Abstract
Objective:
To examine HIV viral suppression during/after pregnancy.
Design:
Prospective observational cohort.
Methods:
We identified pregnancies from 1996 to 2015. We examined HIV RNA viral load (VL), VL suppression (≤500 copies/mL), and antiretroviral therapy (ART) status at pregnancy start, end, and 6 months postpartum. We estimated risk ratios (RRs) and 95% confidence intervals (CIs) for VL nonsuppression.
Results:
Among 253 pregnancies analyzed, 34.8% of women exhibited VL suppression at pregnancy start, 60.1% at pregnancy end, and 42.7% at 6 months postpartum. Median VL (log10 copies/mL) was 2.80 (interquartile range [IQR]: 1.40-3.85) at pregnancy start, 1.70 (IQR: 1.40-2.82) at pregnancy end, and 2.30 (IQR: 1.40-3.86) at postpartum. Risk of postpartum VL nonsuppression was also lower among women on ART and with VL suppression at pregnancy end (versus those not; adjusted RR = 0.30, 95% CI: 0.17-0.53).
Conclusions:
Maintaining VL suppression among US women remains a challenge, particularly during postpartum. Achieving VL suppression earlier during pregnancy benefits women subsequently.
Keywords: HIV, postpartum, pregnancy, viral suppression
Background
In the United States, an estimated 246 372 women were living with diagnosed HIV infection at the end of 2014 and 8328 women were newly diagnosed with HIV in 2014.1 This represents approximately one-fifth of the burden of HIV in the United States. The highest HIV diagnosis rate (9.5 diagnoses per 100 000 persons) was among women aged 25 to 34 years1; in general, women in this age range give birth to 56% of babies born in the United States.2 The number of HIV-infected women giving birth annually in the United States is not regularly determined; however, it was estimated that 8700 HIV-infected women delivered in 2006.3 HIV can be transmitted from mother to child during pregnancy, labor and delivery, and breastfeeding.
The US HIV treatment guidelines and the management of HIV-infected pregnant women have evolved over time to reduce mother-to-child transmission (MTCT) of HIV.4 In 1994, the Pediatric AIDS Clinical Trial Group 076 clinical trial demonstrated that provision of the antiretroviral (ARV) drug zidovudine (ZDV) to pregnant women and their infants reduced MTCT by 68%.5 Within months, the Centers for Disease Control and Prevention (CDC) and US Public Health Service issued guidelines recommending ZDV for pregnant women to prevent MTCT of HIV.6 These guidelines for pregnant women were updated in 1998 to shift from recommending ZDV perinatally to prevent transmission to the child to recommending ARV drugs more broadly (and in combination) perinatally and beyond to both prevent transmission to the child and treat the mother. Similarly, CDC guidelines for HIV testing shifted from recommending voluntary counseling and testing for high-risk pregnant women in 19856 to the routine offer of voluntary counseling and testing for all pregnant women in 19957 and to opt-out testing with inclusion of HIV in the standard testing panel for all pregnant women informally in 20038 and formally in 2006.9
Accordingly, in the United States, from 2005 to 2008, 84% of HIV-infected pregnant women received antiretroviral therapy (ART) prenatally and 85% of women received ART during intrapartum.10 The aim of ART is HIV RNA viral load (VL) suppression such that HIV is undetectable by standard laboratory testing. A recent clinical trial conducted among HIV-infected pregnant women newly initiated on ART from 2002 to 2011 found that 87% had VL suppression at delivery.11,12 Similarly, 79% of HIV-infected women in US Enhanced Perinatal Surveillance (EPS) had VL suppression at delivery.10 In contrast, a recent analysis of EPS data from Philadelphia, Pennsylvania from 2005 to 2011 found that 51% of women had VL suppression at delivery and 31% of women had VL suppression at 1 year postpartum.13 These findings suggest that VL suppression at delivery and postpartum in nonclinical trial settings may be challenging; however, it is unclear whether this represents clinical practice more broadly in the United States.
We examined VL suppression at pregnancy start, pregnancy end, and postpartum among geographically diverse populations of HIV-infected women in clinical care in the United States from 1996 to 2015.
Methods
Study Population
As previously described, the HIV Outpatient Study (HOPS) is a prospective cohort study of over 10 000 HIV-infected adults followed in routine care.14 We analyzed data from HOPS participants seen during 1996 to 2015 at 10 clinics across 8 cities in the United States: Tampa, Florida; Washington, DC; Denver, Colorado (2 sites); San Leandro, California; Chicago, Illinois (2 sites); Stony Brook, New York; Philadelphia, Pennsylvania; and Oakland, California. Data on demographics, risk factors for HIV infection, clinical diagnoses, medication prescriptions, laboratory testing, hospitalizations, and mortality are abstracted from outpatient charts at each visit and entered in an electronic HOPS database. Information was compiled centrally and reviewed and edited before analysis. The HOPS protocol has been approved and renewed annually by the ethical review boards of each participating institution and the CDC. All study participants provided written, informed consent.
We searched the HOPS database for the diagnostic code for pregnancy and corresponding start and end dates to identify all unique pregnancies that occurred between January 1, 1996, and December 2015. Pregnancy diagnosis start and end dates were used to define pregnancy start and pregnancy end; 6 months postpartum was calculated by adding 180 days to pregnancy end date. Main outcomes of interest were VL suppression at pregnancy end and at 6 months postpartum; therefore, we limited our analysis to pregnancies among women who were alive and under observation in the HOPS at both of these time points.
Procedures
We defined VL suppression as HIV RNA plasma levels ≤500 copies/mL to account for variable lower limits of detection of commercially available VL tests widely used during the early calendar period included in our analysis; VLs >500 copies/mL or missing were categorized as unsuppressed, consistent with analyses of data in national surveillance.15 Viral loads that were undetectable were set to the value of lower limit of detection specified for the test used. Viral load at pregnancy start, pregnancy end, and 6 months postpartum was defined as the value obtained within ±90 days and closest to each time point. CD4 T-lymphocyte count/mm3 at each time point was defined similarly. We reviewed prescription start and stop dates to identify the ART regimen used prior to and most proximal to each time point. Baseline participant characteristics considered were maternal age at pregnancy start, self-reported race/ethnicity, education level at enrollment in HOPS, insurance status at pregnancy start, year of pregnancy start, time from HIV diagnosis to pregnancy start, CD4 at pregnancy start, and being on ART and virally suppressed at pregnancy start and pregnancy end.
Statistical Methods
The unit of analysis was pregnancy, with the possibility of inclusion of multiple pregnancies within the same woman. Across the 3 time points (pregnancy start, pregnancy end, and 6 months postpartum), we compared median VL value and proportion of pregnancies among women with VL suppression, stratified by time period of pregnancy start (1996-2004 versus 2005-2015), to explore temporal trends. We also conducted bivariate analyses of the proportion of pregnancies with VL suppression by baseline characteristics. Categorical and continuous variables are presented as proportions and medians with interquartile ranges (IQRs), respectively. Differences between proportions and medians were compared using χ2 and Kruskal-Wallis tests, respectively.
We used modified Poisson regression with generalized estimating equations to estimate risk ratios (RRs) and corresponding 95% confidence intervals (CIs) that account for the correlation between pregnancies within the same woman. Crude, full, and reduced regression models for each baseline characteristic were used to identify potential risk factors for VL nonsuppression at pregnancy end and at 6 months postpartum. Full models were adjusted for all other baseline characteristics as potential confounders. Reduced models for each covariate were adjusted only for factors resulting in ≥10% change in the fully adjusted estimate using a backward elimination method, per purposeful selection of covariates as described by Hosmer and Lemeshow.16,17 An α of .05 was used for statistical testing. All analyses were performed using SAS statistical software, version 9.3 (SAS Institute, Cary, North Carolina).
Results
Baseline Characteristics of Pregnancies
As of December 30, 2015, there were 474 documented pregnancies in HOPS; 338 had a pregnancy start date on or after January 1, 1996, and had a pregnancy end date available (102 pregnancies missing end dates were excluded). Of these, 253 pregnancies were among 176 unique women alive and in HOPS at both pregnancy end and 6 months postpartum; these pregnancies were included in our analysis. The median duration of pregnancy in our study was 8.0 months (IQR: 4.6-9.0) and 48 (19.0%) of pregnancies were <90 days duration.
About half of analyzed pregnancies occurred from 1996 to 2004 (n = 134, 53%); the remaining pregnancies occurred from 2005 to 2015 (n = 119, 47%). The median age of women at pregnancy start was 30 years (IQR: 25-34). Most pregnancies occurred among women who were non-Hispanic black (66.4%), publicly insured (80.2%), and at least high school educated (76.7%). The median time from HIV diagnosis to pregnancy start was 3.4 years (IQR: 1.0-6.6).
Viral Suppression at Pregnancy Start, Pregnancy End, and Postpartum
Among the 253 pregnancies, 184 (72.7%) had a VL documented at pregnancy start, 211 (83.4%) had a VL documented at pregnancy end, and 188 (74.3%) had a VL documented at 6 months postpartum. Viral load documentation was less complete in 1996 to 2004 compared to 2005 to 2015 (67.2% versus 79.0% at pregnancy start, 77.6% versus 89.9% at pregnancy end, and 73.1% versus 75.6% at 6 months postpartum).
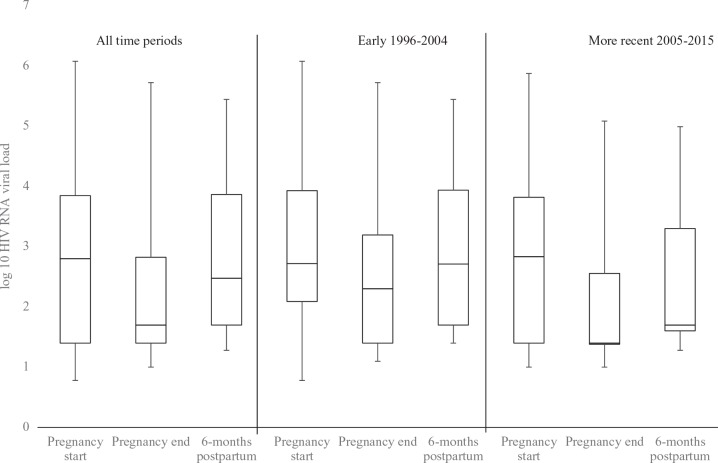
In 34.8% of pregnancies, women had VL suppression at pregnancy start. This percentage increased by pregnancy end to 60.1%, but then decreased by 6 months postpartum to 42.7% (Table 1). This trend was also seen in median log10VL values across the 3 analytical time points from 1996 to 2015 regardless of calendar year: 2.80 (IQR: 1.40-3.85) at pregnancy start, 1.70 (IQR: 1.40-2.82) at pregnancy end, and 2.30 (IQR: 1.40-3.86) at 6 months postpartum, and was similar when stratified by time period (Figure 1).
Table 1.
HIV Viral Suppression at Time Points during and after Pregnancy by Baseline Characteristics, HIV Outpatient Study 1996 to 2015.
| Pregnancy Start | Pregnancy End | 6 Months After Pregnancy End | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Overall | Virally Suppresseda | Virally Nonsuppressedb | P | Virally Suppresseda | Virally Nonsuppressedb | P | Virally Suppresseda | Virally Nonsuppressedb | P | |
| Total pregnancies, n (%) | 253 (100) | 88 (34.8) | 165 (65.2) | <.001 | 152 (60.1) | 101 (39.9) | .001 | 108 (42.7) | 145 (57.3) | .02 |
| Maternal age at pregnancy start, years, median (IQR) | 30 (24.6-34.0) | 29.6 (25.7-33.8) | 28.5 (24.4-34.0) | .14 | 29.6 (24.9-33.8) | 28 (23.8-34.0) | .17 | 29.9 (25.6-35.2) | 28 (24.4-33.0) | .024 |
| Race, n (%) | ||||||||||
| Non-Hispanic black | 168 (66.4) | 49 (29.2) | 119 (70.8) | .008 | 94 (56.0) | 74 (44.1) | .06 | 66 (39.3) | 102 (60.7) | .12 |
| Other race | 85 (33.6) | 39 (45.9) | 46 (54.1) | 58 (68.2) | 27 (31.8) | 42 (49.4) | 43 (50.6) | |||
| Education level, n (%) | ||||||||||
| Less than high school | 59 (23.3) | 23 (39.0) | 36 (61.0) | .44 | 37 (62.7) | 22 (37.3) | .64 | 20 (33.9) | 39 (66.1) | .12 |
| High school or higher | 194 (76.7) | 65 (33.5) | 129 (66.5) | 115 (59.3) | 79 (40.7) | 88 (45.4) | 106 (54.6) | |||
| Insurance, n (%) | ||||||||||
| Private | 50 (19.8) | 21 (42.0) | 29 (58.0) | .23 | 37 (74.0) | 13 (26.0) | .03 | 30 (60.0) | 20 (40.0) | .006 |
| Public or none | 203 (80.2) | 67 (33.0) | 136 (67.0) | 115 (56.7) | 88 (43.4) | 78 (38.4) | 125 (61.6) | |||
| Year of pregnancy start, n (%) | ||||||||||
| 1996-2004 | 134 (53.0) | 44 (32.8) | 90 (67.2) | .49 | 66 (49.3) | 68 (50.8) | <.001 | 47 (35.1) | 87 (64.9) | .009 |
| 2005-2015 | 119 (47.0) | 44 (37.0) | 75 (63.0) | 86 (72.3) | 33 (27.7) | 61 (51.3) | 58 (48.7) | |||
| HIV diagnosis to pregnancy, years, median (IQR) | 3.4 (1.0-6.6) | 4.9 (2.1-7.4) | 3 (0.3-6.0) | <.001 | 2.9 (0.4-6.7) | 4.1 (1.8-6.3) | .22 | 2.8 (0.3-6.9) | 4 (1.5-6.4) | .32 |
| CD4 count at pregnancy start (cell/mm3) | – | 506 (393-683) | 366 (226-580) | <.001 | 462 (330-699) | 370 (235-580) | .04 | 449 (287-695) | 421 (263-609) | .5 |
| ART/viral suppression at preceding time point | ||||||||||
| On ART and virally suppressed | – | – | – | – | 62 (84.9) | 11 (15.1) | <.001 | 75 (52.5) | 68 (47.6) | <.001 |
| Not on ART and virally suppressed | – | – | – | – | 90 (50.0) | 90 (50.0) | 33 (30.0) | 77 (70.0) | ||
Abbreviations: ART, antiretroviral therapy; IQR, interquartile range.
aHIV RNA viral load closest to and within 90 days of time points ≤500 copies/mL.
bHIV RNA viral load closest to and within 90 days of time point >500 or missing.
Figure 1.
Box plot of log10 HIV RNA viral load (VL) at time points during and after pregnancy, HIV Outpatient Study, 1996 to 2015 (N = 253). Not all women have HIV VL documented at all time points: 184 (72.7%) had a VL documented at pregnancy start, 211 (83.4%) at pregnancy end, and 188 (74.3%) at 6 months postpartum.
Viral load suppression at pregnancy end and at 6 months postpartum was more common among pregnancies that occurred in women with private insurance compared with those with public or no insurance (pregnancy end: 74.0% versus 56.7%, P = .03; postpartum: 60.0% versus 38.4%, P = .006; Table 1). Similarly, VL suppression at pregnancy end and at 6 months postpartum was more common in pregnancies that occurred from 2005 to 2015 compared with those that occurred from 1996 to 2004 (pregnancy end: 72.3% versus 49.3%, P < .001; postpartum: 51.3% versus 35.1%, P = .009). Viral load suppression at pregnancy end was less common among non-Hispanic black women compared to women of other race (56.0% versus 68.2%, P = .06), although this difference was not statistically significant. Maternal age at pregnancy start was slightly higher among women who had VL suppression at 6 months postpartum compared to those who did not have VL suppression at 6 months postpartum (29.9 years versus 28.0 years, P = .024). CD4 values at pregnancy start were higher among women with VL suppression at pregnancy end (506 cells/mL, IQR: 393-683 versus 366 cells/mL, IQR: 226-58), but not at 6 months postpartum. Viral load suppression at pregnancy end was more common among women on ART and virally suppressed at pregnancy start compared with women who were not (84.9% versus 50.0%, P < .001); similarly, viral suppression at 6 months postpartum was more common among women on ART and virally suppressed at pregnancy end compared with women who were not (52.5% versus 30.0%, P < .001; Table 1).
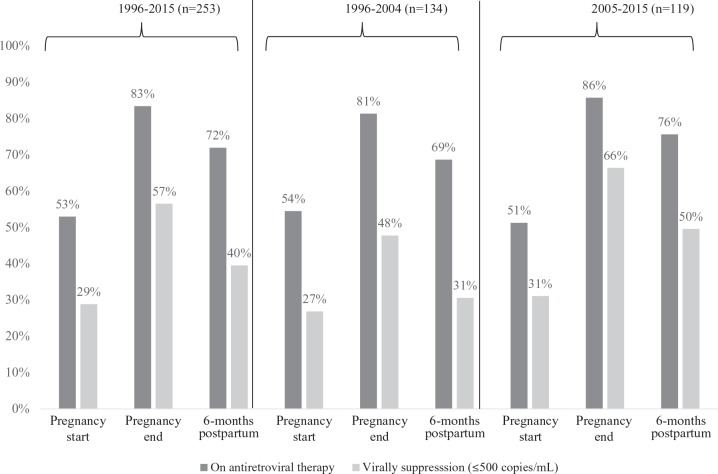
In 53.0% of pregnancies, women were on ART at pregnancy start (Figure 2). This percentage increased by pregnancy end to 83.4%, but then decreased by 6 months postpartum to 71.9%. Additional within-woman analyses revealed that of the 134 (53.0%) women who received ART at pregnancy start, 128 (95.5%) of them remained on ART at pregnancy end; of these 128 women, 114 (89.1%) remained on ART at 6 months postpartum. Of the 119 (47.0%) women not on ART at pregnancy start, 83 (69.8%) were initiated on ART by pregnancy end; of these, 56 (67.5%) remained on ART at 6 months postpartum.
Figure 2.
Viral suppression among women on antiretroviral therapy (ART) at time points during and after pregnancy, HIV Outpatient Study, 1996 to 2015.
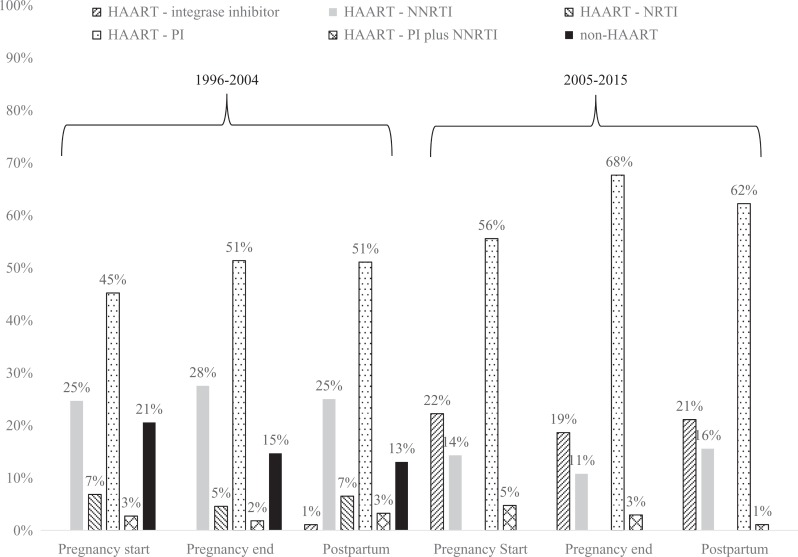
Antiretroviral therapy use at pregnancy end and 6 months postpartum was more frequent among women pregnant from 2005 to 2015 compared with those pregnant from 1996 to 2004 (pregnancy end: 86% versus 81%, postpartum: 76% versus 69%; Figure 2). Similarly, among the subset of women on ART at pregnancy end and at 6 months postpartum, VL suppression was higher among pregnancies that occurred from 2005 to 2015 compared with pregnancies that occurred from 1996 to 2004 (pregnancy end: 66% versus 48%, postpartum: 50% versus 31%; Figure 2). Antiretroviral therapy regimens shifted largely from nucleoside reverse transcriptase inhibitor (NRTI) and non-nucleoside reverse transcriptase inhibitor (NNRTI)-based combinations from 1996 to 2004 to integrase inhibitor and protease inhibitor (PI)-based regimens in 2004 to 2015 (Figure 3).
Figure 3.
Distribution of regimens during and after pregnancy among those on antiretroviral therapy (ART), HIV Outpatient Study, 1996 to 2015 (N = 253).
Independent Correlates of Unsuppressed VL at Pregnancy End and Postpartum
Risk of VL nonsuppression at both pregnancy end (reduced adjusted RR = 0.75, 95% CI: 0.59-0.95) and at 6 months postpartum (reduced adjusted RR = 0.55, 95% CI: 0.38-0.79) was lower among women pregnant from 2005 to 2015 compared to women pregnant from 1996 to 2004. Women on ART and virally suppressed at pregnancy start had lower risk of VL nonsuppression at pregnancy end compared to those not on ART and virally suppressed at pregnancy start (reduced adjusted RR = 0.71, 95% CI: 0.57-0.88); similarly, women on ART and virally suppressed at pregnancy end had lower risk of VL nonsuppression at 6 months postpartum compared to women not on ART and virally suppressed at pregnancy end (reduced adjusted RR = 0.30, 95% CI: 0.17-0.53). Older maternal age at pregnancy start was associated with lower risk of VL nonsuppression (10% reduction in risk per 5-year age increase), but not at 6 months postpartum. Although not statistically significant, the RRs and 95% CIs suggest potential associations between several baseline factors and not being virally suppressed at pregnancy end (less than high school education and public/no insurance) and at 6 months postpartum (public/no insurance; Table 2).
Table 2.
Associations between Baseline Characteristics and Risk of Being HIV Viral Load Nonsuppressed (>500 copies/mL) at Pregnancy End and 6 Months Postpartum, HIV Outpatient Study 1996 to 2015 (N = 253).
| Crude Unadjusted RR (95% CI) | P | Full Adjusted RRa (95% CI) | P | Reduced Adjusted RRb (95% CI) | P | |
|---|---|---|---|---|---|---|
| HIV VL nonsuppression at 6 months postpartum | ||||||
| Maternal age (per 5 years) | 0.90 (0.82-1.00) | .04 | 0.92 (0.83-1.02) | .14 | 0.90 (0.82-1.00) | .04 |
| ≥High school education (versus <high school education) | 0.83 (0.64-1.06) | .13 | 0.88 (0.71-1.10) | .27 | 0.83 (0.64-1.06) | .13 |
| Non-Hispanic black race (versus all other race) | 1.20 (0.92-1.57) | .18 | 1.13 (0.88-1.44) | .34 | 1.11 (0.84-1.46) | .45 |
| Private insurance (versus all public/none) | 0.65 (0.44-0.97) | .034 | 0.76 (0.50-1.15) | .2 | 0.70 (0.47-1.04) | .08 |
| Year of pregnancy ≥2005 (versus <2005) | 0.75 (0.59-0.95) | .018 | 0.77 (0.61-0.97) | .025 | 0.75 (0.59-0.95) | .018 |
| Years since HIV diagnosis (per year) | 1.00 (0.97-1.03) | .78 | 1.02 (0.99-1.06) | .18 | 1.00 (0.97-1.03) | .78 |
| On ART and virally suppressed at pregnancy end (versus not)c | 0.68 (0.55-0.84) | .0004 | 0.78 (0.63-0.96) | .017 | 0.71 (0.57-0.88) | .002 |
| HIV viral nonsuppression at pregnancy end | ||||||
| Maternal age (per 5 years) | 0.93 (0.80-1.08) | .32 | 0.94 (0.82-1.09) | .42 | 0.93 (0.80-1.08) | .32 |
| Non-Hispanic black race (versus all other race) | 1.39 (0.89-2.16) | .15 | 1.30 (0.88-1.93) | .19 | 1.20 (0.79-1.80) | .39 |
| ≥High school education (versus <high school education) | 1.09 (0.70-1.71) | .7 | 1.15 (0.80-1.65) | .46 | 1.09 (0.70-1.71) | .7 |
| Private insurance (versus all public/none) | 0.60 (0.34-1.07) | .08 | 0.71 (0.41-1.22) | .22 | 0.68 (0.40-1.14) | .14 |
| Year of pregnancy ≥2005 (versus <2005) | 0.55 (0.38-0.79) | .001 | 0.52 (0.37-0.73) | <.001 | 0.55 (0.38-0.79) | .001 |
| Years since HIV diagnosis (per year) | 1.02 (0.98-1.06) | .45 | 1.06 (1.01-1.10) | .011 | 1.02 (0.98-1.06) | .45 |
| On ART and virally suppressed at pregnancy start (versus not) | 0.30 (0.17-0.53) | <.001 | 0.32 (0.18-0.55) | <.001 | 0.30 (0.17-0.53) | <.001 |
Abbreviations: ART, antiretroviral therapy; CI, confidence interval; RR, risk ratio; VL, viral load.
aRelative risk was adjusted for all other variables in the table.
bAdjustment sets 6 months postpartum, which specify in curly brackets the variables that were controlled for when deriving reduced RR for each covariate, as per model building criteria described in the Methods: age {null}, education {null}, race {insurance}, insurance {age, ART/viral suppression pregnancy end}, pregnancy year {null}, years since HIV diagnosis {null}, ART/viral suppression at pregnancy end {pregnancy year}; adjustment set pregnancy end: age {null}, race {ART/viral suppression at pregnancy start}, education {null}, insurance {ART/viral suppression at pregnancy start}, pregnancy year {null}, time since HIV diagnosis {null}, ART/viral suppression {null}.
cPersons missing HIV viral load at pregnancy end were categorized as “virally nonsuppressed” at pregnancy end.
Discussion
Among HIV-infected women in routine outpatient care across multiple sites in the United States from 1996 to 2015, we found that VL suppression increased between pregnancy start and pregnancy end, but then subsequently decreased at 6 months postpartum. A similar pattern was also seen in median VL values across the 3 analytical time points. At all 3 time points, VL suppression was suboptimal, given US ART treatment guidelines regarding early ART initiation; even during the most recent calendar period, VL suppression was present in only 72% of women at pregnancy end and 51% women at 6 months postpartum.
Our results suggest that viral suppression at pregnancy end and postpartum in nonclinical trial settings in the United States remains suboptimal. We found that from 1996 to 2015, in 60.1% of pregnancies, women were virally suppressed at pregnancy end; this percentage was higher, 72.3%, in the more recent period from 2005 to 2015. Our findings are similar to those reported from the national EPS (in which 79% of women had VL suppression by pregnancy end)10 and lower than those from a recent clinical trial in the United States (in which 87% of women had VL suppression by pregnancy end).11 In our cohort, nearly a third of women had VL nonsuppression by pregnancy end, implying more substantial risk for MTCT of HIV perinatally, and poorer overall control of HIV infection in the mother compared to women who had VL suppression by pregnancy end.
We also found that from 1996 to 2004, only 42.7% of women had VL suppression at 6 months postpartum; this increased to 51.3% from 2005 to 2015. Even lower rates have been reported from Philadelphia’s EPS from 2005 to 2011, in which only 31% of women had VL suppression at 1 year postpartum.13 Our finding of an increased percentage of women with viral nonsuppression during the postpartum period is concordant with findings from a prospective clinical study (2002-2005) in which 29% of women who had VL suppression at pregnancy end experienced viral rebound (increase of ≥0.7 log copies/mL from third trimester value) by 6 months postpartum.18 This viral rebound may reflect reduced ART adherence during the postpartum period, a phenomenon documented in other studies.19,20 Reasons for reduced ART adherence during the postpartum period have not been well studied21 but may include reduced motivation to adhere to ART after successful prevention of MTCT, changes in schedule and lifestyle due to caregiver responsibilities, less frequent visits with a health-care provider, and challenges with postpartum depression among HIV-infected women. Active postpartum management with coordination of obstetric and HIV provider services may be warranted to strengthen retention and adherence efforts with women during the postpartum period.22
We identified several baseline factors that were associated with VL nonsuppression at pregnancy end and postpartum. Women who were on ART and who had VL suppression at pregnancy start had a 70% lower risk of VL nonsuppression at pregnancy end. Similarly, women who were on ART and had VL suppression at pregnancy end had a 32% lower risk of VL nonsuppression at 6 months postpartum. These findings suggest that it is imperative that HIV-infected women receive effective ART as early as possible; delays in ART initiation or problems with medication adherence may have a carryover effect into later time points during and after pregnancy.12,23 We found women who were older at pregnancy start had lower risk of VL nonsuppression at pregnancy end, 10% lower risk for every additional 5 years increase in age. This finding may reflect an association between older age and better ART adherence reported by others.24,25 Although they were not statistically significant, we also identified high school education and public insurance as potential risk factors for VL nonsuppression at pregnancy end as well as 6 months postpartum. These findings are similar to those of prior studies12,13,25 and suggest that socioeconomically disadvantaged women may be particularly vulnerable to VL nonsuppression during and after delivery.
Antiretroviral therapy use and VL suppression at pregnancy end and 6 months postpartum were more common from 2005 to 2015 compared to 1996 to 2004, indicating improving temporal trends in HIV treatment outcomes. Pregnant women from 2005 to 2015 had 25% lower risk of VL nonsuppression at pregnancy end and 45% lower risk of viral nonsuppression at 6 months postpartum, compared to pregnant women from 1996 to 2004. Furthermore, VL suppression among pregnant women receiving ART from 2005 to 2015 was 38% higher at pregnancy end and 61% higher at postpartum compared to women receiving ART from 1996 to 2004. These trends are not unexpected based on changes over time in guidelines that recommended HIV testing and ART for all pregnant women regardless of the clinical stage of HIV infection of the mother. These trends may also reflect advances in ART regimens over time; we found a shift toward use of integrase inhibitor- and PI-based regimens during pregnancy in 2005 to 2015, which have higher genetic barriers to resistance and fewer adverse drug effects. In addition, more generally, shifts from non-highly-active antiretroviral therapy (HAART) to HAART regimens, and decreased pill burden due to fixed-dose combinations and once-daily regimens, improved ART adherence over time.
Several limitations must be considered in interpreting our findings. First, HOPS is an observational study that relies on abstraction of data that are routinely available in HIV clinical charts—VLs were not measured at regular intervals but rather at the discretion of HIV providers, and we had data on ART prescription but not on ART adherence. Consequently, we were limited in the variables we could consider and adjust for as potential confounders, such as pregnancy outcome (ie, live birth, miscarriage), pregnancy intent, HIV serostatus of baby, postpartum depression, illicit drug use, and breastfeeding status, in our modeling. Second, we categorized women with missing VL or ART as virally nonsuppressed or not receiving ART, respectively. These assumptions are consistent with national surveillance reports from the Medical Monitoring Project; this practice may have resulted in underestimation of VL suppression if VLs were performed and were ≤500 copies/mL, but not documented in the clinical chart. Third, we relied on HIV VL as a biological marker for medication adherence and assumed that VL nonsuppression was due to not being on ART or not being adherent to ARV medications and not due to HIV drug resistance or other factors. Finally, data regarding pregnancies in HIV clinical charts were often limited; therefore, some pregnancies were excluded from our analysis due to missing pregnancy start or end dates, but missing data were likely nondifferential and may have impacted our results only by biasing toward the null.
In conclusion, our findings provide additional and more geographically representative data from the United States that suggest there is room for improvement in timely ART initiation and achievement of VL suppression in women receiving HIV care in the United States, particularly during the postpartum period. Although we identified temporal improvement in VL suppression during more recent years (2005-2015), nearly half of pregnancies during this time period were in women who were virally nonsuppressed at 6 months postpartum. Our findings suggest that earlier effective ART use with consequent achievement of VL suppression benefits pregnant women (and by inference, their neonates), particularly during later time points in pregnancy. Likewise, it is clear that clinical and behavioral interventions that target both providers and patients to ensure timely ART prescription and adherence are needed. In addition, interventions to promote continued ART adherence during the postpartum period are imperative. Although not statistically significant, our findings, similar to other studies, suggest that women of lower socioeconomic status, including those receiving public health insurance or with less than high school education, may be particularly vulnerable and require targeted interventions at delivery and during the postpartum period to promote and support ART adherence.26,27 These interventions will need to be integrated into routine postpartum care of HIV-infected women and may include clinic and home-based visits and support by health-care providers.
Acknowledgments
M.R.P. worked closely with K.B. to design the study. M.R.P. compiled the study database and collaborated with C.A. on statistical analyses. M.R.P. and K.B. participated in writing the manuscript. All authors reviewed and commented on the manuscript and approved its final submission.
Authors’ Note: The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention (CDC).
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The HOPS is funded by the Centers for Disease Control and Prevention (CDC, contract numbers: 200-2001-00133, 200-2006-18797, 200-2011-41872, and 200-2015-63931).
References
- 1. Centers for Disease Control and Prevention. HIV Surveillance Report, 2014. Atlanta, GA: Centers for Disease Control and Prevention; 2015. [Google Scholar]
- 2. Hamilton BE, Martin JA, Osterman MJ, Curtin SC, Matthews TJ. Births: final data for 2014. Natl Vital Stat Rep. 2015;64(12):1–64. [PubMed] [Google Scholar]
- 3. Whitmore SK, Zhang X, Taylor AW, Blair JM. Estimated number of infants born to HIV-infected women in the United States and five dependent areas, 2006. J Acquir Immune Defic Syndr. 2011;57(3):218–222. [DOI] [PubMed] [Google Scholar]
- 4. Jamieson DJ, Clark J, Kourtis AP, et al. Recommendations for human immunodeficiency virus screening, prophylaxis, and treatment for pregnant women in the United States. Am J Obstetr Gynecol. 2007;197(3 suppl):S26–S32. [DOI] [PubMed] [Google Scholar]
- 5. Connor EM, Sperling RS, Gelber R, et al. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. N Engl J Med. 1994,331(18):1173–1180. [DOI] [PubMed] [Google Scholar]
- 6. Centers for Disease Control and Prevention. Recommendations for assisting in the prevention of perinatal transmission of human T-lymphotropic virus type III/lymphadenopathy-associated virus and acquired immunodeficiency syndrome. MMWR Morb Mortal Wkly Rep. 1985;34(48):721–726, 731–732. [PubMed] [Google Scholar]
- 7. Centers for Disease Control and Prevention. US Public Health Service recommendations for human immunodeficiency virus counseling and voluntary testing for pregnant women. MMWR Recomm Rep. 1995;44(RR-7):1–15. [PubMed] [Google Scholar]
- 8. Gerberding J, Jaffe H. Dear Colleague Letter. Atlanta, GA: US Department of Health and Human Services, CDC; 2003. [Google Scholar]
- 9. Branson BM, Handsfield HH, Lampe MA, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55(RR-14):1–17. [PubMed] [Google Scholar]
- 10. Whitmore SK, Taylor AW, Espinoza L, Shouse RL, Lampe MA, Nesheim S. Correlates of mother-to-child transmission of HIV in the United States and Puerto Rico. Pediatrics. 2012;129(1):e74–e81. [DOI] [PubMed] [Google Scholar]
- 11. Katz IT, Leister E, Kacanek D, et al. Factors associated with lack of viral suppression at delivery among highly active antiretroviral therapy-naive women with HIV: a cohort study. Ann Intern Med. 2015;162(2):90–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Katz IT, Shapiro R, Li D, et al. Risk factors for detectable HIV-1 RNA at delivery among women receiving highly active antiretroviral therapy in the women and infants transmission study. J Acquir Immune Defic Syndr. 2010;54(1):27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Adams JW, Brady KA, Michael YL, Yehia BR, Momplaisir FM. Postpartum engagement in HIV care: an important predictor of long-term retention in care and viral suppression. Clin Infect Dis. 2015;61(12):1880–1887. [DOI] [PubMed] [Google Scholar]
- 14. Buchacz K, Baker RK, Moorman AC, et al. Rates of hospitalizations and associated diagnoses in a large multisite cohort of HIV patients in the United States, 1994-2005. AIDS. 2008;22(11):1345–1354. [DOI] [PubMed] [Google Scholar]
- 15. Bradley H, Hall HI, Wolitski RJ, et al. Vital signs: HIV diagnosis, care, and treatment among persons living with HIV—United States, 2011. MMWR Morb Mortal Wkly Rep. 2014;63(47):1113–1117. [PMC free article] [PubMed] [Google Scholar]
- 16. Bursac Z, Gauss CH, Williams DK, Hosmer DW. Purposeful selection of variables in logistic regression. Source Code Biol Med. 2008;3:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hosmer DW, Jr, Lemeshow S, Sturdivant RX. Applied Logistic Regression. New York, NY: John Wiley & Sons; 2013. [Google Scholar]
- 18. Beverly ES, Tierney C, Cohn SE, et al. Postpartum viral load rebound in HIV-1-infected women treated with highly active antiretroviral therapy: AIDS clinical trials group protocol A5150. HIV Clin Trials. 2015;12(1):9–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mellins C, Chu C, Malee K, et al. Adherence to antiretroviral treatment among pregnant and postpartum HIV-infected women. AIDS Care. 2008;20(8):958–968. [DOI] [PubMed] [Google Scholar]
- 20. Nachega JB, Uthman OA, Anderson J, et al. Adherence to antiretroviral therapy during and after pregnancy in low-income, middle-income, and high-income countries: a systematic review and meta-analysis. AIDS. 2012;26(16):2039–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Boehme AK, Davies SL, Moneyham L, Shrestha S, Schumacher J, Kempf MC. A qualitative study on factors impacting HIV care adherence among postpartum HIV-infected women in the rural southeastern USA. AIDS Care. 2014;26(5):574–581. [DOI] [PubMed] [Google Scholar]
- 22. Kapetanovic S, Christensen S, Karim R, et al. Correlates of perinatal depression in HIV-infected women. AIDS Patient Care STDS. 2009;23(2):101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Read PJ, Mandalia S, Khan P, et al. When should HAART be initiated in pregnancy to achieve an undetectable HIV viral load by delivery? AIDS. 2012;26(9):1095–1103. [DOI] [PubMed] [Google Scholar]
- 24. Gordillo V, del Amo J, Soriano V, González-Lahoz J. Sociodemographic and psychological variables influencing adherence to antiretroviral therapy. AIDS. 1999;13(13):1763–1769. [DOI] [PubMed] [Google Scholar]
- 25. Mehta S, Moore RD, Graham NM. Potential factors affecting adherence with HIV therapy. AIDS. 1997;11(14):1665–1670. [DOI] [PubMed] [Google Scholar]
- 26. Higa DH, Marks G, Crepaz N, Liau A, Lyles CM. Interventions to improve retention in HIV primary care: a systematic review of U.S. studies. Curr HIV/AIDS Rep. 2012;9(4):313–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Simoni JM, Amico KR, Pearson CR, Malow R. Strategies for promoting adherence to antiretroviral therapy: a review of the literature. Curr Infect Dis Rep. 2008;10(6):515–521. [DOI] [PMC free article] [PubMed] [Google Scholar]