Abstract
Persons living with HIV (PLHIV) may experience disability. We compared disability among PLHIV in the United States and South Africa and investigated associations with health and demographic characteristics. Secondary analysis of cross-sectional data using medical records and questionnaires including the World Health Organization Disability Assessment Schedule (WHO-DAS) 2.0 12-item version (range: 0-36, with higher scores indicative of more severe disability). Between-country differences for the presence of disability were assessed with logistic regression and differences in severity using multiple regression. Eighty-six percent of US participants reported disability, compared to 51.3% in South Africa. The mean WHO-DAS score was higher in the United States (12.09 ± 6.96) compared to South Africa (8.3 ± 6.27). Participants with muscle pain, depression, or more years since HIV diagnosis were more likely to report disability. Being female or depressed was associated with more severity. Being adherent to anti-retroviral therapy (ART) and employed were associated with less severity. Because muscle pain and depression were predictive factors for disability, treatment of those problems may help mitigate disability in PLHIV.
Keywords: disability, pain, depression
What Do We Already Know about This Topic?
Functional limitation and disability may occur in persons living with HIV (PLHIV); some published studies have begun to explore risk factors for disability in PLHIV.
How Does Your Research Contribute to the Field?
This article is unique in that it compares associations between disability and health or demographic variables in PLHIV across 2 different countries.
What Are Your Research’s Implications toward Theory, Practice, or Policy?
Our findings help confirm that pain, depression, and number of years living with HIV are associated with disability and that these associations exist in cohorts from 2 different countries.
Introduction
HIV continues to be a serious global health issue. Worldwide, about 36.9 million people are living with HIV (PLHIV). Prevalence of HIV infection is 12.6% in South Africa1; in the United States (US) prevalence is 0.34%, with approximately 1.1 million individuals living with HIV.2 For those successfully treated with antiretroviral therapy (ART), HIV is a chronic condition and life expectancies are similar to that of the noninfected population.3 However, living with HIV can be accompanied by multimorbidity and side effects of medications that may have an adverse impact on quality of life and function.4,5 The International Classification of Functioning, Disability and Health (ICF) framework defines disability as a health outcome influenced by an individual’s health condition in the context of environmental and personal factors.6 Applying the ICF framework in the context of HIV may help to examine the nature and extent of disability experience and identify management strategies to address disability among PLHIV. The physical, cognitive, mental, and social health challenges that may occur in PLHIV and multimorbidity may manifest as episodic disability, characterized by unpredictable periods of wellness and illness over time.7,8 Episodes of disability may be exacerbated by extrinsic factors (such as stigma and lack of social support) and intrinsic contextual factors (such as aging and multimorbidity), further complicating the nature and extent of disability experienced by PLHIV.9
Multiple studies from across the globe reported higher rates of impaired physical function and frailty in PLHIV compared to individuals not living with HIV and noted that frailty in PLHIV is associated with longer duration of HIV infection and lower CD4 count.4 In a French study, locomotor function declined over time in PLHIV.10 A study in British Columbia reported high rates of activity limitations (81%) and participation restrictions (91%) in PLHIV.11 A study in the United Kingdom reported 38% of a sample of PLHIV had difficulty in usual activities.12 South African studies reported a high prevalence (62%-82%) of impairments across all body systems13,14 In another South African study, 36% of PLHIV reported moderate to severe functional limitations, with over 80% reportedly having experienced at least 1 impairment, activity limitation, or social participation restriction.15 Depression symptoms were also commonly reported among PLHIV and were associated with functional limitations.15,16 Functional limitations were associated with worse livelihood outcomes in people who experienced disability.17 These studies support the conclusions of a scoping review that found PLHIV experience a range of disability in hyperendemic countries.18 Similarly, a systematic review concluded that HIV is strongly linked to disability in sub-Saharan Africa, demonstrating the global impact of disability on overall health for PLHIV.19 Research on determinants of disability in PLHIV is an emerging area. In the United States, little has been published about disability and its determinants in PLHIV. One US study reported that advanced HIV disease is associated with worse physical function, and that age-associated comorbidity adversely affects physical function.20 Another US study reported that disability in the context of instrumental activities of daily living occurs frequently among middle-aged and older PLHIV and is associated with neurocognitive impairment, socioecomonic factors, and lifestyle factors.21 Furthermore, quality of life and lower limb function were more impaired in PLHIV and HIV-related peripheral neuropathy than in PLHIV without neuropathy.22
In the United States (a relatively high-income country), many individuals with disability receive government financial support. In South Africa (a relatively lower income country), the government disability grant is much smaller than the US version, and only those with moderate to severe disability receive monetary support.23 Although there are many other economic, health-care system and cultural differences between the United States and South Africa, both countries experienced improved access to ART in recent years. To our knowledge, no studies to date have compared functional limitation and disability among PLHIV across countries with disparate socioeconomics and HIV infection rates, such as the United States (a relatively higher income country) and South Africa (a relatively lower income country). Thus, there is a need to examine the nature and extent of disability among PLHIV across these 2 countries. A better understanding of disability may help to identify potential rehabilitation interventions in which to address disability across varying global contexts. The purpose of this study was to describe and compare self-reported functional limitation or disability among PLHIV in the United States and South Africa and to investigate whether certain health or demographic characteristics were associated with self-reported functional limitation or disability among PLHIV.
Methods
We conducted a secondary data analysis to assess disability (ranging from mild functional limitation to severe disability) in adults living with HIV using cross-sectional deidentified data from the South African HIV-Live study (N = 1016) and cross-sectional deidentified data from a US study (N = 127).16,24 In those studies, data were collected from adults living with HIV who attended ambulatory HIV clinics in Gauteng (South Africa) in 2014 to 2015 and Southern New Jersey (United States) in 2015, respectively. Participants were 18 years or older receiving ART at the time of enrollment. In the South African sample, participants did not have any other acute disease upon enrollment; in the US sample, individuals were excluded from the study if they had a current opportunistic infection, dementia, or an uncontrolled psychiatric disorder.
Self-Reported Disability
The WHO-DAS 2.0 questionnaire was used to assess self-reported disability during clinic visits. The WHO-DAS 2.0 is a widely used and validated questionnaire that measures severity of disability.25 In South Africa, investigators used the 12-item version of the WHO-DAS 2.0. In the United States, investigators used the full 36-item version of the WHO-DAS 2.0, from which a 12-item version score was extracted for our comparison. On the 12-item WHO-DAS 2.0, the weighted summary score range is from 0 to 36, with higher scores reflecting a greater number of activity limitations or more severe disability. Because our interest was to capture any level of functional limitation or disability, we considered a score of 1 or greater (i.e. a score of 1-36) to indicate the presence of disability. Although the WHO-DAS 2.0 is a disability measurement tool, there is no widely accepted cutoff score to discriminate between those with and those without disability. Thus, we used “disability” to refer to any score of 1 or greater, given that even a score of 1 is indicative of some degree of functional limitation.
Demographic and Clinical Characteristics
Demographic and clinical characteristic data were obtained from the medical record and study-specific questionnaires (Table 1). Comorbidities recorded in both South Africa and the US sites included tuberculosis, diabetes, depression, and muscle pain. In South Africa, depression was measured using the Center for Epidemiologic Studies Depression Scale; a score ≥10 indicated the presence of depression.26 In the United States, depression was measured using the Beck Depression Inventory II; a score ≥14 indicated the presence of depression.27 We included muscle pain and depression in the analysis because they were commonly measured in both sites and based on the theoretical consideration that pain and depression are comorbidities that have been demonstrated to be associated with disability.28-30 (see Table 1 for an overview of the data sources and variables available from both cohorts used in the analysis.)
Table 1.
Variables from United States and South Africa Samples of Adults Living with HIV Used in the Analysis.
| Variable of Interest | Scale | Source of data: United States | Source of data: South Africa |
|---|---|---|---|
| Demographic characteristics | |||
| Age, years | Ratio | Questionnaire and electronic medical record | Questionnaire |
| Sex (male, female) | Nominal | Questionnaire | Questionnaire |
| Employment status (employed, not employed) | Nominal | Questionnaire | Questionnaire |
| Clinical characteristics | |||
| Number of years since HIV diagnosis | Ratio | Questionnaire | Questionnaire |
| ART adherence (adherent, not adherent) | Nominal | Electronic medical record | Mannheimer Adherence Index |
| CD-4 count, cells/mm3 (most recent) | Ratio | Electronic medical record | Questionnaire and patient record |
| Comorbidities | |||
| Presence of depression | Nominal | Beck Depression Inventory-II | CES-D 10 |
| Presence of tuberculosis | Nominal | Questionnaire and electronic medical record | Questionnaire |
| Presence of diabetes | Nominal | Questionnaire and electronic medical record | Questionnaire |
| Presence of muscle pain | Nominal | Questionnaire | Questionnaire |
| Disability | |||
| Presence of self-reported disability | Nominal | WHODAS 2.0 (12-item extraction from full 36-item version) | WHODAS 2.0, 12 items |
| Severity of self-reported disability | Ratio | WHODAS 2.0 12-item extraction from full 36-item version) | WHODAS 2.0, 12 items |
Abbreviations: CES-D 10, Center for Epidemiologic Studies Depression Scale 10; WHODOS, World Health Organization Disability Assessment Schedule.
We compared demographic, comorbidity, and clinical characteristics between the 2 samples using bivariate statistics to identify potential confounding variables. We additionally compared countries on disability scores bivariately. Because the distribution of the WHO-DAS disability scores was bimodal (i.e. a high proportion of individuals without disability followed by normally distributed scores for those with any amount of disability), we created 2 separate models: one for the presence of any disability (WHO-DAS score = 0 versus WHO-DAS score >0) and another for the degree of severity (among those with WHO-DAS scores >0). We assessed between-country differences for the presence of disability (present versus not) using logistic regression, adjusting for covariates on which the 2 countries differed significantly (P < .05) and variables that were deemed theoretically important for understanding the presence or absence of disability, (i.e. muscle pain, depression, and adherence to ART).14,28-30 Among individuals who reported disability (as indicated by a score of 1 through 36 on the WHO-DAS 2.0 12-item), differences in the severity between the samples were assessed using multiple regression, adjusting for covariates as described above. Although we anticipated that the model would be robust against departures from normality given the sample size, because of the presence of right skew among the South African cohort, we reproduced the abovementioned model using robust estimators to confirm findings from the multiple regression. An a priori α was set at P < .05. Analyses were performed using SPSS 24.0.
Ethical Approval and Informed Consent
This study was approved by the Rutgers Institutional Review Board (IRB; Pro20160000897) and Stockton University IRB (2019051). As this study was a secondary analysis of deidentified data, informed consent was not required by the Rutgers IRB for study approval.
Results
Demographic, clinical characteristic, comorbidity, and disability descriptive data are summarized in Table 2. Compared to the United States, the South African sample had a greater proportion of female participants (72%, n = 732), while the US sample had a greater proportion of male participants (68%, n = 86). A higher proportion of participants were employed in South Africa compared to the United States (38.6%, n = 392 versus 24.6%, n = 31). Participants in South Africa were younger than in the United States (41.8 ± 8.3 years versus 49.6 ± 10.5 years). Concurrent diagnosis of diabetes was higher in the United States compared to South Africa (20.3%, n = 24 versus 2.4%, n = 24), while a diagnosis of tuberculosis was higher in South Africa compared to the United States (25.3%, n = 257 versus 0%, n = 0). Participants in the United States had higher average CD4 counts (629.13 ± 285.56 cells/mm3) compared to participants in South Africa (396.7 ± 263.71 cells/mm3). Given the statistically significant differences between samples (Table 2), we included age, sex, employment status, number of years since HIV diagnosis, diagnosis of diabetes, and diagnosis of tuberculosis in our subsequent regression models. Despite no statistically significant differences between samples, we included depression, muscle pain, and ART adherence in the model based on theoretical considerations that they influence disability.14,28-30 CD4 count, although statistically different between countries, was not included because the mean values were similar from a clinical perspective.
Table 2.
Comparison of Demographic Characteristics, Clinical Characteristics, and Self-Reported Disability Between South African and US Participants.
| South Africa | United States | ||||
|---|---|---|---|---|---|
| n | n (%) or Mean (SD)a | n | n (%) or Mean (SD) | P Valuea | |
| Demographic characteristics | |||||
| Female | 1016 | 732 (72.0%) | 126 | 40 (31.7%) | <.001 |
| Age, years | 1016 | 41.82 (8.28) | 126 | 49.60 (10.52) | <.001 |
| Employed | 1016 | 392 (38.6%) | 126 | 31 (24.6%) | .002 |
| Clinical characteristics | |||||
| Years since HIV diagnosis | 946 | 7.59 (4.59) | 121 | 15.07 (8.42) | <.001 |
| Diabetes | 1016 | 24 (2.4%) | 118 | 24 (20.3%) | <.001 |
| Tuberculosis | 1016 | 257 (25.3%) | 119 | 0 (0%) | <.001 |
| Depression | 1016 | 610 (60.0%) | 123 | 66 (53.7%) | .174 |
| Muscle pain | 1014 | 475 (46.8%) | 117 | 58 (49.6%) | .576 |
| Adherent to ARTb | 1016 | 876 (86.2%) | 121 | 111 (91.7%) | .09 |
| CD4 count, cells/mm3c | 959 | 396.70 (263.71) | 122 | 629.13 (285.56) | <.001 |
| WHO-DAS 2.0 12-item score | |||||
| Presenced (unadjusted) | 1016 | 521 (51.3%) | 127 | 110 (86.6%) | <.001 |
| Severitye,f (unadjusted mean) | 1016 | 8.3 (6.27) | 127 | 12.09 (6.96) | <.001 |
Abbreviations: ART, antiretroviral therapy; CES-D 10, Center for Epidemiologic Studies Depression Scale 10; SD, standard deviation; WHODOS, World Health Organization Disability Assessment Schedule.
aCountry differences for categorical variables computed using χ2 test. Differences for age and self-reported years with HIV computed using independent t tests. Difference for CD4 count computed using Mann-Whitney U test. Difference for disability presence computed using χ2. Difference for disability severity computed using independent t test.
bIn United States, adherent if electronic medical record suggested 90% or higher adherence to ART; in South Africa, adherent if score on CASE Adherence Index score ≥10.31
cFor CD4 counts, values are reported as median (interquartile range).
dDichotomous variable (WHODAS 2.0 12-item score of 0 indicative of no disability; score of 1 or >1 indicative of disability).
eScore from WHODAS 2.0 12-item (range: 0-36, higher scores indicative of more severe disability).
fSeverity scores reported for participants who had a presence of disability (scored ≥1 out of 36 on the WHODAS 2.0 12-item).
Presence of Disability
Presence of disability was 51.3% in South Africa and 86.6% in the US sample. This means that, in the unadjusted model, participants in the United States were 6.15 (95% confidence interval [CI]: 3.46-10.40) times more likely to report some degree disability than participants in South Africa (P < .001; Table 3, unadjusted model). After adjusting for country and demographic, comorbidity, and clinical characteristics (age, sex, employment status, ART adherence, years since HIV diagnosis, tuberculosis, diabetes, depression, and muscle pain; Table 3, adjusted model), presence of disability was 9.76 (95% CI: 4.91-19.41) times higher in the US sample compared to the South African sample. In the adjusted model, females were 61% (odds ratio [OR] = 1.61, 95% CI: 1.19-2.18) more likely to report disability compared with males. Each additional year since HIV diagnosis (i.e. living longer with HIV infection) resulted in a 4.0% (OR = 1.04, 95% CI: 1.01-1.06) increase in the odds of disability. Participants with muscle pain were 82% (OR = 1.82, 95% CI = 1.39-2.39) more likely to report disability compared to those without muscle pain, while individuals with depression were 67% (OR = 1.67, 95% CI = 1.27-2.20) more likely to report disability compared to those without depression (Table 3).
Table 3.
Unadjusted and Adjusted Models of Presence of Disability as Measured by the WHODAS 2.0.
| Odds Ratio | Unadjusted Model | P Value | Odds Ratio | Adjusted Model | P Value | |||
|---|---|---|---|---|---|---|---|---|
| 95% CI for Odds Ratio | 95% CI for Odds Ratio | |||||||
| Lower | Upper | Lower | Upper | |||||
| Country: United Statesa | 6.15 | 3.64 | 10.40 | <.001 | 9.76 | 4.91 | 19.41 | <.001 |
| Sex: femaleb | - | - | - | - | 1.61 | 1.19 | 2.18 | .002 |
| Age: each additional year older | - | - | - | - | 0.99 | 0.98 | 1.01 | .377 |
| Employment status: employed | - | - | - | - | 0.84 | 0.64 | 1.11 | .221 |
| Years since HIV diagnosisc | - | - | - | - | 1.04 | 1.01 | 1.06 | .016 |
| Diabetes | - | - | - | - | 1.38 | 0.63 | 3.02 | .428 |
| Tuberculosis | - | - | - | - | 1.29 | 0.94 | 1.76 | .110 |
| Depressiond | - | - | - | - | 1.67 | 1.27 | 2.20 | <.001 |
| Muscle paine | - | - | - | - | 1.82 | 1.39 | 2.39 | <.001 |
| Adherent to ART | - | - | - | - | 0.69 | 0.48 | 1.01 | .058 |
Abbreviations: ART, antiretroviral therapy; CI, confidence interval; WHODOS, World Health Organization Disability Assessment Schedule.
aUS participants: 6.15× greater risk of disability (unadjusted model); 9.76× greater risk of disability (adjusted model).
bFemale: 61% more likely than male to report disability (adjusted model).
cYears since HIV diagnosis (continuous variable), increased odds of reporting disability of 4% for each additional year (adjusted model).
dIf depressed, 67% more likely to experience disability (adjusted model).
eIf muscle pain, 82% more likely to experience disability (adjusted model).
The boldface values are those that are <0.05, i.e. those that were significant.
Severity of Disability
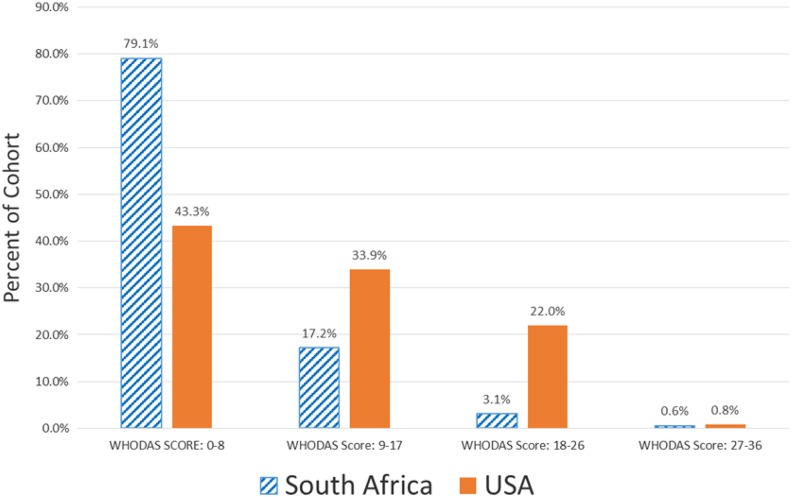
Participants’ WHO-DAS 2.0 scores ranged from 0 to 31 in South Africa and 0 to 29 in the United States. The distribution of WHO-DAS quartile scores by country is presented in Figure 1. In both countries, the largest proportion of participants had scores in 0 to 8 range (indicative of relatively low disability), while the smallest proportion of participants had scores in the 27 to 36 range (indicative of relatively greater disability). Among those who scored a 1 or higher on the WHO-DAS 2.0 (South Africa: n = 521, US: n = 110), the mean WHO-DAS score was significantly (P < .001) higher in the United States (12.09 ± 6.96) compared to South Africa (8.3 ± 6.27). Severity of disability was 4.23 points (95% CI: 2.47-5.99) or 11.6% (on a 0 to 36 point scale), worse in the United States in the adjusted model (Table 4). Being female or depressed was associated with reporting more severe disability (1.56 or 4.3% and 4.31 points or 12.0%, respectively). Being adherent to ART and employed were associated with less severe disability (ART adherence: 1.8 points or 5% less if adherent to ART; employment status: 1.28 points or 3.5% less if employed). The same model, run with robust estimation procedures, yielded the same results and thus is not reported here.
Figure 1.
Distribution of World Health Organization Disability Assessment Schedule (WHODAS) score quartiles by country.
Table 4.
Unadjusted and Adjusted Models for Severity of Disability as Measured by the WHODAS 2.0.
| β Coefficient | Unadjusted Model | P Value | β Coefficient | Adjusted Model | P Value | |||
|---|---|---|---|---|---|---|---|---|
| 95% CI for β | 95% CI for β | |||||||
| Lower | Upper | Lower | Upper | |||||
| Country: United Statesa | 3.79 | 2.47 | 5.11 | <.001 | 4.23 | 2.47 | 5.99 | <.001 |
| Sex: femaleb | – | – | – | – | 1.56 | 0.38 | 2.75 | .010 |
| Age: each additional year older | – | – | – | – | 0.02 | −0.04 | 0.08 | .451 |
| Employment status: employedc | – | – | – | – | −1.28 | −2.38 | −0.17 | .024 |
| Years since HIV diagnosis | – | – | – | – | 0.02 | −0.08 | 0.12 | .675 |
| Diabetes | – | – | – | – | −0.79 | −3.02 | 1.45 | .490 |
| Tuberculosis | – | – | – | – | −0.06 | −1.30 | 1.19 | .928 |
| Depressiond | – | – | – | – | 4.31 | 3.20 | 5.41 | <.001 |
| Muscle pain | – | – | – | – | 0.56 | −0.50 | 1.62 | .298 |
| Adherent to ARTe | – | – | – | – | −1.80 | −3.23 | −0.38 | .013 |
Abbreviations: ART, antiretroviral therapy; β, beta-coefficient; WHODOS, World Health Organization Disability Assessment Schedule.
aUS participants: mean of 3.79 worse WHODAS scores (unadjusted model) compared to South Africa; mean of 4.23 worse WHODAS scores (adjusted model) compared to South Africa.
bFemale: 1.56 points worse WHODAS score than males (adjusted model).
cEmployed: 1.28 points lower on disability score (i.e. less disability; adjusted model).
dDepressed: 4.31 points worse WHODAS score (adjusted model).
eAdherent to ART: 1.8 points less on WHODAS score (i.e. less disability; adjusted model).
The boldface values are those that are <0.05, i.e. those that were significant.
Discussion
To our knowledge, this is the first study to compare the presence and severity of disability among PLHIV in high and low-middle income countries. The differences between samples in mean age, presence of diabetes and tuberculosis, sex distribution, and employment status may be related to differences in the trajectory of the disease over time, differences in cultural, psychosocial, economic, and environmental factors, and differences in access to health care and health-care policy in South Africa and the United States.
Despite the differences in demographic characteristics, clinical characteristics, and comorbidities, a large proportion of participants in both countries (South Africa and the United States) reported experiencing disability. The presence of disability was more frequently reported in the US sample. Similarly, severity of disability was greater in the US sample compared to the South African sample. Comorbidities of depression and muscle pain were frequently reported in both samples. In the combined sample, depression, muscle pain, being female, and living longer with an HIV diagnosis were factors in predicting the presence of disability. Depression and being female were also important factors in the severity of disability.
Some of the variance in disability in our combined sample may be explained by variables not included in this analysis, such as education level, income level, availability of resources, exposure to stressful or traumatic events, other health-related symptoms, social support, lifestyle factors, ART regiments, and length of time on ART. Sex as a predictor of disability presence and severity in our combined sample should be viewed with caution because of the higher number of women in the South African and combined sample. Our analysis was limited to variables that were measured in both countries. Hence, we were able to consider the presence of tuberculosis, diabetes, depression, and muscle pain. Pain from sources other than muscle, such as peripheral nerve or joint, may be factors in disability but was not considered in our analysis. The common data set did not provide information on comorbidities other than those analyzed, and other comorbidities not explored here might play a role in disability. We did not consider the length of time since the onset of disability.
Between-country differences may have impacted disability presence and severity, and thus any inferences about the proportion of disability presence or severity between countries should be made with caution. The between-country differences in the presence and severity of disability may, at least in part, be due to different perceptions and lived experiences and a wide array of factors, including cultural, lifestyle, psychosocial, economic, resource, education, ART regiments, length of time on ART, and health policy differences. For example, 62.4% of participants in the United States were receiving federal government disability benefits. Although some benefits exist through general disability grants in South Africa, they are less accessible and more arduous to acquire.32 It is possible that the US lifestyle is more likely to be sedentary compared to South Africa and that this could affect the perception of functional ability. In addition, there are likely cultural differences in responses to symptoms, the perception of ability or disability, and in resiliency to health challenges.33 South Africans might be more likely to accept certain hardships as “normal” and/or might be more resilient when dealing with health challenges. It is possible that those in the US sample had greater access to transportation services, and thus those in the US sample with more severe disability were more able to travel to the clinic. In addition, there were more men in the US sample, where the most common source of infection is men having sex with men. In South Africa, where the most common route of infection is heterosexual sex, there were more women in the sample. Between-country differences in societal gender roles and social roles may have contributed to the between-country differences in disability presence and severity.
Questions on the WHO-DAS 2.0 might be interpreted differently in the US and South Africa, leading to some instability in measurement properties. Thus, true differences in actual ability or disability among PLHIV between countries may not be as disparate as our data suggest. Our finding of 51.3% for the presence of disability in the South African sample is slightly higher than the value reported by Myezwa et al. in 2016 because we included individuals with a WHO-DAS score of 1, while Myezwa et al. only included those with scores of 2 or higher in their calculation.16 Nevertheless, our results suggest that in both countries, disability is common and is linked to depression and muscle pain among PLHIV in this study. The WHO-DAS 2.0 scores of participants suggested a wide range of disability severity. Our operational definition of disability was a WHO-DAS 12-item score of 1 or higher, and thus, our presence values are inclusive of those with mild as well as severe forms of disability. While there were differences in the presence and severity of disability between countries, presence was high (>50%) in both countries, and the range of WHO-DAS scores suggest that some participants had severe disability. Our data cannot determine the relative proportions of those with clinically important disability. The cross-sectional nature of the study does not allow for us to determine causal inferences between the predictor variables and disability. We cannot infer that the association between depression and pain is unidirectional. More likely, there could be a bidirectional relationship between these factors.
Our findings are consistent with reports of higher prevalence of disability among females in the general population and a greater risk of disability in persons with depression34,35 and with several South African studies that have found associations between depression and disability.14-16 In South African studies, age, sex, length of time on ART, adherence, number of health symptoms, exposure to shocks, mental health, participation, and getting along were factors in predicting disability.14-16
In our study, the high prevalence of depression in participants in both countries suggests that mental health issues including depression should be assessed and treated through appropriate referrals and psychologically informed rehabilitation interventions.36 Treatment of pain, such as muscle pain, should also be emphasized, as pain appeared to be a strong factor in the presence of disability in our study. Other studies also have linked pain to impaired physical function in PLHIV.37 In addition, research has revealed that the experience of pain is increasing with time on ART and that neglecting pain management can adversely impact ART adherence.38 Treatment of depression and pain may be helpful in combating the degree of perceived disability in PLHIV.39 Further research is needed to determine whether treatment of pain and depression can help mitigate activity limitations and disability and improve quality of life among PLHIV or if exposure to rehabilitation interventions can improve pain and depression outcomes. Identification of pain, depression, and functional limitations early in the trajectory of HIV infection should be included in a comprehensive assessment to gain a better understanding of specific health-related needs of PLHIV that extend beyond CD4 count and viral load.
A recently published framework describing the role of physical therapy in the rehabilitation of PLHIV to address disability includes multidimensional, client-centered roles of addressing physical, psychological, and social health domains and contextual factors including aging, comorbidities, competing priorities, continuity of care, stigma, resource security, and social isolation.40 Rehabilitation professionals may use this framework to inform their approach in providing client-centered HIV care that centers on a holistic and long-term approach.41 Future research on HIV and disability in a global context should include a rich and inclusive array of key variables and explore more nuanced domains of disability including sensory functioning, cognition, mobility, self-care, interpersonal relationships, household functions, work, and participation in society. In addition, research on disability with PLHIV should consider that disability may be episodic in nature,42 as described by Solomon et al., who found that disability may increase, decrease, remain stable, or fluctuate over time in PLHIV.8 When treating individuals, appreciation for their episodic trajectory of disability can inform interventions geared to mitigating worsening of disability or frequency of episodes. Given the cross-sectional nature of our study, we were unable to determine the episodic nature of disability over time; hence, this is an area of further study.
Limitations of this study included our unbalanced sample size of participants between countries and lack of an uninfected control group. Because we used data from the 12-item version of the WHO-DAS 2.0, more granular data about specific domains of disability were not available in this secondary analysis. The South African sample was much larger and thus less prone to sampling bias. Both countries used samples of convenience as determined by participants reporting to specific HIV clinics for their care. Thus, these results may not be generalizable to other parts of the countries or continents but might rather reflect disability more regionally (Southern New Jersey and Gauteng).
Conclusions
Disability presence and severity exists among PLHIV in both US and South Africa. In both contexts, muscle pain, depression, and numbers of years since HIV diagnosis were predictive factors for the presence of disability. Greater severity of disability was associated with depression, while less severity of disability was associated with ART adherence and employment. Rehabilitation, specifically physical therapy, has a role in identifying and addressing pain and depression to help mitigate disability experienced by PLHIV.
Acknowledgments
Medical Research Council (MRC), South Africa; Rutgers—School of Health Professions—Dean’s Office; University of Witwatersrand—School of Therapeutic Sciences; Garden State Infectious Disease Associates (Kennedy Health System), Voorhees, New Jersey; Graduate Research Assistants: Quang Tran, Megan Meszaros, and Adam Huynh (Rutgers); Jocelyn Bixler and Lauren Fitzpatrick (Stockton University); Adedayo Tunde Ajidahun (University of Witwatersrand).
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Kelly O’Brien is supported by a Canada Research Chair in Episodic Disability and Rehabilitation.
ORCID iD: David Kietrys  https://orcid.org/0000-0002-8032-478X
https://orcid.org/0000-0002-8032-478X
References
- 1. Statistics South Africa. 2017. ww.statssa.gov.za/?m=2017 Accessed May 2019.
- 2. CDC.gov. HIV Surveillance Report. 2016. https://www.cdc.gov/hiv/library/reports/hiv-surveillance.html. Accessed May 2019
- 3. Samji H, Cescon A, Hogg RS, et al. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One. 2013;8(12):e81355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Erlandson KM, Allshouse AA, Jankowski CM, Mawhinney S, Kohrt WM, Campbell TB. Relationship of physical function and quality of life among persons aging with HIV infection. AIDS. 2014;28(13):1939–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Erlandson KM, Schrack JA, Jankowski CM, Brown TT, Campbell TB. Functional impairment, disability, and frailty in adults aging with HIV-infection. Curr HIV/AIDS Rep. 2014;11(3):279–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stucki G. International Classification of Functioning, Disability, and Health (ICF): a promising framework and classification for rehabilitation medicine. Am J Phys Med Rehabil. 2005;84(10):733–740. [DOI] [PubMed] [Google Scholar]
- 7. O’Brien KK, Bayoumi AM, Strike C, Young NL, Davis AM. Exploring disability from the perspective of adults living with HIV/AIDS: development of a conceptual framework. Health Qual Life Outcomes. 2008;6:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Solomon P, O’Brien KK, Nixon S, Letts L, Baxter L, Gervais N. Trajectories of episodic disability in people aging with HIV: a longitudinal qualitative study. J Int Assoc Provid AIDS Care. 2018;17:2325958218759210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. O’Brien KK, Davis AM, Strike C, Young NL, Bayoumi AM. Putting episodic disability into context: a qualitative study exploring factors that influence disability experienced by adults living with HIV/AIDS. J Int AIDS Soc. 2009;12(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Richert L, Brault M, Mercie P, et al. Decline in locomotor functions over time in HIV-infected patients. AIDS. 2014;28(10):1441–1449. [DOI] [PubMed] [Google Scholar]
- 11. Rusch M, Nixon S, Schilder A, Braitstein P, Chan K, Hogg RS. Prevalence of activity limitation among persons living with HIV/AIDS in British Columbia. Can J Public Health. 2004;95(6):437–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Harding R, Clucas C, Lampe FC, et al. What factors are associated with patient self-reported health status among HIV outpatients? A multi-centre UK study of biomedical and psychosocial factors. AIDS Care. 2012;24(8):963–971. [DOI] [PubMed] [Google Scholar]
- 13. Myezwa H, Stewart A, Musenge E, Nesara P. Assessment of HIV-positive in-patients using the international classification of functioning, disability and health (ICF) at Chris Hani Baragwanath Hospital, Johannesburg. Afr J AIDS Res. 2009;8(1):93–105. [DOI] [PubMed] [Google Scholar]
- 14. Myezwa H, Hanass-Hancock J, Ajidahun AT, Carpenter B. Disability and health outcomes–from a cohort of people on long-term antiretroviral therapy. SAHARA-J. 2018;15(1):50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hanass-Hancock J, Myezwa H, Carpenter B. Disability and living with HIV: baseline from a cohort of people on long term ART in South Africa. PLoS One. 2015;10(12):e0143936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Myezwa H, Hanass-Hancock J, Pautz N, Smith R, Carpenter B. Investigating the interaction between disability and depressive symptoms in the era of widespread access to ART. J AIDS Clin Res. 2016;7(7). [Google Scholar]
- 17. Hanass-Hancock J, Misselhorn A, Carpenter B, Myezwa H. Determinants of livelihood in the era of widespread access to ART. AIDS Care. 2017;29(1):32–39. [DOI] [PubMed] [Google Scholar]
- 18. Hanass-Hancock J, Regondi I, Van Egeraat L, Nixon S. HIV-related disability in HIV hyper-endemic countries: a scoping review. World J AIDS. 2013;3(03):257. [Google Scholar]
- 19. Banks LM, Zuurmond M, Ferrand R, Kuper H. The relationship between HIV and prevalence of disabilities in sub-Saharan Africa: systematic review. Trop Med Int Health. 2015;20(4):411–429. [DOI] [PubMed] [Google Scholar]
- 20. Oursler KK, Goulet JL, Crystal S, et al. Association of age and comorbidity with physical function in HIV-infected and uninfected patients: results from the veterans aging cohort study. AIDS patient care and STDs. 2011;25(1):13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Johs NA, Wu K, Tassiopoulos K, et al. Disability among middle-aged and older persons with human immunodeficiency virus infection. Clin Infect Dis. 2017;65(1):83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Galantino ML, Kietrys DM, Parrott JS, Stevens ME, Stevens AM, Condoluci DV. Quality of life and self-reported lower extremity function in adults with HIV-related distal sensory polyneuropathy. Phys Ther. 2014;94(10):1455–1466. [DOI] [PubMed] [Google Scholar]
- 23. Hanass-Hancock J, McKenzie TC. People with disabilities and income-related social protection measures in South Africa: where is the gap? Afr J Disabil. 2017;6:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kietrys DM, Galaniton ML, Parrott JS, et al. Predictors of self-reported disability in people living with HIV. Paper presented at: 8th International Workshop on HIV & Aging; New York, NY; 2017. [Google Scholar]
- 25. Üstün TB, Chatterji S, Kostanjsek N, et al. Developing the World Health Organization disability assessment schedule 2.0. Bull World Health Organ. 2010;88(11):815–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang W, O’Brien N, Forrest JI, et al. Validating a shortened depression scale (10 item CES-D) among HIV-positive people in British Columbia, Canada. PLoS One. 2012;7(7):e40793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Segal DL, Coolidge FL, Cahill BS, O’Riley AA. Psychometric properties of the Beck Depression Inventory—II (BDI-II) among community-dwelling older adults. Behav Modif. 2008;32(1):3–20. [DOI] [PubMed] [Google Scholar]
- 28. Garbi Mde O, Hortense P, Gomez RR, da Silva Tde C, Castanho AC, Sousa FA. Pain intensity, disability and depression in individuals with chronic back pain. Rev Lat Am Enfermagem. 2014;22(4):569–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Campbell P, Hope K, Dunn KM. The pain, depression, disability pathway in those with low back pain: a moderation analysis of health locus of control. J Pain Res. 2017;10:2331–2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Probst T, Neumeier S, Altmeppen J, Angerer M, Loew T, Pieh C. Depressed mood differentially mediates the relationship between pain intensity and pain disability depending on pain duration: a moderated mediation analysis in chronic pain patients. Pain Res Manag. 2016;2016:3204914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mannheimer S, Mukherjee R, Hirschhorn L, et al. The CASE adherence index: a novel method for measuring adherence to antiretroviral therapy. AIDS Care. 2006;18(7):853–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Govender V, Fried J, Birch S, Chimbindi N, Cleary S. Disability Grant: a precarious lifeline for HIV/AIDS patients in South Africa. BMC Health Serv Res. 2015;15(1):227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Earnshaw VA, Bogart LM, Dovidio JF, Williams DR. Stigma and racial/ethnic HIV disparities: moving toward resilience. Am Psychol. 2013;68(4):225–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Leveille SG, Resnick HE, Balfour J. Gender differences in disability: evidence and underlying reasons. Aging (Milano). 2000;12(2):106–112. [DOI] [PubMed] [Google Scholar]
- 35. Penninx BW, Leveille S, Ferrucci L, van Eijk JT, Guralnik JM. Exploring the effect of depression on physical disability: longitudinal evidence from the established populations for epidemiologic studies of the elderly. Am J Public Health. 1999;89(9):1346–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wilson S, Chaloner N, Osborn M, Gauntlett-Gilbert J. Psychologically informed physiotherapy for chronic pain: patient experiences of treatment and therapeutic process. Physiotherapy. 2017;103(1):98–105. [DOI] [PubMed] [Google Scholar]
- 37. Merlin JS, Westfall AO, Chamot E, et al. Pain is independently associated with impaired physical function in HIV-infected patients. Pain Med. 2013;14(12):1985–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Farrant L, Gwyther L, Dinat N, Mmoledi K, Hatta N, Harding R. Maintaining wellbeing for South Africans receiving ART: the burden of pain and symptoms is greater with longer ART exposure. S Afr Med J. 2014;104(2):119–123. [DOI] [PubMed] [Google Scholar]
- 39. Merlin JS, Selwyn PA, Treisman GJ, Giovanniello AG. Chronic Pain and HIV: A Pracical Approach. West Sussex, England: Wiley Blackwell; 2016. [Google Scholar]
- 40. deBoer H, Andrews M, Cudd S, et al. Where and how does physical therapy fit? Integrating physical therapy into interprofessional HIV care [published online ahead of print March 13, 2018]. Disabil Rehabil. 2018. [DOI] [PubMed] [Google Scholar]
- 41. Nixon SA, Bond V, Solomon P, et al. Optimism alongside new challenges: using a rehabilitation framework to explore experiences of a qualitative longitudinal cohort of people living with HIV on antiretroviral treatment in Lusaka, Zambia. AIDS Care. 2018;30(3):312–317. [DOI] [PubMed] [Google Scholar]
- 42. O’Brien KK, Hanna S, Gardner S, et al. Validation of the Episodic Disability Framework with adults living with HIV. Disabil Rehabil. 2014;36(4):319–329. [DOI] [PubMed] [Google Scholar]