Abstract
The high volume production compound bisphenol A (BPA) is of environmental concern largely because of its estrogenic activity. Consequently, BPA analogues have been synthesized to be considered as replacement molecules for BPA. These analogues need to be thoroughly evaluated for their estrogenic activity. Here, we combined mechanism zebrafish-based assays to examine estrogenic and anti-estrogenic activities of BPA and two of its analogues, bisphenol AF (BPAF) and bisphenol C (BPC) in vitro and in vivo. In vitro reporter cell lines were used to investigate agonistic and antagonistic effects of the three bisphenols on the three zebrafish estrogen receptors. The transgenic Tg(5×ERE:GFP) and Cyp19a1b-GFP zebrafish lines were then used to analyze estrogenic and anti-estrogenic responses of the three bisphenols in vivo. BPA, BPAF and BPC were agonists with different potencies for the three zebrafish estrogen receptors in vitro. The potent zfERα-mediated activity of BPA and BPAF in vitro resulted in vivo by activation of GFP expression in zebrafish larvae in the heart (zfERα-dependent) at lower concentrations, and in the liver (zfERβ-dependent) at higher concentrations. BPC induced zfERβ-mediated luciferase expression in vitro, and the zfERβ agonism led to activation of GFP expression in the liver and the brain in vivo. In addition, BPC acted as a full antagonist on zfERα, and completely inhibited estrogen-induced GFP expression in the heart of the zebrafish larvae. To summarize, applying a combination of zebrafish-based in vitro and in vivo methods to evaluate bisphenol analogues for estrogenic activity will facilitate the prioritization of these chemicals for further analysis in higher vertebrates as well as the risk assessment in humans.
Keywords: zebrafish, bisphenol, estrogen receptor, transgenic fish, reporter cell lines
Introduction
The environmental pollutant bisphenol A (BPA) has been widely studied during the past decade. BPA has since the 1950s been used to make epoxy resin and polycarbonate plastics, and it is one of the highest volume chemicals produced worldwide. BPA is found in products such as reusable food and beverage containers, lining of cans, dental materials, computers, and thermal paper. Through its estrogenic activity, BPA has been shown to cause deleterious effects in laboratory animals, such as abnormal fetal development (Palanza et al., 2008), alterations in sex differentiation (Rubin et al., 2006), reproduction (Honma et al., 2002), brain function and mood (Leranth et al., 2008; Elsworth et al., 2013) as well as promotion of certain types of cancers (Markey et al., 2001; Munoz-de-Toro et al., 2005; Ho et al., 2006). In humans, epidemiological studies have shown that high BPA levels are linked to heart disease, diabetes and liver toxicity ((Lang et al., 2008) and reviewed in (Vandenberg et al., 2012)). Food, indoor air, indoor dust, wastewater and dermal absorption are all important sources of human exposure to BPA (Vandenberg et al., 2007; Zalko et al., 2011). In addition, BPA is commonly found as a contaminant in wastewater, and may as such have deleterious effects on wildlife (Santos et al., 2016; Xu et al., 2016).
Concurrent with the increasing awareness of potential health problems linked to BPA, a search for safer alternatives has begun. Two of these BPA alternatives, bisphenol AF (BPAF) and bisphenol C (BPC), are the focus of this study. BPAF is a fluorinated derivative of BPA, and it is used both as a crosslinking agent and for polymer synthesis in plastics industry. It has an annual estimated production of 10,000–500,000 pounds in the United States (NTP-NIEHS, 2008). BPAF has been detected in wastewater from water treatment plants and was also shown to be resistant to biodegradation (Sun et al., 2017). BPC is used in the preparation of flame-resistant polycarbonates, and it has also been detected in wastewater (Cesen et al., 2018).
In humans, BPA interferes with estrogen signaling mediated by the two estrogen receptors, ERα and ERβ. The canonical estrogen signaling pathway involves estrogen binding to the ligand binding domain (LBD) of the ERs, translocation of the ER-ligand complexes into the nucleus, followed by recruitment of co-regulator complexes and docking to the estrogen response element (ERE) of target gene promoters (reviewed in (Nilsson et al., 2001)). Similarly to BPA, in vitro studies show that several bisphenol analogues are able to bind and activate the human ERs [(Delfosse et al., 2012; Delfosse et al., 2014; Grimaldi et al., 2019) and reviewed in (Chen et al., 2016)]. BPAF has been repeatedly reported to activate ER-dependent transcription at lower concentrations than BPA and other bisphenol derivatives in vitro ((Delfosse et al., 2012) and reviewed in (Chen et al., 2016)). While fewer studies have investigated BPC, this chlorinated analogue has been described to have stronger affinity for the ERs than BPA, acting as a full agonist on MCF7 proliferation, and as both an agonist and antagonist in HELN (HeLa ER negzative cells stably transfected by the ERE-Luc plasmid) cells stably-expressing the human ERs (HELN hERs cells) (Delfosse et al., 2012).
Zebrafish (Danio rerio) is an in vivo model to study environmental pollutants. There are three ERs (zfERα, zfERβ2 and zfERβ1) in zebrafish, with an approximate 50% amino-acid sequence identity to the human ER orthologues (Menuet et al., 2002). We have previously generated cell lines stably-transfected with the zfER subtypes to study the potential estrogenic activity of environmental pollutants in zebrafish (Pinto et al., 2014). We demonstrated with these cell models that mammalian estrogenic ligands also modulate the activity of the zfERs, although the selectivity and potency of the ligand often varied for the human and zebrafish orthologues. In zebrafish, the three ERs are all expressed from embryonic stages to adulthood, but with different temporal and organ distributions (Bardet et al., 2002; Chandrasekar et al., 2010; Mouriec et al., 2009). zfERα is detected in the heart valves of the developing zebrafish larvae, while the zfERβ1 subtype is robustly expressed in the liver (Gorelick et al., 2014). In the developing brain, zebrafish embryos express detectable levels of zfERβ2 and zfERβ1, but not zfERα, in different brain regions. The expression of cyp19a1b gene was temporally correlated with that of zfERβ2 and zfERβ1 (Mouriec et al., 2009) suggesting that these receptors drive the cyp19a1b expression.
During the past few years, several transgenic zebrafish models have been developed to investigate the cell- and/or tissue-specific effects of chemicals on estrogen signaling pathways in developing fish. For instance, in transgenic zebrafish model containing five EREs (Tg(5×ERE:GFP)) (Gorelick and Halpern, 2011), the GFP expression is clearly detected in tissues expressing the estrogen receptors. Thus, compounds with in vitro selectivity for zfERα and zfERβs induce expression of the fluorescent protein in the heart and liver, respectively (Gorelick et al., 2016). For example, PPT specifically activates the zfERβs receptors in vitro and induces GFP expression only in the liver of zebrafish larvae, whereas the pan-agonist 17α-ethinylestradiol induces GFP in both heart and liver (Gorelick and Halpern, 2011; Pinto et al., 2014; Gorelick et al., 2016). Another reporter fish model, the transgenic cyp19a1b-GFP zebrafish, expresses GFP under the control of the ER-regulated cyp19a1b (brain aromatase) gene in radial glial cells in the brain (Tong et al., 2009). This fish model has been thoroughly evaluated for assessing the estrogenic activity of a panel of environmental contaminants - including natural and synthetics hormones, pharmaceuticals, pesticides, industrial chemicals- in embryos and allowed to demonstrate that numerous contaminants were able to disrupt the brain-specific expression of the cyp19a1b gene in an ER-dependent manner (Brion et al., 2012; Cano-Nicolau, et al., 2016; Le Fol et al., 2017).
In this article, we combined in vitro and in vivo zebrafish mechanism-based reporter gene assays as screening tools to assess the estrogenic properties of BPA, BPAF and BPC. HELN cells expressing the zfERs (HELN zfERs cells) were first used to evaluate the capacity of the selected bisphenols to bind to the three zfERs subtypes and to transactivate zfERs to induce luciferase activity. To further evaluate their estrogenic properties at the organism level, (Tg(5×ERE:GFP)) and tg(cyp19a1b-GFP) zebrafish embryos were exposed to BPA, BPAF and BPC and the tissue-specific estrogenic responses in the brain, heart and liver of zebrafish embryos were observed and/or quantified and interpreted in light of their in vitro estrogenic activities towards the zfER subtypes.
Materials and Methods
Materials
Tissue culture plates were obtained from Greiner Bio-one (Monroe, NC, USA), and media was purchased from Invitrogen (Grand Island, NY, USA). Luciferin (sodium salt) was purchased from Promega (Charbonnières, France). The chemicals 17β-estradiol (E2), 17α-ethinylestradiol (EE2), bisphenol A (CAS no. 80–05-7), bisphenol AF (BPAF; CAS no. 1478–61-1), and bisphenol C (BPC; CAS no. 14868–03-2) were purchased from Sigma-Aldrich (Saint-Quentin Fallavier, France). ICI 182,780 (7α,17β-[9[(4,4,5,5,5-pentafluoropentyl)sulfinyl]nonyl]estra-1,3,5(10)-triene-3,17-diol, fulvestrant) was obtained from Zeneca (Macclesfield, UK), and 3,17-Dihydroxy-19-nor-17α-pregna-1,3,5(10)-triene-21,16α-lactone (16α-LE2) was a kind gift of Peter Muhn from Research Laboratories of Schering AG (Berlin, Germany). Compounds were dissolved in dimethyl sulfoxide (DMSO) as 10−2 M stock solutions.
Cell lines
The establishment and culturing of the HELN zfERα (Esr1), zfERβ1 (Esr2b) and zfERβ2 (Esr2a) reporter cell lines have been previously described (Pinto et al. 2014). Briefly, the HELN-zfERα, β1 and β2 cell lines were obtained by transfection of HELN cells (HeLa cells stably transfected with the ERE-βGlobin-Luc-SVNeo plasmid) (Delfosse et al., 2012) by the corresponding pSG5-puro plasmids (pSG5-zfERα-puro, pSG5-zfERβ1-puro and pSG5-zfERβ2-puro, respectively).
The HELN zfER cells were maintained in phenol red-free Dulbecco’s Modified Eagle’s Medium/F12 (DMEM/F12) and supplemented with 5% dextran-coated charcoal-treated fetal bovine serum (FBS-DCC), 1% antibiotic (penicillin/streptomycin), geneticin (1 mg/mL) and puromycin (0.25 μg/mL) in a 5% CO2 humidified atmosphere at 37°C.
zfERs binding assays
The assay for measuring zfER binding affinity has been previously described (Pinto et al., 2014). Briefly, the HELN-zfERs cell lines were treated with 3 nM [3H]-E2 in the absence or in the presence of chemicals for 4h at 28°C. At the end of the incubation period, unbound material was aspirated, cells washed with PBS, and [3H]-bound radioactivity counted. Protein concentrations were used to normalize bound radioactivity values expressed in desintegrations per minute (dpm). Experiments were performed in quadruplicate in at least two independent experiments.
Transactivation assays
Chemicals successive dilutions were performed in cell culture medium, using the same protocol for treatments as described previously (Pinto et al., 2014). The compounds were added 8 h after seeding cells into 96-well plates, and cells were incubated with compounds for 16h at 28°C. Luciferase assays were performed as previously published (Pinto et al., 2014), and experiments were performed in quadruplicates in three independent experiments. Data were expressed as percentage of the maximal activity induced by E2 10 nM. EC50 values were measured using GraphPad Prism (GraphPad Software Inc). Datas were analyzed for significant differences using one-way ANOVA followed by Dunnet’s post-comparison test (vs.control) (***p<0.001, **p<0.01, *p<0.05). Differences were considered statistically significant at P <0.05.
Zebrafish maintenance and transgenic zebrafish Tg(5xERE:GFP) treatment
Transgenic fish Tg(5×ERE:GFP) (Gorelick and Halpern, 2011) were used according to the maintenance and experimental protocols approved by the Institutional Animal Care and Use Committee at the University of Houston. Adult zebrafish were maintained and fed as described previously (Hao et al., 2013).
Embryos from several pairs of adult fish were collected after spawning and cultured in a Petri dish in E3 embryo medium (5 mM NaCl, 0.17 mM KCl, 0.33 mM CaCl2, 0.33 mM MgSO4) at 28.5°C with 14:10 LD. For fluorescent imaging experiments, Tg(5xERE:GFP) zebrafish embryos were incubated in E3 medium with 0.003% 1-phenyl-2-thiourea (PTU) (Sigma-Aldrich, St. Louis, MO) starting at 24h post fertilization (hpf) to inhibit pigment formation. Samples of 10 zebrafish larvae at 3 days post fertilization (dpf) were treated for 24 h with EE2, 16α-LE2 and bisphenols in 24-well plate, and transferred to 96-well plates at a density of one larva per well for imaging. EE2, 16α-LE2, ICI 182,780 and bisphenols in 100% DMSO were diluted in embryo media, and vehicle concentration did not exceed 0.2% DMSO in treatments. Vehicle only was used as a negative control. The larvae were imaged under 10X magnification lenses using a fluorescent microscope (Olympus IX51) equipped with an Olympus XM10 camera (Olympus, Center Valley, PA).
Treatment of cyp19a1b-GFP transgenic zebrafish embryos
Newly fertilized transgenic cyp19a1b-GFP zebrafish eggs were collected and treated as previously described (Brion et al., 2012). Briefly, 20 embryos (< 4 h post-fertilization) were exposed from 0 to 4 dpf to DMSO (0.01% v:v), 0.05 nM EE2 (used as positive control) or a range of concentrations of BPA, BPAF or BPC. The exposure medium was renewed totally every 24 h. At the end of the exposure period, the zebrafish embryos were collected for in vivo fluorescence imaging of the brain. Each 4 dpf-old zebrafish was photographed in dorsal view under a fluorescence microscope connected to an AxioCam Mrm camera (Zeiss GmbH, Gottingen, Germany). All photographs were taken with the same parameters. The fluorescence of the regions of interest was then quantified by image analysis using an ImageJ macro as previously described (Brion et al., 2012).
RNA extraction and quantitative real-time PCR
Zebrafish larvae were treated with bisphenols and controls from 4 dpf to 6 dpf. Treatments were repeated once at 5 dpf. Total RNA from 40 pooled embryos were collected at 6 dpf using Trizol (Invitrogen Corporation, Carlsbad, CA) and RNeasy spin columns (Qiagen, Chatsworth, CA) according to manufacturer’s protocols. DNase I (Qiagen, Chatsworth, CA) was used to remove any DNA in the column. RNA concentration was measured using NanoDrop 1000 spectrophotometer (Agilent Technologies, Palo Alto, CA) and 100 ng of total RNA was used for cDNA synthesis using Superscript III reverse transcriptase (Invitrogen Corporation, Carlsbad, CA) with random hexamer primers.
Expression of Cyp19a1b and Vtg1 were measured by qPCR using an ABI 7500 Fast Real-Time PCR system (Applied Biosystems, Foster City, CA) with iTaq Universal SYBR Green Supermix (Bio-Rad, Hercules, CA) according to the manufacturer’s protocol. Primers were synthesized by Integrated DNA Technologies, Inc (Coralville, IA). Primer sequences were for vtg1 Forward (5’−3’) ACTACCAACTGGCTGCTTAC and Reverse (5’−3’) ACCATCGGCACAGATCTTC; and for cyp19a1b Forward (5’−3’) AAAGAGTTACTAATAAAGATCCACCGGTAT and Reverse (5’−3’) TCCACAAGCTTTCCCATTTCA (Hao et al., 2013). Expression levels were normalized to zebrafish β-actin using the ΔΔCt method. Student’s t-test was performed for statistical analysis using GraphPad Prism version 7 (GraphPad Software, La Jolla, CA).
Results
Transcriptional activity of bisphenols mediated by zfERs in vitro
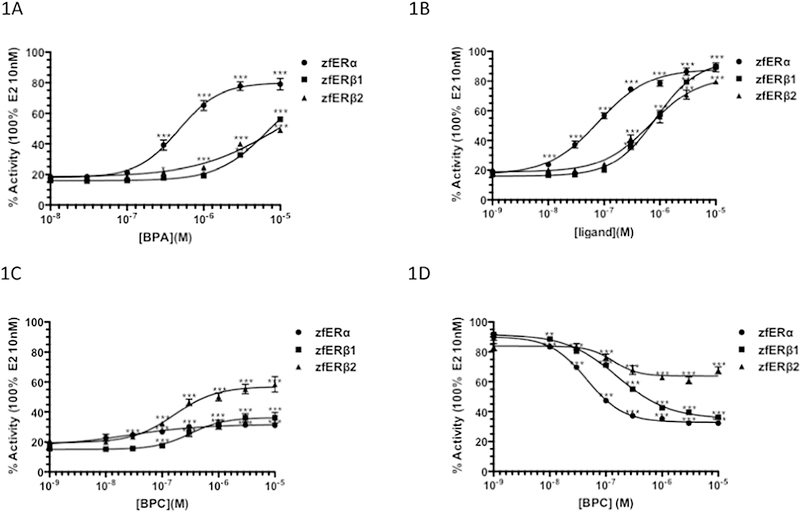
The transcriptional activity of BPA, BPAF and BPC have been reported for human estrogen receptors in HELN cells (Delfosse et al, 2012). To investigate the activity of BPA and its analogues on zebrafish ERs, we treated the HELN cell lines expressing the zfERα, zfERβ1 and zfERβ2 receptors and an ERE-driven luciferase reporter with increasing concentrations of the bisphenols. BPA and BPAF activated the three zfER reporter cells, reaching approximately 53–89% of E2-induced luciferase expression (Figure 1A, B). These bisphenols displayed higher selectivity for zfERα, than for the zfERβ subtypes. BPAF was more potent than BPA in inducing luciferase expression, and displayed approximately 3.1–5.6-fold greater potency on the three zfER cells than BPA, with EC50s of 84, 758 and 897 nM for zfERα, zfERβ1 and zfERβ2, respectively (Table 1). BPC activated both zfERα and zfERβ1 at low concentrations, but the luciferase activity reached only 31 and 36% of the maximal luciferase activity, respectively. BPC induced transcription to 59% of maximal activation on zfERβ2 (Figure 1C). The measured EC50s and maximal activation for the BPA, BPAF, BPC and controls are shown in Table 1.
Fig. 1.
Agonistic and antagonistic activity of bisphenols in HELN zfER reporter cell lines. (A-C) Dose-response curves for BPA, BPAF, and BPC in HELN zfER reporter cell lines. Cells were incubated for16h with various concentrations of BPA, BPAF, and BPC. (D) Dose responsive curves of BPC in HELN zfER reporter cell lines. Cells were incubated for 16h with various concentrations of BPC in presence of in presence of 1 nM E2. Data were expressed as percentage of the maximal activity induced by E2 10 nM. EC50 values were measured using GraphPad Prism (GraphPad Software Inc). Datas were analyzed for significant differences using one-way ANOVA followed by Dunnet’s post-comparison test (vs.control) (***p<0.001, **p<0.01, *p<0.05).
Table 1.
Effective concentration (EC50 in nM), IC50 in nM), relative estrogenic potency (REP) and maximal activity (%) of 17β-E2, 16α-LE2, BPA and the bisphenol analogues on the zfERs.
| zfERα | zfERβ1 | zfERβ2 | ||||
|---|---|---|---|---|---|---|
| Basal activity | 18.8 ± 0.3 | 16.5 ± 0.7 | 20.2 ± 0.6 | |||
| Agonism | ||||||
| Ligand | EC50 (REP) | Maximal activity | EC50 | Maximal activity | EC50 | Maximal activity |
| E2 | 0.08 ± 0.03 (1) | 100 | 0.04 ± 0.01 (1) | 100 | 0.12 ± 0.03 (1) | 100 |
| 16α-LE2 | 0.65 ± 0.1 (1.2 10−1) | 100 | 271 ± 45 (1.5 10−4) | 100 | 69.5 ± 17.5 (1.7 10−3) | 100 |
| BPA | 469 ± 42 (1.7 10−4) | 79 | 3326 ± 381 (1.2 10−5) | 53.1 | 2800 ± 562 (4.3 10−5) | 56.1 |
| BPAF | 84 ± 27 (9.5 10−4) | 89.3 | 758 ± 131 (5.3 10−5) | 89.6 | 897 ± 268 (1.3 10−4) | 79.6 |
| BPC | 25 ± 7.5 (3.2 10−3) | 31 | 316 ± 110 (1.3 10−4) | 36.1 | 167 ± 72 (7.2 10−4) | 58.5 |
| Antagonism | ||||||
| IC50 | IC50 | IC50 | ||||
| BPC | 46 ± 6.4 | 160 ± 42 | 136 ± 75 | |||
Values are the mean ± standard deviations from three separate experiments. Maximal activity is expressed as percentage of luciferase activity induced by 10 nM E2. REPs were determined as the ratio of E2 EC50 to that of bisphenol.
BPA, BPAF and BPC were identified as partial agonists in HELN hERs cells (Delfosse et al., 2012). The partial bisphenols induced-luciferase activity in the human ER reporter cells and the marginal effect of BPC on zfERα and zfERβ1 (figure 1C) suggest that BPC can act as an antagonist for these zfERs. We therefore evaluated the ability of BPC to antagonize the activity of E2. We found that BPC clearly reduced E2-induced transactivation in the zfERα and zfERβ cell lines with IC50s of 46, 160 and 136 nM on zfERα, zfERβ1 and zfERβ2, respectively (Figure 1D; Table 1) thereby revealing a stronger antagonistic activity of BPC towards zfERα and zfERβ1.
The selectivity of BPA, BPAF and BPC for zfERα was confirmed in competitive binding assays with radiolabeled E2 (Table 2). As previously demonstrated for the selective zfERα compound 16α-LE2 (Pinto et al., 2014), bisphenols displayed stronger binding affinity for zfERα compared to the zfERβ subtypes. Additionally, BPAF and BPC displaced radiolabeled E2 at lower concentrations than BPA, confirming the stronger affinity of BPAF and BPC for the zebrafish ERs relative to BPA (Table 2).
Table 2.
Dissociation constant (Kd) values for E2, 16α-LE2, BPA, BPAF and BPC on the zfERs. Values are the mean ± standard deviations from three separate experiments.
| zfERα) | zfERβ1 | zfERβ2 | |
|---|---|---|---|
| Ligands | Kd (nM) | Kd (nM) | Kd (nM) |
| E2 | 2.6 ± 0.6 | 1.1 ± 0.2 | 2.6 ± 0.7 |
| 16α-LE2 | 9.4 ± 0.9 | 1109 ± 126 | 351 ± 44 |
| BPA | 1245 ± 265 | 10040 ± 2919 | 6884 ± 1270 |
| BPAF | 428 ± 85 | 1023 ± 184 | 1993 ± 628 |
| BPC | 78 ± 16 | 678 ± 124 | 402 ± 89 |
Activation of estrogen signaling by bisphenols in tg ERE-GFP embryos
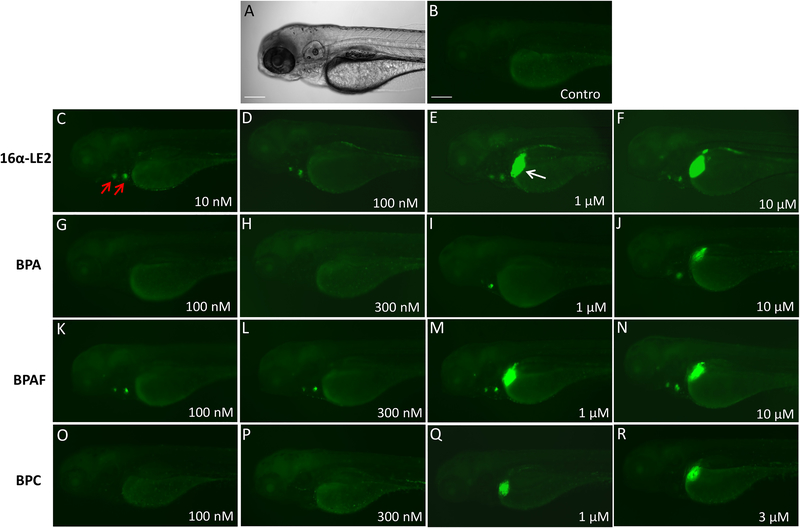
To assess the estrogenic effects of BPA, BPAF and BPC in vivo in zebrafish embryos, the transgenic fish line Tg(5xERE:GFP) containing 5xERE upstream of a c-fos promoter and a GFP reporter was used (Gorelick and Halpern, 2011). In order to qualitatively investigate the tissue-specific activity of the bisphenols, we treated 3 dpf zebrafish larvae for 24 h with BPA, BPAF and BPC (Figure 2). The ligand 16α-LE2, whose zfERα selectivity in vitro is associated with in vivo GFP expression exclusively in the heart valves at lower doses (10–100 nM) (Figure 2C–D), was used to compare the tissue-specific effects resulting from treatment with the bisphenols. The zfERα-selective agonist activity of BPA and BPAF was confirmed in the zebrafish embryos. At lower concentrations, BPA and BPAF only activated GFP expression in the heart valves, while higher doses of the ligands were required for induction of liver expression (Figure 2G–J and 2K–N). Treatment with BPC induced GFP expression in the liver at 1 μM, and no heart GFP expression was observed, likely due to its only marginal activity on zfERα (Figure 2O–R).
Fig. 2.
In vivo estrogenic activities of bisphenols on ERE reporter fish. Tg (ERE:GFP) larvae were treated with 16α-LE2 or bisphenols at 3 dpf for 24 h. Images were taken at 4 dpf. Lateral view showing GFP expression in the heart valves (red arrows) and liver (white arrow). A. Bright-field image; B. GFP fluorescence image of vehicle-treated zebrafish larvae; C-F. GFP images of zebrafish larvae exposed to increasing concentrations of 16α-LE2; G-J. GFP images of zebrafish treated with BPA; K-N. Images of zebrafish exposed to BPAF; O-R. GFP images of zebrafish exposed to BPC. Scale bars, 200 μm.
The stronger potency of BPAF and BPC on the zfERs compared to BPA observed in our experiments vitro was confirmed in vivo. Treatment with 100 nM BPAF induced GFP expression in the heart, whereas a dose of 1 μM BPA was required to achieve a similar effect in the zebrafish embryos. At the hepatic level, GFP expression was induced after exposure to 1 μM BPAF or 1 μM BPC, while BPA was only able to activate hepatic GFP expression at 10 μM (Figure 2G–R). Therefore, the responses observed in vivo seems to reflect both the estrogenic activity reported in vitro as well as the selectivity of the compounds towards the zfERs. Thus, the stronger potencies of BPAF and BPC compared to BPA seen in vitro was also observed at the in vivo level.
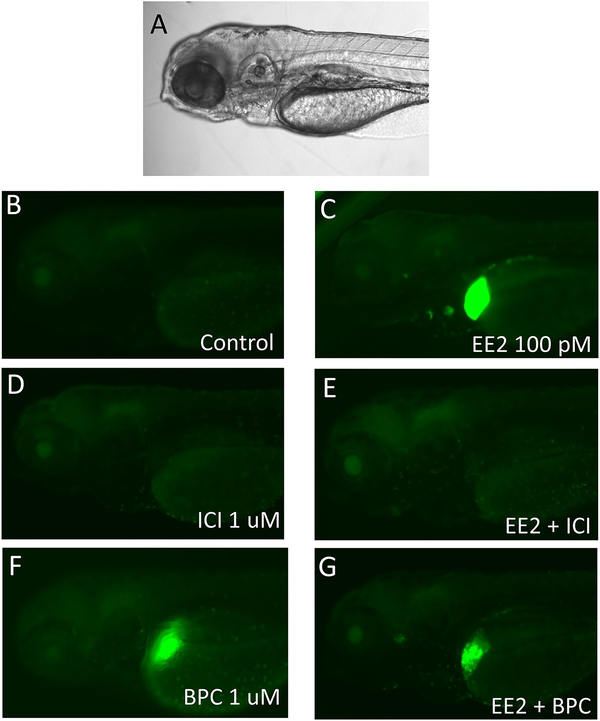
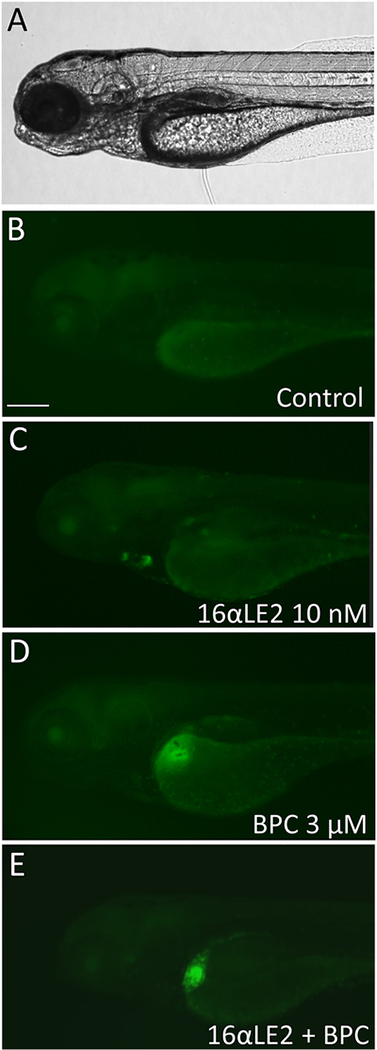
To investigate the antagonist activity of BPC in vivo, we co-treated zebrafish embryos with the estrogenic ligand EE2, which is more stable than E2 in vivo, or 16α-LE2 in combination with BPC (Figure 3 and Figure 4). Treatment with 100 pM EE2 induced GFP expression both in the heart and liver of the embryos, in accordance with its pan-agonist ER activity (Figure 3C) (Pinto et al., 2014; Gorelick et al., 2016). The pure antagonist ICI 182,780 completely blocked both heart and liver GFP expression (Figure 3E). Treatment with 1 μM BPC induced GFP expression in the liver of the embryos in accordance with its zfERβ2 activity. Co-treatment of EE2 with 1 μM BPC completely blocked EE2-induced GFP heart expression whereas it only slightly reduced EE2-induced GFP liver expression (Figure 3G). Likewise, BPC was capable of completely inhibiting the GFP expression induced by 10 nM 16α-LE2 in the heart (Figure 4). These results are in accordance with the in vitro antagonist/agonist activity of BPC, indicating that BPC is an antagonist on zfERα and partial agonist on zfERβs in vivo.
Fig. 3.
Anti-estrogenic effects of BPC on ERE reporter fish co-treated with EE2. A. Bright-field image; B. GFP fluorescence image of vehicle-treated zebrafish larvae; C. GFP images of zebrafish larvae exposed to 100 pm EE2; D. Zebrafish exposed to 1 μM ICI 182,780; E. GFP images of zebrafish co-treated with EE2 and ICI; F. Images of zebrafish exposed to 1 μM BPC; G. GFP images of zebrafish co-treated with EE2 and BPC. Scale bars, 100 μm.
Fig. 4.
Anti-estrogenic effects of BPC on ERE reporter fish co-treated with 16α-LE2. A. Bright- field image; B. GFP fluorescence image of vehicle-treated zebrafish larvae; C. GFP images of zebrafish larvae exposed to 10 nM 16α-LE2; D. GFP images of zebrafish exposed to 3 μM BPC; E. GFP images of zebrafish larvae co-treated with 16α-LE2 and BPC. Scale bars, 200 μm.
Activation of the brain aromatase promoter in the transgenic cyp19a1b- GFP zebrafish embryos by the three bisphenols
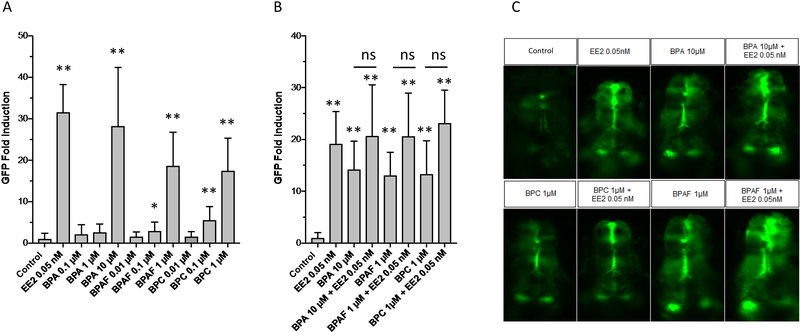
To assess the effect of bisphenols on the expression of the estrogen-sensitive brain aromatase gene, the fluorescence signal in the developing brain of transgenic cyp19a1b-GFP zebrafish embryos was quantified after a 4-day treatment with BPA, BPAF and BPC. All bisphenols induced GFP expression in the brain of the zebrafish embryos (Figure 5A–C). BPA at 10 μM promoted a 28-fold induction in the GFP signal, whereas no or very weak activity was observed at lower concentrations. Exposure to BPAF or BPC at 1 μM was sufficient to promote a 18-fold induction in the GFP expression, approximately, and a 2.9-fold and 5.5-fold GFP activation was observed at 0.1 μM for BPAF and BPC, respectively (Figure 5A).
Fig. 5.
In vivo estrogenic activity of bisphenols in the cyp19a1b-GFP zebrafish. A. Concentration-dependent induction of GFP by BPA, BPAF and BPC in cyp19a1b-GFP transgenic larvae after exposure to the bisphenols from 0 to 4 dpf. Values are expressed as mean +/− SEM (n = 4–13). B. Effect of BPA, BPAF and BPC on EE2-mediated GFP induction in cyp19a1b-GFP transgenic larva after exposure from 0 to 4 dpf. Values are expressed as mean +/− SEM (n = 8–14). C. Fluorescence images of cyp19a1b-GFP transgenic larvae treated with 0.05 nM EE2, 10 μM BPA, 1 μM BPAF and 1 μM BPC alone and co-treatments of EE2 with the bisphenols. Images were taken in the dorsal view, anterior side to the top.
To assess the potential for the bisphenols to inhibit estrogen-mediated activation of the brain aromatase promoter, the cyp19a1b-GFP zebrafish embryos were treated with 0.05 nM EE2 in the presence of the bisphenols. Under these experimental conditions, BPA, BPAF and BPC were unable to inhibit the EE2-mediated induction of GFP in the zebrafish embryos. These results indicate that BPA, BPAF and BPC act as agonists to induce brain aromatase expression within the radial glial cell context but that the bisphenols lack of ER antagonist activity on the brain aromatase of zebrafish embryos (Figure 5B–C).
Endogenous estrogen target gene activation by bisphenols in vivo
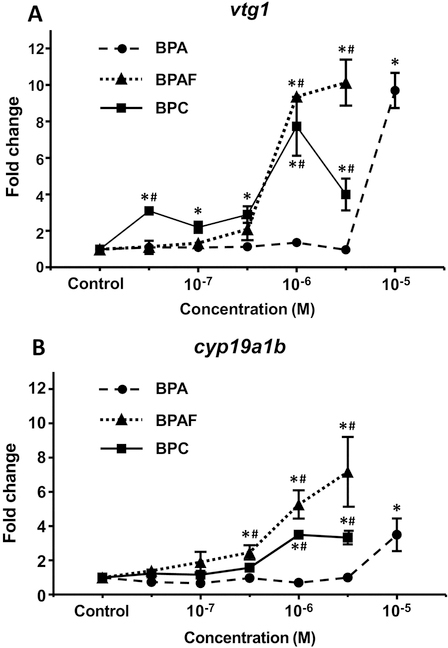
To analyze the effect of BPA, BPAF and BPC exposure on the expression of endogenous estrogen target genes, we performed quantitative PCR. We investigated the expression of the estrogen-sensitive markers vtg1 gene, highly expressed in the liver, and the brain cyp19a1b. In concordance with the in vivo GFP expression data, BPAF and BPC activated vtg1 and cyp19a1b at lower concentrations than BPA (clearly detected at 1 μM vs. 10 μM, respectively) (Figures 6A and 6B). We conclude that the in vivo endogenous activation of estrogenic target genes by BPA analogs is in accordance with both the brain and liver in vivo GFP activation.
Fig. 6.
Endogenous estrogen target gene activation by bisphenols in vivo. Concentration-dependent induction of vtg1 (A) and cyp19a1b (B) mRNA levels after treatment of zebrafish embryos with BPA, BPAF and BPC from 4 to 6 dpf. Transcript levels were normalized to zebrafish β-actin using the ΔΔCt method.
Discussion
Evidence in the literature showing that exposure to BPA causes harmful health effects in humans and wildlife has prompted a search for safer bisphenol alternatives. We assessed the effects of BPA, BPAF and BPC using zfER reporter cell lines, and found that BPAF and BPC in comparison to BPA have a stronger binding affinity for the zebrafish ERs, and a lower EC50 to activate them. In vitro, BPAF and BPA activated luciferase expression mediated by the three zebrafish estrogen receptors, with stronger affinity for zfERα. This zfERα selectivity was confirmed by the preferential activation of the GFP reporter in the heart valves in vivo in zebrafish larvae. BPC only minimally stimulated the activity of zfERα in vitro, and partially activated zfERβ-mediated transcription to levels ranging from 40–60% of the activity of E2. In accordance with its agonist profile in vitro, exposure to BPC induced GFP expression in the brain and in the liver of the zebrafish larvae. Additionally, the stronger affinity of BPAF and BPC for the zebrafish receptors compared to BPA in vitro was confirmed in vivo, as lower concentrations of these bisphenol analogues were necessary to activate liver and brain GFP expression, as well as promote the induction of vtg1 and cyp19a1b gene expression in zebrafish embryos.
The estrogenic activities of bisphenols have previously been analyzed for human ERs in reporter cell lines (Kitamura et al., 2005; Matsushima et al., 2010; Delfosse et al., 2012; Chen et al., 2016; Grimaldi et al., 2019). The pronounced agonist activity of BPAF has been previously reported for human ERs, with several studies demonstrating that BPAF is more estrogen-active than BPA. In HELN cells expressing the human ERs, both BPAF and BPC are more potent ligands than BPA (Delfosse et al., 2012). Bisphenols show a slightly better affinity for human ERp. This opposite ER selectivity profile between human and zebrafish ERs also observed for several other xenoestrogens, could be due to differences in the constitution and interaction with amino acids in the LBD of human and zebrafish receptors (Pinto et al., 2014).
The in vivo estrogenic activity of bisphenols have been studied in fish and rodents. In adult male medaka, BPAF and BPC are more potent than BPA in inducing hepatic upregulation of the expression of vtg and other estrogenic biomarkers (Yamaguchi et al., 2015). Moreover, the bisphenols induce uterine weight gain in ovariectomized rats, and the uterotrophic effect is more pronounced for BPC and BPAF compared to BPA (Conley et al., 2016). These in vivo results are in accordance with our results from in vitro and transgenic zebrafish models, and provide substantial data to support that BPC and BPAF have a stronger ability than BPA to disrupt estrogen signaling across different vertebrate species.
The present study is, to our knowledge, the first to demonstrate that the chlorinated bisphenol analogue, BPC, has pronounced antagonistic zfERα activity in zebrafish both in vitro and in vivo. Exposure to BPC resulted in clear inhibition of the EE2- and 16α-LE2-induced GFP expression in the heart of the zebrafish larvae, in accordance with its zfERα-mediated antagonist activity in the HELN reporter cells. The outcomes of activation or inactivation of estrogen signaling by bisphenols on the development and physiology of the zebrafish heart are unknown. However, it has been reported that estrogenic ligands can alter the basal heart rate in zebrafish embryos. Exposure to estradiol and ICI 182,780 resulted in increased heart rate in zebrafish, an effect that was attributed to modulation of the G protein-coupled estrogen receptor (GPER) pathway (Romano et al., 2016), while treatment with the anti-estrogen tamoxifen caused a decrease in the heart rate of zebrafish embryos (Xia et al., 2016). BPA treatment has been shown to impair the cardiac contractile performance of rodent hearts, an effect associated with altered intracellular calcium handling (Posnack et al., 2015).
Although ER antagonist activity was observed for BPC in the ERE-driven activation of fluorescence in the zebrafish heart, BPC, as well as BPA and BPAF, were unable to inhibit the EE2-induced brain aromatase promoter. zfERβ2 has been reported to be the main ER subtype detected in the brain during zebrafish early developmental stages, while zfERα and zfERβ1 mRNA are in contrast much lower (Mouriec et al., 2009). Studies have also shown that morpholino knockdown of zfERβ2, but not zfERα or zfERβ1, completely blocks estrogen-mediated upregulation of the aromatase mRNA (Griffin et al., 2013). This data suggests that zfERβ2 is the main subtype controlling the activation of brain aromatase during zebrafish early development. The results with BPC support this hypothesis, as BPC would be expected to inhibit the estrogen-mediated activation of aromatase in the cyp19a1b-GFP zebrafish embryos if aromatase expression was indeed controlled by zfERα. Inferring from this data, BPC will act as both an agonist and antagonist in vivo in a tissue-specific manner.
In addition to perturbing the ER pathway, BPAF and BPC have been reported to interact with other endocrine targets (Delfosse et al., 2014; Grimaldi et al., 2019; Lei et al., 2017; Wang et al., 2014). The increasing and widespread use of these bisphenol analogues and their ability to interact with endocrine molecular targets at lower concentrations than BPA pose a serious concern associated with potential health risks for humans and wildlife. Implementation of green chemistry practices for identification of safer alternatives will improve characterization of chemical hazard and should be useful to guide manufacturers on more sustainable bisphenol alternatives.
In conclusion, we show that a combined strategy using cell-based assays and transgenic zebrafish models can be used to thoroughly assess estrogenic properties of bisphenol derivatives. This approach allows for the quick screening of both estrogenic and anti-estrogenic activities of environmental pollutants, and are sensitive tools to inform dose-response relationships and tissue-specific effects. Characterization of species differences in response to estrogenic compounds can be addressed in the HELN cells expressing human or zebrafish ERs. The transgenic zebrafish models could also be used to address the influence of metabolism and pharmacokinetic parameters on chemical activity. Moreover, these tools are amenable to high-throughput screening, and can be incorporated into screening platforms currently applied to inform perturbations in ER signaling, such as the ones of the U.S. Environmental Protection Agency (EPA)’s ToxCast/Tox21 program.
Highlights.
In vitro/in vivo estrogenic activity of BPA, BPAF and BPC in zebrafish
Selectivity on zfERα of bisphenols
Antagonism on zfERα of BPC
Relevance of an integrated strategy to assess EDCs and their substitutes
Acknowledgements
We thank Dr. Daniel A. Gorelick at the Baylor School of Medicine, Houston, TX, for the gift of the Tg(5×ERE:GFP) fish line.
Funding Information
This work was supported by grants from the U.S. Environmental Protection Agency [Grant # R- 834289], National Institutes of Health [Grant # R21ES020036] (MB/JAG), the French Agency for Food, Environmental and Occupational Health & Safety (ANSES-EVALPE), the French National Research Agency (ANR-NEWPLAST) (PB), the DRC 59 (FB and SA) and the Robert A. Welch Foundation (E-0004) (JÅG). The views expressed in the article do not necessarily reflect the views of the funding agencies.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bardet PL, Horard B, Robinson-Rechavi M, Laudet V, Vanacker JM, 2002. Characterization of oestrogen receptors in zebrafish (Danio rerio). Journal of molecular endocrinology 28, 153–163. [DOI] [PubMed] [Google Scholar]
- Brion F, Le Page Y, Piccini B, Cardoso O, Tong SK, Chung BC, Kah O, 2012. Screening estrogenic activities of chemicals or mixtures in vivo using transgenic (cyp19a1b-GFP) zebrafish embryos. Plos One 7, e36069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano-Nicolau J, Valliant C, Pellegrini E, Charlier TD, Kah O, Coumailleau P, 2016. Estrogenic Effects of Several BPA Analogs in the Developing Zebrafish Brain. Front Neurosci 10, 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesen M, Lenarcic K, Mislej V, Levstek M, Kovacic A, Cimrmancic B, Uranjek N, Kosjek T, Heath D, Dolenc MS, Heath E, 2018. The occurrence and source identification of bisphenol compounds in wastewaters. Sci Total Environ 616–617, 744–752. [DOI] [PubMed] [Google Scholar]
- Chandrasekar G, Archer A, Gustafsson JA, Andersson Lendahl M, 2010. Levels of 17beta-estradiol receptors expressed in embryonic and adult zebrafish following in vivo treatment of natural or synthetic ligands. PloS one 5, e9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Kannan K, Tan HL, Zheng ZG, Feng YL, Wu Y, Widelka M, 2016. Bisphenol Analogues Other Than BPA: Environmental Occurrence, Human Exposure, and Toxicity-A Review. Environ Sci Technol 50, 5438–5453. [DOI] [PubMed] [Google Scholar]
- Conley JM, Hannas BR, Furr JR, Wilson VS, Gray LE, 2016. A Demonstration of the Uncertainty in Predicting the Estrogenic Activity of Individual Chemicals and Mixtures From an In Vitro Estrogen Receptor Transcriptional Activation Assay (T47D-KBluc) to the In Vivo Uterotrophic Assay Using Oral Exposure. Toxicol Sci 153, 382–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfosse V, Grimaldi M, Pons JL, Boulahtouf A, le Maire A, Cavailles V, Labesse G, Bourguet W, Balaguer P, 2012. Structural and mechanistic insights into bisphenols action provide guidelines for risk assessment and discovery of bisphenol A substitutes. Proceedings of the National Academy of Sciences of the United States of America 109, 14930–14935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfosse V, Grimaldi M, le Maire A, Bourguet W, Balaguer P, 2014. Nuclear Receptor Profiling of Bisphenol-A and Its Halogenated Analogues. Vitam Horm 94, 229–251. [DOI] [PubMed] [Google Scholar]
- Elsworth JD, Jentsch JD, Vandevoort CA, Roth RH, Jr DE, Leranth C, 2013. Prenatal exposure to bisphenol A impacts midbrain dopamine neurons and hippocampal spine synapses in non-human primates. Neurotoxicology 35, 113–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelick DA, Halpern ME, 2011. Visualization of Estrogen Receptor Transcriptional Activation in Zebrafish. Endocrinology 152, 2690–2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelick DA, Iwanowicz LR, Hung AL, Blazer VS, Halpern ME, 2014. Transgenic Zebrafish Reveal Tissue-Specific Differences in Estrogen Signaling in Response to Environmental Water Samples. Environ Health Persp 122, 356–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelick DA, Pinto CL, Hao R, Bondesson M, 2016. Use of Reporter Genes to Analyze Estrogen Response: The Transgenic Zebrafish Model. Methods in molecular biology 1366, 315–325. [DOI] [PubMed] [Google Scholar]
- Griffin LB, January KE, Ho KW, Cotter KA, Callard GV, 2013. Morpholino-mediated knockdown of ERalpha, ERbetaa, and ERbetab mRNAs in zebrafish (Danio rerio) embryos reveals differential regulation of estrogen-inducible genes. Endocrinology 154, 4158–4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimaldi M, Boulahtouf A, Toporova L, Balaguer P, 2019. Functional profiling of bisphenols for nuclear receptors. Toxicology, 420, 39–45. [DOI] [PubMed] [Google Scholar]
- Hao RX, Bondesson M, Singh AV, Riu A, McCollum CW, Knudsen TB, Gorelick DA, Gustafsson JA, 2013. Identification of Estrogen Target Genes during Zebrafish Embryonic Development through Transcriptomic Analysis. Plos One 8, e79020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho SM, Tang WY, Belmonte de Frausto J, Prins GS, 2006. Developmental exposure to estradiol and bisphenol A increases susceptibility to prostate carcinogenesis and epigenetically regulates phosphodiesterase type 4 variant 4. Cancer research 66, 5624–5632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honma S, Suzuki A, Buchanan DL, Katsu Y, Watanabe H, Iguchi T, 2002. Low dose effect of in utero exposure to bisphenol A and diethylstilbestrol on female mouse reproduction. Reproductive toxicology 16, 117–122. [DOI] [PubMed] [Google Scholar]
- Kitamura S, Suzuki T, Sanoh S, Kohta R, Jinno N, Sugihara K, Yoshihara S, Fujimoto N, Watanabe H, Ohta S, 2005. Comparative study of the endocrine-disrupting activity of bisphenol A and 19 related compounds. Toxicological sciences : an official journal of the Society of Toxicology 84, 249–259. [DOI] [PubMed] [Google Scholar]
- Lang IA, Galloway TS, Scarlett A, Henley WE, Depledge M, Wallace RB, Melzer D, 2008. Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults. JAMA : the journal of the American Medical Association 300, 1303–1310. [DOI] [PubMed] [Google Scholar]
- Le Fol V, Ait-Aissa S, Sonavane M, Porcher JM, Balaguer P, Cravedi JP, Zalko D, Brion F, 2017. In vitro and in vivo estrogenic activity of BPA, BPF and BPS in zebrafish-specific assays. Ecotoxicol Environ Saf,142: 150–156. [DOI] [PubMed] [Google Scholar]
- Lei B, Xu J, Peng W, Wen Y, Zeng X, Yu Z, Wang Y, Chen T, 2017. In vitro profiling of toxicity and endocrine disrupting effects of bisphenol analogues by employing MCF-7 cells and two-hybrid yeast bioassay. Environmental toxicology 32, 278–289. [DOI] [PubMed] [Google Scholar]
- Leranth C, Hajszan T, Szigeti-Buck K, Bober J, MacLusky NJ, 2008. Bisphenol A prevents the synaptogenic response to estradiol in hippocampus and prefrontal cortex of ovariectomized nonhuman primates. Proceedings of the National Academy of Sciences of the United States of America 105, 14187–14191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markey CM, Luque EH, Munoz De Toro M, Sonnenschein C, Soto AM, 2001. In utero exposure to bisphenol A alters the development and tissue organization of the mouse mammary gland. Biology of reproduction 65, 1215–1223. [DOI] [PubMed] [Google Scholar]
- Matsushima A, Liu X, Okada H, Shimohigashi M, Shimohigashi Y, 2010. Bisphenol AF is a full agonist for the estrogen receptor ERalpha but a highly specific antagonist for ERbeta. Environ Health Perspect 118, 1267–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menuet A, Pellegrini E, Anglade I, Blaise O, Laudet V, Kah O, Pakdel F, 2002. Molecular characterization of three estrogen receptor forms in zebrafish: binding characteristics, transactivation properties, and tissue distributions. Biology of reproduction 66, 1881–1892. [DOI] [PubMed] [Google Scholar]
- Mouriec K, Lareyre JJ, Tong SK, Le Page Y, Vaillant C, Pellegrini E, Pakdel F, Chung BC, Kah O, Anglade I, 2009. Early regulation of brain aromatase (cyp19a1b) by estrogen receptors during zebrafish development. Dev Dyn 238, 2641–2651. [DOI] [PubMed] [Google Scholar]
- Munoz-de-Toro M, Markey CM, Wadia PR, Luque EH, Rubin BS, Sonnenschein C, Soto AM, 2005. Perinatal exposure to bisphenol-A alters peripubertal mammary gland development in mice. Endocrinology 146, 4138–4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson S, Makela S, Treuter E, Tujague M, Thomsen J, Andersson G, Enmark E, Pettersson K, Warner M, Gustafsson JA, 2001. Mechanisms of estrogen action. Physiological reviews 81, 1535–1565. [DOI] [PubMed] [Google Scholar]
- NTP-NIEHS, 2008. Chemical Information Profile for Bisphenol AF.
- Palanza P, Gioiosa L, vom Saal FS, Parmigiani S, 2008. Effects of developmental exposure to bisphenol A on brain and behavior in mice. Environmental research 108, 150–157. [DOI] [PubMed] [Google Scholar]
- Pinto C, Grimaldi M, Boulahtouf A, Pakdel F, Brion F, Ait-Aissa S, Cavailles V, Bourguet W, Gustafsson JA, Bondesson M, Balaguer P, 2014. Selectivity of natural, synthetic and environmental estrogens for zebrafish estrogen receptors. Toxicol Appl Pharm 280, 60–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posnack NG, Brooks D, Chandra A, Jaimes R, Sarvazyan N, Kay M, 2015. Physiological response of cardiac tissue to bisphenol a: alterations in ventricular pressure and contractility. Am J Physiol-Heart C 309, H267–H275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano S, Edwards H, Souder J, Cui X, Gorelick D, 2017. G protein-coupled estrogen receptor regulates heart rate in zebrafish embryos. PLOS genetics 13, 11007069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin BS, Lenkowski JR, Schaeberle CM, Vandenberg LN, Ronsheim PM, Soto AM, 2006. Evidence of altered brain sexual differentiation in mice exposed perinatally to low, environmentally relevant levels of bisphenol A. Endocrinology 147, 3681–3691. [DOI] [PubMed] [Google Scholar]
- Santos JM, Putt DA, Jurban M, Joiakim A, Friedrich K, Kim H, 2016. Differential BPA levels in sewage wastewater effluents from metro Detroit communities. Environmental monitoring and assessment 188, 585. [DOI] [PubMed] [Google Scholar]
- Sun Q, Wang Y, Li Y, Ashfaq M, Dai L, Xie X, Yu CP, 2017. Fate and mass balance of bisphenol analogues in wastewater treatment plants in Xiamen City, China. Environ Pollut 225, 542–549. [DOI] [PubMed] [Google Scholar]
- Tong SK, Mouriec K, Kuo MW, Pellegrini E, Gueguen MM, Brion F, Kah O, Chung BC, 2009. A cyp19a1b-gfp (aromatase B) transgenic zebrafish line that expresses GFP in radial glial cells. Genesis 47, 67–73. [DOI] [PubMed] [Google Scholar]
- Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV, 2007. Human exposure to bisphenol A (BPA). Reproductive toxicology 24, 139–177. [DOI] [PubMed] [Google Scholar]
- Vandenberg LN, Chahoud I, Heindel JJ, Padmanabhan V, Paumgartten FJ, Schoenfelder G, 2012. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Ciencia & saude coletiva 17, 407–434. [DOI] [PubMed] [Google Scholar]
- Wang S, Rijk JC, Besselink HT, Houtman R, Peijnenburg AA, Brouwer A, Rietjens IM, Bovee TF, 2014. Extending an in vitro panel for estrogenicity testing: the added value of bioassays for measuring antiandrogenic activities and effects on steroidogenesis. Toxicol Sci 141, 78–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia L, Zheng L, Zhou JL, 2016. Transcriptional and morphological effects of tamoxifen on the early development of zebrafish (Danio rerio). J Appl Toxicol 36, 853–862. [DOI] [PubMed] [Google Scholar]
- Xu G, Ma S, Tang L, Sun R, Xiang J, Xu B, Bao Y, Wu M, 2016. Occurrence, fate, and risk assessment of selected endocrine disrupting chemicals in wastewater treatment plants and receiving river of Shanghai, China. Environ Sci Pollut Res Int 23, 25442–25450. [DOI] [PubMed] [Google Scholar]
- Yamaguchi A, Ishibashi H, Arizono K, Tominaga N, 2015. In vivo and in silico analyses of estrogenic potential of bisphenol analogs in medaka (Oryzias latipes) and common carp (Cyprinus carpio). Ecotox Environ Safe 120, 198–205. [DOI] [PubMed] [Google Scholar]
- Zalko D, Jacques C, Duplan H, Bruel S, Perdu E, 2011. Viable skin efficiently absorbs and metabolizes bisphenol A. Chemosphere 82, 424–430. [DOI] [PubMed] [Google Scholar]