processes from organogenesis to cancer metastasis.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
The work in Wei Guo laboratory is supported by NIH grant GM111128.
References
- [1].Wu B, Guo W. The exocyst at a glance. J Cell Sci 2015; 128:2957-64; PMID:26240175; https://doi.org/ 10.1242/jcs.156398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Pfeffer SR. Transport-vesicle targeting: tethers before SNAREs. Nat Cell Biol 1999; 1:E17-22; PMID:10559876; https://doi.org/ 10.1038/8967 [DOI] [PubMed] [Google Scholar]
- [3].Das A, Guo W. Rabs and the exocyst in ciliogenesis, tubulogenesis and beyond. Trends Cell Biol 2011; 21:383-6; https://doi.org/ 10.1016/j.tcb.2011.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Liu J, Zuo X, Yue P, Guo W. Phosphatidylinositol 4,5-bisphosphate mediates the targeting of the exocyst to the plasma membrane for exocytosis in mammalian cells. Mol Biol Cell 2007; 18:4483-92; PMID:17761530; https://doi.org/ 10.1091/mbc.E07-05-0461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].He B, Xi F, Zhang X, Zhang J, Guo W. Exo70 interacts with phospholipids and mediates the targeting of the exocyst to the plasma membrane. EMBO J 2007; 26:4053-65; PMID:17717527; https://doi.org/ 10.1038/sj.emboj.7601834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hertzog M, Monteiro P, Le Dez G, Chavrier P. Exo70 subunit of the exocyst complex is involved in adhesion-dependent trafficking of caveolin-1. PLoS One 2012; 7:e52627; PMID:23300727; https://doi.org/ 10.1371/journal.pone.0052627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Navarro A, Anand-Apte B, Parat MO. A role for caveolae in cell migration. FASEB J 2004; 18:1801-11; PMID:15576483; https://doi.org/ 10.1096/fj.04-2516rev [DOI] [PubMed] [Google Scholar]
- [8].Balasubramanian N, Meier JA, Scott DW, Norambuena A, White MA, Schwartz MA. RalA-exocyst complex regulates integrin-dependent membrane raft exocytosis and growth signaling. Curr Biol 2010; 20:75-9; https://doi.org/ 10.1016/j.cub.2009.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Spiczka KS, Yeaman C. Ral-regulated interaction between Sec 5 and paxillin targets Exocyst to focal complexes during cell migration. J Cell Sci 2008; 121:2880-91; PMID:18697830; https://doi.org/ 10.1242/jcs.031641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Grindstaff KK YC, Anandasabapathy N, Hsu SC, Rodriguez-Boulan E, Scheller RH, Nelson WJ. Sec 6/8 complex is recruited to cell-cell contacts and specifies transport vesicle delivery to the basal-lateral membrane in epithelial cells. Cell 1998; 93:731-40; PMID:9630218; https://doi.org/ 10.1016/S0092-8674(00)81435-X [DOI] [PubMed] [Google Scholar]
- [11].Park KM, Fogelgren B, Zuo X, Kim J, Chung DC, Lipschutz JH. Exocyst Sec 10 protects epithelial barrier integrity and enhances recovery following oxidative stress, by activation of the MAPK pathway. Am J Physiol Renal Physiol 2010; 298:F818-26; PMID:20053792; https://doi.org/ 10.1152/ajprenal.00596.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Yeaman C, Grindstaff KK, Nelson WJ. Mechanism of recruiting Sec 6/8 (exocyst) complex to the apical junctional complex during polarization of epithelial cells. sJ Cell Sci 2004; 117:559-70; PMID:14709721; https://doi.org/ 10.1242/jcs.00893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Oztan A SM, Weisz OA, Bradbury NA, Hsu SC, Goldenring JR, Yeaman C, Apodaca G. Exocyst requirement for endocytic traffic directed toward the apical and basolateral poles of polarized MDCK cells. Mol Biol Cell 2007; 18:3978-92; PMID:17686995; https://doi.org/ 10.1091/mbc.E07-02-0097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Andersen NJ YC. Sec 3-containing exocyst complex is required for desmosome assembly in mammalian epithelial cells. Mol Biol Cell 2010; 21:152-64; PMID:19889837; https://doi.org/ 10.1091/mbc.E09-06-0459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Xiong X XQ, Huang Y, Singh RD, Anderson R, Leof E, Hu J, Ling K. An association between type Iγ PI4P 5-kinase and Exo70 directs E-cadherin clustering and epithelial polarization. Mol Biol Cell 2012; 23:87-98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lu H, Liu J, Liu S, Zeng J, Ding D, Carstens RP, Cong Y, Xu X, Guo W. Exo70 isoform switching upon epithelial-mesenchymal transition mediates cancer cell invasion. Dev Cell 2013; 27:560-73; https://doi.org/ 10.1016/j.devcel.2013.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Krause M GA. Steering cell migration: lamellipodium dynamics and the regulation of directional persistence. Nat Rev Mol Cell Biol 2014; 15:577-90; PMID:25145849; https://doi.org/ 10.1038/nrm3861 [DOI] [PubMed] [Google Scholar]
- [18].Zuo X, Zhang J, Zhang Y, Hsu SC, Zhou D, Guo W. Exo70 interacts with the Arp2/3 complex and regulates cell migration. Nat Cell Biol 2006; 8:1383-8; PMID:17086175; https://doi.org/ 10.1038/ncb1505 [DOI] [PubMed] [Google Scholar]
- [19].Liu J, Zhao Y, Sun Y, He B, Yang C, Svitkina T, Goldman YE, Guo W. Exo70 stimulates the Arp2/3 complex for lamellipodia formation and directional cell migration. Curr Biol 2012; 22:1510-5; https://doi.org/ 10.1016/j.cub.2012.05.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Biondini M S-DA, Paul-Gilloteaux P, Zago G, Arslanhan MD, Waharte F, Formstecher E, Hertzog M, Yu J, Guerois R, Gautreau A, Scita G, Camonis J, Parrini MC. Direct interaction between Exocyst and Wave complexes promotes cell protrusions and motility. J Cell Sci 2016; 129:3756-69; PMID:27591259; https://doi.org/ 10.1242/jcs.187336 [DOI] [PubMed] [Google Scholar]
- [21].Zhao Y, Liu J, Yang C, Capraro BR, Baumgart T, Bradley RP, Ramakrishnan N, Xu X, Radhakrishnan R, Svitkina T, et al.. Exo70 generates membrane curvature for morphogenesis and cell migration. Dev Cell 2013; 26:266-78; https://doi.org/ 10.1016/j.devcel.2013.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Thapa N, Sun Y, Schramp M, Choi S, Ling K, Anderson RA. Phosphoinositide signaling regulates the exocyst complex and polarized integrin trafficking in directionally migrating cells. Dev Cell 2012; 22:116-30; https://doi.org/ 10.1016/j.devcel.2011.10.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Artym VV, Zhang Y, Seillier-Moiseiwitsch F, Yamada KM, Mueller SC. Dynamic interactions of cortactin and membrane type 1 matrix metalloproteinase at invadopodia: defining the stages of invadopodia formation and function. Cancer Res 2006; 66:3034-43; PMID:16540652; https://doi.org/ 10.1158/0008-5472.CAN-05-2177 [DOI] [PubMed] [Google Scholar]
- [24].Liu J YP, Artym VV, Mueller SC, Guo W. The role of the exocyst in matrix metalloproteinase secretion and actin dynamics during tumor cell invadopodia formation. Mol Biol Cell 2009; 20:3763-71; PMID:19535457; https://doi.org/ 10.1091/mbc.E08-09-0967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Adamo JE RG, Brennwald P. The Rho GTPase Rho3 has a direct role in exocytosis that is distinct from its role in actin polarity. Mol Biol Cell 1999; 10:4121-33; https://doi.org/ 10.1091/mbc.10.12.4121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Adamo JE, Moskow JJ, Gladfelter AS, Viterbo D, Lew DJ, Brennwald PJ. Yeast Cdc42 functions at a late step in exocytosis, specifically during polarized growth of the emerging bud. J Cell Biol 2001; 155:581-92; PMID:11706050; https://doi.org/ 10.1083/jcb.200106065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Dong G, Hutagalung AH, Fu C, Novick P, Reinisch KM. The structures of exocyst subunit Exo70p and the Exo84p C-terminal domains reveal a common motif. Nat Struct Mol Biol 2005; 12:1094-100; PMID:16249794; https://doi.org/ 10.1038/nsmb1017 [DOI] [PubMed] [Google Scholar]
- [28].Inoue M CL, Hwang J, Chiang SH, Saltiel AR. The exocyst complex is required for targeting of Glut4 to the plasma membrane by insulin. Nature 2003; 422:629-33; PMID:12687004; https://doi.org/ 10.1038/nature01533 [DOI] [PubMed] [Google Scholar]
- [29].Pommereit D, Wouters FS. An NGF-induced Exo70-TC10 complex locally antagonises Cdc42-mediated activation of N-WASP to modulate neurite outgrowth. J Cell Sci 2007; 120:2694-705; PMID:17635999; https://doi.org/ 10.1242/jcs.03475 [DOI] [PubMed] [Google Scholar]
- [30].Dupraz S, Grassi D, Bernis ME, Sosa L, Bisbal M, Gastaldi L, Jausoro I, Cáceres A, Pfenninger KH, Quiroga S. The TC10-Exo70 complex is essential for membrane expansion and axonal specification in developing neurons. J Neurosci 2009; 29:13292-301; PMID:19846717; https://doi.org/ 10.1523/JNEUROSCI.3907-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ren J, Guo W. ERK1/2 regulate exocytosis through direct phosphorylation of the exocyst component Exo70. Dev Cell 2012; 22:967-78; https://doi.org/ 10.1016/j.devcel.2012.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lu H, Liu S, Zhang G, Kwong LN, Zhu Y, Miller JP, Hu Y, Zhong W, Zeng J, Wu L, et al.. Oncogenic BRAF-mediated melanoma cell invasion. Cell Rep 2016; 15:2012-24; https://doi.org/ 10.1016/j.celrep.2016.04.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Vukašinović N, Žárský V. Tethering Complexes in the Arabidopsis Endomembrane System. Front Cell Dev Biol 2016; 4:46; PMID:27243010; https://doi.org/ 10.3389/fcell.2016.00046 [DOI] [PMC free article] [PubMed] [Google Scholar]


