ABSTRACT
Although biosynthetic trafficking can function constitutively, it also functions specifically for certain developmental processes. These processes require either a large increase to biosynthesis or the biosynthesis and targeted trafficking of specific players. We review the conserved molecular mechanisms that direct biosynthetic trafficking, and discuss how their genetic disruption affects animal development. Specifically, we consider Arf small G proteins, such as Arf1 and Sar1, and their coat effectors, COPI and COPII, and how these proteins promote biosynthetic trafficking for cleavage of the Drosophila embryo, the growth of neuronal dendrites and synapses, extracellular matrix secretion for bone development, lumen development in epithelial tubes, notochord and neural tube development, and ciliogenesis. Specific need for the biosynthetic trafficking system is also evident from conserved CrebA/Creb3-like transcription factors increasing the expression of secretory machinery during several of these developmental processes. Moreover, dysfunctional trafficking leads to a range of developmental syndromes.
Keywords: animal development, Arf GAPs; Arf GEFs, Arf small G proteins, biosynthetic trafficking, endoplasmic reticulum, Golgi apparatus, vesicle coat proteins
Introduction
Biosynthetic trafficking builds and renews all cells. This general role can be viewed as constitutive. However, certain developmental processes require specific responses of the secretory system. For example, a particular developmental process may require a significant increase to total secretory output, as is the case when the Drosophila embryo increases its plasma membrane (PM) content by ∼25-fold in a few hours,1 or when the vertebrate embryo produces and secretes huge amounts of extracellular matrix (ECM) for bone development.2 Alternately, the development of polarized cells requires specific proteins to enter the biosynthetic trafficking system and then be targeted to PM sub-domains.3 Here, we review developmental processes with specific dependencies on biosynthetic trafficking and the molecular mechanisms involved. We focus on the Arf family of small G proteins, their upstream regulators and their downstream effectors. Implications for developmental diseases and disorders are also highlighted.
An overview of biosynthetic trafficking and its regulation by Arf family small G proteins
The biosynthetic pathway involves a complex network of membrane bound organelles that deliver macromolecules to the PM and extracellular space (Fig. 1A). The endoplasmic reticulum (ER) is an extensive, partially tubulated membrane network that is continuous with the outer nuclear membrane and spans a large volume of the cytosol.4 Newly synthesized proteins translocate from ribosomes into the lumen of the ER, a process coordinated with protein folding and membrane integration.5,6 From the ER, proteins are trafficked to the Golgi apparatus. The Golgi is a system of membrane compartments arranged in flattened stacks referred to as cisternae, with cis cisterna engaged in ER trafficking and trans cisterna directing transport toward the PM.7-9
Figure 1.
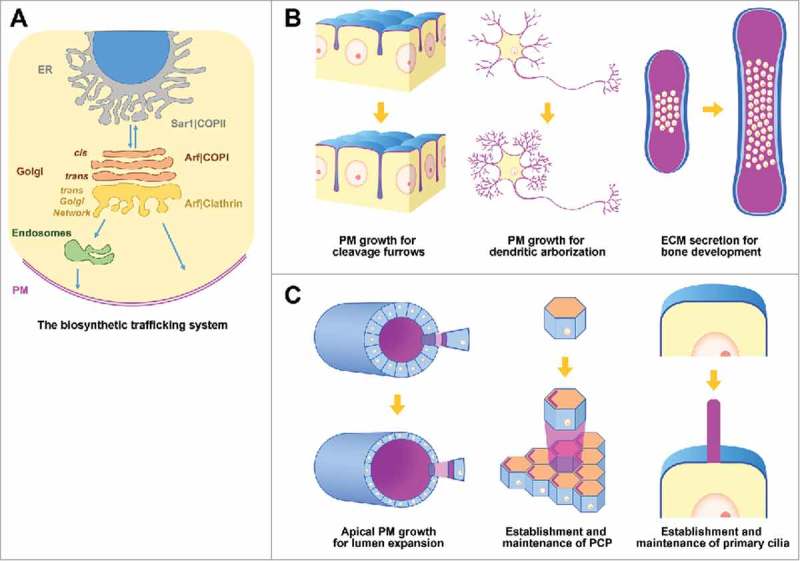
Roles of biosynthetic trafficking for animal development. (A) The biosynthetic secretory pathway and its molecular regulators. The Sar1 small G protein promotes anterograde traffic from the endoplasmic reticulum (ER) by promoting the assembly of COPII-coated vesicles. At the Golgi, Arf1, Arf4, and Arf5 regulate retrograde COPI vesicle trafficking. At the trans Golgi network (TGN), Arfs assemble clathrin-coated vesicles to deliver biomolecules to endosomal compartments and the plasma membrane (PM). (B) Developmental processes under high secretory demand. Left, delivery of membrane to cleavage furrows promotes their ingression for embryo cleavage. Middle, PM growth promotes dendritic growth and arborisation. Right, secretion of ECM promotes bone development. (C) Developmental processes regulated by biosynthetic trafficking in polarized epithelial cells. Left, increases in apical membrane and apical ECM secretion promotes lumen growth in epithelial tubes. Middle, trafficking of polarity determinants promotes the establishment and maintenance of planar cell polarity. Right, biosynthetic trafficking promotes the formation and maintenance of primary cilia. See main text for details.
Trafficking at the ER and Golgi is regulated by the Arf family of small G proteins. Anterograde vesicle transport from the ER to the Golgi is regulated by family member Sar1.10,11 Sar1 organizes COPII coated vesicles for budding of cargo from ER exit sites.9,12 Sar1 is activated at ER membranes by its guanine nucleotide exchange factor (GEF) Sec 12. Sar1-GTP then recruits the cargo adaptor complex of Sec 23 and Sec 24, which recruits the COPII coat components Sec 13 and Sec 31. Sec 23 is also a GAP for Sar1. In a negative feedback loop, coat assembly induces Sec 23 activity leading to Sar1 GTP hydrolysis and coat disassembly.
Outward material flow is, in part, counterbalanced by retrograde transport within Golgi stacks and from the Golgi to the ER. This retrograde transport retrieves transport machinery for re-use. The COPI coat mediates the budding events involved and also contains adaptor proteins for recruiting cargo.9 COPI is recruited to Golgi membranes by the small G proteins Arf1, Arf4, or Arf5. At the Golgi, the Arf GEFs GBF1 and BIG1/2 activate Arf small G proteins and interact with COPI subunits and cargoes. Similar to the regulation of COPII coats, Arf GAPs induce the GTP hydrolysis of Arfs for COPI coat disassembly and recycling.10,11,13
For trafficking to the PM, cargo-ladened vesicles exit the Golgi from the trans Golgi network (TGN), a dynamic membrane compartment associated with multiple clathrin adaptors responsible for the sorting of cargoes into secretory vesicles destined for endosomes and the PM.8 Various classes of adaptors are found at the TGN, including the AP1 family, the GGA family, Epsin related proteins, and the exomer complex.8 Under the regulation of different small G proteins from the Arf and Rab families, including Arf1, Arf4, Arf-like-1 (Arl-1), Rab6, and Rab8, adaptors connect cargo proteins to clathrin coated pits that bud from the Golgi.10,11,14
Membrane supply for cleavage of the Drosophila embryo
The early Drosophila embryo develops as a syncytium.1,15,16 After 9 nuclear divisions at the center of the embryo, nuclei move to the embryo periphery and continue their synchronous nuclear divisions without cell division. At the periphery, the nuclear divisions become coordinated with phases of PM growth. From interphase to metaphase, partial cleavage furrows ingress around each nuclear compartment, providing anchorage for mitotic spindles and preventing the collision of neighboring spindles. From metaphase to telophase, the furrows regress, and for the next cycle, new furrows form around each daughter nuclei. This synchronized growth and dissolution of PM furrows occurs through cycles 10–13. At cycle 14, furrow ingression is dramatically increased and not reversed. Instead, the furrows become the lateral and basal PM of ∼6000 columnar cells. These PM growth periods increase total PM area by ∼25-fold to form the blastoderm (Fig. 1B).
Exocytic trafficking is a major contributor to the inward PM growth of cleavage furrows. Although not cellularized, each nucleus of the syncytial embryo organizes its own ER and Golgi membrane systems.17 The activity of Golgi Arf-GEFs is needed for furrow ingression,17 and Arf1 localizes to the Golgi and is needed for furrow ingression,18 Golgi organization and Golgi COPI coats.19 Additionally, Arl1 promotes the Golgi localization of the golgin Lava Lamp,20 which is important for dynein-mediated translocation of Golgi elements toward the embryo surface where the PM is found.21,22 Post-Golgi trafficking to the PM depends on the exocyst complex. The exocyst complex localizes to PM domains near the embryo surface,23 where new membrane inserts.24 The small G protein RalA localizes to the PM and is required for recruiting the exocyst complex.25 Both RalA and the exocyst appear to recruit Rab8-positive Golgi vesicles and Rab11 vesicles to the PM.25,26 These studies outline a biosynthetic pathway critical for this developmental stage of high plasma membrane growth.
Recently, the Arf GAP Asap was found to promote Arf1 localization at the Golgi for furrow ingression.19 Although an Asap-Arf1 interaction site contributed to furrow biosynthesis, no evidence for Asap at the Golgi was found. Instead, Asap localized primarily to the PM, and thus may displace Arf1-GTP from this or other post-Golgi membranes for recycling to the Golgi. Such recycling may optimize Golgi output to meet the high demand of furrow biosynthesis. Intriguingly, Asap becomes sequestered to the nuclear region just before the onset of furrow regression, a period also marked by mild alterations to Golgi structure. Thus, cell cycle regulation of Asap localization may help couple Golgi output with the furrow ingression-regression cycles of the early embryo.
Membrane supply for dendrite and synapse growth
Neurons can gain a huge surface area to form axons and dendrites for cell-cell communication throughout the body. Most neurons develop multiple branched dendritic extensions that are capable of integrating various incoming signals.27 The secretory pathway plays a significant role in dendritic growth and arborisation28 (Fig. 1B). In particular, the Sar1-COPII axis is required for dendritic growth. Removal of Sar1 from either Drosophila neurons or cultured rat hippocampus neurons leads to Golgi morphology defects and reduction of dendritic extensions, without effects on axonal growth.29 The position of the Golgi is normally polarized within neurons such that post-Golgi trafficking is directed toward growing dendrites.30 Moreover, neurons differ from other cells in that they possess individual structures termed Golgi outposts that are enriched in dendrites versus axonal extensions.29,30 Perturbations of Golgi Arf GEFs or Arf1 reduce dendritic growth.30 Arf4 has also been implicated in dendritic growth of the hippocampus, as mice heterozygous for a Arf4 null allele have behavioral and cognitive disabilities that correlate with abnormal dendritic growth, and Arf4 loss or gain leads to reduced or increased dendritic growth, respectively, in culture.31 Thus, similar to the syncytial Drosophila embryo, biosynthetic machinery is essential for the total membrane growth requirement of dendritic networks.
Additionally, biosynthetic trafficking contributes to neuronal synapses, the specialized membrane domains responsible for the release, detection and uptake of neurotransmitters.32 In Drosophila, Arl1 and the Arf-GEF Gartenzwerg (Garz; a GBF-1 homolog) promote Arfaptin function at the Golgi, and the 3 proteins act together for the development of synapse numbers.33
ECM supply for bone development
Bones are essential for the structure and movement of vertebrates. Most bones develop through endochondral ossification.2,34 Mesenchymal cells migrate to sites of bone development and differentiate into chondrocytes to deposit a cartilage template. Ossification is then mediated by osteoblasts, which mineralize the cartilaginous template. These steps require massive amounts of ECM secretion (Fig. 1B).
For the deposition of cartilage, chondrocytes secrete ECM components including specific collagens and proteoglycans.2 Mutations affecting these ECM components are associated with developmental diseases such as skeletal dysplasias.35,36 Significantly, disruptions to biosynthetic trafficking also leads to skeletal development defects.36 For the ER, mutations affecting components of the COPII coat (Sec 13, Sec 23a, Sec 24d, or the small G protein Sar1b) are all associated with impaired skeletal development. In particular, the autosomal recessive syndrome cranio-facial-sutural dysplasia (CLSD) maps to a mutation affecting a conserved phenylalanine residue of Sec 23a.37 Fibroblasts from CLSD patients display abnormally enlarged ER membranes that contain increased and often excessively tubulated ER exit sites.37,38 In vitro experiments have also shown that the disease mutant of Sec 23a is unable to recruit members of the outer COPII coat, Sec 13 and Sec 31.38 Moreover, a nonsense mutation producing an early stop codon in zebrafish Sec 23a leads to malformations of craniofacial cartilage associated with abnormal ECM deposition.39 Similarly, depletion of Sec 13 in human fibroblasts impairs collagen secretion, and loss of Sec 13 in zebrafish leads to craniofacial abnormalities resembling those with Sec 23a disruption.40 Sar1b knock-down in zebrafish embryos also leads to abnormal craniofacial skeletal development with retention of collagen in intracellular compartments, abnormalities accompanied by additional multi-organ effects.41 A second autosomal recessive skeletal disorder, osteogenesis imperfecta, has been linked to mutations of the COPII coat component Sec 24d.42,43 Fibroblasts from patients with this disorder display enlarged ER membranes that retain ECM components,42 and similarly, zebrafish and medaka mutants of Sec 24d display distended chondrocyte ER membranes, sub-cellular retention of ECM components, and skeletal malformations.44,45
As for ER machinery, Golgi trafficking machinery has been recently implicated in bone disorders.46 Heterozygosity for a mutation of the ARCN1 gene, encoding the δ subunit of the COPI coatomer, was linked to human craniofacial syndromes. Knockdown of ARCN1 in fibroblasts induces the ER stress response and disrupts ECM transport, and Arf GEF inhibition phenocopies the ARCN1 knockdown, implicating COPI recruitment by Arf GEFs and Arfs.
Secretion for lumen development in an epithelial tube
The development of epithelial tubes is essential for animal physiology. The Drosophila tracheal system is an excellent model for tube morphogenesis in vivo. It is a segmented and hierarchical network of air-filled tubes that deliver oxygen throughout the body.47,48 An essential element of a tube is its lumen. Polarized secretion of apical PM determinants, such as the transmembrane protein Crumbs, and lumen ECM materials plays an essential role in lumen expansion and regulation.49,50 (Fig. 1C). In the Drosophila tracheal system, sar1 mutants display ER and Golgi morphology disruptions, sub-cellular retention of tube lumen materials, and decreased tube lumen diameters.51 Disruptions of COPII secretory machinery phenocopy these tracheal disruptions.51,52 Thus anterograde trafficking via COPII vesicles seems to be required for lumen expansion. Whether the secretion of a specific COPII cargo or simply general trafficking is required remains unknown. However, mutations affecting the COPII component Sec 24 were shown to have cell autonomous effects on lumen development,52 and ER export of the apical membrane determinant Crumbs requires Sar1 and COPII.53
At the Golgi, trafficking through Arf1 and the COPI complex also plays an essential role in lumen expansion. Without the COPI components γCOP or δCOP, Golgi and ER membrane organization becomes irregular, secretion of luminal proteins is disrupted, Crumbs levels at the apical PM are lower, and tube diameter decreases.54 The Arf GEF Garz is also needed for tracheal tube development. Garz normally localizes to the cis Golgi of tracheal cells, and in the absence of Garz, Golgi localization of Arf1 and COPI coatomer are both dramatically reduced, and ER-Golgi organization is compromised.55,56
Exocytosis for notochord and neural tube development
In chordates, the notochord plays important structural and signaling activities. The notochord is derived from chordamesoderm which elongates along the anterior-posterior body axis through cell-cell intercalation and secretes ECM for its structural integrity.57,58
The cell-cell intercalation events that elongate the notochord depend on PM domains gaining distinct molecular composition polarized in the plane of the tissue (planar cell polarity; PCP)59 (Fig. 1C). Recent work indicates that Arf1 and its effector AP-1 are required for controlling PCP in different tissues of Drosophila and zebrafish embryos. Disrupting Arf1 or AP-1 activity leads to PCP defects in the Drosophila wing. Arf1 and AP-1 colocalize to trans Golgi membranes of the cells involved and promote the biosynthetic trafficking of the PCP protein Frizzled.60 Interestingly, expression of a constitutively active Arf1 construct in zebrafish embryos resulted in abnormal organization of the notochord and shortening of body length.60 In addition to its elongation, the notochord must also secrete ECM to support body structure. In zebrafish, loss of COPI subunits or Arf GEF activity leads to notochord defects associated with disrupted ER-Golgi structure and defects in ECM secretion.61 Thus, Arfs and biosynthetic trafficking may be important for both the PCP and the ECM of the notochord.
PCP, cell-cell intercalation and asymmetric cell division also organizes the neural tube in vertebrate embryos. The neural tube is derived from the neural plate, a portion of the ectoderm located dorsally to the notochord that gives rise to the central nervous system.62 The COPII coat component Sec 24b is critical for the trafficking of the PCP protein Vangl2 and proper neural tube development in mice,63,64 and 4 mutant variants of the Sec 24b gene have been linked to neural tube closure defects in humans.65 Loss of Sec 23a in mice also causes severe neural tube opening and embryo lethality, although the neural tube defect observed in Sec 23a mutant embryos arises from a reopening of a closed neural tube rather than failure of primary closure.66
Exocytosis for ciliogenesis
Primary cilia are microtubule-based, PM protrusions with a specific membrane composition. Cilia mediate developmental signaling, and mutations affecting cilia structure lead to a class of diseases called ciliopathies. Trafficking of specific cargo to the base of cilia is essential for ciliogenesis and cilia function.67,68 (Fig. 1C). A clear example of trafficking from the Golgi to the primary cilium has been documented for rhodopsin transport in frog photoreceptor cells69 At the Golgi, Arf4 and its Arf GAP ASAP1 help recruit rhodopsin as vesicle cargo.70,71 Arf4 and ASAP1 directly bind rhodopsin through 2 different protein sequence motifs.70,71 Moreover, ASAP1 acts as a scaffold to organize multiple proteins for rhodopsin trafficking. ASAP1 binds Rab11 and its interacting partner FIP3, and recruits the Rab GEF Rabin8 which in turn binds and activates Rab8.70-72 Rab8 and Rab11 target rhodopsin-containing vesicles to the cilia, apparently by tethering of the vesicle to the cilium base through the exocyst complex component Sec 15.69 Thus, an Arf-Rab cascade directs rhodopsin trafficking from the Golgi to the primary cilium of photoreceptor cells.
Transcriptional regulation of biosynthetic machinery during development
Biosynthetic trafficking must be linked to transcription and translation of the cargo being transported. Moreover, a conserved transcription factor family plays a widespread role in increasing the expression of the secretory machinery. The CrebA/Creb3-like transcription factors recognize a consensus motif within enhancer regions of genes for secretory machinery and promote their expression.73 This expression is critical for animal development. As examples, CrebA drives expression of Sar1 and COPII components to promote dendritic growth in Drosophila,74 Creb3l2/BBF2H7 promotes transcription of COPII coat genes for ECM secretion and skeletal development in mice and zebrafish,75 and CrebA induces secretory gene expression for tube morphogenesis and secretion of the Drosophila salivary gland.73,76 Widespread use of a conserved transcriptional mechanism for the expression of biosynthetic trafficking machinery indicates the importance of induced biosynthetic trafficking for specific developmental processes.
Concluding remarks
We have reviewed how specific developmental processes rely on increases to total biosynthetic output or on the biosynthetic trafficking of specific proteins. The membrane trafficking machinery involved can be upregulated for particular developmental processes, and is coordinated locally by Arf family small G proteins. Such trafficking is critical for major developmental processes, and abnormal trafficking results in developmental syndromes.
Numerous questions remain. For many developmental processes, it is unclear whether the biosynthetic trafficking of specific cargo is critical. In such cases, the phenotypes resulting from removal of trafficking machinery should mimic those resulting from removal of cargo. How such cargo finds its final destination would also require definition. For cases in which a general increase to biosynthesis is required the amount of biosynthetic machinery could become limiting. How widely used are CrebA/Creb3-like transcription factors for elevating expression of biosynthetic trafficking machinery, and do distinct transcriptional programs exist for such increases? How is the Golgi positioned for polarized secretion in different cell types? How prevalent are specific developmental roles of trafficking machinery isoforms, and are such roles due to tissue-specific expression or unique protein activities of the isoforms? Moreover, post-translation modifications and interactions with cargo can modify the behavior of biosynthetic trafficking machinery,77 but have not been evaluated during animal development. Thus many avenues are open for pursuing a fuller understanding of the biosynthetic trafficking that underpins animal development.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Dr. Tao Jiang for critical reading of the paper and we thank Eugene Bak for his assistance with illustrations.
Funding
Our research in this area is supported by a Canadian Institutes of Health Research operating grant (MOP82829).
References
- [1].Lecuit T. Junctions and vesicular trafficking during Drosophila cellularization. J Cell Sci 2004; 117:3427-33; PMID:15252125; http://dx.doi.org/ 10.1242/jcs.01312 [DOI] [PubMed] [Google Scholar]
- [2].Melrose J, Shu C, Whitelock JM, Lord MS. The cartilage extracellular matrix as a transient developmental scaffold for growth plate maturation. Matrix Biol 2016; 52-54:363-83; PMID:26807757; http://dx.doi.org/ 10.1016/j.matbio.2016.01.008 [DOI] [PubMed] [Google Scholar]
- [3].Rodriguez-Boulan E, Macara IG. Organization and execution of the epithelial polarity programme. Nat Rev Mol Cell Bio 2014; 15:225-42; http://dx.doi.org/ 10.1038/nrm3775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Westrate LM, Lee JE, Prinz WA, Voeltz GK. Form follows function: the importance of endoplasmic reticulum shape. Annu Rev Biochem 2015; 84:791-811; PMID:25580528; http://dx.doi.org/ 10.1146/annurev-biochem-072711-163501 [DOI] [PubMed] [Google Scholar]
- [5].Mandon EC, Trueman SF, Gilmore R. Protein Translocation across the rough endoplasmic reticulum. Cold Spring Harb Perspect Biol 2013; 5; pii: a013342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Braakman I, Hebert DN. Protein folding in the endoplasmic reticulum. Cold Spring Harb Perspect Biol 2013; 5:a013201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Nakamura N, Wei JH, Seemann J. Modular organization of the mammalian Golgi apparatus. Curr Opin Cell Biol 2012; 24:467-74; PMID:22726585; http://dx.doi.org/ 10.1016/j.ceb.2012.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Guo YS, Sirkis DW, Schekman R. Protein Sorting at the trans-Golgi Network. Ann Rev Cell Dev Biol 2014; 30:169-206; http://dx.doi.org/ 10.1146/annurev-cellbio-100913-013012 [DOI] [PubMed] [Google Scholar]
- [9].Brandizzi F, Barlowe C. Organization of the ER-Golgi interface for membrane traffic control. Nat Rev Mol Cell Bio 2013; 14:382-92; http://dx.doi.org/ 10.1038/nrm3588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Donaldson JG, Jackson CL. ARF family G proteins and their regulators: roles in membrane transport, development and disease (vol 12, pg 362, 2011). Nat Rev Mol Cell Bio 2011; 12:362-75; http://dx.doi.org/ 10.1038/nrm3117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Gillingham AK, Munro S. The small G proteins of the arf family and their regulators. Ann Rev Cell Dev Biol 2007; 23:579-611; http://dx.doi.org/ 10.1146/annurev.cellbio.23.090506.123209 [DOI] [PubMed] [Google Scholar]
- [12].Zanetti G, Pahuja KB, Studer S, Shim S, Schekman R. COPII and the regulation of protein sorting in mammals. Nat Cell Biol 2012; 14:20-8; http://dx.doi.org/ 10.1038/ncb2390 [DOI] [PubMed] [Google Scholar]
- [13].Spang A, Shiba Y, Randazzo PA. Arf GAPs: Gatekeepers of vesicle generation. Febs Lett 2010; 584:2646-51; PMID:20394747;http://dx.doi.org/ 10.1016/j.febslet.2010.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Bio 2009; 10:513-25; http://dx.doi.org/ 10.1038/nrm2728 [DOI] [PubMed] [Google Scholar]
- [15].Lee DM, Harris TJ. Coordinating the cytoskeleton and endocytosis for regulated plasma membrane growth in the early embryo. Bioarchitecture 2014; 4:68-74; PMID:24874871; http://dx.doi.org/ 10.4161/bioa.28949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Foe VE, Alberts BM. Studies of nuclear and cytoplasmic behaviour during the five mitotic cycles that precede gastrulation in Drosophila embryogenesis. J Cell Sci 1983; 61:31-70; PMID:6411748 [DOI] [PubMed] [Google Scholar]
- [17].Frescas D, Mavrakis M, Lorenz H, Delotto R, Lippincott-Schwartz J. The secretory membrane system in the Drosophila syncytial blastoderm embryo exists as functionally compartmentalized units around individual nuclei. J Cell Biol 2006; 173:219-30; PMID:16636144; http://dx.doi.org/ 10.1083/jcb.200601156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lee DM, Rodrigues FF, Yu CG, Swan M, Harris TJ. PH Domain-Arf G Protein Interactions Localize the Arf-GEF Steppke for Cleavage Furrow Regulation in Drosophila. PLoS One 2015; 10:e0142562; PMID:26556630; http://dx.doi.org/ 10.1371/journal.pone.0142562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Rodrigues FF, Shao W, Harris TJC. The Arf GAP Asap promotes Arf1 function at the Golgi for cleavage furrow biosynthesis in Drosophila. Mol Biol Cell 2016; 27:3143-55; PMID:27535433; http://dx.doi.org/ 10.1091/mbc.E16-05-0272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Eisman RC, Stewart N, Miller D, Kaufman TC. Centrosomin's beautiful sister (cbs) encodes a GRIP-domain protein that marks Golgi inheritance and functions in the centrosome cycle in Drosophila. J Cell Sci 2006; 119:3399-412; PMID:16882688; http://dx.doi.org/ 10.1242/jcs.03088 [DOI] [PubMed] [Google Scholar]
- [21].Sisson JC, Field C, Ventura R, Royou A, Sullivan W. Lava lamp, a novel peripheral golgi protein, is required for Drosophila melanogaster cellularization. J Cell Biol 2000; 151:905-18; PMID:11076973; http://dx.doi.org/ 10.1083/jcb.151.4.905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Papoulas O, Hays TS, Sisson JC. The golgin Lava lamp mediates dynein-based Golgi movements during Drosophila cellularization. Nat Cell Biol 2005; 7:612-8; PMID:15908943; http://dx.doi.org/ 10.1038/ncb1264 [DOI] [PubMed] [Google Scholar]
- [23].Murthy M, Teodoro RO, Miller TP, Schwarz TL. Sec 5, a member of the exocyst complex, mediates Drosophila embryo cellularization. Development 2010; 137:2773-83; PMID:20630948; http://dx.doi.org/ 10.1242/dev.048330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lecuit T, Wieschaus E. Polarized insertion of new membrane from a cytoplasmic reservoir during cleavage of the Drosophila embryo. J Cell Biol 2000; 150:849-60; PMID:10953008; http://dx.doi.org/ 10.1083/jcb.150.4.849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Holly RM, Mavor LM, Zuo ZY, Blankenship JT. A rapid, membrane-dependent pathway directs furrow formation through RalA in the early Drosophila embryo. Development 2015; 142:2316-28; PMID:26092850; http://dx.doi.org/ 10.1242/dev.120998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Mavor LM, Miao H, Zuo ZY, Holly RM, Xie Y, Loerke D, Blankenship JT. Rab8 directs furrow ingression and membrane addition during epithelial formation in Drosophila melanogaster. Development 2016; 143:892-903; PMID:26839362; http://dx.doi.org/ 10.1242/dev.128876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Lefebvre JL, Sanes JR, Kay JN. Development of dendritic form and function. Annu Rev Cell Dev Bi 2015; 31:741-77; http://dx.doi.org/ 10.1146/annurev-cellbio-100913-013020 [DOI] [PubMed] [Google Scholar]
- [28].Ehlers MD. Dendritic trafficking for neuronal growth and plasticity. Biochem Soc T 2013; 41:1365-82; http://dx.doi.org/ 10.1042/BST20130081 [DOI] [PubMed] [Google Scholar]
- [29].Ye B, Zhang Y, Song W, Younger SH, Jan LY, Jan YN. Growing dendrites and axons differ in their reliance on the secretory pathway. Cell 2007; 130:717-29; PMID:17719548;http://dx.doi.org/ 10.1016/j.cell.2007.06.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Horton AC, Racz B, Monson EE, Lin AL, Weinberg RJ, Ehlers MD. Polarized secretory trafficking directs cargo for asymmetric dendrite growth and morphogenesis. Neuron 2005; 48:757-71; PMID:16337914; http://dx.doi.org/ 10.1016/j.neuron.2005.11.005 [DOI] [PubMed] [Google Scholar]
- [31].Jain S, Yoon SY, Zhu L, Brodbeck J, Dai J, Walker D, Huang Y. Arf4 determines dentate gyrus-mediated pattern separation by regulating dendritic spine development. Plos One 2012; 7:e46340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Harris KP, Littleton JT. Transmission, development, and plasticity of synapses. Genetics 2015; 201:345-75; PMID:26447126; http://dx.doi.org/ 10.1534/genetics.115.176529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Chang L, Kreko-Pierce T, Eaton BA. The guanine exchange factor Gartenzwerg and the small GTPase Arl1 function in the same pathway with Arfaptin during synapse growth. Biol Open 2015; 4:947-53; PMID:26116655; http://dx.doi.org/ 10.1242/bio.011262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Berendsen AD, Olsen BR. Bone development. Bone 2015; 80:14-8; PMID:26453494; http://dx.doi.org/ 10.1016/j.bone.2015.04.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Bateman JF, Boot-Handford RP, Lamande SR. Genetic diseases of connective tissues: cellular and extracellular effects of ECM mutations. Nat Rev Genet 2009; 10:173-83; PMID:19204719; http://dx.doi.org/ 10.1038/nrg2520 [DOI] [PubMed] [Google Scholar]
- [36].Unlu G, Levic DS, Melville DB, Knapik EW. Trafficking mechanisms of extracellular matrix macromolecules: Insights from vertebrate development and human diseases. Int J Biochem Cell B 2014; 47:57-67; http://dx.doi.org/ 10.1016/j.biocel.2013.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Boyadjiev SA, Fromme JC, Ben J, Chong SS, Nauta C, Hur DJ, Zhang G, Hamamoto S, Schekman R, Ravazzola M, et al.. Cranio-lenticulo-sutural dysplasia is caused by a SEC23A mutation leading to abnormal endoplasmic-reticulumto-Golgi trafficking. Nat Genet 2006; 38:1192-7; PMID:16980979; http://dx.doi.org/ 10.1038/ng1876 [DOI] [PubMed] [Google Scholar]
- [38].Fromme JC, Ravazzola M, Hamamoto S, A-Balwi M, Eyaid W, Boyadjiev SA, Cosson P, Schekman R, Orci L. The genetic basis of a craniofacial disease provides insight into COPII coat assembly. Dev Cell 2007; 13:623-34; PMID:17981132; http://dx.doi.org/ 10.1016/j.devcel.2007.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Lang MR, Lapierre LA, Frotscher M, Goldenring JR, Knapik EW. Secretory COPII coat component Sec23a is essential for craniofacial chondrocyte maturation. Nat Genet 2006; 38:1198-203; PMID:16980978; http://dx.doi.org/ 10.1038/ng1880 [DOI] [PubMed] [Google Scholar]
- [40].Townley AK, Feng Y, Schmidt K, Carter DA, Porter R, Verkade P, Stephens DJ. Efficient coupling of Sec 23-Sec 24 to Sec 13-Sec 31 drives COPII-dependent collagen secretion and is essential for normal craniofacial development. J Cell Sci 2008; 121:3025-34; PMID:18713835; http://dx.doi.org/ 10.1242/jcs.031070 [DOI] [PubMed] [Google Scholar]
- [41].Levic D, Minkel JR, Wang WD, Rybski WM, Melville DB, Knapik EW. Animal model of Sar1b deficiency presents lipid absorption deficits similar to Anderson disease. J Mol Med 2015; 93:165-76; PMID:25559265; http://dx.doi.org/ 10.1007/s00109-014-1247-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Garbes L, Kim K, Riess A, Hoyer-Kuhn H, Beleggia F, Bevot A, Kim MJ, Huh YH, Kweon HS, Savarirayan R, et al.. Mutations in SEC24D, Encoding a Component of the COPII Machinery, Cause a Syndromic Form of Osteogenesis Imperfecta. Am J Hum Genet 2015; 96:432-9; PMID:25683121; http://dx.doi.org/ 10.1016/j.ajhg.2015.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Moosa S, Chung BHY, Tung JYL, Altmuller J, Thiele H, Nurnberg P, Netzer C, Nishimura G, Wollnik B. Mutations in SEC24D cause autosomal recessive osteogenesis imperfecta. Clin Genet 2016; 89:517-9; http://dx.doi.org/ 10.1111/cge.12678 [DOI] [PubMed] [Google Scholar]
- [44].Sarmah S, Barrallo-Gimeno A, Melville DB, Topczewski J, Solnica-Krezel L, Knapik EW. Sec24D-dependent transport of extracellular matrix proteins is required for zebrafish skeletal morphogenesis. Plos One 2010; 5:e10367; PMID:20442775; http://dx.doi.org/ 10.1371/journal.pone.0010367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Ohisa S, Inohaya K, Takano Y, Kudo A. sec24d encoding a component of COPII is essential for vertebra formation, revealed by the analysis of the medaka mutant, vbi. Dev Biol 2010; 342:85-95; PMID:20346938; http://dx.doi.org/ 10.1016/j.ydbio.2010.03.016 [DOI] [PubMed] [Google Scholar]
- [46].Izumi K, Brett M, Nishi E, Drunat S, Tan ES, Fujiki K, Lebon S, Cham B, Masuda K, Arakawa M, et al.. ARCN1 mutations cause a recognizable craniofacial syndrome due to COPI-mediated transport defects. Am J Hum Genet 2016; 99:451-9; PMID:27476655; http://dx.doi.org/ 10.1016/j.ajhg.2016.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Schottenfeld J, Song YJ, Ghabrial AS. Tube continued: morphogenesis of the Drosophila tracheal system. Curr Opin Cell Biol 2010; 22:633-9; PMID:20739171; http://dx.doi.org/ 10.1016/j.ceb.2010.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Maruyama R, Andrew DJ. Drosophila as a model for epithelial tube formation. Dev Dynam 2012; 241:119-35; http://dx.doi.org/ 10.1002/dvdy.22775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Martin-Belmonte F, Rodriguez-Fraticelli AE. Acquisition of membrane polarity in epithelial tube formation: patterns, signaling pathways, molecular mechanisms, and disease. Int Rev Cel Mol Bio 2009; 274:129-82 [DOI] [PubMed] [Google Scholar]
- [50].Dong B, Hayashi S. Shaping of biological tubes by mechanical interaction of cell and extracellular matrix. Curr Opin Genet Dev 2015; 32:129-34; PMID:25819978; http://dx.doi.org/ 10.1016/j.gde.2015.02.009 [DOI] [PubMed] [Google Scholar]
- [51].Tsarouhas V, Senti KA, Jayaram SA, Tiklova K, Hemphala J, Adler J, Samakovlis C. Sequential pulses of apical epithelial secretion and endocytosis drive airway maturation in Drosophila. Dev Cell 2007; 13:214-25; PMID:17681133; http://dx.doi.org/ 10.1016/j.devcel.2007.06.008 [DOI] [PubMed] [Google Scholar]
- [52].Forster D, Armbruster K, Luschnig S. Sec 24-dependent secretion drives cell-autonomous expansion of tracheal tubes in drosophila. Curr Biol 2010; 20:62-8; PMID:20045324; http://dx.doi.org/ 10.1016/j.cub.2009.11.062 [DOI] [PubMed] [Google Scholar]
- [53].Kumichel A, Kapp K, Knust E. A Conserved Di-basic motif of drosophila crumbs contributes to efficient ER export. Traffic 2015; 16:604-16; PMID:25753515; http://dx.doi.org/ 10.1111/tra.12273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Jayaram SA, Senti KA, Tiklova K, Tsarouhas V, Hemphala J, Samakovlis C. COPI vesicle transport is a common requirement for tube expansion in drosophila. Plos One 2008; 3:e1964; PMID:18398480; http://dx.doi.org/ 10.1371/journal.pone.0001964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Armbruster K, Luschnig S. The Drosophila Sec 7 domain guanine nucleotide exchange factor protein Gartenzwerg localizes at the cis-Golgi and is essential for epithelial tube expansion. J Cell Sci 2012; 125:1318-28; PMID:22349697; http://dx.doi.org/ 10.1242/jcs.096263 [DOI] [PubMed] [Google Scholar]
- [56].Wang SS, Meyer H, Ochoa-Espinosa A, Buchwald U, Onel S, Altenhein B, Heinisch JJ, Affolter M, Paululat A. GBF1 (Gartenzwerg)-dependent secretion is required for Drosophila tubulogenesis. J Cell Sci 2012; 125:461-72; PMID:22302994; http://dx.doi.org/ 10.1242/jcs.092551 [DOI] [PubMed] [Google Scholar]
- [57].Corallo D, Trapani V, Bonaldo P. The notochord: structure and functions. Cell Mol Life Sci 2015; 72:2989-3008; PMID:25833128; http://dx.doi.org/ 10.1007/s00018-015-1897-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Stemple DL. Structure and function of the notochord: an essential organ for chordate development. Development 2005; 132:2503-12; PMID:15890825; http://dx.doi.org/ 10.1242/dev.01812 [DOI] [PubMed] [Google Scholar]
- [59].Wallingford JB. Planar cell polarity and the developmental control of cell behavior in vertebrate embryos. Ann Rev Cell Dev Biol 2012; 28:627-53; http://dx.doi.org/ 10.1146/annurev-cellbio-092910-154208 [DOI] [PubMed] [Google Scholar]
- [60].Carvajal-Gonzalez JM, Balmer S, Mendoza M, Dussert A, Collu G, Roman AC, Weber U, Ciruna B, Mlodzik M. The clathrin adaptor AP-1 complex and Arf1 regulate planar cell polarity in vivo. Nat Commun 2015; 6:8720; PMID:26548801; http://dx.doi.org/ 10.1038/ncomms7751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Coutinho P, Parsons MJ, Thomas KA, Hirst EMA, Saude L, Campos I, Williams PH, Stemple DL. Differential requirements for COPI transport during vertebrate early development. Dev Cell 2004; 7:547-58; PMID:15469843; http://dx.doi.org/ 10.1016/j.devcel.2004.07.020 [DOI] [PubMed] [Google Scholar]
- [62].Araya C, Ward LC, Girdler GC, Miranda M. Coordinating cell and tissue behavior during zebrafish neural tube morphogenesis. Dev Dynam 2016; 245:197-208; http://dx.doi.org/ 10.1002/dvdy.24304 [DOI] [PubMed] [Google Scholar]
- [63].Merte J, Jensen D, Wright K, Sarsfield S, Wang YS, Schekman R, Ginty DD. Sec 24b selectively sorts Vangl2 to regulate planar cell polarity during neural tube closure. Nat Cell Biol 2010; 12:41-U94; PMID:19966784; http://dx.doi.org/ 10.1038/ncb2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Wansleeben C, Feitsma H, Montcouquiol M, Kroon C, Cuppen E, Meijlink F. Planar cell polarity defects and defective Vangl2 trafficking in mutants for the COPII gene Sec 24b. Development 2010; 137:1067-73; PMID:20215345; http://dx.doi.org/ 10.1242/dev.041434 [DOI] [PubMed] [Google Scholar]
- [65].Yang XY, Zhou XY, Wang QQ, Li H, Chen Y, Lei YP, Ma XH, Kong P, Shi Y, Jin L, et al.. Mutations in the COPII vesicle component gene SEC24B are associated with human neural tube defects. Hum Mutat 2013; 34:1094-101; PMID:23592378; http://dx.doi.org/ 10.1002/humu.22338 [DOI] [PubMed] [Google Scholar]
- [66].Zhu M, Tao JY, Vasievich MP, Wei W, Zhu GJ, Khoriaty RN, Zhang B. Neural tube opening and abnormal extraembryonic membrane development in SEC23A deficient mice. Sci Rep-Uk 2015; 5:15471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Madhivanan K, Aguilar RC. Ciliopathies: The Trafficking Connection. Traffic 2014; 15:1031-56; PMID:25040720; http://dx.doi.org/ 10.1111/tra.12195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Sung CH, Leroux MR. The roles of evolutionarily conserved functional modules in cilia-related trafficking. Nat Cell Biol 2013; 15:1387-97; PMID:24296415; http://dx.doi.org/ 10.1038/ncb2888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Wang J, Deretic D. Molecular complexes that direct rhodopsin transport to primary cilia. Prog Retin Eye Res 2014; 38:1-19; PMID:24135424; http://dx.doi.org/ 10.1016/j.preteyeres.2013.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Wang J, Morita Y, Mazelova J, Deretic D. The Arf GAP ASAP1 provides a platform to regulate Arf4-and Rab11-Rab8-mediated ciliary receptor targeting. Embo J 2012; 31:4057-71; PMID:22983554; http://dx.doi.org/ 10.1038/emboj.2012.253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Mazelova J, Astuto-Gribble L, Inoue H, Tam BM, Schonteich E, Prekeris R, Moritz OL, Randazzo PA, Deretic D. Ciliary targeting motif VxPx directs assembly of a trafficking module through Arf4. Embo J 2009; 28:183-92; PMID:19153612; http://dx.doi.org/ 10.1038/emboj.2008.267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Wang J, Deretic D. The Arf and Rab11 effector FIP3 acts synergistically with ASAP1 to direct Rabin8 in ciliary receptor targeting. J Cell Sci 2015; 128:1375-85; PMID:25673879; http://dx.doi.org/ 10.1242/jcs.162925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Fox RM, Hanlon CD, Andrew DJ. The CrebA/Creb3-like transcription factors are major and direct regulators of secretory capacity. J Cell Biol 2010; 191:479-92; PMID:21041443; http://dx.doi.org/ 10.1083/jcb.201004062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Iyer SC, Iyer EPR, Meduri R, Rubaharan M, Kuntimaddi A, Karamsetty M, Cox DN. Cut, via CrebA, transcriptionally regulates the COPII secretory pathway to direct dendrite development in Drosophila. J Cell Sci 2013; 126:4732-45; PMID:23902691; http://dx.doi.org/ 10.1242/jcs.131144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Melville DB, Montero-Balaguer M, Levic DS, Bradley K, Smith JR, Hatzopoulos AK, Knapik EW. The feelgood mutation in zebrafish dysregulates COPII-dependent secretion of select extracellular matrix proteins in skeletal morphogenesis. Dis Model Mech 2011; 4:763-76; PMID:21729877; http://dx.doi.org/ 10.1242/dmm.007625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Abrams EW, Andrew DJ. CrebA regulates secretory activity in the Drosophila salivary gland and epidermis. Development 2005; 132:2743-58; PMID:15901661; http://dx.doi.org/ 10.1242/dev.01863 [DOI] [PubMed] [Google Scholar]
- [77].Venditti R, Wilson C, De Matteis MA. Exiting the ER: what we know and what we don't. Trends Cell Biol 2014; 24:9-18; PMID:24076263; http://dx.doi.org/ 10.1016/j.tcb.2013.08.005 [DOI] [PubMed] [Google Scholar]


