Abstract
Osteoarthritis (OA) is a chronic condition that impacts quality of life and functionality for which consumers often seek dietary supplements to provide some relief. The purpose of this double-blind, placebo-controlled clinical trial was to assess the safety and efficacy of a water-soluble chicken eggshell membrane hydrosylate (WSEM) dietary supplement (BiovaFlex®) 450 mg daily on knee function, mobility, and general health and well-being in 88 adults with OA randomized into intervention (n = 44) or placebo (n = 44) groups. Outcomes were assessed periodically over 12 weeks, including the Western Ontario McMaster Osteoarthritis Index (WOMAC), the six-minute walk test (6MWT), knee range of motion (ROM) testing, and safety. Normalized analysis (improvement over baseline) showed that the poorest initial performers benefited the greatest from the WSEM by day 5 in the 6MWT, with the rest of the population showing significant improvement over placebo by week 12. The normalized WOMAC Stiffness score was also significantly improved over placebo by day 5 (P < .05). Without normalization, no statistically significant improvements were seen in WOMAC, 6MWT, and ROM testing. The Product was also found to be safe in this study. In conclusion, daily consumption of WSEM significantly enhanced average individual physical capacity (walking distance and ability), reduced stiffness by the fifth day of supplementation with the greatest benefit seen by the most compromised individuals, and was maintained over 12 weeks. A WSEM dietary supplement may offer a safe option for relief from symptoms and increased mobility for those with OA.
Keywords: cartilage, chondroitin, hyaluronic acid, collagen, glucosamine, dietary supplement, osteoarthritis, water soluble egg shell membrane
Introduction
Osteoarthritis (OA) is a chronic joint condition that affects millions of people worldwide, with significant impact on quality of life, activities of daily living, and health care costs.1–3 According to The World Health Organization (WHO) Global Burden of Disease Study 2010, hip and knee OA is the 11th leading cause of disability and is predicted to increase.4 There is no standard for treatment and what is available is typically focused more on pain alleviation by a combination of pharmacological and nonpharmacological approaches rather than curative treatment. Even when pain alleviation protocols are prescribed, only half of the patients experience pain reduction.5 Some improvements may be achieved by weight-loss and physical activities, including physical therapy; however, this often does not lead to a full alleviation of symptoms.6 Although surgery is often the only option for more severe cases it is not always an option due to cost or co morbidities.7,8 Therefore, there is a need to investigate alternative therapeutics and nutritional supplements as an option for symptom management. Perhaps one of the most known nutritional supplement combinations to be investigated is chondroitin-sulfate, glucosamine, and hyaluronic acid.4–6 In a double-blind, placebo-controlled study, subjects taking a herbal extract in combination with hyaluronate and glucosamine for 8 weeks demonstrated a faster return to joint health and greater quality of life.9 In another study, the effect of UP1306, a standardized, proprietary extract of Morus alba and Acacia catechu, compared with glucosamine chondroitin was evaluated in 135 adults with OA of the knee in a randomized, placebo-controlled, 12-week study. Similar improvements were found for the UP1306 group and the placebo group based on the Western Ontario McMaster Osteoarthritis Index (WOMAC) scales (discomfort, stiffness, and activities of daily living) as well as range of motion (ROM) and distance walked measures. Degradation of cartilage as measured by urinary C-telopeptides of type II collagen was significantly less at 12 weeks in subjects given UP1306. The authors suggested that early treatment with UP1306 might act to prevent joint cartilage damage.10 A pilot clinical trial investigated the effect of daily supplementation with oral hyaluronic acid extracted from chicken combs (Hyal-Joint®, Bioberica, Spain). Twenty subjects aged ≥40 years with knee OA (pain for at least 15 days in the previous month, symptoms present for ≥6 months, Kellgren/Lawrence score ≥2) participated in a randomized, double-blind, placebo-controlled trial. Ten subjects received the study product (80 mg/day) and 10 received placebo for 8 weeks. The WOMAC scale and a quality-of-life questionnaire (the Short Form-36 [SF-36v2®]) were administered at baseline and after 4 and 8 weeks of treatment. The results for subjects in both groups showed statistically significant improvements in WOMAC Pain, Stiffness, and Physical Function subscales, and in the aggregate WOMAC score, with higher magnitudes of change in the treatment group for WOMAC Physical Function and Total symptoms. Within-group changes indicated significant improvement for both groups on the SF-36v2 at 4 and 8 weeks; greater change occurred in the treatment group for bodily pain and social functioning. The results indicated that daily supplementation with oral hyaluronic acid from a natural extract of chicken combs was useful in enhancing several markers of quality of life in adults with OA of the knee.11
Eggshell membrane (EM) is a naturally rich source of combined protein, elastin, collagen, glucosamine, chondroitin, and hyaluronic acid and has been studied as a treatment for management of symptoms related to OA. EM's high content of bioactive components, as well as properties of moisture retention and biodegradability, suggests that it has potential use for clinical,12 cosmetic, nutraceutical, and nanotechnology applications.13 Studies have demonstrated that EM leads to a reduction in the production of pro-inflammatory cytokines, such as interleukin-1 beta and tumor necrosis factor-alpha both in vitro14 and in vivo.15 EM has been shown to improve recovery from exercise-induced joint pain (day 8), decrease stiffness (day 4), and reduce discomfort immediately after exercise (stiffness, day 7) in postmenopausal women taking 500 mg once daily for 2 weeks. In the same study, a decrease in the cartilage degradation biomarker CTX-II occurred.16 Two 1-month, open-label, pilot clinical studies with 11 and 28 subjects, respectively, were conducted to evaluate the efficacy and safety of EM for the relief of pain and discomfort associated with joint and connective tissue disorders. The results indicated that 500 mg of EM taken once daily significantly reduced pain, both within 7 days and over 30 days.17 A follow-up, randomized, double-blind study of 67 subjects revealed that 500 mg of EM taken once per day significantly reduced joint pain and stiffness compared with placebo within 10 days and maintained over 60 days.18
Water-Soluble Eggshell Membrane (WSEM) is a novel, patented, water-soluble version of EM (United States Patent Numbers: 7,584,909, 8,056,844, 8,197,852, 8,211,477, and 8,425,943) containing naturally occurring collagen, elastin, glycosaminoglycans (GAGs), and proteins, which typically are essential for maintaining healthy articular cartilage and the surrounding synovium. GAGs, one of the components of WSEM, are large, linear polysaccharides constructed of repeating disaccharide units with the primary configurations containing an amino sugar (either GlcNAc or GalNAc) and a uronic acid (either glucuronic acid and/or iduronic acid). Examples include hyaluronan, chondroitin, dermatan, heparin/heparan, and keratan, some of which have been shown to benefit joint health in multiple studies.9–11,13,15–18
In a randomized, double-blind, placebo-controlled crossover study of 22 adults with joint pain/stiffness, Jensen et al. reported that supplementation of 450 mg WSEM (BiovaFlex®) for 4 weeks led to a significant improvement in quality of life, physical function,18 ROM of the shoulder (P < .01), neck, spine, hips, and knees compared with placebo (P < .05). Physical activity levels were significantly higher after WSEM than after placebo consumption (P < .05). Subgroup analysis of those who participated in the study in the winter season showed improvement of lower back pain after 5 days of WSEM consumption compared with placebo consumption (P < .05).17 These findings demonstrated efficacy of the intervention, deserving continued exploration and follow-up.
Therefore, to further evaluate the WSEM product, this randomized, double-blind, placebo-controlled trial was undertaken, specifically in adults who were experiencing symptoms associated with OA of the knee to the extent that it was impacting quality of life, yet not severe enough to warrant prescription medication.
Materials and Methods
Study design
This was a randomized, double-blind, placebo-controlled, parallel-design, prospective study conducted at a single research site located in Springfield, Missouri, USA (QPS-BioKinetics). The purpose of the study was to assess the safety and efficacy of the study product [BiovaFlex—Study Product, 450mg daily] on knee function, mobility, and general health and well-being as measured by changes in vitals and by changes in general blood work in subjects with OA at specific time points over an 85-day (12-week) period. The dose was selected based on previously established studies. In general, after undergoing phone- and in-person screening, and after signing an informed consent, subjects were randomized in a 1:1 manner to either study product or placebo group. The study (CSP#87117) was approved by the Bio-Kinetic Clinical Applications Institutional Review Board on November 17, 2018 and executed by QPS Missouri (Springfield, MO).
The study consisted of adult subjects recruited by local advertisements in the community aged 35–65 years (inclusive) with a body mass index (BMI) <35 kg/m2 (normal weight through Class I obesity). To confirm the presence of OA, the subjects, by self-report, had to have experienced knee pain for at least 15 of the prior 30-day before screening as well as other symptoms of knee pain for at least 6 months, with at least 3 of the following indicators as taken from the American College of Rheumatology Clinical Classification Criteria for OA diagnosis of the knee: be older than 50 years of age; report less than 30 min of morning stiffness; crepitus on active motion; bony tenderness; bony enlargement; and/or no palpable warmth of synovium. Other than the indication of OA, subjects had no known egg allergy, and no clinically significant medical conditions based on physical examination, medical history, and screening laboratory results. Further, subjects could not have undergone intra-articular treatment in either knee (e.g., visco-supplementation with hyaluronic acid products or corticosteroid injections) within 12 weeks of randomization.19
The subjects were advised to stop any use of over-the-counter nonsteroidal anti-inflammatory drugs or pain relief products for at least 14 days before the randomization visit. In addition, the subjects were advised to stop the use of acetaminophen for 24 h before the randomization visit to permit an accurate baseline assessment of the WOMAC scale and its subscales.
All subjects were assessed at the baseline visit by the Western Ontario McMaster University Osteoarthritis Index (WOMAC), physical performance—by the six-minute walk test (6MWT) (weight-bearing exercise), and joint ROM for the knee by using a standard goniometer. In addition, all subjects utilized a daily diary to record any use of rescue medication between study visits (rescue medicine, 500 mg acetaminophen to no greater than 2000 mg allowed). Subjects underwent repeat clinic visit testing on days 5, 28, 56, and 84 after the randomization visit.
Study assessments
WOMAC is widely used in the evaluation of overall well-being and hip and knee OA.20 This study concentrated on knee OA. The WOMAC consists of a self-administered questionnaire of 24 items divided into 3 subscales—Pain (5 items): during walking, using stairs, in bed, sitting or lying, and standing upright; Stiffness (2 items): after first waking and later in the day; and Physical function (17 items): using stairs, rising from sitting, standing, bending, walking, getting in/out of a car, shopping, putting on/taking off socks, rising from bed, lying in bed, getting in/out of bath, sitting, getting on/off toilet, heavy domestic duties, and light domestic duties. The study subjects described their current experience by checking one of the five numbered boxes that formed a rating scale, which varied from 0 = none, 1 = slight, 2 = moderate, 3 = very, and 4 = extremely. In the beginning of the study, subjects answered the WOMAC and this was considered the baseline. For all study visits after baseline, subjects were asked to reflect back to baseline that is now termed “since starting this study” for scoring the WOMAC.20,21
The 6MWT is a submaximal exercise test that entails measurement of distance walked over a span of 6 min.22,23 This test measures functional ability to ambulate in a weight-bearing situation. The total distance covered over the 6 min is documented, allowing for comparisons over time and between individuals in a cohort, to determine any positive, neutral, or negative impacts of an intervention. Incremental distances over baseline for individuals were also calculated at each time point and were correlated to the individual's baseline performance.
The ROM testing included a standardized goniometer (Jamar Goniometer 12 1/2″—Model 7541; Patterson Medical, Warrenville, IL) that was also employed for objective evaluation of the ROM of the OA knee under zero stress conditions.24 Subjects were measured for both flexion and extension. While the subject is supine on the examining table with the head of the table elevated to about 25–30°, the leg with the affected knee is extended by support under the ankle so that the subject's knee is raised slightly from the table. Space is provided for the subject to fully extend their knee actively with the angle of maximum extension measured on the third and final attempt. After the knee extension measurement is taken (on the third trial), the bolster is removed, and the subject is asked to place their foot as close as possible to their buttocks (foot on the table). The subject is given three trials to flex their knee as far as possible without using their hands to assist them; the knee flexion measurement is taken on the third trial.
Intervention products
This study evaluated Study Product Water-Soluble Eggshell Membrane Capsules (450 mg/day, CAS# 227025-36-6; Biova, LLC, Johnson, IA) and matching placebo containing the same excipients but without the active study product. Both study product and placebo were manufactured by Uckele Health & Nutrition (Contract Manufacturer, Blissfield, MI) for the study Sponsor, Biova, LLC. Subjects were dosed once daily for the study duration. Compliance was measured by dichotomous questionnaire and the standard return pill-count method.
Safety assessment
Safety was monitored throughout the course of the study by monitoring of blood pressure, liver function, kidney function, blood sugar, general immunity, and red blood cell activity (comprehensive metabolic panel, complete count with differential urinalysis); physical exam, subjective complaints, observed events, and adverse events (AEs) were coded by MedDRA. All these were analyzed by appropriate statistical techniques.
Statistical analysis
All analyses were conducted and presented with descriptive statistics. For population statistics, the paired-t test or Wilcoxon sign-rank test was used, as appropriate to the data type, to compare the population measurements of both groups at each time point. The distributions of the individual changes from baseline within each group were also compared at all time points except the baseline. Fisher's exact test was utilized to compare the difference in proportions between groups when the sample size was small; otherwise, a chi-square test was employed. Significance was set at the 0.05 level (P < .05), with noted confidence intervals findings noted. A nonhierarchal statistical approach was utilized to treat each endpoint of interest separately as an independent endpoint of interest.
In addition to the population statistics described earlier, we tested the hypothesis that the performance of the worst initial performers within each group would improve more than those with the highest initial performance. This is a correlative test that may only be performed on continuum objective data (e.g., 6MWT or goniometer). Where the range of a normally distributed population narrows, the standard deviation (SD) (slope of the distribution on a probability plot) gets smaller and can be compared by t-test with that of another population.25,26 Further, the incremental change in performance of each individual within a treatment cohort can be correlated to their initial objective measured performance.27,28 If the corresponding slope of that correlation is statistically different from zero, then the hypothesis that the worst performing individuals within the group see the most benefit is verified.28,29 Similarly, the relative improvement seen in the worst performers can be compared between the two treatment cohorts by the t-test of the two performance improvement correlations. This comparative statistical technique, defined herein as the Correlative Improvement Over Baseline Performance (CIOBP) test, was applied to both the 6MWT and goniometer data sets.25–29
Results
Study recruitment
This study recruited from local advertisements in the community and screened 125 subjects to qualify and randomize 88 (2 cohorts of 44 subjects per group). With a total of 80 completing patients entering this two-treatment study, the probability was calculated to be 90% that the study would detect a treatment difference at a two-sided 0.05 significance level if the true difference between treatments is 0.735 times the SD. With the same sample size, the probability is 80% that the study will detect a treatment difference at a two-sided 0.05 significance level if the true difference between treatments is 0.635 times the SD. The mean age of study participants was 53.3 ± 7.61 years. The study population was 72% female and 28% male. The mean BMI was 28.20 ± 2.73 kg/m2.
Efficacy results
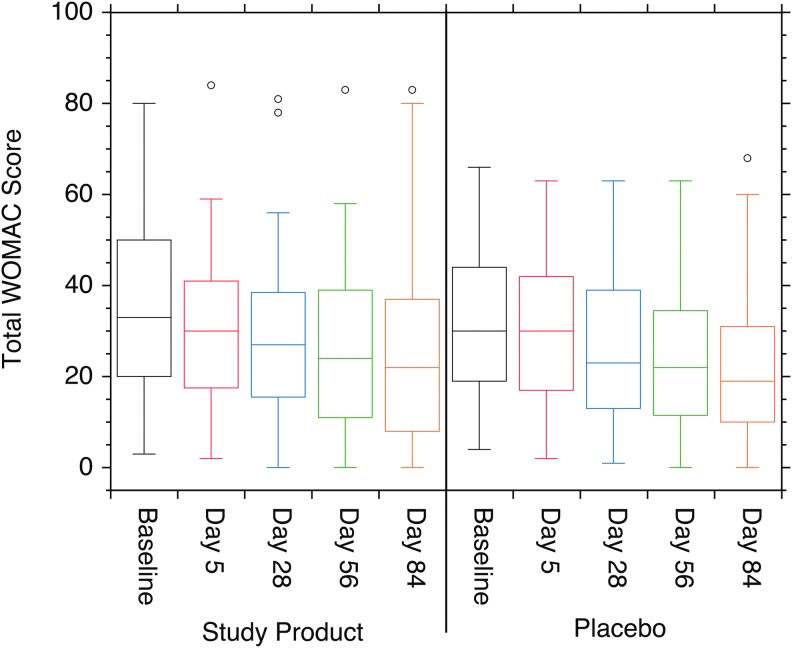
The WOMAC self-assessment test was used to measure the quality of life with pain/soreness, stiffness, physical function, and a composite score of these three subscales. Similar improvements in the composite WOMAC score and each of the subscale scores are seen in both the Study Product and Placebo groups for all quality-of-life measures (Fig. 1). However, these improvements were not statistically different between the two cohorts at any time point.
FIG. 1.
Box plots of the total WOMAC score for both the Study Product and Placebo cohorts. Both cohorts showed moderate improvement in scores over the course of the study, but pairwise comparisons showed no significant differences between the two cohorts at any time point (P > .05). WOMAC, Western Ontario McMaster Osteoarthritis Index.
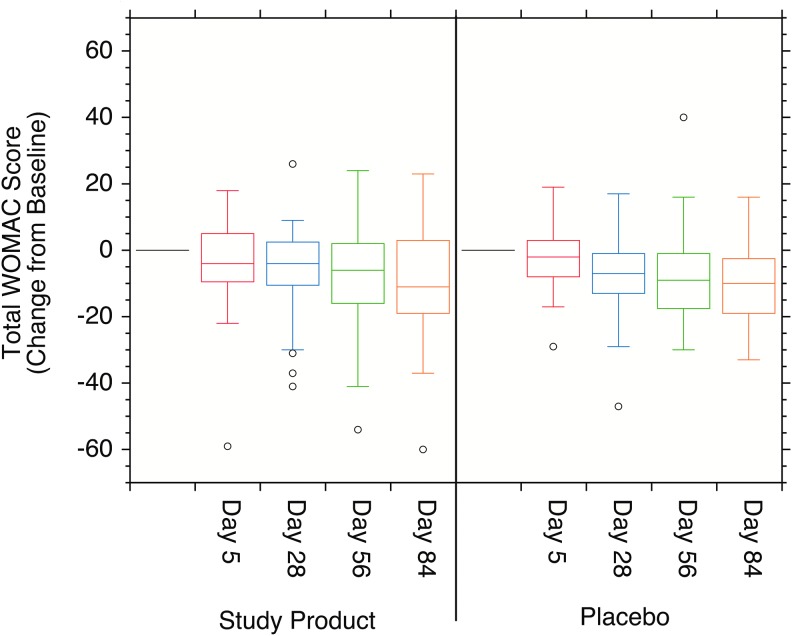
Because WOMAC is a self-assessment lacking a common objective scale, an attempt was made to normalize these data by looking at the individual changes from baseline at each time point (Fig. 2). The baseline composite WOMAC scores (SD) were 35.4 (19.69) and 31.6 (16.35) for the Study Product and Placebo cohorts, respectively. The mean changes from baseline for the composite WOMAC scores were −3.6 (12.87), −6.0 (13.08), −8.7 (15.96), and −9.9 (16.77) for days 5, 28, 57, and 86, respectively, of the Study Product cohort. The comparable composite mean change from baseline scores were −1.9 (9.38), −6.8 (12.33), −8.4 (13.75), and −9.9 (12.89) for the Placebo cohort. The mean change in the composite WOMAC score was statistically different from baseline in the Study Product cohort at all subsequent visits, 3 (day 5), 4 (day 28), 5 (day 57), and 6 (day 86) by t-test. The mean change from baseline in the Placebo cohort was not statistically different from baseline in visit 3 (day 5). It was statistically different from baseline in visits 4 (day 28), 5 (day 57), and 6 (day 86) by t-test. These data, however, showed no statistically significant differences between the two cohorts by the Wilcoxon Rank-Sum test.
FIG. 2.
Box plots of the individual improvements over baseline at each time point for the total WOMAC self-assessment. The means of the individual changes over baseline for the Study Product cohort were statistically different from baseline at all time points (P < .05). The Placebo cohort showed no statistically significant differences over baseline at any time point by either statistical test (P > .05).
WOMAC Pain Subscale
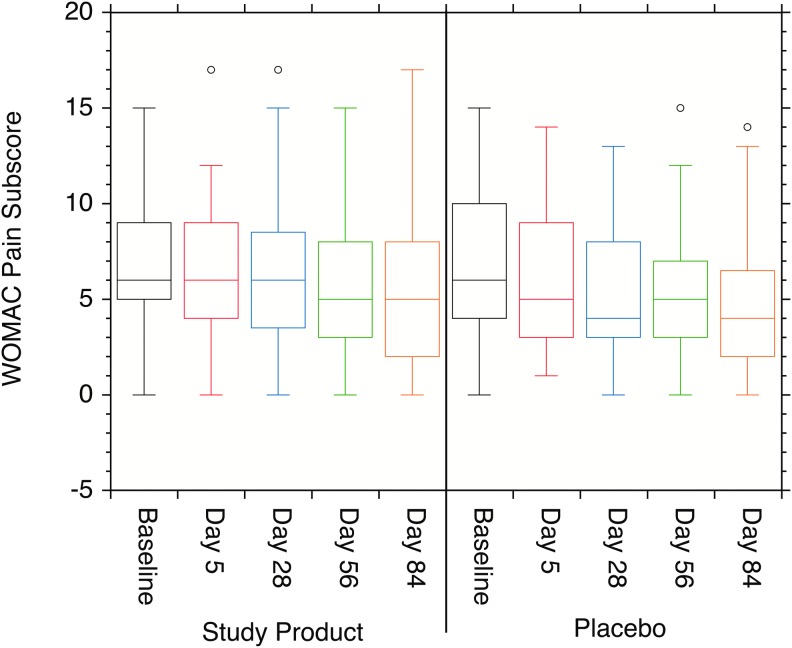
In the Study Product population, the mean score (SD) of pain at baseline (Visit 2) was 7.0 (3.44) in the Study Product group and 6.6 (3.73) in the Placebo group. The Pain subscale score distributions are shown in Fig. 3). The mean change from baseline (SD) in the Study Product group was −0.5 (2.39) at Visit 3 (day 5), −0.8 (3.38) at Visit 4, −1.5 (3.48) at Visit 5, and −1.9 (3.35) at Visit 6 (Fig. 4). The mean change from baseline (SD) in the Placebo group was −0.6 (2.73) at Visit 3, −1.4 (3.11) at Visit 4, −1.8 (3.42) at Visit 5, and −2.0 (3.14) at Visit 6. When compared with baseline, statistically significant decreases were observed at Visits 5 and 6 in the Study Product group and at Visits 4, 5, and 6 in the Placebo group. When compared between groups, no statistically significant difference was reported during the study period (P > .05).
FIG. 3.
Box plots of the individual WOMAC pain subscale scores. No statistically significant changes were observed in the data either between the Study Product and Placebo cohorts or over baseline within each study cohort (P > .05).
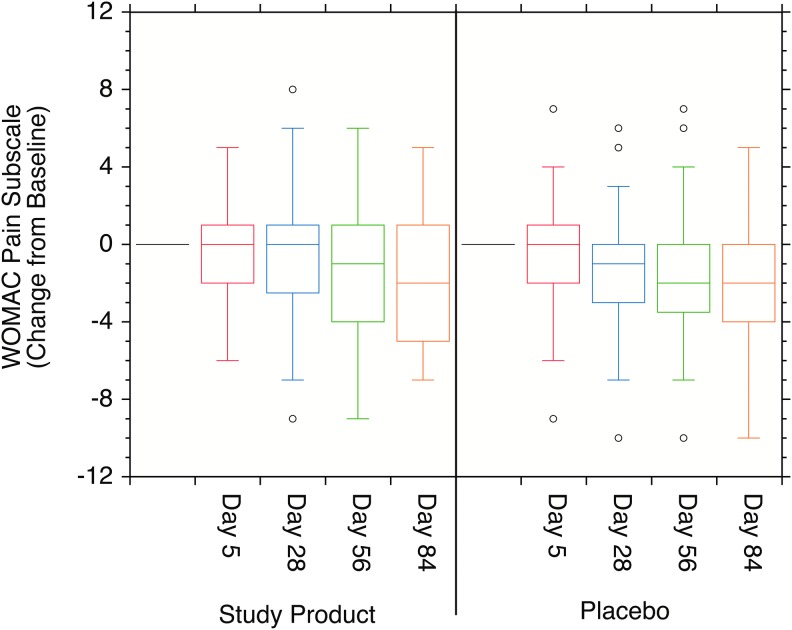
FIG. 4.
Box plots of the WOMAC change over baseline pain subscale scores. The changes in the individual scores over baseline were statistically significant for both the Study Product and Placebo cohorts at days 56 and 84 (P < .05), and for the Placebo cohort at day 28, but not statistically different from each other at any time point (P > .05).
WOMAC Stiffness Subscale
In the ITT population, the mean score (SD) of stiffness at baseline (Visit 2) was 3.9 (1.66) in the Study Product group and 3.6 (1.56) in the Placebo group, with the population distributions shown in Table 1 for each time point. Chi-squared analysis of the Study Product cohort suggests that a significant stiffness improvement was observed by day 5 (P = .0181), but not at later time points. The mean change from baseline (SD) in the Study Product group was −0.6 (0.97) at Visit 3, −0.7 (1.70) at Visit 4, −1.2 (1.83) at Visit 5, and −1.3 (1.86) at Visit 6, with the change-over-baseline score distributions for each cohort shown in Table 2. The mean change from baseline (SD) in the Placebo group was −0.1 (1.17) at Visit 3, −0.8 (1.59) at Visit 4, −1.0 (1.73) at Visit 5, and −1.3 (1.76) at Visit 6. Change from baseline was significantly enhanced (five times better) in the Study Product group by day 5 (significantly less stiffness) as compared with the Placebo group [(0.6) − (0.1)/(0.1) × 100 = 500%; P < .05]. In addition, when compared with baseline, statistically significant decreases were observed from Visit 3 to Visit 6 in the Study Product group and from Visit 4 to Visit 6 in the Placebo group. When compared between groups, no other statistically significant difference was reported during the study period.
Table 1.
Population Distributions in Western Ontario McMaster Osteoarthritis Index Stiffness Subscale Scores for the Study Product and Placebo Cohorts
| No. in Study Product Cohort | |||||
|---|---|---|---|---|---|
| Score 0–8 | Baseline | Day 5 | Day 28 | Day 56 | Day 84 |
| 0–1 | 3 | 5 | 7 | 12 | 13 |
| 2 | 4 | 10 | 12 | 9 | 10 |
| 3 | 10 | 11 | 4 | 3 | 4 |
| 4 | 12 | 8 | 10 | 9 | 5 |
| 5 | 8 | 4 | 3 | 3 | 6 |
| 6 | 3 | 3 | 5 | 5 | 1 |
| 7–8 | 3 | 2 | 2 | 0 | 2 |
| χ2P-score | .3887 | .0181 | .2118 | .3137 | .1676 |
| No. in Placebo Cohort | |||||
|---|---|---|---|---|---|
| Score 0–8 | Baseline | Day 5 | Day 28 | Day 56 | Day 84 |
| 0–1 | 5 | 8 | 8 | 11 | 11 |
| 2 | 3 | 6 | 12 | 9 | 15 |
| 3 | 12 | 5 | 8 | 9 | 4 |
| 4 | 12 | 11 | 11 | 9 | 8 |
| 5 | 8 | 8 | 4 | 2 | 3 |
| 6 | 5 | 6 | 2 | 3 | 2 |
| 7–8 | 0 | 1 | 0 | 0 | 0 |
Chi-squared statistical analysis suggests a statistical improvement in the Study Product cohort over Placebo at day 5.
Table 2.
Population Distributions in the Change-Over-Baseline Scores for the Western Ontario McMaster Osteoarthritis Index Stiffness Subscale
| No. in Study Product Cohort | |||||
|---|---|---|---|---|---|
| Score (−6 to 3) | Baseline | Day 5 | Day 28 | Day 56 | Day 84 |
| −6 to −3 | 1 | 7 | 10 | 9 | |
| −2 | 7 | 12 | 9 | 10 | |
| −1 | 16 | 4 | 6 | 10 | |
| 0 | 14 | 10 | 8 | 3 | |
| 1 | 4 | 3 | 5 | 8 | |
| 2–3 | 1 | 5 | 3 | 1 | |
| χ2P-score | 0.0365 | 0.1263 | 0.6975 | 0.0068 | |
| No. in Placebo Cohort | |||||
|---|---|---|---|---|---|
| Score (0–8) | Baseline | Day 5 | Day 28 | Day 56 | Day 84 |
| −6 to −3 | 1 | 6 | 9 | 11 | |
| −2 | 3 | 7 | 7 | 8 | |
| −1 | 13 | 11 | 8 | 8 | |
| 0 | 14 | 15 | 12 | 11 | |
| 1 | 10 | 4 | 5 | 3 | |
| 2–3 | 4 | 2 | 2 | 2 | |
Chi-squared analysis confirms that the Study Product cohort showed significant improvement over Placebo at both day 5 (confirming the results of Table 1) and day 84.
WOMAC Physical function Subscale
In the Study Product population, the mean score (SD) of physical function at baseline (Visit 2) was 24.4 (15.60) in the Study Product group and 21.4 (12.39) in the Placebo group. The mean change from baseline (SD) in the Study Product group was −2.6 (11.10) at Visit 3, −4.5 (9.80) at Visit 4, −6.0 (12.24) at Visit 5, and −6.6 (12.93) at Visit 6. The mean change from baseline (SD) in the Study Product Placebo group was −1.2 (7.20) at Visit 3, −4.6 (9.73) at Visit 4, −5.6 (10.29) at Visit 5, and −6.7(9.53) at Visit 6. When compared with baseline, statistically significant decreases were observed from Visit 4 to Visit 6 in both Study Product and Placebo groups. When compared between groups, no statistically significant difference was reported during the study period.
Six-minute walk test
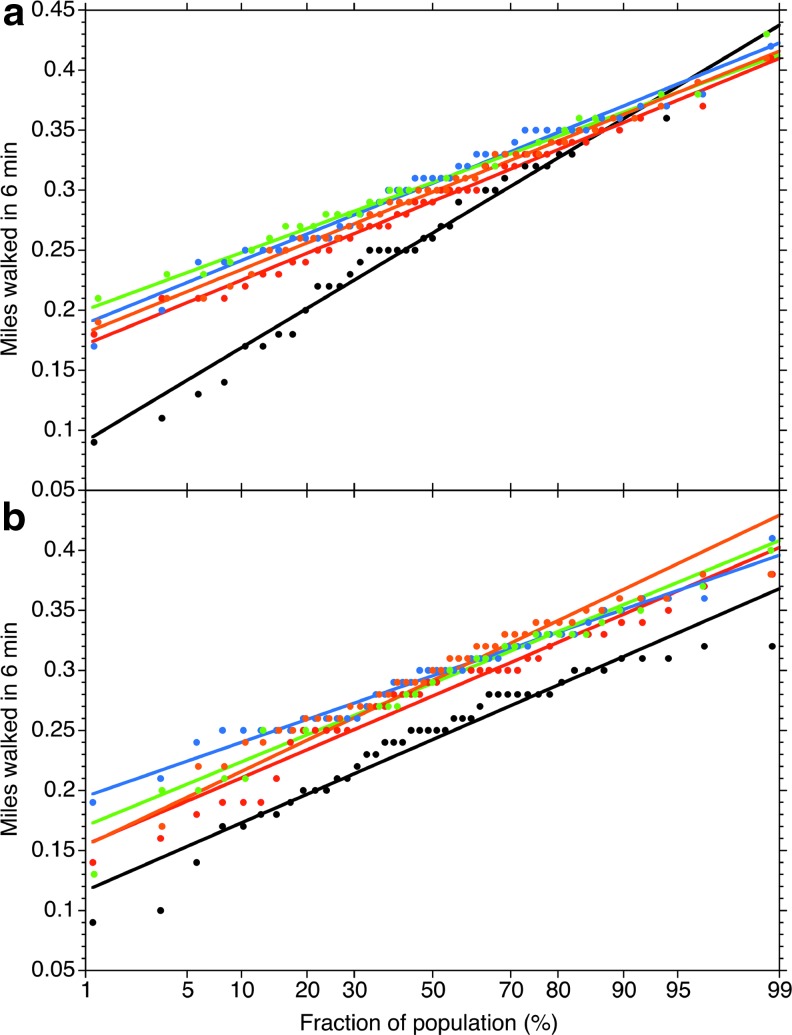
The aim of the test was to have the subject walk as fast as comfortably possible for 6 min. The distance covered over the full 6 min was recorded and documented. The test conducted on day 0 was before the first dose and provides a baseline for comparisons for change from baseline. The probability plots (Fig. 5) show the cumulative population distribution at each time point for each cohort. The linearity of the probability plots shows that the distances walked by the populations were normally distributed at each time point. The average distance walked at each time point was the same between the Study Product and Placebo cohorts within 95% confidence at all time points (Table 3). The average of the individual improvements over baseline, however, shows that the entire study population improves significantly (P = .0273) over that of the Placebo control by day 84 (Table 3).
FIG. 5.
The population distributions of 6MWT results for the (a) Study Product and (b) Placebo cohorts. 6MWT, six-minute walk test.
Table 3.
Walk Test (Miles Walked in 6 Min)
| Population mean (SD) | Average individual improvement over baseline (SD) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Time (days) | Study Product | n | Placebo | n | Difference Placebo to Study Product P-score | Study Product | n | Placebo | n | Difference Placebo to Study Product P-score |
| 0 | 0.268 (0.0720) | 42 | 0.242 (0.0557) | 44 | .1267 | — | 42 | — | 44 | |
| 5 | 0.293 (0.0504) | 42 | 0.279 (0.0544) | 44 | .2907 | 0.025 (0.0543) | 42 | 0.036 (0.0418) | 44 | .1796 |
| 28 | 0.309 (0.0466) | 42 | 0.295 (0.0436) | 44 | .3106 | 0.041 (0.0554) | 42 | 0.053 (0.0483) | 44 | .1770 |
| 56 | 0.306 (0.0453) | 41 | 0.289 (0.0520) | 43 | .1134 | 0.036 (0.0670) | 41 | 0.045 (0.0629) | 43 | .3196 |
| 84 | 0.299 (0.0499) | 41 | 0.299 (0.0472) | 42 | .9947 | 0.029 (0.0587) | 41 | 0.054 (0.0595) | 42 | .0273* |
Statistically significant between the groups.
Although the mean group performance did not appear to change, the variance in miles walked (as measured by the slope of the probability curves in Fig. 5) changed considerably from the baseline (day 0) to that observed at day 5 for the Study Product group. The baseline SD for the Study Product group was 0.0744 ± 0.0018 mi and was 0.0512 ± 0.0008 mi at day 5, which is different from the baseline at P < .00001. This variance difference over baseline persists throughout all the other time points for the Study Product group. No such difference is observed (P = .338) in the variance of the Placebo group between day 5 (0.0532 ± 0.0019 mi) and day 0 (0.0541 ± 0.0021 mi), or between day 5 and any of the other time points. As the distributions merge toward the higher performing end of the distributions, and the widest variance is seen at the lower initial performing end of the distribution, these data suggest a narrowing of the population distribution toward the lowest performing end of the distribution in the Study Product group. This result coupled with the average individual performance improvement seen by day 84 (Table 3) strongly suggests that the Study Product provides the most immediate benefit to the most initially compromised individuals but will eventually benefit walking functions in all individuals experiencing OA in the knee.
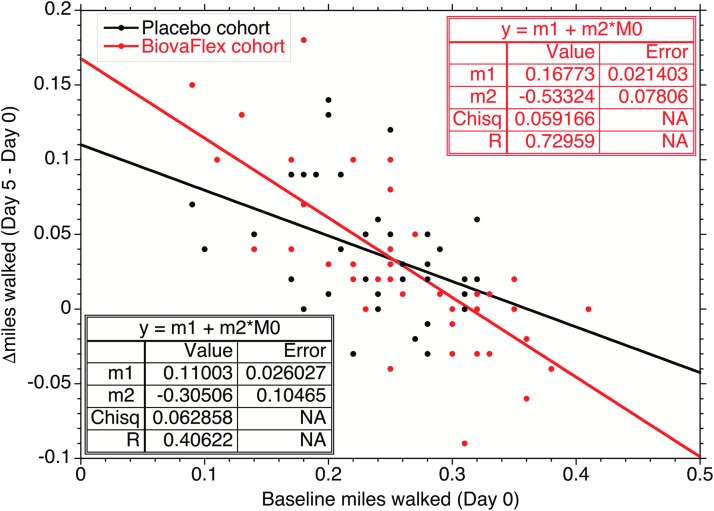
These results are corroborated by CIOBP analysis in which individual benefits from the study product are correlated to baseline performance (Fig. 6). After 5 days of Study Product use, the individual performance difference over baseline for the Study Product users (slope of the correlation, −0.533 ± 0.078) was statistically greater than zero (P < .00001) and greater than that of the Placebo group (−304 ± 0.106, P = .004). The Placebo group also showed statistical improvement over the first 5 days but not to as large an extent as the Study Product group, which might reflect the benefits of increased exercise over their normal activity. These data suggest that those with demonstrably compromised load-bearing performance can expect to experience significant improvement with Study Product use within 5 days, and that performance improvement will persist over time with continued use. However, those without demonstrable performance impairment will see little immediate benefit from using Study Product but do still appear to improve with Study Product versus Placebo over longer time (Table 1). The data demonstrate that there was a 75% relative improvement for the Study Product group over the Placebo group [(−0.53274) − (0.30365)/(0.030364) × 100 = 75.45%].
FIG. 6.
Observed change in miles walked by individuals over the initial 5-day period as a function of their initial (day 0) performance. The correlation of improvement with initial performance for the Study Product is statistically valid at >99.9% confidence (P-score <.0001), and for Placebo, at 99.3% confidence (P-score = .0065). The increase in the correlation slope was greater for the Study Product group than the control (Placebo) group at >98% confidence (P-score = .01981).
Range of ROM testing (goniometer)
Knee extension and knee flexion were measured by a goniometer. When compared with baseline, a statistically significant increase was observed at Visit 4 for knee flexion in the Study Product group. The mean change from baseline (SD) in the Study Product group at Visit 4 was −0.2 (4.75) (P < .05); the mean change from baseline (SD) in the Placebo group at Visit 4 was −0.3 (3.65) (P > .05). When compared for study duration between groups, no statistically significant difference was reported in knee extension and flexion.
Safety results
During the study period, 17 AEs were reported in 9 subjects (20.5%) of the Study Product group and 6 AEs were reported in 6 subjects (13.6%) of the Placebo group. This comparative difference was not statistically significant (P > .05). There was one SAE reported in one subject (2.3%) of the Study Product group in this study. All AEs were analyzed by groups. Of the 17 AEs reported in 9 subjects of the Study Product group, the severity of all AEs was mild and these included nasopharyngitis (6.8%), diarrhea (2.3%), nausea (2.3%), vomiting (2.3%), chest pain (2.3%), influenza-like illness (2.3%), abnormal hepatic function (2.3%), irregular heart rate (2.3%), dehydration (2.3%), dizziness (2.3%), headache (2.3%), poor-quality sleep (2.3%), cough (2.3%), rhinorrhea (2.3%), and rash (2.3%). Regarding the causality or relationship of AEs to Study Product, two Study Products potentially related to AEs such as headache and poor-quality sleep were reported in two subjects. One SAE such as dehydration was reported in one subject and this subject withdrew from the study. The SAE revealed itself as the study subject was hospitalized for acute and chronic alcohol toxicity, which was considered unrelated to the study product. Of the six AEs reported in six subjects of the Placebo group, these were abdominal pain (2.3%), flatulence (2.3%), nausea (2.3%), labyrinthitis (2.3%), hypoaesthesia (2.3%), and sinus operation (2.3%). Regarding the severity of AE incidence, the severity for abdominal pain and hypoaesthesia was moderate, plus the notation of four AEs measured was mild (flatulence, nausea, labyrinthitis, and sinus operation). Two placebo-related AEs such as abdominal pain and nausea were reported in two subjects, and the dose of placebo was discontinued due to one placebo-related AE (nausea). No SAE was reported in the Placebo group. Overall, no subject in either group had clinically significant abnormality or concerns in physical examination, vital signs, hematology, and chemistry assessment during the study period. The study did not find cause for safety concern of either the Study Product or Placebo.
Discussion
Discomfort may impact joint mobility and impact our ability to exercise, carry out typical tasks of daily living, especially if occurring at the level of the hips, knees, lower back, and so forth. Impaired joint mobility may also impact quality of life. It is for these reasons that the results of this study hold interest. This study was able to determine that for those who have a harder time ambulating because of their joint discomfort/OA, the WSEM Study Product could significantly enhance physical capacity (ability to walk and cover distance) as well as feelings of stiffness (joint stiffness) to a greater and more meaningful degree than the placebo product. Medicinal studies with OA have previously found that it is those with the most severe forms of the condition that achieve the greatest clinically relevant improvement.31 Our results are consistent with this previous finding. This early relief or improvement may help spur a person to continued practices for maintaining improved joint health. In this study, which included adults with OA, it was decided to examine whether the OA interacts with and impacts the ability to ambulate (physical activity) as well as quality of life. Based on their initial results, subjects were classified by their ability to do load-bearing work (ambulate efficiently); it was found that subjects with initial apparent, compromised load-bearing performance had significant improvement with the administration of the study product within 5 days (the 6MWT and WOMAC-stiffness score, both significantly improved), and that the performance improvement was maintained in the 6MWT over the course of the study.
Those who were worse off physically (poorer performers on the 6MWT) experienced a meaningful improvement in their physical abilities and quality of life (perceived stiffness) compared with those who were less physically compromised (as detailed by 6MWT and WOMAC-stiffness scores on day 5, P < .05). Overall, when compared with baseline, statistically significant decreases were observed until the end of the study in subscales and total score of WOMAC in both study groups and there was no statistically significant difference between groups (P > .05). The study product group had some improvement in knee function when compared with baseline and showed significant improvement in physical performance, mobility, and joint stiffness in 5 days when compared with the placebo (P < .05) across outcomes listed. These improvements appeared to be maintained over the ∼12 weeks of the study. The consumption of Study Product and Placebo over the 12 weeks of the study (looking at end of study data vs. baseline) was found not to differently effect WOMAC pain, WOMAC physical function, the 6MWT, or ROM.
The fact that the findings of this study are supported by prior published research using this direct tested Study Product (BiovaFlex) or one in the same food category (egg shell membrane) should bolster the strength of the findings due to consistency of the results.16–18,30,31 In addition, with the understanding that GAGs are a component of the WSEM (e.g., chondroitin and hyaluronan), there are also prior published data demonstrating the benefits of GAGs for quality of life and joint function and ability in those with joint health issues or OA; hence, the findings of this study appear well supported.9–11,30,31
From a safety perspective, this 12-week study results supported safety as measured by blood tests (metabolic panel, complete blood count with differential), vitals (systolic and diastolic blood pressure, heart rate), subjective statements by the participant or direct observation or assessment by the study physician for both Study Product and the Placebo. There were no signals of any human safety concerns.
Limitations of this study may include an OA population that was not defined by grade (ACR criteria), not specifically recruiting people with radiographic moderate to severe OA. There is an unequal distribution of women to men, which might be considered a weakness but is known of the prevalence of knee OA in that it is more prevalent in women. A further weakness is that we did not collect any biomarkers related to inflammation or bone or cartilage health. Future studies may consider adding more outcome-based objective markers for detailing efficacy.
In conclusion, this study found that for those who are physically compromised in their ability to ambulate (walk slower due to their joint discomfort or pain), 450 mg of the Study Product (BiovaFlex) had a positive impact on functional capacity (ability to ambulate) as well as on perceived joint stiffness within the initial 5 days of dosing. This improvement was also considered meaningful as it was maintained over the course of the study (12 weeks).
Acknowledgments
Funding: This research was funded by Biova LLC, Johnson, Iowa, with a research grant to QPS-Missouri, USA.
Author Contributions
D.K. (who worked at QPS at the time of this study) was responsible for study design (along with other QPS-Missouri scientists), study execution, and article writing and editing. S.H. is the corresponding author and was responsible for article writing and editing. L.S. is a paid consultant of Biova, LLC.
Author Disclosure Statement
D.K. and S.H. report receiving funding support from Biova (study sponsor) for writing this article. At the time of the study, D.K. was an employee of QPS. L.V.S. is a paid consultant of Biova.
References
- 1. Hunter DJ, Schofield D, Callander E: The individual and socioeconomic impact of osteoarthritis. Lancet Nat Rev Rheumatol 2014;10:437–441 [DOI] [PubMed] [Google Scholar]
- 2. Global Burden of Disease Study 2013 Collaborators: Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: A systematic analysis for the Global Burden of Disease Study. Lancet 2013;386:743–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vos T, Flaxman AD, Naghavi M, et al. : Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: A systematic analysis for the Global Burden of Disease Study. Lancet 2012;380:2163–2196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Turk DC, Wilson HD, Cahana A: Treatment of chronic non-cancer pain. Lancet 2011;377:2226–2235 [DOI] [PubMed] [Google Scholar]
- 5. Messier SP, Loeser RF, Miller GD, et al. : Exercise and dietary weight loss in overweight and obese older adults with knee osteoarthritis: The arthritis, diet, and activity promotion trial. Arthritis Rheum 2004;50:1501–1510 [DOI] [PubMed] [Google Scholar]
- 6. Karsdal MA, Christiansen C, Ladel C, Henriksen K, Kraus VB, Bay-Jensen AC: Osteoarthritis—A case for personalized health care? Osteoarthritis Cartilage 2014;22:7–16 [DOI] [PubMed] [Google Scholar]
- 7. Tonge DP, Pearson MJ, Jones SW: The hallmarks of osteoarthritis and the potential to develop personalised disease-modifying pharmacological therapeutics. Osteoarthritis Cartilage 2014;22:609–621 [DOI] [PubMed] [Google Scholar]
- 8. Bucci LR, Sheldon E, Schwartz H: Comparison of glucosamine and a proprietary dietary supplement for symptoms of knee osteoarthritis. Poster Presentation. Abstract: P194. Osteoarthritis Cartilage 2005;13(A):S98 [Google Scholar]
- 9. Kalman DS, Hewling SJ: The effects of Morus alba and Acacia catechu on quality of life and overall function in adults with osteoarthritis of the knee. J Nutr Metab 2017;2017:4893104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kalman DS, Heimer M, Valdeon A, Schwartz H, Sheldon E: Effect of a natural extract of chicken combs with a high content of hyaluronic acid (Hyal-Joint®) on pain relief and quality of life in subjects with knee osteoarthritis: A pilot randomized double-blind placebo-controlled trial. Nutr J 2008;7:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Maeda K, Sasaki Y: An experience of hen-egg membrane as a biological dressing. Burns Incl Therm Inj 1982;8:313–316 [DOI] [PubMed] [Google Scholar]
- 12. Cordeiro CM, Hincke MT: Recent patents on eggshell: Shell and membrane applications. Recent Pat Food Nutr Agric 2011;3:1–8 [DOI] [PubMed] [Google Scholar]
- 13. Benson KF, Ruff KJ, Jensen GS: Effects of Natural Eggshell Membrane (NEM) on cytokine production in cultures of peripheral blood mononuclear cells: Increased suppression of tumor necrosis factor-α levels after in vitro digestion. J Med Food 2012;15:360–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ruff KJ, DeVore DP: Reduction of pro-inflammatory cytokines in rats following 7-day oral supplementation with a proprietary eggshell membrane-derived product. Mod Res Inflamm 2014;3:19–25 [Google Scholar]
- 15. Ruff KJ, Morrison D, Duncan SA, Back M, Aydogan C, Theodosakis J: Beneficial effects of natural eggshell membrane versus placebo in exercise-induced joint pain, stiffness, and cartilage turnover in healthy, postmenopausal women. Clin Interv Aging 2018;13:285–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ruff KJ, DeVore DP, Leu MD, Robinson MA: Eggshell membrane: A possible new natural therapeutic for joint and connective tissue disorders. Results from two open-label human clinical studies. Clin Interv Aging 2009;4:235–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ruff KJ, Winkler A, Jackson RW, DeVore DP, Ritz BW: Eggshell membrane in the treatment of pain and stiffness from osteoarthritis of the knee: A randomized, multicenter, double-blind, placebo-controlled clinical study. Clin Rheumatol 2009;28:907–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jensen GS, Lenninger MR, Beaman JL, Taylor R, Benson KF: Support of joint function, range of motion, and physical activity levels by consumption of a water-soluble egg membrane hydrolyzate. J Med Food 2015;18:1042–1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Borenstein D, Brandt K, Christy W, et al. : Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum 1986;29:1039–1049 [DOI] [PubMed] [Google Scholar]
- 20. Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW: Validation study of WOMAC: A health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 1988;15:1833–1840 [PubMed] [Google Scholar]
- 21. American College of Rheumatology, 2018. https://www.rheumatology.org/I-Am-A/Rheumatologist/Research/Clinician-Researchers/Western-Ontario-McMaster-Universities-Osteoarthritis-Index-WOMAC (accessed August7, 2018)
- 22. Guyatt GH, Sullivan MJ, Thompson PJ: The 6-minute walk: A new measure of exercise capacity in patients with chronic heart failure. Can Med Assoc J 1985;132:919–923 [PMC free article] [PubMed] [Google Scholar]
- 23. Six Minute Walk Test. American College of Rheumatology, 2018. https://www.rheumatology.org/I-Am-A/Rheumatologist/Research/Clinician-Researchers/Six-Minute-Walk-Test-SMWT (accessed August7, 2018)
- 24. Steultjens MP, Dekker J, van Baar ME, Oostendorp RA, Bijlsma JW: Range of joint motion and disability in patients with osteoarthritis of the knee or hip. Rheumatology 2000;39:955–961 [DOI] [PubMed] [Google Scholar]
- 25. Chambers J, Cleveland W, Kleiner B, Tukey P: Graphical Methods for Data Analysis. Wadsworth, Belmont, California, 1983 [Google Scholar]
- 26. Bland M: An Introduction to Medical Statistics, 3rd ed. Oxford University Press, Oxford, 2000 [Google Scholar]
- 27. Andrade JM, Estevez-Perez MG: Statistical comparison of the slopes of two regression lines: A tutorial. Anal Chim Acta 2014;838:1–12 [DOI] [PubMed] [Google Scholar]
- 28. Donahue RM: A summary statistic for measuring change from baseline. J Biopharm Stat 1997;7:287–299 [DOI] [PubMed] [Google Scholar]
- 29. Morita M, Yamada K, Date H, Hayakawa K, Sakurai H, Yamada H: Efficacy of chondroitin sulfate for painful knee osteoarthritis: A One-Year, Randomized, Double-Blind, Multicenter Clinical Study in Japan. Biol Pharm Bull 2018;41:163–171 [DOI] [PubMed] [Google Scholar]
- 30. Galluccio F, Barskova T, Cerinic MM: Short-term effect of the combination of hyaluronic acid, chondroitin sulfate, and keratin matrix on early symptomatic knee osteoarthritis. Eur J Rheumatol 2015;2:106–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tubach F, Ravaud P, Baron G, et al. : Evaluation of clinically relevant changes in patient reported outcomes in knee and hip osteoarthritis: the minimal clinically important improvement. Ann Rheum Dis 2005;64:29–33 [DOI] [PMC free article] [PubMed] [Google Scholar]