Abstract
Background:
This observational study describes implementation of HIV retesting of HIV-negative women in prevention of mother-to-child transmission (PMTCT) services in Zambia.
Methods:
Uptake of retesting and PMTCT services were compared across age, parity, and weeks of gestation at the time of the first HIV test, antiretrovirals regime, and HIV early diagnosis results from infants born to HIV-positive mothers.
Results:
A total of 19 090 pregnant women were tested for HIV at their first antenatal visit, 16 838 tested HIV-negative and were offered retesting 3 months later: 11 339 (67.3%) were retested; of those, 55 (0.5%) were HIV positive. Uptake of the PMTCT package by women HIV positive at retest was not different but HIV-exposed infants born to women who retested HIV positive were infected at a higher rate (11.1%) compared to those born to women who tested HIV positive at their initial test (3.2%).
Conclusion:
We suggest rigorously (1) measuring the proportion of MTCT attributable to women who seroconvert during pregnancy and possibly adjust PMTCT approaches and (2) addressing the substantial loss to follow-up of HIV-negative pregnant women before HIV retesting.
Keywords: prevention of mother-to-child transmission, HIV testing and counseling, Zambia
What Do We Already Know about This Topic?
Retesting of previously tested HIV-negative pregnant women is a recommended practice in many countries, including Zambia, but its evaluation has been limited.
How Does Your Research Contribute to the Field?
This article contributes to the understanding of the extent to which seroconverting pregnant women contribute to mother-to-child transmission of HIV.
What Are Your Research’s Implications toward Theory, Practice, or Policy?
Our observations would inform the prevention of mother-to-child transmission strategy and policy in Zambia or in countries with similar profiles; questions to consider might be “what should be the best approach to prevent mother-to-child transmission to minimize the risk of HIV vertical transmission from seroconverting women.”
Background
Vertical transmission remains the principal route of new pediatric HIV infections despite the availability of effective interventions for the prevention of mother-to-child transmission (PMTCT) of HIV. In the last decade in Zambia, as in many other low- and middle-income countries, considerable improvements in PMTCT have been observed: Services are increasingly available in primary health centers (PHCs) and a growing proportion of HIV-positive pregnant women are accessing efficient combination antiretroviral treatment (ART).1 The goal to eliminate new pediatric HIV infection by 2015 was missed by most countries and very few countries are on track toward elimination of new cases of pediatric HIV.2
Progress toward elimination of new pediatric HIV infection is hindered by a number of factors including the suboptimal uptake and low retention on ART among pregnant and breastfeeding women.1 Another factor thought to hinder PMTCT efforts is newly acquired HIV infection during pregnancy or breastfeeding period.3–5 Literature suggests that pregnant women have a higher risk of acquiring HIV infection than the general population6–8; this risk is likely due to sexual behavior, pregnancy itself is the result of “unprotected” sex. Incident HIV infection during pregnancy is associated with higher risk of mother-to-child transmission of HIV (MTCT).9 Diagnosing newly acquired HIV infections at the programmatic level is very challenging, and the realistic alternative is to repeat HIV testing among clients previously found to be HIV negative. The Ministry of Health of Zambia recommends that pregnant women previously tested HIV negative be retested every 3 months after the initial test until delivery.10
Despite the need for early diagnosis and initiation of treatment for pregnant women, data are not systematically collected, reported, and analyzed on the implementation of this recommendation.
Objective
The objective of this study was 4-fold: (1) determine to what extent women previously tested HIV negative are being retested, (2) determine the rate of HIV infection in the specific group of women with a previous HIV-negative test who were retested, (3) describe how HIV-incident pregnant women (and their infants) accessed PMTCT services, and (4) determine the MTCT rate among infants born to women who seroconverted during pregnancy.
Methods
Study Context and Population
Zambia is an HIV hyperendemic country; in 2015, the estimated HIV prevalence among adults (aged 15-49 years) was 13.3%; in the same age group, HIV prevalence is higher among women (15.1%) than among men (11.3%). HIV is twice as prevalent in urban (18.2%) than in rural (9.1%) areas.11 According to the Joint United Nations Programme on HIV and AIDS, in 2015 an estimated 4700 children were newly infected with HIV in Zambia; one of the highest national figures of new pediatric HIV infections.1
In response to the HIV epidemic threat, strong leadership from the Government of Zambia and a partnership with United States Agency for International Development (USAID) resulted in a decentralized HIV/AIDS program. In addition, there has been a visible integration of HIV-related interventions, particularly PMTCT, into routine maternal, neonatal and child health care (MNCH).12,13
The USAID support has been implemented through various initiatives, including the Zambia Prevention Care and Treatment Partnership (ZPCT) project since 2005. This article reports on a study conducted in 10 MNCH clinics that have integrated PMTCT interventions. The interventions are based on the Zambian PMTCT guidelines, which recommend retesting HIV-negative pregnant and breastfeeding women every 3 to 6 months.13 All HIV-positive pregnant women (at initial test or at retest) were offered a combination ART for the entire period of pregnancy and breastfeeding. The 10 MNCH clinics were rural PHCs that have been receiving technical support from the ZPCT project. They were selected because they had the highest volume of clients among the PHCs supported by the ZPCT project. To shorten the recruitment time, the authors decided to select health facilities that registered an average of 20 or more new pregnant women for first antenatal visits per month.
The study population consisted of all pregnant women and their infants registered in antenatal clinics, labor and delivery settings, and postnatal service points from 10 selected health facilities supported by ZPCT between February 2010 and December 2013 in 5 provinces. Four of the PHCs were in Copperbelt province, 3 in Central province, 1 in Luapula province, 1 in North province, and 1 in North-Western province. All 10 health facilities had PMTCT services integrated into MNCH services.
HIV Testing and Retesting Methodology
At their first antenatal care visit, all pregnant women were offered HIV testing and counseling (HTC) on an “opt out” basis, meaning all women were tested for HIV unless they explicitly refused. The HTC was performed by trained providers: nurses or midwives or lay PMTCT counselors. As per the national guidelines, the HIV testing algorithm is a serial rapid HIV testing, and the description here refers to the active guidelines at the time of the study. The first-line test used was Determine; if the Determine test result was negative, the HIV status was considered negative; when Determine result was positive, the second-line test, Uni-Gold was used; if Uni-Gold test result, Trinity Biotech was also positive, the result was considered positive; if Uni-Gold test result was negative (discordant with Determine test result), a third-line test, Bioline, was used as a tiebreaker.13 The retesting of previously tested HIV-negative women was performed by the same providers and followed the same algorithm as the initial HIV testing.
Design
This is a descriptive observational study of HIV testing and retesting activities within MNCH settings. We analyzed all HIV test and retest results as well as age, parity, and gestational age at the time of the first HIV tests; antiretrovirals regime received by HIV-positive women; and feeding method and HIV early diagnosis results from infants born to HIV-positive mothers.
Data Collection, Entry, and Analysis
The study used data routinely collected by PHC for integrated PMTCT services—integrated antenatal register, delivery, and postpartum registers as well as HIV-exposed infants registers. The specific data elements were entered into dedicated data extraction article-based registers by health facility personnel at the selected health facilities; the personnel were trained on the use of data extraction tools and were regularly supervised by the ZPCT provincial technical officers to ensure good quality data collection. The article-based register was double entered into an Excel 2016 database by the data clerks and checked for accuracy by the ZPCT provincial monitoring and evaluation (M&E) officer before being sent to the ZPCT central office in Lusaka.
Frequencies and percentage distributions of key variables were generated. Differences in proportion were tested using the χ2 test. Continuous variables were described as mean or median depending on the distribution of the data. Incidence rate was estimated from the number of seroconversions and total person-years of follow-up. For the incidence rate calculations, exposure time was calculated as the interval between time of enrollment until last HIV-negative test for non-seroconverters and until the time of HIV infection for seroconverters, which was estimated as the midpoint between the last negative HIV rapid test result and the first positive HIV rapid test result.
P values below .05 were considered statistically significant. Statistical analysis was performed with STATA, StataCorp (version 12).
Ethical Approval and Informed Consent
Ethical approval was granted by the ethical review board and the Ministry of Health of Zambia (reference: 2010-AUG-001) and the FHI 360’s Protection of Human Subjects Committee (reference: OIRE/5/25/10).
The ethical review board and the Ministry of Health of Zambia as well as the FHI 360’s Protection of Human Subjects Committee judged that informed consent was not needed. No names or personal identifier of individual patients were collected. There was no participant ID or other means that could link data to participants on the electronic data set. Data analysis was performed anonymously by a team that did not have contact with the participants or with their identifiable information.
Results
Study Population and Follow-up Overview
A total of 19 090 pregnant women were registered for the initial antenatal clinic visit, ranging in age from 10 to 49 years with a median of 23 years (interquartile range [IQR]: 20-28). Their parity ranged from 0 to 14 with a median of 1 (IQR: 0-3). The median gestational age at the time of the first antenatal clinical visit was 22 weeks (IQR: 18-26).
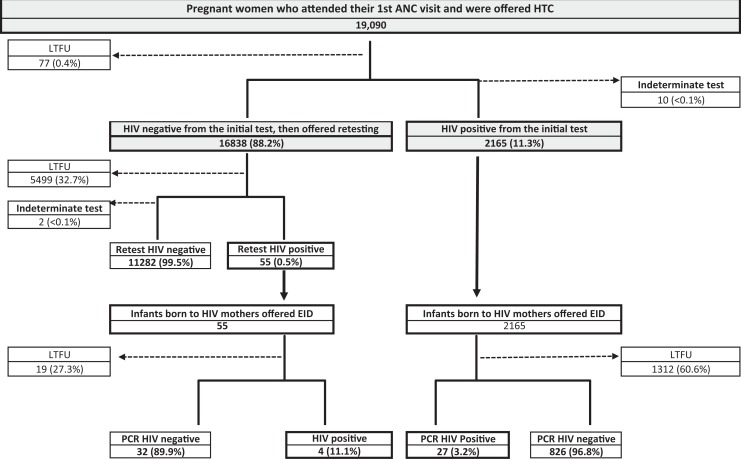
The pregnant women who registered for the initial antenatal clinic visit (n = 19 090) were offered HTC or screening for an HIV-positive status; 16 838 (88.2%) of these tested HIV negative. The proportions of loss to follow-up (LTFU) and indeterminate results were negligible, 0.4% (n = 77) and <0.1% (n = 10), respectively. A total of 2165 (11.3%) pregnant women were determined to be HIV positive at that initial antenatal screening (see Figure 1).
Figure 1.
Study population and follow-up overview.
A total of 2220 infants born to HIV-positive mothers were recorded: 2165 born to HIV-positive mothers from the initial HIV test and 55 to mothers who were HIV positive at the retest.
Throughout the cascade various degrees of LTFU were registered. The biggest LTFU (60.6%) was observed among infants born to mothers found HIV positive at the initial HIV test between offer of early infant diagnosis and actual dried blood collection. See Figure 1 for further details.
Retesting and LTFU
All HIV-negative women (16 838) were offered HIV retesting and counseling; the retest was to be conducted every 3 months before delivery. A sizeable proportion of women—32.7% (n = 5499)—was considered LTFU as they did not return for subsequent antenatal clinic visits. Women LTFU (before retesting) tended to be of older age (≥35), higher parity (≥4), and in late gestational age (≥28 week pregnancy; see Table 1).
Table 1.
Comparative Description of LTFU before Retest by Age, Parity, and Gestational Age.
| Comparing key maternal variables between pregnant women that retested and those who were loss to follow-up (LTFU) before retest (N = 16 838) | |||
|---|---|---|---|
| Variables | Retested (%) | LTFU (%) | P Value |
| Age group (years) | |||
| ≤24 (n = 9622) | 6444 (67.0) | 3178 (33.0) | |
| 25-34 (n = 5922) | 4094 (69.1) | 1828 (30.9) | <.001 |
| ≥35 (n = 1294) | 801 (61.9) | 493 (38.1) | |
| Parity category | |||
| 0 (n = 5527) | 3940 (71.3) | 1587 (28.8) | |
| 1-3 (n = 8402) | 5790 (68.9) | 2612 (31.1) | <.001 |
| ≥4 (n = 2909) | 1609 (55.3) | 1300 (44.7) | |
| Gestational categorya | |||
| <28-week pregnancy (n = 10 587) | 7256 (68.5) | 3331 (31.5) | <.001 |
| ≥28-week pregnancy (n = 2032) | 866 (42.6) | 1166 (57.4) | |
aData on weeks of gestation could not be reconstructed for 4219 pregnant women.
Median gestational age at the time of retesting was 34 weeks, ranging from 5 to 40 weeks. Very few (2.4%) were retested at the time of delivery. The median time between the previous HIV-negative test and the HIV retest (regardless of the HIV retest results) was 93 days, ranging from 30 to 245 days. Authors could not explain the wide range of the lapse of time between the first HIV-negative test and the retesting despite the recommendation to implement retesting every 3 months.
Incident HIV Infection
The study defined incident HIV as a positive HIV test in a woman who had a documented previous HIV negative test. A total of 55 (0.49%) of 11 282 women (previously tested HIV negative) retested HIV positive. Observation time was 5195.4 person-years. HIV incidence was estimated at 1.06 per 100 person-years. The median time between the previous HIV-negative test and the HIV-positive one was 97 days, ranging from 32 to 276 days. Authors believed that this wide range is the results of wide range of the lapse of time between the first HIV-negative test and the retesting.
We observed that in Luapula Province, 2.1% of women retested HIV positive, while 0.6%, 0.4%, and 0.4%, did so in Central, Copperbelt, and North-Western Provinces, respectively. In Northern Province, none retested HIV positive. The small proportion of those who retested HIV positive did not allow for any meaningful statistical analysis to compare incidence between provinces.
Discussion
Published literature on retesting of previously tested HIV-negative participants stressed the importance of retesting but to our knowledge, our analysis is the first to describe HIV retesting in the context of PMTCT programs and particularly in Zambia.4,5,14–16 Our findings reflect the landscape of MNCH and HIV programs in Zambia.
We found that retesting, which is part of the integrated PMTCT and MNCH interventions, is affected by the significant dropout along the MNCH and PMTCT care cascade. Indeed, 32.7% of pregnant women were lost to follow-up after their first HIV-negative test, meaning they could not be retested. According to the most recent Zambia Demographic and Health Survey 2013-2014, approximately 45% of pregnant women are lost from the antenatal follow-up.11 Although the magnitude of LTFU observed in the context of this study (32.7%) appears lower than the reported LTFU of pregnant women in the context of general antenatal care (ANC) (45%), the later mirrors and probably explains the first.
From our analysis, the estimated HIV incidence was 1.06 per 100 person-years slightly higher than the previously reported HIV incidence in Zambia, which was reported as 0.80 per 100 person-years in 2012.17 A systematic review and meta-analysis that did not find a significantly higher incidence among pregnant women as compared to the general population.3 A similar prospective study in Uganda and Zimbabwe also concluded that neither pregnancy nor lactation placed women at increased risk of HIV-1 acquisition.5 Our study was not powered to measure the HIV incidence; thus, we cannot compare our findings to the cited publications and draw a meaningful conclusion.
We could not explain the higher proportion of pregnant women who retested HIV positive in Luapula (2.1%) compared to 0% to 0.6% in the 4 other provinces; the overall low proportion of women who retested HIV positive did not allow for meaningful statistical analysis.
Pregnant women who seroconverted were more than likely cases of acute HIV infection. Indeed, the average CD4 count was significantly higher among women who retested HIV positive (median: 570 cells/mm3) than those who tested HIV positive at the initial contact (median: 390 cells/mm3), a proxy indicating that those who retested HIV positive were in an earlier stage of HIV infection (see Table 2).
Table 2.
Key PMTCT Interventions Received by Categories of Women (“Retested” versus “Initially” Tested HIV+).
| Among Women Who Were HIV Positive at Retest (n = 55) | Among Women Who Were HIV Positive at the Initial Test (n = 2165) |
|---|---|
Access to ARVs for PMTCT; of the 55:
|
Access to ARVs for PMTCT; of the 2165:
|
| A total of 24 (43.6%) women had a CD4 test performed and
results available; the median CD4 count was 571 cells/mm3 (IQR:
444-773). Of their HIV-exposed infants, 4 (7.3%) received ARV prophylaxis and 39 (70.9%) were reportedly exclusively breastfed for the first 6 months of life. A total of 36 exposed infants accessed HIV “early infant diagnosis” (EID) and had their results available. The median age at the time of the dried blood collection for EID was 10 weeks rangingd from 2 to 58 weeks; 4 (11.1%) were HIV positive of the 36 EID results. |
A total of 1759 (81.2%) women had a CD4 test performed and
results available; the median CD4 count was 395 cells/mm3 (IQR:
271-550). Of their HIV-exposed infants, 27 (1.3%) received ARV prophylaxis and 906 (41.8%) were reportedly exclusively breastfed for the first 6 months of life. A total of 853 exposed infants accessed EID and had their results available. The median age at the time of the dried blood collection for EID was 7 weeks rangingd from 2 to 111 weeks; 27 (3.2%) were HIV positive of the 853 EID results. |
Abbreviations: ARV, antiretrovirals; EID, early infant diagnosis; IQR, interquartile range; PMTCT, prevention of mother-to-child transmission; sdNVP, single-dose nevirapine.
a This consists of zidovudine (AZT) 300 mg + nevirapine (NVP) twice a day.
b This is a highly active antiretroviral therapy comprised of AZT + lamivudine (3TC) + NVP.
c sdNVP is a single dose of NVP (200 mg) at the onset of labor.
dAuthors believe that this huge range is the reflection of the known huge turnaround time between the blood sample collection and the availability of results to the health providers.
The overall proportion of HIV-exposed infants LTFU prior to accessing early HIV diagnosis was remarkably high at 60% (1331/2220). That proportion of LTFU was higher for infants born to HIV-positive women at initial screening (60.6% or 1312/2165) compared to LTFU of infants born to mothers identified HIV positive at retesting (27.3% of 19/55).
Because such a large proportion of participants were LTFU, we could not run meaningful statistical analysis to compare MTCT rates between infants born to mothers tested HIV positive at their initial antenatal visit and those born to mothers retested HIV positive. Women who were 35 years of age or older, with a parity 4 or higher, and who came for ANC at 28 weeks of gestation or later were less likely to be retested for HIV during their pregnancy. We could not find a plausible explanation for that observation. The overall substantial LTFU is possibly the result of pregnant women not attending the number antenatal visits as recommended by the Zambian norms and guidelines.
Based on available data, we observed a higher proportion of MTCT among infants of mothers who retested HIV positive—11.1% (4/36)—than in infants born to mothers who had tested HIV positive at their initial visit—3.2% (27/853; see Table 2). We suggest that the difference in the MTCT rates is consistent with other studies, which found higher MTCT rates when HIV infection is acquired during pregnancy.3,9,18
The major limitation of our study is the huge proportion of LTFU throughout the cascade of the integrated PMTCT and MNCH. The small number of women that seroconverted (55) limited our capacity to assess the MTCT rates associated with incident HIV infection. We believe that this is a call to actively analyze the capacity of programs to implement HIV retesting and (for those found HIV positive) linkage to care, as one of the criteria for performance of PMTCT services.
Conclusion
In rural Zambia, in the 10 health facilities studied, retesting of previously HIV-negative women was offered but only 2 of 3 women received that service. Women who were 35 years of age or older, with a parity 4 or higher, and who came for ANC at 28 weeks of gestation or later were less likely to be retested for HIV during their pregnancy. Incidence of HIV infection observed was comparable to the known HIV incidence in Zambia (0.80 per 100 person-years).
With observed vertical transmission rates of 11.1% and 3.2% from mothers with “incident” and “non-incident” HIV infection, respectively, we suggest that studies be conducted to rigorously measure the proportion of MTCT attributable to women who seroconvert during pregnancy in program settings. We also suggest that PMTCT programs continue to offer retesting and thrive to improve its uptake. Efforts to reduce LTFU along the cascade of MNCH and PMTCT should also be considered to optimize PMTCT.
Acknowledgements
The authors would like to acknowledge Shelly Amieva and Paige Zaitlin for proof-reading and editing the manuscript.
Authors’ Note: Data can be made available upon request but is not included in the submission at this time. Per FHI 360 policy, it would require permission from the country office and the Zambia Prevention, Care and Treatment Partnership, which includes the Ministry of Health and the Ministry of Community Development and Mother and Child Health.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was made possible thanks to funding from FHI 360.
ORCID iD: Justin Mandala  https://orcid.org/0000-0002-3878-0693
https://orcid.org/0000-0002-3878-0693
References
- 1. United Nations International Children’s Emergency Fund. For Every Child, End AIDS—Seventh Stocktaking Report. New York, NY: UNICEF; 2016. [Google Scholar]
- 2. Joint United Nations Programme on HIV/AIDS. Global AIDS response progress reporting 2016 Geneva, Switzerland: UNAIDS; 2016. [Google Scholar]
- 3. Drake AL, Wagner A, Richardson B, John-Stewart G. Incident HIV during pregnancy and postpartum and risk of mother-to-child HIV transmission: a systematic review and meta-analysis. PLoS Med. 2014;11(2):e1001608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Moodley D, Esterhuizen T, Reddy L, et al. Incident HIV infection in pregnant and lactating women and its effect on mother-to-child transmission in South Africa. J Infect Dis. 2011;203(9):1231–1234. [DOI] [PubMed] [Google Scholar]
- 5. Morrison CS, Wang J, Van Der Pol B, Padian N, Salata RA, Richardson BA. Pregnancy and the risk of HIV-1 acquisition among women in Uganda and Zimbabwe. AIDS. 2007;21(8):1027–1034. [DOI] [PubMed] [Google Scholar]
- 6. De Schacht C, Mabunda N, Ferreira OC, et al. High HIV incidence in the postpartum period sustains vertical transmission in settings with generalized epidemics: a cohort study in Southern Mozambique. J Int AIDS Soc. 2014;17:18808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Feldblum PJ, Enosse S, Dube K, et al. HIV prevalence and incidence in a cohort of women at higher risk for HIV acquisition in Chókwè, southern Mozambique. PLoS One. 2014;9(5): e97547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. De Schacht C, Hoffman HJ, Mabunda N, et al. High rates of HIV seroconversion in pregnant women and low reported levels of HIV testing among male partners in Southern Mozambique: results from a mixed methods study. PLoS One. 2014;9(12): e115014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brenner BG, Roger M, Routy JP, et al. High rates of forward transmission events after acute/early HIV-1 infection. J Infect Dis. 2007;195(7):951–959. [DOI] [PubMed] [Google Scholar]
- 10. Ministry of Health Z. Zambia Consolidated Guidelines for Treatment and Prevention of HIV. Lusaka, Zambia: Ministry of Health Z; 2016. [Google Scholar]
- 11. Central Statistical Office Lusaka Zambia MoHLZ, University of Zambia Teaching Hospital Virology Laboratory Lusaka Zambia, University of Zambia Department of Population Studies Lusaka Zambia, Tropical Diseases Research Centre Ndola Zambia, The DHS Program ICF International Zambia Demographic and Health Survey 2013-14. Rockville, MD; 2015. [Google Scholar]
- 12. Ministry of Health Z. National Protocol Guidelines: Integrated Prevention of Mother to Child Transmission of HIV. Lusaka, Zambia: Ministry of Health Z; 2007. [Google Scholar]
- 13. Ministry of Health Z. Zambia Consolidated Guidelines for Treatment and Prevention of HIV Infection. Lusaka, Zambia: Ministry of Health Z; 2013. [Google Scholar]
- 14. Kinuthia J, Drake AL, Matemo D, et al. HIV acquisition during pregnancy and postpartum is associated with genital infections and partnership characteristics. AIDS. 2015;29(15):2025–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Moodley D, Esterhuizen TM, Pather T, Chetty V, Ngaleka L. High HIV incidence during pregnancy: compelling reason for repeat HIV testing. AIDS. 2009;23(10):1255–1259. [DOI] [PubMed] [Google Scholar]
- 16. Gray RH, Li X, Kigozi G, et al. Increased risk of incident HIV during pregnancy in Rakai, Uganda: a prospective study. Lancet. 2005;366(9492):1182–1188. [DOI] [PubMed] [Google Scholar]
- 17. Republic of Zambia. Zambia Country Report, Monitoring the Declaration of Commitment on HIV and AIDS and the Universal Access. Biennial Report Submitted to the United Nations General Assembly Special Session on HIV and AIDS National AIDS Council. March 31, 2014. Lusaka, Zambia: Republic of Zambia; 2014. [Google Scholar]
- 18. Patterson KB, Leone PA, Fiscus SA, et al. Frequent detection of acute HIV infection in pregnant women. AIDS. 2007;21(17):2303–2308. [DOI] [PubMed] [Google Scholar]