Abstract
Background
Infants born preterm (before 37 weeks' gestation) have poorer outcomes than infants at term, particularly if born before 32 weeks. Early cord clamping has been standard practice over many years, and enables quick transfer of the infant to neonatal care. Delayed clamping allows blood flow between the placenta, umbilical cord and baby to continue, and may aid transition. Keeping baby at the mother's side enables neonatal care with the cord intact and this, along with delayed clamping, may improve outcomes. Umbilical cord milking (UCM) is proposed for increasing placental transfusion when immediate care for the preterm baby is needed. This Cochrane Review is a further update of a review first published in 2004 and updated in 2012.
Objectives
To assess the effects on infants born at less than 37 weeks' gestation, and their mothers of: 1) delayed cord clamping (DCC) compared with early cord clamping (ECC) both with immediate neonatal care after cord clamping; 2) DCC with immediate neonatal care with cord intact compared with ECC with immediate neonatal care after cord clamping; 3) DCC with immediate neonatal care after cord clamping compared with UCM; 4) UCM compared with ECC with immediate neonatal care after cord clamping.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group Trials Register, ClinicalTrials.gov, the WHO International Clinical Trials Registry Platform (ICTRP) (10 November 2017), and reference lists of retrieved studies. We updated the search in November 2018 and added nine new trial reports to the awaiting classification section to be assessed at the next update.
Selection criteria
Randomised controlled trials (RCTs) comparing delayed with early clamping of the umbilical cord (with immediate neonatal care after cord clamping or with cord intact) and UCM for births before 37 weeks' gestation. Quasi‐RCTs were excluded.
Data collection and analysis
Two review authors independently assessed trials for inclusion and risk of bias, extracted data and checked them for accuracy. Random‐effects are used in all meta‐analyses. Review authors assessed the certainty of the evidence using the GRADE approach.
Main results
This update includes forty‐eight studies, involving 5721 babies and their mothers, with data available from 40 studies involving 4884 babies and their mothers. Babies were between 24 and 36+6 weeks' gestation at birth and multiple births were included. The data are mostly from high‐income countries. Delayed clamping ranged between 30 to 180 seconds, with most studies delaying for 30 to 60 seconds. Early clamping was less than 30 seconds and often immediate. UCM was mostly before cord clamping but some were milked after cord clamping. We undertook subgroup analysis by gestation and type of intervention, and sensitivity analyses by low risk of selection and attrition bias.
All studies were high risk for performance bias and many were unclear for other aspects of risk of bias. Certainty of the evidence using GRADE was mostly low, mainly due to imprecision and unclear risk of bias.
Delayed cord clamping (DCC) versus early cord clamping (ECC) both with immediate neonatal care after cord clamping (25 studies, 3100 babies and their mothers)
DCC probably reduces the number of babies who die before discharge compared with ECC (average risk ratio (aRR) 0.73, 95% confidence interval (CI) 0.54 to 0.98, 20 studies, 2680 babies (moderate certainty)).
No studies reported on 'Death or neurodevelopmental impairment' in the early years'.
DCC may make little or no difference to the number of babies with severe intraventricular haemorrhage (IVH grades 3 and 4) (aRR 0.94, 95% CI 0.63 to 1.39, 10 studies, 2058 babies, low certainty) but slightly reduces the number of babies with any grade IVH (aRR 0.83, 95% CI 0.70 to 0.99, 15 studies, 2333 babies, high certainty).
DCC has little or no effect on chronic lung disease (CLD) (aRR 1.04, 95% CI 0.94 to 1.14, 6 studies, 1644 babies, high certainty).
Due to insufficient data, we were unable to form conclusions regarding periventricular leukomalacia (PVL) (aRR 0.58, 95% CI 0.26 to 1.30, 4 studies, 1544 babies, low certainty) or maternal blood loss of 500 mL or greater (aRR 1.14, 95% CI 0.07 to 17.63, 2 studies, 180 women, very low certainty).
We identified no important heterogeneity in subgroup or sensitivity analyses.
Delayed cord clamping (DCC) with immediate neonatal care with cord intact versus early cord clamping (ECC) (one study, 276 babies and their mothers)
There are insufficient data to be confident in our findings, but DCC with immediate neonatal care with cord intact may reduce the number of babies who die before discharge, although the data are also compatible with a slight increase in mortality, compared with ECC (aRR 0.47, 95% CI 0.20 to 1.11, 1 study, 270 babies, low certainty). DCC may also reduce the number of babies who die or have neurodevelopmental impairment in early years (aRR 0.61, 95% CI 0.39 to 0.96, 1 study, 218 babies, low certainty). There may be little or no difference in: severe IVH; all grades IVH; PVL; CLD; maternal blood loss ≥ 500 mL, assessed as low certainty mainly due to serious imprecision.
Delayed cord clamping (DCC) with immediate neonatal care after cord clamping versus umbilical cord milking (UCM) (three studies, 322 babies and their mothers) and UCM versus early cord clamping (ECC) (11 studies, 1183 babies and their mothers)
There are insufficient data for reliable conclusions about the comparative effects of UCM compared with delayed or early clamping (mostly low or very low certainty).
Authors' conclusions
Delayed, rather than early, cord clamping may reduce the risk of death before discharge for babies born preterm. There is insufficient evidence to show what duration of delay is best, one or several minutes, and therefore the optimum time to clamp the umbilical cord remains unclear. Whilst the current evidence supports not clamping the cord before 30 seconds at preterm births, future trials could compare different lengths of delay. Immediate neonatal care with the cord intact requires further study, and there are insufficient data on UCM.
The nine new reports awaiting further classification may alter the conclusions of the review once assessed.
Keywords: Female; Humans; Infant, Newborn; Pregnancy; Infant, Premature; Infant, Premature/growth & development; Umbilical Cord; Blood Transfusion; Blood Transfusion/statistics & numerical data; Cerebral Hemorrhage; Cerebral Hemorrhage/prevention & control; Delivery, Obstetric; Placental Circulation; Placental Circulation/physiology; Pregnancy Outcome; Premature Birth; Randomized Controlled Trials as Topic; Time Factors
Plain language summary
Does delaying cord clamping or using cord milking at birth improve the health of babies born too early?
What is the issue?
In this Cochrane Review, we set out to determine if delayed cord clamping or umbilical cord milking improves the health outcomes for babies born before 37 weeks' gestation. These interventions were compared with early cord clamping.
Why is it important?
Babies born before 37 weeks, or preterm, have poorer health outcomes than babies born at term, particularly if they are born before 32 weeks. Babies born preterm can experience problems with the functioning of many of their major organs including their lungs, gut and hearts. They have a greater risk of dying or having long‐term problems such as cerebral palsy. After birth, the babies may need blood transfusions and drugs to strengthen their heart contractions (inotropes) and to raise their blood pressure. It is important to try to find ways of improving the health of these tiny babies.
Early clamping of the umbilical cord has been standard practice over many years. It allows the baby to be transferred quickly to care from a specialised team of doctors either at the side of the room or in another room. Yet, delayed clamping for half to three or more minutes allows continuing blood flow between the mother and her baby, and this may help the baby to adjust to breathing air. Squeezing blood along the umbilical cord towards the baby (milking the cord), can boost the baby's blood volume, and this may improve the baby's health. We wanted to see if there are any benefits or harms from either waiting to clamp or milking the cord.
What evidence did we find?
We collected and analysed all relevant studies to answer this question (date of search: November 2017). Our updated review included 40 studies which provided data on 4884 babies and their mothers. Studies were undertaken across the world, but mostly in high‐income countries. Births were in hospitals which practiced early clamping. For many outcomes there were insufficient data to be really confident of our findings.
1) For delayed cord clamping (with immediate care of the baby after cord clamping) compared with early cord clamping, we found it likely that fewer babies died before discharge (20 studies, 2680 babies). Also, fewer babies may have had any bleeding in the brain (15 studies, 2333 babies), but there was probably no difference in the numbers of babies with very serious brain bleeds (10 studies, 2058 babies).
2) Only one study of 276 babies and their mothers provided data on delayed cord clamping with immediate care of the baby beside the mother with cord intact compared with early cord clamping. This study was small and did not identify any marked differences in health outcomes.
3) For delayed cord clamping (with immediate care of the baby after cord clamping) versus cord milking, there were insufficient data (three studies, 322 babies) to make comparisons between outcomes.
4) For cord milking versus early cord clamping, we found 11 studies providing data with 1183 babies and their mothers. Again, there were insufficient data to make clear comparisons on outcomes.
What does this mean?
Delayed cord clamping probably reduced the risk of death for babies born preterm. Early cord clamping probably causes harm. No studies showed what length of delay was best, and only a few studies followed babies for health outcomes in early childhood. There is insufficient evidence for reliable conclusions on providing immediate care for the baby beside the mother with the cord intact. Similarly, there is insufficient evidence for reliable conclusions on cord milking. Further studies are in progress.
Summary of findings
Summary of findings for the main comparison. DCC with immediate neonatal care after cord clamping compared to ECC (subgroup analysis by gestation) for health problem or population.
| DCC with immediate neonatal care after cord clamping compared to ECC (subgroup analysis by gestation) for health problem or population | ||||||
| Patient or population: babies born preterm, and their mothers Setting: hospital births mostly in high‐income countries Intervention: delayed cord clamping (DCC) with immediate neonatal care after cord clamping Comparison: early cord clamping (ECC) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with ECC (subgroup analysis by gestation) | Risk with DCC with immediate neonatal care after cord clamping | |||||
| Death of baby (up to discharge) | Study population | RR 0.73 (0.54 to 0.98) | 2680 (20 RCTs) | ⊕⊕⊕⊝ MODERATE 1 2 | ||
| 74 per 1000 | 54 per 1000 (40 to 72) | |||||
| Death or neurodevelopmental impairment in early years | Study population | ‐ | (0 studies) | ‐ | ||
| see comment | see comment | |||||
| Severe intraventricular haemorrhage (IVH grades 3, 4) | Study population | RR 0.94 (0.63 to 1.39) | 2058 (10 RCTs) | ⊕⊕⊝⊝ LOW 3 4 | ||
| 48 per 1000 | 45 per 1000 (30 to 66) | |||||
| Intraventricular haemorrhage (IVH, all grades) | Study population | RR 0.83 (0.70 to 0.99) | 2333 (15 RCTs) | ⊕⊕⊕⊕ HIGH 5 6 | ||
| 187 per 1000 | 155 per 1000 (131 to 185) | |||||
| Periventricular leukomalacia (PVL) | Study population | RR 0.58 (0.26 to 1.30) | 1544 (4 RCTs) | ⊕⊕⊝⊝ LOW 7 | ||
| 22 per 1000 | 13 per 1000 (6 to 28) | |||||
| Chronic lung disease (CLD) ‐ oxygen supplement at 36 weeks (corrected for gestation) | Study population | RR 1.04 (0.94 to 1.14) | 1644 (6 RCTs) | ⊕⊕⊕⊕ HIGH 8 | ||
| 494 per 1000 | 514 per 1000 (464 to 563) | |||||
| Maternal blood loss of 500 mL or greater | Study population | RR 1.14 (0.07 to 17.63) | 180 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 9 10 | ||
| 11 per 1000 | 12 per 1000 (1 to 188) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Although many of the included studies have unclear risk of bias, the large trial which provided 80% of the data is low risk of bias. No downgrade.
2 Number of participants = 2680 and OIS > 11,000 (ref Tarnow‐Mordi 2017); number of events 171 less than the 300 calculated for confidence in findings; upper confidence interval close to the line of no difference. Downgrade 1.
3 25% of data comes from studies where the risk of bias is unclear or high, however, the large study which provides 70% of data are low risk of bias. No downgrade.
4 Number of participants 2083; number of events 86 (< 300 generally required); CI crosses line of no difference. Downgrade 2.
5 78% of data coming from studies of low risk of bias including the large study which is of low risk of bias. No downgrade.
6 Number of participants 2333; number of events 409. No downgrade.
7 Number of participants 1544 and number of events 26 (well below generally required 300). Downgrade 2.
8 98% of data comes from trials of low risk of selection bias, including 1 large well‐conducted trial. No downgrade.
9 Although Selection bias is low risk of bias, incomplete outcome data is high risk of bias. Downgrade 1.
10 Only 180 women and 2 events. Downgrade 2.
Summary of findings 2. DCC with immediate neonatal care with cord intact compared to ECC in babies born preterm.
| DCC with immediate neonatal care with cord intact compared to ECC in babies born preterm | ||||||
| Patient or population: babies born preterm, and their mothers Setting: hospital births in UK Intervention: delayed cord clamping (DCC) with immediate neonatal care with cord intact Comparison: early cord clamping (ECC) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with ECC (subgroup analysis by gestation) | Risk with DCC with immediate neonatal care with cord intact | |||||
| Death of baby (up to discharge) | Study population | RR 0.47 (0.20 to 1.11) | 270 (1 RCT) | ⊕⊕⊝⊝ LOW 1 | ||
| 111 per 1000 | 52 per 1000 (22 to 123) | |||||
| Death or neurodevelopmental impairment at age 2 to 3 years | Study population | RR 0.61 (0.39 to 0.96) | 218 (1 RCT) | ⊕⊕⊝⊝ LOW 2 | ||
| 340 per 1000 | 207 per 1000 (133 to 326) | |||||
| Severe intraventricular haemorrhage (IVH grades 3, 4) | Study population | RR 0.84 (0.29 to 2.45) | 266 (1 RCT) | ⊕⊕⊝⊝ LOW 3 | ||
| 53 per 1000 | 45 per 1000 (15 to 130) | |||||
| Intraventricular haemorrhage (IVH, all grades) | Study population | RR 0.90 (0.64 to 1.26) | 266 (1 RCT) | ⊕⊕⊝⊝ LOW 4 | ||
| 356 per 1000 | 320 per 1000 (228 to 449) | |||||
| Periventricular leukomalacia (PVL) | Study population | RR 0.86 (0.32 to 2.31) | 266 (1 RCT) | ⊕⊕⊝⊝ LOW 5 | ||
| 61 per 1000 | 52 per 1000 (19 to 140) | |||||
| Chronic lung disease (CLD) ‐ oxygen supplement at 36 weeks (corrected for gestation) | Study population | RR 0.95 (0.66 to 1.37) | 249 (1 RCT) | ⊕⊕⊝⊝ LOW 6 | ||
| 325 per 1000 | 309 per 1000 (215 to 445) | |||||
| Maternal blood loss of 500 mL or greater | Study population | RR 0.94 (0.72 to 1.22) | 254 (1 RCT) | ⊕⊕⊝⊝ LOW 7 8 | ||
| 476 per 1000 | 447 per 1000 (343 to 580) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Only one small study (N = 270); wide CI crossing line of no effect and very few events (n = 22). Downgrade 2.
2 Only one small study (N = 218); wide CI crossing line of no effect and very few events (n = 59). Downgrade 2.
3 Only one small study (N = 266); wide CI crossing line of no effect and very few events (n = 13). Downgrade 2.
4 Only one small study (N = 266); wide CI crossing line of no effect and few events (n = 90). Downgrade 2.
5 Only one small study (N = 266); wide CI crossing line of no effect and very few events (n = 15). Downgrade 2.
6 Only one small study (N = 249); wide CI crossing line of no effect and few events (n = 79). Downgrade 2.
7 High risk of bias through not being able to blind clinicians or women and this outcome. Downgrade 1.
8 Only one small study (N = 254); wide CI crossing line of no effect and few events (n = 117). Downgrade 1.
Summary of findings 3. DCC with immediate neonatal care after cord clamping compared to UCM in babes born preterm.
| DCC with immediate neonatal care after cord clamping compared to UCM in babies born preterm | ||||||
| Patient or population: babies born preterm, and their mothers Setting: hospital births mostly in high‐income countries Intervention: delayed cord clamping (DCC) with immediate neonatal care after cord clamping Comparison: umbilical cord milking (UCM). | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with UCM (subgroup analysis by gestation) | Risk with DCC with immediate neonatal care after cord clamping | |||||
| Death of baby (up to discharge) | Study population | RR 2.14 (0.93 to 4.93) | 322 (3 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | ||
| 44 per 1000 | 94 per 1000 (41 to 216) | |||||
| Death or neurodevelopmental impairment at age 2 to 3 years | Study population | RR 1.67 (0.78 to 3.57) | 195 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 3 4 | ||
| 162 per 1000 | 270 per 1000 (126 to 577) | |||||
| Severe intraventricular haemorrhage (IVH grades 3, 4) | Study population | RR 2.63 (0.11 to 61.88) | 58 (1 RCT) | ⊕⊕⊝⊝ LOW 5 6 | ||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Intraventricular haemorrhage (IVH, all grades) | Study population | RR 1.32 (0.55 to 3.17) | 125 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 7 8 | ||
| 129 per 1000 | 170 per 1000 (71 to 409) | |||||
| Periventricular leukomalacia (PVL) | Study population | not estimable | 58 (1 RCT) | ⊕⊕⊝⊝ LOW 9 10 | ||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Chronic lung disease (CLD) ‐ oxygen supplement at 36 weeks (corrected for gestation) | Study population | RR 1.53 (0.43 to 5.48) | 125 (2 RCTs) | ⊕⊕⊝⊝ LOW 11 12 | ||
| 48 per 1000 | 74 per 1000 (21 to 265) | |||||
| Maternal blood loss of 500 mL or greater | Study population | ‐ | (0 studies) | ‐ | ||
| see comment | see comment | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Risk of bias: two out of three studies were low risk of bias for sequence generation, allocation concealment and incomplete outcome data and provided over 90% of data. No downgrade.
2 Imprecision: small number of participants (N = 322); very few events (n = 24) and wide 95% CI crossing line of no difference. Downgrade 2.
3 One study providing over 70% of data was high risk of attrition bias and selective outcome reporting bias. Downgrade 1.
4 Wide CI crossing line of no difference, small number of participants (N = 195) and few events (n = 41). Downgrade 2.
5 One small study ‐ low risk of bias. No downgrade.
6 Small sample size (N = 58), only 1 event and wide 95% CI crossing line of no difference. Downgrade 2.
7 One study providing over 50% of data was unclear for selection bias. Downgrade 1.
8 Small sample size (N = 125), few events (n = 19) and wide 95% CI crossing line of no difference. Downgrade 2.
9 Risk of bias: low for sequence generation, allocation concealment and incomplete outcome data. No downgrade.
10 Imprecision: small sample size (N = 58) and no events. Downgrade 2.
11 One study provided 82% of the data were assessed as low risk of bias. No downgrade.
12 Small sample size (N = 125), very few events (n = 9) and wide 95% CI crossing line of no difference. Downgrade 2.
Summary of findings 4. UCM compared to ECC in babies born preterm.
| UCM compared to ECC in babies born preterm | ||||||
| Patient or population: babies born preterm, and their mothers. Setting: hospital births mostly in high‐income countries. Intervention: umbilical cord milking(UCM) Comparison: early cord clamping (ECC). | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with ECC (subgroup analysis by gestation) | Risk with UCM | |||||
| Death of baby (up to discharge) | Study population | RR 0.81 (0.47 to 1.41) | 931 (9 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | ||
| 60 per 1000 | 48 per 1000 (28 to 84) | |||||
| Death or neurodevelopmental impairment at age 2 to 3 years | Study population | ‐ | (0 studies) | ‐ | ||
| see comment | see comment | |||||
| Severe intraventricular haemorrhage (IVH grades 3, 4) | Study population | RR 0.75 (0.39 to 1.45) | 618 (6 RCTs) | ⊕⊕⊝⊝ LOW 3 4 | ||
| 64 per 1000 | 48 per 1000 (25 to 93) | |||||
| Intraventricular haemorrhage (IVH, all grades) | Study population | RR 0.85 (0.62 to 1.18) | 716 (8 RCTs) | ⊕⊕⊕⊝ MODERATE 5 6 | ||
| 270 per 1000 | 230 per 1000 (168 to 319) | |||||
| Periventricular leukomalacia (PVL) | Study population | RR 0.63 (0.15 to 2.63) | 315 (3 RCTs) | ⊕⊕⊝⊝ LOW 7 8 | ||
| 31 per 1000 | 20 per 1000 (5 to 82) | |||||
| Chronic lung disease (CLD) ‐ oxygen supplement at 36 weeks (corrected for gestation) | Study population | RR 1.03 (0.64 to 1.66) | 682 (7 RCTs) | ⊕⊕⊝⊝ LOW 9 10 11 | ||
| 198 per 1000 | 204 per 1000 (127 to 329) | |||||
| Maternal blood loss of 500 mL or greater | Study population | not estimable | 200 (1 RCT) | ⊕⊕⊝⊝ LOW 12 13 | ||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Five out of nine studies were low risk of selection bias and provided over 50% of data. No downgrade.
2 Not many events (n = 50) out of 931 babies, and wide 95% CI crossing line of no difference. Downgraded 2
3 Three out of six studies were low risk of selection bias and provided over 50% of data. No downgrade
4 Not a large sample size (N = 618), few events (n = 36) and wide 95% CI crossing line of no difference. Downgrade 2.
5 Four out of eight studies were low risk of selection bias and contributed over 50% of data. No downgrade
6 Wide CI crossing line of no difference. Not a large sample size (N = 716). 181 events. Downgrade 1.
7 Two out of three studies were low risk of selection bias and provided over 60% of data. No downgrade.
8 Small sample size (N = 315), very few events (n = 8) and wide 95% CI crossing line of no difference. Downgrade 2.
9 Four out of seven studies were low risk of selection bias and provided over 60% of data. No downgrade.
10 Heterogeneity I2 = 50%. Downgrade 1.
11 Wide CI crossing line of no difference. Not a large sample size (N = 682). 141 events. Downgrade 1.
12 Risk of bias: low for sequence generation, allocation concealment and incomplete outcome data. No downgrade.
13 Imprecision: small sample size (N = 200) and no events. Downgrade 2.
Background
Being born too early (preterm birth) has a major impact on survival and quality of life for the child, on psychosocial and emotional stress on the family, and on costs for health services and society (Bhutta 2002; Saigal 2008; Zeitlin 2008). Infants born very preterm, before 32 weeks' gestation, have the highest risk. For example, in the UK infant mortality (deaths in the first year of life) for babies born very preterm is 144 deaths per 1000 live births, compared to 1.8 deaths per 1000 live births at term (Moser 2007). Although only 1.4% of live births in the UK are very preterm, these babies account for 51% of infant deaths (Moser 2007).
The costs of neonatal care for infants born very preterm are also high, and these babies are often in neonatal intensive care units for many weeks and sometimes months. In the UK, for example, duration of hospital stay for infants born before 28 weeks is 85 times that for term births, and hospital inpatient costs are £15,000 ($21,000) higher; for those born at 28 to 31 weeks, it is 16 times and £12,000 ($17,000), respectively (Petrou 2003). Having a baby born very preterm is a difficult and distressing time for the parents (Sawyer 2013).
Amongst children born very preterm who survive, morbidity is also high compared to those born at term (Zeitlin 2008) as around 5% develop cerebral palsy, and those without severe disability have a two‐fold or greater increased risk for developmental, cognitive, and behavioural difficulties (Bhutta 2002; Saigal 2008). These impairments may persist into adolescence and early adulthood (Aarnoudse‐Moens 2009; Anderson 2003).
Description of the condition
Physiology
At birth, if the umbilical cord is not clamped, blood flow between the baby and placenta may continue for several minutes (Boere 2015; Dawes 1968; Farrar 2011; Vijayaselvi 2015). This umbilical flow is part of the physiological transition from the fetal to the neonatal circulation, and for very preterm infants may improve resilience during this transition (Bhatt 2013; Committee 2012; Gunther 1957). ‘Placental transfusion’ refers to the net transfer of blood to the baby between birth and cord clamping.
For term births, umbilical blood flow is usually complete by two minutes, but may continue for up to five minutes (Boere 2015; Farrar 2011). This gives a term infant, on average, an additional 80 mL to 100 mL of blood and can contribute a third to a quarter of the neonatal blood volume at birth (Dawes 1968; Farrar 2011). The additional plasma from placental transfusion is quickly lost to the circulation, leaving a high red cell mass which is broken down and the iron stored. For term births, delayed (or deferred) cord clamping improves iron stores at age six to 12 months (Chaparro 2006; McDonald 2013). Although the physiology of placental transfusion at preterm birth is less well understood, the relative contribution to blood volume and red cell mass of delayed cord clamping may be greater than for those born at term, as a higher proportion of the intrauterine blood volume (blood in the baby, cord and placenta) is sequestered in the placenta. At 30 weeks' gestation, for example, about half the intrauterine blood is in the baby and half is in the cord and placenta; by term, this rises to two‐thirds being in the baby. Nevertheless, at preterm birth placental transfusion may take longer than at term (Aladangady 2006; Saigal 1972).
Transition at birth from intra‐uterine to extra‐uterine life
At birth, the infant must move from fetal circulation to his/her own independent circulation. Therefore, as the baby is born the umbilical circulation slows and pulmonary vascular resistance falls, rapidly increasing pulmonary blood flow. Continued flow in the umbilical vein and arteries at birth may be part of the physiological mechanisms assisting the baby as it makes this transition to the neonatal circulation, potentially helping to stabilise blood pressure and support cardiovascular changes (Duley 2015; Gunther 1957; Mercer 2002). For preterm infants, the mechanisms for these circulatory changes may not be fully developed and so they may take longer. Immediate cord clamping may restrict the infant’s ability to deal with the transition to the neonatal circulation. Whilst most healthy babies at term may adapt without major consequences, for those born preterm, or with their cardio‐respiratory circulation already impaired, there may be an impact on clinical outcome.
A common complication of being born preterm is fluctuating and low blood pressure during the first days of life, which contributes to the risk of bleeding into the brain (intraventricular haemorrhage); if severe, this can be life threatening or lead to long‐term problems. Delaying cord clamping was first suggested for babies born very preterm based on the hypothesis that it might reduce hypotension and stabilise blood pressure, thereby reducing the risk of intraventricular haemorrhage and its consequences (Hofmeyr 1988). Thus, if the preterm babies blood pressure is stable and in the normal range for their age, their adaptation to extra‐uterine life should be easier to achieve.
Lessons learned from animal studies
Recent work with preterm lambs born by caesarean section supports the hypothesis that delaying cord clamping until the neonatal circulation is established may benefit cardiovascular function (Bhatt 2013). Starting ventilation at birth and waiting until respiration was established before clamping the cord improved cardiovascular function compared with immediate clamping and then ventilating the lambs (Bhatt 2013). Ventilation with deferred cord clamping was associated with improved pulmonary blood flow, and less variability in carotid artery pressure, carotid artery blood flow and heart rate. This suggests the mechanisms for improvement in cardiorespiratory function may be a more stable haemodynamic transition, rather than increased neonatal blood volumes. Improved understanding of the physiology of placental transfusion and neonatal transition is leading to calls for a more physiological approach to umbilical cord clamping, based on whether the infant has aerated lungs and established respiration, rather than any specific timing for cord clamping (Hooper 2015).
Description of the intervention
Standard approach to the third stage of labour: active management
The third stage of labour is the time between birth of the baby and complete delivery of the placenta. Due to the separation of the placenta, the mother will experience some degree of blood loss after the birth of the baby. If the uterus does not contract well after birth, heavy blood loss may occur and this can endanger the life of the mother. Immediate cord clamping was widely implemented in the 1960s, as part of a package of care known as 'active management of the third stage of labour' (Begley 2019; Prendiville 1989). To clamp the cord, two clamps are placed close together on the cord, and the cord is cut between them. This stops flow in the umbilical vein towards the baby, and in the two umbilical arteries towards the placenta. Arterial pulsation is muscular, and not related to blood flow. The aim of active management of third stage was to reduce maternal blood loss after the birth, in particular postpartum haemorrhage (blood loss of 500 mL or more) (Begley 2019).
The traditional components of active management are a prophylactic uterotonic drug, immediate cord clamping and controlled cord traction (Prendiville 1989). Immediate cord clamping and controlled cord traction were included due to the concerns that the administration of the uterotonic drug might lead to ‘over transfusion’ of the baby, and that the placenta might become trapped in the contracted uterus. Concern that delaying cord clamping might lead to ‘over‐transfusion’ of the baby is understandable, as ergometrine was used at that time. With the modern use of less potent drugs, such as syntocinon, the concern is much less, but is still apparent (Oddie 2014), and becomes irrelevant if administration of the uterotonic drug is after the cord is clamped.
More recently, re‐evaluation of the individual components of active management has made clear that, whilst uterotonic drugs do indeed reduce the risk of postpartum haemorrhage (Gallos 2018; Salati 2019), controlled cord traction does not offer significant additional benefit (Hofmeyr 2015). Similarly, timing of cord clamping at term births does not appear to have any substantive effect on the risk of postpartum haemorrhage, and delaying cord clamping may be beneficial for the infant (McDonald 2013).
The introduction of active management of the third stage of labour coincided with the advent of neonatal medicine (Aflaifel 2012). Hence, for preterm births, it became standard practice that once the cord was cut the baby was handed to the neonatal team, who transferred the infant to the resuscitation equipment either at the side of the delivery room, or in an adjacent room (O'Donnell 2017). Mother and baby were, therefore, separated at birth and, as the baby was quickly taken to the neonatal unit, often mothers did not see or touch their baby until much later (Arnold 2013).
Alternative approaches for timing of cord clamping
Delayed (deferred) cord clamping
There is no consensus about the definition of early or immediate cord clamping, nor of delayed or deferred clamping for preterm birth. As discussed above, a specific timing may not be ideal, and a physiologic approach may be more appropriate. Previously, immediate clamping for preterm birth was generally defined as within 15 to 20 seconds, but more recently up to 30 seconds (NICE 2015), or 60 seconds (WHO 2014) have become more widely accepted. For delayed cord clamping, particularly between 30 and 45 seconds has often been used as the definition for delayed cord clamping for very preterm births, at up to three minutes for late preterm births (Rabe 2012). However, timing of cord clamping for very preterm infants was often determined by neonatal guidance to provide prompt ventilation support (Perlman 2010).
After birth whilst the cord is intact, umbilical flow will be ‘physiological’ if the infant is either level with the mother's bed (i.e. level with the placenta) or level with her abdomen. For term births, lowering the baby by 20 cm appears to increase the volume of placental transfusion (Yao 1969). However, for preterm lambs although raising or lowering the lamb caused small transient effect on umbilical (both vein and arteries) and cerebral flow, this was not associated in any net change in the volume of placental transfusion (Hooper 2017). A recent randomised trial has also reported that for term births, whether the infant was level with the mother’s vagina or abdomen did not influence birthweight, and so did not appear to influence placental transfusion (Vain 2014).
Umbilical cord milking (UCM)
Umbilical cord ‘milking’ or ‘stripping’ is when the cord is pinched between the thumb and forefingers, and then gently squeezed to push cord blood towards the baby. The cord is then released and the ‘milking repeated' (typically, a 20 cm length of cord is ‘milked’ between two and four times, each done for about two seconds, before clamping) (Hosono 2008; Rabe 2011). Sometimes the cord is milked after the cord is cut (Kumar 2015). This technique is sometimes used as an alternative to delaying cord clamping when the baby requires immediate stabilisation and resuscitation at birth (Al‐Wassia 2015).
Immediate neonatal care with cord intact
Recently, strategies to care for the infant without clamping the cord have been developed (Batey 2017; Hutchon 2008; Katheria 2017a; Knol 2018; Weeks 2015). Providing initial neonatal care and stabilisation with the umbilical cord intact allows cord clamping to be delayed for longer, even for infants requiring immediate resuscitation (CORD Pilot 2018).
An adjustable mobile trolley specially designed to allow neonatal care to be provided beside the mother and with the cord intact is available. This has a platform on which the baby is placed that can reach close to the mother's perineum at vaginal births or can be draped to allow access at caesarean section (Katheria 2017a; Weeks 2013). However, the usual resuscitation equipment can easily be adapted to provide the same care with cord intact at both vaginal and caesarean births (Batey 2017; Schoonakker 2013). They also allow the baby to be cared for at birth beside the mother, which is valued by parents and appears to be acceptable to clinicians (Sawyer 2015; Thomas 2014; Yoxall 2015). Providing neonatal care with the cord intact requires training and a multidisciplinary team approach (Batey 2017).
How the intervention might work
Delayed (deferred) cord clamping
For healthy term births the benefits of delaying umbilical cord clamping are largely related to an increase in neonatal blood volume (placental transfusion) (McDonald 2013). For preterm births, the physiology is more complex, and allowing longer for transition to the neonatal circulation may be as important as any placental transfusion (Hooper 2015; Kluckow 2015). Potential benefits for delayed, rather than immediate, cord clamping at preterm birth will depend on the gestation at birth but may include a reduction in the risk of intraventricular haemorrhage (Hofmeyr 1988), blood transfusion, respiratory distress (Linderkamp 1978), and respiratory support (Holland 1991; Hudson 1990; Kinmond 1993).
Potential side effects such as the baby getting cold (hypothermia) and delay in providing stabilisation and resuscitation, when needed, are not directly related to the timing of cord clamping per se, and can potentially be overcome by changing neonatal practice and providing neonatal care at birth beside the mother and, if needed, with the cord intact. As well as comparing the benefits and risks during the first few days and weeks of life for alternative policies for timing of cord clamping for preterm births, it is also important to assess whether any short‐term effects are reflected in long‐term outcomes (Tarnow‐Mordi 2014).
Umbilical cord milking (UCM)
The rationale for UCM is that it enables blood to be directed into the baby more quickly at birth than waiting for this to happen physiologically (Hosono 2008; Rabe 2011; Tarnow‐Mordi 2014). Cord milking is, therefore, proposed as an alternative to delayed cord clamping, allowing rapid transfer of blood from the placenta to the baby and earlier access for thermal and respiratory support. This is based on the assumption that an increase in placental transfusion is the main benefit of delayed cord clamping, which can be used by the baby to fill their lung circulation, whereas it has been hypothesised that the circulatory disruption following cord milking may be similar to that following immediate cord clamping (Blank 2018).
Immediate neonatal care with cord intact
Immediate cord clamping for preterm infants, particularly those very preterm, is often to allow rapid access to the baby for clinical assessment and/or resuscitation. The timing of delayed clamping has usually been a balance between allowing some placental transfusion and the need to transfer the baby for neonatal care. An alternative strategy is to change neonatal practice and provide neonatal care beside the mother and, if needed, with the cord intact (Batey 2017; CORD Pilot 2018; Katheria 2017a; Knol 2018). Studies assessing delayed cord clamping with immediate neonatal care available with the cord intact are able to recruit higher‐risk babies requiring immediate resuscitation at birth, a group excluded from previous research (CORD Pilot 2018; Manley 2017).
Why it is important to do this review
There remains huge uncertainty in this area of care. The comparative benefits and harms of delayed rather than early clamping of the umbilical cord for the preterm infant (less than 37 weeks' gestation) has been the subject of much debate, and the optimal timing for clamping the cord remains unclear and requires further research (Poscencheg 2015). Leaving the cord unclamped for longer at preterm births, to allow the cardio‐respiratory changes associated with transition to the neonatal circulation to be supported by umbilical flow, may conflict with a perceived need for immediate resuscitation, which usually takes place away from the mother. UCM is a possible alternative approach but also requires assessment of the current evidence (Katheria 2017b; Poscencheg 2015).
For low‐income settings, where the availability of specialist neonatal care is often limited, the balance of benefits and harms associated with alternative policies for timing of cord clamping may be different (Manley 2017).
As the potential benefits and harms of alternative policies for timing of cord clamping are different at term compared with preterm, term births are covered by a separate Cochrane Review (McDonald 2013).
This review will be of interest to obstetricians, midwives, neonatologists as well as pregnant women and their partners. This Cochrane Review is a further update of a review first published in 2004 and updated in 2012.
Objectives
To assess the effects on infants born at less than 37 weeks' gestation, and their mothers of: 1) delayed cord clamping compared with early cord clamping both with immediate neonatal care after cord clamping; 2) delayed cord clamping with immediate neonatal care with cord intact compared with early cord clamping with immediate neonatal care after cord clamping; 3) delayed cord clamping with immediate neonatal care after cord clamping compared with umbilical cord milking (UCM); 4) UCM compared with early cord clamping with immediate neonatal care after cord clamping.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials. Cluster‐randomised trials were eligible for inclusion but none were identified. Quasi‐randomised trials were not included.
Types of participants
Preterm infants born before 37 completed weeks' gestation and their mothers.
Types of interventions
Delayed umbilical cord clamping (DCC ‐ after 30 seconds or more) versus early umbilical cord clamping (ECC ‐ less than 30 seconds). This could be with or without oxytocin, with or without the baby held above or below the level of the placenta, and with or without milking of the cord towards the infant. In this update of the review we have also considered studies examining umbilical cord milking (UCM) and delayed cord clamping with immediate neonatal care with the cord intact.
For this review comparisons will include:
delayed cord clamping with immediate neonatal care after cord clamping versus early cord clamping;
delayed cord clamping with immediate neonatal care with cord intact versus early cord clamping;
delayed cord clamping with immediate neonatal care after cord clamping versus umbilical cord milking;
umbilical cord milking versus early cord clamping.
Comparisons of different lengths of delay in cord clamping will be included at a future update.
Types of outcome measures
We searched the COMET database (http://www.comet‐initiative.org/) to see if a core outcome set (COS) had been developed for outcomes in preterm birth. We only found reference to COS for preventing preterm birth in the CROWN Initiative (http://www.crown‐initiative.org/tag/preterm‐birth/) and also in a further publication on prevention of preterm birth (Meher 2014). We have, therefore, in discussion amongst co‐authors chosen the primary and secondary outcomes below.
Primary outcomes
For the baby
Death of the baby: at or before discharge from hospital
Death or neurodevelopmental impairment in early childhood (around two to three years)
Severe intraventricular haemorrhage (IVH) ‐ ultrasound diagnosis grades three and four
IVH ‐ all grades
Periventricular leukomalacia (PVL)
Chronic lung disease (CLD) ‐ or chronic pulmonary disease (CPD) ‐ assessed by oxygen supplementation at 36 weeks (corrected for gestation)
For the mother
Postpartum haemorrhage (blood loss of 500 mL or greater)
Secondary outcomes
For the baby
Condition at birth
Low Apgar score as defined by trialists (generally < eight at five minutes)
Temperature < 360 within one hour of birth
Respiratory
Respiratory distress syndrome (RDS)
Respiratory support (ventilator or continuous positive airway pressure (CPAP))
Duration of respiratory support ‐ continuous data
Surfactant treatment ‐ for severe RDS
Home oxygen
Cardiovascular
Treatment for patent ductus arteriosus (medical and/or surgical)
Inotropic support for hypotension during the first 24 hours of life
Mean arterial blood pressure in early hours after birth
Central nervous system
IVH grades one and two
Hydrocephalus
Neurodevelopmental impairment in early childhood (around two to three years)
Cerebral palsy
Gastrointestinal
Necrotising enterocolitis (NEC) confirmed by X‐ray or laparotomy
Haematological
Blood transfusion in infant
Volume of blood transfused ‐ continuous data
Haemoglobin (Hb) within first 24 hours ‐ continuous data
Hyperbilirubinaemia (treated by phototherapy)
Other
Late sepsis (after three days or as defined by trialists)
Treatment for retinopathy of prematurity (RoP)
Severe visual impairment
Length of infant stay in neonatal intensive care unit (NICU)
For the mother
Prolonged third stage (> 30 minutes) (denominator = vaginal births)
Manual removal of the placenta (denominator = vaginal births)
Blood transfusion
Postpartum infection
Rhesus‐isoimmunisation
Psychological well‐being
Bonding with the infant
Breastfeeding initiation
Fully breastfed or mixed feeding at infant discharge
Mothers' anxieties
Mothers' views
For the father
Psychological well‐being
Bonding with the infant
Fathers' anxieties
Fathers' views
Search methods for identification of studies
The following methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Electronic searches
For this update, we searched Cochrane Pregnancy and Childbirth’s Trials Register by contacting their Information Specialist (10 November 2017). We updated this search in November 2018. The results of the updated search have not yet been fully incorporated (see: Results of the search for full details).
The Trials Register is a database containing over 25,000 reports of controlled trials in the field of pregnancy and childbirth. It represents over 30 years of searching. For full current search methods used to populate Pregnancy and Childbirth’s Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL; the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link.
Briefly, Cochrane Pregnancy and Childbirth’s Trials Register is maintained by their Information Specialist and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Search results are screened by two people and the full text of all relevant trial reports identified through the searching activities described above is reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set that has been fully accounted for in the relevant review sections (Included studies; Excluded studies; Studies awaiting classification; Ongoing studies).
In addition, we searched ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) for unpublished, planned and ongoing trial reports (8 November 2018) using the search methods detailed in Appendix 1.
Searching other resources
We searched the reference lists of retrieved studies.
We did not apply any language or date restrictions.
Data collection and analysis
For methods used in the previous version of this review, seeElbourne 1995; Rabe 2004; Rabe 2012.
For this update, the following methods were used for assessing the reports that were identified as a result of the updated search and we went back over the trial reports in the 2012 publication to allocate to the appropriate comparison and subgroup and to update their risk of bias.
Selection of studies
Two review authors independently assessed for inclusion all the potential studies we identified as a result of the search strategy. We resolved any disagreement through discussion or, if required, we consulted the third review author.
Data extraction and management
We designed an updated form to extract data. For eligible studies, two review authors extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, we consulted the third review author. We entered data into Review Manager software (RevMan 2014) and checked for accuracy.
When information regarding any of the above was unclear, we attempted to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion, or by involving a third assessor.
(1) Random sequence generation (checking for possible selection bias)
We have described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We have described for each included study the method used to conceal allocation to interventions prior to assignment and have assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias (we considered a study to be unclear for risk of bias for allocation concealment if the study was unclear on sequence generation).
(3.1) Blinding of participants and personnel (checking for possible performance)
Blinding participants and staff to the types of interventions considered in this review may not be feasible, but it may be possible to blind outcome assessors for at least some of the outcomes reported. We have described for each included study the methods used, if any, to achieve blinding. We considered studies to be at low risk of bias if they were blinded, or if we judged that the lack of blinding would be unlikely to affect results.
We assessed the methods as:
low risk of bias for participants and personnel;
high risk of bias for participants and personnel;
unclear risk of bias for participants and personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We have assessed methods used to blind outcome assessment as:
low risk of bias for outcome assessors;
high risk of bias for outcome assessors;
unclear risk of bias for outcome assessors.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We have described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We have stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or was supplied by the trial authors, we re‐included missing data in the analyses which we undertook.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We have described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so could not be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We have described for each included study any important concerns we have about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias:
low risk of other bias;
high risk of other bias;
unclear whether there is risk of other bias.
(7) Overall risk of bias
We have made explicit judgements about whether studies are at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it was likely to impact on the findings. We explored the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
In addition, we have collected data on funding source for the individual studies and whether there was a declaration of interest by the individual authors.
Assessing the certainty of the evidence using GRADE
For this update the certainty of the evidence was assessed using the GRADE approach as outlined in the GRADE handbook in order to assess the certainty of the body of evidence relating to the following outcomes for the main comparisons.
Delayed cord clamping with immediate neonatal care after cord clamping versus early cord clamping
Delayed cord clamping with immediate neonatal care with cord intact versus early cord clamping
Delayed cord clamping with immediate neonatal care after cord clamping versus umbilical cord milking
Umbilical cord milking versus early cord clamping
Outcomes
Death of the baby: at or before discharge from hospital
Death or neurosensory disability in early childhood (around two to three years)
Severe IVH ‐ ultrasound diagnosis grade three and four
IVH ‐ all grades
PVL
CPD ‐ or CLD ‐ assessed by oxygen supplementation at 36 weeks (corrected for gestation)
Maternal postpartum haemorrhage (blood loss of 500 mL or greater)
We used GradePro 2015 Guideline Development Tool to import data from Review Manager 5.3 (RevMan 2014) in order to create ’Summary of findings’ tables. A summary of the intervention effect and a measure of quality or certainty for each of the above outcomes were produced using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, indirectness, imprecision and publication bias) to assess the certainty of the body of evidence for each outcome. The evidence was downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we have presented results as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we used the mean difference if outcomes were measured in the same way between trials. We used the standardised mean difference to combine trials that measured the same outcome, but used different methods.
Unit of analysis issues
Cluster‐randomised trials
Had we found any, we would have included cluster‐randomised trials in the analyses along with individually‐randomised trials. If in future updates we do include cluster‐randomised trials, we will adjust their sample sizes using the methods described in the Handbook (Higgins 2011) using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there was little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely. We will also acknowledge heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate the effects of the randomisation unit.
Cross‐over trials
Due to the nature of the studied interventions cross‐over designs are not possible.
Other unit of analysis issues
Other unit of analysis issues could include, e.g. multiple pregnancies or more than two treatment groups, which need specialist statistical analysis. However, these type of trials have, so far, not been reported for cord clamping interventions.
Dealing with missing data
For included studies, we noted levels of attrition. We had planned to explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis but we felt there were insufficient data to assess this.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses, and all participants were analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial was the number of women or babies randomised minus any participants whose outcomes were known to be missing. However, where babies who died after randomisation have been excluded by trial authors, we have, where possible, re‐included them in the numerators and denominations for the outcome of 'Death', if appropriate and the data were available.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We regarded heterogeneity as substantial if a Tau² was greater than zero and either an I² was greater than 30% or there was a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
Where there were 10 or more studies in the meta‐analysis, we investigated reporting biases (such as publication bias) using funnel plots for primary outcomes only. We assessed funnel plot asymmetry visually. If asymmetry was suggested by a visual assessment, we planned to perform exploratory analyses to investigate it. For most outcomes in this review too few studies contributed data to carry out these planned analyses.
For all meta‐analyses we ordered studies according to weight so that we would be able to identify any obvious differences in effect associated with smaller studies.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2014). We used random‐effect meta‐analysis for combining data as we considered it was reasonable to assume that there was clinical heterogeneity due to the large variation in the timing of delayed cord clamping between the included studies and where the baby was placed during this time (whether gravity could affect movement of blood to the baby). There was also variation in how umbilical cord milking was undertaken, either before of after cutting the cord. These variations led us to consider that the underlying treatment effects would differ between trials.
Subgroup analysis and investigation of heterogeneity
For this update, the subgroup analyses from the previous version of the review (position of the baby relative to the level of the placenta before cord clamping; whether the woman was given oxytocin as a uterotonic drug before cord clamping: milking of the cord: vaginal birth or caesareans section; gestational age at birth) were replaced because there were insufficient data available to provide appropriate information. Instead we assessed the following subgroups in this update.
By gestational age at birth: 1) < 32 to 34 weeks; 2) > 32 to 34 weeks; 3) mixed gestation or not reported.
By type of delay intervention: 1) DCC at < one minute with baby level with uterus and placenta; 2) DCC at < one minute with baby low (+ gravity); 3) DCC at one to two minutes with baby level with uterus and placenta; 4) DCC at one to two minutes with baby low (+ gravity); 5) DCC at > two minutes with baby level with uterus and placenta; 6) DCC at > two minutes with baby low (+ gravity); 7) unclear or mixed interventions.
By type of milking intervention: 1) cord intact during UCM; 2) cord cut before UCM; 3) unclear or not reported.
We planned to undertake subgroup analyses on all outcomes.
We assessed differences between subgroups by inspection of the subgroups’ confidence intervals with non‐overlapping confidence intervals suggesting a statistically significant difference in treatment effect between the subgroups. Where sufficient data were available, we carried out more formal statistical tests to assess differences between subgroups by applying the interaction tests available in RevMan 2014.
Sensitivity analysis
The previous review (Rabe 2012) used adequate allocation concealment as a criterion for sensitivity analysis, however in this update, we felt the other aspects of risk of bias were equally important. Hence, we undertook sensitivity analysis by excluding studies at unclear or high risk of bias based on selection bias (sequence generation and allocation concealment) and attrition bias (incomplete outcome data), including only studies at low risk of bias for these domains. We carried out this analysis for primary outcomes only.
Results
Description of studies
Results of the search
See: Figure 1
1.
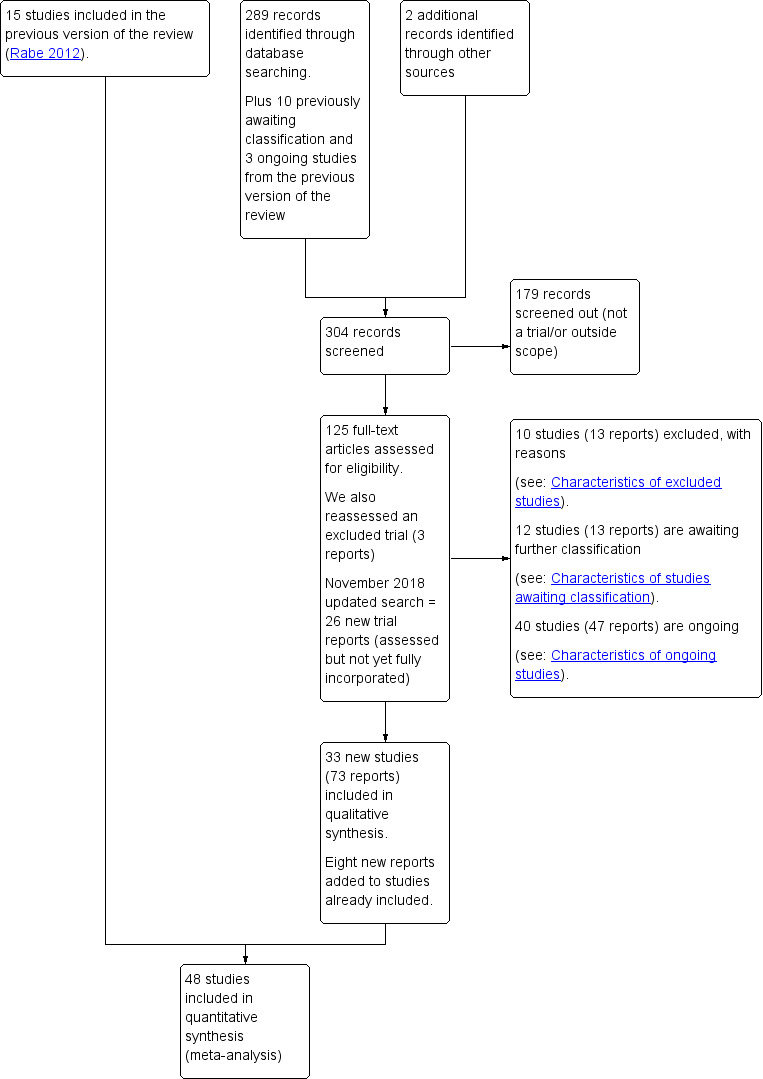
Study flow diagram.
For this update, we retrieved 291 new citations from the search conducted in November 2017 and also reassessed the 10 awaiting classification and three ongoing trials from the previous version of the review (Rabe 2012). We also reassessed one previously excluded study (three reports). We included 33 new studies (73 reports) and added six new reports to previously included studies. We excluded 10 new studies (13 reports). We added three studies (four reports) to Studies awaiting classification and 29 to Ongoing studies (32 reports).
We updated the search in November 2018 and retrieved 26 new trial reports. Two of these were additional reports of included studies with no new data so the references have been added under the main study (Katheria 2015; Tarnow‐Mordi 2017). Six are new studies to be fully assessed at the next update (Kazemi 2017; Leal 2018; Li 2018; Ram Mothan 2018; Song 2017; Weeks 2018). Three are additional reports of included studies and the new data will be added at the next update (Das 2018a; El‐Naggar 2018; Wang 2018). The remaining 15 reports refer to 11 ongoing studies and have been added to Ongoing studies (Aghai 2018; Allam 2018; Gupta 2018; Hao 2018; Jomjak 2018; Katheria 2018; Liu 2018; Mirzaeian 2018; Nour 2018a; Nour 2018b; Shahgheibi 2018).
This update now includes 48 studies (Characteristics of included studies), with 20 studies excluded (Characteristics of excluded studies), 12 studies awaiting classification (Characteristics of studies awaiting classification), and 40 ongoing studies (Characteristics of ongoing studies).
Included studies
We now include 48 studies (involving 5721 babies and their mothers) in this update, see Characteristics of included studies for more detail of participants and interventions, gestational age, mode of birth, positioning of the infant and type of intervention. There were no usable data in eight of the included studies (Aladangady 2006; Das 2018; Dhaliwal 2014; Malik 2013; Nelle 1998; Pongmee 2010; Rana 2017; Sekhavat 2008). Thus 40 studies provided data on 4884 babies and their mothers (Alan 2014; Armanian 2017; Backes 2016; Baenziger 2007; Chu 2011; CORD Pilot 2018; Dai 2014; Datta 2017; Dipak 2017; Dong 2016; Elimian 2014; El‐Naggar 2016; Gokmen 2011; Hofmeyr 1988; Hofmeyr 1993; Hosono 2008; Hosono 2015; Josephsen 2014; Katheria 2014; Katheria 2015; Kilicdag 2016; Kinmond 1993; Krueger 2015; Kugelman 2007; Kumar 2015; March 2013; McDonnell 1997; Mercer 2003; Mercer 2006; Mercer 2016; Oh 2011; Rabe 2000; Rabe 2011; Ranjit 2015; Salae 2016; Shi 2017; Strauss 2008; Tarnow‐Mordi 2017; Tiemersma 2015; Ultee 2008).
The studies either enrolled women giving birth preterm and their babies, or they enrolled babies born preterm, so between 24 and 36+6 weeks' gestation. We included studies with singleton and multiple pregnancies and those with babies being born vaginally and by caesarean. For some studies, the unit of randomisation was the baby, but for most mother‐infant pairs were randomised. There was some inconsistency in both the intervention and the control procedures between studies, and wide variation in outcome measures reported.
Ten studies included multiple births (Alan 2014; CORD Pilot 2018; El‐Naggar 2016; Gokmen 2011; Katheria 2014; Katheria 2015; Kinmond 1993; Kugelman 2007; McDonnell 1997; Tarnow‐Mordi 2017). Three studies probably included twins as they reported excluding twin‐twin transfusion, or monochorionic/amniotic, (Pongmee 2010; Ranjit 2015; Shi 2017). Nine studies were unclear as to whether they included multiple births or not (Chu 2011; Dhaliwal 2014; Hofmeyr 1988; Hofmeyr 1993; Kilicdag 2016; Malik 2013; Nelle 1998; Sekhavat 2008; Strauss 2008). The remainder of studies were restricted to singleton births.
Studies were conducted between 1988 and 2017. Some studies did not report the dates when they were undertaken, and these tended to be older studies. For 18 studies recruitment began between 2011 and 2015; between 2006 and 2010 for seven studies; between 2000 and 2005 for four studies, and between 1988 and 1999 for four studies (Characteristics of included studies).
The 48 studies were undertaken in 19 countries covering high‐, middle‐ and low‐income countries:
13 in USA (Backes 2016; Elimian 2014; Josephsen 2014; Katheria 2014; Katheria 2015; Krueger 2015; March 2013; Mercer 2003; Mercer 2006; Mercer 2016; Oh 2011; Strauss 2008; Tarnow‐Mordi 2017);
seven in India (Das 2018; Dhaliwal 2014; Datta 2017; Dipak 2017; Kumar 2015; Rana 2017; Ranjit 2015);
four in the UK (Aladangady 2006; CORD Pilot 2018; Kinmond 1993; Rabe 2011);
three in Canada (Chu 2011; El‐Naggar 2016; Tarnow‐Mordi 2017);
three in South Africa (Hofmeyr 1988; Hofmeyr 1993; Tiemersma 2015);
three in Turkey (Alan 2014; Gokmen 2011; Kilicdag 2016);
two in Australia (McDonnell 1997; Tarnow‐Mordi 2017);
two in Germany (Nelle 1998; Rabe 2000);
two in Iran (Armanian 2017; Sekhavat 2008);
two in Japan (Hosono 2008; Hosono 2015);
two in Pakistan (Malik 2013; Tarnow‐Mordi 2017);
two in Thailand (Pongmee 2010; Salae 2016);
one study involved France (Tarnow‐Mordi 2017);
one in Israel (Kugelman 2007);
one in the Netherlands (Ultee 2008);
one study involved Northern Ireland (Tarnow‐Mordi 2017);
one study involved New Zealand (Tarnow‐Mordi 2017);
one in Switzerland (Baenziger 2007).
The largest in the review was undertaken across seven countries: Australia, Canada, France, New Zealand, Northern Ireland, Pakistan and USA (Tarnow‐Mordi 2017).
Sources of trial funding
Sources of funding were reported in 23 studies, but in 25 studies there was nothing reported regarding funding. See Characteristics of included studies.
Trialists declaration of interest
Declarations of interest were reported as "none" in 21 studies. One study reported association for a number of authors with the development of a small mobile resuscitation trolley, which was later marketed as Life‐Start, but those involved had no further relationship with the manufacturer (CORD Pilot 2018). One study reported the declarations of interest were with the journal and we have not described these (Tarnow‐Mordi 2017). Twenty‐five studies did not report on declarations of interest of trialists. See Characteristics of included studies.
Interventions compared
A. Delayed cord clamping (DCC) with immediate neonatal care after cord clamping versus early cord clamping (ECC) (Comparisons 1 and 2)
Thirty studies involving 3651 babies and their mothers addressed this question. Five studies provided no data for the outcomes in this review (Dhaliwal 2014; Malik 2013; Nelle 1998; Rana 2017; Sekhavat 2008). This left 25 studies, involving 3100 babies and their mothers, which contributed data to this comparison (Armanian 2017; Backes 2016; Baenziger 2007; Chu 2011; Dai 2014; Datta 2017; Dipak 2017; Dong 2016; Gokmen 2011; Hofmeyr 1988; Hofmeyr 1993; Kinmond 1993; Kugelman 2007; McDonnell 1997; Mercer 2003; Mercer 2006; Oh 2011; Rabe 2000; Ranjit 2015; Salae 2016; Shi 2017; Strauss 2008; Tarnow‐Mordi 2017; Tiemersma 2015; Ultee 2008).
The studies were undertaken in the following countries: Australia (McDonnell 1997; Tarnow‐Mordi 2017); Canada (Chu 2011; Tarnow‐Mordi 2017); France (Tarnow‐Mordi 2017); Germany (Nelle 1998; Rabe 2000); India (Dhaliwal 2014; Datta 2017; Dipak 2017; Malik 2013; Rana 2017; Ranjit 2015); Iran (Armanian 2017; Sekhavat 2008); Israel (Kugelman 2007); the Netherlands (Ultee 2008); New Zealand (Tarnow‐Mordi 2017); Northern Ireland (Tarnow‐Mordi 2017); Pakistan (Malik 2013; Tarnow‐Mordi 2017); South Africa (Hofmeyr 1988; Hofmeyr 1993; Tiemersma 2015); Switzerland (Baenziger 2007), Thailand (Salae 2016), Turkey (Gokmen 2011); USA (Backes 2016; Mercer 2003; Mercer 2006; Oh 2011; Strauss 2008; Tarnow‐Mordi 2017); and UK (Kinmond 1993).
Of the studies providing data, 16 studies recruited births before 32 to 34 weeks' gestation (Armanian 2017; Backes 2016; Baenziger 2007; Chu 2011; Dipak 2017; Dong 2016; Gokmen 2011; Hofmeyr 1988; Kinmond 1993; Kugelman 2007; McDonnell 1997; Mercer 2003; Mercer 2006; Oh 2011; Rabe 2000; Tarnow‐Mordi 2017). Three studies recruited births after 32 to 34 weeks' gestation (Datta 2017Salae 2016; Ultee 2008). Six studies recruited births at mixed gestation or the gestation was unclear (Dai 2014; Hofmeyr 1993; Ranjit 2015; Shi 2017; Strauss 2008; Tiemersma 2015).
Of the studies providing data, two studies examined DCC for less than one minute whilst keeping the baby level with the placenta (Datta 2017; McDonnell 1997). Eight studies examined DCC for less than one minute and held the baby low during this time (Backes 2016; Dong 2016; Kinmond 1993; Kugelman 2007; Mercer 2003; Mercer 2006; Oh 2011; Rabe 2000). Two studies examined DCC for between one and two minutes holding the baby level with the placenta during this time (Hofmeyr 1993; Salae 2016). Four studies examined DCC for between one and two minutes whilst holding the baby low (Baenziger 2007; Dipak 2017; Strauss 2008; Tarnow‐Mordi 2017). Three studies examined DCC by more than two minutes whilst holding the baby level with the placenta (Ranjit 2015; Tiemersma 2015; Ultee 2008). There were no studies which examined DCC for more than two minutes whilst holding the baby low. Six studies included mixed interventions for DCC (Armanian 2017; Chu 2011; Dai 2014; Gokmen 2011; Hofmeyr 1988; Shi 2017).
B. Delayed cord clamping (DCC) with immediate neonatal care with cord intact versus early cord clamping (ECC) (Comparisons 3 and 4)
Two studies involving 322 babies and their mothers addressed this question (Aladangady 2006; CORD Pilot 2018) both were undertaken in the UK, but only one study involving 276 babies and 261 mothers (twin pregnancies were included) provided data (CORD Pilot 2018).
In this one study (CORD Pilot 2018), women were randomised if they were expected to give birth before 32 weeks' gestation. The study compared cord clamping after at least two minutes with clamping within 20 seconds. In the DCC group, immediate neonatal care was provided by the mother's side with the cord intact and this enabled babies requiring immediate resuscitation at birth to be included. For the ECC group, immediate neonatal care was after cord clamping. Babies were placed at the level of the mother's abdomen for vaginal births or the anterior thigh for caesarean sections (Characteristics of included studies).
C. Delayed cord clamping (DCC) with immediate neonatal care after cord clamping versus umbilical cord milking (UCM) (Comparisons 5 and 6)
Three studies involving 325 babies and their mothers addressed this question (Katheria 2015; Krueger 2015; Rabe 2011) and all provided data for this review.
The studies were undertaken in the following countries: two studies in the USA (Katheria 2015; Krueger 2015), and one in the UK (Rabe 2011).
All three studies included babies born before 32 to 34 weeks' gestation.
Two studies looked at delaying cord clamping for 30 seconds (Krueger 2015; Rabe 2011) whilst holding the baby low. One study carried out DCC for 45 seconds or more with the baby held low (Katheria 2015).
D. Umbilical cord milking (UCM) versus early cord clamping (ECC) (Comparisons 7 and 8)
Thirteen studies involving 1423 babies and their mothers addressed this question. Two studies did not provide data on outcomes in this review (Das 2018; Pongmee 2010). So 11 studies involving 1183 babies and their mothers provided data (Alan 2014; Elimian 2014; El‐Naggar 2016; Hosono 2008; Hosono 2015; Josephsen 2014; Katheria 2014; Kilicdag 2016; Kumar 2015; March 2013; Mercer 2016).
The studies were undertaken in the following countries: the USA (Elimian 2014; Josephsen 2014; Katheria 2014; Kilicdag 2016; March 2013; Mercer 2016); India (Das 2018; Kumar 2015); Japan (Hosono 2008; Hosono 2015); Canada (El‐Naggar 2016); Thailand (Pongmee 2010) and Turkey (Alan 2014).
Ten studies providing data included babies expected or born at less than 32 to 34 weeks' gestation (Alan 2014; Elimian 2014; El‐Naggar 2016; Hosono 2008; Hosono 2015; Josephsen 2014; Katheria 2014; Kilicdag 2016; March 2013; Mercer 2016). One study providing data included babies born after 32 to 34 weeks' gestation (Kumar 2015).
Eight studies providing data undertook UCM with the cord intact (Alan 2014; Elimian 2014; El‐Naggar 2016; Hosono 2008; Katheria 2014; Kilicdag 2016; March 2013; Mercer 2016). Two studies providing data undertook UCM after the cord had been clamped and cut (Hosono 2015; Kumar 2015). In one study, it was unclear when cord milking took place relative to clamping (Josephsen 2014).
Excluded studies
In this update, we excluded 20 studies because of a number of reasons including: studies were on babies born at term; studies were not randomised or were quasi‐randomised controlled trials; the definitions of delay and early did not fit our criteria (Characteristics of excluded studies).
Risk of bias in included studies
Overall, summaries of assessments of bias in included studies are set out in Figure 2 and Figure 3, and details of the assessments made on risk of bias are reported under Characteristics of included studies are briefly described below.
2.
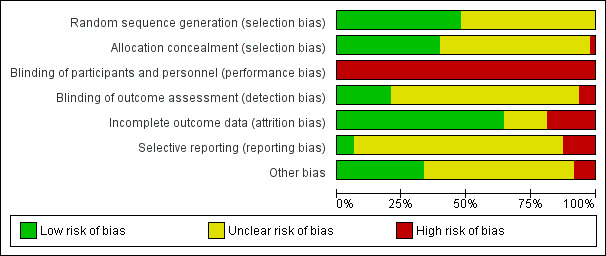
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
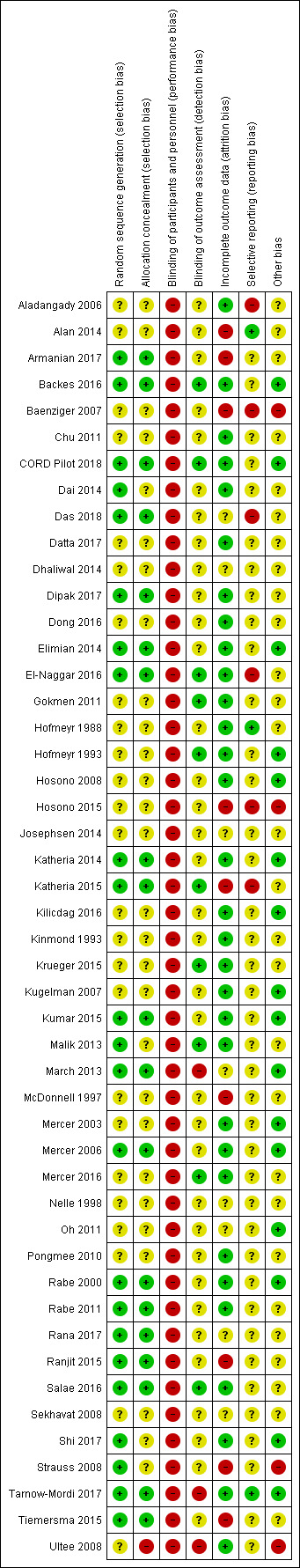
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The method used to generate the randomisation sequence in the included studies was generally not well described, with only 23 out of 48 studies meeting the criteria for low risk of bias. Similarly with allocation concealment, only 19 out of 48 studies met the criteria for low risk of bias. In addition, only 19 out of 48 studies met the criteria for low risk of selection bias (both sequence generation and allocation concealment). The remaining studies were unclear with one at high risk of bias from allocation concealment. See Figure 3.
Blinding
Performance bias
For this type of intervention the blinding of staff present at the birth to group allocation is not possible, and so this is considered high risk of bias across all studies. Figure 3; Characteristics of included studies.
Detection bias
We considered 10 studies were at low risk of detection bias as researchers blinded clinicians to the assessments of the clinical outcomes. Three studies were assessed as high risk of detection bias as authors said they had not been able to blind these assessments. The remaining studies were unclear. Figure 3; Characteristics of included studies.
Incomplete outcome data
For the data in the included studies collected soon after the birth, loss to follow‐up was generally not a problem. Thirty‐one studies were assessed as low risk of attrition bias, nine were considered high risk and the remaining eight studies were unclear. The post randomisation exclusions in a number of the included studies mean that some results are difficult to interpret. Figure 3; Characteristics of included studies.
Selective reporting
For most of the included studies only published data were available to us, and we did not have access to trial registration reports or study protocols. Under these circumstances, we were not able to assess whether authors had omitted to report findings for all of their pre‐specified outcomes. We did identify six studies where we assessed reporting bias to be high risk. Figure 3; Characteristics of included studies.
Other potential sources of bias
In most of the included studies, it was unclear whether there were other biases or not, and most studies reported similar baseline risks in the two groups. We assessed 16 studies as low risk of bias for other aspects of the studies and four studies were assessed as high risk ‐ mainly due to uneven denominators in the groups, post randomisation exclusions due to low Apgar scores, babies needing resuscitation, protocol violations and trial stopped early because of interim analysis (less than 50% of planned recruitment) Figure 3; Characteristics of included studies.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
This update includes 48 studies involving 5721 babies and their mothers. However, eight studies provided no data for our analyses and so the update has 40 studies which provided data involving 4884 babies and their mothers (Characteristics of included studies). We have four main comparisons with two subgroup analyses for each covering gestation and types of interventions.
We used random‐effect meta‐analysis for combining data for all analyses. We considered it was reasonable to assume that there was clinical heterogeneity due to the large variation in the timing of DCC between the included studies and where the baby was placed during this time (whether gravity could affect movement of blood to the baby). There was also variation in how umbilical cord milking (UCM) was undertaken, either before of after cutting the cord. These variations led us to consider that the underlying treatment effects would differ between trials.
A. Delayed cord clamping (DCC) with immediate neonatal care after cord clamping versus early cord clamping (ECC) (Comparisons 1 ‐ subgroup analysis by gestation: Comparison 2 ‐ subgroup analysis by type of intervention)
We identified 30 studies for this comparison, although five provided no data (Dhaliwal 2014; Malik 2013; Nelle 1998; Rana 2017; Sekhavat 2008). Twenty‐five studies, involving 3100 babies and their mothers, contributed data (Armanian 2017; Backes 2016; Baenziger 2007; Chu 2011; Dai 2014; Datta 2017; Dipak 2017; Dong 2016; Gokmen 2011; Hofmeyr 1988; Hofmeyr 1993; Kinmond 1993; Kugelman 2007; McDonnell 1997; Mercer 2003; Mercer 2006; Oh 2011; Rabe 2000; Ranjit 2015; Salae 2016; Shi 2017; Strauss 2008; Tarnow‐Mordi 2017; Tiemersma 2015; Ultee 2008). Studies were undertaken in a range of countries and most studies included babies less than 32 to 34 weeks' gestation. The studies covered a variety of timings of cord clamping and where the baby was held (Characteristics of included studies).
Main outcomes
1. Death of baby (up to discharge)
DCC with immediate neonatal care provided after cord clamping probably reduces the risk of the baby dying before discharge compared with ECC, (average risk ratio (RR) 0.73, 95% confidence interval (CI) 0.54 to 0.98, 20 studies, 2680 babies, random‐effects models; Analysis 1.1; Analysis 2.1). However, the CI is wide. The certainty of the evidence was assessed as 'moderate', downgraded due to imprecision (Table 1).
1.1. Analysis.
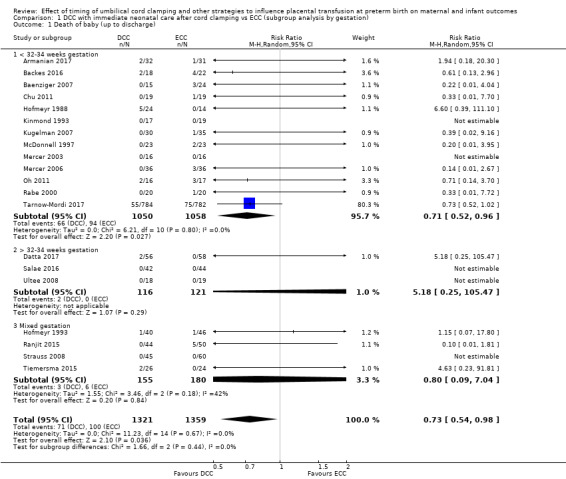
Comparison 1 DCC with immediate neonatal care after cord clamping vs ECC (subgroup analysis by gestation), Outcome 1 Death of baby (up to discharge).
2.1. Analysis.
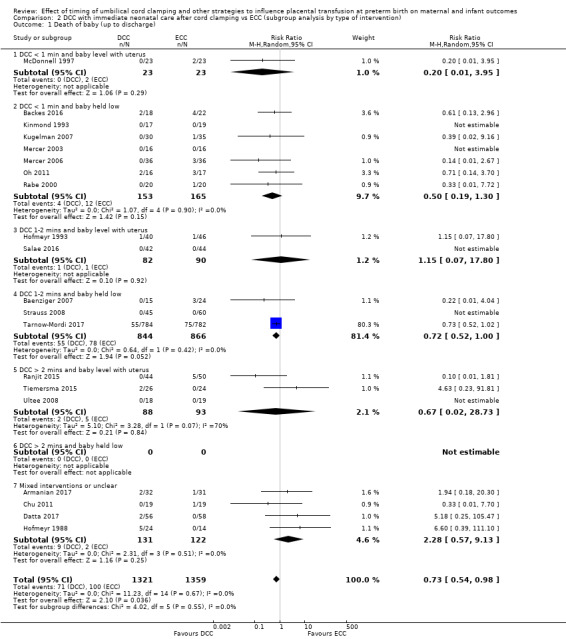
Comparison 2 DCC with immediate neonatal care after cord clamping vs ECC (subgroup analysis by type of intervention), Outcome 1 Death of baby (up to discharge).
There was no evidence of overall heterogeneity (Tau2 = 0, Chi2 P = 0.67, I2 = 0%) nor of differences between the subgroups by gestation or by types of intervention (Analysis 1.1; Analysis 2.1). We found no evidence of missing studies according to visual assessment of the funnel plot (Figure 4).
4.
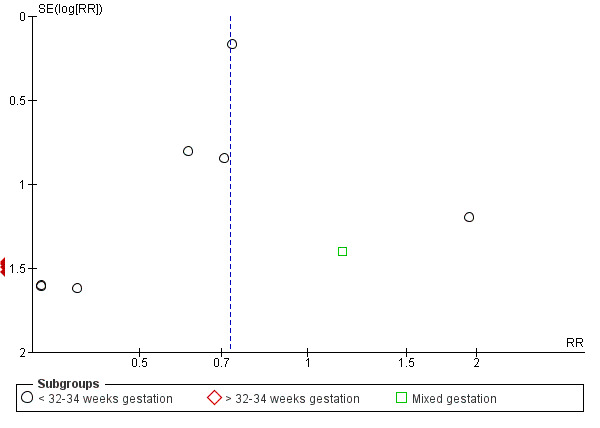
Funnel plot of comparison: 1 DCC with immediate neonatal care after cord clamping vs ECC (subgroup analysis by gestation), outcome: 1.1 Death of baby (up to discharge).
2. Death or neurodevelopmental impairment in early years
No studies assessed this composite outcome (Analysis 1.2; Analysis 2.2).
3. Severe intraventricular haemorrhage (IVH grades 3, 4)
We found insufficient evidence for reliable conclusions about the effect on the risk of severe IVH between the two interventions in this comparison (average RR 0.94, 95% CI 0.63 to 1.39, 10 studies, 2058 babies, random‐effects model; Analysis 1.3; Analysis 2.3). The certainty of the evidence was assessed as 'low', downgraded due to serious imprecision (Table 1).
1.3. Analysis.
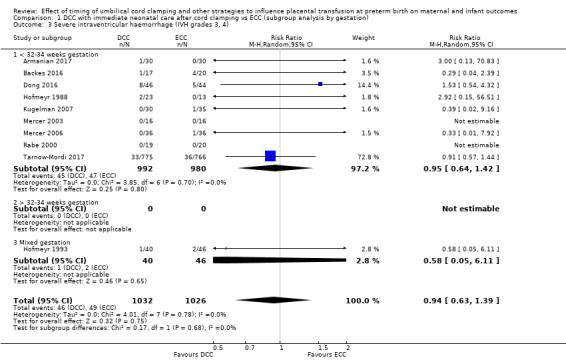
Comparison 1 DCC with immediate neonatal care after cord clamping vs ECC (subgroup analysis by gestation), Outcome 3 Severe intraventricular haemorrhage (IVH grades 3, 4).
2.3. Analysis.
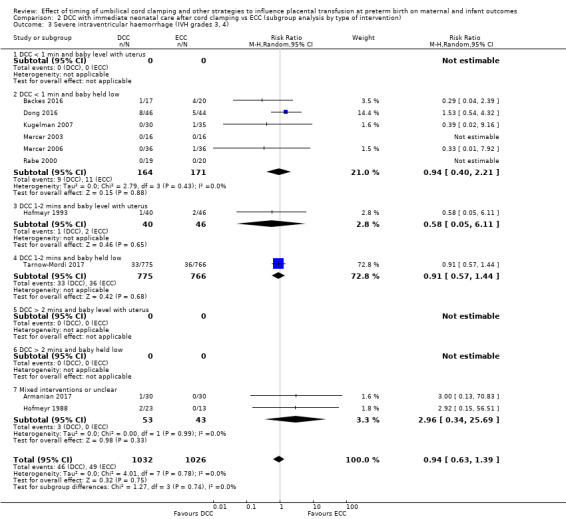
Comparison 2 DCC with immediate neonatal care after cord clamping vs ECC (subgroup analysis by type of intervention), Outcome 3 Severe intraventricular haemorrhage (IVH grades 3, 4).
There was no evidence of overall heterogeneity (Tau2 = 0, Chi2 P = 0.78, I2 = 0%) nor of differences between the subgroups by gestation or by types of intervention (Analysis 1.3; Analysis 2.3). We found no evidence of missing studies according to visual assessment of the funnel plot (Figure 5).
5.
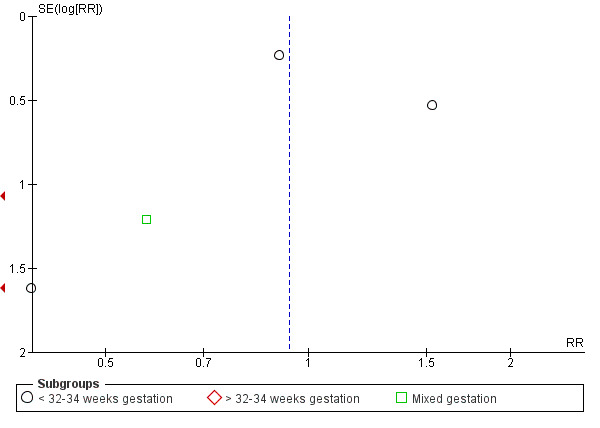
Funnel plot of comparison: 1 DCC with immediate neonatal care after cord clamping vs ECC (subgroup analysis by gestation), outcome: 1.3 Severe intraventricular haemorrhage (IVH grades 3, 4).
4. Intraventricular haemorrhage (IVH, all grades)
DCC is associated with a modest reduction in risk of any IVH (all grades) compared with ECC (average RR 0.83, 95% CI 0.70 to 0.99, 15 studies, 2333 babies, random‐effects model; Analysis 1.4; Analysis 2.4). The certainty of the evidence was assessed as 'high'. However, the large Australian trial (Tarnow‐Mordi 2017), which contributes 68% of the data showed no difference in this outcome, so it is likely the reduction comes from small studies of unclear risk of bias (Table 1).
1.4. Analysis.
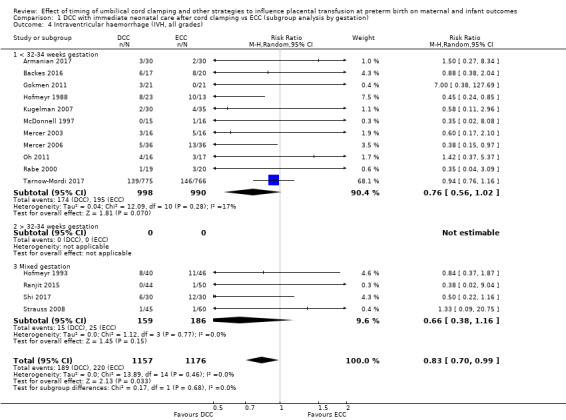
Comparison 1 DCC with immediate neonatal care after cord clamping vs ECC (subgroup analysis by gestation), Outcome 4 Intraventricular haemorrhage (IVH, all grades).
2.4. Analysis.
Comparison 2 DCC with immediate neonatal care after cord clamping vs ECC (subgroup analysis by type of intervention), Outcome 4 Intraventricular haemorrhage (IVH, all grades).
There was no evidence of overall heterogeneity (Tau2 = 0, Chi2 P = 0.46, I2 = 0%) nor of differences between the subgroups by gestation or by types of intervention (Analysis 1.4; Analysis 2.4). We found no evidence of missing studies according to visual assessment of the funnel plot (Figure 6).
6.
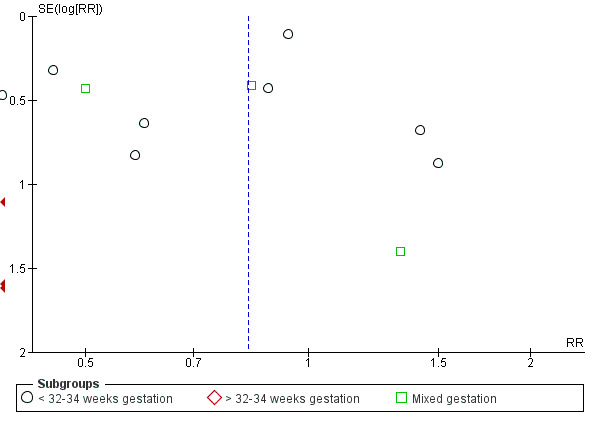
Funnel plot of comparison: 1 DCC with immediate neonatal care after cord clamping vs ECC (subgroup analysis by gestation), outcome: 1.4 Intraventricular haemorrhage (IVH, all grades).
5. Periventricular leukomalacia (PVL)
There was insufficient evidence for reliable conclusions about the effect on the risk of PVL associated with DCC compared with ECC (average RR 0.58, 95% CI 0.26 to 1.30, 4 studies, 1544 babies, random‐effects model; Analysis 1.5; Analysis 2.5). The certainty of the evidence was assessed as 'low', downgraded due to serious imprecision (Table 1).
1.5. Analysis.
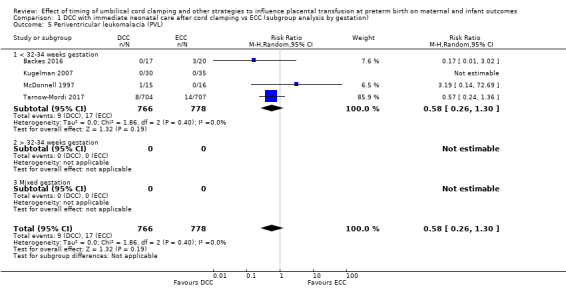
Comparison 1 DCC with immediate neonatal care after cord clamping vs ECC (subgroup analysis by gestation), Outcome 5 Periventricular leukomalacia (PVL).
2.5. Analysis.
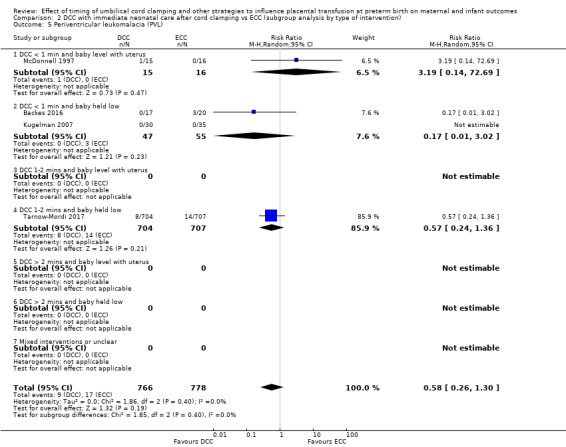
Comparison 2 DCC with immediate neonatal care after cord clamping vs ECC (subgroup analysis by type of intervention), Outcome 5 Periventricular leukomalacia (PVL).
There was no evidence of overall heterogeneity (Tau2 = 0, Chi2P = 0.40, I2 = 0%) (Analysis 1.5; Analysis 2.5). Subgroup analysis by gestation was not possible because all three trials included babies of less than 32 to 34 weeks' gestation (Analysis 1.5). There was no evidence of differences in the subgroups by types of intervention (Analysis 2.5).
6. Chronic lung disease (CLD) ‐ oxygen supplement at 36 weeks (corrected for gestation)
There was little or no difference identified between delayed clamping (with immediate neonatal care after cord clamping) and ECC (average RR 1.04, 95% CI 0.94 to 1.14, 6 studies, 1644 babies, random‐effects model; Analysis 1.6; Analysis 2.6). The certainty of the evidence was assessed as 'high' (Table 1).
1.6. Analysis.
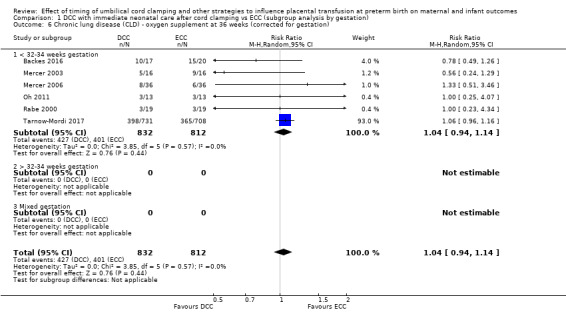
Comparison 1 DCC with immediate neonatal care after cord clamping vs ECC (subgroup analysis by gestation), Outcome 6 Chronic lung disease (CLD) ‐ oxygen supplement at 36 weeks (corrected for gestation).
2.6. Analysis.
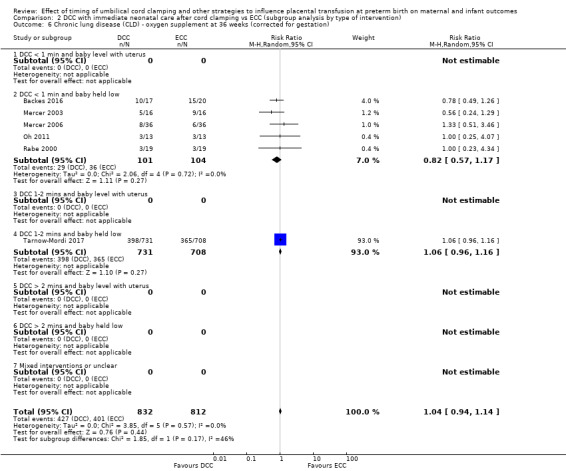
Comparison 2 DCC with immediate neonatal care after cord clamping vs ECC (subgroup analysis by type of intervention), Outcome 6 Chronic lung disease (CLD) ‐ oxygen supplement at 36 weeks (corrected for gestation).
There was no heterogeneity identified (Tau2 = 0; Chi2 P = 0.57, I2 = 0%) (Analysis 1.6; Analysis 2.6). Subgroup analysis by gestation was not possible because all six trials included babies of less than 32 to 34 weeks' gestation (Analysis 1.6). There was no evidence of differences in the subgroups by types of intervention (Analysis 2.6).
7. Maternal blood loss of 500 mL or greater
Most studies did not report outcome data for the mother. There was insufficient evidence for reliable conclusions about the comparative effects of DCC compared with ECC (average RR 1.14, 95% CI 0.07 to 17.63, 2 studies, 180 women, random‐effects model; Analysis 1.7; Analysis 2.7). The certainty of the evidence was assessed as 'very low', downgraded due to risk of bias and serious imprecision (Table 1).
1.7. Analysis.
Comparison 1 DCC with immediate neonatal care after cord clamping vs ECC (subgroup analysis by gestation), Outcome 7 Maternal blood loss of 500 mL or greater.
2.7. Analysis.
Comparison 2 DCC with immediate neonatal care after cord clamping vs ECC (subgroup analysis by type of intervention), Outcome 7 Maternal blood loss of 500 mL or greater.
We found no heterogeneity and were unable to undertake subgroup analysis due to insufficient data (Analysis 1.7; Analysis 2.7).
Other important outcomes for the baby
For the following outcomes, unless stated otherwise below, we found no evidence of heterogeneity. Similarly, unless stated otherwise, where there are more than 10 studies, we found no evidence of missing studies according to visual assessments of funnel plots.
Condition at birth
There is probably little or no difference between DCC (with immediate neonatal care after cord clamping) compared with ECC on the following outcomes assessing the baby's condition at birth.
Low Apgar score (generally < eight at five minutes): (average RR 1.07, 95% CI 0.70 to 1.63, 4 studies, 1721 babies, random‐effects model) (Analysis 1.18; Analysis 2.18), (very low‐certainty evidence, downgraded due to unclear risk of bias and serious imprecision).
Temperature below 36oC within an hour of birth: there were no babies who had a temperature below 36oC in the one study of 86 babies which reported this outcome (Analysis 1.23; Analysis 2.23).
1.18. Analysis.
Comparison 1 DCC with immediate neonatal care after cord clamping vs ECC (subgroup analysis by gestation), Outcome 18 Low Apgar as defined by trialists (generally < 8 at 5 mins).
2.18. Analysis.
Comparison 2 DCC with immediate neonatal care after cord clamping vs ECC (subgroup analysis by type of intervention), Outcome 18 Low Apgar as defined by trialists (generally < 8 at 5 mins).
1.23. Analysis.
Comparison 1 DCC with immediate neonatal care after cord clamping vs ECC (subgroup analysis by gestation), Outcome 23 Temperature < 36.0oC within 1 hour of birth.
2.23. Analysis.
Comparison 2 DCC with immediate neonatal care after cord clamping vs ECC (subgroup analysis by type of intervention), Outcome 23 Temperature < 36.0oC within 1 hour of birth.
Respiratory
It is uncertain whether there is any clinically important difference between DCC (with immediate neonatal care after cord clamping) compared with ECC on the following outcomes assessing the baby's respiratory function.
Respiratory distress syndrome (RDS): (average RR 1.09, 95% CI 0.86 to 1.38, 7 studies, 457 babies, random‐effects model) (Analysis 1.10; Analysis 2.10), (very low‐certainty evidence, downgraded due to unclear risk of bias and severe imprecision).
Respiratory support: (average RR 0.95, 95% CI 0.77 to 1.16, 6 studies, 325 babies, random‐effects model) (Analysis 1.11; Analysis 2.11), (moderate‐certainty evidence, downgraded due to imprecision).
Duration of respiratory support (in days): (mean difference (MD) ‐0.60, 95% CI ‐3.04 to 1.84, 1 study, 42 babies) (Analysis 1.12; Analysis 2.12), (very low‐certainty evidence, downgraded due to unclear risk of bias and severe imprecision).
Surfactant treatment for severe RDS: (average RR 0.80, 95% CI 0.50 to 1.28, 3 studies, 145 babies, random‐effects model) (Analysis 1.13; Analysis 2.13), (very low‐certainty evidence, downgraded due to unclear risk of bias and severe imprecision).
Home oxygen: (RR 0.47, 95% CI 0.06 to 3.72, 2 studies, 101 babies) (Analysis 1.27; Analysis 2.27), (very low‐certainty evidence, downgraded due to unclear risk of bias and severe imprecision).
1.10. Analysis.
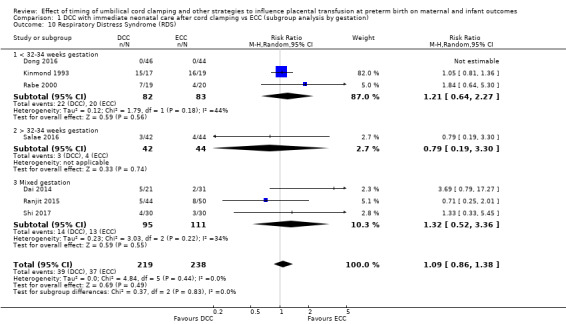
Comparison 1 DCC with immediate neonatal care after cord clamping vs ECC (subgroup analysis by gestation), Outcome 10 Respiratory Distress Syndrome (RDS).
2.10. Analysis.
Comparison 2 DCC with immediate neonatal care after cord clamping vs ECC (subgroup analysis by type of intervention), Outcome 10 Respiratory Distress Syndrome (RDS).
1.11. Analysis.
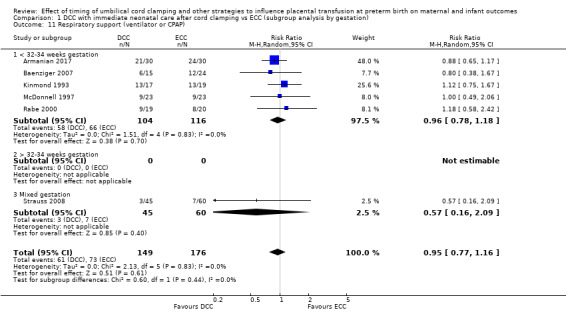
Comparison 1 DCC with immediate neonatal care after cord clamping vs ECC (subgroup analysis by gestation), Outcome 11 Respiratory support (ventilator or CPAP).
2.11. Analysis.
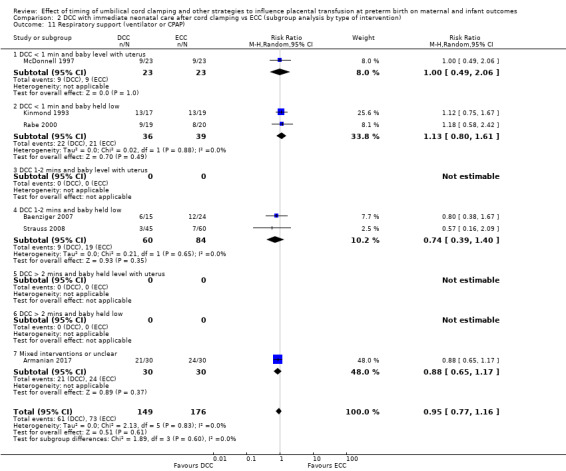
Comparison 2 DCC with immediate neonatal care after cord clamping vs ECC (subgroup analysis by type of intervention), Outcome 11 Respiratory support (ventilator or CPAP).
1.12. Analysis.
Comparison 1 DCC with immediate neonatal care after cord clamping vs ECC (subgroup analysis by gestation), Outcome 12 Duration of respiratory support (in days).
2.12. Analysis.
Comparison 2 DCC with immediate neonatal care after cord clamping vs ECC (subgroup analysis by type of intervention), Outcome 12 Duration of respiratory support.
1.13. Analysis.
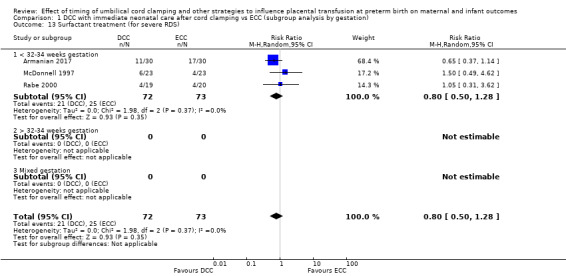
Comparison 1 DCC with immediate neonatal care after cord clamping vs ECC (subgroup analysis by gestation), Outcome 13 Surfactant treatment (for severe RDS).
2.13. Analysis.
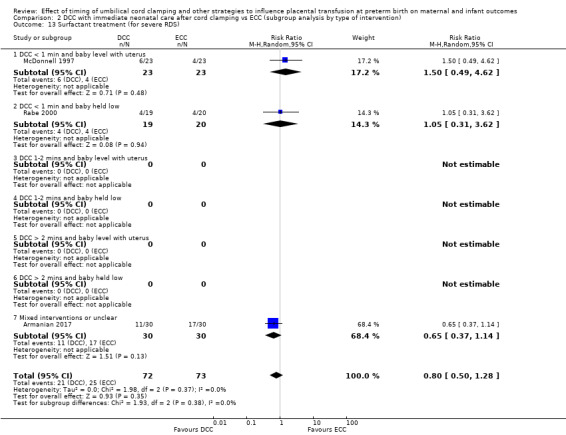
Comparison 2 DCC with immediate neonatal care after cord clamping vs ECC (subgroup analysis by type of intervention), Outcome 13 Surfactant treatment (for severe RDS).
1.27. Analysis.
Comparison 1 DCC with immediate neonatal care after cord clamping vs ECC (subgroup analysis by gestation), Outcome 27 Home oxygen.
2.27. Analysis.
Comparison 2 DCC with immediate neonatal care after cord clamping vs ECC (subgroup analysis by type of intervention), Outcome 27 Home oxygen.
Cardiovascular
DCC (with immediate neonatal care after cord clamping) may slightly improve a baby's arterial blood pressure compared with ECC.
Mean arterial blood pressure in the early hours after birth (in mm Hg): (average MD 2.87, 95% CI 1.09 to 4.64, 4 studies, 208 babies, random‐effects model) (Analysis 1.25; Analysis 2.25), (low‐certainty evidence, downgraded due to serious imprecision).
1.25. Analysis.
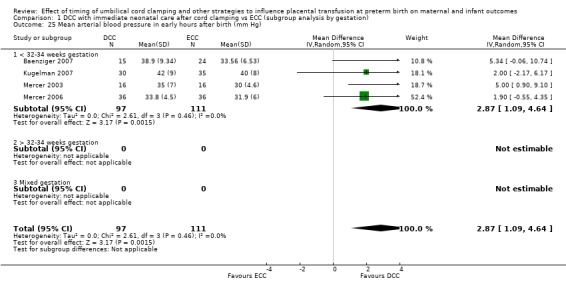
Comparison 1 DCC with immediate neonatal care after cord clamping vs ECC (subgroup analysis by gestation), Outcome 25 Mean arterial blood pressure in early hours after birth (mm Hg).
2.25. Analysis.
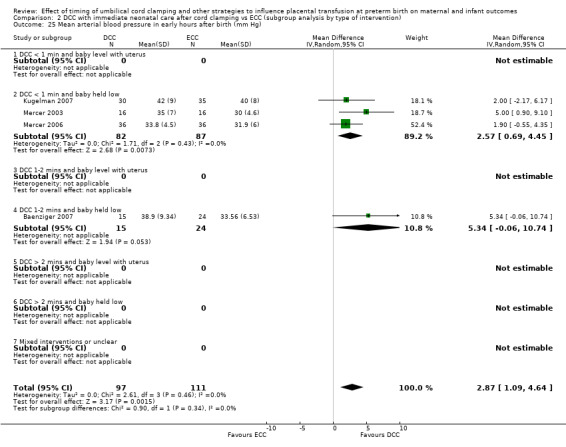
Comparison 2 DCC with immediate neonatal care after cord clamping vs ECC (subgroup analysis by type of intervention), Outcome 25 Mean arterial blood pressure in early hours after birth (mm Hg).
There is probably no difference between DCC (with immediate neonatal care after cord clamping) compared with ECC for:
Treatment for patent ductus arteriosis (PDA) (medical and/or surgical): average RR 1.12, 95% CI 0.99 to 1.26, 10 studies, 2046 babies, random‐effects model) (Analysis 1.14; Analysis 2.14), (moderate‐certainty evidence, downgraded because some trials seem to be missing according to visual assessment of the funnel plot, with potential for publication bias).
1.14. Analysis.
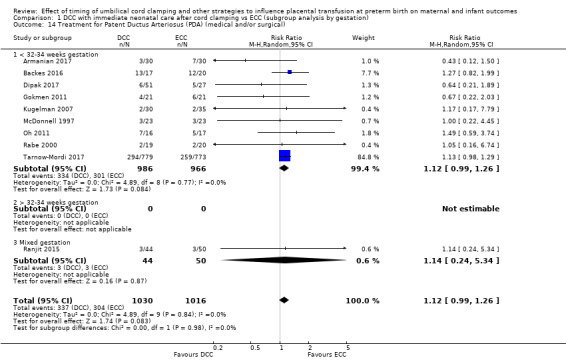
Comparison 1 DCC with immediate neonatal care after cord clamping vs ECC (subgroup analysis by gestation), Outcome 14 Treatment for Patent Ductus Arteriosus (PDA) (medical and/or surgical).
2.14. Analysis.
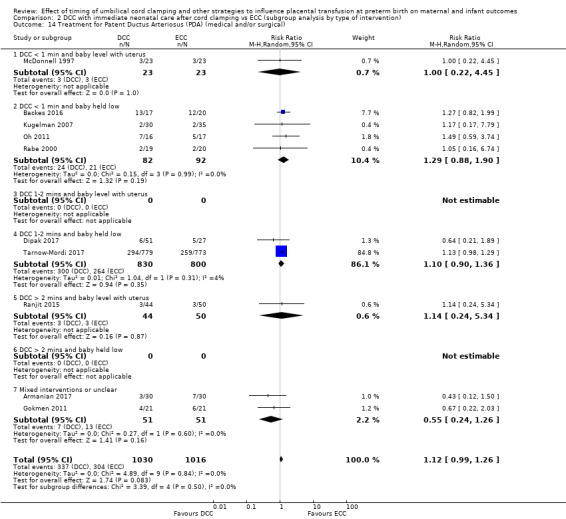
Comparison 2 DCC with immediate neonatal care after cord clamping vs ECC (subgroup analysis by type of intervention), Outcome 14 Treatment for Patent Ductus Arteriosus (PDA) (medical and/or surgical).
It is uncertain whether DCC (with immediate neonatal care after cord clamping) reduces the use of inotropes or not, compared with ECC.
Inotropics for low blood pressure during first 24 hours of life: (RR 0.37, 95% CI 0.17 to 0.81, 5 studies, 250 babies, random‐effects model) (Analysis 1.17; Analysis 2.17), (very low‐certainty evidence, downgraded due to unclear risk of bias and serious imprecision), (Analysis 1.17; Analysis 2.17).
1.17. Analysis.
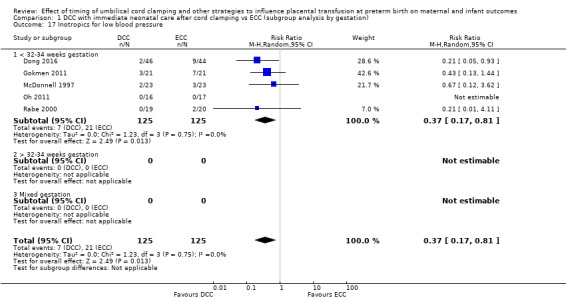
Comparison 1 DCC with immediate neonatal care after cord clamping vs ECC (subgroup analysis by gestation), Outcome 17 Inotropics for low blood pressure.
2.17. Analysis.
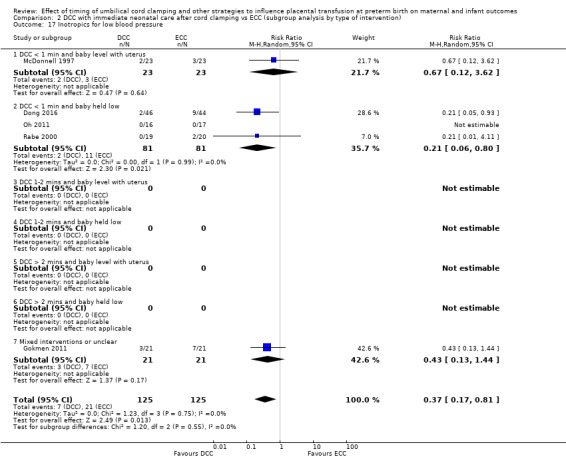
Comparison 2 DCC with immediate neonatal care after cord clamping vs ECC (subgroup analysis by type of intervention), Outcome 17 Inotropics for low blood pressure.
Central nervous system
DCC (with immediate neonatal care after cord clamping) may reduce the number of babies with IVH grades one and two compared with ECC, or there may be no difference.
IVH (grades one and two): (average RR 0.72, 95% CI 0.51 to 1.02, 9 studies, 1968 babies, random‐effects model) (Analysis 1.8; Analysis 2.8), (high‐certainty evidence).
1.8. Analysis.
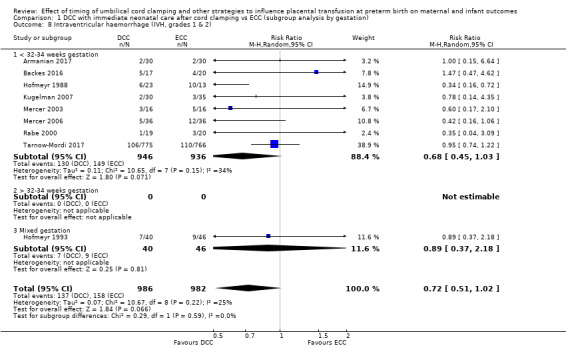
Comparison 1 DCC with immediate neonatal care after cord clamping vs ECC (subgroup analysis by gestation), Outcome 8 Intraventricular haemorrhage (IVH, grades 1 & 2).
2.8. Analysis.
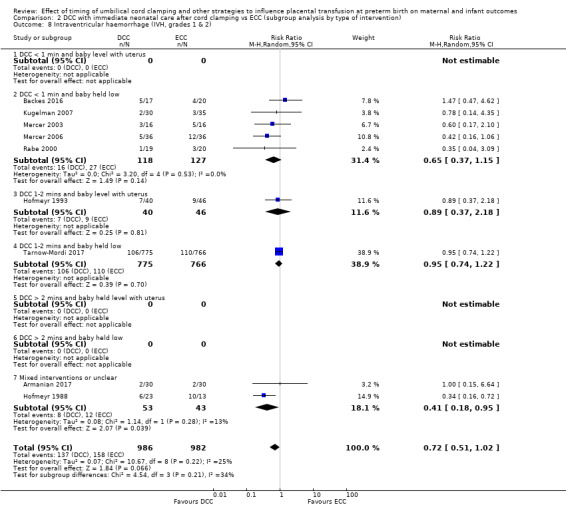
Comparison 2 DCC with immediate neonatal care after cord clamping vs ECC (subgroup analysis by type of intervention), Outcome 8 Intraventricular haemorrhage (IVH, grades 1 & 2).
Neurodevelopmental impairment at around two to three years was not assessed in any of the studies. We expect some of these studies will systematically gather the data on this outcome when the babies in their trials reach the appropriate age. We will report these data when they become available.
Gastrointestinal
DCC (with immediate neonatal care after cord clamping) probably makes little difference to the incidence of necrotising enterocolitis (NEC) compared with ECC.
NEC (confirmed by X‐ray or laparotomy): (average RR 0.91, 95% CI 0.64 to 1.28, 11 studies, 2010 babies, random‐effects model) (Analysis 1.9; Analysis 2.9), (moderate‐certainty evidence, downgraded for imprecision).
1.9. Analysis.
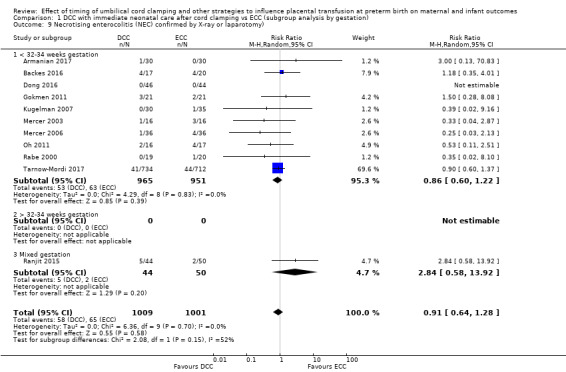
Comparison 1 DCC with immediate neonatal care after cord clamping vs ECC (subgroup analysis by gestation), Outcome 9 Necrotising enterocolitis (NEC) confirmed by X‐ray or laparotomy).
2.9. Analysis.
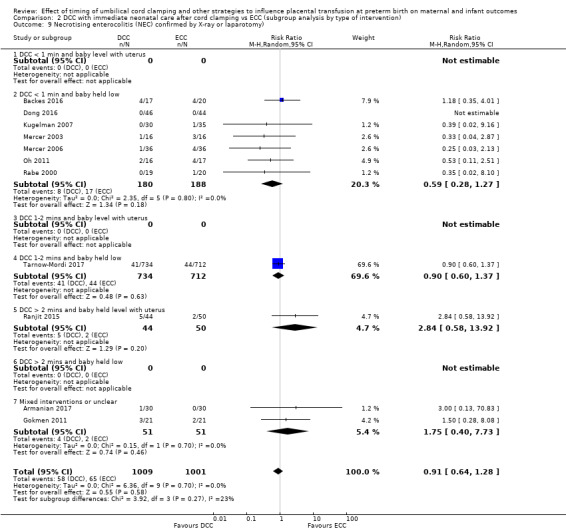
Comparison 2 DCC with immediate neonatal care after cord clamping vs ECC (subgroup analysis by type of intervention), Outcome 9 Necrotising enterocolitis (NEC) confirmed by X‐ray or laparotomy).
Haematological
DCC (with immediate neonatal care after cord clamping) makes little or no difference to the following haematological outcomes.
Hyperbilirubinemia (treated by phototherapy): (average RR 1.05, 95% CI 0.95 to 1.16, 8 studies, 495 babies, random‐effects model) (Analysis 1.16; Analysis 2.16), (high‐certainty evidence).
Volume of blood transfused (in mL): (MD ‐6.00, 95% CI ‐26.11 to 14.11, 1 study, 72 babies) (Analysis 1.20; Analysis 2.20), (low‐certainty evidence, downgraded for serious imprecision).
Haemoglobin (Hb) within first 24 hours of birth (in g/dL): (MD 0.80, 95% CI ‐0.02 to 1.62, 1 study, 42 babies) (Analysis 1.24; Analysis 2.24), (very low‐certainty evidence, downgraded for unclear risk of bias and serious imprecision).
1.16. Analysis.
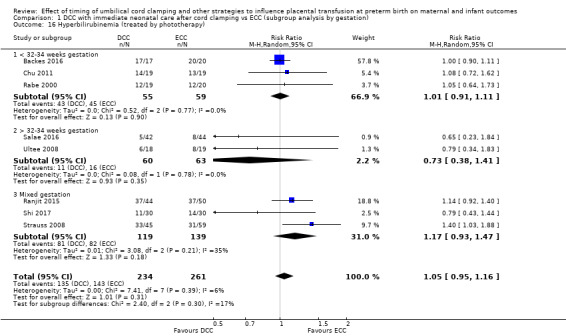
Comparison 1 DCC with immediate neonatal care after cord clamping vs ECC (subgroup analysis by gestation), Outcome 16 Hyperbilirubinemia (treated by phototherapy).
2.16. Analysis.
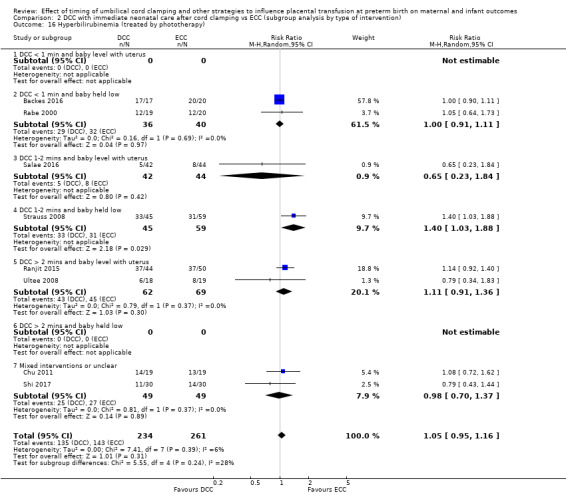
Comparison 2 DCC with immediate neonatal care after cord clamping vs ECC (subgroup analysis by type of intervention), Outcome 16 Hyperbilirubinemia (treated by phototherapy).
1.20. Analysis.
Comparison 1 DCC with immediate neonatal care after cord clamping vs ECC (subgroup analysis by gestation), Outcome 20 Volume of blood transfused (mL).
2.20. Analysis.
Comparison 2 DCC with immediate neonatal care after cord clamping vs ECC (subgroup analysis by type of intervention), Outcome 20 Volume of blood transfused (mL).
1.24. Analysis.
Comparison 1 DCC with immediate neonatal care after cord clamping vs ECC (subgroup analysis by gestation), Outcome 24 Hb within 1st 24 hour of birth (g/dL).
2.24. Analysis.
Comparison 2 DCC with immediate neonatal care after cord clamping vs ECC (subgroup analysis by type of intervention), Outcome 24 Hb within 1st 24 hour of birth (g/dL).
DCC (with immediate neonatal care after cord clamping) probably reduces the need for blood transfusion in the infant compared with ECC.
Blood transfusion in infant: (average RR 0.66, 95% CI 0.50 to 0.86, 11 studies, 2280 babies, random‐effects model). There was some evidence of heterogeneity (Tau2 = 0.06, Chi2 P = 0.10, I2 = 39%) (Analysis 1.19; Analysis 2.19), (moderate‐certainty evidence, downgraded as some studies seemed to be missing, suggesting potential publication bias).
1.19. Analysis.
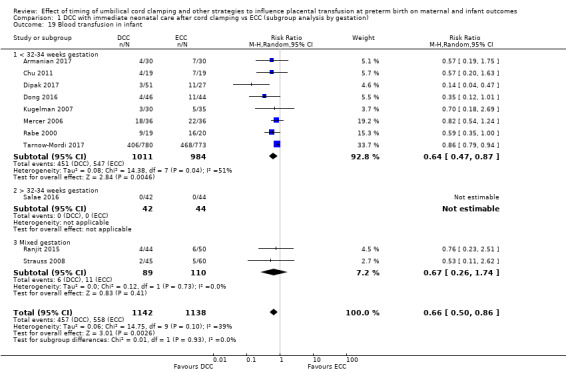
Comparison 1 DCC with immediate neonatal care after cord clamping vs ECC (subgroup analysis by gestation), Outcome 19 Blood transfusion in infant.
2.19. Analysis.
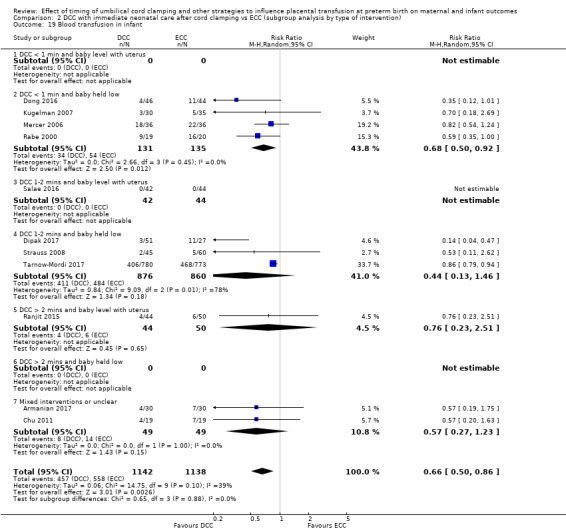
Comparison 2 DCC with immediate neonatal care after cord clamping vs ECC (subgroup analysis by type of intervention), Outcome 19 Blood transfusion in infant.
Additional outcomes
DCC (with immediate neonatal care after cord clamping) probably makes little or no difference to the following additional outcomes.
Treatment for retinopathy of prematurity: (average RR 0.83, 95% CI 0.62 to 1.12, 8 studies, 1827 babies, random‐effects model), (Analysis 1.15; Analysis 2.15), (moderate‐certainty evidence, downgraded for imprecision).
Late sepsis (after three days or as defined by trialists): (average RR 0.79, 95% CI 0.56 to 1.10, 10 studies, 2017 babies, random‐effects model). There was some evidence of heterogeneity (Tau2 = 0.11, Chi2 P = 0.06, I2 = 44%) but we found no strong evidence of publication bias. (Analysis 1.21; Analysis 2.21), (low‐certainty evidence, downgraded for heterogeneity and imprecision).
Fully breastfed or mixed feeding at infant discharge: (average RR 1.11, 95% CI 1.00 to 1.23, 1 study, 94 babies), (Analysis 1.39; Analysis 2.39), (very low‐certainty evidence, downgraded for risk of bias and serious imprecision).
1.15. Analysis.
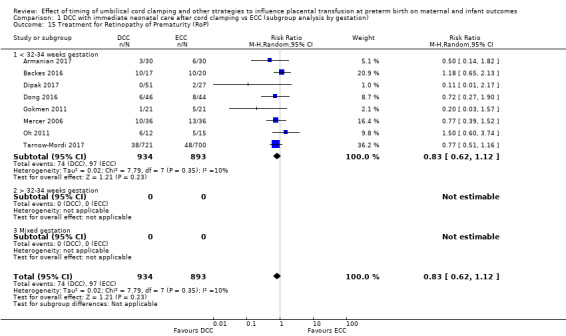
Comparison 1 DCC with immediate neonatal care after cord clamping vs ECC (subgroup analysis by gestation), Outcome 15 Treatment for Retinopathy of Prematurity (RoP).
2.15. Analysis.
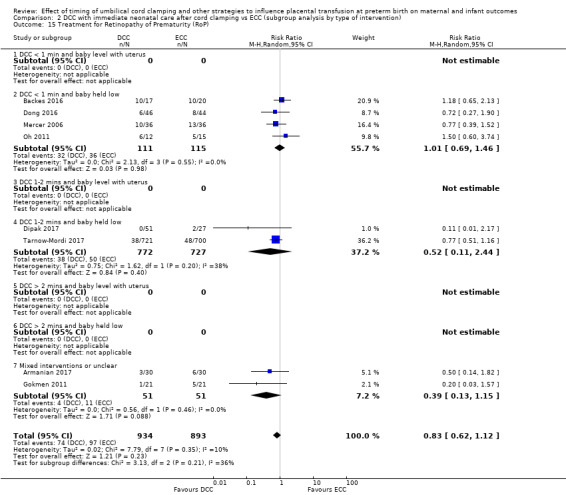
Comparison 2 DCC with immediate neonatal care after cord clamping vs ECC (subgroup analysis by type of intervention), Outcome 15 Treatment for Retinopathy of Prematurity (RoP).
1.21. Analysis.
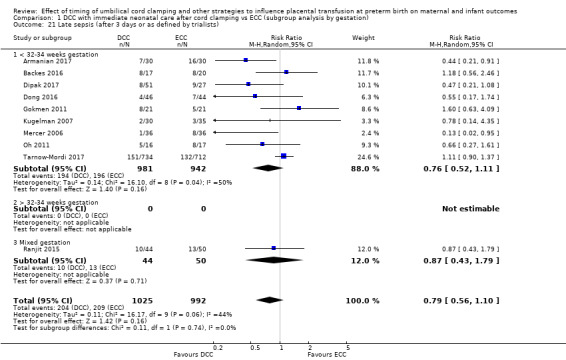
Comparison 1 DCC with immediate neonatal care after cord clamping vs ECC (subgroup analysis by gestation), Outcome 21 Late sepsis (after 3 days or as defined by trialists).
2.21. Analysis.
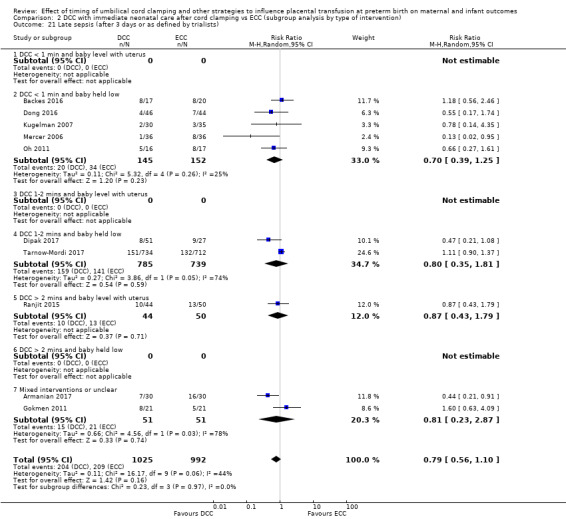
Comparison 2 DCC with immediate neonatal care after cord clamping vs ECC (subgroup analysis by type of intervention), Outcome 21 Late sepsis (after 3 days or as defined by trialists).
1.39. Analysis.
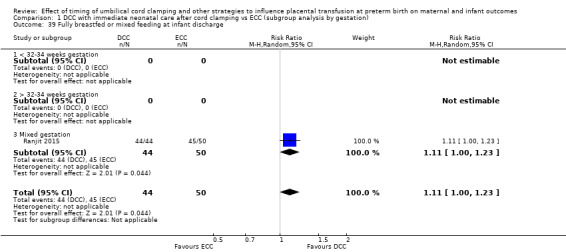
Comparison 1 DCC with immediate neonatal care after cord clamping vs ECC (subgroup analysis by gestation), Outcome 39 Fully breastfed or mixed feeding at infant discharge.
2.39. Analysis.
Comparison 2 DCC with immediate neonatal care after cord clamping vs ECC (subgroup analysis by type of intervention), Outcome 39 Fully breastfed or mixed feeding at infant discharge.
There were no data on severe visual impairment.
Other important outcomes for the mother
DCC (with immediate neonatal care after cord clamping) may make little or no difference to the number of mothers having blood transfusions following the birth (RR 0.67, 95% CI 0.36 to 1.24, 1 study, 1176 mothers), (low‐certainty evidence, downgraded for serious imprecision) (Analysis 1.33; Analysis 2.33).
1.33. Analysis.
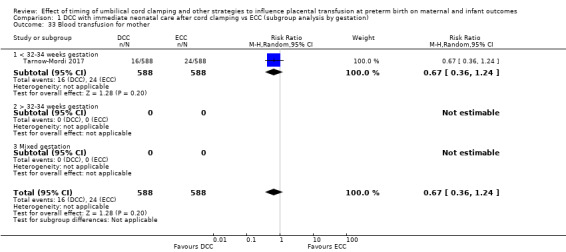
Comparison 1 DCC with immediate neonatal care after cord clamping vs ECC (subgroup analysis by gestation), Outcome 33 Blood transfusion for mother.
2.33. Analysis.
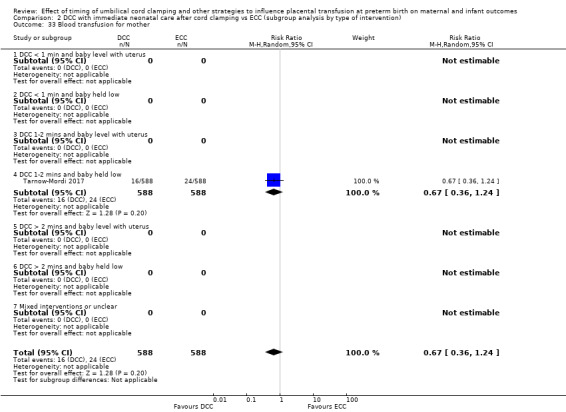
Comparison 2 DCC with immediate neonatal care after cord clamping vs ECC (subgroup analysis by type of intervention), Outcome 33 Blood transfusion for mother.
We found no included studies reporting other data on our secondary outcomes for mothers.
Other important outcomes for the father
None of the included studies reported results for any of our secondary outcomes for fathers (psychological well‐being, bonding with the infant, anxiety, and fathers’ views).
Sensitivity analysis (Comparison 9)
We undertook sensitivity analyses on primary outcomes only with the five studies assessed as low risk of bias for selection bias and incomplete outcome data (Backes 2016; Mercer 2006; Rabe 2000; Salae 2016; Tarnow‐Mordi 2017). We found no real differences in the overall findings.
B. Delayed cord clamping (DCC) with immediate neonatal care with cord intact versus early cord clamping (ECC) (Comparison 3 ‐ subgroup analysis by gestation: comparison 4 ‐ subgroup by type of intervention)
We found two studies addressing this comparison (Aladangady 2006; CORD Pilot 2018) but only one study involving 276 babies and 261 mothers (twin pregnancies were included) provided data (CORD Pilot 2018). This study was undertaken in eight maternity units in the UK, and women were included if they were expected to give birth at less than 32 weeks' gestation.
The intervention of DCC was to wait for at least two minutes, with the baby held level. Immediate neonatal care was provided at the mother's side so that care could be given with the cord intact. This was compared with ECC, namely, before 20 seconds after birth. Immediate neonatal care was given in this group after the cord was cut. Six women and babies were excluded after randomisation because they gave birth after 36 weeks' gestation. One mother whose baby died withdrew consent so data are provided on infant death only and on no other outcomes.
With only one study it was not possible to assess heterogeneity nor to undertake subgroup analyses.
Main outcomes
1. Death of baby (up to discharge)
Although the point estimate suggests DCC (with immediate neonatal care with cord intact) may reduce baby deaths up to discharge compared with ECC (with immediate neonatal care after cord clamping) (RR 0.47, 95% CI 0.20 to 1.11, 1 study, 270 babies) (Analysis 3.1; Analysis 4.1), the CI includes the possibility of a small increase in mortality. Low‐certainty evidence, downgraded due to serious imprecision (Table 2).
3.1. Analysis.
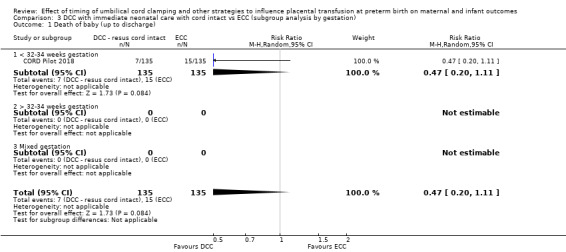
Comparison 3 DCC with immediate neonatal care with cord intact vs ECC (subgroup analysis by gestation), Outcome 1 Death of baby (up to discharge).
4.1. Analysis.
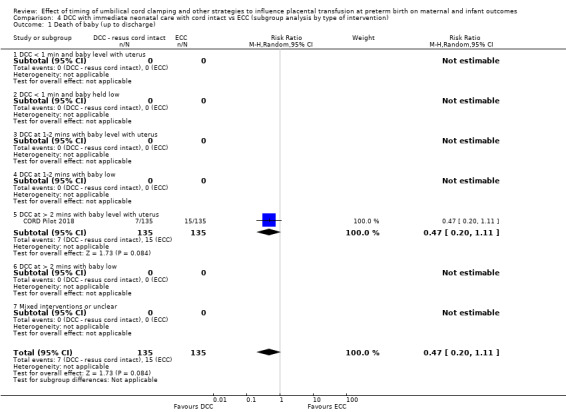
Comparison 4 DCC with immediate neonatal care with cord intact vs ECC (subgroup analysis by type of intervention), Outcome 1 Death of baby (up to discharge).
2. Death or neurodevelopmental impairment at age two to three years
DCC (with immediate neonatal care with cord intact) may reduce the composite outcome of 'death or neurodevelopmental impairment at two to three years' compared with ECC (with immediate neonatal care after cord clamping): (RR 0.61, 95% CI 0.39 to 0.96, 1 study, 218 babies) (Analysis 3.2; Analysis 4.2). However, the CI is wide and so the clinical certainty of any such reduction is uncertain. Low‐certainty evidence, downgraded due to serious imprecision (Table 2).
3.2. Analysis.
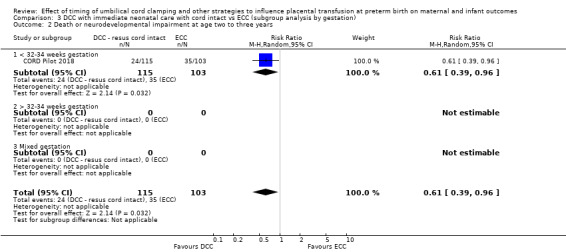
Comparison 3 DCC with immediate neonatal care with cord intact vs ECC (subgroup analysis by gestation), Outcome 2 Death or neurodevelopmental impairment at age two to three years.
4.2. Analysis.
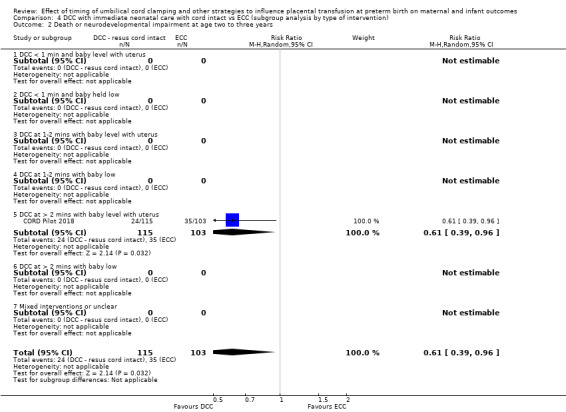
Comparison 4 DCC with immediate neonatal care with cord intact vs ECC (subgroup analysis by type of intervention), Outcome 2 Death or neurodevelopmental impairment at age two to three years.
3. Severe intraventricular haemorrhage (IVH grades 3, 4)
DCC (with immediate neonatal care with cord intact) may make little or no difference to the number of babies with severe IVH compared with early cord clamping (with immediate neonatal care after cord clamping): (RR 0.84, 95% CI 0.29 to 2.45, 1 study, 266 babies) (Analysis 3.3; Analysis 4.3). Low‐certainty evidence, downgraded due to serious imprecision (Table 2).
3.3. Analysis.
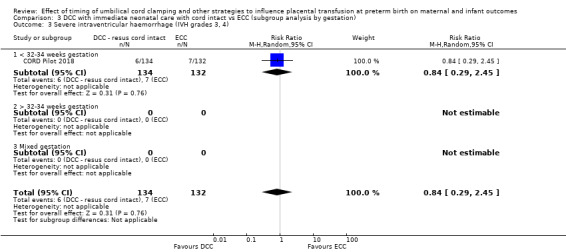
Comparison 3 DCC with immediate neonatal care with cord intact vs ECC (subgroup analysis by gestation), Outcome 3 Severe intraventricular haemorrhage (IVH grades 3, 4).
4.3. Analysis.
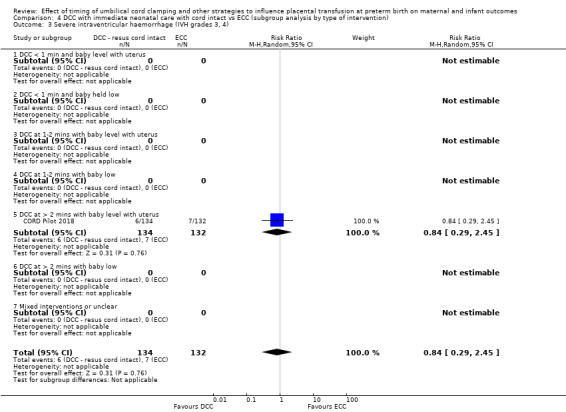
Comparison 4 DCC with immediate neonatal care with cord intact vs ECC (subgroup analysis by type of intervention), Outcome 3 Severe intraventricular haemorrhage (IVH grades 3, 4).
4. Intraventricular haemorrhage (IVH, all grades)
DCC (with immediate neonatal care with cord intact) may make little or no difference to the number of babies with any grade of IVH compared with early cord clamping (with immediate neonatal care after cord clamping) (RR 0.90, 95% CI 0.64 to 1.26, 1 study, 266 babies). Low‐certainty evidence, downgraded due to serious imprecision (Analysis 3.4; Analysis 4.4) (Table 2).
3.4. Analysis.
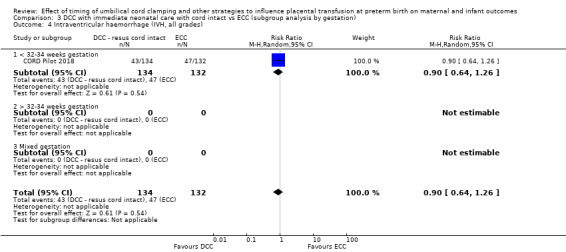
Comparison 3 DCC with immediate neonatal care with cord intact vs ECC (subgroup analysis by gestation), Outcome 4 Intraventricular haemorrhage (IVH, all grades).
4.4. Analysis.
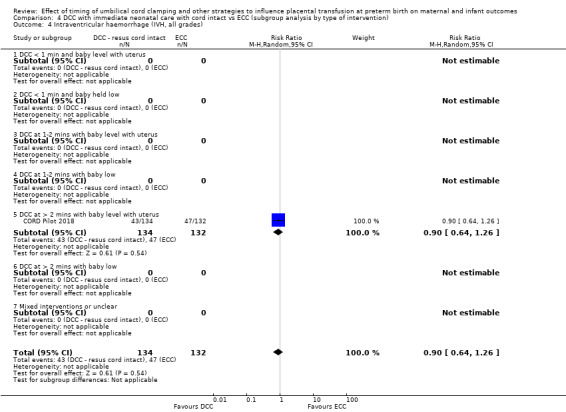
Comparison 4 DCC with immediate neonatal care with cord intact vs ECC (subgroup analysis by type of intervention), Outcome 4 Intraventricular haemorrhage (IVH, all grades).
5. Periventricular leukomalacia (PVL)
DCC (with immediate neonatal care with cord intact) may make little or no difference to the number of babies with PVL compared with early cord clamping (with immediate neonatal care after cord clamping) (RR 0.86, 95% CI 0.32 to 2.31, 1 study, 266 babies) (Analysis 3.5; Analysis 4.5). Low‐certainty evidence, downgraded due to serious imprecision (Table 2).
3.5. Analysis.
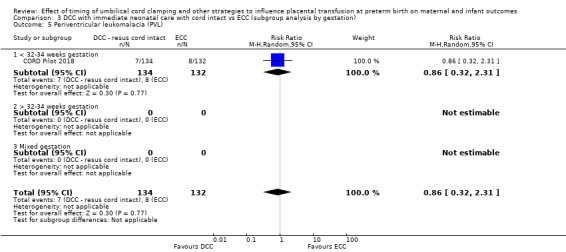
Comparison 3 DCC with immediate neonatal care with cord intact vs ECC (subgroup analysis by gestation), Outcome 5 Periventricular leukomalacia (PVL).
4.5. Analysis.
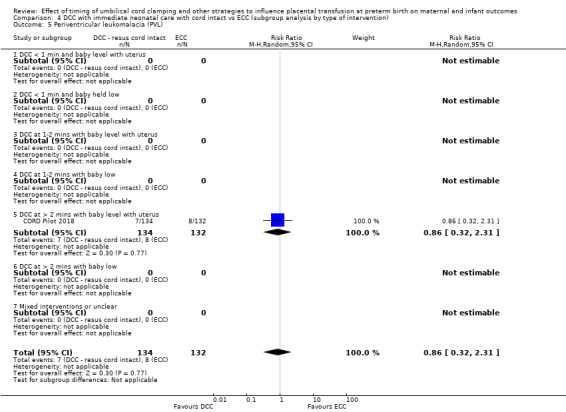
Comparison 4 DCC with immediate neonatal care with cord intact vs ECC (subgroup analysis by type of intervention), Outcome 5 Periventricular leukomalacia (PVL).
6. Chronic lung disease (CLD) ‐ oxygen supplement at 36 weeks (corrected for gestation)
DCC (with immediate neonatal care with cord intact) may make little or no difference to the number of babies with CLD compared with early cord clamping (with immediate neonatal care after cord clamping) (RR 0.95, 95% CI 0.66 to 1.37, 1 study, 249 babies) (Analysis 3.6; Analysis 4.6). Low‐certainty evidence, downgraded due to serious imprecision (Table 2).
3.6. Analysis.
Comparison 3 DCC with immediate neonatal care with cord intact vs ECC (subgroup analysis by gestation), Outcome 6 Chronic lung disease (CLD) ‐ oxygen supplement at 36 weeks (corrected for gestation).
4.6. Analysis.
Comparison 4 DCC with immediate neonatal care with cord intact vs ECC (subgroup analysis by type of intervention), Outcome 6 Chronic lung disease (CLD) ‐ oxygen supplement at 36 weeks (corrected for gestation).
7. Maternal blood loss of 500 mL or greater
DCC (with immediate neonatal care with cord intact) may make little or no difference to the number of mothers with blood loss of 500 mL or greater compared with early cord clamping (with immediate neonatal care after cord clamping) (RR 0.94, 95% CI 0.72 to 1.22, 1 study, 254 women) (Analysis 3.7; Analysis 4.7). Low‐certainty evidence, downgraded due to risk of bias and imprecision (Table 2).
3.7. Analysis.
Comparison 3 DCC with immediate neonatal care with cord intact vs ECC (subgroup analysis by gestation), Outcome 7 Maternal blood loss of 500 mL or greater.
4.7. Analysis.
Comparison 4 DCC with immediate neonatal care with cord intact vs ECC (subgroup analysis by type of intervention), Outcome 7 Maternal blood loss of 500 mL or greater.
Other important outcomes for the baby
There is insufficient evidence for any reliable conclusions about the comparative effects of DCC (with immediate neonatal care with cord intact) and ECC (with immediate neonatal care after cord clamping) on the following outcomes.
Condition at birth
Temperature < 36.0oC within one hour of birth: (RR 1.20, 95% CI 0.61 to 2.33, 1 study, 266 babies) (Analysis 3.23; Analysis 4.23), (low‐certainty evidence, downgraded for serious imprecision).
3.23. Analysis.
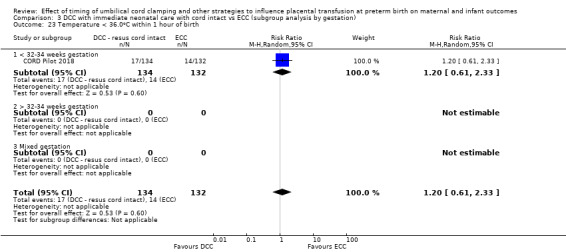
Comparison 3 DCC with immediate neonatal care with cord intact vs ECC (subgroup analysis by gestation), Outcome 23 Temperature < 36.0oC within 1 hour of birth.
4.23. Analysis.
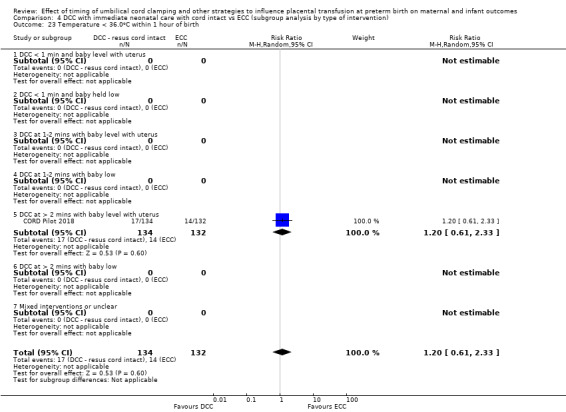
Comparison 4 DCC with immediate neonatal care with cord intact vs ECC (subgroup analysis by type of intervention), Outcome 23 Temperature < 36.0oC within 1 hour of birth.
There were no data on low Apgar scores.
Respiratory
Respiratory support (ventilator or continuous positive airway pressure (CPAP)): (RR 0.96, 95% CI 0.84 to 1.09, 1 study, 266 babies) (Analysis 3.11; Analysis 4.11), (moderate‐certainty evidence, downgraded for imprecision).
3.11. Analysis.
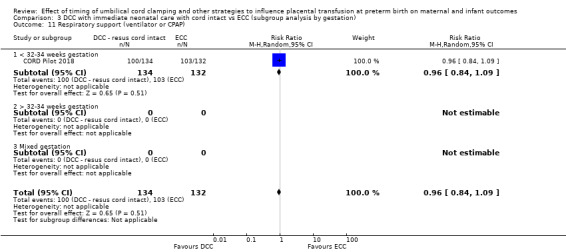
Comparison 3 DCC with immediate neonatal care with cord intact vs ECC (subgroup analysis by gestation), Outcome 11 Respiratory support (ventilator or CPAP).
4.11. Analysis.
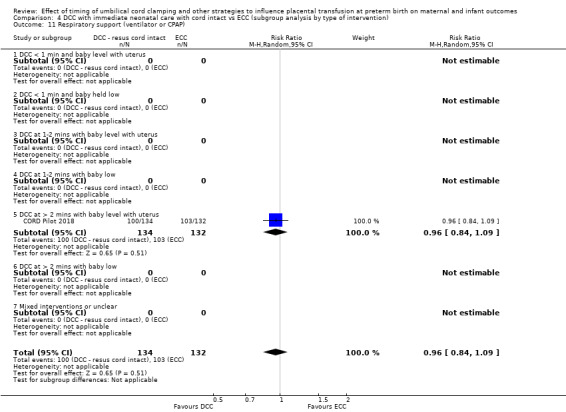
Comparison 4 DCC with immediate neonatal care with cord intact vs ECC (subgroup analysis by type of intervention), Outcome 11 Respiratory support (ventilator or CPAP).
There were no data on: RDS, duration of respiratory support, surfactant treatment and home oxygen.
Cardiovascular
Treatment for patent ductus arteriosus: (RR 0.99, 95% CI 0.56 to 1.74, 1 study, 266 babies) (Analysis 3.14; Analysis 4.14), (low‐certainty evidence, downgraded for serious imprecision).
3.14. Analysis.
Comparison 3 DCC with immediate neonatal care with cord intact vs ECC (subgroup analysis by gestation), Outcome 14 Treatment for Patent Ductus Arteriosus (PDA) (medical and/or surgical).
4.14. Analysis.
Comparison 4 DCC with immediate neonatal care with cord intact vs ECC (subgroup analysis by type of intervention), Outcome 14 Treatment for Patent Ductus Arteriosus (PDA) (medical and/or surgical).
There were no data on: inotropic support for hypotension nor mean arterial blood pressure.
Central nervous system
IVH (grades 1 and 2): (RR 0.91, 95% CI 0.63 to 1.33, 1 study, 266 babies) (Analysis 3.8; Analysis 4.8), (low‐certainty evidence, downgraded for serious imprecision).
Hydrocephalus: (RR 0.99, 95% CI 0.14 to 6.89, 1 study, 266 babies) (Analysis 3.22; Analysis 4.22), (low‐certainty evidence, downgraded for serious imprecision).
Neurodevelopmental impairment at two to three years: (RR 0.75, 95% CI 0.41 to 1.39, 1 study, 218 babies) (Analysis 3.28; Analysis 4.28), (low‐certainty evidence, downgraded for serious imprecision).
3.8. Analysis.
Comparison 3 DCC with immediate neonatal care with cord intact vs ECC (subgroup analysis by gestation), Outcome 8 Intraventricular haemorrhage (IVH, grades 1 & 2).
4.8. Analysis.
Comparison 4 DCC with immediate neonatal care with cord intact vs ECC (subgroup analysis by type of intervention), Outcome 8 Intraventricular haemorrhage (IVH, grades 1 & 2).
3.22. Analysis.
Comparison 3 DCC with immediate neonatal care with cord intact vs ECC (subgroup analysis by gestation), Outcome 22 Hydrocephalus.
4.22. Analysis.
Comparison 4 DCC with immediate neonatal care with cord intact vs ECC (subgroup analysis by type of intervention), Outcome 22 Hydrocephalus.
3.28. Analysis.
Comparison 3 DCC with immediate neonatal care with cord intact vs ECC (subgroup analysis by gestation), Outcome 28 Neurodevelopmental impairment at age two to three years.
4.28. Analysis.
Comparison 4 DCC with immediate neonatal care with cord intact vs ECC (subgroup analysis by type of intervention), Outcome 28 Neurodevelopmental impairment at age two to three years.
There were no data on: cerebral palsy (CP).
Gastrointestinal
NEC (confirmed by X‐ray or laparotomy): (RR 1.58, 95% CI 0.53 to 4.69, 1 study, 266 babies) (Analysis 3.9; Analysis 4.9), (low‐certainty evidence, downgraded for serious imprecision).
3.9. Analysis.
Comparison 3 DCC with immediate neonatal care with cord intact vs ECC (subgroup analysis by gestation), Outcome 9 Necrotising enterocolitis (NEC) confirmed by X‐ray or laparotomy).
4.9. Analysis.
Comparison 4 DCC with immediate neonatal care with cord intact vs ECC (subgroup analysis by type of intervention), Outcome 9 Necrotising enterocolitis (NEC) confirmed by X‐ray or laparotomy).
Haematological
Blood transfusion in infant: (RR 0.91, 95% CI 0.71 to 1.17, 1 study, 266 babies) (Analysis 3.19; Analysis 4.19), (moderate‐certainty evidence, downgraded for imprecision).
Hyperbilirubinemia (treated by phototherapy): (RR 1.01, 95% CI 0.94 to 1.09, 1 study, 266 babies) (Analysis 3.16; Analysis 4.16), (moderate‐certainty evidence, downgraded for imprecision).
3.19. Analysis.
Comparison 3 DCC with immediate neonatal care with cord intact vs ECC (subgroup analysis by gestation), Outcome 19 Blood transfusion in infant.
4.19. Analysis.
Comparison 4 DCC with immediate neonatal care with cord intact vs ECC (subgroup analysis by type of intervention), Outcome 19 Blood transfusion in infant.
3.16. Analysis.
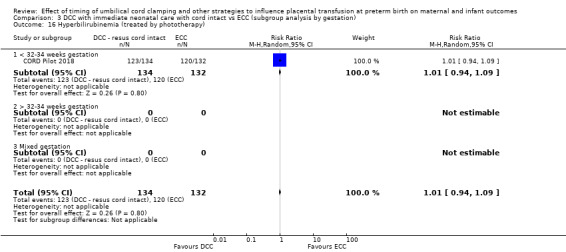
Comparison 3 DCC with immediate neonatal care with cord intact vs ECC (subgroup analysis by gestation), Outcome 16 Hyperbilirubinemia (treated by phototherapy).
4.16. Analysis.
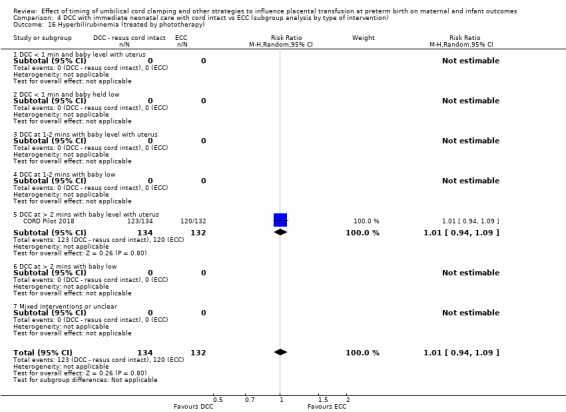
Comparison 4 DCC with immediate neonatal care with cord intact vs ECC (subgroup analysis by type of intervention), Outcome 16 Hyperbilirubinemia (treated by phototherapy).
There were no data on: volume of blood transfused to baby nor Hb within the first 24 hours.
Other infant outcomes
Treatment for retinopathy of prematurity (RoP): (RR 0.93, 95% CI 0.28 to 3.13, 1 study, 249 babies) (Analysis 3.15; Analysis 4.15), (low‐certainty evidence, downgraded for serious imprecision).
Late sepsis (after three days or as defined by trialists): (RR 0.89, 95% CI 0.72 to 1.09, 1 study, 266 babies) (Analysis 3.21; Analysis 4.21), (moderate‐certainty evidence, downgraded for imprecision).
Fully breastfed or mixed feeding at infant discharge: (RR 0.98, 95% CI 0.79 to 1.22, 1 study, 248 babies) (Analysis 3.39; Analysis 4.39), (moderate‐certainty evidence, downgraded for imprecision).
3.15. Analysis.
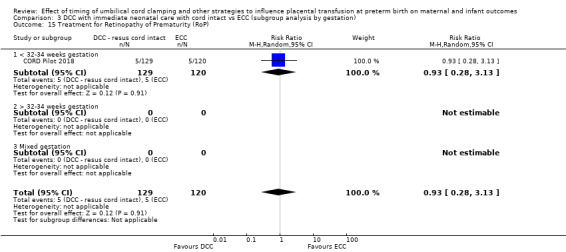
Comparison 3 DCC with immediate neonatal care with cord intact vs ECC (subgroup analysis by gestation), Outcome 15 Treatment for Retinopathy of Prematurity (RoP).
4.15. Analysis.
Comparison 4 DCC with immediate neonatal care with cord intact vs ECC (subgroup analysis by type of intervention), Outcome 15 Treatment for Retinopathy of Prematurity (RoP).
3.21. Analysis.
Comparison 3 DCC with immediate neonatal care with cord intact vs ECC (subgroup analysis by gestation), Outcome 21 Late sepsis (after 3 days or as defined by trialists).
4.21. Analysis.
Comparison 4 DCC with immediate neonatal care with cord intact vs ECC (subgroup analysis by type of intervention), Outcome 21 Late sepsis (after 3 days or as defined by trialists).
3.39. Analysis.
Comparison 3 DCC with immediate neonatal care with cord intact vs ECC (subgroup analysis by gestation), Outcome 39 Fully breastfed or mixed feeding at infant discharge.
4.39. Analysis.
Comparison 4 DCC with immediate neonatal care with cord intact vs ECC (subgroup analysis by type of intervention), Outcome 39 Fully breastfed or mixed feeding at infant discharge.
There were no data on: severe visual impairment nor length of infant stay in neonatal intensive care units (NICUs).
Other important outcomes for the mother
Manual removal of placenta (denominator = vaginal births): (RR 0.99, 95% CI 0.32 to 3.04, 1 study, 105 women) (Analysis 3.31; Analysis 4.31), (low‐certainty evidence, downgraded for serious imprecision).
Prolonged third stage (> 30 minutes) (denominator = vaginal births): (RR 0.79, 95% CI 0.24 to 2.64, 1 study, 105 women) (Analysis 3.32; Analysis 4.32), (low‐certainty evidence, downgraded for serious imprecision).
Blood transfusion for mother: (RR 1.59, 95% CI 0.39 to 6.51, 1 study, 254 women) (Analysis 3.33; Analysis 4.33), (low‐certainty evidence, downgraded for serious imprecision).
Postpartum infection in mother: (RR 1.12, 95% CI 0.73 to 1.72, 1 study, 254 women) (Analysis 3.34; Analysis 4.34), (low‐certainty evidence, downgraded for serious imprecision).
3.31. Analysis.
Comparison 3 DCC with immediate neonatal care with cord intact vs ECC (subgroup analysis by gestation), Outcome 31 Manual removal of placenta (denominator = vaginal births).
4.31. Analysis.
Comparison 4 DCC with immediate neonatal care with cord intact vs ECC (subgroup analysis by type of intervention), Outcome 31 Manual removal of placenta (denominator = vaginal births).
3.32. Analysis.
Comparison 3 DCC with immediate neonatal care with cord intact vs ECC (subgroup analysis by gestation), Outcome 32 Prolonged third stage (>30 minutes) (denominator = vaginal births).
4.32. Analysis.
Comparison 4 DCC with immediate neonatal care with cord intact vs ECC (subgroup analysis by type of intervention), Outcome 32 Prolonged third stage (>30 minutes) (denominator = vaginal births).
3.33. Analysis.
Comparison 3 DCC with immediate neonatal care with cord intact vs ECC (subgroup analysis by gestation), Outcome 33 Blood transfusion for mother.
4.33. Analysis.
Comparison 4 DCC with immediate neonatal care with cord intact vs ECC (subgroup analysis by type of intervention), Outcome 33 Blood transfusion for mother.
3.34. Analysis.
Comparison 3 DCC with immediate neonatal care with cord intact vs ECC (subgroup analysis by gestation), Outcome 34 Postpartum infection in mother.
4.34. Analysis.
Comparison 4 DCC with immediate neonatal care with cord intact vs ECC (subgroup analysis by type of intervention), Outcome 34 Postpartum infection in mother.
There were no data on: Rhesus‐isoimmunisation; psychological well‐being, bonding with the infant, breastfeeding initiation, fully breastfed or mixed feeding at discharge, mothers' anxieties not mothers' views.
Other important outcomes for the father
None of the included studies reported results for any of our secondary outcomes for fathers (psychological well‐being, bonding with the infant, anxiety, and fathers’ views).
Sensitivity analysis (Comparison 10)
We were unable to undertake a sensitivity analysis as there was only one study providing data for this comparison. This study (CORD Pilot 2018) was assessed as low risk of bias for selection bias and incomplete outcome data.
C. Delayed cord clamping (DCC) with immediate neonatal care after cord clamping versus Umbilical cord milking (UCM) (Comparison 5 ‐ subgroup analysis by gestation: Comparison 6 ‐ subgroup analysis by type of intervention)
Three studies involving 322 babies and their mothers contributed data to this comparison (Katheria 2015; Krueger 2015; Rabe 2011). Studies were undertaken in the USA and UK. All included babies expected to be born before 32 to 34 weeks' gestation. DCC was for 30 or 45 seconds, all with the baby held low. There were insufficient data to undertake subgroup analyses.
Main outcomes
Unless otherwise stated, we found no evidence on heterogeneity. Subgroup analyses by gestation or by type of intervention were not possible as the three studies included babies of the same gestation (at less than 32 to 34 weeks) and the same type of intervention (DCC of less then one minute with the baby held low).
We found insufficient evidence of a difference between DCC (with immediate neonatal care after cord clamping) compared with UCM for the main outcomes.
1. Death of baby (up to discharge)
DCC (with immediate neonatal care after cord clamping) may make little or no difference compared with UCM in the number of babies who died before discharge (average RR 2.14, 95% CI 0.93 to 4.93, 3 studies, 322 babies, random‐effects model) (Analysis 5.1; Analysis 6.1). Low‐certainty evidence, downgraded due to serious imprecision (Table 3).
5.1. Analysis.
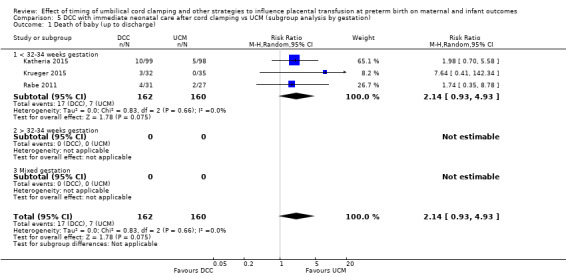
Comparison 5 DCC with immediate neonatal care after cord clamping vs UCM (subgroup analysis by gestation), Outcome 1 Death of baby (up to discharge).
6.1. Analysis.
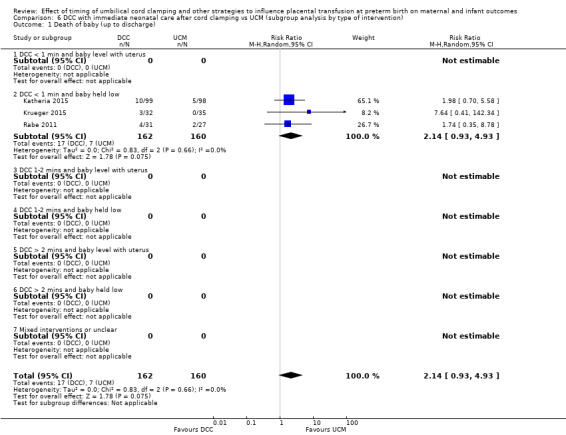
Comparison 6 DCC with immediate neonatal care after cord clamping vs UCM (subgroup analysis by type of intervention), Outcome 1 Death of baby (up to discharge).
2. Death or neurodevelopmental impairment at age two to three years
It is uncertain whether DCC (with immediate neonatal care after cord clamping) reduces the number of babies with death or neurodevelopmental impairment at two years compared with UCM (RR 1.67, 95% CI 0.78 to 3.57, 2 studies, 195 babies) (Analysis 5.2; Analysis 6.2). Very low‐certainty evidence, downgraded for risk of bias and serious imprecision (Table 3).
5.2. Analysis.
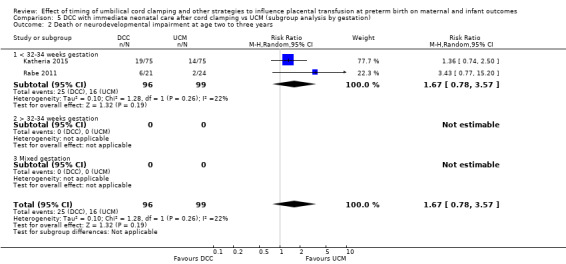
Comparison 5 DCC with immediate neonatal care after cord clamping vs UCM (subgroup analysis by gestation), Outcome 2 Death or neurodevelopmental impairment at age two to three years.
6.2. Analysis.
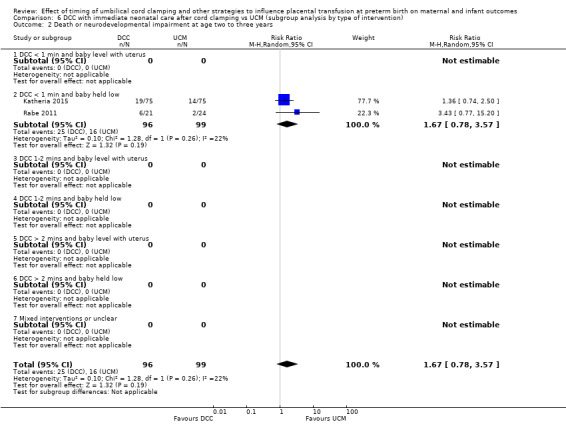
Comparison 6 DCC with immediate neonatal care after cord clamping vs UCM (subgroup analysis by type of intervention), Outcome 2 Death or neurodevelopmental impairment at age two to three years.
3. Severe intraventricular haemorrhage (IVH grades 3, 4)
DCC (with immediate neonatal care after cord clamping) may make little or no difference compared with UCM (RR 2.63, 95% CI 0.11 to 61.88, 1 study, 58 babies) (Analysis 5.3; Analysis 6.3). Low‐certainty evidence, downgraded for serious imprecision (Table 3).
5.3. Analysis.
Comparison 5 DCC with immediate neonatal care after cord clamping vs UCM (subgroup analysis by gestation), Outcome 3 Severe intraventricular haemorrhage (IVH grades 3, 4).
6.3. Analysis.
Comparison 6 DCC with immediate neonatal care after cord clamping vs UCM (subgroup analysis by type of intervention), Outcome 3 Severe intraventricular haemorrhage (IVH grades 3, 4).
4. Intraventricular haemorrhage (IVH, all grades)
DCC (with immediate neonatal care after cord clamping) makes little or no difference compared with UCM for all grades of IVH (average RR 1.32, 95% CI 0.55 to 3.17, 2 studies, 125 babies, random‐effects model) (Analysis 5.4; Analysis 6.4). Very low‐certainty evidence, downgraded for risk of bias and serious imprecision (Table 3).
5.4. Analysis.
Comparison 5 DCC with immediate neonatal care after cord clamping vs UCM (subgroup analysis by gestation), Outcome 4 Intraventricular haemorrhage (IVH, all grades).
6.4. Analysis.
Comparison 6 DCC with immediate neonatal care after cord clamping vs UCM (subgroup analysis by type of intervention), Outcome 4 Intraventricular haemorrhage (IVH, all grades).
5. Periventricular leukomalacia (PVL)
We identified one study involving 58 babies and none of the babies was identified with periventricular leukomalacia (Analysis 5.5; Analysis 6.5). Low‐certainty evidence, downgraded for serious imprecision (Table 3).
5.5. Analysis.
Comparison 5 DCC with immediate neonatal care after cord clamping vs UCM (subgroup analysis by gestation), Outcome 5 Periventricular leukomalacia (PVL).
6.5. Analysis.
Comparison 6 DCC with immediate neonatal care after cord clamping vs UCM (subgroup analysis by type of intervention), Outcome 5 Periventricular leukomalacia (PVL).
6. Chronic lung disease (CLD) ‐ oxygen supplement at 36 weeks (corrected for gestation)
DCC (with immediate neonatal care after cord clamping) may make little or no difference to the incidence of chronic lung disease compared with UCM (average RR 1.53, 95% CI 0.43 to 5.48, 2 studies, 125 babies, random‐effects model) (Analysis 5.6; Analysis 6.6). Low‐certainty evidence, downgraded fro serious imprecision (Table 3).
5.6. Analysis.
Comparison 5 DCC with immediate neonatal care after cord clamping vs UCM (subgroup analysis by gestation), Outcome 6 Chronic lung disease (CLD) ‐ oxygen supplement at 36 weeks (corrected for gestation).
6.6. Analysis.
Comparison 6 DCC with immediate neonatal care after cord clamping vs UCM (subgroup analysis by type of intervention), Outcome 6 Chronic lung disease (CLD) ‐ oxygen supplement at 36 weeks (corrected for gestation).
7. Maternal blood loss of 500 mL or greater
No studies assessed this outcome (Analysis 5.7; Analysis 6.7).
Other important outcomes for the baby
DCC (with immediate neonatal care after cord clamping) may make little or no difference to the following outcomes compared with UCM.
Condition at birth
There were no data on: low Apgar score; temperature < 360 within one hour of birth.
Respiratory
Duration of respiratory support (in days): (MD 1.80, 95% CI ‐2.01 to 5.61, 1 study, 67 babies) (Analysis 5.12; Analysis 6.12), (very low‐certainty evidence, downgraded for unclear risk of bias and serious imprecision).
Surfactant treatment (for severe RDS): (RR 1.19, 95% CI 0.66 to 2.13, 1 study, 58 babies) (Analysis 5.13; Analysis 6.13), (low‐certainty evidence, downgraded for serious imprecision).
Home oxygen: (RR 0.29, 95% CI 0.01 to 6.88, 1 study, 58 babies) (Analysis 5.27; Analysis 6.27), (low‐certainty evidence, downgraded for serious imprecision).
5.12. Analysis.
Comparison 5 DCC with immediate neonatal care after cord clamping vs UCM (subgroup analysis by gestation), Outcome 12 Duration of respiratory support (days).
6.12. Analysis.
Comparison 6 DCC with immediate neonatal care after cord clamping vs UCM (subgroup analysis by type of intervention), Outcome 12 Duration of respiratory support (days).
5.13. Analysis.
Comparison 5 DCC with immediate neonatal care after cord clamping vs UCM (subgroup analysis by gestation), Outcome 13 Surfactant treatment (for severe RDS).
6.13. Analysis.
Comparison 6 DCC with immediate neonatal care after cord clamping vs UCM (subgroup analysis by type of intervention), Outcome 13 Surfactant treatment (for severe RDS).
5.27. Analysis.
Comparison 5 DCC with immediate neonatal care after cord clamping vs UCM (subgroup analysis by gestation), Outcome 27 Home oxygen.
6.27. Analysis.
Comparison 6 DCC with immediate neonatal care after cord clamping vs UCM (subgroup analysis by type of intervention), Outcome 27 Home oxygen.
There were no data on: RDS; respiratory support (ventilator or CPAP).
Cardiovascular
There were no data on: treatment for patent ductus arteriosus (PDA); Inotropic support for hypotension; mean arterial blood pressure in early hours after birth.
Central nervous system
IVH (grades 1 and 2): (average RR 1.74 (95% CI 0.48 to 6.30, 1 study, 58 babies, random‐effects) (Analysis 5.8; Analysis 6.8), (low‐certainty evidence, downgraded for serious imprecision).
Hydrocephalus: one study involving 58 babies looked at this outcome and there were no babies in either group with hydrocephalus (Analysis 5.22; Analysis 6.22).
Neurodevelopmental impairment at age two to three years: (average RR 1.18, 95% CI 0.04 to 32.88, 2 studies, 174 infants, random‐effects model) (Analysis 5.28; Analysis 6.28), (very low‐certainty evidence, downgraded for unclear risk of bias and serious imprecision).
Cerebral palsy: one study involving 39 infants assessed this outcome and found no cases of cerebral palsy amongst the 39 infants (Analysis 5.30; Analysis 6.30).
5.8. Analysis.
Comparison 5 DCC with immediate neonatal care after cord clamping vs UCM (subgroup analysis by gestation), Outcome 8 Intraventricular haemorrhage (IVH, grades 1 & 2).
6.8. Analysis.
Comparison 6 DCC with immediate neonatal care after cord clamping vs UCM (subgroup analysis by type of intervention), Outcome 8 Intraventricular haemorrhage (IVH, grades 1 & 2).
5.22. Analysis.
Comparison 5 DCC with immediate neonatal care after cord clamping vs UCM (subgroup analysis by gestation), Outcome 22 Hydrocephalus.
6.22. Analysis.
Comparison 6 DCC with immediate neonatal care after cord clamping vs UCM (subgroup analysis by type of intervention), Outcome 22 Hydrocephalus.
5.28. Analysis.
Comparison 5 DCC with immediate neonatal care after cord clamping vs UCM (subgroup analysis by gestation), Outcome 28 Neurodevelopmental impairment at age two to three years.
6.28. Analysis.
Comparison 6 DCC with immediate neonatal care after cord clamping vs UCM (subgroup analysis by type of intervention), Outcome 28 Neurodevelopmental impairment at age two to three years.
5.30. Analysis.
Comparison 5 DCC with immediate neonatal care after cord clamping vs UCM (subgroup analysis by gestation), Outcome 30 Cerebral palsy (CP).
6.30. Analysis.
Comparison 6 DCC with immediate neonatal care after cord clamping vs UCM (subgroup analysis by type of intervention), Outcome 30 Cerebral palsy (CP).
Gastrointestinal
NEC confirmed by X‐ray or laparotomy): (RR 3.48 95% CI 0.41 to 29.31, 1 study, 58 babies) (Analysis 5.9; Analysis 6.9), (low‐certainty evidence, downgraded for serious imprecision).
5.9. Analysis.
Comparison 5 DCC with immediate neonatal care after cord clamping vs UCM (subgroup analysis by gestation), Outcome 9 Necrotising enterocolitis (NEC) confirmed by X‐ray or laparotomy).
Haematological
Blood transfusion in infant: (RR 0.77, 95% CI 0.48 to 1.22, 1 study, 58 babies) (Analysis 5.19; Analysis 6.19), (low‐certainty evidence, downgraded for serious imprecision).
Hb within first 24 hours of birth (in g/dL): (MD ‐0.20, 95% CI ‐1.57 to 1.17, 1 study, 58 babies) (Analysis 5.24; Analysis 6.24), (low‐certainty evidence, downgraded for serious imprecision).
5.19. Analysis.
Comparison 5 DCC with immediate neonatal care after cord clamping vs UCM (subgroup analysis by gestation), Outcome 19 Blood transfusion in infant.
6.19. Analysis.
Comparison 6 DCC with immediate neonatal care after cord clamping vs UCM (subgroup analysis by type of intervention), Outcome 19 Blood transfusion in infant.
5.24. Analysis.
Comparison 5 DCC with immediate neonatal care after cord clamping vs UCM (subgroup analysis by gestation), Outcome 24 Hb within 1st 24 hour of birth (g/dL).
6.24. Analysis.
Comparison 6 DCC with immediate neonatal care after cord clamping vs UCM (subgroup analysis by type of intervention), Outcome 24 Hb within 1st 24 hour of birth (g/dL).
There were no data on: volume of blood transfused; hyperbilirubinaemia (treated by phototherapy).
Other outcomes
Treatment for retinopathy of prematurity (RoP): (RR 0.73, 95% CI 0.23 to 2.35, 1 study, 67 babies) (Analysis 5.15; Analysis 6.15), (very low‐certainty evidence, downgraded for risk of bias and serious imprecision).
Late sepsis (after three days or as defined by trialists): (RR 0.87, 95% CI 0.06 to 13.27, 1 study, 58 babies) (Analysis 5.21; Analysis 6.21), (low‐certainty evidence, downgraded for serious imprecision).
Severe visual impairment: one study involving 39 babies assessed this outcome but found no cases of visual impairment amongst the 39 babies (Analysis 5.29; Analysis 6.29).
5.15. Analysis.
Comparison 5 DCC with immediate neonatal care after cord clamping vs UCM (subgroup analysis by gestation), Outcome 15 Treatment for Retinopathy of Prematurity (RoP).
6.15. Analysis.
Comparison 6 DCC with immediate neonatal care after cord clamping vs UCM (subgroup analysis by type of intervention), Outcome 15 Treatment for Retinopathy of Prematurity (RoP).
5.21. Analysis.
Comparison 5 DCC with immediate neonatal care after cord clamping vs UCM (subgroup analysis by gestation), Outcome 21 Late sepsis (after 3 days or as defined by trialists).
6.21. Analysis.
Comparison 6 DCC with immediate neonatal care after cord clamping vs UCM (subgroup analysis by type of intervention), Outcome 21 Late sepsis (after 3 days or as defined by trialists).
5.29. Analysis.
Comparison 5 DCC with immediate neonatal care after cord clamping vs UCM (subgroup analysis by gestation), Outcome 29 Severe visual impairment.
6.29. Analysis.
Comparison 6 DCC with immediate neonatal care after cord clamping vs UCM (subgroup analysis by type of intervention), Outcome 29 Severe visual impairment.
There were no data on: length of infant stay in NICU.
Other important outcomes for the mother
None of the included studies reported on any of our secondary outcomes for mothers.
Other important outcomes for the father
None of the included studies reported results for any of our secondary outcomes for fathers (psychological well‐being, bonding with the infant, anxiety, and fathers’ views).
Sensitivity analysis (Comparison 11)
We undertook sensitivity analyses on primary outcomes only with the one study assessed as low risk of bias for selection bias and incomplete outcome data (Rabe 2011). We found no real differences in the overall findings.
D. Umbilical cord milking (UCM) versus early cord clamping (ECC) (Comparison 7 ‐ subgroup analysis by gestation: Comparison 8 ‐ subgroup analysis by type of intervention)
We identified 13 studies addressing this question. Two studies provided no data (Das 2018; Pongmee 2010) leaving 11 studies involving 1183 babies and their mothers providing data for this comparison (Alan 2014; Elimian 2014; El‐Naggar 2016; Hosono 2008; Hosono 2015; Josephsen 2014; Katheria 2014; Kilicdag 2016; Kumar 2015; March 2013; Mercer 2016).
There was a range of countries involved in the studies which provided data; five studies were undertaken in the USA (Elimian 2014; Josephsen 2014; Katheria 2014; March 2013; Mercer 2016); two in Japan (Hosono 2008; Hosono 2015); two in Turkey (Alan 2014; Kilicdag 2016); one in Canada (El‐Naggar 2016); one in India (Kumar 2015) and one in Thailand (Pongmee 2010).
Most studies included babies being born at less than 32 to 34 weeks' gestation and in most studies the cord was milked whilst still intact.
There were insufficient data to assess if there were any differences in the subgroups, either by gestation or by type of intervention.
Main outcomes
1. Death of baby (up to discharge)
UCM compared with ECC may make little or no difference to the number of babies who died before discharge (average RR 0.81, 95% CI 0.47 to 1.41, 9 studies, 931 babies, random‐effects model) (Analysis 7.1; Analysis 8.1). Low‐certainty evidence, downgraded for serious imprecision (Table 4).
7.1. Analysis.
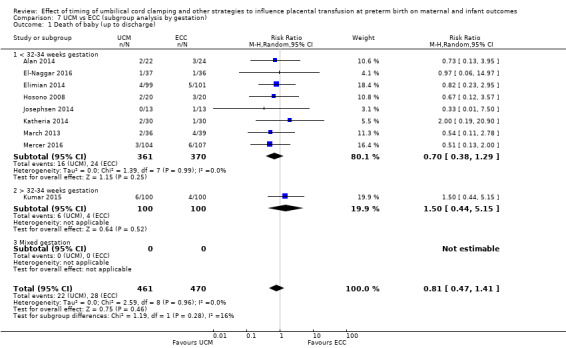
Comparison 7 UCM vs ECC (subgroup analysis by gestation), Outcome 1 Death of baby (up to discharge).
8.1. Analysis.
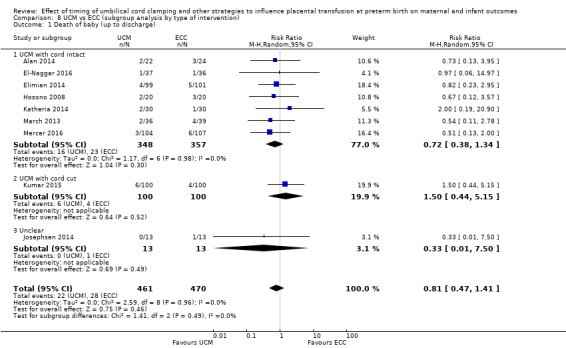
Comparison 8 UCM vs ECC (subgroup analysis by type of intervention), Outcome 1 Death of baby (up to discharge).
There was no evidence of overall heterogeneity (Tau2 = 0, Chi2 P = 0.96, I2 = 0%). (Analysis 7.1; Analysis 8.1).
2. Death or neurodevelopmental impairment at age two to three years
No studies assessed this composite outcome (Analysis 7.2; Analysis 8.2).
3. Severe intraventricular haemorrhage (IVH grades 3, 4)
UCM compared with ECC may make little or no difference to the incidence of severe IVH (average RR 0.75, 95% CI 0.39 to 1.45, 6 studies, 618 babies) (Analysis 7.3; Analysis 8.3). Low‐certainty evidence, downgraded for serious imprecision (Table 4).
7.3. Analysis.
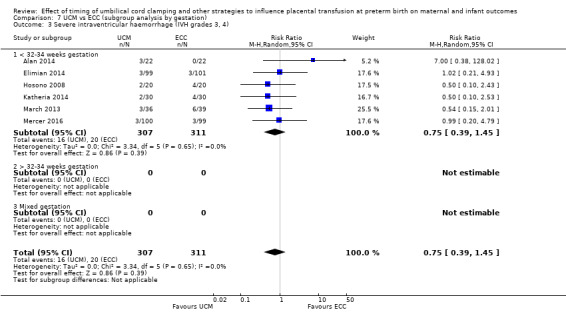
Comparison 7 UCM vs ECC (subgroup analysis by gestation), Outcome 3 Severe intraventricular haemorrhage (IVH grades 3, 4).
8.3. Analysis.
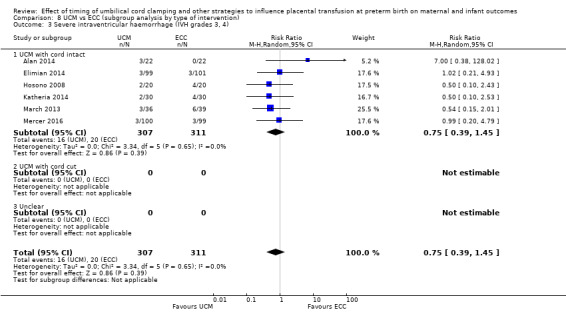
Comparison 8 UCM vs ECC (subgroup analysis by type of intervention), Outcome 3 Severe intraventricular haemorrhage (IVH grades 3, 4).
There was no evidence of overall heterogeneity (Tau2 = 0, Chi2 P = 0.65, I2 = 0%) (Analysis 7.3; Analysis 8.3).
4. Intraventricular haemorrhage (IVH, all grades)
UCM probably makes little of no difference to the incidence of all grades of IVH compared with ECC (average RR 0.85, 95% CI 0.62 to 1.18, 8 studies, 716 babies, random‐effects model) (Analysis 7.4; Analysis 8.4). Moderate‐certainty evidence, downgraded for imprecision (Table 4).
7.4. Analysis.
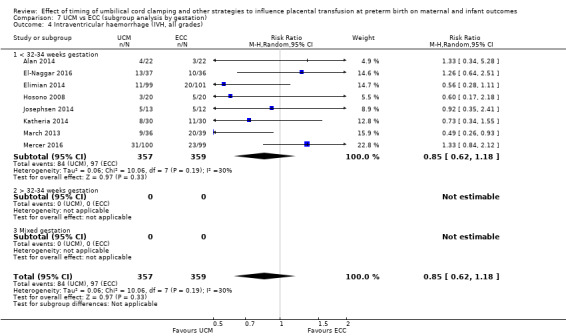
Comparison 7 UCM vs ECC (subgroup analysis by gestation), Outcome 4 Intraventricular haemorrhage (IVH, all grades).
8.4. Analysis.
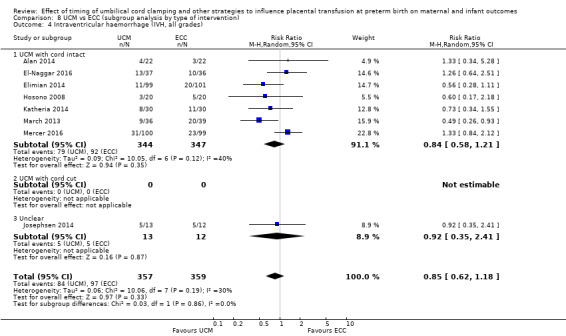
Comparison 8 UCM vs ECC (subgroup analysis by type of intervention), Outcome 4 Intraventricular haemorrhage (IVH, all grades).
There was some evidence of heterogeneity (Tau2 = 0.06, Chi2 P = 0.19, I2 = 30%) (Analysis 7.4; Analysis 8.4).
5. Periventricular leukomalacia (PVL)
UCM compared with ECC may make little or no difference to the incidence of PVL (average RR 0.63, 95% CI 0.15 to 2.63, 3 studies, 315 babies, random‐effects model) (Analysis 7.5; Analysis 8.5). Low‐certainty evidence, downgraded for serious imprecision (Table 4).
7.5. Analysis.
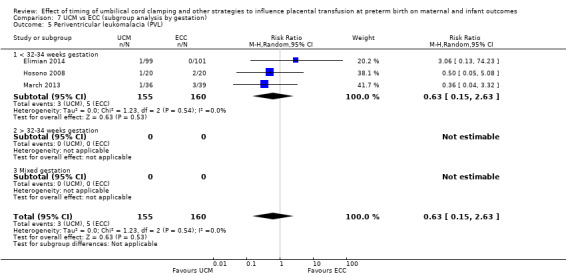
Comparison 7 UCM vs ECC (subgroup analysis by gestation), Outcome 5 Periventricular leukomalacia (PVL).
8.5. Analysis.
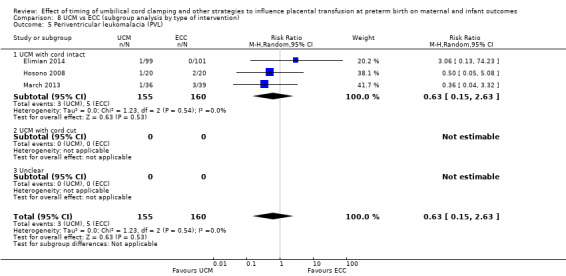
Comparison 8 UCM vs ECC (subgroup analysis by type of intervention), Outcome 5 Periventricular leukomalacia (PVL).
There was no evidence of overall heterogeneity (Tau2 = 0, Chi2 P = 54, I2 = 0%) (Analysis 7.5; Analysis 8.5).
6. Chronic lung disease (CLD) ‐ oxygen supplement at 36 weeks (corrected for gestation)
UCM compared with ECC may make little or no difference to the incidence of CLD (RR 1.03, 95% CI 0.64 to 1.66, 7 studies, 682 babies, random‐effects model) (Analysis 7.6; Analysis 8.6). Low‐certainty evidence, downgraded due to heterogeneity and imprecision (Table 4).
7.6. Analysis.
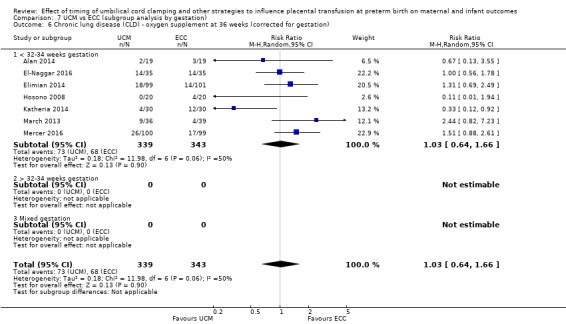
Comparison 7 UCM vs ECC (subgroup analysis by gestation), Outcome 6 Chronic lung disease (CLD) ‐ oxygen supplement at 36 weeks (corrected for gestation).
8.6. Analysis.
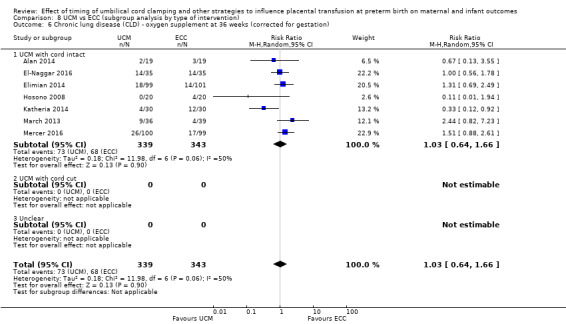
Comparison 8 UCM vs ECC (subgroup analysis by type of intervention), Outcome 6 Chronic lung disease (CLD) ‐ oxygen supplement at 36 weeks (corrected for gestation).
There was some evidence of heterogeneity (Tau2 = 0.18, Chi2 P = 0.06, I2 = 50%) (Analysis 7.6; Analysis 8.6).
7. Maternal blood loss of 500 mL or greater
We identified one study, with 200 women, relevant to this outcome. There were no women who had a blood loss of 500 mL or greater (Analysis 7.7; Analysis 8.7). Low‐certainty evidence, downgraded for serious imprecision (Table 4).
7.7. Analysis.
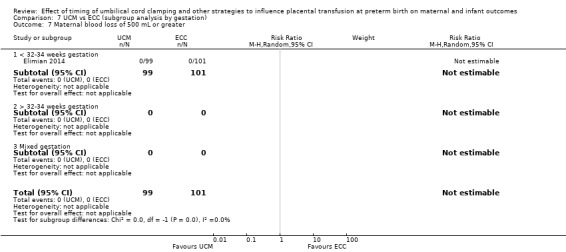
Comparison 7 UCM vs ECC (subgroup analysis by gestation), Outcome 7 Maternal blood loss of 500 mL or greater.
8.7. Analysis.
Comparison 8 UCM vs ECC (subgroup analysis by type of intervention), Outcome 7 Maternal blood loss of 500 mL or greater.
Other important outcomes for the baby
Condition at birth
There was no evidence of a difference for:
low Apgar as defined by trialists (generally < eight at five minutes): (average RR 1.03, 95% CI 0.67 to 1.60, 2 studies, 398 babies, random‐effects model) (Analysis 7.18; Analysis 8.18), (low‐certainty evidence, downgraded for serious imprecision).
7.18. Analysis.
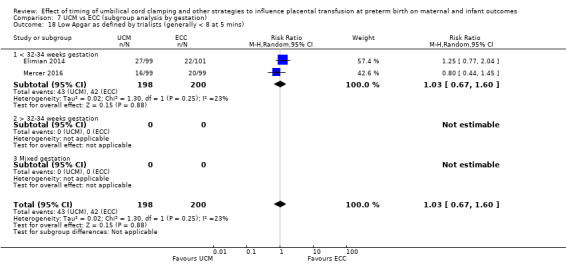
Comparison 7 UCM vs ECC (subgroup analysis by gestation), Outcome 18 Low Apgar as defined by trialists (generally < 8 at 5 mins).
8.18. Analysis.
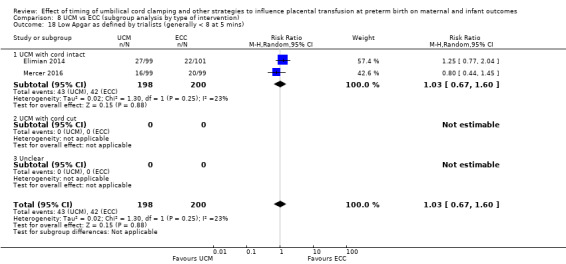
Comparison 8 UCM vs ECC (subgroup analysis by type of intervention), Outcome 18 Low Apgar as defined by trialists (generally < 8 at 5 mins).
No studies assessed temperature < 360C at 24 hours.
Respiratory
We found no evidence of a difference in the following respiratory outcomes.
RDS: (average RR 1.05, 95% CI 0.83 to 1.32, 4 studies, 515 babies, random‐effects model). There is some evidence of heterogeneity (Tau2 = 0.03, Chi2 P = 0.10, I2 = 53%) (Analysis 7.10; Analysis 8.10), (low‐certainty evidence, downgraded for heterogeneity and imprecision).
Respiratory support (ventilator or CPAP): (average RR 1.04, 95% CI 0.74 to 1.47, 2 studies, 129 babies, random‐effects model). There is some evidence of heterogeneity (Tau2 = 0.04, Chi2 P = 0.14, I2 = 54%) (Analysis 7.11; Analysis 8.11), (moderate‐certainty evidence, downgraded for heterogeneity and imprecision).
Duration of respiratory support (in days): (MD 2.80, 95% CI ‐9.78 to 15.38, 1 study, 199 babies) (Analysis 7.12; Analysis 8.12), (very low‐certainty evidence, downgraded for risk of bias and serious imprecision).
Surfactant treatment (for severe RDS): (average RR 1.13, 95% CI 0.81 to 1.58, 5 studies, 433 babies, random‐effects model). There is some evidence of heterogeneity (Tau2 = 0.10, Chi2 P = 0.0004, I2 = 81%) (Analysis 7.13; Analysis 8.13), (low‐certainty evidence, downgraded for heterogeneity and imprecision).
Home oxygen: (RR 0.89, 95% CI 0.38 to 2.10, 1 study, 199 babies) (Analysis 7.27; Analysis 8.27), (very low‐certainty evidence, downgraded for risk of bias and serious imprecision).
7.10. Analysis.
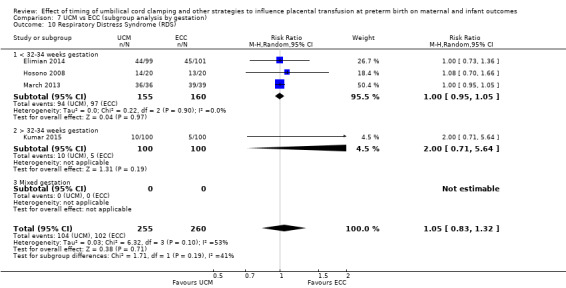
Comparison 7 UCM vs ECC (subgroup analysis by gestation), Outcome 10 Respiratory Distress Syndrome (RDS).
8.10. Analysis.
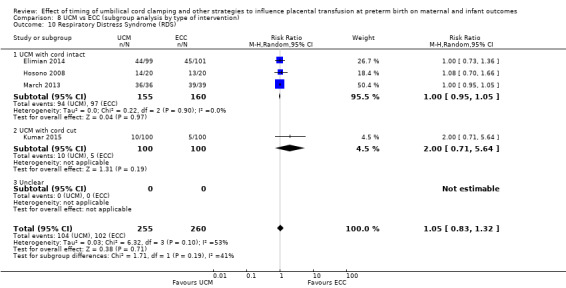
Comparison 8 UCM vs ECC (subgroup analysis by type of intervention), Outcome 10 Respiratory Distress Syndrome (RDS).
7.11. Analysis.
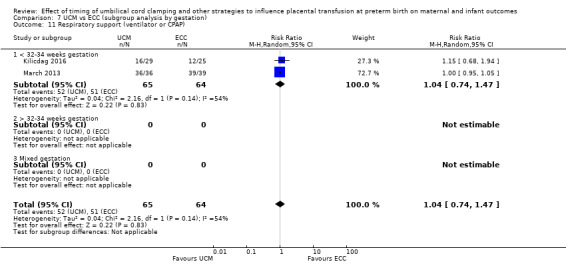
Comparison 7 UCM vs ECC (subgroup analysis by gestation), Outcome 11 Respiratory support (ventilator or CPAP).
8.11. Analysis.
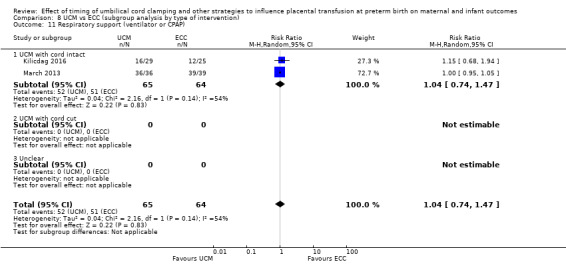
Comparison 8 UCM vs ECC (subgroup analysis by type of intervention), Outcome 11 Respiratory support (ventilator or CPAP).
7.12. Analysis.
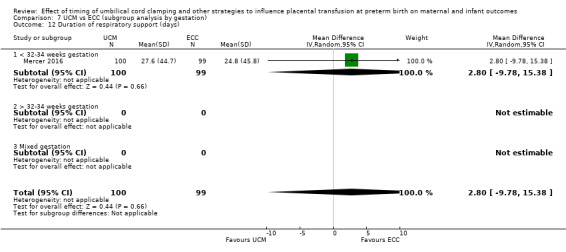
Comparison 7 UCM vs ECC (subgroup analysis by gestation), Outcome 12 Duration of respiratory support (days).
8.12. Analysis.
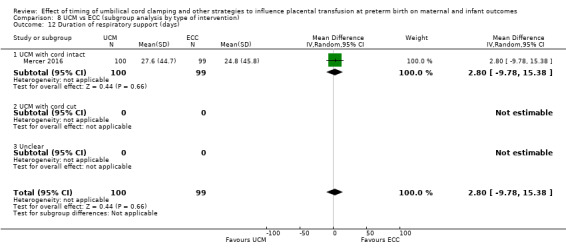
Comparison 8 UCM vs ECC (subgroup analysis by type of intervention), Outcome 12 Duration of respiratory support (days).
7.13. Analysis.
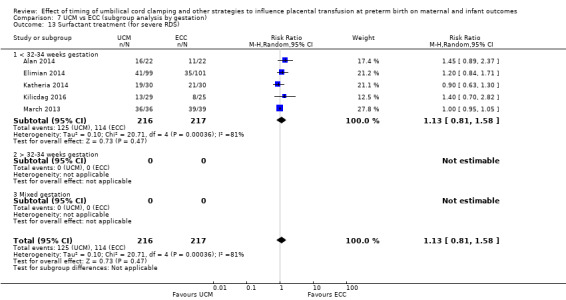
Comparison 7 UCM vs ECC (subgroup analysis by gestation), Outcome 13 Surfactant treatment (for severe RDS).
8.13. Analysis.
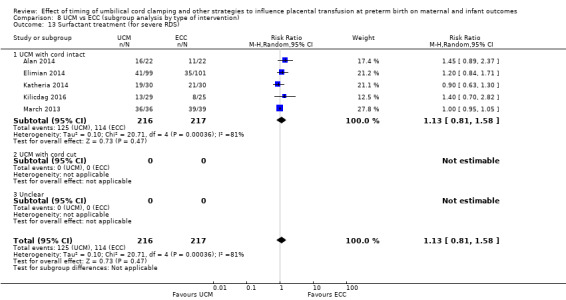
Comparison 8 UCM vs ECC (subgroup analysis by type of intervention), Outcome 13 Surfactant treatment (for severe RDS).
7.27. Analysis.
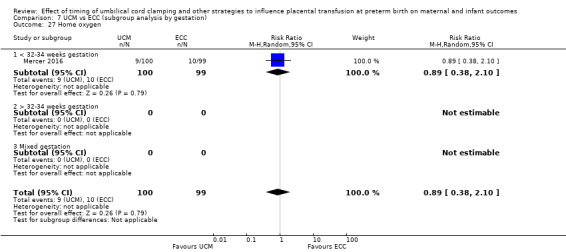
Comparison 7 UCM vs ECC (subgroup analysis by gestation), Outcome 27 Home oxygen.
8.27. Analysis.
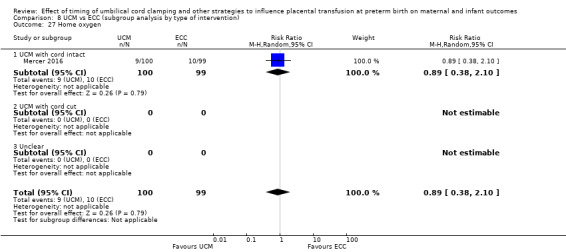
Comparison 8 UCM vs ECC (subgroup analysis by type of intervention), Outcome 27 Home oxygen.
Cardiovascular
We found no evidence of a difference for the following cardiovascular outcomes.
Treatment for patent ductus arteriosus (PDA) (medical and/or surgical): (average RR 1.00, 95% CI 0.73 to 1.38, 5 studies, 411 babies) and there was no evidence of heterogeneity (Analysis 7.14; Analysis 8.14), (moderate‐certainty evidence, downgraded for imprecision).
Inotropics for low blood pressure in first 24 hours: (average RR 0.61, 95% CI 0.36 to 1.04, 3 studies, 300 babies, random‐effects model). There is some evidence of heterogeneity (Tau2 = 0.09, Chi2 P = 0.19, I2 = 41%) (Analysis 7.17; Analysis 8.17), (very low‐certainty evidence, downgraded for heterogeneity and serious imprecision).
Mean arterial blood pressure: (average MD 0.38, 95% CI ‐1.33 to 2.09, 2 studies, 408 babies, random‐effects model), and there was no evidence of heterogeneity (Analysis 7.25; Analysis 8.25), (low‐certainty evidence, downgraded for risk of bias and imprecision).
7.14. Analysis.
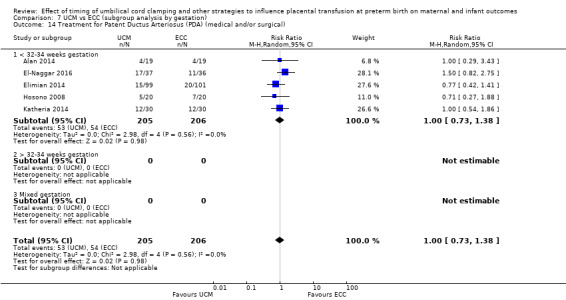
Comparison 7 UCM vs ECC (subgroup analysis by gestation), Outcome 14 Treatment for Patent Ductus Arteriosus (PDA) (medical and/or surgical).
8.14. Analysis.
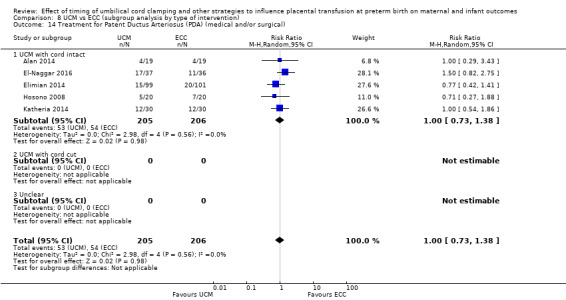
Comparison 8 UCM vs ECC (subgroup analysis by type of intervention), Outcome 14 Treatment for Patent Ductus Arteriosus (PDA) (medical and/or surgical).
7.17. Analysis.
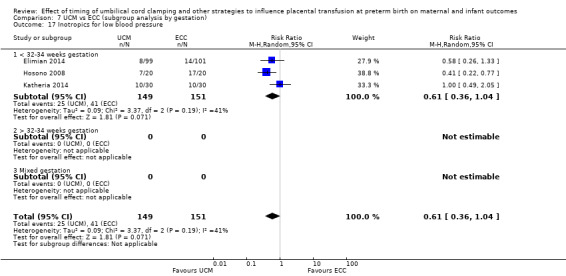
Comparison 7 UCM vs ECC (subgroup analysis by gestation), Outcome 17 Inotropics for low blood pressure.
8.17. Analysis.
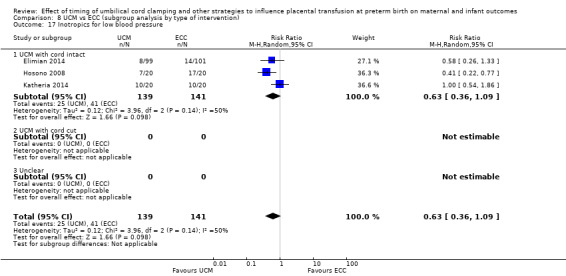
Comparison 8 UCM vs ECC (subgroup analysis by type of intervention), Outcome 17 Inotropics for low blood pressure.
7.25. Analysis.
Comparison 7 UCM vs ECC (subgroup analysis by gestation), Outcome 25 Mean arterial blood pressure.
8.25. Analysis.
Comparison 8 UCM vs ECC (subgroup analysis by type of intervention), Outcome 25 Mean arterial blood pressure (subgrouped by time after birth).
Central nervous system
We found no evidence of a difference for the following central nervous system outcomes.
IVH (grades one and two): (average RR 0.74, 95% CI 0.44 to 1.25, 6 studies, 618 babies, random‐effects model). There is some evidence of heterogeneity (Tau2 = 0.15, Chi2 P = 0.14, I2 = 40%) (Analysis 7.8; Analysis 8.8), (low‐certainty evidence, downgraded for heterogeneity and imprecision).
Neurodevelopmental impairment at age two to three years: (average RR 1.25, 95% CI 0.49 to 3.17, 2 studies, 187 infants). Heterogeneity was unclear (Tau2 = 0.06, Chi2 P = 0.28, I2 = 14%) (Analysis 7.28; Analysis 8.28), (very low certainty evidence, downgraded for risk of bias and serious imprecision).
Cerebral palsy: (average RR 0.70, 95% CI 0.05 to 10.63, 2 studies, 286 infants, random‐effects model). There is clear evidence of heterogeneity (Tau2 = 3.43, Chi2 P = 0.003, I2 = 89%) (Analysis 7.30; Analysis 8.30), (very low‐certainty evidence, downgraded for risk of bias, heterogeneity and serious imprecision).
7.8. Analysis.
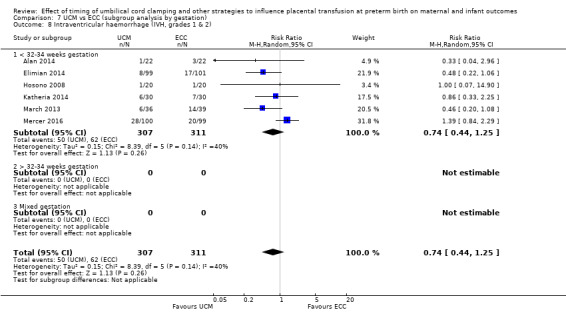
Comparison 7 UCM vs ECC (subgroup analysis by gestation), Outcome 8 Intraventricular haemorrhage (IVH, grades 1 & 2).
8.8. Analysis.
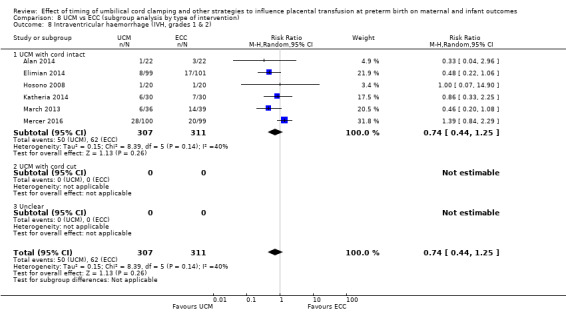
Comparison 8 UCM vs ECC (subgroup analysis by type of intervention), Outcome 8 Intraventricular haemorrhage (IVH, grades 1 & 2).
7.28. Analysis.
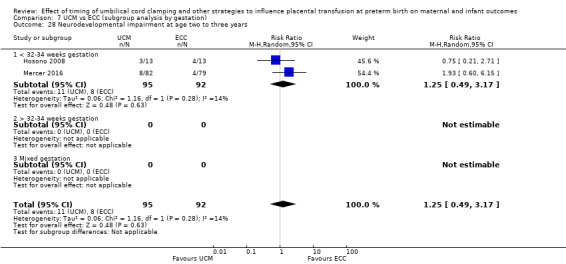
Comparison 7 UCM vs ECC (subgroup analysis by gestation), Outcome 28 Neurodevelopmental impairment at age two to three years.
8.28. Analysis.
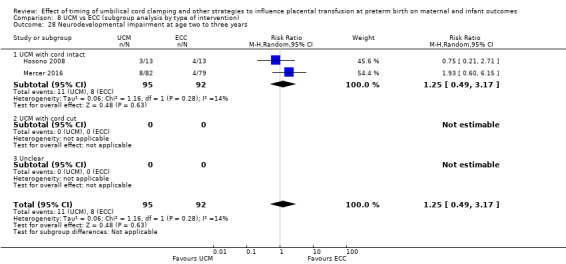
Comparison 8 UCM vs ECC (subgroup analysis by type of intervention), Outcome 28 Neurodevelopmental impairment at age two to three years.
7.30. Analysis.
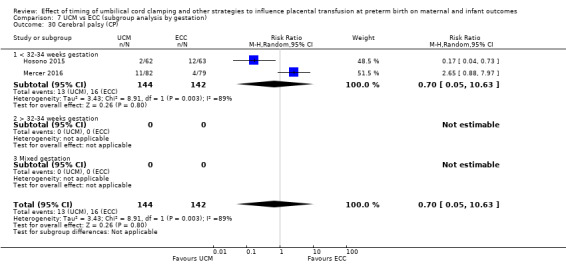
Comparison 7 UCM vs ECC (subgroup analysis by gestation), Outcome 30 Cerebral palsy (CP).
8.30. Analysis.
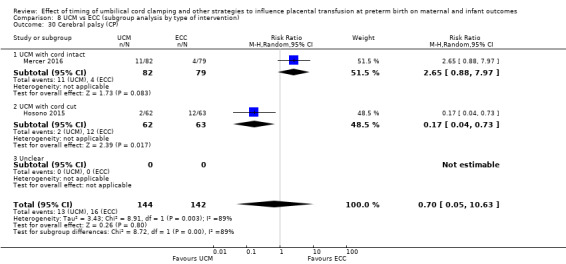
Comparison 8 UCM vs ECC (subgroup analysis by type of intervention), Outcome 30 Cerebral palsy (CP).
There were no data on hydrocephalus.
Gastrointestinal
We found no evidence of a difference for the following.
NEC confirmed by X‐ray or laparotomy: (average RR 0.75, 95% CI 0.41 to 1.38, 6 studies, 616 babies, random‐effects model) (low‐certainty evidence, downgraded for serious imprecision), (Analysis 7.9; Analysis 8.9).
7.9. Analysis.
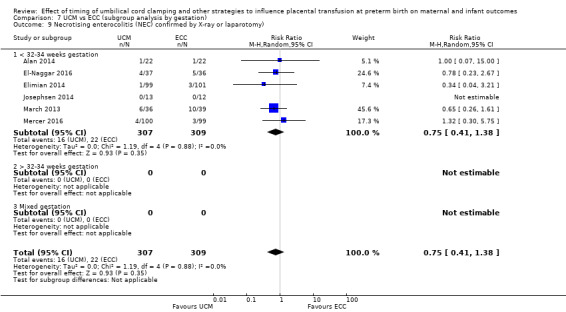
Comparison 7 UCM vs ECC (subgroup analysis by gestation), Outcome 9 Necrotising enterocolitis (NEC) confirmed by X‐ray or laparotomy).
8.9. Analysis.
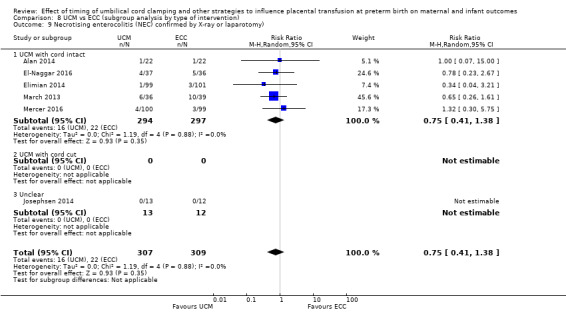
Comparison 8 UCM vs ECC (subgroup analysis by type of intervention), Outcome 9 Necrotising enterocolitis (NEC) confirmed by X‐ray or laparotomy).
Haematological
We found a possible benefit for UCM over ECC for:
blood transfusion in infant: (average RR 0.71, 95% CI 0.57 to 0.89, 6 studies, 567 babies, random‐effects model). There is some evidence of heterogeneity (Tau2 = 0.03, Chi2 P = 0.13, I2 = 41) (Analysis 7.19; Analysis 8.19), (very low‐certainty evidence, downgraded for risk of bias, heterogeneity and imprecision).
7.19. Analysis.
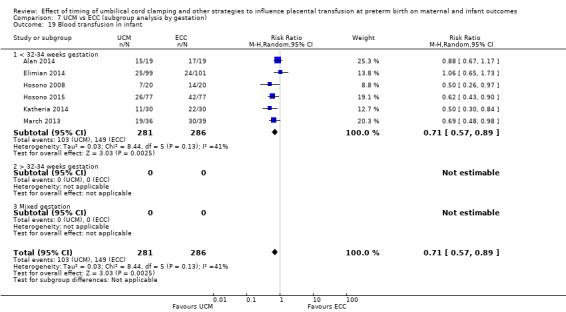
Comparison 7 UCM vs ECC (subgroup analysis by gestation), Outcome 19 Blood transfusion in infant.
8.19. Analysis.
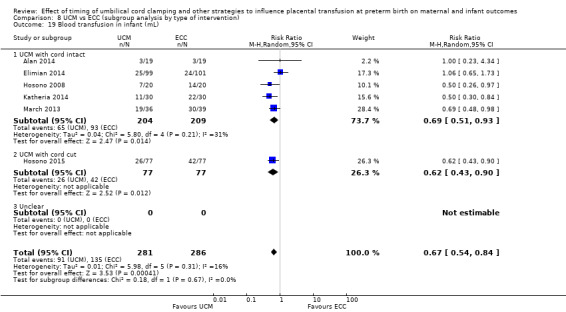
Comparison 8 UCM vs ECC (subgroup analysis by type of intervention), Outcome 19 Blood transfusion in infant (mL).
We found no evidence for a difference for the following haematological outcomes.
Hyperbilirubinaemia (treated by phototherapy): (average RR 1.39, 95% CI 0.73 to 2.63, 3 studies, 475 babies, random‐effects model). There is clear evidence of heterogeneity (Tau2 = 0.28, Chi2 P < 0.00001, I2 = 94) (Analysis 7.16; Analysis 8.16), (very low‐certainty evidence, downgraded for high heterogeneity and imprecision).
Volume of blood transfused (in mL): (MD ‐19.00, 95% CI ‐39.61 to 1.61, 1 study, 199 babies), (very low‐certainty evidence, downgraded for risk of bias and serious imprecision) (Analysis 7.20; Analysis 8.20).
Hb within first 24 hours of birth (in g/dL): (average MD 0.84, 95% CI 0.54 to 1.14, 7 studies, 905 babies, random‐effects model). There was no evidence of heterogeneity (Analysis 7.24; Analysis 8.24), (moderate‐certainty evidence, downgraded for risk of bias).
7.16. Analysis.
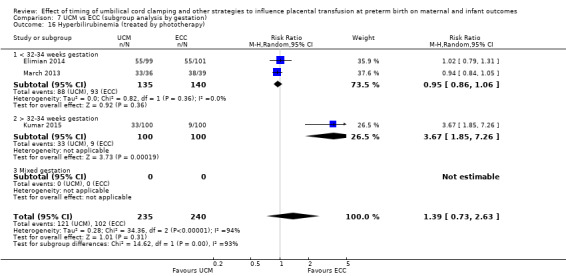
Comparison 7 UCM vs ECC (subgroup analysis by gestation), Outcome 16 Hyperbilirubinemia (treated by phototherapy).
8.16. Analysis.
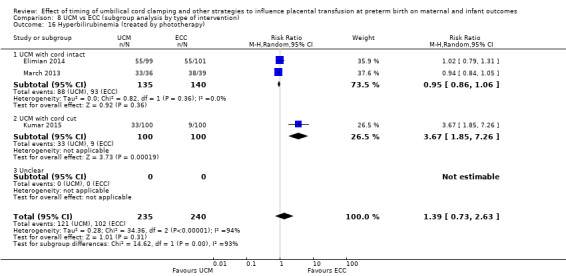
Comparison 8 UCM vs ECC (subgroup analysis by type of intervention), Outcome 16 Hyperbilirubinemia (treated by phototherapy).
7.20. Analysis.
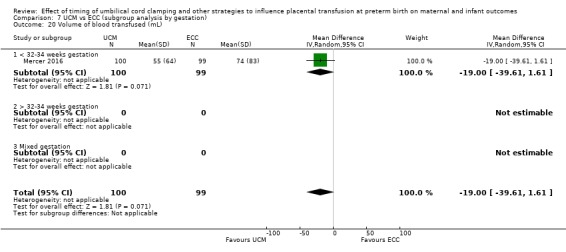
Comparison 7 UCM vs ECC (subgroup analysis by gestation), Outcome 20 Volume of blood transfused (mL).
8.20. Analysis.
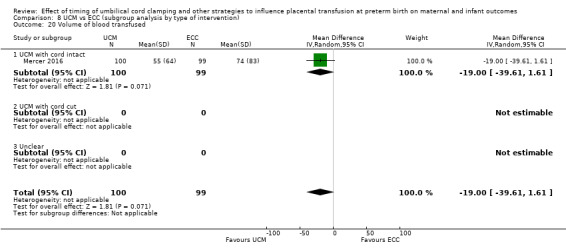
Comparison 8 UCM vs ECC (subgroup analysis by type of intervention), Outcome 20 Volume of blood transfused.
7.24. Analysis.
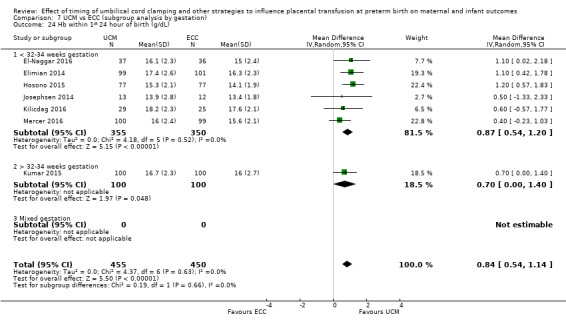
Comparison 7 UCM vs ECC (subgroup analysis by gestation), Outcome 24 Hb within 1st 24 hour of birth (g/dL).
8.24. Analysis.
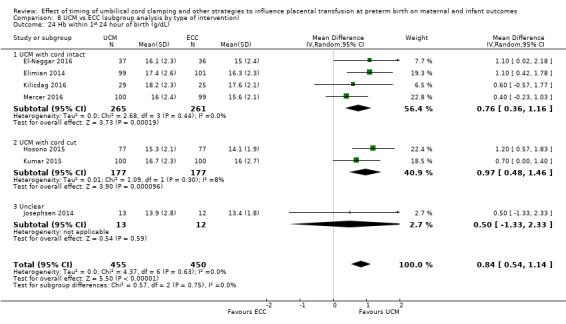
Comparison 8 UCM vs ECC (subgroup analysis by type of intervention), Outcome 24 Hb within 1st 24 hour of birth (g/dL).
Other outcomes
We found no evidence of a difference for the following.
Treatment for retinopathy of prematurity (RoP): (average RR 0.95, 95% CI 0.76 to 1.19, 5 studies, 274 babies, random‐effects model). There was no evidence of heterogeneity (Analysis 7.15; Analysis 8.15), (low‐certainty evidence, downgraded for serious imprecision).
Late sepsis (after three days or as defined by trialists): (average RR 0.87, 95% CI 0.64 to 1.19, 4 studies, 385 babies, random‐effects model). There was no evidence of heterogeneity (Analysis 7.21; Analysis 8.21), (very low‐certainty evidence, downgraded for risk of bias and serious imprecision).
Length of infant stay in NICU (in weeks): (MD 5.30, 95% CI ‐5.49 to 16.09, 1 study, 199 babies), (Analysis 7.26; Analysis 8.26), (low‐certainty evidence, downgraded for risk of bias and imprecision).
Severe visual impairment: we found one study involving 125 infants and there were no infants with severe visual impairment (Analysis 7.29; Analysis 8.29).
7.15. Analysis.
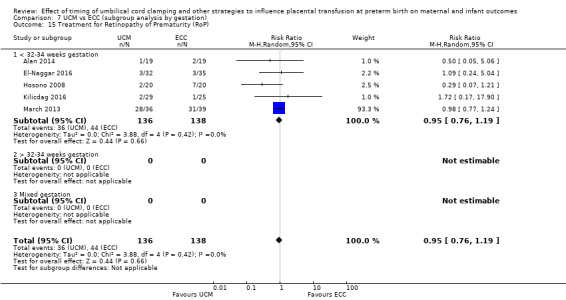
Comparison 7 UCM vs ECC (subgroup analysis by gestation), Outcome 15 Treatment for Retinopathy of Prematurity (RoP).
8.15. Analysis.
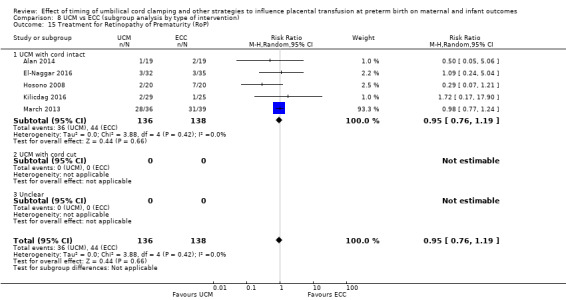
Comparison 8 UCM vs ECC (subgroup analysis by type of intervention), Outcome 15 Treatment for Retinopathy of Prematurity (RoP).
7.21. Analysis.
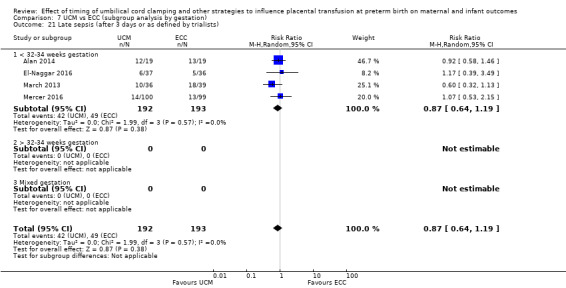
Comparison 7 UCM vs ECC (subgroup analysis by gestation), Outcome 21 Late sepsis (after 3 days or as defined by trialists).
8.21. Analysis.
Comparison 8 UCM vs ECC (subgroup analysis by type of intervention), Outcome 21 Late sepsis (after 3 days or as defined by trialists).
7.26. Analysis.
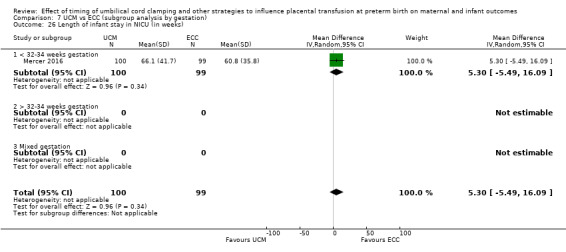
Comparison 7 UCM vs ECC (subgroup analysis by gestation), Outcome 26 Length of infant stay in NICU (in weeks).
8.26. Analysis.
Comparison 8 UCM vs ECC (subgroup analysis by type of intervention), Outcome 26 Length of infant stay in NICU.
7.29. Analysis.
Comparison 7 UCM vs ECC (subgroup analysis by gestation), Outcome 29 Severe visual impairment.
8.29. Analysis.
Comparison 8 UCM vs ECC (subgroup analysis by type of intervention), Outcome 29 Severe visual impairment.
Other important outcomes for the mother
None of the included studies reported on any of the secondary outcomes for mothers.
Other important outcomes for the father
None of the included studies reported results for any of our secondary outcomes for fathers.
Sensitivity analysis (Comparison 12)
We undertook sensitivity analyses on primary outcomes only with the four studies assessed as low risk of bias for selection bias and incomplete outcome data (Elimian 2014; El‐Naggar 2016; Katheria 2014; Kumar 2015). We found no real differences in the overall findings.
Discussion
Summary of main results
This updated review now includes 48 studies, with data from 40 studies involving 4884 babies and their mothers. Studies were conducted mostly in high‐income countries but with some from low‐ and middle‐income countries. Nevertheless, for almost all the studies there appeared to be access to a neonatal intensive care unit (although this was not always specified in the trial reports). Largely, the babies in this review were born before 32 to 34 weeks' gestation. Multiple births were included in some of the studies. Few studies report outcomes for the mother.
For this update, we have separated the different interventions for influencing umbilical flow and placental transfusion, as the impact on the physiology of neonatal transition and placental transfusion may be different (Blank 2018; Hooper 2017; Manley 2017). Studies evaluating alternative policies for timing of cord clamping and those evaluating umbilical cord milking are separated, as are those where immediate neonatal care, if required, is given with the cord intact during delayed clamping. For delayed clamping, the timing of clamping has often been determined by the balance between allowing some delay and the imperative to cut the cord to allow transfer of the baby for neonatal care. When immediate neonatal care is available with the cord intact, however, babies requiring resuscitation at birth can be recruited. These babies at high risk are often excluded from trials where neonatal care is after cord clamping, or if they are recruited they do not receive the intervention.
Delayed cord clamping with immediate neonatal care after clamping versus early cord clamping
This comparison now includes data from 25 studies involving 3100 babies and their mothers. In these studies, delayed clamping was between 30 seconds and three minutes, with some studies waiting until pulsation ceased. Below 32 weeks' gestation, clamping was largely between 30 seconds and 60 seconds, however. The trials where they waited until pulsation ceased also included term babies, and gestation of the preterm births is unclear. Early clamping in these studies was before 30 seconds and most studies specified immediate or within 10 seconds. The largest trial contributing over half the data in this comparison was a well‐conducted study co‐ordinated from Australia involving 25 centres across seven countries, and including 1634 babies and their mothers (Tarnow‐Mordi 2017). This study recruited babies born before 30 weeks' gestation.
Delayed cord clamping with immediate neonatal care after clamping, probably reduces the relative risk of a baby dying before discharge by 27% (95% confidence interval (CI) 2% to 46%) and a probable 2% reduction in absolute risk (95% CI 1% reduction to 3% reduction) compared with early clamping (moderate‐certainty evidence. No studies reported death or neurosensory disability in early childhood. There were insufficient data for reliable conclusions about comparative effects on severe intraventricular haemorrhage (IVH) (grades 3‐4) (low‐certainty evidence). However, delayed clamping was associated with a 17% reduction in the relative risk of any IVH (grades 1‐4) (95% CI 1% reduction to 30% reduction) and a 3% reduction in absolute risk (95% CI 1% reduction to 7% reduction) (high‐certainty evidence). Delayed cord clamping has little or no effect on chronic lung disease (CLD) (high‐certainty evidence). There was insufficient evidence for reliable conclusions about the comparative effects on periventricular leukomalacia (PVL) and maternal blood loss of 500 mL or greater.
For our secondary outcomes, delayed cord clamping with immediate neonatal care after clamping was associated with a 28% reduction in the relative risk of IVH (grade 1‐2) (95% CI 2% increase to 49% reduction). The relative risk of having inotropes as treatment for low blood pressure was also lower when cord clamping was delayed rather than early, although few trials reported this outcome. The relative risk of having a blood transfusion was reduced by 34% (95% CI 14% reduction to 50% reduction; absolute risk 9% reduction, 95% CI 3% to 16% reduction). Mean arterial blood pressure shortly after birth was higher for babies allocated delayed clamping, although data are only reported for 208 babies. We found insufficient evidence to be able to draw meaningful conclusions on the other secondary outcome measures we assessed.
Delayed cord clamping with immediate neonatal care, if needed, with cord intact versus early cord clamping
This comparison included one study that provided data involving 276 babies and their mothers. The study was conducted at eight centres in the UK. Recruitment was of women expected to give birth before 32 weeks' gestation.
We found insufficient data for identifying possible differences between the allocated groups for any of the primary outcomes of this review, namely: baby death before discharge; death or neurodevelopmental impairment in the early years; severe IVH (grade 3‐4); any IVH (grade 1‐4); PVL; CLD or maternal blood loss of 500 mL or greater. However, this study was a feasibility study and so not powered to assess effectiveness. For baby deaths before discharge, the point estimate for the relative risk favours delayed cord clamping with immediate neonatal care, if needed, with cord intact, being a 53% reduction with 95% CI ranging from an 11% increase to an 80% reduction. Promising evidence, but requiring confirmation in larger trials. For secondary outcomes, we again found insufficient data for reliable conclusions.
Delayed cord clamping with immediate neonatal care after clamping versus umbilical cord milking
This comparison included three studies (undertaken in the USA and UK) involving 322 babies. We found no meaningful conclusions could be drawn on possible differences between the allocated groups for any of the primary outcomes in this review namely: baby death before discharge; death or neurodevelopmental impairment in the early years; severe IVH (grade 3‐4); any IVH (grade 1‐4); PVL; CLD or maternal blood loss of 500 mL or greater. However, for baby deaths before discharge, the point estimate for the relative risk favours umbilical cord milking, being a two‐fold increase in death for DCC with 95% CI a 7% reduction to a nearly five‐fold increase but of low‐certainty evidence, requiring confirmation in larger trials. All babies were born before 32 to 34 weeks' gestation. There are insufficient data for reliable conclusions about any secondary outcomes.
Umbilical cord milking versus early cord clamping
This comparison included 11 studies providing data involving 1183 babies. The studies were undertaken in a range of countries, and all but one recruited babies born before 32 weeks' gestation. We found insufficient evidence to draw meaningful conclusions between the allocated groups for any of the primary outcomes in this review namely: baby death before discharge; death or neurodevelopmental impairment in the early years; severe IVH (grade 3‐4); any IVH (grade 1‐4); PVL; CLD or maternal blood loss of 500 mL or greater. There may possibly be fewer babies having blood transfusions but this is very low‐certainty evidence, other secondary outcomes did not identify any other differences.
Overall
There is some consistency in our findings in that early cord clamping, whether compared against delayed cord clamping with immediate neonatal care after cord clamping or against delayed cord clamping with immediate neonatal care with cord intact, or against umbilical cord milking, appears to lead to more baby deaths. Although more data are needed, early clamping appears overall to be harmful.
Further studies are needed to find the optimum care for preterm neonates.
Overall completeness and applicability of evidence
There are many gaps in the data for this review. This is due to a variety of factors. For example, many trials reported laboratory data rather than clinical outcomes; not all report the same outcomes or measured the same outcome in different ways; a number of studies excluded babies who died after randomisation, both neonatal deaths and babies stillborn who were alive at randomisation; one study reported outcomes for caesarean births, despite recruiting caesarean and vaginal births (Katheria 2015), and another only reported outcomes for babies admitted to neonatal intensive care unit (Armanian 2017). Also, there are few long‐term follow‐up data on outcomes in early childhood, not enough for meaningful conclusions. This should improve as the large multicentre Australian trial is conducting follow‐up (Tarnow‐Mordi 2017).
Many trials in this review explicitly excluded babies requiring immediate resuscitation at birth. If babies allocated delayed clamping were considered to need immediate resuscitation at birth, often they did not receive the intervention and sometimes were excluded from the analysis of outcome. Only one trial provided data from a more generalisable population of babies, as immediate neonatal care, if needed, was provided with the cord intact (CORD Pilot 2018). Hence, results from this review should be applied with caution to babies requiring resuscitation. Guidelines for newborn life support often recommend early cord clamping if resuscitation is required (Wyllie 2015). Recently, a delay of 30 to 45 seconds, or umbilical cord milking have been proposed as an alternative (Sweet 2017).
One difficulty in understanding applicability of the evidence for clinical practice is that there is little consistency in the interventions. For example, the studies use different definitions for delayed cord clamping, ranging from 30 seconds to three minutes (and a few were until pulsation ceases). The outcome may differ for different interventions, and for the same intervention at different gestational ages. Similarly, there is variation in the definition of umbilical cord milking.
Although there is insufficient evidence as to whether providing immediate neonatal care beside the mother with the cord intact impacts on outcomes, evidence from qualitative interviews with parents suggest they appreciate neonatal care being provided beside the mother (Sawyer 2015). Providing neonatal care beside the mother, irrespective of whether with cord intact, appears to be acceptable to clinicians (Thomas 2014; Yoxall 2015), but requires training and a multidisciplinary approach (Batey 2017). Further research should assess the benefits and adverse effects of neonatal care beside the mother, irrespective of whether care is provided with cord intact.
As the evidence in this review comes primarily from high‐income countries, and appears to be all from settings with access to neonatal intensive care, the results may not be generalisable to settings without such access, particularly in low‐income countries. Delaying cord clamping may be associated with greater benefit in settings without access to expert neonatal care in the delivery room or a neonatal intensive care unit after birth. Further research in such settings should therefore be a priority.
There are insufficient data for reliable conclusions about the comparative effects of umbilical cord milking compared with any policy for timing of cord clamping. However, as the evidence is now suggesting benefit for delayed cord clamping, the priority is to compare cord milking with delayed clamping.
In order to determine the optimal policy for influencing umbilical cord flow and placental transfusion at preterm birth, we need to understand more about the underlying physiology. Work with lambs has suggested that a more physiological approach based on the onset of respiration may be preferable to any arbitrary fixed timing for cord clamping (Bhatt 2013; Kluckow 2015; Te Pas 2018), and that umbilical cord milking causes haemodynamic disturbance and does not provide an increase in placental transfusion (Blank 2018).
Quality of the evidence
Most of the studies were small and only reported a few clinical outcomes. No studies could blind the clinicians to the allocated intervention, as this was unrealistic because the nature of the intervention of supporting placental transition for the preterm baby, and only a few studies reported if outcome assessment was blind to the allocation. For baby death, blinding of the allocation is probably not critical, unless babies considered to be stillborn were excluded as potential for bias would be if knowledge of the allocation influenced the decision as to whether the baby had shown any signs of life at birth.
Overall, the risk of bias for trials in this review was unclear for selection bias (sequence generation and allocation concealment) and attrition bias (incomplete outcome data), with only 12 of the 48 studies being assessed as low risk of bias for these parameters (see Figure 2; Figure 3) (Backes 2016; CORD Pilot 2018; Dipak 2017; Elimian 2014; El‐Naggar 2016; Katheria 2014; Kumar 2015; Mercer 2006; Rabe 2000; Rabe 2011; Salae 2016; Tarnow‐Mordi 2017). However, the large trials were at lower risk of bias.
We used GRADEPro software to assess the certainty, or quality, of the evidence (CoE or QoE) for each primary and secondary outcome, but reported 'Summary of findings' tables for the main four comparisons for the seven primary outcomes.
For delayed cord clamping with immediate neonatal care after cord clamping versus early cord clamping, we assessed death as moderate‐certainty evidence; severe IVH and PVL as low‐certainty evidence; any IVH and CLD as high‐certainty evidence and maternal haemorrhage as very low‐certainty evidence (Table 1). Reasons for downgrading included limitations in study design and imprecision.
For delayed cord clamping and immediate neonatal care with cord intact compared with early cord clamping, we assessed all the primary outcomes as low‐certainty evidence (Table 2). Reasons for downgrading included mainly limitations in terms of imprecision ‐ the evidence was from a single small study with few events and wide CIs crossing the line of no effect.
For delayed cord clamping versus umbilical cord milking, we assessed death, severe IVH and CLD as low‐certainty evidence; death or neurodevelopmental impairment in early years and all IVH as very low‐certainty evidence (Table 3). PVL was not estimable, but assessed as low certainty, and there were no data on maternal haemorrhage. Reasons for downgrading included limitations in study design and imprecision.
For umbilical cord milking versus early cord clamping, we assessed death, severe IVH, PVL, CLD and maternal blood loss as low‐certainty evidence and all IVH at moderate‐certainty evidence (Table 4). There were no data on death or neurodevelopmental impairment in early years nor on maternal haemorrhage. Reasons for downgrading included limitations in study design, inconsistency and imprecision.
Potential biases in the review process
We are aware of potential biases in the reviewing process; and we took some steps to minimise bias (such as data extraction which was carried out by two review authors independently). We also acknowledge that we would have been better to have published an updated protocol prior to undertaking this update. Nevertheless, changes to the protocol were agreed by the review team prior to commencing the update. Three of the review authors (HR; GG; LD) were also trial authors on three of the included studies (CORD Pilot 2018; Rabe 2000; Rabe 2011). HR and LD did not assess or extract data on their own studies and GG did not assess or extract data on the study on which she was a co‐applicant.
Agreements and disagreements with other studies or reviews
A recent systematic review and meta‐analysis compares delayed versus early cord clamping for preterm birth, and is similar to our Comparison 1 and has similar findings (Fogarty 2018). However, there are some differences. We used random‐effects models for our meta‐analyses as we considered the variation in the study designs sufficient to believe the relative risks might vary between studies; we have a separate comparison for studies where immediate neonatal care is given, if needed, with the cord intact; we have found and were able to obtain slightly different studies in our searches. Although, both reviews used the Cochrane methodology, we vary in our assessments of risk of bias with Fogarty 2018 assessing risk of bias as lower for most of the studies. Our reasoning on risk of bias are explained in the Characteristics of included studies. Also, where we assessed the certainty of the evidence using GRADE as mostly low to moderate (due mainly to imprecision), Fogarty and colleagues assessed the certainty of the evidence as high. Our reasoning for our assessments is explained in the Notes in Table 1. As Fogarty 2018 included studies of delayed cord clamping, pooling those where immediate neonatal care was after cord clamping or with the cord intact, they have a larger cohort of babies. In our Cochrane Review, we separated studies where immediate neonatal care was after cord clamping from studies where immediate neonatal care was with the cord intact, as they may have different benefits and harms, which may be relevant to informing future guidance for clinical practice.
An earlier systematic review (Backes 2014) compared delayed cord clamping or umbilical cord milking with early cord clamping for babies less than 32 weeks' gestation. This review reported a reduction in mortality, fewer blood transfusions and less IVH (all grades) for babies allocated delayed cord clamping or umbilical cord milking, rather than early clamping. As discussed above, we consider delayed cord clamping and umbilical cord milking to be interventions with different potential benefits and harms. Hence, we did not combine them in our analysis, instead advocating head‐to‐head comparison of delayed clamping and cord milking to compare their effects.
Similarly, the systematic review Ghavam 2014 also included delayed cord clamping or umbilical cord milking in the same comparison arm. The objective of this review was to compare long‐term neurodevelopment (at age 18 to 24 months). However, there were insufficient data for reliable conclusions about neurodevelopment. Short‐term outcomes reported included: better blood pressure control and haemoglobin levels, fewer babies having blood transfusions, fewer babies with IVH (all grades), and fewer babies with late sepsis for babies with either delayed cord clamping or umbilical cord milking.
The review Rabe 2008 undertook a systematic review of babies less than 37 weeks' gestation comparing a brief delay in cord clamping (> 30 seconds) compared with early cord clamping (< 30 seconds). This review was superseded by the previous version of this Cochrane Review (Rabe 2012).
Finally, one systematic review (Al‐Wassia 2015) included trials of umbilical cord milking for both term and preterm births and combined early and delayed clamping for the control group. As the benefits and harms of early and delayed clamping may be different, we consider it inappropriate to combine data for these interventions.
Authors' conclusions
Implications for practice.
Delaying cord clamping appears to be beneficial at premature births. The mechanism of the probable reduced infant deaths before discharge is not known, but the demonstrated effects on blood transfusions and blood pressure stability seem to suggest that the benefits are haemodynamic.
The evidence of a probable reduction in infant death before discharge with delayed cord clamping compared with early clamping comes from a substantial body of evidence, 20 studies involving 2680 babies, seems to be consistent (I2 = 0%), and indicating a 27% reduction in infant death. These data include one large multicentre trial from Australia involving 1634 babies and their mothers (Tarnow‐Mordi 2017). Across the review, there is a consistent favouring of delayed cord clamping being beneficial and immediate clamping tending to cause harm, however, the optimal time for delaying cord clamping is not known.
The use of bedside resuscitation to allow a longer delay in cord clamping whilst not delaying immediate care is an attractive option. Whilst the one study that provided data on this technique was underpowered (CORD Pilot 2018), their results are consistent with those of delayed cord clamping with immediate neonatal care after clamping.
There are insufficient data on umbilical cord milking either compared with early cord clamping or compared with delayed cord clamping.
There are, as yet, insufficient data for reliable conclusions on long‐term follow‐up and neurological development in early childhood. These data are important, not only to demonstrate whether any benefit in short‐term outcome is reflected in subsequent neurodevelopment, but also to provide adequate reassurance about safety (Marlow 2015).
The nine new reports awaiting further classification may alter the conclusions of the review once assessed (Das 2018a; El‐Naggar 2018; Kazemi 2017; Leal 2018; Li 2018; Ram Mothan 2018; Song 2017; Wang 2018; Weeks 2018).
Implications for research.
Whilst the current evidence supports not clamping the cord before 30 seconds at preterm births, the optimum time to clamp the umbilical cord remains unclear. Thus, trials could compare clamping at 30 to 60 seconds with a longer delay. At preterm birth, time for the cardiovascular and respiratory changes may be more important than placental transfusion. Therefore, the priority could be to evaluate an adequate delay in cord clamping, and to provide immediate neonatal care, if needed, with the cord intact. This will allow recruitment of babies requiring immediate resuscitation at birth, a group largely excluded from the current evidence. Further research to improve understanding of the physiology of neonatal transition, and how it varies with gestation, would help determine the optimal policy for delayed clamping to evaluate in future trials.
Trials should no longer compare umbilical cord milking with cord clamping before 30 seconds at preterm births. Cord milking should be compared with delayed cord clamping, and ideally such studies should await assessment of the optimal policy for delayed clamping. The mode of umbilical cord milking with the cord intact should be well defined in future studies.
Future trials should be high quality, and report the methods they used in sufficient detail to allow assessment of the risk of bias. They should all report clinically important outcomes, particularly the primary outcomes and main secondary outcomes listed in this review. This should include reporting outcomes for the mother, and outcomes in early childhood for the babies. Trials should be powered on clinical outcomes rather than laboratory measures. As the benefits and hazards of alternative policies for timing of cord clamping may be different in low‐ and middle‐income settings with no access to neonatal intensive care, where mortality and morbidity is highest, trials in these settings are a particular priority.
Understanding parents’ and clinicians' views and experiences of delayed cord clamping and providing immediate neonatal care with the cord intact is also important, and should include follow‐up sometime after the birth.
The number of new trials being conducted to compare alternative policies for cord clamping and umbilical cord milking at preterm birth (see Characteristics of ongoing studies) is growing fast, with over 20 registered in the two years 2016‐2017. These trials are largely small and being undertaken with little standardisation of the interventions being compared or the outcomes being collected. Results from this updated Cochrane Review should guide the choice of promising interventions for future evaluation. Meta‐analysis using individual participant data from each trial maximises statistical power and enables reliable subgroup analyses to be undertaken. Trialists for many of the planned and recently published studies have agreed to share their data for a prospective meta‐analysis (Duley 2014).
What's new
| Date | Event | Description |
|---|---|---|
| 10 November 2017 | New citation required and conclusions have changed | We updated the search (November 2017) and included 33 new studies. The data now show that compared with early cord clamping, delayed cord clamping probably reduces the risk of infant death before hospital discharge. |
| 10 November 2017 | New search has been performed | Gillian Gyte has joined the team. We have separated delayed cord clamping from umbilical cord milking and included a new delayed intervention where immediate neonatal care is given with the cord intact. We have re‐visited the outcomes and modified them to focus more on clinical outcomes (See Differences between protocol and review). New subgroups are gestation and type of intervention (see Differences between protocol and review). We have extended the definition of 'low risk' for sensitivity analyses to include sequence generation, allocation concealment and incomplete outcome data. We have restructured the 'Plain language summary' to incorporate standardised headings. Four new 'Summary of findings' tables have been incorporated. We updated the search in November 2018 and identified 26 new reports. The references have been assessed but not fully incorporated into the review. Two of these were additional reports of included studies with no new data so the references have been added under the main study (Katheria 2015; Tarnow‐Mordi 2017). Six are new studies to be fully assessed at the next update (Kazemi 2017; Leal 2018; Li 2018; Ram Mothan 2018; Song 2017; Weeks 2018). Three are additional reports of included studies and the new data will be added at the next update (Das 2018a; El‐Naggar 2018; Wang 2018). The remaining 15 reports refer to 11 ongoing studies and have been added to Ongoing studies (Aghai 2018; Allam 2018; Gupta 2018; Hao 2018; Jomjak 2018; Katheria 2018; Liu 2018; Mirzaeian 2018; Nour 2018a; Nour 2018b; Shahgheibi 2018). |
History
Protocol first published: Issue 3, 2001 Review first published: Issue 4, 2004
| Date | Event | Description |
|---|---|---|
| 16 January 2012 | New citation required and conclusions have changed | This updated review is based on an search carried out in May 2011. We have now included 15 studies and the weight of the evidence is greater. New authors have helped to update the review. We updated the search in June 2012 and added results to Studies awaiting classification for consideration in the next update. |
| 31 December 2011 | New search has been performed | Search updated in May 2011, eight new studies added with 437 mother and infant pairs. Subgroup analyses added for cord milking. Methods updated in line with the new Cochrane Handbook. |
| 30 November 2009 | Amended | Search updated. Thirteen reports added to Studies awaiting classification. |
| 28 February 2009 | Amended | Converted to new review format. |
| 1 May 2008 | New citation required and conclusions have changed | Substantive amendment. |
Notes
The title of the previously published protocol was 'Early versus delayed cord clamping in preterm infants'.
Acknowledgements
Diane Elbourne who undertook the first Cohrane Review on this topic (Elbourne 1995).
Therese Dowswell who provided considerable input into the 2012 publication (Rabe 2012).
Graham Reynolds for his editorial and clinical contributions to previous versions of this review.
W Oh, M McDonnell, M Nelle, S Kinmond, J Mercer, N Aladangady A Katheria and H Rabe who kindly provided additional information regarding their studies. The information about randomisation for the trials by W Oh and M Nelle was directly obtained from the authors. The review authors thank the authors for supplying the information.
Jon Dorling, Donna Winterbank‐Scott, Lambert Felix, Anna Cuthbert all provided help with data extraction of information from the studies. Aidan Tan provided very helpful translations of the three Chinese papers.
As part of the pre‐publication editorial process, this review has been commented on by five peers (an editor and four referees who are external to the editorial team) and the Group's Statistical Adviser. The authors are grateful to the following peer reviewers for their time and comments: Prof NJ Shaw, Liverpool Women's Hospital; Andrew D Weeks, University of Liverpool; Jamie B Warren MD MPH, Oregon Health & Science University; Serena Xodo MD, Clinic of Obstetrics and Gynecology, Academic Hospital of Udine, Udine (Italy).
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Search methods ‐ ICTRP and ClinicalTrials.gov
ICTRP
cord AND clamp
cord and clamping
cord AND milking
cord AND stripping
ClinicalTrials.gov
Advanced search
Interventional studies | cord clamping
Interventional studies | cord milking
Interventional studies | cord stripping
Data and analyses
Comparison 1. DCC with immediate neonatal care after cord clamping vs ECC (subgroup analysis by gestation).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death of baby (up to discharge) | 20 | 2680 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.54, 0.98] |
| 1.1 < 32‐34 weeks gestation | 13 | 2108 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.52, 0.96] |
| 1.2 > 32‐34 weeks gestation | 3 | 237 | Risk Ratio (M‐H, Random, 95% CI) | 5.18 [0.25, 105.47] |
| 1.3 Mixed gestation | 4 | 335 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.09, 7.04] |
| 2 Death or neurodevelopmental impairment in early years | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.1 < 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Severe intraventricular haemorrhage (IVH grades 3, 4) | 10 | 2058 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.63, 1.39] |
| 3.1 < 32‐34 weeks gestation | 9 | 1972 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.64, 1.42] |
| 3.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 Mixed gestation | 1 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.05, 6.11] |
| 4 Intraventricular haemorrhage (IVH, all grades) | 15 | 2333 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.70, 0.99] |
| 4.1 < 32‐34 weeks gestation | 11 | 1988 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.56, 1.02] |
| 4.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Mixed gestation | 4 | 345 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.38, 1.16] |
| 5 Periventricular leukomalacia (PVL) | 4 | 1544 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.26, 1.30] |
| 5.1 < 32‐34 weeks gestation | 4 | 1544 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.26, 1.30] |
| 5.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Chronic lung disease (CLD) ‐ oxygen supplement at 36 weeks (corrected for gestation) | 6 | 1644 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.94, 1.14] |
| 6.1 < 32‐34 weeks gestation | 6 | 1644 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.94, 1.14] |
| 6.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Maternal blood loss of 500 mL or greater | 2 | 180 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.07, 17.63] |
| 7.1 < 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 > 32‐34 weeks gestation | 1 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.3 Mixed gestation | 1 | 94 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.07, 17.63] |
| 8 Intraventricular haemorrhage (IVH, grades 1 & 2) | 9 | 1968 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.51, 1.02] |
| 8.1 < 32‐34 weeks gestation | 8 | 1882 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.45, 1.03] |
| 8.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.3 Mixed gestation | 1 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.37, 2.18] |
| 9 Necrotising enterocolitis (NEC) confirmed by X‐ray or laparotomy) | 11 | 2010 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.64, 1.28] |
| 9.1 < 32‐34 weeks gestation | 10 | 1916 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.60, 1.22] |
| 9.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.3 Mixed gestation | 1 | 94 | Risk Ratio (M‐H, Random, 95% CI) | 2.84 [0.58, 13.92] |
| 10 Respiratory Distress Syndrome (RDS) | 7 | 457 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.86, 1.38] |
| 10.1 < 32‐34 weeks gestation | 3 | 165 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.64, 2.27] |
| 10.2 > 32‐34 weeks gestation | 1 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.19, 3.30] |
| 10.3 Mixed gestation | 3 | 206 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [0.52, 3.36] |
| 11 Respiratory support (ventilator or CPAP) | 6 | 325 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.77, 1.16] |
| 11.1 < 32‐34 weeks gestation | 5 | 220 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.78, 1.18] |
| 11.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.3 Mixed gestation | 1 | 105 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.16, 2.09] |
| 12 Duration of respiratory support (in days) | 1 | 42 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐3.04, 1.84] |
| 12.1 < 32‐34 weeks gestation | 1 | 42 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐3.04, 1.84] |
| 12.2 > 32‐34 weeks gestation | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.3 Mixed gestation | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Surfactant treatment (for severe RDS) | 3 | 145 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.50, 1.28] |
| 13.1 < 32‐34 weeks gestation | 3 | 145 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.50, 1.28] |
| 13.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Treatment for Patent Ductus Arteriosus (PDA) (medical and/or surgical) | 10 | 2046 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.99, 1.26] |
| 14.1 < 32‐34 weeks gestation | 9 | 1952 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.99, 1.26] |
| 14.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.3 Mixed gestation | 1 | 94 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.24, 5.34] |
| 15 Treatment for Retinopathy of Prematurity (RoP) | 8 | 1827 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.62, 1.12] |
| 15.1 < 32‐34 weeks gestation | 8 | 1827 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.62, 1.12] |
| 15.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Hyperbilirubinemia (treated by phototherapy) | 8 | 495 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.95, 1.16] |
| 16.1 < 32‐34 weeks gestation | 3 | 114 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.91, 1.11] |
| 16.2 > 32‐34 weeks gestation | 2 | 123 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.38, 1.41] |
| 16.3 Mixed gestation | 3 | 258 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.93, 1.47] |
| 17 Inotropics for low blood pressure | 5 | 250 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.17, 0.81] |
| 17.1 < 32‐34 weeks gestation | 5 | 250 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.17, 0.81] |
| 17.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Low Apgar as defined by trialists (generally < 8 at 5 mins) | 4 | 1721 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.70, 1.63] |
| 18.1 < 32‐34 weeks gestation | 3 | 1637 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.62, 1.62] |
| 18.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.3 Mixed gestation | 1 | 84 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [0.53, 3.31] |
| 19 Blood transfusion in infant | 11 | 2280 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.50, 0.86] |
| 19.1 < 32‐34 weeks gestation | 8 | 1995 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.47, 0.87] |
| 19.2 > 32‐34 weeks gestation | 1 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19.3 Mixed gestation | 2 | 199 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.26, 1.74] |
| 20 Volume of blood transfused (mL) | 1 | 72 | Mean Difference (IV, Random, 95% CI) | ‐6.0 [‐26.11, 14.11] |
| 20.1 < 32‐34 weeks gestation | 1 | 72 | Mean Difference (IV, Random, 95% CI) | ‐6.0 [‐26.11, 14.11] |
| 20.2 > 32‐34 weeks gestation | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20.3 Mixed gestation | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 21 Late sepsis (after 3 days or as defined by trialists) | 10 | 2017 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.56, 1.10] |
| 21.1 < 32‐34 weeks gestation | 9 | 1923 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.52, 1.11] |
| 21.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 21.3 Mixed gestation | 1 | 94 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.43, 1.79] |
| 22 Hydrocephalus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 22.1 < 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 22.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 22.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 23 Temperature < 36.0oC within 1 hour of birth | 1 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 23.1 < 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 23.2 > 32‐34 weeks gestation | 1 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 23.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 24 Hb within 1st 24 hour of birth (g/dL) | 1 | 42 | Mean Difference (IV, Random, 95% CI) | 0.80 [‐0.02, 1.62] |
| 24.1 < 32‐34 weeks gestation | 1 | 42 | Mean Difference (IV, Random, 95% CI) | 0.80 [‐0.02, 1.62] |
| 24.2 > 32‐34 weeks gestation | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 24.3 Mixed gestation | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 25 Mean arterial blood pressure in early hours after birth (mm Hg) | 4 | 208 | Mean Difference (IV, Random, 95% CI) | 2.87 [1.09, 4.64] |
| 25.1 < 32‐34 weeks gestation | 4 | 208 | Mean Difference (IV, Random, 95% CI) | 2.87 [1.09, 4.64] |
| 25.2 > 32‐34 weeks gestation | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 25.3 Mixed gestation | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26 Length of infant stay in NICU | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26.1 < 32‐34 weeks gestation | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26.2 > 32‐34 weeks gestation | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26.3 Mixed gestation | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 27 Home oxygen | 2 | 101 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.06, 3.72] |
| 27.1 < 32‐34 weeks gestation | 2 | 101 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.06, 3.72] |
| 27.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 27.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 28 Neurodevelopmental impairment at age two to three years (Baileys 11 MDI < 70) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 28.1 < 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 28.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 28.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29 Severe visual impairment | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29.1 < 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 30 Cerebral palsy (CP) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 30.1 < 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 30.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 30.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 31 Manual removal of placenta (denominator = vaginal births) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 31.1 < 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 31.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 31.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 32 Prolonged third stage (> 30 minutes) (denominator = vaginal births) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 32.1 < 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 32.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 32.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 33 Blood transfusion for mother | 1 | 1176 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.36, 1.24] |
| 33.1 < 32‐34 weeks gestation | 1 | 1176 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.36, 1.24] |
| 33.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 33.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 34 Postpartum infection in mother | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 34.1 < 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 34.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 34.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35 Rhesus isoimmunisation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35.1 < 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 36 Psychological well being in mother | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 36.1 < 32‐34 weeks gestation | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 36.2 > 32‐34 weeks gestation | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 36.3 Mixed gestation | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 37 Bonding | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 37.1 < 32‐34 weeks gestation | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 37.2 > 32‐34 weeks gestation | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 37.3 Mixed gestation | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 38 Breastfeeding initiation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 38.1 < 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 38.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 38.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 39 Fully breastfed or mixed feeding at infant discharge | 1 | 94 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [1.00, 1.23] |
| 39.1 < 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 39.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 39.3 Mixed gestation | 1 | 94 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [1.00, 1.23] |
| 40 Maternal anxiety | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 40.1 < 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 40.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 40.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 41 Mothers' views | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 41.1 < 32‐34 weeks gestation | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 41.2 > 32‐34 weeks gestation | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 41.3 Mixed gestation | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2. DCC with immediate neonatal care after cord clamping vs ECC (subgroup analysis by type of intervention).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death of baby (up to discharge) | 20 | 2680 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.54, 0.98] |
| 1.1 DCC < 1 min and baby level with uterus | 1 | 46 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.01, 3.95] |
| 1.2 DCC < 1 min and baby held low | 7 | 318 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.19, 1.30] |
| 1.3 DCC 1‐2 mins and baby level with uterus | 2 | 172 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.07, 17.80] |
| 1.4 DCC 1‐2 mins and baby held low | 3 | 1710 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.52, 1.00] |
| 1.5 DCC > 2 mins and baby level with uterus | 3 | 181 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.02, 28.73] |
| 1.6 DCC > 2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.7 Mixed interventions or unclear | 4 | 253 | Risk Ratio (M‐H, Random, 95% CI) | 2.28 [0.57, 9.13] |
| 2 Death or neurodevelopmental impairment at age two to three years | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 DCC < 1 min and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 DCC 1‐2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.4 DCC 1‐2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.5 DCC > 2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.6 DCC > 2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.7 Mixed interventions or unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Severe intraventricular haemorrhage (IVH grades 3, 4) | 10 | 2058 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.63, 1.39] |
| 3.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 DCC < 1 min and baby held low | 6 | 335 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.40, 2.21] |
| 3.3 DCC 1‐2 mins and baby level with uterus | 1 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.05, 6.11] |
| 3.4 DCC 1‐2 mins and baby held low | 1 | 1541 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.57, 1.44] |
| 3.5 DCC > 2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.6 DCC > 2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.7 Mixed interventions or unclear | 2 | 96 | Risk Ratio (M‐H, Random, 95% CI) | 2.96 [0.34, 25.69] |
| 4 Intraventricular haemorrhage (IVH, all grades) | 15 | 2333 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.70, 0.99] |
| 4.1 DCC < 1 min and baby level with uterus | 1 | 31 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.02, 8.08] |
| 4.2 DCC < 1 min and baby held low | 6 | 278 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.41, 1.06] |
| 4.3 DCC 1‐2 mins and baby level with uterus | 1 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.37, 1.87] |
| 4.4 DCC 1‐2 mins and baby held low | 2 | 1646 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.76, 1.16] |
| 4.5 DCC > 2 mins and baby level with uterus | 1 | 94 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.02, 9.04] |
| 4.6 DCC > 2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.7 Mixed interventions or unclear | 4 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.31, 1.42] |
| 5 Periventricular leukomalacia (PVL) | 4 | 1544 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.26, 1.30] |
| 5.1 DCC < 1 min and baby level with uterus | 1 | 31 | Risk Ratio (M‐H, Random, 95% CI) | 3.19 [0.14, 72.69] |
| 5.2 DCC < 1 min and baby held low | 2 | 102 | Risk Ratio (M‐H, Random, 95% CI) | 0.17 [0.01, 3.02] |
| 5.3 DCC 1‐2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.4 DCC 1‐2 mins and baby held low | 1 | 1411 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.24, 1.36] |
| 5.5 DCC > 2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.6 DCC > 2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.7 Mixed interventions or unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Chronic lung disease (CLD) ‐ oxygen supplement at 36 weeks (corrected for gestation) | 6 | 1644 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.94, 1.14] |
| 6.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 DCC < 1 min and baby held low | 5 | 205 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.57, 1.17] |
| 6.3 DCC 1‐2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.4 DCC 1‐2 mins and baby held low | 1 | 1439 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.96, 1.16] |
| 6.5 DCC > 2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.6 DCC > 2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.7 Mixed interventions or unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Maternal blood loss of 500 mL or greater | 2 | 180 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.07, 17.63] |
| 7.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 DCC < 1 min and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.3 DCC 1‐2 mins and baby level with uterus | 1 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.4 DCC 1‐2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.5 DCC > 2 mins and baby held level with uterus | 1 | 94 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.07, 17.63] |
| 7.6 DCC > 2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.7 Mixed interventions or unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Intraventricular haemorrhage (IVH, grades 1 & 2) | 9 | 1968 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.51, 1.02] |
| 8.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 DCC < 1 min and baby held low | 5 | 245 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.37, 1.15] |
| 8.3 DCC 1‐2 mins and baby level with uterus | 1 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.37, 2.18] |
| 8.4 DCC 1‐2 mins and baby held low | 1 | 1541 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.74, 1.22] |
| 8.5 DCC > 2 mins and baby held level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.6 DCC > 2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.7 Mixed interventions or unclear | 2 | 96 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.18, 0.95] |
| 9 Necrotising enterocolitis (NEC) confirmed by X‐ray or laparotomy) | 11 | 2010 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.64, 1.28] |
| 9.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 DCC < 1 min and baby held low | 7 | 368 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.28, 1.27] |
| 9.3 DCC 1‐2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.4 DCC 1‐2 mins and baby held low | 1 | 1446 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.60, 1.37] |
| 9.5 DCC > 2 mins and baby held level with uterus | 1 | 94 | Risk Ratio (M‐H, Random, 95% CI) | 2.84 [0.58, 13.92] |
| 9.6 DCC > 2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.7 Mixed interventions or unclear | 2 | 102 | Risk Ratio (M‐H, Random, 95% CI) | 1.75 [0.40, 7.73] |
| 10 Respiratory Distress Syndrome (RDS) | 6 | 367 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.86, 1.38] |
| 10.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 DCC < 1 min and baby held low | 2 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.64, 2.27] |
| 10.3 DCC 1‐2 mins and baby level with uterus | 1 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.19, 3.30] |
| 10.4 DCC 1‐2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.5 DCC > 2 mins and baby held level with uterus | 1 | 94 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.25, 2.01] |
| 10.6 DCC > 2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.7 Mixed interventions or unclear | 2 | 112 | Risk Ratio (M‐H, Random, 95% CI) | 2.12 [0.75, 5.99] |
| 11 Respiratory support (ventilator or CPAP) | 6 | 325 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.77, 1.16] |
| 11.1 DCC < 1 min and baby level with uterus | 1 | 46 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.49, 2.06] |
| 11.2 DCC < 1 min and baby held low | 2 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.80, 1.61] |
| 11.3 DCC 1‐2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.4 DCC 1‐2 mins and baby held low | 2 | 144 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.39, 1.40] |
| 11.5 DCC > 2 mins and baby held level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.6 DCC > 2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.7 Mixed interventions or unclear | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.65, 1.17] |
| 12 Duration of respiratory support | 1 | 42 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐3.04, 1.84] |
| 12.1 DCC < 1 min and baby level with uterus | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 DCC < 1 min and baby held low | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.3 DCC 1‐2 mins and baby level with uterus | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.4 DCC 1‐2 mins and baby held low | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.5 DCC > 2 mins and baby level with uterus | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.6 DCC > 2 mins and baby held low | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.7 Mixed interventions or unclear | 1 | 42 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐3.04, 1.84] |
| 13 Surfactant treatment (for severe RDS) | 3 | 145 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.50, 1.28] |
| 13.1 DCC < 1 min and baby level with uterus | 1 | 46 | Risk Ratio (M‐H, Random, 95% CI) | 1.5 [0.49, 4.62] |
| 13.2 DCC < 1 min and baby held low | 1 | 39 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.31, 3.62] |
| 13.3 DCC 1‐2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.4 DCC 1‐2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.5 DCC > 2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.6 DCC > 2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.7 Mixed interventions or unclear | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.37, 1.14] |
| 14 Treatment for Patent Ductus Arteriosus (PDA) (medical and/or surgical) | 10 | 2046 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.99, 1.26] |
| 14.1 DCC < 1 min and baby level with uterus | 1 | 46 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.22, 4.45] |
| 14.2 DCC < 1 min and baby held low | 4 | 174 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.88, 1.90] |
| 14.3 DCC 1‐2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.4 DCC 1‐2 mins and baby held low | 2 | 1630 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.90, 1.36] |
| 14.5 DCC > 2 mins and baby level with uterus | 1 | 94 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.24, 5.34] |
| 14.6 DCC > 2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.7 Mixed interventions or unclear | 2 | 102 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.24, 1.26] |
| 15 Treatment for Retinopathy of Prematurity (RoP) | 8 | 1827 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.62, 1.12] |
| 15.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 DCC < 1 min and baby held low | 4 | 226 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.69, 1.46] |
| 15.3 DCC 1‐2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.4 DCC 1‐2 mins and baby held low | 2 | 1499 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.11, 2.44] |
| 15.5 DCC > 2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.6 DCC > 2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.7 Mixed interventions or unclear | 2 | 102 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.13, 1.15] |
| 16 Hyperbilirubinemia (treated by phototherapy) | 8 | 495 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.95, 1.16] |
| 16.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.2 DCC < 1 min and baby held low | 2 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.91, 1.11] |
| 16.3 DCC 1‐2 mins and baby level with uterus | 1 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.23, 1.84] |
| 16.4 DCC 1‐2 mins and baby held low | 1 | 104 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [1.03, 1.88] |
| 16.5 DCC > 2 mins and baby level with uterus | 2 | 131 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.91, 1.36] |
| 16.6 DCC > 2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.7 Mixed interventions or unclear | 2 | 98 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.70, 1.37] |
| 17 Inotropics for low blood pressure | 5 | 250 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.17, 0.81] |
| 17.1 DCC < 1 min and baby level with uterus | 1 | 46 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.12, 3.62] |
| 17.2 DCC < 1 min and baby held low | 3 | 162 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.06, 0.80] |
| 17.3 DCC 1‐2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.4 DCC 1‐2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.5 DCC > 2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.6 DCC > 2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.7 Mixed interventions or unclear | 1 | 42 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.13, 1.44] |
| 18 Low Apgar as defined by trialists (generally < 8 at 5 mins) | 4 | 1721 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.70, 1.63] |
| 18.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.2 DCC < 1 min and baby held low | 1 | 39 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.29, 1.96] |
| 18.3 DCC 1‐2 mins and baby level with uterus | 1 | 84 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [0.53, 3.31] |
| 18.4 DCC 1‐2 mins and baby held low | 1 | 1560 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.59, 1.83] |
| 18.5 DCC > 2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.6 DCC > 2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.7 Mixed interventions or unclear | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 5.4 [0.31, 93.42] |
| 19 Blood transfusion in infant | 11 | 2280 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.50, 0.86] |
| 19.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19.2 DCC < 1 min and baby held low | 4 | 266 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.50, 0.92] |
| 19.3 DCC 1‐2 mins and baby level with uterus | 1 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19.4 DCC 1‐2 mins and baby held low | 3 | 1736 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.13, 1.46] |
| 19.5 DCC > 2 mins and baby level with uterus | 1 | 94 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.23, 2.51] |
| 19.6 DCC > 2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19.7 Mixed interventions or unclear | 2 | 98 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.27, 1.23] |
| 20 Volume of blood transfused (mL) | 1 | 72 | Mean Difference (IV, Random, 95% CI) | ‐6.0 [‐26.11, 14.11] |
| 20.1 DCC < 1 min and baby level with uterus | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20.2 DCC < 1 min and baby held low | 1 | 72 | Mean Difference (IV, Random, 95% CI) | ‐6.0 [‐26.11, 14.11] |
| 20.3 DCC 1‐2 mins and baby level with uterus | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20.4 DCC 1‐2 mins and baby held low | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20.5 DCC > 2 mins and baby level with uterus | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20.6 DCC > 2 mins and baby held low | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20.7 Mixed interventions or unclear | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 21 Late sepsis (after 3 days or as defined by trialists) | 10 | 2017 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.56, 1.10] |
| 21.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 21.2 DCC < 1 min and baby held low | 5 | 297 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.39, 1.25] |
| 21.3 DCC 1‐2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 21.4 DCC 1‐2 mins and baby held low | 2 | 1524 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.35, 1.81] |
| 21.5 DCC > 2 mins and baby level with uterus | 1 | 94 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.43, 1.79] |
| 21.6 DCC > 2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 21.7 Mixed interventions or unclear | 2 | 102 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.23, 2.87] |
| 22 Hydrocephalus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 22.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 22.2 DCC < 1 min and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 22.3 DCC 1‐2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 22.4 DCC 1‐2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 22.5 DCC > 2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 22.6 DCC > 2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 22.7 Mixed interventions or unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 23 Temperature < 36.0oC within 1 hour of birth | 1 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 23.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 23.2 DCC < 1 min and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 23.3 DCC 1‐2 mins and baby level with uterus | 1 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 23.4 DCC 1‐2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 23.5 DCC > 2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 23.6 DCC > 2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 23.7 Mixed interventions or unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 24 Hb within 1st 24 hour of birth (g/dL) | 1 | 42 | Mean Difference (IV, Random, 95% CI) | 0.80 [‐0.02, 1.62] |
| 24.1 DCC < 1 min and baby level with uterus | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 24.2 DCC < 1 min and baby held low | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 24.3 DCC 1‐2 mins and baby level with uterus | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 24.4 DCC 1‐2 mins and baby held low | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 24.5 DCC > 2 mins and baby level with uterus | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 24.6 DCC > 2 mins and baby held low | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 24.7 Mixed interventions or unclear | 1 | 42 | Mean Difference (IV, Random, 95% CI) | 0.80 [‐0.02, 1.62] |
| 25 Mean arterial blood pressure in early hours after birth (mm Hg) | 4 | 208 | Mean Difference (IV, Random, 95% CI) | 2.87 [1.09, 4.64] |
| 25.1 DCC < 1 min and baby level with uterus | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 25.2 DCC < 1 min and baby held low | 3 | 169 | Mean Difference (IV, Random, 95% CI) | 2.57 [0.69, 4.45] |
| 25.3 DCC 1‐2 mins and baby level with uterus | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 25.4 DCC 1‐2 mins and baby held low | 1 | 39 | Mean Difference (IV, Random, 95% CI) | 5.34 [‐0.06, 10.74] |
| 25.5 DCC > 2 mins and baby level with uterus | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 25.6 DCC > 2 mins and baby held low | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 25.7 Mixed interventions or unclear | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26 Length of infant stay in NICU | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26.1 DCC < 1 min and baby level with uterus | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26.2 DCC < 1 min and baby held low | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26.3 DCC 1‐2 mins and baby level with uterus | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26.4 DCC 1‐2 mins and baby held low | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26.5 DCC > 2 mins and baby level with uterus | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26.6 DCC > 2 mins and baby held low | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26.7 Mixed interventions or unclear | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 27 Home oxygen | 2 | 101 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.06, 3.72] |
| 27.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 27.2 DCC < 1 min and baby held low | 2 | 101 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.06, 3.72] |
| 27.3 DCC 1‐2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 27.4 DCC 1‐2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 27.5 DCC > 2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 27.6 DCC > 2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 27.7 Mixed interventions or unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 28 Neurodevelopmental impairment at age two to three years | 0 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 28.1 DCC < 1 min and baby level with uterus | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 28.2 DCC < 1 min and baby held low | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 28.3 DCC 1‐2 mins and baby level with uterus | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 28.4 DCC 1‐2 mins and baby held low | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 28.5 DCC > 2 mins and baby level with uterus | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 28.6 DCC > 2 mins and baby held low | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 28.7 Mixed interventions or unclear | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 29 Severe visual impairment | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29.2 DCC < 1 min and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29.3 DCC 1‐2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29.4 DCC 1‐2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29.5 DCC > 2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29.6 DCC > 2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29.7 Mixed interventions or unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 30 Cerebral palsy (CP) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 30.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 30.2 DCC < 1 min and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 30.3 DCC 1‐2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 30.4 DCC 1‐2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 30.5 DCC > 2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 30.6 DCC > 2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 30.7 Mixed interventions or unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 31 Manual removal of placenta (denominator = vaginal births) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 31.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 31.2 DCC < 1 min and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 31.3 DCC 1‐2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 31.4 DCC 1‐2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 31.5 DCC > 2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 31.6 DCC > 2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 31.7 Mixed interventions or unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 32 Prolonged third stage (>30 minutes) (denominator = vaginal births) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 32.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 32.2 DCC < 1 min and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 32.3 DCC 1‐2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 32.4 DCC 1‐2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 32.5 DCC > 2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 32.6 DCC > 2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 32.7 Mixed interventions or unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 33 Blood transfusion for mother | 1 | 1176 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.36, 1.24] |
| 33.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 33.2 DCC < 1 min and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 33.3 DCC 1‐2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 33.4 DCC 1‐2 mins and baby held low | 1 | 1176 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.36, 1.24] |
| 33.5 DCC > 2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 33.6 DCC > 2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 33.7 Mixed interventions or unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 34 Postpartum infection in mother | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 34.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 34.2 DCC < 1 min and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 34.3 DCC 1‐2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 34.4 DCC 1‐2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 34.5 DCC > 2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 34.6 DCC > 2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 34.7 Mixed interventions or unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35 Rhesus isoimmunisation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35.2 DCC < 1 min and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35.3 DCC 1‐2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35.4 DCC 1‐2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35.5 DCC > 2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35.6 DCC > 2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35.7 Mixed interventions or unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 36 Psychological well being in mother | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 36.1 DCC < 1 min and baby level with uterus | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 36.2 DCC < 1 min and baby held low | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 36.3 DCC 1‐2 mins and baby level with uterus | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 36.4 DCC 1‐2 mins and baby held low | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 36.5 DCC > 2 mins and baby level with uterus | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 36.6 DCC > 2 mins and baby held low | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 36.7 Mixed interventions or unclear | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 37 Bonding | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 37.1 DCC < 1 min and baby level with uterus | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 37.2 DCC < 1 min and baby held low | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 37.3 DCC 1‐2 mins and baby level with uterus | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 37.4 DCC 1‐2 mins and baby held low | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 37.5 DCC > 2 mins and baby level with uterus | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 37.6 DCC > 2 mins and baby held low | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 37.7 Mixed interventions or unclear | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 38 Breastfeeding initiation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 38.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 38.2 DCC < 1 min and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 38.3 DCC 1‐2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 38.4 DCC 1‐2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 38.5 DCC > 2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 38.6 DCC > 2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 38.7 Mixed interventions or unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 39 Fully breastfed or mixed feeding at infant discharge | 1 | 94 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [1.00, 1.23] |
| 39.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 39.2 DCC < 1 min and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 39.3 DCC 1‐2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 39.4 DCC 1‐2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 39.5 DCC > 2 mins and baby level with uterus | 1 | 94 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [1.00, 1.23] |
| 39.6 DCC > 2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 39.7 Mixed interventions or unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 40 Maternal anxiety | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 40.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 40.2 DCC < 1 min and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 40.3 DCC 1‐2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 40.4 DCC 1‐2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 40.5 DCC > 2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 40.6 DCC > 2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 40.7 Mixed interventions or unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 41 Mothers' views | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 41.1 DCC < 1 min and baby level with uterus | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 41.2 DCC < 1 min and baby held low | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 41.3 DCC 1‐2 mins and baby level with uterus | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 41.4 DCC 1‐2 mins and baby held low | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 41.5 DCC > 2 mins and baby level with uterus | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 41.6 DCC > 2 mins and baby held low | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 41.7 Mixed interventions or unclear | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 42 Neurosensory disability at 7 months (Bailey's MDI < 70) ‐ not prespecified | 2 | 73 | Risk Ratio (M‐H, Random, 95% CI) | 1.64 [0.66, 4.09] |
| 42.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 42.2 DCC < 1 min and baby held low | 2 | 73 | Risk Ratio (M‐H, Random, 95% CI) | 1.64 [0.66, 4.09] |
| 42.3 DCC 1‐2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 42.4 DCC 1‐2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 42.5 DCC > 2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 42.6 DCC > 2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 42.7 Mixed interventions or unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
2.42. Analysis.
Comparison 2 DCC with immediate neonatal care after cord clamping vs ECC (subgroup analysis by type of intervention), Outcome 42 Neurosensory disability at 7 months (Bailey's MDI < 70) ‐ not prespecified.
Comparison 3. DCC with immediate neonatal care with cord intact vs ECC (subgroup analysis by gestation).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death of baby (up to discharge) | 1 | 270 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.20, 1.11] |
| 1.1 < 32‐34 weeks gestation | 1 | 270 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.20, 1.11] |
| 1.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Death or neurodevelopmental impairment at age two to three years | 1 | 218 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.39, 0.96] |
| 2.1 < 32‐34 weeks gestation | 1 | 218 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.39, 0.96] |
| 2.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Severe intraventricular haemorrhage (IVH grades 3, 4) | 1 | 266 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.29, 2.45] |
| 3.1 < 32‐34 weeks gestation | 1 | 266 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.29, 2.45] |
| 3.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Intraventricular haemorrhage (IVH, all grades) | 1 | 266 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.64, 1.26] |
| 4.1 < 32‐34 weeks gestation | 1 | 266 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.64, 1.26] |
| 4.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Periventricular leukomalacia (PVL) | 1 | 266 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.32, 2.31] |
| 5.1 < 32‐34 weeks gestation | 1 | 266 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.32, 2.31] |
| 5.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Chronic lung disease (CLD) ‐ oxygen supplement at 36 weeks (corrected for gestation) | 1 | 249 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.66, 1.37] |
| 6.1 < 32‐34 weeks gestation | 1 | 249 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.66, 1.37] |
| 6.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Maternal blood loss of 500 mL or greater | 1 | 254 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.72, 1.22] |
| 7.1 < 32‐34 weeks gestation | 1 | 254 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.72, 1.22] |
| 7.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Intraventricular haemorrhage (IVH, grades 1 & 2) | 1 | 266 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.63, 1.33] |
| 8.1 < 32‐34 weeks gestation | 1 | 266 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.63, 1.33] |
| 8.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Necrotising enterocolitis (NEC) confirmed by X‐ray or laparotomy) | 1 | 266 | Risk Ratio (M‐H, Random, 95% CI) | 1.58 [0.53, 4.69] |
| 9.1 < 32‐34 weeks gestation | 1 | 266 | Risk Ratio (M‐H, Random, 95% CI) | 1.58 [0.53, 4.69] |
| 9.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Respiratory Distress Syndrome (RDS) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.1 < 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Respiratory support (ventilator or CPAP) | 1 | 266 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.84, 1.09] |
| 11.1 < 32‐34 weeks gestation | 1 | 266 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.84, 1.09] |
| 11.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Duration of respiratory support | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.1 < 32‐34 weeks gestation | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 > 32‐34 weeks gestation | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.3 Mixed gestation | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Surfactant treatment (for severe RDS) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.1 < 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Treatment for Patent Ductus Arteriosus (PDA) (medical and/or surgical) | 1 | 266 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.56, 1.74] |
| 14.1 < 32‐34 weeks gestation | 1 | 266 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.56, 1.74] |
| 14.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Treatment for Retinopathy of Prematurity (RoP) | 1 | 249 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.28, 3.13] |
| 15.1 < 32‐34 weeks gestation | 1 | 249 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.28, 3.13] |
| 15.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Hyperbilirubinemia (treated by phototherapy) | 1 | 266 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.94, 1.09] |
| 16.1 < 32‐34 weeks gestation | 1 | 266 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.94, 1.09] |
| 16.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Inotropics for low blood pressure | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.1 < 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Low Apgar as defined by trialists (generally < 8 at 5 mins) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.1 < 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Blood transfusion in infant | 1 | 266 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.71, 1.17] |
| 19.1 < 32‐34 weeks gestation | 1 | 266 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.71, 1.17] |
| 19.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20 Volume of blood transfused (mL) | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20.1 < 32‐34 weeks gestation | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20.2 > 32‐34 weeks gestation | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20.3 Mixed gestation | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 21 Late sepsis (after 3 days or as defined by trialists) | 1 | 266 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.72, 1.09] |
| 21.1 < 32‐34 weeks gestation | 1 | 266 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.72, 1.09] |
| 21.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 21.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 22 Hydrocephalus | 1 | 266 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.14, 6.89] |
| 22.1 < 32‐34 weeks gestation | 1 | 266 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.14, 6.89] |
| 22.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 22.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 23 Temperature < 36.0oC within 1 hour of birth | 1 | 266 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.61, 2.33] |
| 23.1 < 32‐34 weeks gestation | 1 | 266 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.61, 2.33] |
| 23.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 23.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 24 Hb within 1st 24 hour of birth (g/dL) | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 24.1 < 32‐34 weeks gestation | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 24.2 > 32‐34 weeks gestation | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 24.3 Mixed gestation | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 25 Mean arterial blood pressure (subgrouped by time after birth) | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 25.1 < 32‐34 weeks gestation | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 25.2 Mixed gestation | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 25.3 > 32‐34 weeks gestation | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26 Length of infant stay in NICU | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26.1 < 32‐34 weeks gestation | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26.2 > 32‐34 weeks gestation | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26.3 Mixed gestation | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 27 Home oxygen | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 27.1 < 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 27.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 27.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 28 Neurodevelopmental impairment at age two to three years | 1 | 218 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.41, 1.39] |
| 28.1 < 32‐34 weeks gestation | 1 | 218 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.41, 1.39] |
| 28.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 28.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29 Severe visual impairment | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29.1 < 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 30 Cerebral palsy (CP) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 30.1 < 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 30.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 30.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 31 Manual removal of placenta (denominator = vaginal births) | 1 | 105 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.32, 3.04] |
| 31.1 < 32‐34 weeks gestation | 1 | 105 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.32, 3.04] |
| 31.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 31.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 32 Prolonged third stage (>30 minutes) (denominator = vaginal births) | 1 | 105 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.24, 2.64] |
| 32.1 < 32‐34 weeks gestation | 1 | 105 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.24, 2.64] |
| 32.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 32.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 33 Blood transfusion for mother | 1 | 254 | Risk Ratio (M‐H, Random, 95% CI) | 1.59 [0.39, 6.51] |
| 33.1 < 32‐34 weeks gestation | 1 | 254 | Risk Ratio (M‐H, Random, 95% CI) | 1.59 [0.39, 6.51] |
| 33.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 33.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 34 Postpartum infection in mother | 1 | 254 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.73, 1.72] |
| 34.1 < 32‐34 weeks gestation | 1 | 254 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.73, 1.72] |
| 34.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 34.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35 Rhesus isoimmunisation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35.1 < 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 36 Psychological well being in mother | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 36.1 < 32‐34 weeks gestation | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 36.2 > 32‐34 weeks gestation | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 36.3 Mixed gestation | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 37 Bonding | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 37.1 < 32‐34 weeks gestation | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 37.2 > 32‐34 weeks gestation | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 37.3 Mixed gestation | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 38 Breastfeeding initiation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 38.1 < 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 38.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 38.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 39 Fully breastfed or mixed feeding at infant discharge | 1 | 248 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.79, 1.22] |
| 39.1 < 32‐34 weeks gestation | 1 | 248 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.79, 1.22] |
| 39.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 39.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 40 Maternal anxiety | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 40.1 < 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 40.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 40.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 41 Mothers' views | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 41.1 < 32‐34 weeks gestation | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 41.2 > 32‐34 weeks gestation | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 41.3 Mixed gestation | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 4. DCC with immediate neonatal care with cord intact vs ECC (subgroup analysis by type of intervention).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death of baby (up to discharge) | 1 | 270 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.20, 1.11] |
| 1.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 DCC < 1 min and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 DCC at 1‐2 mins with baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.4 DCC at 1‐2 mins with baby low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.5 DCC at > 2 mins with baby level with uterus | 1 | 270 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.20, 1.11] |
| 1.6 DCC at > 2 mins with baby low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.7 Mixed interventions or unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Death or neurodevelopmental impairment at age two to three years | 1 | 218 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.39, 0.96] |
| 2.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 DCC < 1 min and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 DCC at 1‐2 mins with baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.4 DCC at 1‐2 mins with baby low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.5 DCC at > 2 mins with baby level with uterus | 1 | 218 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.39, 0.96] |
| 2.6 DCC at > 2 mins with baby low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.7 Mixed interventions or unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Severe intraventricular haemorrhage (IVH grades 3, 4) | 1 | 266 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.29, 2.45] |
| 3.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 DCC < 1 min and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 DCC at 1‐2 mins with baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.4 DCC at 1‐2 mins with baby low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.5 DCC at > 2 mins with baby level with uterus | 1 | 266 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.29, 2.45] |
| 3.6 DCC at > 2 mins with baby low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.7 Mixed interventions or unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Intraventricular haemorrhage (IVH, all grades) | 1 | 266 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.64, 1.26] |
| 4.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 DCC < 1 min and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 DCC at 1‐2 mins with baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.4 DCC at 1‐2 mins with baby low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.5 DCC at > 2 mins with baby level with uterus | 1 | 266 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.64, 1.26] |
| 4.6 DCC at > 2 mins with baby low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.7 Mixed interventions or unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Periventricular leukomalacia (PVL) | 1 | 266 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.32, 2.31] |
| 5.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 DCC < 1 min and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 DCC at 1‐2 mins with baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.4 DCC at 1‐2 mins with baby low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.5 DCC at > 2 mins with baby level with uterus | 1 | 266 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.32, 2.31] |
| 5.6 DCC at > 2 mins with baby low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.7 Mixed interventions or unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Chronic lung disease (CLD) ‐ oxygen supplement at 36 weeks (corrected for gestation) | 1 | 249 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.66, 1.37] |
| 6.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 DCC < 1 min and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 DCC at 1‐2 mins with baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.4 DCC at 1‐2 mins with baby low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.5 DCC at > 2 mins with baby level with uterus | 1 | 249 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.66, 1.37] |
| 6.6 DCC at > 2 mins with baby low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.7 Mixed interventions or unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Maternal blood loss of 500 mL or greater | 1 | 254 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.72, 1.22] |
| 7.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 DCC < 1 min and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.3 DCC at 1‐2 mins with baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.4 DCC at 1‐2 mins with baby low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.5 DCC at > 2 mins with baby level with uterus | 1 | 254 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.72, 1.22] |
| 7.6 DCC at > 2 mins with baby low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.7 Mixed interventions or unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Intraventricular haemorrhage (IVH, grades 1 & 2) | 1 | 266 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.63, 1.33] |
| 8.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 DCC < 1 min and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.3 DCC at 1‐2 mins with baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.4 DCC at 1‐2 mins with baby low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.5 DCC at > 2 mins with baby level with uterus | 1 | 266 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.63, 1.33] |
| 8.6 DCC at > 2 mins with baby low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.7 Mixed interventions or unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Necrotising enterocolitis (NEC) confirmed by X‐ray or laparotomy) | 1 | 266 | Risk Ratio (M‐H, Random, 95% CI) | 1.58 [0.53, 4.69] |
| 9.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 DCC < 1 min and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.3 DCC at 1‐2 mins with baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.4 DCC at 1‐2 mins with baby low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.5 DCC at > 2 mins with baby level with uterus | 1 | 266 | Risk Ratio (M‐H, Random, 95% CI) | 1.58 [0.53, 4.69] |
| 9.6 DCC at > 2 mins with baby low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.7 Mixed interventions or unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Respiratory Distress Syndrome (RDS) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 DCC < 1 min and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.3 DCC at 1‐2 mins with baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.4 DCC at 1‐2 mins with baby low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.5 DCC at > 2 mins with baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.6 DCC at > 2 mins with baby low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.7 Mixed interventions or unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.8 Mixed interventions or unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Respiratory support (ventilator or CPAP) | 1 | 266 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.84, 1.09] |
| 11.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 DCC < 1 min and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.3 DCC at 1‐2 mins with baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.4 DCC at 1‐2 mins with baby low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.5 DCC at > 2 mins with baby level with uterus | 1 | 266 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.84, 1.09] |
| 11.6 DCC at > 2 mins with baby low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.7 Mixed interventions or unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Duration of respiratory support | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.1 DCC < 1 min and baby level with uterus | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 DCC < 1 min and baby held low | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.3 DCC 1‐2 mins and baby level with uterus | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.4 DCC 1‐2 mins and baby held low | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.5 DCC > 2 mins and baby level with uterus | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.6 DCC > 2 mins and baby held low | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.7 Mixed interventions or unclear | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Surfactant treatment (for severe RDS) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 DCC < 1 min and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.3 DCC at 1‐2 mins with baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.4 DCC at 1‐2 mins with baby low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.5 DCC at > 2 mins with baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.6 DCC at > 2 mins with baby low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.7 Mixed interventions or unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Treatment for Patent Ductus Arteriosus (PDA) (medical and/or surgical) | 1 | 266 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.56, 1.74] |
| 14.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 DCC < 1 min and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.3 DCC at 1‐2 mins with baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.4 DCC at 1‐2 mins with baby low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.5 DCC at > 2 mins with baby level with uterus | 1 | 266 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.56, 1.74] |
| 14.6 DCC at > 2 mins with baby low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.7 Mixed interventions or unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Treatment for Retinopathy of Prematurity (RoP) | 1 | 249 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.28, 3.13] |
| 15.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 DCC < 1 min and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.3 DCC at 1‐2 mins with baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.4 DCC at 1‐2 mins with baby low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.5 DCC at > 2 mins with baby level with uterus | 1 | 249 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.28, 3.13] |
| 15.6 DCC at > 2 mins with baby low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.7 Mixed interventions or unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Hyperbilirubinemia (treated by phototherapy) | 1 | 266 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.94, 1.09] |
| 16.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.2 DCC < 1 min and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.3 DCC at 1‐2 mins with baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.4 DCC at 1‐2 mins with baby low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.5 DCC at > 2 mins with baby level with uterus | 1 | 266 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.94, 1.09] |
| 16.6 DCC at > 2 mins with baby low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.7 Mixed interventions or unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Inotropics for low blood pressure | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 DCC < 1 min and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.3 DCC at 1‐2 mins with baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.4 DCC at 1‐2 mins with baby low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.5 DCC at > 2 mins with baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.6 DCC at > 2 mins with baby low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.7 Mixed interventions or unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Low Apgar as defined by trialists (generally < 8 at 5 mins) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.2 DCC < 1 min and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.3 DCC at 1‐2 mins with baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.4 DCC at 1‐2 mins with baby low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.5 DCC at > 2 mins with baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.6 DCC at > 2 mins with baby low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.7 Mixed interventions or unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Blood transfusion in infant | 1 | 266 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.71, 1.17] |
| 19.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19.2 DCC < 1 min and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19.3 DCC at 1‐2 mins with baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19.4 DCC at 1‐2 mins with baby low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19.5 DCC at > 2 mins with baby level with uterus | 1 | 266 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.71, 1.17] |
| 19.6 DCC at > 2 mins with baby low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19.7 Mixed interventions or unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20 Volume of blood transfused | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20.1 DCC < 1 min and baby level with uterus | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20.2 DCC < 1 min and baby held low | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20.3 DCC 1‐2 mins and baby level with uterus | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20.4 DCC 1‐2 mins and baby held low | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20.5 DCC > 2 mins and baby level with uterus | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20.6 DCC > 2 mins and baby held low | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20.7 Mixed interventions or unclear | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 21 Late sepsis (after 3 days or as defined by trialists) | 1 | 266 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.72, 1.09] |
| 21.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 21.2 DCC < 1 min and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 21.3 DCC at 1‐2 mins with baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 21.4 DCC at 1‐2 mins with baby low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 21.5 DCC at > 2 mins with baby level with uterus | 1 | 266 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.72, 1.09] |
| 21.6 DCC at > 2 mins with baby low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 21.7 Mixed interventions or unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 22 Hydrocephalus | 1 | 266 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.14, 6.89] |
| 22.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 22.2 DCC < 1 min and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 22.3 DCC at 1‐2 mins with baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 22.4 DCC at 1‐2 mins with baby low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 22.5 DCC at > 2 mins with baby level with uterus | 1 | 266 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.14, 6.89] |
| 22.6 DCC at > 2 mins with baby low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 22.7 Mixed interventions or unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 23 Temperature < 36.0oC within 1 hour of birth | 1 | 266 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.61, 2.33] |
| 23.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 23.2 DCC < 1 min and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 23.3 DCC at 1‐2 mins with baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 23.4 DCC at 1‐2 mins with baby low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 23.5 DCC at > 2 mins with baby level with uterus | 1 | 266 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.61, 2.33] |
| 23.6 DCC at > 2 mins with baby low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 23.7 Mixed interventions or unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 24 Hb within 1st 24 hour of birth (g/dL) | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 24.1 DCC < 1 min and baby level with uterus | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 24.2 DCC < 1 min and baby held low | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 24.3 DCC 1‐2 mins and baby level with uterus | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 24.4 DCC 1‐2 mins and baby held low | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 24.5 DCC > 2 mins and baby level with uterus | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 24.6 DCC > 2 mins and baby held low | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 24.7 Mixed interventions or unclear | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 25 Mean arterial blood pressure (subgrouped by time after birth) | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 25.1 DCC < 1 min and baby level with uterus | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 25.2 DCC < 1 min and baby held low | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 25.3 DCC 1‐2 mins and baby level with uterus | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 25.4 DCC 1‐2 mins and baby held low | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 25.5 DCC > 2 mins and baby level with uterus | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 25.6 DCC > 2 mins and baby held low | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 25.7 Mixed interventions or unclear | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26 Length of infant stay in NICU | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26.1 DCC < 1 min and baby level with uterus | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26.2 DCC < 1 min and baby held low | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26.3 DCC 1‐2 mins and baby level with uterus | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26.4 DCC 1‐2 mins and baby held low | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26.5 DCC > 2 mins and baby level with uterus | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26.6 DCC > 2 mins and baby held low | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26.7 Mixed interventions or unclear | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 27 Home oxygen | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 27.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 27.2 DCC < 1 min and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 27.3 DCC at 1‐2 mins with baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 27.4 DCC at 1‐2 mins with baby low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 27.5 DCC at > 2 mins with baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 27.6 DCC at > 2 mins with baby low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 27.7 Mixed interventions or unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 28 Neurodevelopmental impairment at age two to three years | 1 | 194 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.06, 1.64] |
| 28.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 28.2 DCC < 1 min and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 28.3 DCC at 1‐2 mins with baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 28.4 DCC at 1‐2 mins with baby low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 28.5 DCC at > 2 mins with baby level with uterus | 1 | 194 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.06, 1.64] |
| 28.6 DCC at > 2 mins with baby low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 28.7 Mixed interventions or unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29 Severe visual impairment | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29.2 DCC < 1 min and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29.3 DCC at 1‐2 mins with baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29.4 DCC at 1‐2 mins with baby low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29.5 DCC at > 2 mins with baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29.6 DCC at > 2 mins with baby low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29.7 Mixed interventions or unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 30 Cerebral palsy (CP) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 30.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 30.2 DCC < 1 min and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 30.3 DCC at 1‐2 mins with baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 30.4 DCC at 1‐2 mins with baby low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 30.5 DCC at > 2 mins with baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 30.6 DCC at > 2 mins with baby low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 30.7 Mixed interventions or unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 31 Manual removal of placenta (denominator = vaginal births) | 1 | 105 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.32, 3.04] |
| 31.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 31.2 DCC < 1 min and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 31.3 DCC at 1‐2 mins with baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 31.4 DCC at 1‐2 mins with baby low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 31.5 DCC at > 2 mins with baby level with uterus | 1 | 105 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.32, 3.04] |
| 31.6 DCC at > 2 mins with baby low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 31.7 Mixed interventions or unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 32 Prolonged third stage (>30 minutes) (denominator = vaginal births) | 1 | 105 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.24, 2.64] |
| 32.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 32.2 DCC < 1 min and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 32.3 DCC at 1‐2 mins with baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 32.4 DCC at 1‐2 mins with baby low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 32.5 DCC at > 2 mins with baby level with uterus | 1 | 105 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.24, 2.64] |
| 32.6 DCC at > 2 mins with baby low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 32.7 Mixed interventions or unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 33 Blood transfusion for mother | 1 | 254 | Risk Ratio (M‐H, Random, 95% CI) | 1.59 [0.39, 6.51] |
| 33.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 33.2 DCC < 1 min and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 33.3 DCC at 1‐2 mins with baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 33.4 DCC at 1‐2 mins with baby low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 33.5 DCC at > 2 mins with baby level with uterus | 1 | 254 | Risk Ratio (M‐H, Random, 95% CI) | 1.59 [0.39, 6.51] |
| 33.6 DCC at > 2 mins with baby low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 33.7 Mixed interventions or unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 34 Postpartum infection in mother | 1 | 254 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.73, 1.72] |
| 34.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 34.2 DCC < 1 min and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 34.3 DCC at 1‐2 mins with baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 34.4 DCC at 1‐2 mins with baby low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 34.5 DCC at > 2 mins with baby level with uterus | 1 | 254 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.73, 1.72] |
| 34.6 DCC at > 2 mins with baby low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 34.7 Mixed interventions or unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35 Rhesus isoimmunisation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35.2 DCC < 1 min and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35.3 DCC at 1‐2 mins with baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35.4 DCC at 1‐2 mins with baby low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35.5 DCC at > 2 mins with baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35.6 DCC at > 2 mins with baby low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35.7 Mixed interventions or unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 36 Psychological well being in mother | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 36.1 DCC < 1 min and baby level with uterus | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 36.2 DCC < 1 min and baby held low | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 36.3 DCC 1‐2 mins and baby level with uterus | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 36.4 DCC 1‐2 mins and baby held low | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 36.5 DCC > 2 mins and baby level with uterus | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 36.6 DCC > 2 mins and baby held low | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 36.7 Mixed interventions or unclear | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 37 Bonding | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 37.1 DCC < 1 min and baby level with uterus | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 37.2 DCC < 1 min and baby held low | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 37.3 DCC 1‐2 mins and baby level with uterus | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 37.4 DCC 1‐2 mins and baby held low | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 37.5 DCC > 2 mins and baby level with uterus | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 37.6 DCC > 2 mins and baby held low | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 37.7 Mixed interventions or unclear | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 38 Breastfeeding initiation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 38.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 38.2 DCC < 1 min and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 38.3 DCC at 1‐2 mins with baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 38.4 DCC at 1‐2 mins with baby low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 38.5 DCC at > 2 mins with baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 38.6 DCC at > 2 mins with baby low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 38.7 Mixed interventions or unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 39 Fully breastfed or mixed feeding at infant discharge | 1 | 248 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.79, 1.22] |
| 39.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 39.2 DCC < 1 min and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 39.3 DCC at 1‐2 mins with baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 39.4 DCC at 1‐2 mins with baby low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 39.5 DCC at > 2 mins with baby level with uterus | 1 | 248 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.79, 1.22] |
| 39.6 DCC at > 2 mins with baby low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 39.7 Mixed interventions or unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 40 Maternal anxiety | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 40.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 40.2 DCC < 1 min and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 40.3 DCC at 1‐2 mins with baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 40.4 DCC at 1‐2 mins with baby low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 40.5 DCC at > 2 mins with baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 40.6 DCC at > 2 mins with baby low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 40.7 Mixed interventions or unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 41 Mothers' views | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 41.1 DCC < 1 min and baby level with uterus | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 41.2 DCC < 1 min and baby held low | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 41.3 DCC 1‐2 mins and baby level with uterus | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 41.4 DCC 1‐2 mins and baby held low | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 41.5 DCC > 2 mins and baby level with uterus | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 41.6 DCC > 2 mins and baby held low | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 41.7 Mixed interventions or unclear | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 5. DCC with immediate neonatal care after cord clamping vs UCM (subgroup analysis by gestation).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death of baby (up to discharge) | 3 | 322 | Risk Ratio (M‐H, Random, 95% CI) | 2.14 [0.93, 4.93] |
| 1.1 < 32‐34 weeks gestation | 3 | 322 | Risk Ratio (M‐H, Random, 95% CI) | 2.14 [0.93, 4.93] |
| 1.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Death or neurodevelopmental impairment at age two to three years | 2 | 195 | Risk Ratio (M‐H, Random, 95% CI) | 1.67 [0.78, 3.57] |
| 2.1 < 32‐34 weeks gestation | 2 | 195 | Risk Ratio (M‐H, Random, 95% CI) | 1.67 [0.78, 3.57] |
| 2.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Severe intraventricular haemorrhage (IVH grades 3, 4) | 1 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 2.63 [0.11, 61.88] |
| 3.1 < 32‐34 weeks gestation | 1 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 2.63 [0.11, 61.88] |
| 3.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Intraventricular haemorrhage (IVH, all grades) | 2 | 125 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [0.55, 3.17] |
| 4.1 < 32‐34 weeks gestation | 2 | 125 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [0.55, 3.17] |
| 4.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Periventricular leukomalacia (PVL) | 1 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 < 32‐34 weeks gestation | 1 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Chronic lung disease (CLD) ‐ oxygen supplement at 36 weeks (corrected for gestation) | 2 | 125 | Risk Ratio (M‐H, Random, 95% CI) | 1.53 [0.43, 5.48] |
| 6.1 < 32‐34 weeks gestation | 2 | 125 | Risk Ratio (M‐H, Random, 95% CI) | 1.53 [0.43, 5.48] |
| 6.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Maternal blood loss of 500 mL or greater | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.1 < 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Intraventricular haemorrhage (IVH, grades 1 & 2) | 1 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 1.74 [0.48, 6.30] |
| 8.1 < 32‐34 weeks gestation | 1 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 1.74 [0.48, 6.30] |
| 8.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Necrotising enterocolitis (NEC) confirmed by X‐ray or laparotomy) | 1 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 3.48 [0.41, 29.31] |
| 9.1 < 32‐34 weeks gestation | 1 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 3.48 [0.41, 29.31] |
| 9.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Respiratory Distress Syndrome (RDS) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.1 < 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Respiratory support (ventilator or CPAP) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.1 < 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Duration of respiratory support (days) | 1 | 67 | Mean Difference (IV, Random, 95% CI) | 1.80 [‐2.01, 5.61] |
| 12.1 < 32‐34 weeks gestation | 1 | 67 | Mean Difference (IV, Random, 95% CI) | 1.80 [‐2.01, 5.61] |
| 12.2 > 32‐34 weeks gestation | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.3 Mixed gestation | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Surfactant treatment (for severe RDS) | 1 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.66, 2.13] |
| 13.1 < 32‐34 weeks gestation | 1 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.66, 2.13] |
| 13.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Treatment for Patent Ductus Arteriosus (PDA) (medical and/or surgical) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.1 < 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Treatment for Retinopathy of Prematurity (RoP) | 1 | 67 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.23, 2.35] |
| 15.1 < 32‐34 weeks gestation | 1 | 67 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.23, 2.35] |
| 15.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Hyperbilirubinemia (treated by phototherapy) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.1 < 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Inotropics for low blood pressure | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.1 < 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Low Apgar as defined by trialists (generally < 8 at 5 mins) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.1 < 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Blood transfusion in infant | 1 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.48, 1.22] |
| 19.1 < 32‐34 weeks gestation | 1 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.48, 1.22] |
| 19.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20 Volume of blood transfused | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20.1 < 32‐34 weeks gestation | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20.2 > 32‐34 weeks gestation | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20.3 Mixed gestation | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 21 Late sepsis (after 3 days or as defined by trialists) | 1 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.06, 13.27] |
| 21.1 < 32‐34 weeks gestation | 1 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.06, 13.27] |
| 21.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 21.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 22 Hydrocephalus | 1 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 22.1 < 32‐34 weeks gestation | 1 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 22.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 22.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 23 Temperature < 36.0oC within 1 hour of birth | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 23.1 < 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 23.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 23.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 24 Hb within 1st 24 hour of birth (g/dL) | 1 | 58 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐1.57, 1.17] |
| 24.1 < 32‐34 weeks gestation | 1 | 58 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐1.57, 1.17] |
| 24.2 > 32‐34 weeks gestation | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 24.3 Mixed gestation | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 25 Mean arterial blood pressure (subgrouped by time after birth) | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 25.1 < 32‐34 weeks gestation | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 25.2 > 32‐34 weeks gestation | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 25.3 Mixed gestation | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26 Length of infant stay in NICU | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26.1 < 32‐34 weeks gestation | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26.2 > 32‐34 weeks gestation | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26.3 Mixed gestation | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 27 Home oxygen | 1 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.01, 6.88] |
| 27.1 < 32‐34 weeks gestation | 1 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.01, 6.88] |
| 27.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 27.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 28 Neurodevelopmental impairment at age two to three years | 2 | 174 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.04, 32.88] |
| 28.1 < 32‐34 weeks gestation | 2 | 174 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.04, 32.88] |
| 28.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 28.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29 Severe visual impairment | 1 | 39 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29.1 < 32‐34 weeks gestation | 1 | 39 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 30 Cerebral palsy (CP) | 1 | 39 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 30.1 < 32‐34 weeks gestation | 1 | 39 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 30.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 30.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 31 Manual removal of placenta (denominator = vaginal births) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 31.1 < 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 31.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 31.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 32 Prolonged third stage (> 30 minutes) (denominator = vaginal births) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 32.1 < 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 32.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 32.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 33 Blood transfusion for mother | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 33.1 < 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 33.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 33.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 34 Postpartum infection in mother | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 34.1 < 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 34.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 34.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35 Rhesus isoimmunisation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35.1 < 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 36 Psychological well being in mother | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 36.1 < 32‐34 weeks gestation | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 36.2 > 32‐34 weeks gestation | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 36.3 Mixed gestation | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 37 Bonding | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 37.1 < 32‐34 weeks gestation | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 37.2 > 32‐34 weeks gestation | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 37.3 Mixed gestation | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 38 Breastfeeding initiation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 38.1 < 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 38.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 38.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 39 Fully breastfed or mixed feeding at infant discharge | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 39.1 < 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 39.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 39.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 40 Maternal anxiety | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 40.1 < 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 40.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 40.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 41 Mothers' views | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 41.1 < 32‐34 weeks gestation | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 41.2 > 32‐34 weeks gestation | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 41.3 Mixed gestation | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 6. DCC with immediate neonatal care after cord clamping vs UCM (subgroup analysis by type of intervention).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death of baby (up to discharge) | 3 | 322 | Risk Ratio (M‐H, Random, 95% CI) | 2.14 [0.93, 4.93] |
| 1.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 DCC < 1 min and baby held low | 3 | 322 | Risk Ratio (M‐H, Random, 95% CI) | 2.14 [0.93, 4.93] |
| 1.3 DCC 1‐2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.4 DCC 1‐2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.5 DCC > 2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.6 DCC > 2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.7 Mixed interventions or unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Death or neurodevelopmental impairment at age two to three years | 2 | 195 | Risk Ratio (M‐H, Random, 95% CI) | 1.67 [0.78, 3.57] |
| 2.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 DCC < 1 min and baby held low | 2 | 195 | Risk Ratio (M‐H, Random, 95% CI) | 1.67 [0.78, 3.57] |
| 2.3 DCC 1‐2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.4 DCC 1‐2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.5 DCC > 2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.6 DCC > 2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.7 Mixed interventions or unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Severe intraventricular haemorrhage (IVH grades 3, 4) | 1 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 2.63 [0.11, 61.88] |
| 3.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 DCC < 1 min and baby held low | 1 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 2.63 [0.11, 61.88] |
| 3.3 DCC 1‐2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.4 DCC 1‐2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.5 DCC > 2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.6 DCC > 2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.7 Mixed interventions or unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Intraventricular haemorrhage (IVH, all grades) | 2 | 125 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [0.55, 3.17] |
| 4.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 DCC < 1 min and baby held low | 2 | 125 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [0.55, 3.17] |
| 4.3 DCC 1‐2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.4 DCC 1‐2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.5 DCC > 2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.6 DCC > 2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.7 Mixed interventions or unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Periventricular leukomalacia (PVL) | 1 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 DCC < 1 min and baby held low | 1 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 DCC 1‐2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.4 DCC 1‐2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.5 DCC > 2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.6 DCC > 2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.7 Mixed interventions or unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Chronic lung disease (CLD) ‐ oxygen supplement at 36 weeks (corrected for gestation) | 2 | 125 | Risk Ratio (M‐H, Random, 95% CI) | 1.53 [0.43, 5.48] |
| 6.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 DCC < 1 min and baby held low | 2 | 125 | Risk Ratio (M‐H, Random, 95% CI) | 1.53 [0.43, 5.48] |
| 6.3 DCC 1‐2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.4 DCC 1‐2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.5 DCC > 2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.6 DCC > 2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.7 Mixed interventions or unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Maternal blood loss of 500 mL or greater | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 DCC < 1 min and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.3 DCC 1‐2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.4 DCC 1‐2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.5 DCC > 2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.6 DCC > 2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.7 Mixed interventions or unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Intraventricular haemorrhage (IVH, grades 1 & 2) | 1 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 1.74 [0.48, 6.30] |
| 8.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 DCC < 1 min and baby held low | 1 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 1.74 [0.48, 6.30] |
| 8.3 DCC 1‐2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.4 DCC 1‐2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.5 DCC > 2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.6 DCC > 2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.7 Mixed interventions or unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Necrotising enterocolitis (NEC) confirmed by X ray or laparotomy) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 DCC < 1 min and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.3 DCC 1‐2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.4 DCC 1‐2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.5 DCC > 2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.6 DCC > 2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.7 Mixed interventions or unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Respiratory Distress Syndrome (RDS) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 DCC < 1 min and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.3 DCC 1‐2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.4 DCC 1‐2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.5 DCC > 2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.6 DCC > 2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.7 Mixed interventions or unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Respiratory support (ventilator or CPAP) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 DCC < 1 min and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.3 DCC 1‐2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.4 DCC 1‐2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.5 DCC > 2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.6 DCC > 2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.7 Mixed interventions or unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Duration of respiratory support (days) | 1 | 67 | Mean Difference (IV, Random, 95% CI) | 1.80 [‐2.01, 5.61] |
| 12.1 DCC < 1 min and baby level with uterus | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 DCC < 1 min and baby held low | 1 | 67 | Mean Difference (IV, Random, 95% CI) | 1.80 [‐2.01, 5.61] |
| 12.3 DCC 1‐2 mins and baby level with uterus | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.4 DCC 1‐2 mins and baby held low | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.5 DCC > 2 mins and baby level with uterus | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.6 DCC > 2 mins and baby held low | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.7 Mixed interventions or unclear | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Surfactant treatment (for severe RDS) | 1 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.66, 2.13] |
| 13.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 DCC < 1 min and baby held low | 1 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.66, 2.13] |
| 13.3 DCC 1‐2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.4 DCC 1‐2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.5 DCC > 2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.6 DCC > 2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.7 Mixed interventions or unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Treatment for Patent Ductus Arteriosus (PDA) (medical and/or surgical) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 DCC < 1 min and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.3 DCC 1‐2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.4 DCC 1‐2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.5 DCC > 2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.6 DCC > 2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.7 Mixed interventions or unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Treatment for Retinopathy of Prematurity (RoP) | 1 | 67 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.23, 2.35] |
| 15.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 DCC < 1 min and baby held low | 1 | 67 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.23, 2.35] |
| 15.3 DCC 1‐2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.4 DCC 1‐2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.5 DCC > 2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.6 DCC > 2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.7 Mixed interventions or unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Hyperbilirubinemia (treated by phototherapy) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.2 DCC < 1 min and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.3 DCC 1‐2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.4 DCC 1‐2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.5 DCC > 2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.6 DCC > 2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.7 Mixed interventions or unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Inotropics for low blood pressure | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 DCC < 1 min and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.3 DCC 1‐2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.4 DCC 1‐2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.5 DCC > 2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.6 DCC > 2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.7 Mixed interventions or unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Low Apgar as defined by trialists (generally < 8 at 5 mins) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.2 DCC < 1 min and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.3 DCC 1‐2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.4 DCC 1‐2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.5 DCC > 2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.6 DCC > 2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.7 Mixed interventions or unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Blood transfusion in infant | 1 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.48, 1.22] |
| 19.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19.2 DCC < 1 min and baby held low | 1 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.48, 1.22] |
| 19.3 DCC 1‐2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19.4 DCC 1‐2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19.5 DCC > 2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19.6 DCC > 2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19.7 Mixed interventions or unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20 Volume of blood transfused | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20.1 DCC < 1 min and baby level with uterus | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20.2 DCC < 1 min and baby held low | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20.3 DCC 1‐2 mins and baby level with uterus | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20.4 DCC 1‐2 mins and baby held low | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20.5 DCC > 2 mins and baby level with uterus | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20.6 DCC > 2 mins and baby held low | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20.7 Mixed interventions or unclear | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 21 Late sepsis (after 3 days or as defined by trialists) | 1 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.06, 13.27] |
| 21.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 21.2 DCC < 1 min and baby held low | 1 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.06, 13.27] |
| 21.3 DCC 1‐2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 21.4 DCC 1‐2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 21.5 DCC > 2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 21.6 DCC > 2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 21.7 Mixed interventions or unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 22 Hydrocephalus | 2 | 116 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 22.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 22.2 DCC < 1 min and baby held low | 2 | 116 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 22.3 DCC 1‐2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 22.4 DCC 1‐2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 22.5 DCC > 2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 22.6 DCC > 2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 22.7 Mixed interventions or unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 23 Temperature < 36.0oC within 1 hour of birth | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 23.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 23.2 DCC < 1 min and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 23.3 DCC 1‐2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 23.4 DCC 1‐2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 23.5 DCC > 2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 23.6 DCC > 2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 23.7 Mixed interventions or unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 24 Hb within 1st 24 hour of birth (g/dL) | 1 | 58 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐1.57, 1.17] |
| 24.1 DCC < 1 min and baby level with uterus | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 24.2 DCC < 1 min and baby held low | 1 | 58 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐1.57, 1.17] |
| 24.3 DCC 1‐2 mins and baby level with uterus | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 24.4 DCC 1‐2 mins and baby held low | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 24.5 DCC > 2 mins and baby level with uterus | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 24.6 DCC > 2 mins and baby held low | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 24.7 Mixed interventions or unclear | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 25 Mean arterial blood pressure (subgrouped by time after birth) | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 25.1 DCC < 1 min and baby level with uterus | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 25.2 DCC < 1 min and baby held low | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 25.3 DCC 1‐2 mins and baby level with uterus | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 25.4 DCC 1‐2 mins and baby held low | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 25.5 DCC > 2 mins and baby level with uterus | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 25.6 DCC > 2 mins and baby held low | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 25.7 Mixed interventions or unclear | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26 Length of infant stay in NICU | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26.1 DCC < 1 min and baby level with uterus | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26.2 DCC < 1 min and baby held low | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26.3 DCC 1‐2 mins and baby level with uterus | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26.4 DCC 1‐2 mins and baby held low | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26.5 DCC > 2 mins and baby level with uterus | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26.6 DCC > 2 mins and baby held low | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26.7 Mixed interventions or unclear | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 27 Home oxygen | 1 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.01, 6.88] |
| 27.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 27.2 DCC < 1 min and baby held low | 1 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.01, 6.88] |
| 27.3 DCC 1‐2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 27.4 DCC 1‐2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 27.5 DCC > 2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 27.6 DCC > 2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 27.7 Mixed interventions or unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 28 Neurodevelopmental impairment at age two to three years | 2 | 174 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.04, 32.88] |
| 28.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 28.2 DCC < 1 min and baby held low | 2 | 174 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.04, 32.88] |
| 28.3 DCC 1‐2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 28.4 DCC 1‐2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 28.5 DCC > 2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 28.6 DCC > 2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 28.7 Mixed interventions or unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29 Severe visual impairment | 1 | 39 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29.2 DCC < 1 min and baby held low | 1 | 39 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29.3 DCC 1‐2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29.4 DCC 1‐2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29.5 DCC > 2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29.6 DCC > 2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29.7 Mixed interventions or unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 30 Cerebral palsy (CP) | 1 | 39 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 30.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 30.2 DCC < 1 min and baby held low | 1 | 39 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 30.3 DCC 1‐2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 30.4 DCC 1‐2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 30.5 DCC > 2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 30.6 DCC > 2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 30.7 Mixed interventions or unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 31 Manual removal of placenta (denominator = vaginal births) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 31.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 31.2 DCC < 1 min and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 31.3 DCC 1‐2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 31.4 DCC 1‐2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 31.5 DCC > 2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 31.6 DCC > 2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 31.7 Mixed interventions or unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 32 Prolonged third stage (>30 minutes) (denominator = vaginal births) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 32.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 32.2 DCC < 1 min and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 32.3 DCC 1‐2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 32.4 DCC 1‐2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 32.5 DCC > 2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 32.6 DCC > 2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 32.7 Mixed interventions or unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 33 Blood transfusion for mother | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 33.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 33.2 DCC < 1 min and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 33.3 DCC 1‐2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 33.4 DCC 1‐2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 33.5 DCC > 2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 33.6 DCC > 2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 33.7 Mixed interventions or unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 34 Postpartum infection in mother | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 34.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 34.2 DCC < 1 min and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 34.3 DCC 1‐2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 34.4 DCC 1‐2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 34.5 DCC > 2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 34.6 DCC > 2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 34.7 Mixed interventions or unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35 Rhesus isoimmunisation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35.2 DCC < 1 min and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35.3 DCC 1‐2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35.4 DCC 1‐2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35.5 DCC > 2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35.6 DCC > 2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35.7 Mixed interventions or unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 36 Psychological well being in mother | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 36.1 DCC < 1 min and baby level with uterus | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 36.2 DCC < 1 min and baby held low | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 36.3 DCC 1‐2 mins and baby level with uterus | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 36.4 DCC 1‐2 mins and baby held low | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 36.5 DCC > 2 mins and baby level with uterus | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 36.6 DCC > 2 mins and baby held low | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 36.7 Mixed interventions or unclear | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 37 Bonding | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 37.1 DCC < 1 min and baby level with uterus | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 37.2 DCC < 1 min and baby held low | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 37.3 DCC 1‐2 mins and baby level with uterus | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 37.4 DCC 1‐2 mins and baby held low | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 37.5 DCC > 2 mins and baby level with uterus | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 37.6 DCC > 2 mins and baby held low | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 37.7 Mixed interventions or unclear | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 38 Breastfeeding initiation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 38.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 38.2 DCC < 1 min and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 38.3 DCC 1‐2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 38.4 DCC 1‐2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 38.5 DCC > 2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 38.6 DCC > 2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 38.7 Mixed interventions or unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 39 Fully breastfed or mixed feeding at infant discharge | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 39.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 39.2 DCC < 1 min and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 39.3 DCC 1‐2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 39.4 DCC 1‐2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 39.5 DCC > 2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 39.6 DCC > 2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 39.7 Mixed interventions or unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 40 Maternal anxiety | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 40.1 DCC < 1 min and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 40.2 DCC < 1 min and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 40.3 DCC 1‐2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 40.4 DCC 1‐2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 40.5 DCC > 2 mins and baby level with uterus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 40.6 DCC > 2 mins and baby held low | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 40.7 Mixed interventions or unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 41 Mothers' views | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 41.1 DCC < 1 min and baby level with uterus | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 41.2 DCC < 1 min and baby held low | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 41.3 DCC 1‐2 mins and baby level with uterus | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 41.4 DCC 1‐2 mins and baby held low | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 41.5 DCC > 2 mins and baby level with uterus | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 41.6 DCC > 2 mins and baby held low | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 41.7 Mixed interventions or unclear | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 7. UCM vs ECC (subgroup analysis by gestation).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death of baby (up to discharge) | 9 | 931 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.47, 1.41] |
| 1.1 < 32‐34 weeks gestation | 8 | 731 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.38, 1.29] |
| 1.2 > 32‐34 weeks gestation | 1 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 1.5 [0.44, 5.15] |
| 1.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Death or neurodevelopmental impairment at age two to three years | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.1 < 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Severe intraventricular haemorrhage (IVH grades 3, 4) | 6 | 618 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.39, 1.45] |
| 3.1 < 32‐34 weeks gestation | 6 | 618 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.39, 1.45] |
| 3.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Intraventricular haemorrhage (IVH, all grades) | 8 | 716 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.62, 1.18] |
| 4.1 < 32‐34 weeks gestation | 8 | 716 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.62, 1.18] |
| 4.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Periventricular leukomalacia (PVL) | 3 | 315 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.15, 2.63] |
| 5.1 < 32‐34 weeks gestation | 3 | 315 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.15, 2.63] |
| 5.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Chronic lung disease (CLD) ‐ oxygen supplement at 36 weeks (corrected for gestation) | 7 | 682 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.64, 1.66] |
| 6.1 < 32‐34 weeks gestation | 7 | 682 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.64, 1.66] |
| 6.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Maternal blood loss of 500 mL or greater | 1 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.1 < 32‐34 weeks gestation | 1 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Intraventricular haemorrhage (IVH, grades 1 & 2) | 6 | 618 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.44, 1.25] |
| 8.1 < 32‐34 weeks gestation | 6 | 618 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.44, 1.25] |
| 8.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Necrotising enterocolitis (NEC) confirmed by X‐ray or laparotomy) | 6 | 616 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.41, 1.38] |
| 9.1 < 32‐34 weeks gestation | 6 | 616 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.41, 1.38] |
| 9.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Respiratory Distress Syndrome (RDS) | 4 | 515 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.83, 1.32] |
| 10.1 < 32‐34 weeks gestation | 3 | 315 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.95, 1.05] |
| 10.2 > 32‐34 weeks gestation | 1 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.71, 5.64] |
| 10.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Respiratory support (ventilator or CPAP) | 2 | 129 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.74, 1.47] |
| 11.1 < 32‐34 weeks gestation | 2 | 129 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.74, 1.47] |
| 11.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Duration of respiratory support (days) | 1 | 199 | Mean Difference (IV, Random, 95% CI) | 2.80 [‐9.78, 15.38] |
| 12.1 < 32‐34 weeks gestation | 1 | 199 | Mean Difference (IV, Random, 95% CI) | 2.80 [‐9.78, 15.38] |
| 12.2 > 32‐34 weeks gestation | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.3 Mixed gestation | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Surfactant treatment (for severe RDS) | 5 | 433 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.81, 1.58] |
| 13.1 < 32‐34 weeks gestation | 5 | 433 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.81, 1.58] |
| 13.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Treatment for Patent Ductus Arteriosus (PDA) (medical and/or surgical) | 5 | 411 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.73, 1.38] |
| 14.1 < 32‐34 weeks gestation | 5 | 411 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.73, 1.38] |
| 14.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Treatment for Retinopathy of Prematurity (RoP) | 5 | 274 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.76, 1.19] |
| 15.1 < 32‐34 weeks gestation | 5 | 274 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.76, 1.19] |
| 15.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Hyperbilirubinemia (treated by phototherapy) | 3 | 475 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [0.73, 2.63] |
| 16.1 < 32‐34 weeks gestation | 2 | 275 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.86, 1.06] |
| 16.2 > 32‐34 weeks gestation | 1 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 3.67 [1.85, 7.26] |
| 16.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Inotropics for low blood pressure | 3 | 300 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.36, 1.04] |
| 17.1 < 32‐34 weeks gestation | 3 | 300 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.36, 1.04] |
| 17.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Low Apgar as defined by trialists (generally < 8 at 5 mins) | 2 | 398 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.67, 1.60] |
| 18.1 < 32‐34 weeks gestation | 2 | 398 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.67, 1.60] |
| 18.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Blood transfusion in infant | 6 | 567 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.57, 0.89] |
| 19.1 < 32‐34 weeks gestation | 6 | 567 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.57, 0.89] |
| 19.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20 Volume of blood transfused (mL) | 1 | 199 | Mean Difference (IV, Random, 95% CI) | ‐19.0 [‐39.61, 1.61] |
| 20.1 < 32‐34 weeks gestation | 1 | 199 | Mean Difference (IV, Random, 95% CI) | ‐19.0 [‐39.61, 1.61] |
| 20.2 > 32‐34 weeks gestation | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20.3 Mixed gestation | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 21 Late sepsis (after 3 days or as defined by trialists) | 4 | 385 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.64, 1.19] |
| 21.1 < 32‐34 weeks gestation | 4 | 385 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.64, 1.19] |
| 21.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 21.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 22 Hydrocephalus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 22.1 < 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 22.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 22.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 23 Temperature < 36.0oC within 1 hour of birth | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 23.1 < 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 23.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 23.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 24 Hb within 1st 24 hour of birth (g/dL) | 7 | 905 | Mean Difference (IV, Random, 95% CI) | 0.84 [0.54, 1.14] |
| 24.1 < 32‐34 weeks gestation | 6 | 705 | Mean Difference (IV, Random, 95% CI) | 0.87 [0.54, 1.20] |
| 24.2 > 32‐34 weeks gestation | 1 | 200 | Mean Difference (IV, Random, 95% CI) | 0.70 [0.00, 1.40] |
| 24.3 Mixed gestation | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 25 Mean arterial blood pressure | 2 | 408 | Mean Difference (IV, Random, 95% CI) | 0.38 [‐1.33, 2.09] |
| 25.1 < 32‐34 weeks gestation | 1 | 208 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐2.17, 2.17] |
| 25.2 > 32‐34 weeks gestation | 1 | 200 | Mean Difference (IV, Random, 95% CI) | 1.0 [‐1.76, 3.76] |
| 25.3 Mixed gestation | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26 Length of infant stay in NICU (in weeks) | 1 | 199 | Mean Difference (IV, Random, 95% CI) | 5.30 [‐5.49, 16.09] |
| 26.1 < 32‐34 weeks gestation | 1 | 199 | Mean Difference (IV, Random, 95% CI) | 5.30 [‐5.49, 16.09] |
| 26.2 > 32‐34 weeks gestation | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26.3 Mixed gestation | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 27 Home oxygen | 1 | 199 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.38, 2.10] |
| 27.1 < 32‐34 weeks gestation | 1 | 199 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.38, 2.10] |
| 27.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 27.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 28 Neurodevelopmental impairment at age two to three years | 2 | 187 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.49, 3.17] |
| 28.1 < 32‐34 weeks gestation | 2 | 187 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.49, 3.17] |
| 28.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 28.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29 Severe visual impairment | 1 | 125 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29.1 < 32‐34 weeks gestation | 1 | 125 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 30 Cerebral palsy (CP) | 2 | 286 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.05, 10.63] |
| 30.1 < 32‐34 weeks gestation | 2 | 286 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.05, 10.63] |
| 30.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 30.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 31 Manual removal of placenta (denominator = vaginal births) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 31.1 < 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 31.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 31.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 32 Prolonged third stage (> 30 minutes) (denominator = vaginal births) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 32.1 < 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 32.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 32.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 33 Blood transfusion for mother | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 33.1 < 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 33.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 33.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 34 Postpartum infection in mother | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 34.1 < 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 34.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 34.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35 Rhesus isoimmunisation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35.1 < 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 36 Psychological well being in mother | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 36.1 < 32‐34 weeks gestation | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 36.2 > 32‐34 weeks gestation | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 36.3 Mixed gestation | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 37 Bonding | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 37.1 < 32‐34 weeks gestation | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 37.2 > 32‐34 weeks gestation | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 37.3 Mixed gestation | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 38 Breastfeeding initiation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 38.1 < 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 38.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 38.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 39 Fully breastfed or mixed feeding at infant discharge | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 39.1 < 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 39.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 39.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 40 Maternal anxiety | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 40.1 < 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 40.2 > 32‐34 weeks gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 40.3 Mixed gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 41 Mothers' views | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 41.1 < 32‐34 weeks gestation | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 41.2 > 32‐34 weeks gestation | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 41.3 Mixed gestation | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 8. UCM vs ECC (subgroup analysis by type of intervention).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death of baby (up to discharge) | 9 | 931 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.47, 1.41] |
| 1.1 UCM with cord intact | 7 | 705 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.38, 1.34] |
| 1.2 UCM with cord cut | 1 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 1.5 [0.44, 5.15] |
| 1.3 Unclear | 1 | 26 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 7.50] |
| 2 Death or neurodevelopmental impairment at age two to three years | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.1 UCM with cord intact | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 UCM with cord cut | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Severe intraventricular haemorrhage (IVH grades 3, 4) | 6 | 618 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.39, 1.45] |
| 3.1 UCM with cord intact | 6 | 618 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.39, 1.45] |
| 3.2 UCM with cord cut | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 Unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Intraventricular haemorrhage (IVH, all grades) | 8 | 716 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.62, 1.18] |
| 4.1 UCM with cord intact | 7 | 691 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.58, 1.21] |
| 4.2 UCM with cord cut | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Unclear | 1 | 25 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.35, 2.41] |
| 5 Periventricular leukomalacia (PVL) | 3 | 315 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.15, 2.63] |
| 5.1 UCM with cord intact | 3 | 315 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.15, 2.63] |
| 5.2 UCM with cord cut | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 Unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Chronic lung disease (CLD) ‐ oxygen supplement at 36 weeks (corrected for gestation) | 7 | 682 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.64, 1.66] |
| 6.1 UCM with cord intact | 7 | 682 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.64, 1.66] |
| 6.2 UCM with cord cut | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 Unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Maternal blood loss of 500 mL or greater | 1 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.1 UCM with cord intact | 1 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 UCM with cord cut | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.3 Unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Intraventricular haemorrhage (IVH, grades 1 & 2) | 6 | 618 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.44, 1.25] |
| 8.1 UCM with cord intact | 6 | 618 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.44, 1.25] |
| 8.2 UCM with cord cut | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.3 Unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Necrotising enterocolitis (NEC) confirmed by X‐ray or laparotomy) | 6 | 616 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.41, 1.38] |
| 9.1 UCM with cord intact | 5 | 591 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.41, 1.38] |
| 9.2 UCM with cord cut | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.3 Unclear | 1 | 25 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Respiratory Distress Syndrome (RDS) | 4 | 515 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.83, 1.32] |
| 10.1 UCM with cord intact | 3 | 315 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.95, 1.05] |
| 10.2 UCM with cord cut | 1 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.71, 5.64] |
| 10.3 Unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Respiratory support (ventilator or CPAP) | 2 | 129 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.74, 1.47] |
| 11.1 UCM with cord intact | 2 | 129 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.74, 1.47] |
| 11.2 UCM with cord cut | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.3 Unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Duration of respiratory support (days) | 1 | 199 | Mean Difference (IV, Random, 95% CI) | 2.80 [‐9.78, 15.38] |
| 12.1 UCM with cord intact | 1 | 199 | Mean Difference (IV, Random, 95% CI) | 2.80 [‐9.78, 15.38] |
| 12.2 UCM with cord cut | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.3 Unclear | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Surfactant treatment (for severe RDS) | 5 | 433 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.81, 1.58] |
| 13.1 UCM with cord intact | 5 | 433 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.81, 1.58] |
| 13.2 UCM with cord cut | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.3 Unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Treatment for Patent Ductus Arteriosus (PDA) (medical and/or surgical) | 5 | 411 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.73, 1.38] |
| 14.1 UCM with cord intact | 5 | 411 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.73, 1.38] |
| 14.2 UCM with cord cut | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.3 Unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Treatment for Retinopathy of Prematurity (RoP) | 5 | 274 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.76, 1.19] |
| 15.1 UCM with cord intact | 5 | 274 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.76, 1.19] |
| 15.2 UCM with cord cut | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.3 Unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Hyperbilirubinemia (treated by phototherapy) | 3 | 475 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [0.73, 2.63] |
| 16.1 UCM with cord intact | 2 | 275 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.86, 1.06] |
| 16.2 UCM with cord cut | 1 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 3.67 [1.85, 7.26] |
| 16.3 Unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Inotropics for low blood pressure | 3 | 280 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.36, 1.09] |
| 17.1 UCM with cord intact | 3 | 280 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.36, 1.09] |
| 17.2 UCM with cord cut | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.3 Unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Low Apgar as defined by trialists (generally < 8 at 5 mins) | 2 | 398 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.67, 1.60] |
| 18.1 UCM with cord intact | 2 | 398 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.67, 1.60] |
| 18.2 UCM with cord cut | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.3 Unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Blood transfusion in infant (mL) | 6 | 567 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.54, 0.84] |
| 19.1 UCM with cord intact | 5 | 413 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.51, 0.93] |
| 19.2 UCM with cord cut | 1 | 154 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.43, 0.90] |
| 19.3 Unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20 Volume of blood transfused | 1 | 199 | Mean Difference (IV, Random, 95% CI) | ‐19.0 [‐39.61, 1.61] |
| 20.1 UCM with cord intact | 1 | 199 | Mean Difference (IV, Random, 95% CI) | ‐19.0 [‐39.61, 1.61] |
| 20.2 UCM with cord cut | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20.3 Unclear | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 21 Late sepsis (after 3 days or as defined by trialists) | 4 | 385 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.64, 1.19] |
| 21.1 UCM with cord intact | 4 | 385 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.64, 1.19] |
| 21.2 UCM with cord cut | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 21.3 Unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 22 Hydrocephalus | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 22.1 UCM with cord intact | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 22.2 UCM with cord cut | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 22.3 Unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 23 Temperature < 36.0oC within 1 hour of birth | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 23.1 UCM with cord intact | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 23.2 UCM with cord cut | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 23.3 Unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 24 Hb within 1st 24 hour of birth (g/dL) | 7 | 905 | Mean Difference (IV, Random, 95% CI) | 0.84 [0.54, 1.14] |
| 24.1 UCM with cord intact | 4 | 526 | Mean Difference (IV, Random, 95% CI) | 0.76 [0.36, 1.16] |
| 24.2 UCM with cord cut | 2 | 354 | Mean Difference (IV, Random, 95% CI) | 0.97 [0.48, 1.46] |
| 24.3 Unclear | 1 | 25 | Mean Difference (IV, Random, 95% CI) | 0.5 [‐1.33, 2.33] |
| 25 Mean arterial blood pressure (subgrouped by time after birth) | 2 | 408 | Mean Difference (IV, Random, 95% CI) | 0.38 [‐1.33, 2.09] |
| 25.1 UCM with cord intact | 1 | 208 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐2.17, 2.17] |
| 25.2 UCM with cord cut | 1 | 200 | Mean Difference (IV, Random, 95% CI) | 1.0 [‐1.76, 3.76] |
| 25.3 Unclear | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26 Length of infant stay in NICU | 1 | 199 | Mean Difference (IV, Random, 95% CI) | 5.30 [‐5.49, 16.09] |
| 26.1 UCM with cord intact | 1 | 199 | Mean Difference (IV, Random, 95% CI) | 5.30 [‐5.49, 16.09] |
| 26.2 UCM with cord cut | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26.3 Unclear | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 27 Home oxygen | 1 | 199 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.38, 2.10] |
| 27.1 UCM with cord intact | 1 | 199 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.38, 2.10] |
| 27.2 UCM with cord cut | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 27.3 Unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 28 Neurodevelopmental impairment at age two to three years | 2 | 187 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.49, 3.17] |
| 28.1 UCM with cord intact | 2 | 187 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.49, 3.17] |
| 28.2 UCM with cord cut | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 28.3 Unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29 Severe visual impairment | 1 | 125 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29.1 UCM with cord intact | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29.2 UCM with cord cut | 1 | 125 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29.3 Unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 30 Cerebral palsy (CP) | 2 | 286 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.05, 10.63] |
| 30.1 UCM with cord intact | 1 | 161 | Risk Ratio (M‐H, Random, 95% CI) | 2.65 [0.88, 7.97] |
| 30.2 UCM with cord cut | 1 | 125 | Risk Ratio (M‐H, Random, 95% CI) | 0.17 [0.04, 0.73] |
| 30.3 Unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 31 Manual removal of placenta (denominator = vaginal births) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 31.1 UCM with cord intact | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 31.2 UCM with cord cut | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 31.3 Unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 32 Prolonged third stage (>30 minutes) (denominator = vaginal births) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 32.1 UCM with cord intact | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 32.2 UCM with cord cut | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 32.3 Unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 33 Blood transfusion for mother | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 33.1 UCM with cord intact | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 33.2 UCM with cord cut | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 33.3 Unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 34 Postpartum infection in mother | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 34.1 UCM with cord intact | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 34.2 UCM with cord cut | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 34.3 Unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35 Rhesus isoimmunisation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35.1 UCM with cord intact | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35.2 UCM with cord cut | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35.3 Unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 36 Psychological well being in mother | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 36.1 UCM with cord intact | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 36.2 UCM with cord cut | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 36.3 Unclear | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 37 Bonding | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 37.1 UCM with cord intact | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 37.2 UCM with cord cut | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 37.3 Unclear | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 38 Breastfeeding initiation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 38.1 UCM with cord intact | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 38.2 UCM with cord cut | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 38.3 Unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 39 Fully or mixed feeding at infant discharge | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 39.1 UCM with cord intact | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 39.2 UCM with cord cut | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 39.3 Unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 40 Maternal anxiety | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 40.1 UCM with cord intact | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 40.2 UCM with cord cut | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 40.3 Unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 41 Mothers' views | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 41.1 UCM with cord intact | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 41.2 UCM with cord cut | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 41.3 Unclear | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 9. DCC with immediate neonatal care after cord clamping vs ECC (low risk of bias).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death of baby (up to discharge) | 5 | 1804 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.51, 0.97] |
| 2 Death or neurodevelopmental impairment in early years | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Severe intraventricular haemorrhage (IVH grades 3, 4) | 4 | 1689 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.54, 1.32] |
| 4 Intraventricular haemorrhage (IVH, all grades) | 4 | 1689 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.52, 1.18] |
| 5 Periventricular leukomalacia (PVL) | 2 | 1448 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.23, 1.19] |
| 6 Chronic lung disease (CLD) ‐ oxygen supplement at 36 weeks (corrected for gestation) | 4 | 1587 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.95, 1.15] |
| 7 Maternal blood loss of 500 mL or greater | 1 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
9.1. Analysis.
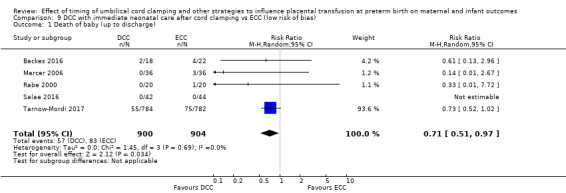
Comparison 9 DCC with immediate neonatal care after cord clamping vs ECC (low risk of bias), Outcome 1 Death of baby (up to discharge).
9.3. Analysis.
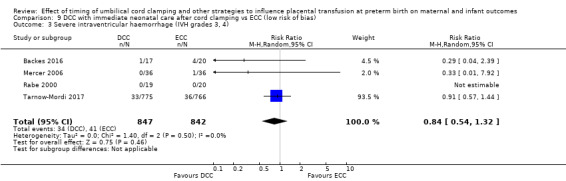
Comparison 9 DCC with immediate neonatal care after cord clamping vs ECC (low risk of bias), Outcome 3 Severe intraventricular haemorrhage (IVH grades 3, 4).
9.4. Analysis.
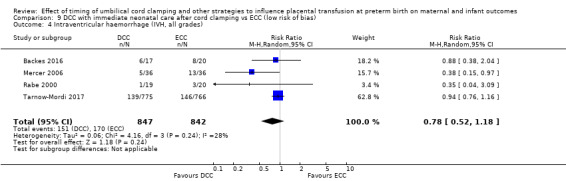
Comparison 9 DCC with immediate neonatal care after cord clamping vs ECC (low risk of bias), Outcome 4 Intraventricular haemorrhage (IVH, all grades).
9.5. Analysis.
Comparison 9 DCC with immediate neonatal care after cord clamping vs ECC (low risk of bias), Outcome 5 Periventricular leukomalacia (PVL).
9.6. Analysis.
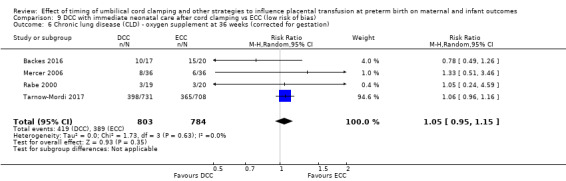
Comparison 9 DCC with immediate neonatal care after cord clamping vs ECC (low risk of bias), Outcome 6 Chronic lung disease (CLD) ‐ oxygen supplement at 36 weeks (corrected for gestation).
9.7. Analysis.
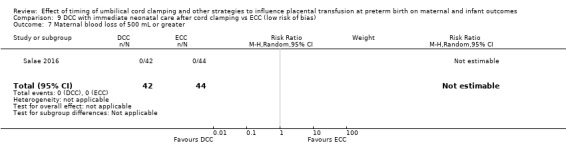
Comparison 9 DCC with immediate neonatal care after cord clamping vs ECC (low risk of bias), Outcome 7 Maternal blood loss of 500 mL or greater.
Comparison 10. DCC with immediate neonatal care with cord intact vs ECC (low risk of bias).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death of baby (up to discharge) | 1 | 270 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.20, 1.11] |
| 2 Death or neurodevelopmental impairment in early years | 1 | 218 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.39, 0.96] |
| 3 Severe intraventricular haemorrhage (IVH grades 3, 4) | 1 | 266 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.29, 2.45] |
| 4 Intraventricular haemorrhage (IVH, all grades) | 1 | 266 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.64, 1.26] |
| 5 Periventricular leukomalacia (PVL) | 1 | 266 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.32, 2.31] |
| 6 Chronic lung disease (CLD) ‐ oxygen supplement at 36 weeks (corrected for gestation) | 1 | 249 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.66, 1.37] |
| 7 Maternal blood loss of 500 mL or greater | 1 | 254 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.72, 1.22] |
10.1. Analysis.
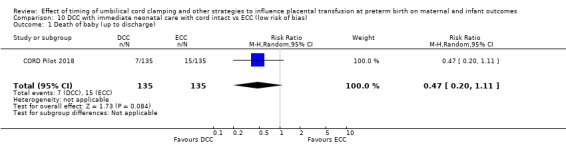
Comparison 10 DCC with immediate neonatal care with cord intact vs ECC (low risk of bias), Outcome 1 Death of baby (up to discharge).
10.2. Analysis.
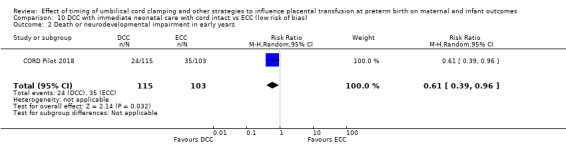
Comparison 10 DCC with immediate neonatal care with cord intact vs ECC (low risk of bias), Outcome 2 Death or neurodevelopmental impairment in early years.
10.3. Analysis.
Comparison 10 DCC with immediate neonatal care with cord intact vs ECC (low risk of bias), Outcome 3 Severe intraventricular haemorrhage (IVH grades 3, 4).
10.4. Analysis.
Comparison 10 DCC with immediate neonatal care with cord intact vs ECC (low risk of bias), Outcome 4 Intraventricular haemorrhage (IVH, all grades).
10.5. Analysis.
Comparison 10 DCC with immediate neonatal care with cord intact vs ECC (low risk of bias), Outcome 5 Periventricular leukomalacia (PVL).
10.6. Analysis.
Comparison 10 DCC with immediate neonatal care with cord intact vs ECC (low risk of bias), Outcome 6 Chronic lung disease (CLD) ‐ oxygen supplement at 36 weeks (corrected for gestation).
10.7. Analysis.
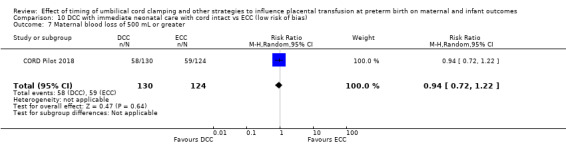
Comparison 10 DCC with immediate neonatal care with cord intact vs ECC (low risk of bias), Outcome 7 Maternal blood loss of 500 mL or greater.
Comparison 11. DCC with immediate neonatal care after cord clamping vs UCM (low risk of bias).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death of baby (up to discharge) | 1 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 1.74 [0.35, 8.78] |
| 2 Death or neurodevelopmental impairment in early years | 1 | 45 | Risk Ratio (M‐H, Random, 95% CI) | 3.43 [0.77, 15.20] |
| 3 Severe intraventricular haemorrhage (IVH grades 3, 4) | 1 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 2.63 [0.11, 61.88] |
| 4 Intraventricular haemorrhage (IVH, all grades) | 1 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 2.03 [0.58, 7.09] |
| 5 Periventricular leukomalacia (PVL) | 1 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Chronic lung disease (CLD) ‐ oxygen supplement at 36 weeks (corrected for gestation) | 1 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.28, 4.73] |
| 7 Maternal blood loss of 500 mL or greater | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
11.1. Analysis.
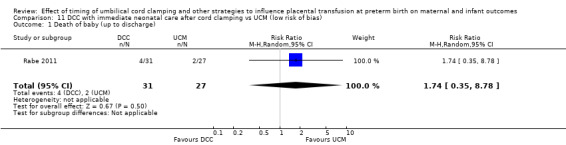
Comparison 11 DCC with immediate neonatal care after cord clamping vs UCM (low risk of bias), Outcome 1 Death of baby (up to discharge).
11.2. Analysis.
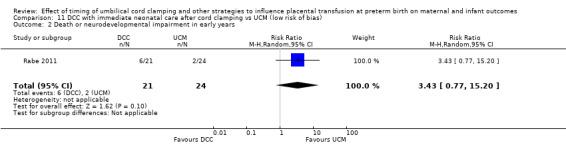
Comparison 11 DCC with immediate neonatal care after cord clamping vs UCM (low risk of bias), Outcome 2 Death or neurodevelopmental impairment in early years.
11.3. Analysis.
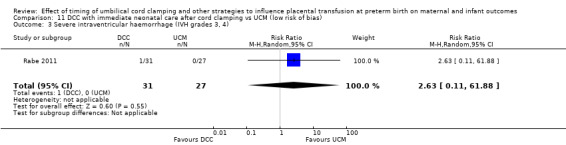
Comparison 11 DCC with immediate neonatal care after cord clamping vs UCM (low risk of bias), Outcome 3 Severe intraventricular haemorrhage (IVH grades 3, 4).
11.4. Analysis.
Comparison 11 DCC with immediate neonatal care after cord clamping vs UCM (low risk of bias), Outcome 4 Intraventricular haemorrhage (IVH, all grades).
11.5. Analysis.
Comparison 11 DCC with immediate neonatal care after cord clamping vs UCM (low risk of bias), Outcome 5 Periventricular leukomalacia (PVL).
11.6. Analysis.
Comparison 11 DCC with immediate neonatal care after cord clamping vs UCM (low risk of bias), Outcome 6 Chronic lung disease (CLD) ‐ oxygen supplement at 36 weeks (corrected for gestation).
Comparison 12. UCM vs ECC (low risk of bias).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death of baby (up to discharge) | 4 | 533 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.53, 2.62] |
| 2 Death or neurodevelopmental impairment in early years | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Severe intraventricular haemorrhage (IVH grades 3, 4) | 2 | 260 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.23, 2.23] |
| 4 Intraventricular haemorrhage (IVH, all grades) | 3 | 333 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.50, 1.31] |
| 5 Periventricular leukomalacia (PVL) | 1 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 3.06 [0.13, 74.23] |
| 6 Chronic lung disease (CLD) ‐ oxygen supplement at 36 weeks (corrected for gestation) | 3 | 330 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.44, 1.64] |
| 7 Maternal blood loss of 500 mL or greater | 1 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
12.1. Analysis.
Comparison 12 UCM vs ECC (low risk of bias), Outcome 1 Death of baby (up to discharge).
12.3. Analysis.
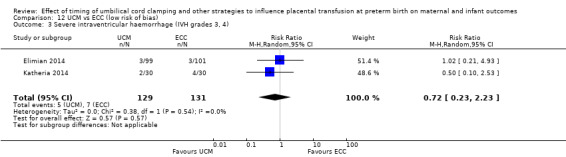
Comparison 12 UCM vs ECC (low risk of bias), Outcome 3 Severe intraventricular haemorrhage (IVH grades 3, 4).
12.4. Analysis.
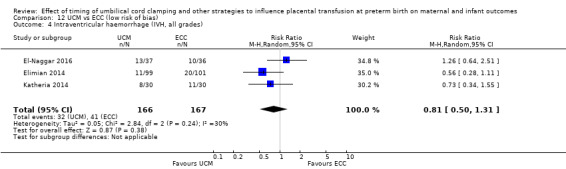
Comparison 12 UCM vs ECC (low risk of bias), Outcome 4 Intraventricular haemorrhage (IVH, all grades).
12.5. Analysis.
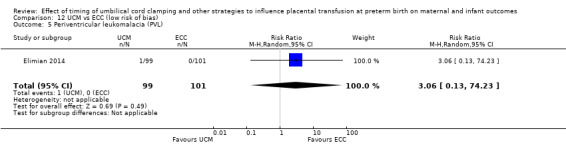
Comparison 12 UCM vs ECC (low risk of bias), Outcome 5 Periventricular leukomalacia (PVL).
12.6. Analysis.
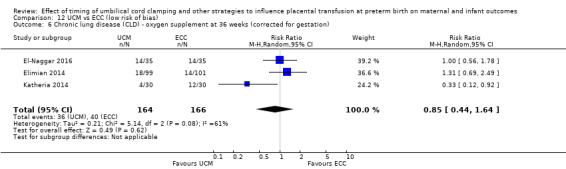
Comparison 12 UCM vs ECC (low risk of bias), Outcome 6 Chronic lung disease (CLD) ‐ oxygen supplement at 36 weeks (corrected for gestation).
12.7. Analysis.
Comparison 12 UCM vs ECC (low risk of bias), Outcome 7 Maternal blood loss of 500 mL or greater.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aladangady 2006.
| Methods | Randomised controlled trial, stratified randomisation list for gestational age group (24‐26, 27‐29, 30‐32 weeks) and mode of birth (vaginal/caesarean). | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: DCC
Comparator: ECC
Additional information
Comparison 3 DCC with neonatal resuscitation with cord intact (subgroup by gestation) Subgroup 1: < 32‐34 weeks' gestation Comparison 4 DCC with neonatal resuscitation with cord intact (subgroup by type of intervention) Subgroup 7: mixed intervention |
|
| Outcomes |
Primary outcome
Other outcomes
|
|
| Notes |
Setting: Tertiary Perinatal Centre, Queen Mother’s Hospital, Glasgow, UK Dates: not reported Declaration of interest: not reported Trial funding source: quote: "Well Being,” a research grant from the Royal College of Obstetricians and Gynaecologists, for invaluable financial assistance." Further information:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Randomisation was performed by a stratified randomisation list, just before delivery”. For stratification gestational age and type of birth "were taken account of". It was not clear how this sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | Randomisation occurred quote: “just before delivery”. There is no information about whether allocation was concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | For this type of intervention blinding participants and the staff present at the birth to the group allocation is not possible. Staff providing care may have modified their behaviour according to randomisation group. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It is not stated whether assessment of outcome was blinded but the outcomes reported (mean fetal blood volume and Hct levels) are objective. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 46 mother‐infant pairs were randomised and all infants appeared to be accounted for in the analysis. Although 3/23 allocated to delayed clamping actually had early clamping (1 due to short cord, 2 asked for by neonatologist) there was an ITT analysis. |
| Selective reporting (reporting bias) | High risk | No clinical outcomes were reported. It was stated that quote: “clinical outcomes were not analyzed", implying they were collected. For Baenziger 2007, which was a subset of the same multicentre study the reported outcomes were quote "blood volume, need for red cell transfusion, and respiratory and neurological complications". |
| Other bias | Unclear risk | The study was stratified to reduce baseline imbalance. |
Alan 2014.
| Methods | Randomised controlled trial | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: UCM
Comparator: ECC
Additional information
Comparison 7 UCM vs ECC (subgroup by gestation) Subgroup 1: < 32‐34 weeks' gestation Comparison 8 UCM vs ECC (subgroup by type of intervention) Subgroup 1: cord intact during UCM |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Setting: Ankara, Turkey Dates: April 2011 to February 2013 Declaration of interest: not reported Trial funding source: not reported Further information:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Just reports 'randomly assigned'. |
| Allocation concealment (selection bias) | Unclear risk | Quote:"...Sequentially numbered sealed nontransparent envelopes...", however, it is not possible to have concealment of allocation if sequence generation is unclear. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote:"The intervention was unmasked for the attending neonatal and obstetric teams in the delivery room." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There is no mention of whether the authors tried to blind outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 48 randomised to 24 each group. UCM: 2 excluded in delivery room for inappropriate milking and 3 excluded later because of death or major bleeding. So N = 19 for analysis – loss of 5/24 = 21% ECC: 2 excluded in delivery room because of death or major bleeding and 3 excluded later because of death or major bleeding. So N = 19 for analysis ‐ loss of 5/24 = 21% |
| Selective reporting (reporting bias) | Low risk | Very comprehensive outcome measures listed in the methods section. |
| Other bias | Unclear risk | Baseline demographics were similar. Trial was small for assessing clinical outcomes, no other biases apparent. |
Armanian 2017.
| Methods | Randomised controlled trial | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: DCC
Comparator: ECC
|
|
| Outcomes |
Primary
Secondary
Comparison 1 DCC with neonatal resuscitation after cord clamping vs ECC (subgroup by gestation) Subgroup 1: < 32‐34 weeks' gestation Comparison 2 DCC with neonatal resuscitation after cord clamping vs ECC (subgroup by type of intervention) Subgroup 7: type of intervention unclear |
|
| Notes |
Setting: Iran Dates: July 2014 to Feb 2015 Funding source: not reported. Quote: "This paper is derived from a research project no. 292270 in the Isfahan University of Medical Sciences." Declaration of interest: not reported Further information:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote:“…table of random numbers…” |
| Allocation concealment (selection bias) | Low risk | Quote:“Central allocation with telephone.” Personal communication from Amir Armanian. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Cannot blind clinicians (confirmed by trial registration form) and it is unclear if women knew or not. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Although Figure 2 reports no loss to follow‐up, the Figure reports:
These exclusions will necessarily have come after randomisation and after cord clamping, so were post‐randomisation exclusions 35/74 + 35 = 35/109 = 32%. We are checking this with A. Armanian |
| Selective reporting (reporting bias) | Unclear risk | All outcomes listed in trial registration were reported on, but there were additional outcomes reported that were not listed in the trial registration form (NEC; RoP). We have not assessed the trial protocol. |
| Other bias | Unclear risk | Very little information on trial methods. |
Backes 2016.
| Methods | Randomised controlled trial | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: DCC
Comparator: ECC
Additional information
Comparison 1 DCC with neonatal resuscitation after cord clamping vs ECC (subgroup by gestation) Subgroup 1: < 32‐34 weeks' gestation Comparison 2 DCC with neonatal resuscitation after cord clamping vs ECC (subgroup by type of intervention) Subgroup 2: DCC at < 1 min with baby low (+ gravity) |
|
| Outcomes | Circulating progenitor cell types in postnatal days 1‐30; IVH grades 3 and 4; Infant mortality; infant Hct. | |
| Notes |
Setting: nationwide Children's Hospital, Ohio State University Wexner Medical Center, Ohio, USA Dates: August 2009 to December 2013 Declaration of interest: quote:"The authors declare no conflict of interest.". Trial funding source: quote:“The present work is supported in part by a grant from the American Heart Association (# 10CRP3730033, CHB) and by internal funding provided by Nationwide Children’s Hospital Research Institute.”. Further information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote:"A random number system was generated by a statistician not involved in the study." |
| Allocation concealment (selection bias) | Low risk | Quote:“Laminated cards for randomization were maintained in sealed, opaque envelopes. Study personnel provided contact information to labor and delivery staff to notify them of potential study participants or the impending delivery of previously enrolled subjects. When called for a subject’s impending delivery, the team member opened the next randomization card..." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Cannnot blind clinicians to intervention, and no information as to whether women knew or not. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote:“None of the study members present at the time of randomization or aware of group assignment participated in the daily clinical care of study patients.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 40 infants enrolled and no losses, although authors did exclude babies who died on delivery suite from their denominator data but we will include these babies in our denominator data as normal. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol. |
| Other bias | Low risk | Baseline characteristics similar (gestational age; gender; small for gestational age; birthweight. No infants assigned to DCC received ECC to expedite resuscitation. No other biases apparent. |
Baenziger 2007.
| Methods | Randomised controlled trial, stratified randomisation list for gestational age group (24‐26, 27‐29, 30‐32 weeks) and mode of birth (vaginal/caesarean). | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: DCC
Comparator: ECC
Additional information
Comparison 1 DCC with neonatal resuscitation after cord clamping vs ECC (subgroup by gestation) Subgroup 1: < 32‐34 weeks' gestation Comparison 2 DCC with neonatal resuscitation after cord clamping vs ECC (subgroup by type intervention) Subgroup 4: DCC at 1‐2 mins with baby low (+ gravity) |
|
| Outcomes | Outcomes: cerebral oxygenation evaluated by NIRS at 4, 24 and 72 hrs of age, mechanical ventilation, death before discharge from hospital. | |
| Notes |
Setting: Zurich, Switzerland Dates: September 1996 to July 1997 Declaration of interest: quote:“The authors have indicated they have no financial relationships relevant to this article to disclose.”. Trial funding source: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Part of the same multicentre study as Aladangady 2006. Described as 'selected randomly and assigned to an experimental group or a control group by a central study co‐ordinator'. The uneven group size (15 vs 24) is discussed as being due to central randomisation for a larger study, and the primary outcome for the larger study was not tissue oxygenation (the primary outcome for this report). This suggests that there may have been post randomisation exclusions of babies who did not have tissue oxygenation measured. |
| Allocation concealment (selection bias) | Unclear risk | Part of the same multicentre study as Aladangady 2006. Described as 'selected randomly and assigned to an experimental group or a control group by a central study co‐ordinator'. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | For this type of intervention blinding participants and the staff present at delivery to group allocation is not possible. Staff providing care may have modified their behaviour according to randomisation group. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Obstetricians were informed of the study allocation, and it was stated that the neonatologist was not aware of the timing of cord clamping. It is not clear whether outcome assessment was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There were missing data for some outcomes. |
| Selective reporting (reporting bias) | High risk | This study was part of a larger multicentre study. The outcome of tissue oxygenation reported here was collected just for this subset, and the text implies have been post randomisation exclusions of infants who did not have tissue oxygenation measured. The outcomes in the main study were quote:"blood volume, need for red cell transfusion, and respiratory and neurological complications", but these data are not reported. |
| Other bias | High risk | Uneven group size although the characteristics of the groups appeared similar. |
Chu 2011.
| Methods | Randomisd controlled trial | |
| Participants |
Inclusion criteria
Exclusion criteria |
|
| Interventions |
Intervention: DCC
Comparator: ECC
Additional information
Comparison 1 DCC with neonatal resuscitation after cord clamping vs ECC (subgroup by gestation) Subgroup 1: < 32‐34 weeks' gestation Comparison 2 DCC with neonatal resuscitation after cord clamping vs ECC (subgroup by type intervention) Subgroup 1: DCC at < 1 min with baby level with uterus and placenta |
|
| Outcomes | IVH, sepsis, anaemia, and hyperbilirubinaemia | |
| Notes |
Setting: Toronto, Canada Dates: not reported Trial funding source: not reported Declaration of interest: not reported Further information:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No information. Clinicians at birth likely to be unblinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses reported |
| Selective reporting (reporting bias) | Unclear risk | We did not assess trial protocol |
| Other bias | Unclear risk | Compliance: 1 protocol violation ‐ not told which group. No other information. Conference abstract only. |
CORD Pilot 2018.
| Methods | Multicentre randomised controlled trial (8 UK maternity units). Stratified by centre with balanced blocks of varying size. | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: DCC with immediate neonatal care with intact cord (DCC‐ICCI)
Comparator: ECC
Both groups
Additional information
Comparison 3 DCC with immediate neonatal care with intact cord (subgroup by gestation) Subgroup 1: < 32‐34 weeks' gestation Comparison 4 DCC with immediate neonatal with intact cord (subgroup by type of intervention) Subgroup 5: DCC at > 2 mins with baby level with uterus and placenta |
|
| Outcomes |
Primary
Secondary Baby
Mother
Father
|
|
| Notes |
Setting: 8 tertiary maternity units in UK, all with NICUs Dates: March 2013 to February 2015 Further information on data included
Declaration of interest: all authors declare no support from any organisation for the submitted work other than the NIHR programme grant; no financial relationships with any organisations that might have an interest in the submitted work in the previous 3 years; the grant funded research included development of a neonatal resuscitation trolley now marketed as ’LifeStart’ and purchased by 2 sites for use in this trial, several authors were involved in development of the trolley but have no further relationship with the manufacturer; no other relationships or activities that could appear to have influenced the submitted work. Trial funding source: this trial is independent research funded by the National Institute for HealthResearch (NIHR) under its Programme Grants for Applied Research funding scheme (RPPG‐0609‐10107). The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health. The funder had no role in study design,conduct, analysis or reporting. Trial coordination was at the Nottingham Clinical Trials Unit (NCTU). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote:“Sequence generation was by computer, stratified by centre with balanced blocks of randomly varying size, created by NCTU.” |
| Allocation concealment (selection bias) | Low risk | Quote:“…sealed consecutively numbered opaque envelope … On the envelope was a label to record the date, time, woman’s initials, her date of birth and gestation. Once this label was completed she was considered randomized, even if the envelope was not opened.” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Attending clinicians could not be blinded and there is no information about whether the mother knew or not. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | For the primary outcomes, death is an objective outcome. Cranial ultrasound scan reports were reviewed by a single assessor blind to the allocated group. Independent adjudication of the ultrasound scans was by 8 trained neonatologists or radiologists, blind to allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6 women gave birth after 35+6 weeks' gestation and were excluded. 1 women withdrew consent and outcome data are only reported for 'death before discharge'. |
| Selective reporting (reporting bias) | Unclear risk | The trial protocol was for a feasibility study and clinical outcomes are unclear. |
| Other bias | Low risk | No other biases apparent. |
Dai 2014.
| Methods | Randomised controlled trial | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: DCC
Comparator: ECC
Additional information
Comparison 1 DCC with neonatal resuscitation after cord clamping vs ECC (subgroup by gestation) Subgroup 3: mixed gestation Comparison 2 DCC with neonatal resuscitation after cord clamping vs ECC (subgroup by type of intervention) Subgroup 7: DCC at < 1 min with timing of delay unclear |
|
| Outcomes |
|
|
| Notes |
Setting: China Dates: not reported Declaration of interest: not reported Funding source: Zhejiang Province Science and Technology Bureau of Science and Technology Research Project (Y20120237) Further information:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | The only information is 'Randomisation was done for the participating mothers using a random number table'. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | There is no mention of blinding though the clinicians at the birth cannot be blinded to the intervention but it unclear if women were blinded or not. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All women who were enrolled completed the study and their data were analysed. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol although all outcomes stated in the methodology in the paper were reported. |
| Other bias | Unclear risk | Generally seems fine. Values were reported without blanket P value statements. However, since information on methodology is limited in the paper and the number of babies in each preterm group are not similar (1 and 31), so we assessed it is unclear on this item. |
Das 2018.
| Methods | Randomised controlled trial ‐ subgroup | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: UCM
Comparator: ECC
Additional information
Comparison 7 UCM vs ECC (subgroup by gestation) Subgroup 1: < 32‐34 weeks' gestation Comparison 2 UCM vs ECC (subgroup by type intervention) Subgroup 2: after cord clamping |
|
| Outcomes |
Primary
Secondary ‐ Incidence of following at 40 weeks postnatal age:
|
|
| Notes |
Setting: tertiary care hospital and the neonatal unit in Northern India Dates: November 2012 to December 2013 Declaration of interest: nothing to disclose Trial funding source: no funding required. Primary sponsor: PGIMER Chandigarh‐160012 (Trial registration form) Other information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “…Random sequence was generated using a secure web based randomization algorithm (http//randomization.com) within two strata separately (30‐31 weeks and 32‐33 weeks) in blocks of variable sizes…” |
| Allocation concealment (selection bias) | Low risk | Quote: “…Allocation was kept concealed by placing the sequence in serially numbered, sealed and opaque envelopes…” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open label |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: “… laboratory person who analyzed the serum ferritin levels was blinded to group allocation…” but it is unclear regarding the clinical outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Data on ferritin seem to be complete. Unclear on clinical outcomes. |
| Selective reporting (reporting bias) | High risk | This is a subgroup of the main trial and also the registration document states many clinical outcomes which are not reported here. |
| Other bias | Unclear risk | No other biases apparent but this is only a subgroup of main trial. |
Datta 2017.
| Methods | Randomised controlled trial ‐ open label | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: DCC
Comparator: ECC
Additional information
Comparison 1: but no usable data DCC with neonatal resuscitation after cord clamping vs ECC (subgroup by gestation) Subgroup 2: > 32‐34 weeks' gestation Comparison 2: but no usable data DCC with neonatal resuscitation after cord clamping vs ECC (subgroup by type intervention) Subgroup 7: mixed intervention or unclear |
|
| Outcomes |
Primary
|
|
| Notes |
Setting: Neonatal Units, Department of Paediatrics and Department of Obstetrics and Gynaecology at Lady Hardinge Medical College, New Delhi, India Dates: November 2011 to April 2013 Declaration of interest: not reported Trial funding source: not reported Further information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “The randomization sequence was generated and implemented by an independent physician into a block size of six patients.” |
| Allocation concealment (selection bias) | Unclear risk | Quote: “Allocation concealment was done using sequentially labelled opaque sealed envelopes.”. however, it is not possible to have concealment of allocation if sequence generation is unclear. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information to suggest they tried to blind outcome assessment ‐ though no data available for the review |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Excluded 6/120 babies, = 5% |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol. |
| Other bias | Unclear risk | Unclear on other biases. |
Dhaliwal 2014.
| Methods | Randomised controlled trial | |
| Participants |
Inclusion criteria
Exclusion criteria |
|
| Interventions |
Intervention: DCC
Comparator: ECC
Additional information
Comparison 1 DCC with neonatal resuscitation after cord clamping vs ECC (subgroup by gestation) Subgroup 2: > 32‐34 weeks' gestation Comparison 2 DCC with neonatal resuscitation after cord clamping vs ECC (subgroup by type intervention) Subgroup 7: mixed intervention or unclear |
|
| Outcomes |
Primary Death and abnormal neurological exam till 40 weeks' gestation Secondary for the baby Neonatal anaemia; blood transfusion; late sepsis; NEC; hyperbilirubinaemia; need for phototherapy; Hct Secondary for the mother PPH; therapeutic uterotonics; MRP; Hb at 48 hrs; ferritin 48 hrs |
|
| Notes |
Setting: Chandigarh, India Dates: not reported Declaration of interest: not reported Trial funding source: not reported Other information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It is not possible to blind the clinicians at the birth. It is not clear if women knew or not. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol |
| Other bias | Unclear risk | Ony a conference abstract, so very little information on the methodology |
Dipak 2017.
| Methods | Randomised controlled trial using variable blocks of 3 and 6 | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention 1: DCC
Intervention 2: DCC + IM ergometrine to mother
We pooled data from Interventions 1 and 2. Comparator: ECC
Additional information
Comparison 1 DCC with neonatal resuscitation after cord clamping vs ECC (subgroup by gestation) Subgroup 1: < 32‐34 weeks' gestation Comparison 4 DCC with neonatal resuscitation after cord clamping vs ECC (subgroup by type intervention) Subgroup 4: DCC at 1‐2 mins with baby low (+ gravity) |
|
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Notes |
Setting: tertiary care hospital, Mumbai, India Dates: October 2012 to September 2013 Declaration of interest: no competing interests reported Trial funding source: Quote: "None". |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “…random number sequence with variable block size of 3 or 6 using a ‘Random Allocation Software’ program... The random allocation sequence was generated by a statistician who was not a part of the study.” |
| Allocation concealment (selection bias) | Low risk | Quote: “The sequence was concealed in serially numbered, opaque, sealed and identical envelopes.” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It was not possible to blind clinicians. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There is no information regarding blinding of outcome assessments. The laboratory data could have been blinded but it is unclear about clinical outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data reported as complete |
| Selective reporting (reporting bias) | Unclear risk | We did not assess trial protocol |
| Other bias | Unclear risk | No other biases apparent but not really clear. |
Dong 2016.
| Methods | Randomised controlled trial | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: DCC
Comparator: ECC
Additional information
Comparison 1 DCC with neonatal resuscitation after cord clamping vs ECC (subgroup by gestation) Subgroup 1: < 32‐34 weeks' gestation Comparison 2 DCC with neonatal resuscitation after cord clamping vs ECC (subgroup by type intervention) Subgroup 2: DCC 45 secs with baby low (+ gravity) |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Setting: China Dates: January to December 2015 Declaration of interest: not reported Trial funding source: Nanjing Medical University Research Funding funded project 2013NJMU134 Further information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of randomisation given; simply a broad statement saying quote: “participants were randomized into the two groups” |
| Allocation concealment (selection bias) | Unclear risk | No description or statement given. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No description or statement given but not possible to blind clinicians, and unclear if women knew. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No description or statement given. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All were followed up. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol though all stated outcomes within the methodology in the paper were reported, either in text or tables. |
| Other bias | Unclear risk | Generally seems fine. Values were reported without blanket P value statements. However, since important information on methodology is missing in the paper we assessed it is unclear on other biases. |
El‐Naggar 2016.
| Methods | Randomised controlled trial | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: UCM
Comparator: ECC
Additional information
Comparison 7 UCM vs ECC (subgroup by gestation) Subgroup 1: < 32‐34 weeks' gestation Comparison 8 UCM vs ECC (subgroup by type of intervention) Subgroup 1: cord intact during UCM |
|
| Outcomes |
Primary
Secondary
Not mentioned in trial registration but in the conference abstracts reporting findings.
|
|
| Notes |
Setting: Canada Dates: November 2011 ‐ 2014 Declaration of interest: not reported Trial funding source: not reported Further information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Trial registration reports: quote: "...a randomization table” |
| Allocation concealment (selection bias) | Low risk | Trial registration reports: quote: "Randomization will be done in variable block sizes and will be concealed by using opaque envelopes prepared ahead of time from a randomization table. Envelopes will be opened before the time of delivery." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Clinicians at birth cannot be blinded and trial registration form says quote: “Single blinded (outcome assessor)”. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Clinicians at birth cannot be blinded and trial registration form says quote: “Single blinded (outcome assessor)”. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants reported on though for some outcome denominators are less. |
| Selective reporting (reporting bias) | High risk | Authors reported more outcomes in the 2 conference abstracts than they said they would in the Trial protocol registration form, and it is unclear if other outcomes have been assessed but not reported. We did not assess the full trial protocol. |
| Other bias | Unclear risk | 2 conference abstracts with very little information on methodology. |
Elimian 2014.
| Methods | Randomised controlled trial | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: UCM
Comparator: ECC
Additional information
Comparison 7 UCM vs ECC (subgroup by gestation) Subgroup 1: < 32‐34 weeks' gestation Comparison 8 UCM vs ECC (subgroup by type of intervention) Subgroup 1: cord intact during UCM |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Setting: Teaching and Research Center of Konya, University of Baskent, Turkey. Dates: September 2008 – April 2009 Declaration of interest: the authors reported no potential conflicts of interest. Trial funding source: sponsor was University of Oklahoma Further information:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Allocation sequence was generated by a computer" |
| Allocation concealment (selection bias) | Low risk | Quote: “The allocation sequence was concealed by using sequentially numbered, opaque, sealed envelopes kept in a central location on labor and delivery.” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open label |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The only blinding was the neuroradiologists who interpreted the cranial ultrasound scans so IVH and PVL are low risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses after randomisation. |
| Selective reporting (reporting bias) | Unclear risk | We checked the publication against the trial registration form, but the form did not list the outcomes to be measured. Requirement for resuscitation was the only unreported outcome. Some outcomes reported in categorical way when continuous data were suggested in methods and could easily have been given. |
| Other bias | Low risk | Used ITT. No difference at baseline for maternal age, height and weight, ethnicity, and selected maternal outcome variables. No other biases apparent. |
Gokmen 2011.
| Methods | Randomised controlled trial | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: DCC
Comparator: ECC
Additional information
Comparison 1 DCC with neonatal resuscitation after cord clamping vs ECC (subgroup by gestation) Subgroup 1: < 32‐34 weeks' gestation Comparison 2 DCC with neonatal resuscitation after cord clamping vs ECC (subgroup by type intervention) Subgroup 1: DCC at < 1 min with baby level with uterus and placenta |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Setting: Teaching and Research Center of Konya, University of Baskent, Turkey. Dates: September 2008 – April 2009 Declaration of interest: quote: "The authors stated that there are no conflicts of interest regarding the publication of this article.". Trial funding source: not reported Further information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation occurred when birth was imminent, with no mention of the random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote:'It was not possible to mask the trial assignment to the neonatal or obstetric team in the delivery room…The subsequent clinical management of the infant was left to the discretion of the attending neonatologist in the NICU.' |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote:"However, the neonatal staff was asked not to record the time in the chart (only randomization code number), and this information was not available to the staff in the NICU… The subsequent clinical management of the infant was left to the discretion of the attending neonatologist in the NICU." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 babies (9%) were excluded and these were not reported by group. Quote:"One infant had significant IUGR, one case was due to placental abruption, and two infants of 24 weeks’ gestation died in the first 12 hours of life." |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol. |
| Other bias | Unclear risk | Baseline characteristics for mother (age; antenatal steroids; PROM; reasons for preterm birth) and baby (birthweight; gestational age; male/female ratio; Apgar scores at 1 and 5 mins) were reported similar. However, pre‐eclampsia occurred in 8 women in ECC and 4 women in DCC arms. There is very little reporting of the methodology on the RCT. |
Hofmeyr 1988.
| Methods | Randomised controlled trial, randomisation cards, stratified by birthweight < 1500 g. | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: DCC
Comparator: ECC
Additional information
Comparison 1 DCC with neonatal resuscitation after cord clamping vs ECC (subgroup by gestation) Subgroup 1: < 32‐34 weeks' gestation Comparison 2 DCC with neonatal resuscitation after cord clamping vs ECC (subgroup by type intervention) Subgroup 3: DCC at 1‐2 mins with baby level with uterus and placenta |
|
| Outcomes | Outcomes: PVH/IVH assessed by cerebral ultrasound 6‐72 hrs after birth, Apgar score at 5 mins, birthweight, systolic blood pressure at 5 mins, cord blood gas, death. | |
| Notes |
Setting: South Africa Dates: not reported Declaration of interest: not reported Trial funding source: not reported Further information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Allocation by quote: “randomisation cards”. |
| Allocation concealment (selection bias) | Unclear risk | Allocation by quote:“randomisation cards”. No further information was provided on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of the intervention was not possible. Knowledge of group allocation may have influenced other aspects of clinician behaviour, and assessment of some outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Cranial ultrasound examination was blind to the allocated group. There is no information about blinding assessment of other outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 38 mother‐infant pairs were randomised. All women and babies appeared to be accounted for in the analyses. |
| Selective reporting (reporting bias) | Low risk | Data for the outcomes listed in methods are reported. Assessment of risk of bias from published paper. |
| Other bias | Unclear risk | There was some baseline imbalance between the groups, suggesting those allocated delayed clamping might have been at higher risk of IVH. |
Hofmeyr 1993.
| Methods | Randomised controlled trial | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: DCC
Comparator: ECC
Additional information
Comparison 1 DCC with neonatal resuscitation after cord clamping vs ECC (subgroup by gestation) Subgroup 3: mixed gestation Comparison 2 DCC with neonatal resuscitation after cord clamping vs ECC (subgroup by type intervention) Subgroup 3: DCC at 1‐2 mins with baby level with uterus and placenta |
|
| Outcomes | Outcomes: death of the baby, PVH/IVH assessed by cerebral ultrasound 6‐72 hrs after birth, Apgar score at 5 mins, cord‐pH, bilirubin. | |
| Notes |
Setting: South Africa Dates: not reported Declaration of interest: not reported Trial funding source: not reported Further information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote:"randomised sealed cards", no further information. |
| Allocation concealment (selection bias) | Unclear risk | Quote:"randomised sealed cards", no further information. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | For this type of intervention blinding participants and the staff present at delivery to group allocation is not possible. Staff providing care may have modified their behaviour according to randomisation group. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Cranial ultrasound scans were blind to the allocated group. Blinding for assessment of other outcomes is not discussed. For death lack of blinding remains low risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants randomised were accounted for in the analysis and analysis was according to randomisation. |
| Selective reporting (reporting bias) | Unclear risk | Bilirubin was reported for only 30 infants. We did not assess the trial protocol. |
| Other bias | Low risk | Groups appeared similar at baseline. No other bias identified. |
Hosono 2008.
| Methods | Randomised controlled trial | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: UCM
Comparator: ECC
Additional information
Comparison 7 UCM vs ECC (subgroup by gestation) Subgroup 1: < 32‐34 weeks' gestation Comparison 8 UCM vs ECC (subgroup by type of intervention) Subgroup 1: cord intact during UCM |
|
| Outcomes | Primary outcomes: not needing transfusion and total number of RBC transfusions. Secondary outcomes: Hb and BP on admission, polycythaemia, IVH, IVH grade 3 or 4, patent ductus, gut perforation, death. | |
| Notes |
Setting: Nihon University Itabashi Hospital, Tokyo, Japan (a single tertiary perinatal centre) Dates: January 2001‐December 2002 Declaration of interest: reports no competing interests. Trial funding source: quote: "This study was supported by ‘‘The Mother and Child Health Foundation’’. Further information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly selected", no further information. |
| Allocation concealment (selection bias) | Unclear risk | Serially numbered opaque envelopes opened just before delivery. It was not stated if any envelopes were unaccounted for, or if they were opened in the correct order. Also as sequence generation is unknown it is possible the next allocation could be predicted. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of the intervention was not possible. Staff providing care may have modified their behaviour according to randomisation group. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Some of the outcomes depended on clinical decisions that may have been affected by knowledge of group status, However, other outcomes are unlikely to have been affected by lack of blinding (e.g. infant death). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 40 mother‐infant pairs were randomised and there was no apparent loss to follow‐up for the babies. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol. |
| Other bias | Low risk | Groups appeared balanced at baseline. Other bias was not apparent. |
Hosono 2015.
| Methods | Randomised controlled trial ‐ multicentre (14 centres) | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: UCM
Comparator: ECC
Additional information
Comparison 7 UCM vs ECC (subgroup by gestation) Subgroup 1: < 32‐34 weeks' gestation Comparison 8 UCM vs ECC (subgroup by type of intervention) Subgroup 2: cord cut before UCM |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Setting: Japan in 14 centres Dates: January 2008 to December 2013 Declaration of interest: not reported. Trial funding source: The Ministry of Health, Labour and Welfare Further information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open – no one is blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 203 women recruited but outcomes on 154 only – lost 24%. Also planned to recruit 534 on power calculation but have stopped recruiting based on interim analysis. |
| Selective reporting (reporting bias) | High risk | There are many outcomes listed in the trial registration form which are not reported on in the conference abstract. Hopefully they will be reported in the full paper. |
| Other bias | High risk | Study quote:“terminated before completion of its planned recruitment of 534 patients based on interim analysis.” Also, conference abstract only so very little information to assess other biases. |
Josephsen 2014.
| Methods | Randomised controlled trial | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: UCM
Comparator: ECC
Additional information
Comparison 7 UCM vs ECC (subgroup by gestation) Subgroup 1: < 32‐34 weeks' gestation Comparison 8 UCM vs ECC (subgroup by type of intervention) Subgroup 3: unclear |
|
| Outcomes | Mean initial Hb; number of blood transfusions in first 28 days; IVH; NEC; mortality | |
| Notes |
Setting: USA Dates: August 2013 Declaration of interest: not reported Trial funding source: not reported Further information:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No information, but clearly clinicians providing the intervention would have known though it is unclear if women knew or not. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol. |
| Other bias | Unclear risk | No information. Only a conference abstract. |
Katheria 2014.
| Methods | Randomised controlled trial, stratified by gestational age (23 to 28 + 6/7) and (29 to 31 + 6/7). | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: UCM
Comparator: ECC
Additional information
Comparison 7 UCM vs ECC (subgroup by gestation) Subgroup 1: < 32‐34 weeks' gestation Comparison 8 UCM vs ECC (subgroup by type of intervention) Subgroup 1: cord intact during UCM |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Trial registration: NCT01434732 Setting: California, USA. Single tertiary centre Dates: 1 February 2011 to 31 January 2013 Declaration of interest: quote: “All authors declare no conflict of interest.” Trial funding source: sponsors: Sharp HealthCare Further information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | No information in publication but personal communication with author reports quote:"computer generated". |
| Allocation concealment (selection bias) | Low risk | Quote: "Infants were randomised by the placement of their information in opaque, sealed envelopes immediately before delivery." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote:"The obstetricians were made aware of the randomizations by the neonatology team before delivery of the infant." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No specific information about the clinical outcomes and who measured those, although there were blinded echocardiographic examinations. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Excluded 8% after randomisation. |
| Selective reporting (reporting bias) | Unclear risk | Outcomes from trial registration reported but added additional outcomes and this may cause bias but unclear. |
| Other bias | Low risk | Demographics – similar between groups. Trial not stopped early. |
Katheria 2015.
| Methods | Randomised controlled trial, stratified by gestation and mode of birth | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: DCC
Comparator: UCM
Additional information
Comparison 5 DCC with neonatal resuscitation after cord clamping vs UCM (subgroup by gestation) Subgroup 1: < 32‐34 weeks' gestation Comparison 6 DCC with neonatal resuscitation after cord clamping vs UCM (subgroup by type of intervention) Subgroup 2: DCC at < 1 min with baby low (+ gravity) |
|
| Outcomes | Primary:
Secondary:
|
|
| Notes |
Setting: California, USA. 2 tertiary centres (Sharp Mary Birch Hospital for Women and Newborns (SMBHWN) and Loma Linda University Medical Center) Dates: interim analysis August 2013 ‐ August 2014. Declaration of interest: “The authors have indicated they have no potential conflicts of interest to disclose.” and“The authors have indicated they have no financial relationships relevant to this article to disclose.” Trial funding source: “All phases of this study were supported by a National Institutes of Health (NIH) grant 5R03HD072934‐02. Funded by the National Institutes of Health (NIH).” Further information:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote:“Computer‐generated randomisation was stratified by age and mode of birth” |
| Allocation concealment (selection bias) | Low risk | Quote: “Infants were randomly assigned by opaque, sealed envelopes immediately before delivery”. Also the envelopes were handed out in a pre‐defined blinded order to provide allocation concealment (personal communication from A Ketheria). |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote:"The obstetricians were made aware of the randomization by the neonatology team immediately before delivery of the infant." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote:"Blinded echocardiograms and head ultrasounds were performed mainly (.90%) by the principal investigator (A.C.K.). None of the investigators performing echocardiograms were involved in the randomization or the recording of the intervention. All images were analyzed and measured offline by using EchoPAC software (GE HealthCare, Horten, Norway) and were analyzed without knowledge of the assigned group by the principal investigator. The blinding was achieved by allowing only the ALS nurse attending the delivery and the obstetrician performing the intervention to be aware of the allocation arm." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only reported outcomes on caesarean births and not the vaginal births (a few outcomes in a supplementary on‐line sheet). |
| Selective reporting (reporting bias) | High risk | Not all outcomes listed in trial protocol are reported on, e.g. omitted admission to NICU and inotropic support, etc. |
| Other bias | Unclear risk | Similar at baseline. ITT but stopped trial following interim analysis. |
Kilicdag 2016.
| Methods | Randomised controlled trial | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: UCM
Comparator: ECC
Additional information
Comparison 7 UCM vs ECC (subgroup by gestation) Subgroup 1: < 32‐34 weeks' gestation Comparison 8 UCM vs ECC (subgroup by type of intervention) Subgroup 1: cord intact during UCM |
|
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Notes |
Setting: probably Turkey Dates: August 2012 ‐ August 2013 Declaration of interest: quote: "The authors report no conflicts of interest" Trial funding source: not reported Further information:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote:"...randomly assigned..." ‐ no further information |
| Allocation concealment (selection bias) | Unclear risk | Quote:“…using sequentially numbered sealed nontransparent envelopes…”, however, it is not possible to have concealment of allocation if sequence generation is unclear. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind clinicians at birth but unclear if women knew or not. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information but laboratory tests likely to be blinded – unclear about the clinical assessments. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 58 randomised. Excluded 4 (8%) because: placental abruption x2; congenital anomaly x1; Rh haemolytic disease x1. Unlikely to bias outcomes. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess trial protocol |
| Other bias | Low risk | No other biases apparent |
Kinmond 1993.
| Methods | Randomised controlled trial | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: regulated cord clamping ‐ included in delayed clamping group (DCC)
Comparator: conventional cord clamping ‐ included in early clamping group (ECC)
Additional information
Comparison 1 DCC with neonatal resuscitation after cord clamping vs ECC (subgroup by gestation) Subgroup 1: < 32‐34 weeks' gestation Comparison 2 DCC with neonatal resuscitation after cord clamping vs ECC (subgroup by type intervention) Subgroup 2: DCC at < 1 min with baby low (+ gravity) |
|
| Outcomes | Outcomes: initial packed red cell volume, peak serum bilirubin, transfusion requirement, respiratory impairment, arterial‐alveolar oxygen ratio, duration of oxygen. | |
| Notes |
Setting: Glasgow, Scotland, UK Dates: not reported Declaration of interest: not reported Trial funding source: not reported Further information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described. |
| Allocation concealment (selection bias) | Unclear risk | Infants were quote:“randomised immediately before delivery by means of sealed envelopes”. Not clear if envelopes opaque or sequentially numbered or that all envelopes were accounted for. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | For this type of intervention blinding participants and the staff present at delivery to group allocation is not possible. Staff providing care may have modified their behaviour according to randomisation group. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There was no mention of blinding in this study, although it is not clear how lack of blinding would have affected those outcomes measured. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All 36 participants were accounted for in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study report. We did not have access to the protocol. |
| Other bias | Unclear risk | The study was quote:"terminated when exogenous surfactant was introduced because this influenced our respiratory outcomes". We considered there was a “chance excess of boys” in the delayed clamping group (13/17 vs 7/19 controls). |
Krueger 2015.
| Methods | Randomised controlled trial | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: DCC
Comparator: UCM
Additional information
Comparison 5 DCC with neonatal resuscitation after cord clamping vs UCM (subgroup by gestation) Subgroup 1: < 32‐34 weeks' gestation Comparison 6 DCC with neonatal resuscitation after cord clamping vs UCM (subgroup by type of intervention) Subgroup 2: DCC at < 1 min with baby held low |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Setting: University of South Alabama Children’s and Women’s Hospital, USA Dates: August 2012 and November 2013. Declaration of interest: authors report no conflict of interest. Trial funding source: not reported Further information:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote:“Randomization was performed with opaque envelopes contained on the labor and delivery unit containing cards with instruction on either delayed cord clamping alone or delayed cord clamping plus cord stripping. An equal number of envelopes were created for each arm and were scrambled by a third‐party registered nurse.” |
| Allocation concealment (selection bias) | Unclear risk | Quote:“Randomization was performed with opaque envelopes contained on the labor and delivery unit containing cards with instruction on either delayed cord clamping alone or delayed cord clamping plus cord stripping. An equal number of envelopes were created for each arm and were scrambled by a third‐party registered nurse.” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind clinicians at birth and it is unclear whether women were blinded or not. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote:The neonatal team was not told which patients were participating in the study, and the randomisation arm was not documented on the infants’ charts. This was done in an effort to avoid alteration in subsequent management and achieve blinding of the care team.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 excluded after randomisation because they did not meet inclusion criteria. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol. |
| Other bias | Unclear risk | Groups were similar in terms of birthweight and gestation. Women were excluded if baby had abnormality or suspected placental abruption – not sure if before or after randomisation. Other possible biases not clear. |
Kugelman 2007.
| Methods | Randomised, controlled trial, stratification by mode of birth and risk of pregnancy (pre‐eclampsia, PIH). | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: DCC
Comparator: ECC
Additional information:
Comparison 1: DCC with neonatal resuscitation after cord clamping vs ECC (subgroup by gestation) Subgroup 1: < 32‐34 weeks' gestation (mostly) Comparison 2: DCC with neonatal resuscitation after cord clamping vs ECC (subgroup by type intervention) Subgroup 2: DCC at < 1 min with baby low (+ gravity) |
|
| Outcomes | Baby death, IVH, PVL, blood transfusion. peak bilirubin, serum complement, immunoglobulins between group, risk, of sepsis, sepsis events, antibiotic therapy. | |
| Notes |
Setting: Haiha, Israel Dates: September 2004 to December 2005 Declaration of interest: not reported Trial funding source: not reported Further information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random assignment quote:“was performed with a system of randomly prepared cards in sealed nontransparent envelopes...”. |
| Allocation concealment (selection bias) | Unclear risk | Random assignment quote:“was performed with a system of randomly prepared cards in sealed nontransparent envelopes...”. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The study was described as masked. However, clinical staff at the birth would be aware of group assignment but staff were asked not to record group status in case notes so neonatal staff were not aware of allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The study was described as masked. However, clinical staff at the birth would be aware of group assignment but staff were asked not to record group status in case notes so neonatal staff were not aware of allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 65 participants were randomised and all appeared to be accounted for in the analyses. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study reports. Outcomes on infection and sepsis not mentioned in first report. Hence unclear whether all outcomes collected have been reported. We did not assess the trial protocol. |
| Other bias | Low risk | Baseline characteristics were similar and there is no evidence of other biases. |
Kumar 2015.
| Methods | Randomised controlled trial | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: UCM
Comparator: ECC
Additional information
Comparison 7 UCM vs ECC (subgroup by gestation) Subgroup 2: > 32‐34 weeks' gestation Comparison 8 UCM vs ECC (subgroup by type of intervention) Subgroup 2: cord cut before UCM |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Setting: Department of Pediatrics and Obstetrics of a tertiary care institute in Northern India. Dates: September 2013 to August 2014 Declaration of interest: no competing interests reported Trial funding source: no funding. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote:“…online generated random number list and assigned even numbers to early cord clamping (control) group and" |
| Allocation concealment (selection bias) | Low risk | Quote:“The numbers were written on small slips and placed in serially numbered opaque sealed envelopes. Sealed envelope was opened by a delivery room staff nurse, just" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No information provided. Clinicians at the birth cannot be blinded but it is unclear if women were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information but most of outcomes are laboratory tests – though there are a few clinical outcomes – so unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | UCM group lost 3/100 and ECC group lost 7/100 for clinical outcomes. So well under 20%. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol |
| Other bias | Low risk | No indication of other biases,. |
Malik 2013.
| Methods | Randomised controlled trial | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: DCC
Comparator: ECC
Additional information
Comparison 1 DCC with neonatal resuscitation after cord clamping vs ECC (subgroup by gestation) Subgroup 2: > 32‐34 weeks' gestation (mostly) Comparison 2 DCC with neonatal resuscitation after cord clamping vs ECC (subgroup by type intervention) Subgroup 7: DCC at > 2 mins ‐ unclear where baby placed |
|
| Outcomes | Hct and polycythaemia (high Hct) | |
| Notes |
No data for this review Setting: Department of Pediatric Medicine, Services Hospital, Lahore and labour room, Services Hospital, Lahore, Pakistan Dates: 8 Jan 2009 to 7 July 2009 Declaration of interest: not reported Trial funding source: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...random number table..." |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Clinicians at birth cannot be blinded – unclear if women blinded or not. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No information but Hct and polycythaemia are the outcomes so unlikely to be influenced by knowledge of group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Appears complete |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol. |
| Other bias | Unclear risk | Very little information on methodology, so unclear about other possible biases. |
March 2013.
| Methods | Randomised controlled trial. Random permuted blocks of 10. | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: UCM
Comparator: ECC
Additional information
Comparison 7 UCM vs ECC (subgroup by gestation) Subgroup 1: < 32‐34 weeks' gestation Comparison 8 UCM vs ECC (subgroup by type of intervention) Subgroup 1: cord intact during UCM |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Setting: East Virginia, USA. Single tertiary centre. Dates: September 2009 to June 2011 Declaration of interest: authors declare no conflicts of interest. Trial funding source: this work was conducted with support from Harvard Catalyst. The Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Award 8UL1TR000170‐05 and financial contributions from the Harvard University and its affiliated academic healthcare centres. Further information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | ‘An independent statistician provided the randomisation sequence.’ Personal communication with Dr March provided the following information: Quote: “A statistician provided random permuted blocks of 10 using a SAS program." |
| Allocation concealment (selection bias) | Low risk | 'Serially numbered opaque envelopes' |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The neonatologists and pediatric support staff were not blinded to treatment assignment given that they were required to be present for the delivery." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "The neonatologists and pediatric support staff were not blinded to treatment assignment given that they were required to be present for the delivery....no notation of study participation was made in the neonate’s chart in order to minimize the possibility that postnatal treatment decisions would be influenced by study participation." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 33.6% of women were excluded because they gave birth beyond 28 weeks' gestation. This was 16 in each group and so we believe this is unlikely to cause serious bias but unclear. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol. |
| Other bias | Low risk | Baseline characteristics were similar in the groups. Compliance: 1 woman in the cord milking group had the cord inadvertently clamped and cut immediately. This was dealt with by ITT. |
McDonnell 1997.
| Methods | Randomised controlled trial, stratified by vaginal or CS, 26 to 29 weeks, 30 to 33 weeks. | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: DCC
Comparator: ECC
Additional information
Comparison 1 DCC with neonatal resuscitation after cord clamping vs ECC (subgroup by gestation) Subgroup 1: < 32‐34 weeks' gestation Comparison 2 DCC with neonatal resuscitation after cord clamping vs ECC (subgroup by type intervention) Subgroup 1: DCC at < 1 min with baby level with uterus and placenta |
|
| Outcomes | Primary outcome: Hct at 4 hrs. Secondary outcomes: Apgar score, temperature on admission, requirement for ventilation, oxygen, surfactant, peak serum bilirubin, inotropic support, cerebral ultrasound, blood transfusion, death | |
| Notes |
Setting: Sydney, Australia Dates: January to December 1994 Declaration of interest: not reported Trial funding source: not reported Further information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence not stated. There was stratification by gestational age and type of delivery. |
| Allocation concealment (selection bias) | Unclear risk | Quote: “sealed opaque envelopes”. Not clear if envelopes numbered and used sequentially. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not mentioned. It is possible that lack of blinding could influence other aspects of care and the recording of outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding was not mentioned. It is possible that lack of blinding could influence other aspects of care and the recording of outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 46 infants were randomised. It was not clear in the publication how many infants were in each randomised group and we understand that personal communication with the authors provided the information and data. For the outcomes, of IVH and PVL there were only 31/46 (67%) of data available though death is reported on all babies. Analysis was according to randomisation group. |
| Selective reporting (reporting bias) | Unclear risk | Assessment of risk of bias from published trial report. Several outcomes were not reported in the brief trial report although the authors offer other data on request. We did not assess the trial protocol. |
| Other bias | Unclear risk | Groups appeared similar at baseline although there were more boys in the immediate clamping group (15 vs 9, denominators not clear). |
Mercer 2003.
| Methods | Randomised controlled trial | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: deferred cord clamping (DCC)
Comparator: ECC
Additional information
Comparison 1 DCC with neonatal resuscitation after cord clamping vs ECC (subgroup by gestation) Subgroup 1: < 32‐34 weeks' gestation Comparison 2 DCC with neonatal resuscitation after cord clamping vs ECC (subgroup by type intervention) Subgroup 2: DCC at < 1 min with baby low (+ gravity) |
|
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Notes |
Setting: USA Dates: October 1998 to March 2001 Declaration of interest: not reported Trial funding source:“Sigma Theta Tau, Epsilon Chapter; University of Rhode Island Foundation and College of Nursing”. Further information:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “system of randomly prepared cards in sealed nontransparent envelopes." |
| Allocation concealment (selection bias) | Unclear risk | Quote: “system of randomly prepared cards in sealed nontransparent envelopes.” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | For this type of intervention blinding participants and the staff present at delivery to group allocation is not possible. Staff providing care may have modified their behaviour according to randomisation group. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It is not clear whether lack of blinding would have had an effect on the outcomes measured. There was an attempt to achieve blinding for some of the outcomes assessed as staff were requested not to record group assignment on case notes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 32 participants were randomised and all appeared to be accounted for in the analysis. 2 babies in the delayed clamping group were not treated according to protocol but they were analysed according to randomisation. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study report. |
| Other bias | Low risk | Groups appeared similar at baseline. Other bias not apparent. |
Mercer 2006.
| Methods | Randomised controlled trial, stratification by gestation: 24‐27 and 28‐32 weeks. | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: DCC
Comparator: ECC
Additional information
Comparison 1 DCC with neonatal resuscitation after cord clamping vs ECC (subgroup by gestation) Subgroup 1: < 32‐34 weeks' gestation Comparison 2 DCC with neonatal resuscitation after cord clamping vs ECC (subgroup by type intervention) Subgroup 2: DCC at < 1 min with baby low (+ gravity) |
|
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Notes |
Setting: Women and Infants Hospital, Providence, Rhode Island, USA Dates: August 2003 to December 2004 Declaration of interest: authors have no financial relationships relevant to this work Trial funding source: this work was supported by National Institutes of Health, National Institure of Nursing Research grant K23 NR00078 Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “A statistician who was not involved in the trial developed a computer‐generated random number system. Block‐stratified randomisation was used...” to take account of gestational age. |
| Allocation concealment (selection bias) | Low risk | Quote: “Two sets of cards labelled for randomisation were enclosed in sequenced, opaque envelopes containing group assignment...” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | For this type of intervention blinding participants and the staff present at delivery to group allocation is not possible. Staff providing care may have modified their behaviour according to randomisation group. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The study was not blinded although the groups status was not recorded on case notes in an attempt to reduce detection bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 72 were randomised (36 in each group). Although there were some protocol violations the analysis was according to randomisation group. There were 3 early deaths in the immediate cord clamping group and these babies were excluded from subsequent analysis as they were no longer eligible to experience outcomes. |
| Selective reporting (reporting bias) | Unclear risk | 'Risk of bias' assessment from published study report. We did not assess the trial protocol. |
| Other bias | Low risk | No baseline imbalance between groups apparent. Quote: "All infants remained in their assigned groups for analyses.” hence ITT. By 7 months there had been 5 deaths, and of the 67 remaining babies 58 were followed up for longer‐term outcomes where there is an inevitable loss to follow‐up. Other bias not identified. |
Mercer 2016.
| Methods | Randomised controlled trial. Block stratified by < 28 weeks or > 28 weeks. | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: UCM
Comparator: ECC
Additional information
Comparison 7 UCM vs ECC (subgroup by gestation) Subgroup 1: < 32‐34 weeks' gestation Comparison 8 UCM vs ECC (subgroup by type of intervention) Subgroup 1: cord intact during UCM |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Setting: Women and Infants’ Hospital of Rhode Island, USA Dates: 15 May 2008 to 30 January 2012 Declaration of interest: authors declare no conflicts of interest Trial funding source: National Institute of Nursing Research and Thrasher Research Fund. Further information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | Sequenced and sealed envelopes identifying the stratification and group assignment on cards were prepared by a statistician not involved in the trial and kept in a locked file box in the labour and delivery unit (personal communication from J Mercer), however, it is not possible to have concealment of allocation if sequence generation is unclear. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The study could not be blinded because of the nature of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Personnel collecting on‐going clinical data and the follow‐up staff completing the developmental assessment remained blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 babies excluded after randomisation 2 from ECC (2/107 = 2%) for congenital or birth trauma and 1 from DCC (1/104 = 1%) for congenital anomaly. At 18 months: DCC 82/100 (82%) ECC 79/99 (79%) |
| Selective reporting (reporting bias) | Unclear risk | Many outcomes where data were collected were not reported in the published paper. We understand we have all the data now (Personal communication with J Mercer). |
| Other bias | Unclear risk | Baseline data similar between groups for parity, public insurance, marital status, antenatal steroids, IUGR, or caesarean delivery rate (data not reported). However, there were significantly more women with PROM/PTL in the DCC group in both cohorts, and more women with pre‐eclampsia (PEC) at admission in the ICC group. (Data not reported.) Authors undertook an additional multi‐logistic regression analysis. Compliance: DCC 89/100 (86%) received DCC. ECC 98/99 (92%) received ECC |
Nelle 1998.
| Methods | Randomised controlled trial. Randomisation by sealed opaque envelopes. | |
| Participants |
Inclusion criteria
Exclusion criteria |
|
| Interventions |
Intervention: DCC
Comparator: ECC
Additional information
Comparison 1 DCC with neonatal resuscitation after cord clamping vs ECC (subgroup by gestation) Subgroup 1: < 32‐34 weeks' gestation Comparison 2 DCC with neonatal resuscitation after cord clamping vs ECC (subgroup by type intervention) Subgroup 2: DCC at < 1 min with baby low (+ gravity) |
|
| Outcomes | Outcomes:
|
|
| Notes |
No data for the review Setting: Germany Dates: not reported Declaration of interest: not reported Trial funding source: not reported Further information:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Sealed, opaque envelopes (information provided by the author). |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. Not clear whether outcomes would be affected by lack of blinding. Other aspects of care may have been affected by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding. Not clear whether outcomes would be affected by lack of blinding. Other aspects of care may have been affected by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not clear whether full data were available for all participants. |
| Selective reporting (reporting bias) | Unclear risk | Reported in brief abstract. |
| Other bias | Unclear risk | Very little information on study methods. |
Oh 2011.
| Methods | Randomised controlled trial. Stratified by mode of birth and centre. 3 centre trial. | |
| Participants |
Inclusion criteria
Exclusion criteria
Additional information 190 women were screened, 97 were eligible and 54 consented. The main reason women who consented were not randomised was logistics, i.e. research staff not present at the birth |
|
| Interventions |
Intervention: DCC
Comparator: ECC
Additional information
Comparison 1 DCC with neonatal resuscitation after cord clamping vs ECC (subgroup by gestation) Subgroup 1: < 32‐34 weeks' gestation Comparison 2 DCC with neonatal resuscitation after cord clamping vs ECC (subgroup by type intervention) Subgroup 2: DCC at < 1 min with baby low (+ gravity) |
|
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Notes |
Setting: USA. 3 centres: 1) University of Alabama, Birmingham, AL. 2) The Rainbow Babies and Children’s Hospital, Cleveland, OH. 3) The Women and Infants Hospital, Providence, RI. Dates: May 2000 to June 2001 Declaration of interest: quote: “The authors declare no conflict of interest” Trial funding source: quote: "The National Institutes of Health and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) provided grant support for the Neonatal Research Network’s Delayed Cord Clamping Study.” Further information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information on sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Quote:“The subject was randomized (per phone call to the RTI (Research Triangle Institute) International Data Coordinating Center) to one of the two groups:…” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The study was not blinded, although it was stated that efforts were made to quote: “avoid revelation of grouping of infants to the attending physicians". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention of whether outcome assessment was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 33 were randomised and all appeared to be accounted for in the analysis, except for the outcome CLD where data were provided on 26/33 (79%) babies. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published reports. The main study paper was published in 2011; the first report (in abstract form) in 2002. It is not clear why so long elapsed before the publication of study findings. We also did not assess the trial protocol. |
| Other bias | Low risk | No baseline imbalance between groups was apparent. Many eligible women were not randomised for logistic reasons. Other bias not identified. Transfusion is mentioned in abstract as frequency and volume but numbers with transfusion are not reported which suggests not all data collected have been reported. Ghavam 2014 reports on long‐term neurodevelopment outcome for 16 babies (8 in each group) with no mention of the methodology for collecting long‐term follow‐up data. |
Pongmee 2010.
| Methods | Randomised controlled trial | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: UCM
Comparator: ECC
Additional information
Comparison 7: but no usable data UCM vs ECC (subgroup by gestation) Subgroup 1: < 32‐34 weeks' gestation Comparison 8: but no usable data UCM vs ECC (subgroup by type of intervention) Subgroup 2: cord cut before UCM |
|
| Outcomes |
Primary outcomes
Secondary outcome
|
|
| Notes |
No usable data Setting: not reported Dates: not reported Declaration of interest: not reported Trial funding source: not reported Further information:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No information, but attending clinicians will have known the group allocation, it is unclear if women knew or not. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data appears to be complete |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol |
| Other bias | Unclear risk | Very little information ‐ conference abstract |
Rabe 2000.
| Methods | Randomised controlled trial | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: DCC
Comparator: ECC
Additional information
Comparison 1 DCC with neonatal resuscitation after cord clamping vs ECC (subgroup by gestation) Subgroup 1: < 32‐34 weeks' gestation Comparison 2 DCC with neonatal resuscitation after cord clamping vs ECC (subgroup by type intervention) Subgroup 2: DCC at < 1 min with baby low (+ gravity) |
|
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Notes |
Setting: Germany Dates: 1997 to 1998 Declaration of interest: none reported Trial funding source: Children's University Hospital of Münster Further information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The sequencing was computer generated. The allocation was done by a staff member not involved in clinical care or the clinical trial (personal communication). |
| Allocation concealment (selection bias) | Low risk | Quote:“by opening a sealed dark envelope”. The sealed dark envelopes were sequentially numbered. The clinician opening the envelope could not predict the allocation (personal communication). |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It was not possible to blind the clinicians at the birth, and it is unclear whether women knew their allocation or not (changed from unclear to high risk). |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There was an attempt to blind outcome assessors (group status was not recorded in notes). It was not clear whether lack of blinding affected clinical care or decisions that may have influenced outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 40 participants were randomised and 39 were included in the analysis. 1 baby in the late clamping group had cord clamping at 30 secs due to clinical concern, and was excluded from the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Assessment of bias from published study report. |
| Other bias | Low risk | Other bias not apparent. Study groups appeared similar at baseline. |
Rabe 2011.
| Methods | Randomised controlled trial | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: DCC
Comparator: UCM
Additional information
Comparison 5 DCC with neonatal resuscitation after cord clamping vs UCM (subgroup by gestation) Subgroup 1: < 32‐34 weeks' gestation Comparison 6 DCC with neonatal resuscitation after cord clamping vs UCM (subgroup by type of intervention) Subgroup 2: DCC at < 1 min with baby low (+ gravity) |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Setting: single tertiary care centre ‐ Royal Sussex County Hospital, Brighton, UK Dates: 2007 to 2009 Declaration of interest: authors reported no potential conflicts of interest. Trial funding source: quote: “Funded by a grant from the Brighton and Sussex University Hospitals Research and Development Directorate.” Also partly funded by National Institute of Health Research under Research for Patient Benefit Programme (PB‐PG‐1208‐18244). Further information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was based on computer‐created tables performed by a person not involved in the trial. The randomization was stratified by gestational age, 24 0/7 to 27 6/7 completed weeks of gestation and 28 0/7 to 32 6/7 weeks of gestation” |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomization allocation cards were kept on the labor ward in sealed opaque envelopes and consecutively numbered. The attending midwife opened the envelope before birth” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unable to blind participants/personnel due to quote: “nature of the interventions” and “routine practice that the neonatal team is directly present in the delivery room”. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unable to blind data collector to allocation group but data retrospectively collected from patient records so difficult to influence numerical data, e.g. Hb or presence/absence of morbidity. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised babies had data available. All exclusions accounted for. No loss to follow‐up and no failures to deliver intended intervention. Except the long‐term follow‐up where data were missing on 14/31 (45%) in DCC and 5/27 (18%) in UCM. Authors report that some parents did not want to come back for the 3.5. year follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes appear to be reported but 2 and 3.5 year neurological data reported in a later paper (Rabe 2015) and it was unclear in the original paper that these data were to be collected. |
| Other bias | Unclear risk | Baseline data on women and babies were similar. Long‐term follow up ‐ data missing on 14/31 (45%) in DCC and 5/27 (18%) in UCM. Authors report that some parents lost interest at 3.5 years as children were doing well and often families were busy. |
Rana 2017.
| Methods | Randomised, parallel group trial | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: DCC
Comparator: ECC
|
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
No usable data for this review Setting: no information ‐ authors live in India Dates: Started 15 April 2014 but no information on completion date though trial reported as complete on trial registration form. Declaration of interest: not reported Trial funding source: Maulana Azad Medical College (Government Medical College), Bahadur Shah Zafar Marg, New Delhi 110002 Further information:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation (information from trial registration). |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, sealed, opaque envelopes (information from trial registration). |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Clinicians cannot be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There is no information to show that the assessments of outcomes were blinded. For lab tests it is likely there was blinding ‐ but unclear for the clinical outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol. |
| Other bias | Unclear risk | There is very little information in this short 'Letter to the Editor'. |
Ranjit 2015.
| Methods | Randomised controlled trial | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: DCC
Comparator: ECC
Additional information
Comparison 1 DCC with neonatal resuscitation after cord clamping vs ECC (subgroup by gestation) Subgroup 3: mixed gestation Comparison 2 DCC with neonatal resuscitation after cord clamping vs ECC (subgroup by type intervention) Subgroup 5: DCC at > 2 mins with baby level with uterus and placenta |
|
| Outcomes |
Primary
Secondary
Definitions
|
|
| Notes |
Setting: tertiary care hospital in South India Dates: May 2010 to November 2010 Declaration of interest: no conflict of interest. Trial funding source: quote: "Role of funding source ‐ None" Further information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Qquote: “…based on computer generated random numbers.” |
| Allocation concealment (selection bias) | Low risk | Quote: “Allocation concealment was achieved by sequentially numbered opaque sealed envelopes containing the codes for intervention” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It is not possible to blind clinicians nor women. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There is no information as to whether there was any attempt to blind outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 6 babies (12%) randomised to DCC group were excluded from the study as they did not receive the intervention due to the need for resuscitation. None were excluded from the ECC group, yet 5 died in the ECC group and no deaths were reported in the DCC group, but there is no information on the 6 who were excluded ‐ it is important to know how many of these died. 4 in the ECC group and 3 in the DCC group were lost for follow‐up. Total exclusion DCC 9/50 (18%) ICC 4/50 (8%). Overall 13% exclusions, but uneven. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol. |
| Other bias | Unclear risk | Baseline characteristics were similar but they excluded 6 babies from DCC because they needed resuscitation. |
Salae 2016.
| Methods | Randomised controlled trial | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: DCC
Comparator: ECC
Additional information
Comparison 1: DCC with neonatal resuscitation after cord clamping vs ECC (subgroup by gestation) Subgroup 2: > 32‐34 weeks' gestation Comparison 2: DCC with neonatal resuscitation after cord clamping vs ECC (subgroup by type intervention) Subgroup 3: DCC at 1‐2 mins with baby level with uterus and placenta |
|
| Outcomes | Primary:
Secondary:
|
|
| Notes |
Setting: Department of Obstetrics and Gynaecology, Thammasat University Hospital, Pathumthani, Thailand Dates: July 2014 – April 2015. Declaration of interest: no conflict of interest Trial funding source: Faculty of Medicine, Thammasat University, Pathumthani, Thailand, Further information:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Statistical computer program. Simple randomisation was used. Sealed envelopes containing numbers which had been generated by a statistical computer program were placed in a box. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes containing numbers – attached to woman’s medical records in an intact manner opened only at start of second stage of labour….The first attending physician at the labour room picked up a sealed envelope, opened at the start of the second stage of labour. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not mentioned but attending clinician cannot be blinded. No information as to whether women knew or not. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not mentioned but most likely blind as main outcome as Hct (a laboratory estimation) and the clinical data likely to be collected by ward staff. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Lost 8/50 (16%) from DCC and 6/50 (12%) from ECC – so unlikely to cause bias. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess trial protocol and paper just documents that it will report maternal and neonatal complications – without specifying. We would expect other data to be collected. |
| Other bias | Unclear risk | Nothing apparent but we would have expected more methodological information to be included. |
Sekhavat 2008.
| Methods | Randomised controlled trial | |
| Participants |
Inclusion criteria
Exclusion criteria |
|
| Interventions |
Intervention group: DCC
Comparator: ECC
Additional information
Comparison 1: but no usable data DCC with neonatal resuscitation after cord clamping vs ECC (subgroup by gestation) Subgroup 1: < 32‐34 weeks' gestation Comparison 2: but no usable data DCC with neonatal resuscitation after cord clamping vs ECC (subgroup by type intervention) Subgroup 7: unclear |
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Notes |
No usable data Setting: Shahid Sedudhu University, Iran. Dates: not reported Declaration of interest: not reported Trial funding source: not reported Further information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind clinicians and unclear whether mothers knew or not. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information on how many had outcome data assessed. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol. |
| Other bias | Unclear risk | Conference abstract only so very little information on which to judge. |
Shi 2017.
| Methods | Randomised controlled trial | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: DCC
Comparator: ECC
Additional information
Comparison 1 DCC with neonatal resuscitation after cord clamping vs ECC (subgroup by gestation) Subgroup 3: gestation unclear Comparison 2: but no usable data DCC with neonatal resuscitation after cord clamping vs ECC (subgroup by type intervention) Subgroup 7: unclear |
|
| Outcomes |
|
|
| Notes |
Setting: ZhengZhou University Third Affiliated Hospital, China Dates: June to October 2015 Funding source: not reported Declaration of interest: not reported Further information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Random number method to allocate groups” |
| Allocation concealment (selection bias) | Unclear risk | Quote: “Random number method to allocate groups” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No statement on blinding although the clinicians at the birth cannot be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No statement on blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No exclusions. All 520 participants completed the study and were analysed. |
| Selective reporting (reporting bias) | Unclear risk | All methodology stated outcomes were reported, either in text or prose but we did not assess the trial protocol. |
| Other bias | Low risk | Seems generally okay, reported values were given (not blanket P values. They used recognised scales like Apgar scores. |
Strauss 2008.
| Methods | Randomised controlled trial. Stratified by gestation (< 30 weeks and > 30 weeks). | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: DCC
Comparator: ECC
Additional information
Comparison 1: DCC with neonatal resuscitation after cord clamping vs ECC (subgroup by gestation) Subgroup 3: mixed gestation Comparison 2: DCC with neonatal resuscitation after cord clamping vs ECC (subgroup by type intervention) Subgroup 4: DCC at 1‐2 mins with baby low (+ gravity) |
|
| Outcomes |
Primary outcome
Secondary outcome
|
|
| Notes |
Setting: USA. Dates: not reported Declaration of interest: R Strauss and D Mock report nothing to disclose in the conference abstract of 2007. Other authors have not reported. Trial funding source: not reported Further information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Table of random numbers. Stratified by gestation (< 30 weeks, > 30 weeks). |
| Allocation concealment (selection bias) | Unclear risk | Quote: “written instructions in sealed envelopes opened immediately before delivery.” Not clear whether envelopes were numbered and opaque. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Because the focus of this trial was on haematological outcomes it is unlikely that lack of blinding of women had an impact on outcomes. However, the focus of the review is clinical outcomes. It is possible staff who were aware of group assignment may had altered other aspects of care. It was stated that laboratory staff were blind to group assignment. Lack of blinding may have influenced other aspects of clinician behaviour and the recording of outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Because the focus of this trial was on haematological outcomes it is unlikely that lack of blinding of women had an impact on outcomes. It is possible staff who were aware of group assignment may had altered other aspects of care. It was stated that laboratory staff were blind to group assignment. Lack of blinding may have influenced other aspects of clinician behaviour and the recording of outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition and missing data were not clearly described. For babies less than 30 weeks' gestation there was major loss to follow‐up (but data for these infants have not been included in the review as they did not undergo true early clamping). Of 105 deliveries after 30 weeks all seemed to be accounted for in the analysis although the authors reported some missing data for some variables. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol . |
| Other bias | High risk | The study groups were not balanced in terms of size (60 in the immediate clamping group and 45 delayed). The reason for uneven group size for births < 30 weeks’ gestation was not explained. |
Tarnow‐Mordi 2017.
| Methods | Randomised controlled trial With minimisation and stratification according to gestational age (< 27 weeks vs ≥ 27 weeks), by centre, and multiple birth status (singleton birth vs. multiple birth). Infants of multiple births underwent randomisation individually. |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: DCC
Comparator: ECC
Additional information
Comparison 1 DCC with neonatal resuscitation after cord clamping vs ECC (subgroup by gestation) Subgroup 1: < 32‐34 weeks' gestation Comparison 2 DCC with neonatal resuscitation after cord clamping vs ECC (subgroup by type intervention) Subgroup 4: DCC at 1‐2 mins with baby low (+ gravity) |
|
| Outcomes |
Primary outcomes
Secondary outcomes
Tertiary outcomes (analyses of which were considered to be hypothesis generating)
|
|
| Notes |
Setting: 25 centres in 7 countries: Australia; New Zealand; Canada; France; Northern Ireland; Pakistan and USA. Led by University of Sydney, Australia. Dates: December 2010 to January 2017 Declaration of interest: disclosure forms provided by the authors are available with the full text of this article at NEJM.org. Trial funding source: supported by the National Health and Medical Research Council (NHMRC) and by the NHMRC Clinical Trials Centre, University of Sydney. Further information:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “…computer generated randomisation…” |
| Allocation concealment (selection bias) | Low risk | Quote: “The computer generated randomisation lists used with the interactive voice response system will be prepared by an independent statistician at the NHMRC Clinical Trials Centre, University of Sydney. The randomisation code will be stored securely by the statistical group at the centre.” (Trial registration form) “Randomization was performed centrally … with the use of an interactive voice‐response system with minimization and with stratification according to gestational age (<27 weeks vs. ≥27 weeks), center, and multiplebirth status (singleton birth vs. multiple birth).” (2017 publication) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Clinicians at the birth cannot be blinded and it is unclear whether women knew or not but women knowing is unlikely to affect outcome assessment. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Qquote: “For practical reasons, no attempt was made to make staff who were diagnosing these morbidities unaware of the timing of cord clamping.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1634 infants randomised. 68 were excluded and 69 had missing data for ≥ 1 component of primary outcome. So overall loss of primary data were 8%. |
| Selective reporting (reporting bias) | Low risk | All outcomes from full trial protocol are reported in the 2017 paper and Supplementary Appendix. |
| Other bias | Low risk | No other biases apparent. |
Tiemersma 2015.
| Methods | Randomised controlled trial | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: DCC
Comparator: ECC
Additional information
Comparison 1 DCC with neonatal resuscitation after cord clamping vs ECC (subgroup by gestation) Subgroup 3: mixed gestation Comparison 2: DCC with neonatal resuscitation after cord clamping vs ECC (subgroup by type intervention) Subgroup 5: DCC at > 2 mins with baby level with uterus and placenta |
|
| Outcomes |
Primary
Secondary
Also:
|
|
| Notes |
Setting: Stanger Provincial Hospital in Stanger, KwaZulu‐Natal, South Africa. Dates: February to October 2012 Declaration of interest: not reported Trial funding source: quote: “This study was supported by the Otto Kranendonk Fund of the Netherlands Society for Tropical Medicine and International Health and Drager Medical South Africa (Pty) Ltd. The funding organisations did not participate in the study design, collection, analysis and interpretation of data. They had no participation either in the writing of the report or in the decision to submit the manuscript for publication.” Further information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Computer generated blocks of 10 participants" |
| Allocation concealment (selection bias) | Low risk | Quote: "...sequentially numbered sealed opaque envelopes. Randomisation cards were not reused in case of post‐randomisation exclusion." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The nature of the intervention prevented us from blinding the study." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "The nature of the intervention prevented us from blinding the study." Not stated whether assessment postnatally was done by blinded assessors or not. If unblinded, unlikely to have influenced Hb/Hct or non‐subjective measures but may have influenced clinical judgement, e.g. regarding hyperviscosity diagnosis in the intervention. However, no diagnoses were made of this in either group – reduced effect of bias. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 77 out of 181 randomised were excluded (42.5%) because birthweight was > 3 kg. also 7/88 (8%) babies in DCC group excluded because they needed resuscitation and had ECC. None in ECC group were excluded for this. |
| Selective reporting (reporting bias) | Unclear risk | Reported data on all primary and secondary objectives mentioned as well as reported non‐significant parameters. However, we have not assessed trial protocol. |
| Other bias | Unclear risk | There were no differences between groups with respect to maternal age, maternal nutritional status, HIV‐positivity, Hb, birthweight, gestational age, gender or cord blood values. Not using ITT because they excluded babies in DCC group who got ECC. |
Ultee 2008.
| Methods | Randomised controlled trial | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: DCC
Comparator: ECC
Additional information
Comparison 1 DCC with neonatal resuscitation after cord clamping vs ECC (subgroup by gestation) Subgroup 2: > 32‐34 weeks' gestation Comparison 2 DCC with neonatal resuscitation after cord clamping vs ECC (subgroup by type intervention) Subgroup 5: DCC at > 2 mins with baby level with uterus and placenta |
|
| Outcomes | Outcomes:
|
|
| Notes |
Setting: the Netherlands Dates: not reported Declaration of interest: report no competing interests. Trial funding source: not reported Further information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “... subjects were randomly assigned ... by pulling the category out of a blinded box with loose papers”. The same person carried out randomisation, delivered clinical care and collected some outcome data". |
| Allocation concealment (selection bias) | High risk | Quote: “... subjects were randomly assigned ... by pulling the category out of a blinded box with loose papers”. The same person carried out randomisation, delivered clinical care and collected some outcome data." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It is not possible to blind clinicians at baby’s birth and it is unclear if women knew or not. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | It was stated that some clinical staff were unaware of groups assignment. However, the same person delivered care and assessed Apgar score and low score was a reason for post‐randomisation exclusion (although this would not have been assessed until AFTER the designated intervention period). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 41 were randomised and outcome data were available for 37 (4/41 = 10% loss). |
| Selective reporting (reporting bias) | Unclear risk | Assessment of bias from published study report. We did not assess trial protocol. |
| Other bias | High risk | Groups appeared similar at baseline. There were 4 post‐randomisation exclusions, 2 because of protocol violations and a further 1 because of a low Apgar score at 1 and 5 mins and 1 for congenital malformation. Exclusion because of an outcome which is assessed after randomisation (low Apgar) raises concern about potential for bias. Appears to be no ITT analysis as they excluded protocol violations. |
BP: blood pressure BPD: bronchopulmonary dysplasia BUN: blood urea nitrogen BW: birthweight cm: centimetres CP: cerebral palsy CS: caesarean section DCC: delayed cord clamping ECC: early cord clamping EPO: erythropoietin hr(s): hour(s) Hb; haemoglobin Hct: haematocrit HR: heart rate IM: intramuscular ITT: intention to treat IU: international unit IUGR: intrauterine growth restriction IV: intravenous IVH: intraventricular haemorrhage LOS: late onset sepsis MCV: mean cell volume MDI: Mental Development Index mins: minutes MRP: manual removal of the placenta n/a: not applicable NICU: neonatal intensive care unit NIPB: non‐invasive blood pressure NIRS: near‐infrared spectroscopy NEC: necrotising enterocolitis OR: odds ratio PDA: Patent ductus arteriosus PIH: pregnancy‐induced hypertension PPH: postpartum haemorrhage PRBC: packed red blood cell PVH: periventricular haemorrhage PVL: periventricular leukomalacia RBC: red blood cell RCT: randomised controlled trial RDS: respiratory distress syndrome Rh: Rhesus RoP: retinopathy of prematurity RR: risk ratio SD: standard deviation sec(s): second(s) SVC: superior vena cava UCM: umbilical cord milking VB: vaginal birth vs: versus
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aitchison | Trial plan only. No data recorded with this citation (Aitchison). |
| Akhtar 2014 | Babies at term not preterm (Akhtar 2014). |
| Ashish 2017 | Does not fit with the objectives of the review as the authors compared 2 different lengths of delayed cord clamping (≥ 180 seconds versus ≤ 60 seconds) (Ashish 2017). |
| Chopra 2016 | Study includes a different population of babies (≥ 35 weeks who were small for gestational age). Although some preterm babies would be included in this study, the mean gestational age of those providing data was around 37.6 weeks and the study was not stratified by gestational age. In addition, there was a loss of 42% of data. |
| Frank 1967 | This was a non‐randomised study in which delayed cord clamping was defined as that performed after the second breath (Frank 1967). |
| Garabedian 2016 | Not an RCT. A cohort study of a continuous series compared with a historical continuous series (Garabedian 2016). |
| Ibrahim 2000 | Randomised trial with adequate concealment. The intervention consisted of a delay in cord clamping of 20 seconds. Control infants had their cords clamped immediately. The study was excluded for the reason that the intervention group at a cord clamping time of less than 30 seconds. Delay of cord clamping was defined in the protocol for this review to be of at least 30 seconds duration (Ibrahim 2000). |
| Katheria 2016 | The comparison is ventilation during delayed cord clamping (V‐DCC) compared with delayed cord clamping alone (DCC only). DCC was 60 seconds in both groups (Katheria 2016). |
| Kattwinkel 2016 | This study is a comparison of ventilation before or after clamping, either with standard ventilation or CPAP. |
| Mungkornkaew 2015 | Does not fit with the objectives of the review as the authors compared 2 different lengths of delayed cord clamping (2 minutes versus 1 minute) (Mungkornkaew 2015). |
| Narendra 1998 | Abstract only, further details on women and study not available from the authors (Narendra 1998). |
| Ruangkit 2015 | Not an RCT but compared with historical cohort (Ruangkit 2015). |
| Saigal 1972 | Sequential allocation procedure, which is not a randomised trial (Saigal 1972). |
| Saigal 1977 | Sequential allocation procedure, which is not a randomised trial (Saigal 1977). |
| Spears 1966 | Randomisation procedure was unclear. Gestational age of the low birthweight infants was not given (Spears 1966). |
| Taylor 1963 | Inadequate randomisation. Largely term infants. DCC > 1 minute vs ECC < 1 minute quote: "...patients were assigned in rotation..." i.e. quasi randomised so excluded. Also report randomisation of premature infants failed (Taylor 1963). |
| Tipwaree 2015 | Study included women and babies at term only (Tipwaree 2015). |
| Yadav 2015 | Study included women and babies at term only (Yadav 2015). |
| Yasmeen 2014 | Does not fit with the objectives of the review as the authors compared 2 different lengths of delayed cord clamping (≥ 3 minutes versus ≤ 1 minute) (Yasmeen 2014). |
| Zisovska 2008 | Quasi‐RCT reported as quote: "...randomised alteratively..." (Zisovska 2008). |
CPAP: continuous positive airway pressure DCC: delayed cord clamping ECC: early cord clamping RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
Das 2018a.
| Methods | Randomised controlled trial |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | New report identified in November 2018 to add to Das 2018 study ‐ to be assessed in next update |
El‐Naggar 2018.
| Methods | Randomised controlled trial |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | New report identified in November 2018 to be added to El‐Naggar 2016 ‐ to be assessed in next update |
Hu 2015.
| Methods | Randomised controlled trial |
| Participants | Babies born between 28 and 35 weeks' gestation. |
| Interventions | Group A: ECC (10 secs) Group B: DCC (30 secs) Group C: DCC (60 secs) Group D; DCC 120 secs) |
| Outcomes | |
| Notes | Seeking full text of thesis. Interlibrary loans service (UK) received no reply from Zhejiang University (November 2017) Conference abstract published in 2017 but no data reported. |
Hua 2010.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Awaiting translation help. |
Kazemi 2017.
| Methods | Randomised controlled trial |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | New study identified in November 2018 ‐ to be assessed in next update |
Leal 2018.
| Methods | Randomised controlled trial |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | New study identified in November 2018 ‐ to be assessed in next update |
Li 2018.
| Methods | Randomised controlled trial |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | New study identified in November 2018 ‐ to be assessed in next update |
Medina 2014.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Awaiting translation from Spanish. Only part translated into English in paper is title "Late clamping of the umbilical cord in premature neonates: The real haemodynamic benefits". Looks like an additional report of Mercer 2006 ‐ awaiting confirmation. |
Ram Mothan 2018.
| Methods | Randomised controlled trial |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | New study identified in November 2018 ‐ to be assessed in next update |
Song 2017.
| Methods | Randomised controlled trial |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | New study identified in November 2018 ‐ to be assessed in next update |
Wang 2018.
| Methods | Randomised controlled trial |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | New report identified in November 2018 to be added to Mercer 2016 ‐ to be assessed in next update |
Weeks 2018.
| Methods | Randomised controlled trial |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | New study identified in November 2018 ‐ to be assessed in next update |
DCC: delayed cord clamping ECC: early cord clamping secs: seconds
Characteristics of ongoing studies [ordered by study ID]
Aghai 2018.
| Trial name or title | Umbilical cord milking in neonates who are depressed at birth (MIDAB) |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | Trial registration: https://clinicaltrials.gov/ct2/show/NCT03657394 |
Al‐Wassia 2016.
| Trial name or title | Deferred cord clamping compared to umbilical cord milking in preterm infants |
| Methods | RCT |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention: DCC
Comparator: Umbilical cord milking (UCM)
Comparison 5: DCC with neonatal resuscitation after cord clamping vs UCM (subgroup by gestation) S1: < 32‐34 weeks' gestation Comparison 6: DCC with neonatal resuscitation after cord clamping vs UCM (subgroup by type of intervention) S3: DCC at 1‐2 mins with baby level with uterus and placenta |
| Outcomes |
Primary IVH at 28 days Secondary Need for resuscitation; Apgar score at 1 minute; Apgar score at 5 minutes; need for blood transfusion during hospital stay; venous Hb; venous Hct; bilirubin; maximum bilirubin level; polycythaemia; RDS; oxygen dependency; need for volume administration; use of inotropes; NEC; mortality in hospital; sepsis |
| Starting date | January 2017 (anticipated end date January 2019) |
| Contact information | Heidi Al‐Wassia, King Abdulaziz University, Jeddah, Saudi Arabia. |
| Notes | Trial Registration: NCT02996799 Trial funding source: Declaration of interest: |
Allam 2018.
| Trial name or title | Delayed fetal cord clamping in premature labour: the effect on fetal haemoglobin, bilirubin and neonatal death, maternal haemoglobin, neonatal ICU admission and postpartum haemorrhage, |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | Trial registration: https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12618000758202 |
Anusha 2017.
| Trial name or title | Early cord clamping versus delayed cord clamping in very low birthweight neonates. |
| Methods | Multi‐centre RCT |
| Participants |
Inclusion criteria Low birthweight babies: < 1500 g. Estimated birthweight antenatally Exclusion criteria |
| Interventions |
Intervention: DCC
Comparator: ECC
Comparison: C1 S1 |
| Outcomes | Haemodynamic stability; blood transfusions; IVH; iron deficiency anaemia; hyperbilirubinaemia; polycythaemia. |
| Starting date | 02/02/2017 |
| Contact information | Anusha S, 1st floor, Department of Pediatrics, WC Block, Kasturba Hospital, Manipal, Udupi, KARNATAKA 576104 India. Tel: 8197188444. Email: anushacoolanu@gmail.com |
| Notes | Setting: India Clinical Trials: CTRI/2017/01/007671 Trial funding source: Declaration of interest: |
Bhriguvanshi 2017.
| Trial name or title | The effects of umbilical cord milking in neonates requiring resuscitation at birth: a randomised controlled trial |
| Methods | RCT |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention: UCM
Comparator: ECC
Comparison |
| Outcomes | Hb and Hct; Apgar scores; cord pH; resuscitation; transfusion; jaundice; phototherapy; inotropes; RDS; NEC; IVH; PVL; CPD; RoP; sepsis; mortality; neurodevelopment |
| Starting date | 31/08/2017 |
| Contact information | Arpita Bhriguvanshi, Department of Pediatrics, King Georges Medical University, Lucknow, UTTAR PRADESH, 226003, India. Telephone: 7376865064, Email: arpime84@gmail.com |
| Notes | Setting: India Trial registration: CTRI/2017/08/009484. Trial funding source: Declaration of interest: |
Bienstock 2011.
| Trial name or title | Milking the umbilical cord versus immediate clamping in preterm infants < 33 weeks: a randomised controlled trial |
| Methods | Randomised controlled trial |
| Participants | Preterm neonates born between 24 0/7 and 32 6/7 |
| Interventions |
Intervention UCM: umbilical cord will be "milked" in direction towards neonate 4 times over the course of 10 minutes Comparator ECC: immediate cord clamping |
| Outcomes |
Primary
Secondary
|
| Starting date | September 2011 |
| Contact information | Contact: Christopher Wayock, Tel: 01 4106145143 Email: cwayock1@jhmi.edu |
| Notes | Trial Registration: NCT01819532 Trial funding source: Declaration of interest: Comparison 3. |
Carroli 2017.
| Trial name or title | Early versus delayed umbilical cord clamping in preterm infants born at less than 31 weeks of gestational age: a study to know which one is better for infant health |
| Methods | A multicentre randomised controlled trial |
| Participants | Women and infants between 24 and 30+6 gestation. Target 700 women and babies. |
| Interventions | DCC (90 secs) vs DCC (30 secs) |
| Outcomes | Sepsis, Apgar, IVH |
| Starting date | 29/06/2015. Expected finish date 30/12/2020. |
| Contact information | Dr Guillermo Carroli, Centro Rosarino de Estudios Perinatales, Moreno 878, 6th Floor, Rosario 2000 Argentina. |
| Notes | Sponsor: Pan American Health Organisation (PAHO) ‐ research organisation ‐ government funded Need new comparison ‐ next update. Trial funding source: Declaration of interest: |
Chamnanvanakij 2015.
| Trial name or title | Effect of delayed cord clamping versus cord milking in infants born at < 34 weeks’ gestation: a randomised controlled trial |
| Methods | Randomised controlled trial |
| Participants | Pregnant women who have preterm labour at 25 to 34 weeks of gestation |
| Interventions |
Intervention DCC: deferred cord clamping (60 seconds) Comparator UCM: umbilical cord milking (3‐4 times) |
| Outcomes |
Primary
Secondary
|
| Starting date | 4 April 2015 |
| Contact information | Sangkae Chamnanvanakij, Department of Pediatrics, Phramongkutklao Hospital 315 Rajavithee Rd, Bangkok, Postal code: 10400, Thailand. Phone: 66850712700. Email: chamnanvanakij@gmail.com |
| Notes | Trial registration: TCTR20150106001 Comparison 2. |
De Paco Matallana 2013.
| Trial name or title | Randomised study of delayed cord clamping versus early cord clamping in preterm infants born between 24 and 34 weeks |
| Methods | Randomised controlled trial |
| Participants | Women who are expected to give birth below 34 weeks of gestation. |
| Interventions |
Intervention DCC: cord clamping 45‐60 seconds after birth Comparator ECC: cord clamping within 10 seconds |
| Outcomes |
Primary
Secondary
|
| Starting date | 01 February 2011 to 01 September 2014 |
| Contact information | Dr Catalina De Paco Matallana, C/Alhelies 4. Edif. Al Andalus 3E El Ranero 30009 Spain. +34676672617 +34676672617 Email: katy.depaco@gmail.com |
| Notes | Trial registration: ISRCTN66018314 Setting: Clinic University Hospital 'Virgen de la Arrixaca', Murcia, Spain Trial funding source: Sistema Murciano de Salud, Spain Declaration of interest: |
Dempsey 2016.
| Trial name or title | Clamping the Umbilical cord In Premature Deliveries (CUPID): a randomised controlled pilot trial |
| Methods | A randomised controlled pilot trial. |
| Participants | Preterm infants |
| Interventions | Intervention 1: DCC (60 secs) Intervention 2: ECC (< 20 secs) Intervention 3: UCM |
| Outcomes | Infant outcomes: ECG brain activity; Apgar; haemodynamics; sepsis; NEC; death. Maternal outcomes: |
| Starting date | 1 July 2015. Expected finish 1 July 2017. |
| Contact information | Prof Eugene Dempsey, Department of Paediatrics and Child Health, Cork University Maternity Hospital, Wilton, Cork, Ireland. |
| Notes | Trial registration: ISRCTN92719670 Trial funding source: University College Cork (research organisation). Declaration of interest: |
Driggers 2013.
| Trial name or title | Delayed umbilical cord clamping versus cord milking in preterm neonate ‐ a randomised, controlled trial |
| Methods | Randomised controlled trial |
| Participants | Singleton or multiples pregnancies in women admitted for medically indicated delivery or in advanced spontaneous preterm labour with imminent delivery at 24 0/7 ‐ 28 6/7 weeks' gestation. Women ages 18 and older |
| Interventions |
Intervention 1 DCC: delay cord clamping for 30 seconds after birth Intervention 2 UCM: milking of the cord 4 times in 10 seconds Comparator ECC: immediate cord clamping |
| Outcomes |
Primary
Secondary
|
| Starting date | December 2011 to January 2013 |
| Contact information | Rita W Driggers, MD, Washington Hospital Center, Georgetown University Hospital |
| Notes | Trial registration: NCT01393834 Sponsors: Medstar Research Institute Declarations of interest: Comparisons 1;2;3 |
Gomaa 2017.
| Trial name or title | The hematologic impact of umbilical cord milking versus deferred cord clamping in premature neonates. a randomised controlled trial |
| Methods | Randomised controlled trial |
| Participants | Premature infants (24 to 35 weeks' gestation) |
| Interventions | DCC (60 secs) versus UCM (5 times) |
| Outcomes | Haematological parameters |
| Starting date | 1 December 2016. Estimated finish date 1 January 2018. |
| Contact information | Mohamed K Gomaa, MD. 00966/0501783606; mekano_1@yahoo.com Hytham Atia, MD. 00966/0538308500; hythamatia@gmail.com |
| Notes | Trial registration: NCT03147846 Setting: Zagazig, Saudi Arabia |
Gupta 2018.
| Trial name or title | Early versus delayed cord clamping in IUGR preterms a randomised controlled study |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | Trial registration: http://www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=25064 |
Haghshenas 2014.
| Trial name or title | Comparative study of the effect of delayed versus early cord clamping on the incidence of intraventricular haemorrhage in preterm neonate |
| Methods | Randomised controlled trial |
| Participants | Premature infants; delivered via caesarean section; gestational age of less 32 weeks; birthweight of less than 1500 g |
| Interventions |
Intervention DCC: cord clamping within 30 to 45 seconds of life Comparator ECC: cord clamping in the first 10 seconds of life |
| Outcomes |
Primary
|
| Starting date | 20 March 2014 to 20 March 2015 |
| Contact information | Mohsen Haghshenas, NICU ward, Ayatollah Rouhani Hospital, Babol University of Medical Sciences, Babol Babol Mazandaran, Islamic Republic Of Iran. Phone: 00981132238290 Email: matia.mojaveri@yahoo.com |
| Notes | Trial registration: IRCT2014091319145N1 Sponsor: Babol University of Medical Sciences |
Hao 2018.
| Trial name or title | Effect of delayed cord clamping versus umbilical cord milking on cerebral blood flow in preterm infant: a randomised, double‐blind controlled trial |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | Trial registration: http://www.chictr.org.cn/showproj.aspx?proj=30981 |
Hemmati 2014.
| Trial name or title | Comparing the effect of delayed versus immediate cord clamping on the incidence of intraventricular haemorrhage (IVH) in preterm neonates with gestational age = 34 weeks in Hafez and Zeynab hospitals from September 2012 to December 2013. |
| Methods | |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention: DCC
Comparator: ECC
|
| Outcomes |
Primary
Secondary
|
| Starting date | September 2012 |
| Contact information | Dr. Fariba Hemmati, Neonatal part, Nemazee Hospital, Zand Street, Shiraz, Fars, Iran, Islamic Republic. Email: hemmatif@sums.ac.ir |
| Notes | IRCT2014031116936N1. Retrospective registration: May 17, 2014 Iran September 2012 to December 2013 |
Holland 1998.
| Trial name or title | Placento‐fetal (autologous) transfusion at birth in infants born preterm: a randomised, controlled trial. |
| Methods | Randomised controlled trial |
| Participants | Infants < 32 weeks' gestation. |
| Interventions |
Intervention: DCC
Comparator:
|
| Outcomes |
Primary outcome
Secondary outcome
|
| Starting date | 1998 |
| Contact information | BM Holland Queen Mother's Hospital Glasgow G3 8SH |
| Notes | Trial completed in 2001. Results not available. 2 centres have published part of their centre's results (Aladangady 2006; Baenziger 2007). |
Isac 2017.
| Trial name or title | Effect of umbilical cord milking of late preterm and term infants on maternal and neonatal outcomes in a tertiary care hospital in South India: a randomised control trial. |
| Methods | Randomised, parallel group, placebo‐controlled trial. Permuted block randomisation, fixed. Sequentially‐numbered, sealed, opaque envelopes. Participant and Investigator blinded. |
| Participants |
Inclusion criteria
Exclusion criteria |
| Interventions |
Intervention: umbilical cord milking (UCM)
Comparator: ECC
|
| Outcomes |
Primary
Secondary
|
| Starting date | 3 November 2017. Estimated duration 1 year. |
| Contact information | Scientific enquiry: Dr Mini Isac, Professor, Dept. of Obstetrics and Gynecology MOSC Medical College Hospital, Kolenchery, Ernakulam, KERALA 682311, India. Email: drminiisac@gmail.com Public enquiry: Anu Anna George, Post Graduate, Dept. of Obstetrics and Gynecology MOSC MMH Kolenchery, Ernakulam, KERALA 682311, India. Email: annamed013@gmail.com |
| Notes | Sample size: 142. |
Jomjak 2018.
| Trial name or title | To compare the effects of delayed versus early cord clamping on neonatal outcomes in preterm (gestational age at >24 weeks to 36+6 weeks) and maternal outcomes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | Trial registration: http://www.clinicaltrials.in.th/index.php?tp=regtrials&menu=trialsearch&smenu=fulltext&task=search&task2=view1&id=3833 |
Katheria 2017.
| Trial name or title | Premature Infants Receiving Milking or Delayed Cord Clamping: PREMOD2 (PREMOD2) and PREMOD2 With Near Infrared Spectroscopy Sub‐study (PREMOD2) |
| Methods | Randomised, parallel assignment. It is not possible to blind the delivering obstetrician, however all other caregivers will be blinded. The procedure will be documented as "placental transfusion" in the delivery summary or admission‐progress notes and all study assessments whether primary (head US) or secondary (neurodevelopmental exams) will be performed by blinded team members. |
| Participants |
Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Intervention: DCC
Comparator: Umbilical cord milking
|
| Outcomes | Primary:
Secondary:
|
| Starting date | June 6, 2017 |
| Contact information | Anup Katheria: Email: anup.katheria@sharp.com Kathy Arnell: Email: kathy.arnell@sharp.com |
| Notes | 'Premature Infants Receiving Milking or Delayed Cord Clamping: PREMOD2' Recruiting 1500 infants. Dates aiming for: June 2017 to Dec 2020 (complete 2022) Setting: Canada, Germany, Ireland, United States Sponsor: Sharp HealthCare Declarations of interest: Substudy: assessing Near IR spectroscopy for cerebral oxygenation in 400 infants. Comparison 5 and 6 |
Katheria 2018.
| Trial name or title | Umbilical Cord Milking in Non‐Vigorous Infants Developmental Follow‐up (MINVIFU) (MINVIFU) |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | Trial registration: https://clinicaltrials.gov/ct2/show/NCT03621943 |
Liu 2018.
| Trial name or title | Delayed cord clamping prevents respiratory distress of infants delivered by selective caesarean section in between 34‐38 weeks of gestational age, a randomised controlled trial |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | Trial registration: http://www.chictr.org.cn/showproj.aspx?proj=30199 |
Martin 2013.
| Trial name or title | Timing of umbilical cord clamping after vaginal or caesarean preterm birth. |
| Methods | Randomised controlled trial |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention: DCC
Comparator: ECC
|
| Outcomes |
Primary
Secondary
|
| Starting date | December 2012 |
| Contact information | James Martin, University of Mississippi Medical Center |
| Notes |
NCT01766908 Completion date: June 2014 |
Mirzaeian 2018.
| Trial name or title | Investigation and comparison of neonatal complications of 2 methods of umbilical cord milking and early cord clamping in neonates |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | Trial registration: https://en.irct.ir/trial/29424 |
Nour 2018a.
| Trial name or title | Effect of delayed cord clamping in preterm neonates with placental insufficiency |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | Trial registration: https://clinicaltrials.gov/ct2/show/NCT03731546 |
Nour 2018b.
| Trial name or title | Impact of umbilical cord milking in preterm neonates with placental insufficiency |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | Trial registration: https://clinicaltrials.gov/ct2/show/NCT03731611 |
Panichkul 2015.
| Trial name or title | Effects of delayed versus early cord clamping in late preterm infants: a randomised controlled trial. |
| Methods | Randomised controlled trial |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention: DCC
Comparator: ECC
|
| Outcomes |
Primary
Secondary
|
| Starting date | Not yet recruiting (as per 6 Jan 2015) |
| Contact information | Prisana Panichkul, Department of Obstetrics and Gynecology, Phramongkutklao Hospital 315 Rajavithee Rd, Bankok 10400, Thailand. Email: boonsuki@gmail.com |
| Notes | TCTR20150107001 Thailand |
Perlman 2015.
| Trial name or title | The effects of delayed cord clamping on postnatal circulatory status in preterm neonates. |
| Methods | Randomised controlled trial |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention: DCC
Comparator: ECC
|
| Outcomes |
Primary
Secondary |
| Starting date | July 2015 |
| Contact information | Jeffrey M Perlman, jmp2007@med.cornell.edu |
| Notes | ClinicalTrials.gov Identifier: NCT02478684 |
Ping 2010.
| Trial name or title | Immediate versus delayed cord clamping on newborns. |
| Methods | Randomised controlled trial several arms. |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention 1: DCC
Intervention 2: DCC
Intervention 3: DCC with stabilisation with cord intact
Comparator: ECC
|
| Outcomes | Not specified in trials register. |
| Starting date | September 2009. |
| Contact information | Dr Zhang Hong Yu, Hainan Medical Centre, China |
| Notes | Ongoing trial. ClinicalTrials.gov Identifier: NCT01029496 |
Puiggros 2014.
| Trial name or title | Umbilical cord milking compared with delayed cord clamping to increase placental transfusion in preterm infants less than 34 weeks' gestation born by caesarean section. Randomised clinical trial |
| Methods | Randomised controlled trial |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention: DCC
Comparator: umbilical cord milking (UCM)
Comparison |
| Outcomes |
Primary
Secondary
|
| Starting date | July 2014 |
| Contact information | Monica Domingo‐Puiggros, MD; Corporacio Sanitaria Parc Tauli, Spain |
| Notes | ClinicalTrials.gov Identifier: NCT02187510 Other study number: CSPTNeonat2014_01 Trial is on‐going but not recruiting as at July 2014 |
Ruangkit 2017.
| Trial name or title | A randomised controlled trial of immediate versus delayed umbilical cord clamping in preterm infants of multiple births |
| Methods | Randomised controlled trial |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention: DCC
Comparator: ECC
|
| Outcomes |
Primary
Secondary
|
| Starting date | 1 March 2016 |
| Contact information | Chayatat Ruangkit, Division of Neonatology, Department of Pediatrics, Faculty of Medicine Ramathibodi Hospital, Bangkok 10400, Thailand. Email: chayatatr@hotmail.com |
| Notes | Trial reg: TCTR20170125001 Funding source: Faculty of Medicine Ramathibodi Hospital Research Fund Declaration of interest: no information Comparisons 1 and 2 |
Shahgheibi 2018.
| Trial name or title | The delayed umbilical cord clamping effects on early outcome in preterm neonates |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | Trial registration: https://en.irct.ir/trial/17924 |
Smith 2014.
| Trial name or title | Delayed clamping and milking the umbilical cord in preterm infants |
| Methods | Randomised controlled trial |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention: DCC
Comparator: UCM
|
| Outcomes |
Primary
Secondary
|
| Starting date | March 2014 to March 2015 |
| Contact information | Kathleen Smith |
| Notes |
Tanthawat 2017.
| Trial name or title | The effect of one‐time umbilical cord milking and early cord clamping in preterm infants: a randomised controlled trial (one‐time umbilical cord milking) |
| Methods | Randomised controlled trial |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention: UCM
Comparator: ECC
|
| Outcomes |
Primary
Secondary
|
| Starting date | 1 March 2016 |
| Contact information | Sopida Tanthawat, Ramathibodi Hospital, Bangkok 10400, Thailand. Email: sopida_tanth@hotmail.com |
| Notes | Recruitment complete ‐ 40 babies enrolled Trial reg: TCTR20170201003 Funding source: Ramathibodi hospital, Mahidol University Declaration of interest: |
Thukral 2016.
| Trial name or title | Comparison of umbilical cord milking with delayed cord clamping in late preterm and term neonates: randomised control trial |
| Methods | Randomised, parallel group, active controlled trial Computer‐generated randomisation; Sequentially‐numbered, sealed, opaque envelopes. Participant and outcome assessor blinded. |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions | Intervention: DCC
Comparator: UCM
|
| Outcomes |
Primary
Secondary
|
| Starting date | 20 June 2016 |
| Contact information | Dr Anu Thukral, AIIMS DELHI AIIMS DELHI, South West, DELHI, 110029, India. Email: dranuthukral@gmail.com Mukul Kumar Mangla, AIIMS DELHI AIIMS DELHI, South West, DELHI, 110029, India. Email: drmanglamukul@yahoo.co.in |
| Notes | Setting: India Primary sponsor: AIIMS DELHI, a research institution and hospital Declaration of interest: Trial completed. |
Upahyay 2014.
| Trial name or title | Umbilical cord milking in preterm newborns and its role in prevention of anaemia in early infancy |
| Methods | Randomised controlled trial |
| Participants |
Inclusion criteria
Exclusion criteria |
| Interventions |
Intervention: umbilical cord milking (UCM)
Comparator: ECC
|
| Outcomes |
Primary
Secondary
|
| Starting date | 1 September 2013 |
| Contact information | AMIT UPADHYAY, DERARTMENT OF PEDIATRICS, LLRM MEDICAL COLLEGE, MEERUT Meerut UTTAR PRADESH 250004 India. Phone 9837405009; Email: anuamit7@rediffmail.com |
| Notes | CTRI/2014/12/005278 (registered retrospectively) |
Varanattu 2017.
| Trial name or title | Effect of intact umbilical cord milking versus immediate cord clamping on neonatal outcomes and first year neurodevelopmental outcomes in very preterm infants ‐ a randomised controlled trial |
| Methods | Randomised parallel assignment |
| Participants |
Inclusion criteria
Exclusion criteria |
| Interventions |
Intervention: UCM
Comparator: ECC
|
| Outcomes |
Primary
Secondary
|
| Starting date | Anticipated 1 September 2017 |
| Contact information | Manoj Varanattu, Email: manojvaranattu@gmail.com Varghese PR, Email: drprvarghese@gmail.com |
| Notes |
Whitehead 2014.
| Trial name or title | Effects of delayed cord clamp and/or indomethacin on preterm infant brain injury. |
| Methods | |
| Participants | |
| Interventions |
Intervention: Comparator: |
| Outcomes |
Primary
Secondary
|
| Starting date | August 2014 |
| Contact information | |
| Notes | ClinicalTrials.gov Identifier: NCT02221219 |
Xie 2017.
| Trial name or title | Study on umbilical cord milking to prevent and decrease the severity of anaemia in preterms |
| Methods | Randomised, parallel assignments |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention: UCM
Comparator: ECC
|
| Outcomes |
Primary
Secondary
|
| Starting date | 30 June 2017 |
| Contact information | Lijuan Xie, director, Email: xlj68115@sina.com |
| Notes | Setting: China Sponsors: Xinhua Hospital, Shanghai Jiao Tong University School of Medicine |
Yared 2015.
| Trial name or title | Delayed cord clamping at 30 vs. 60 seconds for very low birthweight infants: a randomised controlled trial |
| Methods | |
| Participants | |
| Interventions |
Intervention: DCC
Comparator: ECC
|
| Outcomes | |
| Starting date | January 2015 |
| Contact information | Edom Yared, Email: edom.yared@uchospitals.edu |
| Notes | ClinicalTrials.gov Identifier: NCT02337088 |
BPD: bronchopulmonary dysplasia cm: centimetres CPD: chronic pulmonary disease DCC: delayed cord clamping ECC: early cord clamping Hb; haemoglobin Hct: haematocrit IUGR: intrauterine growth restriction IVH: intraventricular haemorrhage NICU: neonatal intensive care unit NEC: necrotising enterocolitis PCV: packed cell volume PVL: periventricular leukomalacia RCT: randomised controlled trial RCV: red cell volume RDS: respiratory distress syndrome Rh: Rhesus RoP: retinopathy of prematurity sec(s): second(s) UCM: umbilical cord milking vs: versus
Differences between protocol and review
We set up separate comparisons for delayed cord clamping and umbilical cord milking.
HR, LD and GG modified the list of outcomes choosing seven primary outcomes to assist the assessment using GRADE software.
We removed the following outcomes: Requirement for resuscitation; Apgar scores at 1,5 and 10 minutes; Use of exogenous surfactant; Days of oxygen dependency; Oxygen dependency at 28 days; Treatment for hyperbilirubinaemia with blood exchange transfusion; Blood counts at six and 12 months of age (haemoglobin and ferritin); Maternal death.
We added the following new outcomes: Apgar < eight at five minutes: Duration of respiratory support; Home oxygen; Mean arterial blood pressure in early hours after birth; Hydrocephalis; Neurosensory disability at two to three years; Cerebral Palsy; Late sepsis; Treatment for retinopathy of prematurity; Severe visual impairment; Length of infant stay in NICU; Maternal blood transfusion; Maternal postpartum infection; Breastfeeding initiation; Fully breastfeeding or mixed breast & formula feeding at discharge.
We changed the following outcomes: 'Maternal blood loss greater than 500 mL' to 'Maternal blood loss of 500 mL or greater'; ‘Hypothermia’ to ‘Temperature < 36o within 1 hour of birth’; ‘Oxygen dependency at 36 weeks to CLD with this definition; Chronic lung disease (Northway Stage two, three or four) to CLD (oxygen dependency at 36 weeks corrected for gestational age)’; ‘Volume (colloid, sodium chloride 0.9%, blood transfusion) administration for hypotension during the first 24 hours of life’ to ‘Blood transfusion in infant’; 'Maternal bonding to infant' to 'Bonding'
Due to lack of data for previously intended subgroups (Position of the baby relative to the placenta; Whether the mother had oxytocin before cord clamping; With or without milking of the cord; Mode of birth), we chose to look at gestation and type of intervention only.
We updated the methods including the use of GRADE as recommended by Cochrane's MECIR standards and incorporated four new 'Summary of findings' tables.
We updated the Plain language summary to reflect the Cochrane Pregnancy and Childbirth Group's guidance on this.
We searched ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) for unpublished, planned and ongoing trial reports.
Contributions of authors
For this update
Gill Gyte (GG) undertook the data extraction and data entry with assistance from Heike Rabe (HR), Jose Diaz‐Rosello (JDR) and Lelia Duley (LD). HR, JDR and LD contributed clinical knowledge and input. GG conducted the GRADE assessments and drafted the results section. Review authors assessed the studies independently. HR and LD did not assess their own studies and GG did not assess the study on which she was a co‐applicant.
For previous versions of the review
Graham Reynolds (GR) prepared the first draft of the protocol and commented on the second draft. HR commented on the first draft of the protocol and wrote the second draft.
All review authors assessed studies independently. HR did not assess her own study. HR and GR entered study data. GR wrote the 'Methodological quality of included studies' section. HR completed all other sections of the review. JDR completed the corrections to the statistics. All three review authors commented on the review and agreed on the conclusion.
For the update of this review, the process of assessing the eligible studies and extracting the data were followed in the same way as described as above. HR updated the data tables and updated the text of the review. JDR and Therese Dowswell (TD) corrected the statistics. TD and LD introduced the risk of bias tables, and revised the text of the review. All review authors agreed on the updated version of the review.
Sources of support
Internal sources
University of Liverpool, UK.
External sources
No sources of support, UK.
Declarations of interest
Heike Rabe is main author for two included studies in this review (Rabe 2000; Rabe 2011). Studies by the contact author, which may be relevant for inclusion in this review, were not assessed by herself but by the co‐authors who, in agreement with the Cochrane Pregnancy and Childbirth group, have named other experts in the field for this purpose.
Jose Diaz‐Rossello ‐ none known.
Lelia Duley has been awarded an NIHR research grant for a programme of work which includes a pilot trial of timing of cord clamping for preterm births (CORD Pilot 2018), and a prospective meta‐analysis.
Gillian Gyte was a co‐applicant on one of the included studies in this review (CORD Pilot 2018). She also has received royalties from John Wiley & Son in respect of ‘A Cochrane Pocket Handbook – Pregnancy and Childbirth' Hofmeyr GJ et al. 2008.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Aladangady 2006 {published and unpublished data}
- Aladangady N, McHugh S, Aitchison TC, Wardrop CA, Holland BM. Infant's blood volume in a controlled trial of placental transfusion at preterm delivery. Pediatrics 2006;117:93‐8. [DOI] [PubMed] [Google Scholar]
Alan 2014 {published data only}
- Alan S, Arsan S, Okulu E, Akin I, Kilic A, Taskin S, et al. Effects of umbilical cord milking on the need for packed red blood cell transfusions and early neonatal haemodynamic adaptation in preterm infants born =1500 g. Archives of Disease in Childhood 2014; Vol. 99:A453‐4. [DOI] [PubMed]
- Alan S, Arsan S, Okulu E, Akin IM, Kilic A, Taskin S, et al. Effects of umbilical cord milking on the need for packed red blood cell transfusions and early neonatal hemodynamic adaptation in preterm infants born </=1500 g: a prospective, randomized, controlled trial. Journal of Pediatric Hematology/Oncology 2014; Vol. 36, issue 8:e493‐e498. [DOI] [PubMed]
Armanian 2017 {published data only}
- Armanian AM, Ghasemi Tehrani H, Ansari M, Ghasemi S. Is "delayed umbilical cord clamping" beneficial for premature newborns?. International Journal of Pediatrics 2017;5:4909‐18. [Google Scholar]
- Armanian M. Is "delayed umbilical cord clamping" beneficial for premature newborns?. en.search.irct.ir/view/22000 (first received 14 February 2015).
Backes 2016 {published data only}
- Backes C, Copeland K, Iams JP, Giannone PJ, Bauer JA. Impact of delayed umbilical cord clamping at the limits of viability. International Conference of Transitional Care; 2013 19th April; Birmingham, UK. 2013.
- Backes CH, Huang H, Iams JD, Bauer JA, Giannone PJ. Timing of umbilical cord clamping among infants born at 22 through 27 weeks' gestation. Journal of Perinatology 2016;36(1):35‐40. [2800931] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backes CH, Huang H, Luce WA, Schanbacher BL, Backes KA, Ehrenberg H, et al. Postnatal progenitor cells pools and delayed umbilical cord clamping at the limits of viability. Pediatric Academic Societies and Asian Society for Pediatric Research Joint Meeting; 2011 April 30‐May 3; Denver, Colorado, USA. 2011:441.
- Huang H, Eastman N, Schanbacher B, Backes C, Giannone P, Bauer JA. [2887.675] Delayed cord clamping improves gross motor skills in extremely premature infants at age 6‐9 months corrected age. Pediatric Academic Societies Annual Meeting; 2016 April 30 ‐ May 3; Baltimore, USA. 2016.
- Huang H, Eastman N, Schanbacher B, Backes C, Giannone P, Bauer JA. [3821.208] Impact of delayed cord clamping on circulating progenitor cells in extremely premature infants. Pediatric Academic Societies Annual Meeting; 2016 April 30 ‐ May 3; Baltimore, USA. 2016.
Baenziger 2007 {published data only}
- Baenziger O, Stolkin F, Keel, M, Siebenthal K, Fauchere JC, Kundu SD, et al. The influence of the timing of cord clamping on postnatal cerebral oxygenation in preterm neonates: a randomized, controlled trial. Pediatrics 2007;119:455‐9. [DOI] [PubMed] [Google Scholar]
Chu 2011 {published data only}
- Chu K, Whittle W, Windrim R, Shah P, Murphy K. The DUC trial: A pilot randomized controlled trial of immediate vs. delayed umbilical cord clamping in preterm infants born between 24 and 32 weeks gestation. American Journal of Obstetrics and Gynecology 2011;204(1 Suppl):S201. [Google Scholar]
CORD Pilot 2018 {published data only}
- Armstrong‐Buisseret L, Powers K, Dorling J, Bradshaw L, Johnson S, Mitchell E. Randomised trial of cord clamping and initial stabilisation at very preterm birth: neurodevelopmental outcomes at age two years (corrected for gestation at birth). NIHR Monograph 2019; Vol. Monograph still to be published as well as paper.
- Ayers S, Sawyer A, Chhoa C, Duley L. Clinicians’ and women’s experiences of two consent pathways in a trial of timing of clamping at very preterm birth: A qualitative study. BJOG: an international journal of obstetrics and gynaecology 2016;123(Suppl 2):151. [Google Scholar]
- Bradshaw LE, Pushpa‐Rajah A, Dorling J, Mitchell EJ, Duley L, for the Cord Pilot Trial Collaborative Group. Cord pilot trial: update to randomised trial protocol. Trials 2015;16:407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorling J, Armstrong‐Buisseret L, Powers K, Bradshaw L, Johnson S, Mitchell E, et al. Randomised trial of delayed cord clamping and initial stabilisation at very preterm birth. Neurodevelopmental outcome at age 2 years CGA. Abstract presented at 7th Congress of the European Academy of Paediatric Societies. Paris, 2018.
- Duley L, Abbott J, Dorling J, Field D, Gyte G, Oddie S, et al. Timing of cord clamping and care at the bedside for very preterm birth: a randomised trial. BJOG: an international journal of obstetrics and gynaecology 2013;120(Suppl s1):159. [Google Scholar]
- Duley L, Dorling J, Pushpa‐Rajah A, Oddie S, Yoxall B, Schoonakker B, et al. Umbilical cord clamping after at least 2 minutes (and neonatal resuscitation with cord intact) versus clamping within 20 seconds: The Cord Pilot Trial, a controlled randomised trial of very preterm births. BJOG: an international journal of obstetrics and gynaecology 2016;123(Suppl 2):60. [Google Scholar]
- Duley L, Dorling J, Pushpa‐Rajah A, Oddie SJ, Yoxall CW, Schoonakker B, et al. Randomised trial of cord clamping and initial stabilisation at very preterm birth. Archives of Disease in Childhood. Fetal and Neonatal Edition 2018; Vol. 103, issue 1:F6‐F14. [PUBMED: 28923985] [DOI] [PMC free article] [PubMed]
- Duley L, Pushpa‐Rajah A. Immediate versus deferred cord clamping for very preterm birth: a pilot randomised trial. Archives of Disease in Childhood. Fetal and Neonatal Edition 2014;99(Suppl 1):A76‐A77, Abstract no: PC.117. [Google Scholar]
- Pushpa‐Rajah A, Bradshaw L, Dorling J, Gyte G, Mitchell EJ, Thornton J, et al. Cord pilot trial ‐ immediate versus deferred cord clamping for very preterm birth (before 32 weeks gestation): study protocol for a randomized controlled trial. Trials 2014;15(1):258. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dai 2014 {published data only}
- Dai S, Jin X, Qi HJ. [Bu tong duan qi shi jian dui xin sheng er yu hou de ying xiang]. Modern Practical Medicine 2014;26:69‐71. [Google Scholar]
Das 2018 {published data only}
- Das B. Placental transfusion in delivery room in preterm neonates 30 to 33 6/7 weeks: an open label randomized controlled trial. http://ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=7688&EncHid=&userName (first received 18 February 2014).
- Das B, Sundaram V, Kumar P. [3842.16] Effect of placental transfusion on serum ferritin levels in moderately preterm neonates. Pediatric Academic Societies Annual Meeting; 2015 April 25‐28; San Diego, California. 2015.
- Das B, Sundaram V, Kumar P, Mordi WT, Dhaliwal LK, Das R. Effect of placental transfusion on iron stores in moderately preterm neonates of 30‐33 weeks gestation. Indian Journal of Pediatrics 2018;85(3):172‐8. [DOI] [PubMed] [Google Scholar]
Datta 2017 {published data only}
- Datta V, Kumar A, Yadav R. A randomized controlled trial to evaluate the role of brief delay in cord clamping in preterm neonates (34‐36 weeks) on short‐term neurobehavioural outcome. Journal of Tropical Pediatrics 2017 [Epub ahead of print]. [DOI] [PubMed]
- Kumar A, Datta V. A randomized controlled trial to evaluate the role of brief delay in cord clamping in preterm neonates (34‐36 weeks) on short term neurodevelopmental outcomes. Pediatric Academic Societies and Asian Society for Pediatric Research Joint Meeting; 2014 May 3‐6; Vancouver, Canada. 2014:Abstract no: 2945.606.
Dhaliwal 2014 {published data only}
- Dhaliwal LK, Anjumunnisa S, Kumar P, Saha PK, Venkataseshan S. Effects of placental transfusion in preterm birth. Journal of Maternal‐Fetal & Neonatal Medicine 2014;27(Suppl 1):369. [Google Scholar]
Dipak 2017 {published data only}
- Kumar Dipak N, Nanavati RN, Kabra NK, Srinivasan A, Ananthan A. Effect of delayed cord clamping on hematocrit, and thermal and hemodynamic stability in preterm neonates: A randomized controlled trial. Indian Pediatrics 2017;54(2):112‐5. [DOI] [PubMed] [Google Scholar]
Dong 2016 {published data only}
- Dong XY, Sun XF, Li MM, Yu ZB, Han SP. [Influence of delayed cord clamping on preterm infants with a gestational age of <32 weeks] [26687]. Zhongguo Dang Dai Er Ke Za Zhi = Chinese Journal of Contemporary Pediatrics 2016;18(7):635‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elimian 2014 {published data only}
- Elimian A, Goodman J, Escobedo M, Nightingale L, Knudtson E, Williams M. A randomized controlled trial of immediate versus delayed cord clamping in the preterm neonate. American Journal of Obstetrics and Gynecology 2013;208(1 Suppl):S22. [DOI] [PubMed] [Google Scholar]
- Elimian A, Goodman J, Escobedo M, Nightingale L, Knudtson E, Williams M. Immediate compared with delayed cord clamping in the preterm neonate. Obstetrics & Gynecology 2014;124(6):1075‐9. [DOI] [PubMed] [Google Scholar]
El‐Naggar 2016 {published data only}
- El‐Naggar W. The effect of cord milking on hemodynamic status of preterm infants. clinicaltrials.gov/ct2/show/record/NCT01487187 (first received 7 December 2011).
- El‐Naggar W, Simpson D, Hussain A, Armson A, Dodds L, Warren A, et al. The effect of umbilical cord milking on hemodynamic status of preterm infants: a randomized controlled trial. Journal of Paediatrics and Child Health 2016;21:e88. [Google Scholar]
- El‐Naggar W, Simpson D, Hussain A, Armson A, Dodds L, Warren A, et al. [3856.517] The effect of umbilical cord milking on hemodynamic status of preterm infants: a randomized controlled trial. Pediatric Academic Societies Annual Meeting; 2016 April 30 ‐ May 3; Baltimore, USA. 2016.
- El‐Naggar W, Simpson D, Hussain A, Warren A, Robin W, McMillan D, et al. The effect of umbilical cord milking on cerebral blood flow of preterm infants: a randomized controlled trial. European Journal of Pediatrics 2016; Vol. 175, issue 11:1698.
Gokmen 2011 {published data only}
- Gokmen Z, Ozkiraz S, Tarcan A, Kozanoglu I, Ozcimen EE, Ozbek N. Effects of delayed umbilical cord clamping on peripheral blood hematopoietic stem cells in premature neonates. Journal of Perinatal Medicine 2011;39:323‐9. [DOI] [PubMed] [Google Scholar]
Hofmeyr 1988 {published data only}
- Hofmeyr GJ, Bolton KD, Bowen DC, Govan JJ. Periventricular/intraventricular haemorrhage and umbilical cord clamping. South African Medical Journal 1988;73:104‐6. [PubMed] [Google Scholar]
- Hofmeyr GJ, Bolton KD, Bowen DC, Govan JJ. Periventricular/intraventricular hemorrhage and umbilical cord clamping. Proceedings of the 10th European Congress of Perinatal Medicine; 1986; Leipzig, Germany. 1986:309.
Hofmeyr 1993 {published data only}
- Hofmeyr GJ, Gobetz L, Bex PJ, Griendt M, Nikodem CV, Skapinker R, et al. Periventricular/intraventricular hemorrhage following early and delayed umbilical cord clamping: a randomized trial. Online Journal of Current Clinical Trials 1993 Doc No 110: [2002 words; 26 paragraphs]. [PubMed]
- Hofmeyr GJ, Gobetz L, Bex PJ, Griendt M, Nikodem VC, Skapinker R, et al. Periventricular/intraventricular haemorrhage following early and delayed umbilical cord clamping. Letter to: Cochrane Pregnancy and Childbirth Group, Oxford 1991.
Hosono 2008 {published data only}
- Ghavam S, Batra D, Mercer J, Kugelman A, Hosono S, Oh W, et al. Effects of placental transfusion in extremely low birthweight infants: meta‐analysis of long‐ and short‐term outcomes. Transfusion 2014;54(4):1192‐8. [DOI] [PubMed] [Google Scholar]
- Hosono S, Mugishima H, Fujita H, Hosono A, Minato M, Okada T, et al. Umbilical cord milking reduces the need for red cell transfusions and improves neonatal adaptation in infants born less than 29 weeks' gestation: a randomized controlled trial. Archives of Disease in Childhood. Fetal and Neonatal Edition 2008;93:F14‐9. [DOI] [PubMed] [Google Scholar]
- Hosono S, Mugishima H, Fujita H, Hosono A, Okada T, Takahashi S, et al. Blood pressure and urine output during the first 120 h of life in infants born at less than 29 weeks' gestation related to umbilical cord milking. Archives of Disease in Childhood Fetal & Neonatal Edition 2009;94(5):F328‐31. [DOI] [PubMed] [Google Scholar]
- Hosono S, Mugishima H, Yonezawa R, Fujita H, Makimoto M, Okada T, et al. The effects of umbilical cord milking on cardio‐pulmonary adaptation in preterm infants. Journal of Maternal‐Fetal and Neonatal Medicine 2008;21(Suppl 1):45. [Google Scholar]
Hosono 2015 {published data only}
- Hosono S. A multicenter randomized control study of the effect of umbilical cord milking in avoiding red cell transfusions in extremely immature infants. upload.umin.ac.jp/cgi‐open‐bin/ctr_e/ctr_view.cgi?recptno=R000001193 (first received 23 January 2008).
- Hosono S, Tamura M, Kusuda S, Hirano S, Fujimura M, Takahashi S. [2765.7] One‐time umbilical cord milking after cord cutting reduces the need for red blood cell transfusion and reduces the mortality rate in extremely preterm infants; a multicenter randomized controlled trial. Pediatric Academic Societies Annual Meeting; 2015 April 25‐28; San Diego, California 2015.
- Hosono S, Tamura M, Kusuda S, Hirano S, Mori R, Fujimura M. [4470.5] Eighteen month corrected age developmental outcomes of extremely preterm infants enrolled in a randomized controlled trial of one‐time umbilical cord milking versus immediate cord clamping. Randomized controlled trial. Pediatric Academic Societies Annual Meeting; 2016 April 30 ‐ May 3; Baltimore, USA. 2016.
Josephsen 2014 {published data only}
- Josephsen J, Vlastos E, Potter S, Al‐Hosni M. Milking the umbilical cord in extreme preterm infants. American Journal of Obstetrics and Gynecology 2014;210(1 Suppl):S403‐4. [Google Scholar]
Katheria 2014 {published data only}
- Katheria A, Blank D, Rich W, Finer N. Umbilical cord milking improves transition in premature infants at birth. PLOS One 2014;9(4):e94085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katheria AC, Leone TA, Woelkers D, Garey DM, Rich W, Finer NN. The effects of umbilical cord milking on hemodynamics and neonatal outcomes in premature neonates. Journal of Pediatrics 2014;164(5):1045‐50. [DOI] [PubMed] [Google Scholar]
Katheria 2015 {published data only}
- Katheria A, Garey D, Truong G, Akshoomoff N, Steen J, Maldonado M, et al. A randomized clinical trial of umbilical cord milking vs delayed cord clamping in preterm infants: Neurodevelopmental outcomes at 22‐26 months of corrected age. Journal of Pediatrics 2018;194:76‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katheria A, NCT03476980. Two year developmental follow‐up for premature infants receiving milking or delayed cord clamping: PREMOD2. https://clinicaltrials.gov/ct2/show/NCT03476980 (first received 26 March 2018). [NCT03476980]
- Katheria AC, Truong G, Cousins L, Oshiro B, Finer NN. Umbilical cord milking versus delayed cord clamping in preterm infants. Pediatrics 2015;136(1):61‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katheria AC, Truong G, Wade R, Nguyen T, Kim S, Arnell K, et al. [2765.6] Umbilical cord milking improves systemic blood flow at cesarean section in premature infants. Pediatric Academic Societies Annual Meeting; 2015 April 25‐28; San Diego, California. 2015.
Kilicdag 2016 {published data only}
- Kilicdag H, Gulcan H, Hanta D, Torer B, Gokmen Z, Ozdemir SI, et al. Is umbilical cord milking always an advantage?. Journal of Maternal‐Fetal & Neonatal Medicine 2016;29(4):615‐8. [DOI] [PubMed] [Google Scholar]
Kinmond 1993 {published and unpublished data}
- Kinmond S, Aitchison TC, Holland BM, Jones JG, Turner TL, Wardrop CA. Umbilical cord clamping and preterm infants: a randomised trial. BMJ 1993;306:172‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinmond S, Aitchison TC, Holland BM, Jones JG, Turner TL, Wardrop CA. Umbilical cord clamping and preterm infants: a randomized trial. International Journal of Gynaecology and Obstetrics 1993;42(3):328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinmond S, Hudson IR, Aitchison T, Holland BM, Turner TL, Jones JG, et al. Placento‐fetal transfusion in preterm infants. Proceedings of the Neonatal Society; 1990 March; London, UK. 1990.
Krueger 2015 {published data only}
- Krueger MS, Eyal FG, Peevy KJ, Hamm CR, Whitehurst RM, Lewis DF. Delayed cord clamping with and without cord stripping: A prospective randomized trial of preterm neonates. American Journal of Obstetrics and Gynecology 2015;212(3):394.e1‐5. [DOI] [PubMed] [Google Scholar]
Kugelman 2007 {published data only}
- Kugelman A, Borenstein‐Levin L, Kessel A, Riskin A, Toubi E, Bader D. Immunologic and infectious consequences of immediate versus delayed umbilical cord clamping in premature infants: A prospective, randomized, controlled study. Journal of Perinatal Medicine 2009;37(3):281‐7. [DOI] [PubMed] [Google Scholar]
- Kugelman A, Borenstein‐Levin L, Riskin A, Chistyakov I, Ohel G, Gonen R, et al. Early versus delayed umbilical cord clamping in premature infants: a prospective, randomized, controlled study. Conference Proceedings, Pediatric Academic Societies Annual Meeting, Toronto, Canada. 2007 May 5‐8.
- Kugelman A, Borenstein‐Levin L, Riskin A, Christyakov I, Ohel G, Gonen R, et al. Immediate versus delayed umbilical cord clamping in premature neonates born < 35 weeks: a prospective, randomized, controlled study. American Journal of Perinatology 2007;24:307‐15. [DOI] [PubMed] [Google Scholar]
Kumar 2015 {published data only}
- Kumar B, Upadhyay A, Garg A, Gothwal S, Yadav AK, Sharma RS. [1580.592] Effect of umbilical cord milking on hematological parameters at 6 weeks of life in preterm newborns. Pediatric Academic Societies Annual Meeting; 2015 April 25‐28; San Diego, California. 2015.
- Kumar B, Upadhyay A, Gothwal S, Jaiswal V, Joshi P, Dubey K. Umbilical cord milking and hematological parameters in moderate to late preterm neonates: a randomized controlled trial. Indian Pediatrics 2015;52(9):753‐7. [DOI] [PubMed] [Google Scholar]
Malik 2013 {published data only}
- Malik AU, Shahnawaz K, Riaz A. Comparison between the efficacy of early and delayed umbilical cord clamping in preterm infants. Pakistan Journal of Medical and Health Sciences 2013;7(4):992‐5. [Google Scholar]
March 2013 {published data only}
- March M, Veciana M, Parson A. The efficacy of umbilical cord milking on the reduction of red blood cell transfusion rates in infants born between 24 and 28 6/7 weeks gestation ‐ A randomized controlled trial. American Journal of Obstetrics and Gynecology 2011;204(1 Suppl):S204. [Google Scholar]
- March MI, Hacker MR, Parson AW, Modest AM, De M. The effects of umbilical cord milking in extremely preterm infants: A randomized controlled trial. Journal of Perinatology 2013;33(10):763‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
McDonnell 1997 {published and unpublished data}
- McDonnell M, Henderson Smart DJ. Delayed umbilical cord clamping in preterm infants: a feasibility study. Journal of Paediatrics and Child Health 1997;33(4):308‐10. [DOI] [PubMed] [Google Scholar]
Mercer 2003 {published and unpublished data}
- Mercer JS, McGrath MM, Hensman A, Silver H, Oh W. Immediate and delayed cord clamping in infants born between 24 and 32 weeks: a pilot randomized controlled trial. Journal of Perinatology 2003;23:466‐72. [DOI] [PubMed] [Google Scholar]
Mercer 2006 {published and unpublished data}
- Mercer JS, Vohr BR, Erickson‐Owens DA, Padbury JF, Oh W. Seven‐month developmental outcomes of very low birth weight infants enrolled in a randomized controlled trial of delayed versus immediate cord clamping. Journal of Perinatology 2010;30(1):11‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer JS, Vohr BR, Erickson‐Owens DA, Padbury JF, Oh W. Seven‐month neurodevelopmental outcomes of infants enrolled in a randomized controlled trial of delayed versus immediate cord clamping. Pediatric Academic Societies Annual Meeting; 2009 May 2‐5; Baltimore, USA. 2009.
- Mercer JS, Vohr BR, McGrath MM, Padbury JF, Wallach M, Oh W. Delayed cord clamping in very preterm infants reduces the incidence of intraventricular hemorrhage and late‐onset sepsis: a randomized, controlled trial. Pediatrics 2006;117(4):1235‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer JS, Vohr BR, Oh W. Delayed cord clamping in very preterm infants reduces the incidence of intraventricular hemorrhage (IVH) and late onset sepsis (LOS) [abstract]. Pediatric Academic Societies Annual Meeting; 2005 May 14‐17; Washington DC, USA. 2005:Abstract no: 2618. [DOI] [PMC free article] [PubMed]
- Sommers R, Stonestreet BS, Laptook A, Yanowitz T, Oh W, Raker C. Hemodynamic effects of delayed umbilical cord clamping in premature infants. Pediatric Academic Societies and Asian Society for Pediatric Research Joint Meeting; 2011 April 30‐May 3; Denver, Colorado, USA. 2011:3535.2.
- Sommers R, Stonestreet BS, Oh W, Laptook A, Yanowitz TD, Raker C, et al. Hemodynamic effects of delayed cord clamping in premature infants. Pediatrics 2012;129(3):e667‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mercer 2016 {published data only}
- Mercer JS, Erickson‐Owens DA, Vohr BR, Tucker R, Oh W, Padbury JF. [2765.8] Delayed cord clamping at birth improves motor scores at 18 to 22 months corrected age: a randomized controlled trial. Pediatric Academic Societies Annual Meeting; 2015 April 25‐28; San Diego, California. 2015.
- Mercer JS, Erickson‐Owens DA, Vohr BR, Tucker RJ, Parker AB, Oh W, et al. Effects of placental transfusion on neonatal and 18 month outcomes in preterm infants: a randomized controlled trial. Journal of Pediatrics 2016; Vol. 168:50‐5. [DOI] [PMC free article] [PubMed]
Nelle 1998 {published and unpublished data}
- Nelle M, Fischer S, Conze S, Beedgen B, Grischke EM, Linderkamp O. Effects of late cord clamping on circulation in prematures (VLBWI). Pediatric Research 1998;44(3):454. [Google Scholar]
Oh 2011 {published and unpublished data}
- Ghavam S, Batra D, Mercer J, Kugelman A, Hosono S, Oh W, et al. Effects of placental transfusion in extremely low birthweight infants: meta‐analysis of long‐ and short‐term outcomes. Transfusion 2014;54(4):1192‐8. [DOI] [PubMed] [Google Scholar]
- Oh W, Carlo WA, Fanaroff AA, McDonald S, Donovan EF, Poole K, et al. Delayed cord clamping in extremely low birth weight infants ‐ a pilot randomised controlled trial. Pediatric Research 2002;51(4 Suppl):365‐6. [Google Scholar]
- Oh W, Fanaroff AA, Carlo WA, Donovan EF, McDonald SA, Poole WK, et al. Effects of delayed cord clamping in very‐low‐birth‐weight infants. Journal of Perinatology 2011;31:S68‐S71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pongmee 2010 {published data only}
- Pongmee P, Nuntnarumit P. Effects of umbilical cord milking on initial hematocrit and the need for blood transfusion in preterm infants. Pediatric Academic Societies Annual Meeting; 2010 May 1‐4; Vancouver, Canada. 2010.
Rabe 2000 {published and unpublished data}
- Rabe H, Hentschel R, Brune T, Hulskamp G, Jorch G. A randomised study of delayed cord clamping: the starting point in treatment of anaemia of prematurity. Prenatal and Neonatal Medicine 1996;1 Suppl 1:174. [Google Scholar]
- Rabe H, Wacker A, Hulskamp G, Hornig‐Franz I, Jorch G. Late cord clamping benefits extrauterine adaptation [abstract]. Pediatric Research 1998;44(3):454. [Google Scholar]
- Rabe H, Wacker A, Hulskamp G, Hornig‐Franz I, Schulze‐Everding A, Harms E, et al. A randomised controlled trial of delayed cord clamping in very low birth weight preterm infants. European Journal of Pediatrics 2000;159(10):775‐7. [DOI] [PubMed] [Google Scholar]
Rabe 2011 {published data only}
- Ayers S, Sawyer A, During C, Rabe H. Parents report positive experiences about enrolling babies in a cord‐related clinical trial before birth. Acta Paediatrica 2015;104:e164‐70. [DOI] [PubMed] [Google Scholar]
- Rabe H. Effects of a slight delay in cord clamping time versus milking the cord in preterm infants. isrctn.com/ISRCTN86296143 (first received 29 September 2006).
- Rabe H, Dhanjal K, Stilton D, Ayers S, Holden D. Parents perception of giving antenatal consent to include their preterm infant into a randomized controlled trial (RCT). Klinische Padiatrie 2010;222(S 01):GNPI_PO_50. [Google Scholar]
- Rabe H, Jewison A, Alvarez JRF, Stilton D, Bradley R, Holden D. Randomized controlled trial (RCT): effects on blood pressure of 4 times milking of the cord versus slight delay of cord clamping in very low birth weight infants (VLBW). Klinische Padiatrie 2010;222(Suppl 1):GNPI_FV_30. [Google Scholar]
- Rabe H, Jewison A, Alvarez RF, Stilton D, Bradley R, Holden D. Randomized controlled trial (RCT) on 4 times milking of the cord versus slight delay of cord clamping in very low birth weight infants (VLBW): effects on circulation. Pediatric Academic Societies Annual Meeting; 2010 May 1‐4; Vancouver, Canada. 2010.
- Rabe H, Jewison A, Fernandez Alvarez R, Crook D, Stilton D, Bradley R, et al. for the Brighton Perinatal Study Group. Milking compared with delayed cord clamping to increase placental transfusion in preterm neonates. Obstetrics & Gynecology 2011;117:205‐11. [DOI] [PubMed] [Google Scholar]
- Rabe H, Sawyer A, Amees P, Ayres S. Neurodevelopmental outcomes at 2 and 3.5 years for very preterm babies enrolled in a randomized trial of milking the umbilical cord versus delayed cord clamping. Neonatology 2016;109:113‐9. [DOI: 10.1159/000441891] [DOI] [PubMed] [Google Scholar]
Rana 2017 {published data only}
- Agarwal K. Delayed versus early umbilical cord clamping in preterm infants of less than 34 weeks of gestation. ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=5900 (first received 4 April 2013).
- Rana A, Agarwal K. Safety of delayed umbilical cord clamping in preterm neonates less than 34 weeks gestation. Indian Journal of Pediatrics 2017;84(5):414. [DOI] [PubMed] [Google Scholar]
Ranjit 2015 {published data only}
- Ranjit T, Nesargi S, Rao PN, Sahoo JP, Ashok C, Chandrakala BS, et al. Effect of early versus delayed cord clamping on hematological status of preterm infants at 6 wk of age. Indian Journal of Pediatrics 2015;82(1):29‐34. [DOI] [PubMed] [Google Scholar]
Salae 2016 {published data only}
- Salae R. Efficacy of delayed versus immediate cord clamping in late preterm newborn followed normal labor: a randomized control trial. clinicaltrials.in.th/index.php?tp=regtrials&menu=trialsearch&smenu=fulltext&task=search&task2=view1&id=1421 (first received 8 June 2015).
- Salae R, Tanprasertkul C, Somprasit C, Bhamarapravatana C, Suwannarurk K. Efficacy of delayed versus immediate cord clamping in late preterm newborns following normal labour: randomised control trial. Journal of the Medical Association of Thailand 2016;99 Suppl 4:S1‐7. [PubMed] [Google Scholar]
- Tanprasertkul C, Salae R, Somprasit C, Bhamarapravatana C, Suwannarurk K. Efficacy of delayed versus immediate cord clamping in late preterm newborns following normal labour: randomised control trial. BJOG: an international journal of obstetrics and gynaecology 2016;123(Suppl 2):86‐7. [PubMed] [Google Scholar]
Sekhavat 2008 {published data only}
- Sekhavat L, Tabatabaii A. Immediate and delayed cord clamping in infants born between 26 and 34 weeks. Journal of Maternal‐Fetal and Neonatal Medicine 2008;21(Suppl 1):181‐2. [Google Scholar]
Shi 2017 {published data only}
- Shi W, Peng J, Chen N. Effect of delayed cord clamping on outcome of delivery. Henan Journal of Preventive Medicine 2017; Vol. 28:85‐7, 93.
Strauss 2008 {published data only}
- Strauss RG, Mock DM. A randomized clinical trial comparing immediate vs delayed clamping of the umbilical cord in preterm infants. Transfusion 2007;47 Suppl:21A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss RG, Mock DM, Johnson KL, Cress GA, Burmeister LF, Zimmermann MB, et al. A randomized clinical trial comparing immediate versus delayed clamping of the umbilical cord in preterm infants: short‐term clinical and laboratory endpoints. Transfusion 2008;48:658‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss RG, Mock MM, Johnson K, Mock NI, Cress G, Knosp L, et al. Circulating rbc volume, measured with biotinylated rbcs, is superior to the hct to document the hematologic effects of delayed versus immediate umbilical cord clamping in preterm neonates. Transfusion 2003;43:1168‐72. [DOI] [PubMed] [Google Scholar]
Tarnow‐Mordi 2017 {published data only}
- Popat H, Galea C, Evans N, Lingwood B, Colditz P, Halliday R, et al. Effect of delayed cord clamping on cerebral oxygenation in preterm infants <30 weeks gestation. Journal of Paediatrics and Child Health 2017;53(Suppl 2):80. [Google Scholar]
- Popat H, Galea C, Evans N, Lingwood B, Colditz PB, Halliday R, et al. Effect of delayed cord clamping on cerebral oxygenation in very preterm infants. Neonatology 2019; Vol. 115, issue 1:13‐20. [DOI] [PubMed]
- Popat H, Mann K, Buchan J, Brown R, Cornthwaite K, Waal K, et al. Australian placental transfusion study echo sub‐study: effect on systemic blood flow. Journal of Paediatrics and Child Health 2015;51(Suppl 1):17. [Google Scholar]
- Popat H, Mann K, Buchan J, Brown R, Cornthwaite K, Waal K, et al. [2765.4] Australian placental transfusion study echo sub‐study: effect on systemic blood flow. Pediatric Academic Societies Annual Meeting; 2015 April 25‐28; San Diego, California. 2015.
- Popat H, Robledo KP, Sebastian L, Evans N, Gill A, Kluckow M, et al. Effect of delayed cord clamping on systemic blood flow: a randomized controlled trial. Journal of Pediatrics. ACTRN12610000633088 2017; Vol. 178:81‐6.e2. [DOI] [PubMed]
- Tarnow‐Mordi W. Which method of placental transfusion should very preterm babies receive at birth? A randomised controlled trial four arm pilot study comparing methods of placental transfusion with standard immediate cord clamping to determine which placental transfusion method delivers the greatest increase in blood volume. anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12609000248268 (first received 17 February 2009).
- Tarnow‐Mordi W, Morris J, Kirby A, Robledo K, Askie L, Brown R, et al. Delayed versus immediate cord clamping in preterm infants. New England Journal of Medicine 2017; Vol. 377, issue 25:2445‐55. [DOI] [PubMed]
Tiemersma 2015 {published data only}
- Tiemersma S, Heistein J, Ruijne R, Lopez G, Lobenstei J, Rheenen P. Delayed cord clamping in South African neonates with expected low birthweight: a randomised controlled trial. Tropical Medicine & International Health 2015;20(2):177‐83. [DOI] [PubMed] [Google Scholar]
Ultee 2008 {published data only}
- Ultee CA, Deure J, Swart J, Lasham C, Baar AL. Delayed cord clamping in preterm infants delivered at 34‐36 weeks' gestation: a randomised controlled trial. Archives of Disease in Childhood. Fetal and Neonatal Edition 2008;93:F20‐3. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Aitchison {unpublished data only}
- Aitchison T, Beattie B, Cameron A, Halliday H, Holland B, Wardrop C. Placento‐fetal (Autologous) transfusion (PFTx) at birth in infants born preterm: a randomised, controlled trial. Personal communication.
Akhtar 2014 {published data only}
- Akhtar S, Bora R. Effect of umbilical cord milking on haemoglobin & serum ferritin levels at six months of age in infants ‐ a randomized controlled trial. Pediatric Academic Societies and Asian Society for Pediatric Research Joint Meeting; 2014 May 3‐6; Vancouver, Canada. 2014:Abstract no: 2945.604.
Ashish 2017 {published data only}
- Ashish KC, Malqvist M, Rana N, Ranneberg LJ, Andersson O. Effect of timing of umbilical cord clamping on anaemia at 8 and 12 months and later neurodevelopment in late pre‐term and term infants; a facility‐based, randomized‐controlled trial in Nepal. BMC Pediatrics 2016;16(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kc A, Rana N, Malqvist M, Jarawka Ranneberg L, Subedi K, Andersson O. Effects of delayed umbilical cord clamping vs early clamping on anemia in infants at 8 and 12 months: a randomized clinical trial. JAMA Pediatrics 2017; Vol. 171, issue 3:264‐70. [DOI] [PubMed]
Chopra 2016 {published data only}
- Chopra A, Kier N, Garg P, Thakur A. [4110.102] Effect of delayed versus early cord clamping on neonatal outcomes and iron stores at 3 months in small for gestational age babies ‐ a randomized controlled trial. Pediatric Academic Societies Annual Meeting; 2016 April 30 ‐ May 3; Baltimore, USA. 2016.
- Chopra A, Kler N, Thakur A, Garg P. Delayed vs early cord clamping in small for gestational age infants >35 weeks gestation: A randomized controlled trial. Journal of Pediatric Gastroenterology and Nutrition 2016; Vol. 62, issue Suppl 1:657.
Frank 1967 {published data only}
- Frank DJ, Gabriel M. Timing of cord ligation and newborn respiratory distress. American Journal of Obstetrics and Gynecology 1967;97:1142‐4. [DOI] [PubMed] [Google Scholar]
Garabedian 2016 {published data only}
- Garabedian C, Rakza T, Drumez E, Poleszczuk M, Ghesquiere L, Wibaut B, et al. Benefits of delayed cord clamping in red blood cell alloimmunization. Pediatrics 2016;137(3):e20153236. [DOI] [PubMed] [Google Scholar]
Ibrahim 2000 {published data only}
- Ibrahim HM, Krouskop RW, Lewis DF, Dhanireddy R. Placental transfusion: umbilical cord clamping in preterm infants. Journal of Perinatology 2000;120:351‐4. [DOI] [PubMed] [Google Scholar]
Katheria 2016 {published data only}
- Katheria A, Poeltler D, Durham J, Steen J, Rich W, Arnell K, et al. [4470.5] Eighteen‐month corrected age developmental outcomes of extremely preterm infants enrolled in a randomized controlled trial of one‐time umbilical milking versus immediate cord clamping. Randomized controlled trial. Pediatric Academic Societies Annual Meeting; 2016 April 30 ‐ May 3; Baltimore, USA. 2016.
Kattwinkel 2016 {published data only}
- Kattwinkel J. VentFiirst: A multicenter RCT of assisted ventilation during delayed cord clamping for extremely preterm infants. https://clinicaltrials.gov/ct2/show/record/NCT02742454 2016.
Mungkornkaew 2015 {published data only}
- Mungkornkaew S, Siwadune T. The difference of hematocrit in term and preterm vaginal births in different timing of delayed cord clamping. Thai Journal of Obstetrics and Gynaecology 2015;23:223‐30. [Google Scholar]
Narendra 1998 {published data only}
- Narendra A, Beckett C, Aitchinson T, Kyle E, Coutts J, Turner T, et al. Is it possible to promote placental transfusion (PTFx) at preterm delivery?. Pediatric Research 1998;44:454A. [Google Scholar]
Ruangkit 2015 {published data only}
Saigal 1972 {published data only}
- Saigal S, O'Neill A, Surainder Y, Chua LB, Usher R. Placental transfusion and hyperbilirubinemia in the premature. Pediatrics 1972;49(3):406‐19. [PubMed] [Google Scholar]
Saigal 1977 {published data only}
- Saigal S, Usher RH. Symptomatic neonatal plethora. Biology of the Neonate 1977;32:62‐72. [DOI] [PubMed] [Google Scholar]
Spears 1966 {published data only}
- Spears RL, Anderson GV, Brotman S, Farrier J, Kwan J, Masto A, et al. The effect of early vs late cord clamping on signs of respiratory distress. American Journal of Obstetrics and Gynecology 1966;95:564‐8. [DOI] [PubMed] [Google Scholar]
Taylor 1963 {published data only}
- Taylor P, Bright N, Birchard E. Effect of early vs delayed clamping of the umbilical cord on the clinical condition of the newborn infant. American Journal of Obstetrics and Gynecology 1963;86:893‐8. [DOI] [PubMed] [Google Scholar]
Tipwaree 2015 {published data only}
- Tipwaree S. The effect of umbilical cord milking in term infants followed by cesarean section delivery: a randomized controlled trial. clinicaltrials.in.th/index.php?tp=regtrials&menu=trialsearch&smenu=fulltext&task=search&task2=view1&id=1404 (first received 28 May 2015).
Yadav 2015 {published data only}
- Yadav AK, Upadhyay A, Gothwal S, Dubey K, Mandal U, Yadav CP. Comparison of three types of intervention to enhance placental redistribution in term newborns: randomized control trial. Journal of Perinatology 2015;35:720‐4. [DOI] [PubMed] [Google Scholar]
- Yadav AK, Upadhyay A, Jaiswal V, Gupta NK, Sharma RS, Gothwal S, et al. [2765.5] To compare the effect of delayed cord clamping, umbilical cord milking and delayed cord clamping with milking on haematological parameters in term neonates. Pediatric Academic Societies Annual Meeting; 2015 April 25‐28; San Diego, California. 2015.
Yasmeen 2014 {published data only}
- Yasmeen S, Shahidullah M, Mannan MA, Dey AC, Chowdhury RB, Haque AQMS, et al. Iron status in early versus delayed cord clamping groups of preterm neonates delivered in a tertiary level hospital. Journal of Armed Forces Medical College Bangladesh 2014;10:62‐5. [Google Scholar]
Zisovska 2008 {published data only}
- Zisovska E, Lazarevska L, Spasova L, Zivkovik J. The effect of delayed cord clamping on the need for blood transfusion. Transfusion Alternatives in Transfusion Medicine 2008;10(Suppl 1):25, Abstract no. P17. [Google Scholar]
References to studies awaiting assessment
Das 2018a {published data only}
- Das B, Sundaram V, Tarnow‐Mordi W, Ghadge A, Dhaliwal LK, Kumar P. Placental transfusion in preterm neonates of 30‐33 weeks' gestation: a randomized controlled trial. Journal of Perinatology 2018;38(5):496‐504. [CTRI/2014/02/004414] [DOI] [PubMed] [Google Scholar]
El‐Naggar 2018 {published data only}
Hu 2015 {published data only}
- Hu X, Xu X. The Effects of Different Cord Clamping Time in Preterm Infants by Vaginal Delivery [Master's Degree Thesis]. Zhejiang University, 2015. [Google Scholar]
- Hu X, Xu X. The effects of different cord clamping time in vaginal birth preterm infants. 31st International Confederation of Midwives Triennial Congress. Midwives ‐ Making a Difference in the World; 2017 June 18‐22; Toronto, Canada. 2017:Abstract no: A12.02.
Hua 2010 {published data only}
- Hua SP, Zhang HY, Zhou H, Zhang SH, Chen W, Xie CL. Effect of time of clamping umbilical cord on outcome of mothers and newborns. Journal of Hainan Medical College 2010;16:1572‐5. [Google Scholar]
Kazemi 2017 {published data only}
- Kazemi MV, Akbarianrad Z, Zahedpasha Y, Haghshenas Mojaveri M, Mehraein R. Effects of delayed cord clamping on intraventricular hemorrhage in preterm infants. Iranian Journal of Pediatrics 2017;27(5):e6570. [Google Scholar]
Leal 2018 {published data only}
Li 2018 {published data only}
Medina 2014 {published data only}
- Medina IMF. Late clamping of the umbilical cord in premature neonates: The real haemodynamic benefits. Enfermeria Clinica 2014;24(5):305‐8. [DOI] [PubMed] [Google Scholar]
Ram Mothan 2018 {published data only}
Song 2017 {published data only}
- Song SY, Kim Y, Kang BH, Yoo HJ, Lee M. Safety of umbilical cord milking in very preterm neonates: a randomized controlled study. Obstetrics & Gynecology Science 2017;60(6):527‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2018 {published data only}
Weeks 2018 {published data only}
- Weeks A, Bewley S. Improbable, but plausible, research study: a randomised controlled trial of premature cord clamping vs. neonatal venesection to achieve routine prophylactic neonatal red cell reduction. Journal of the Royal Society of Medicine 2018;111(8):270‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
Aghai 2018 {published data only}
- Aghai ZH, Katheria A, NCT03657394. Short‐term outcomes of umbilical cord milking in term and late preterm neonates who are depressed at birth. https://clinicaltrials.gov/ct2/show/NCT03657394 (first received 5 September 2018). [NCT03657394]
- Aghai ZH, Katheria A, NCT03681314. Long‐term outcomes of umbilical cord milking in term and late preterm neonates who are depressed at birth. https://clinicaltrials.gov/ct2/show/NCT03681314 (first received 24 September 2018). [NCT03681314]
- Aghai ZH, Katheria A, NCT03682042. Long‐term outcomes of umbilical cord milking in term and late preterm neonates with moderate to severe hypoxic ischemic encephalopathy. https://clinicaltrials.gov/ct2/show/NCT03682042 (first received 24 September 2018). [NCT03682042]
Allam 2018 {published data only}
- Allam N, ACTRN12618000758202p. Delayed fetal cord clamping in premature labour: the effect on fetal haemoglobin, bilirubin and neonatal death, maternal haemoglobin, neonatal ICU admission and postpartum haemorrhage. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12618000758202 (first received 7 May 2018).
Al‐Wassia 2016 {published data only}
- NCT02996799, Al‐Wassia H. Efficacy and safety of deferred umbilical cord clamping compared to umbilical cord milking in preterm infants: a randomized clinical trial. clinicaltrials.gov/ct2/show/record/NCT02996799 (first received 3 December 2016).
Anusha 2017 {published data only}
- Anusha S, CTRI/2017/01/007671. Early cord clamping versus delayed cord clamping in very low birth weight neonates. ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=16653 (first received 11 January 2017).
Bhriguvanshi 2017 {published data only}
- Bhriguvanshi A. The effects of umbilical cord milking in neonates requiring resuscitation at birth: a randomized controlled trial. ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=18641 (first received 24 August 2017).
Bienstock 2011 {published data only}
- Bienstock J. Milking the umbilical cord versus immediate clamping in pre‐term infants <33 weeks: a randomized controlled trial. ClinicalTrials.gov (http://clinicaltrials.gov/) [accessed 3 July 2015] 2011.
Carroli 2017 {published data only}
- Carroli G, ISRCTN12219110. Early compared to delayed umbilical cord clamping in very small prematurely born babies: a study to know which one is better for infant health. isrctn.com/ISRCTN12219110 (first received 30 March 2017).
Chamnanvanakij 2015 {published data only}
- Chamnanvanakij S. Effect of delayed cord clamping versus cord milking in infants born at <34 weeks’ gestation: a randomized controlled trial. Thai Clinical Trials Registry (http://www.clinicaltrials.in.th/) [accessed 3 July 2015] [accessed 3 July 2015] 2015.
Dempsey 2016 {published data only}
- Dempsey E, ISRCTN92719670. Clamping the umbilical cord in premature deliveries (CUPID). http://www.isrctn.com/ISRCTN92719670 (first received 25 January 2016).
De Paco Matallana 2013 {published data only}
- Paco Matallana C. DElayed COrd CLAmping versus early cord clamping in preterm infants born between 24 and 34 weeks. isrctn.com/ISRCTN66018314 (first received 6 October 2013).
Driggers 2013 {published data only}
- Driggers RW. Delayed umbilical cord clamping versus cord milking in preterm neonate ‐ a randomized, controlled trial. ClinicalTrials.gov (http://clinicaltrials.gov/) [accessed 3 July 2015] 2013.
Gomaa 2017 {published data only}
- Gomaa M, NCT03147846. The hematologic impact of umbilical cord milking versus deferred cord clamping in premature neonates. a randomized controlled trial. clinicaltrials.gov/ct2/show/record/NCT03147846 (first received 10 May 2017).
Gupta 2018 {published data only}
- Gupta A, CTRI/2018/08/015204. Early versus delayed cord clamping in IUGR preterms a randomised controlled study. http://www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=25064 (first received 6 August 2018).
Haghshenas 2014 {published data only}
- Haghshenas M. Comparative study of the effect of delayed versus early cord clamping on the incidence of intra ventricular hemorrhage in preterm neonate. Iranian Registry of Clinical Trials (http://www.irct.ir/) [accessed 3 July 2015] 2014.
Hao 2018 {published data only}
- Hao P, ChiCTR1800018366. Effect of delayed cord clamping versus umbilical cod milking on cerebral blood flow in preterm infant: a randomized, double‐blind controlled trial. http://www.chictr.org.cn/showproj.aspx?proj=30981 (first received 13 September 2018).
Hemmati 2014 {published data only}
- Hemmati F. Comparing the effect of delayed versus immediate cord clamping on the incidence of intraventricular hemorrhage (IVH) in preterm neonates with gestational age =34 weeks in Hafez & Zeynab hospitals from September 2012 to December 2013. Iranian Registry of Clinical Trials (http://www.irct.ir/) [accessed 3 July 2015] 2014.
Holland 1998 {published data only (unpublished sought but not used)}
- Holland BM. Placento‐fetal (autologous) transfusion at birth in infants born preterm: a randomised controlled trial. Personal communication 1998.
Isac 2017 {published data only}
- Isac M. Effect of umbilical cord milking of late preterm and term infants on maternal and neonatal outcomes in a tertiary care hospital in South India: a randomized control trial. ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=19631 (first received 3 October 2017).
Jomjak 2018 {published data only}
- Jomjak P, TCTR20180817001. To compare the effects of delayed versus early cord clamping on neonatal outcomes in preterm (gestational age at >24 weeks to 36+6 weeks) and maternal outcomes. http://www.clinicaltrials.in.th/index.php?tp=regtrials&menu=trialsearch&smenu=fulltext&task=search&task2=view1&id=3833 (first received 14 August 2018).
Katheria 2017 {published data only}
- Katheria A, NCT03019367. Premature infants receiving milking or delayed cord clamping: randomized controlled multicenter non‐inferiority trial. clinicaltrials.gov/ct2/show/NCT03019367 (first received 12 January 2017).
- Katheria A, NCT03145142. PREMOD2 NIRS substudy ‐ a randomized trial of cerebral oxygen saturation in infants randomized to umbilical cord milking or delayed cord clamping. clinicaltrials.gov/ct2/show/record/NCT03145142 (first received 9 May 2017).
Katheria 2018 {published data only}
- Katheria A, NCT03621943. Umbilical cord milking in non‐vigorous infants developmental followup (MINVIFU). https://clinicaltrials.gov/ct2/show/NCT03621943 (first received 9 August 2018). [NCT03621943]
- Katheria A, NCT03621956. Umbilical cord milking in non‐vigorous infants ‐ NIRS Sub‐study (MINVI_NIRS). https://clinicaltrials.gov/ct2/show/NCT03621956 (first received 9 August 2018). [NCT03621956]
- Katheria A, NCT03631940. Umbilical cord milking in non‐vigorous infants. https://clinicaltrials.gov/ct2/show/NCT03631940 (first received 15 August 2018). [NCT03631940]
Liu 2018 {published data only}
- Liu J, ChiCTR1800017865. Delayed cord clamping prevents respiratory distress of infants delivered by selective cesarean section in between 34‐38 weeks of gestational age, a randomized controlled trial. http://www.chictr.org.cn/showproj.aspx?proj=30199 (1first received 8 August 2018). [ChiCTR1800017865]
Martin 2013 {published data only}
- Martin J. Timing of umbilical cord clamping after vaginal or cesarean preterm birth. ClinicalTrials.gov (http://clinicaltrials.gov/) [accessed 4 June 2015] 2013.
Mirzaeian 2018 {published data only}
- Mirzaeian S, IRCT20180201038586N1. Investigation and comparison of neonatal complications of two methods of umbilical cord milking and early cord clamping in neonates. https://en.irct.ir/trial/29424 (first received 9 March 2018).
Nour 2018a {published data only}
- Nour I, NCT03731546. Effect of delayed cord clamping in preterm neonates with placental insufficiency. https://clinicaltrials.gov/ct2/show/NCT03731546 (first received 6 November 2018). [NCT03731546]
Nour 2018b {published data only}
- Nour I, NCT03731611. Impact of umbilical cord milking in preterm neonates with placental insufficiency. https://clinicaltrials.gov/ct2/show/NCT03731611 (first received 6 November 2018). [NCT03731611]
Panichkul 2015 {published data only}
- Panichkul P. Effects of delayed versus early cord clamping in late preterm infants: a randomized controlled trial. Thai Clinical Trials Registry http://www.clinicaltrials.in.th/ [accessed 28 June 2015] 2015.
Perlman 2015 {published data only}
- Perlman J. The effects of delayed cord clamping on postnatal circulatory status in preterm neonates. ClinicalTrials.gov (http://clinicaltrials.gov/) [accessed 3 July 2015] 2015.
- Spaight M, McKinsey S, Perlman J. [375] A randomized study of delayed cord clamping (DCC) in preterm neonates; preliminary observations. Pediatric Academic Societies Annual Meeting; 2016 April 30 ‐ May 3; Baltimore, USA. 2016.
- Spaight M, McKinsey S, Perlman J. [4110.103] A randomized study of delayed cord clamping (DCC) in preterm neonates; preliminary observations. Pediatric Academic Societies Annual Meeting; 2016 April 30 ‐ May 3; Baltimore, USA. 2016.
Ping 2010 {published data only}
- Ping HS. Immediate vs delayed cord clamping on newborns (no). ClinicalTrials.gov (http://clinicaltrials.gov/) (accessed 12.05.2010) 2010.
Puiggros 2014 {published data only}
- Puiggros MD. Umbilical cord milking compared with delayed cord clamping to increase placental transfusion in preterm infants less than 34 weeks' gestation born by cesarean section. randomised clinical trial. ClinicalTrials.gov (http://clinicaltrials.gov/) [accessed 3 July 2015] 2014.
Ruangkit 2017 {published data only}
- Ruangkit C, TCTR20170125001. A randomized controlled trial of immediate versus delayed umbilical cord clamping in preterm infants of multiple births. clinicaltrials.in.th/index.php?tp=regtrials&menu=trialsearch&smenu=fulltext&task=search&task2=view1&id=2189 (first received 25 January 2017).
Shahgheibi 2018 {published data only}
- Shahgheibi S, IRCT20141208020249N2. The delayed umbilical cord clamping effects on early outcome in preterm neonates. https://en.irct.ir/trial/17924 (first received 1 July 2018).
Smith 2014 {published data only}
- Smith K. Delayed clamping and milking the umbilical cord in preterm infants. ClinicalTrials.gov [accessed 25 May 2014] 2014.
Tanthawat 2017 {published data only}
- Tanthawat S, TCTR20170201003. The effect of one‐time umbilical cord milking and early cord clamping in preterm infants: a randomized controlled trial (one‐time umbilical cord milking). clinicaltrials.in.th/index.php?tp=regtrials&menu=trialsearch&smenu=fulltext&task=search&task2=view1&id=2347 (first received 1 February 2017).
Thukral 2016 {published data only}
- Thukral A, CTRI/2016/11/007470. Comparison of umbilical cord milking with delayed cord clamping in late preterm and term neonates: randomized control trial. www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=14204 (first received 16 November 2016).
Upahyay 2014 {published data only}
- Upahyay A. Effect of umbilical cord milking on hematological parameters at 6 weeks of life in pre term newborns: a randomised control trial. ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=10367 (first received 11 December 2014).
Varanattu 2017 {published data only}
- Varanattu M, NCT03200301. Effect of intact umbilical cord milking versus immediate cord clamping on neonatal outcomes and first year neurodevelopmental outcomes in very preterm infants ‐ a randomised controlled trial. clinicaltrials.gov/ct2/show/record/NCT03200301 (first received 27 June 2017).
Whitehead 2014 {published data only}
- Whitehead V. Effects of delayed cord clamp and/or indomethacin on preterm infant brain injury. clinicaltrials.gov/ct2/show/NCT02221219 (first received 20 August 2014).
Xie 2017 {published data only}
- Xie L, NCT03023917. The study on umbilical cord milking to prevent and decrease the severity of anemia in preterms‐‐a multi‐center randomized controlled trial. clinicaltrials.gov/ct2/show/record/NCT03023917 (first received 18 January 2017).
Yared 2015 {published data only}
- Yared E, NCT02337088. Delayed cord clamping at 30 vs. 60 seconds for very low birth weight infants: a randomized controlled trial. clinicaltrials.gov/ct2/show/NCT02337088 (first received 13 January 2015).
Additional references
Aarnoudse‐Moens 2009
- Aarnoudse‐Moens CS, Weisglas‐Kuperus N, Goudoever JB, Oosterlaan J. Meta‐analysis of neurobehavioral outcomes in very preterm and/or very low birth weight children. Pediatrics 2009;124(2):717‐28. [DOI] [PubMed] [Google Scholar]
Aflaifel 2012
- Aflaifel N, Weeks A. Push, pull, squeeze, clamp: 100 years of changes in the management of the third stage of labour as described by Ten Teachers. BMJ 2012;345:e8270. [DOI: 10.1136/bmj.e8270] [DOI] [PubMed] [Google Scholar]
Al‐Wassia 2015
- Al‐Wassia H, Shah PS. Efficacy and safety of umbilical cord milking at birth: a systematic review and meta‐analysis. Journal of the American Medical Association Pediatrics 2015;169(1):18‐25. [DOI] [PubMed] [Google Scholar]
Anderson 2003
- Anderson P, Doyle LW. Neurobehavioral outcomes of school‐age children born extremely low birth weight or very preterm in the 1990s. JAMA 2003;289(24):3264‐72. [DOI] [PubMed] [Google Scholar]
Arnold 2013
- Arnold L, Sawyer A, Rabe H, Abbott J, Gyte G, Duley L, et al. Parents' first moments with their very preterm babies: a qualitative study. BMJ Open 2013;3:e002487. [DOI: 10.1136/bmjopen-2012-002487] [DOI] [PMC free article] [PubMed] [Google Scholar]
Backes 2014
- Backes CH, Rivera BK, Haque U, Bridge JA, Smith CV, Hutchon DJR, et al. Placental transfusion strategies in very preterm neonates: a systematic review and meta‐analysis. Obstetrics & Gynecology 2014;124(1):47‐56. [DOI] [PubMed] [Google Scholar]
Batey 2017
- Batey N, Yoxall CW, Fawke JA, Duley L, Dorling J. Fifteen‐minute consultation: stabilisation of the high‐risk newborn infant beside the mother. Archives of Disease in Childhood. Education and Practice Edition 2017;102(5):235–8. [DOI] [PubMed] [Google Scholar]
Begley 2019
- Begley CM, Gyte GML, Devane D, McGuire W, Weeks A, Biesty LM. Active versus expectant management for women in the third stage of labour. Cochrane Database of Systematic Reviews 2019, Issue 2. [DOI: 10.1002/14651858.CD007412.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bhatt 2013
- Bhatt S, Alison BJ, Wallace EM, Crossley KJ, Gill AW, Kluckow M, et al. Delaying cord clamping until ventilation onset improves cardiovascular function at birth in preterm lambs. Journal of Physiology 2013;591(Pt 8):2113‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bhutta 2002
- Bhutta AT, Cleves MA, Casey PH, Cradock MM, Anand KJ. Cognitive and behavioral outcomes of school‐aged children who were born preterm: a meta‐analysis. JAMA 2002;288(6):728‐37. [DOI] [PubMed] [Google Scholar]
Blank 2018
- Blank DA, Polglase GR, Kluckow M, Gill AW, Crossley KJ, Moxham A, et al. Haemodynamic effects of umbilical cord milking in premature sheep during the neonatal transition. Archives of Disease in Childhood. Fetal and Neonatal Edition 2018;103(6):F539‐46. [DOI: 10.1136/archdischild-2017-314005] [DOI] [PMC free article] [PubMed] [Google Scholar]
Boere 2015
- Boere I, Roest AA, Wallace E, Harkel AD, Haak MC, Morley CJ, et al. Umbilical blood flow patterns directly after birth before delayed cord clamping. Archives of Disease in Childhood. Fetal and Neonatal Edition 2015;100:F121‐5. [DOI: 10.1136/archdischild-2014-307144] [DOI] [PubMed] [Google Scholar]
Chaparro 2006
- Chaparro CM, Neufeld LM, Alavez GT, Cedillo RE, Dewey KG. Effect of timing of umbilical cord clamping on iron status in Mexican infants: a randomised controlled trial. Lancet 2006;367:1997‐2004. [DOI] [PubMed] [Google Scholar]
Committee 2012
- Committee on Obstetric Practice, American College of Obstetricians and Gynecologists. Committee Opinion No.543: Timing of umbilical cord clamping after birth. Obstetrics and Gynecology 2012;120(6):1522‐6. [DOI: 10.1097/01.AOG.0000423817.47165.48] [DOI] [PubMed] [Google Scholar]
Dawes 1968
- Dawes GS. Chapter 13. Foetal and Neonatal Physiology; a Comparative Study of the Changes At Birth. Chicago, Year Book Medical Publishers, 1968. [Google Scholar]
Duley 2014
- Duley L, Askie L, Yang M. Cord clamping and placental transfusion at preterm birth prospective meta‐analysis. http://www.crd.york.ac.uk/prospero/display_record.asp?ID=CRD42013004405 2014; Vol. Accessed 1 June 2014.
Duley 2015
- Duley L, Gyte G. When should the umbilical cord be clamped?. BMJ 2015;351:h4206. [DOI: 10.1136/bmj.h4206] [DOI] [PubMed] [Google Scholar]
Farrar 2011
- Farrar D, Airey R, Law GR, Tuffnell D, Cattle B, Duley L. Measuring placental transfusion for term births: weighing babies with cord intact. British Journal of Obstetrics and Gynaecology 2011;118(1):70‐5. [DOI] [PubMed] [Google Scholar]
Fogarty 2018
- Fogarty M, Osborn DA, Askie L, Seidler AL, Hunter K, Lui K, et al. Delayed versus early umbilical cord clamping for preterm infants: a systematic review and meta‐analysis. American Journal of Obstetrics and Gynecology 2018;218(1):1‐18. [DOI: 10.1016/j.ajog.2017] [DOI] [PubMed] [Google Scholar]
Gallos 2018
- Gallos ID, Papadopoulou A, Man R, Athanasopoulos N, Tobias A, Price MJ, et al. Uterotonic agents for preventing postpartum haemorrhage: a network meta‐analysis. Cochrane Database of Systematic Reviews 2018, Issue 12. [DOI: 10.1002/14651858.CD011689.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ghavam 2014
- Ghavam S, Batra D, Mercer J, Kugelman A, Hosono S, Oh W, et al. Effects of placental transfusion in extremely low birthweight infants: meta‐analysis of long‐ and short‐term outcomes. Transfusion 2014;54:1192‐8. [DOI] [PubMed] [Google Scholar]
GradePro 2015 [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version Accessed 19 February 2018. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Gunther 1957
- Gunther M. The transfer of blood between baby and placenta in the minutes after birth. Lancet 1957;272(6982):1277‐80. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hofmeyr 2015
- Hofmeyr GJ, Mshweshwe NT, Gulmezoglu AM. Controlled cord traction for the third stage of labour. Cochrane Database of Systematic Reviews 2015, Issue 1. [DOI: 10.1002/14651858.CD008020.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Holland 1991
- Holland BM, Wardrop CA. Anaemias of the preterm infant. In: Turner TL editor(s). Perinatal Haematological Problems. Chichester, UK: Wiley, 1991:121‐35. [Google Scholar]
Hooper 2015
- Hooper SB, Polglase GR, Pas AB. A physiological approach to the timing of umbilical cord clamping at birth. Archives of Disease in Childhood. Fetal and Neonatal Edition 2015;100(4):F355‐60. [DOI: 10.1136/archdischild-2013-305703] [DOI] [PubMed] [Google Scholar]
Hooper 2017
- Hooper SB, Crossley KJ, Zahra VA, Vonderen J, Moxham A, Gill AW, et al. Effect of body position and ventilation on umbilical artery and venous blood flows during delayed umbilical cord clamping in preterm lambs. Archives of Disease in Childhood. Fetal and Neonatal Edition 2017;102(4):F312‐9. [DOI: 10.1136/archdischild-2016-311159] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hudson 1990
- Hudson IRB, Holland BM, Jones JG, Turner TL, Wardrop CA. First day total circulating red cell volume (RCV) predicts outcome in preterm infants. Pediatric Research 1990;27(4 Pt 2):209A. [Google Scholar]
Hutchon 2008
- Hutchon DJ, Thakur I. Resuscitate with the placental circulation intact. Archives of Disease in Childhood 2008;93(5):451. [PubMed] [Google Scholar]
Katheria 2017a
- Katheria AC, Brown MK, Rich W, Arnell K. Providing a placental transfusion in newborns who need resuscitation. Frontiers in Pediatrics 2017;5:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Katheria 2017b
- Katheria AC, Lakshminrusimha S, Rabe H, McAdams R, Mercer JS. Placental transfusion – a review. Journal of Perinatology 2017;37(2):105‐11. [DOI: 10.1038/jp.2016.151] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kluckow 2015
- Kluckow M, Hooper SB. Using physiology to guide time to cord clamping. Seminars in Fetal & Neonatal Medicine 2015;20(4):225‐31. [DOI: 10.1016/j.siny.2015.03.002] [DOI] [PubMed] [Google Scholar]
Knol 2018
- Knol R, Brouwer E, Vernooij AS, Klumper FJ, DeKoninck P, Hooper SB, et al. Clinical aspects of incorporating cord clamping into stabilisation of preterm infants. Archives of Disease in Childhood. Fetal and Neonatal Edition 2018;103:F493–7. [DOI: 10.1136/archdischild-2018-314947] [DOI] [PMC free article] [PubMed] [Google Scholar]
Linderkamp 1978
- Linderkamp O, Versmold HT, Fendel H, Reigel KP, Betke K. Association of neonatal respiratory distress with birth asphyxia and deficiency of red cell mass in premature infants. European Journal of Paediatrics 1978;129:167‐73. [DOI] [PubMed] [Google Scholar]
Manley 2017
- Manley BJ, Owen LS, Hooper SB, Jacobs SE, Cheong JL, Doyle LW, et al. Towards evidence‐based resuscitation of the newborn infant. Lancet 2017;389(10079):1639‐48. [DOI: 10.1016/S0140-6736(17)30547-0] [DOI] [PubMed] [Google Scholar]
Marlow 2015
- Marlow N. Is survival and neurodevelopmental impairment at 2 years of age the gold standard outcome for neonatal studies?. Archives of Disease in Childhood. Fetal and Neonatal Edition 2015;100(1):F82‐F4. [DOI] [PMC free article] [PubMed] [Google Scholar]
McDonald 2013
- McDonald SJ, Middleton P, Dowswell T, Morris PS. Effect of timing of umbilical cord clamping of term infants on maternal and neonatal outcomes. Cochrane Database of Systematic Reviews 2013, Issue 7. [DOI: 10.1002/14651858.CD004074.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Meher 2014
- Meher S, Alfirevic Z. Choice of primary outcomes in randomised trials and systematic reviews evaluating interventions for preterm birth prevention: a systematic review. British Journal of Obstetrics and Gynaecology 2014;121:1188‐96. [DOI] [PubMed] [Google Scholar]
Mercer 2002
- Mercer JS, Skovgaard RL. Neonatal transitional physiology: a new paradigm. Journal of Perinatal & Neonatal Nursing 2002;15(4):56‐75. [DOI] [PubMed] [Google Scholar]
Moser 2007
- Moser K, Macfarlane A, Chow YH, Hilder L, Dattani N. Introducing new data on gestation‐specific infant mortality among babies born in 2005 in England and Wales. Health Statistics Quarterly / Office for National Statistics 2007;35:13‐27. [PubMed] [Google Scholar]
NICE 2015
- National Institute for Health and Care Excellence. Preterm labour and birth. NICE Guideline. London, 2015. [PubMed]
O'Donnell 2017
- O'Donnell CP. The timing of cord clamping for preterm infants. New England Journal of Medicine 2017;377(25):2488‐9. [DOI: 10.1056/NEJMe1714522] [DOI] [PubMed] [Google Scholar]
Oddie 2014
- Oddie S, Rhodes P. Barriers to deferred cord clamping in preterm infants. Archives of Disease in Childhood. Fetal and Neonatal Edition 2014;99(5):F391‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Perlman 2010
- Perlman JM, Wyllie J, Kattwinkel J, Atkins DL, Chameides L, Goldsmith JP, et al. Part 11: Neonatal Resuscitation: 2010 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Circulation 2010;122(16 suppl 2):S516‐538. [DOI] [PubMed] [Google Scholar]
Petrou 2003
- Petrou S, Mehta Z, Hockley C, Cook‐Mozaffari P, Henderson J, Goldacre M. The impact of preterm birth on hospital inpatient admissions and costs during the first 5 years of life. Pediatrics 2003;112(6 Pt 1):1290‐7. [DOI] [PubMed] [Google Scholar]
Poscencheg 2015
- Poscencheg M, Kirpalani H. Placental transfusion at birth: do we have all the answers?. Journal of the American Medical Association Pediatrics 2015;169(1):9‐11. [DOI] [PubMed] [Google Scholar]
Prendiville 1989
- Prendiville WJ, Elbourne DR. Care during the third stage of labour. In: Chalmers I, Enkin M, Keirse MJNC editor(s). Effective Care in Pregnancy and Childbirth. Vol. 2, Oxford: Oxford University Press, 1989:1145‐69. [Google Scholar]
Rabe 2008
- Rabe H, Reynolds G, Diaz‐Rossello J. A systematic review and meta‐analysis of a brief delay in clamping the umbilical cord of preterm infants. Neonatology 2008;93:138‐44. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Saigal 2008
- Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet 2008;371(9608):261‐9. [DOI] [PubMed] [Google Scholar]
Salati 2019
- Salati JA, Leathersich SJ, Williams MJ, Cuthbert A, Tolosa JE. Prophylactic oxytocin for the third stage of labour to prevent postpartum haemorrhage. Cochrane Database of Systematic Reviews 2019, Issue 4. [DOI: 10.1002/14651858.CD001808.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sawyer 2013
- Sawyer A, Rabe H, Abbott J, Gyte G, Duley L, Ayers S. Parents’ experiences and satisfaction with care during the birth of their very preterm baby: a qualitative study. British Journal of Obstetrics and Gynaecology 2013;120(5):637–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sawyer 2015
- Sawyer A, Ayers S, Bertullies S, Thomas M, Weeks AD, Yoxall CW, et al. Providing immediate neonatal care and resuscitation at birth beside the mother: parents' views, a qualitative study. BMJ Open 2015;5(9):e008495. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schoonakker 2013
- Schoonakker BD, Dorling J, Oddie S, Batra D, Grace N, Duley L. Bedside resuscitation of preterm infants with cord intact is achievable using standard resuscitaire. European Society for Pediatric Research; Oporto, Portugual 2013. 2013;Available at p430 of:http://www.mcaevents.org/t/files/9349_abstract_book_‐_25sett13‐it‐it.pdf. [Google Scholar]
Sweet 2017
- Sweet DG, Carnielli V, Greisen G, Hallman M, Ozek E, Plavka R, et al. European Consensus Guidelines on the Management of Respiratory Distress Syndrome ‐ 2016 Update. Neonatology 2017;111(2):107‐25. [DOI] [PubMed] [Google Scholar]
Tarnow‐Mordi 2014
- Tarnow‐Mordi WO, Duley L, Field D, Marlow N, Morris J, Newnham J, et al. Timing of cord clamping in very preterm infants: more evidence is needed. American Journal of Obstetrics and Gynecology 2014;211(2):118‐23. [DOI] [PubMed] [Google Scholar]
Te Pas 2018
- Pas AB. Timing is everything. Archives of Disease in Childhood. Fetal and Neonatal Edition 2018;103(1):F2‐F3. [DOI] [PubMed] [Google Scholar]
Thomas 2014
- Thomas M, Yoxall C, Weeks A, Duley L. Providing newborn resuscitation at the mother's bedside: assessing the safety, usability and acceptability of a mobile trolley. BMC Pediatrics 2014;14:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vain 2014
- Vain NE, Satragno DS, Gorenstein AN, Gordillo JE, Berazategui JP, Alda MG, et al. Effect of gravity on volume of placental transfusion: a multicentre, randomised, non‐inferiority trial. Lancet 2014;384(9939):235‐40. [DOI: 10.1016/S0140-6736(14)60197-5] [DOI] [PubMed] [Google Scholar]
Vijayaselvi 2015
- Vijayaselvi R, Abraham A, Kumar M, Kuruvilla A, Mathews J, Duley L. Measuring umbilical flow and placental transfusion for preterm births: weighing babies at 33‐36 weeks gestation with cord intact. 1st congress of joint European Neonatal Societies. Budapest, 2015.
Weeks 2013
- Weeks AD, Peake M, Yoxall W. The Bedside Assessment, Stabilisation and Initial Cardiorespiratory Support (BASICS) trolley: enabling neonatal resuscitation with an intact cord. RCOG World Congress. Liverpool, 2013.
Weeks 2015
- Weeks AD, Watt P, Yoxall CW, Gallagher A, Burleigh A, Bewley S, et al. Innovation in immediate neonatal care: development of the Bedside Assessment, Stabilisation and Initial Cardiorespiratory Support (BASICS) trolley. BMJ Innovations 2015;1:53‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2014
- World Health Organization (WHO). Delayed umbilical cord clamping for improved maternal and infant health and nutrition outcomes. World Health Organization 2014; Vol. Guideline. [PubMed]
Wyllie 2015
- Wyllie J, Bruinenberg J, Roehr CC, Rudiger M, Trevisanuto D, Urlesberger B. Resuscitation and support of transition of babies at birth. European Resuscitation Council Guidelines for Resuscitation 2015: Section 7 2015;95:249‐63. [DOI] [PubMed] [Google Scholar]
Yao 1969
- Yao AC, Lind J. Effect of gravity on placental transfusion. Lancet 1969;2(7619):505‐8. [DOI] [PubMed] [Google Scholar]
Yoxall 2015
- Yoxall CW, Ayers S, Sawyer A, Bertullies S, Thomas M, Weeks A, et al. Providing immediate neonatal care and resuscitation at birth beside the mother: clinicians' views, a qualitative study. BMJ Open 2015;5(9):e008494. [DOI: 10.1136/bmjopen-2015-008494] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zeitlin 2008
- Zeitlin J, Draper ES, Kollee L, Milligan D, Boerch K, Agostino R, et al. Differences in rates and short‐term outcome of live births before 32 weeks of gestation in Europe in 2003: results from the MOSAIC cohort. Pediatrics 2008;121(4):e936‐44. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Elbourne 1995
- Elbourne DR. Early cord clamping in preterm infants. [revised 22 June 1993]. In: Enkin MW, Keirse MJNC, Renfrew MJ, Neilson JP, Crowther C (eds.) Pregnancy and Childbirth Module. In: The Cochrane Pregnancy and Childbirth Database [database on disk and CDROM]. The Cochrane Collaboration; Issue 2, Oxford: Update Software; 1995.
Rabe 2001
- Rabe H, Reynolds GJ. Delayed cord clamping in preterm infants. Cochrane Database of Systematic Reviews 2001, Issue 3. [DOI: 10.1002/14651858.CD003248] [DOI] [PubMed] [Google Scholar]
Rabe 2004
- Rabe H, Reynolds GJ, Diaz‐Rosello JL. Early versus delayed umbilical cord clamping in preterm infants. Cochrane Database of Systematic Reviews 2004, Issue 4. [DOI: 10.1002/14651858.CD003248.pub2] [DOI] [PubMed] [Google Scholar]
Rabe 2012
- Rabe H, Diaz‐Rossello JL, Duley L, Dowswell T. Effect of timing of umbilical cord clamping and other strategies to influence placental transfusion at preterm birth on maternal and infant outcomes. Cochrane Database of Systematic Reviews 2012, Issue 8. [DOI: 10.1002/14651858.CD003248.pub3] [DOI] [PubMed] [Google Scholar]


