Abstract
The benefits of “early” antiretroviral therapy (ART; ie, initiation when CD4 ≥500 cells/mm3) are now well accepted as reflected in the removal of the CD4-based eligibility from new ART guidelines by the World Health Organization (WHO). However, neither the “treat-all” strategy recommendations presented in the guidelines nor the HIV care cascade goals in the Joint United Nations Programme on HIV/AIDS (UNAIDS) 90-90-90 targets adequately address the issue of ART timing. Our recent study on “immediate” ART (ie, ≤30 days after HIV diagnosis) adds important evidence demonstrating the real and meaningful benefits of rapid ART initiation even among those who have CD4 ≥500 cells/mm3. We call on WHO and UNAIDS to consider this research and encourage a shift from the treat-all strategy to an “immediately-treat-all” strategy, and from a slow, fragmented, complicated, multistep HIV care cascade to a fast, easy, and simple cascade with effectiveness measures that incorporate the important aspect of time.
Keywords: HIV, early, immediate, antiretroviral therapy, China
Comment on Zhao Y, Wu Z, McGoogan JM, Shi CX, Li A, Dou Z, Ma Y, Qin Q, Brookmeyer R, Detels R, Montaner JSG. Immediate antiretroviral therapy decreases mortality among patients with high CD4 counts in China: a nationwide, retrospective cohort study. Clin Infect Dis. 2018;66(5):727-734.
The World Health Organization (WHO) issued the second edition of its Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection in 2016 (hereafter referred to as the WHO Consolidated Guidelines).1 Therein, a thorough review of a large body of moderate- to high-quality evidence is presented, demonstrating that “early” antiretroviral therapy (ART) has meaningful individual-level clinical benefit (ie, reduced morbidity and mortality), community-level public health benefit (ie, reduced transmission), and broader programmatic, social, and economic benefit (eg, reduced health system burden, improved productivity). Early ART is defined as ART initiation prior to CD4+ T-lymphocyte count falling below 500 cells/mm3 (Box 1), and as such, the WHO eliminated traditional CD4-based ART eligibility requirements in late 2015. Thus, ART is now recommended for all people living with HIV (PLWH) regardless of CD4 level or clinical stage.1
Box 1.
Definitions Relevant to the Timing of ART Initiation.
Late presentation/diagnosis—Diagnosis of HIV infection only after CD4 counts have fallen below 200 cells/mm3 or symptoms of advanced HIV disease or AIDS are present.
Early ART—Initiation of ART before CD4 falls below 500 cells/mm3, regardless of the time interval between diagnosis date and ART initiation date (ie, “early” timing with respect to the disease progression time line).
Immediate ART—Initiation of ART immediately after diagnosis, regardless of CD4 count (ie, “immediate” timing with respect to the care cascade timeline), emphasizing the shortest time interval possible between diagnosis date and ART initiation date.
Abbreviation: ART, antiretroviral therapy.
This “treat-all” strategy has helped drive progress toward the Joint United Nations Programme on HIV/AIDS (UNAIDS) 90-90-90 targets for ending the HIV epidemic—90% of PLWH know their status, 90% of PLWH who know their status are on treatment, and 90% of PLWH on treatment are virally suppressed by 2020.2,3 Globally, 79% of PLWH who knew their status were on ART as of mid-20184—a major accomplishment, and yet, a major shortfall at the same time. An enormous number of the PLWH on ART today did not start early. In fact, many are still diagnosed late and present for treatment only after the onset of symptoms. By this time, their CD4 counts have already dropped below the 200 cells/mm3 mark and many have already entered advanced HIV disease or AIDS. As a result, these individuals are unable to take advantage of the benefits of early ART and, not surprisingly, tend to experience higher mortality and elevated health care costs.1
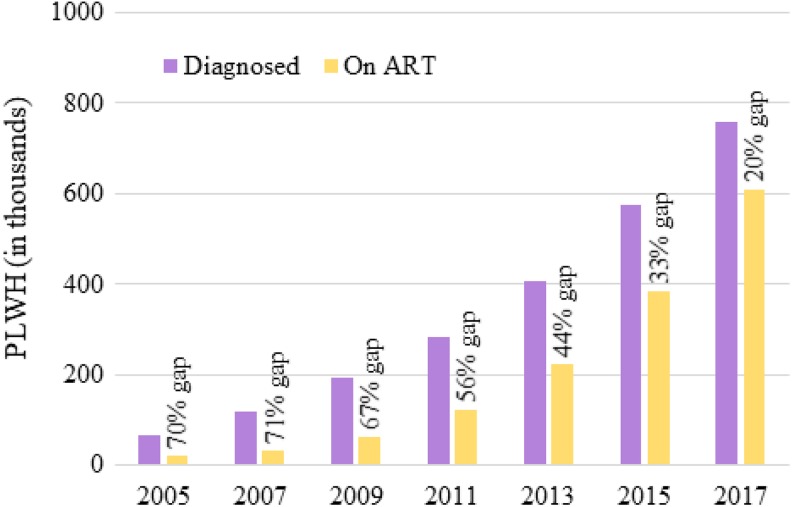
Undoubtedly, the right thing to do for these late presenters is to immediately offer treatment. However, do we know that immediate treatment is not the right thing to do for all PLWH? After all, the dramatic attrition from the HIV care continuum in the pre-ART period has long been well-documented in many settings, and the importance of limiting this attrition is underscored by the UNAIDS 90-90-90 targets.2,3 In China, for instance, until as recently as 2011, more than 50% of diagnosed PLWH were not on treatment. The vast majority of these individuals were lost to follow-up (Figure 1).5–7 Successfully linking PLWH to care and retaining PLWH in care is important for improving outcomes of all kinds. Perhaps, at a minimum, this could be achieved via reducing the pre-ART period, meaning offering ART immediately after diagnosis.
Figure 1.
Graphical representation of a portion of China’s HIV care continuum highlighting the treatment gap caused by lengthy pre-ART periods (ie, the time between diagnosis and treatment initiation). For example, in 2013, there were nearly 200 000 PLWH who were diagnosed with HIV infection but still not on ART, a treatment gap of 44%. Adapted with permission from Ma and colleagues.5 ART, antiretroviral therapy; PLWH, people living with HIV.
Thus, we (and others) began investigating the potential benefits of immediate ART for all. In contrast to early ART, which focuses on immune function decline as a proxy for estimating the time interval from infection to treatment, “immediate” ART focuses on the actual time interval from diagnosis with HIV infection to the start of treatment, regardless of CD4 count or clinical status (Box 1).
Fortunately, a relatively large number of PLWH in China were already receiving ART regardless of CD4 count by 2012, 3 years ahead of the WHO guideline revision.1 Beginning in 2008, China’s National Free Antiretroviral Treatment Program began adding exceptions to the ART eligibility criterion of CD4 ≤350 cells/mm3 that was standard of care at that time. First, all patients with coinfections and all pregnant women, and then all individuals in serodiscordant relationships, were made eligible regardless of CD4 count.8 Then, in 2012, a series of cluster randomized trials were launched for the purpose of evaluating interventions intended to promote earlier diagnosis and faster ART initiation regardless of CD4 count among key, high-risk populations including men who have sex with men (MSM), serodiscordant couples, people who inject drugs (PWID), and female sex workers (FSWs). Hence, we already had a large pool of PLWH who initiated ART prior to their CD4 counts falling below 500 cells/mm3, and for each of these individuals, we had HIV diagnosis and treatment information as well as routine health and death information.
So, we conducted 2 large, retrospective, observational cohort studies of ART timing among PLWH in China.9,10 The first aimed to evaluate mortality and causes of death. In this study, we enrolled more than 34 500 PLWH who were diagnosed with HIV infection between January 2012 and June 2014 and had baseline CD4 results greater than 500 cells/mm3. We found a 63% reduction in mortality among those in our cohort who received immediate ART (ie, within 30 days of diagnosis) with 95% of deaths attributed to non-AIDS causes.9 We then aimed to evaluate treatment failure.10 In this study, we enrolled more than 123 000 PLWH who were diagnosed between January 2011 and December 2015. We found that those who received immediate ART (ie, within 30 days of diagnosis) had significantly reduced rates of treatment dropout and virological failure (ie, viral load >400 copies/mL). Delay of only 31 to 90 days was associated with a 35% increased risk of virological failure and delay of 91 to 365 days was associated with 33% greater risk of treatment dropout and 66% greater risk of virological failure.10
To prospectively evaluate the benefit of compressing the overall interval between initial HIV-reactive screening result and receipt of HIV treatment regardless of CD4 count, we also conducted 2 trials of 2 different structural interventions.11,12 Firstly, using a pre-/post-intervention design (January to December 2010 and January to December 2011), we evaluated a simplified test-and-treat procedure, whereby HIV confirmatory testing, CD4 testing, and ART initiation were performed concurrently. Among the more than 1000 participants enrolled and followed, rates of baseline CD4 testing within 30 days of diagnosis increased from 61% to 97%, rates of ART initiation increased from 49% to 89%, time from diagnosis to ART initiation decreased from a median of 53 days to a median of 5 days, and mortality fell by 62%.11 Secondly, using a cluster randomized, controlled trial design, we evaluated the effect of a streamlined (ie, faster, simpler, and less fragmented) testing and linkage to care intervention that included rapid, point-of-care HIV screening and CD4 testing with in-parallel viral load testing. Nearly 500 participants were enrolled in 2014 and followed for 1 year. We found 20 times greater odds of testing completeness within 30 days of initially screening HIV-reactive, 3 times greater odds of ART initiation within 90 days, and 56% reduced mortality at 12 months. Although not a main outcome measure of the study, median time from first screening HIV-reactive to ART initiation was cut by nearly 70%.7,12
Taken together, these results demonstrate that immediate ART initiation under otherwise standard-of-care conditions and ART provided in a streamlined HIV care algorithm both individually provide significant and meaningful clinical benefits even for those who are not diagnosed late and even under real-world conditions in a middle-income country setting like China.9–12 Moreover, reduction of the pre-ART period in China likely has other benefits as well. One may be mitigation of some of the myriad reasons for losses to follow-up during the pre-ART period, which has been documented in the China setting.6 Another may be prevention of confusion and misunderstanding about when to return for treatment (if it is not initiated immediately), which has been reported anecdotally by ART providers as a major cause of loss to follow-up in the pre-ART period. Additionally, increased frequency of contact with health care providers for PLWH on ART likely improves overall health and well-being and facilitates timely referrals to ancillary services (eg, methadone maintenance and needle/syringe exchange for PWID, treatment for other sexually transmitted infections for FSW, MSM, and others, psychosocial support services for those experiencing stigma and discrimination, and specialized counseling and care for those struggling with mental illness). Finally, we have found that the streamlined approach to accelerated HIV diagnosis and treatment is highly cost-effective—while more patients will be treated over a longer period of time, which will increase cost over standard of care, the benefit is substantial in terms of quality-adjusted life years, deaths averted, and new HIV infections averted.13
The results of our studies are supported by others on immediate and/or accelerated ART initiation at all CD4 levels in other low- and middle-income countries, which evaluated outcomes including ART uptake, time to ART initiation, retention in care/loss to follow-up, immunological response, viral suppression, onward transmission, mortality, and safety (ie, adverse events reported).14–18 Furthermore, a recently published meta-analysis of 12 studies in mostly high-income country settings published between 2000 and 2017 found that for PLWH with CD4 counts still above 500 cells/mm3, immediate ART initiation reduced risk of death and progression to AIDS and increased odds of immunological recovery and viral suppression.19 Finally, a review of 13 observational studies on ART initiated during the acute or early chronic infection phases found that many markers of innate and adaptive immune activation, microbial translocation and systemic activation, and viral persistence were all significantly reduced.20
Although the WHO Consolidated Guidelines mention that “efforts should be made to reduce the time between HIV diagnosis and ART initiation,” this is not given the recognition afforded by a recommendation and is rather labeled as only a “good practice statement.” Antiretroviral therapy is described as a “non-emergency intervention” that is “rarely urgent” and may be risky as PLWH who begin ART before they are ready may struggle with adherence or have adverse outcomes.1 Concern is also raised in the WHO Consolidated Guidelines,1 and elsewhere,21 about some PLWH obtaining ART ahead of those who more urgently need it, and these authors argue for clinical prioritization of PLWH for ART receipt in low-resource settings.
However, a recent, large, global study of the timing of ART initiation failed to find evidence of “sicker” patients being “crowded out” when ART eligibility was expanded,22 and we strongly disagree with this approach of clinical prioritization—it is a step backward and does a disservice to PLWH in low-resource settings,23 and PLWH in high-resource settings as well. We believe that all PLWH urgently need ART. As much and as quickly as possible, the pre-ART period must be shortened, ART delivery must be simplified, and ART coverage must be expanded. The individual, community, programmatic, health system, and societal and economic benefits of immediate ART deserve to be addressed in the next revision of the WHO Consolidated Guidelines. Furthermore, they warrant consideration as we approach the 2020 deadline for the UNAIDS 90-90-90 target. The current target focuses solely on the proportion of PLWH who achieve the 3 main HIV care continuum steps,2 but growing evidence indicates that the time it takes for PLWH to achieve each step is a similarly important measure of the effectiveness of the HIV care continuum.24–26 We propose that the next iteration of the target includes ambitious time components; for example, 90% of all PLWH diagnosed while CD4 level is still above 500 cells/mm 3 (to address late presentation), 90% of diagnosed PLWH on treatment within 30 days of diagnosis (to realize the full benefits of immediate ART for all), and 90% of treated PLWH virologically suppressed within 9 months of initiation (to meet ART’s ultimate goal).
The next phase in our collective effort against the global HIV epidemic will be just as difficult as the previous ones—as always, it will require commitment, focus, determination, investment, and innovation—but without deliberately challenging ourselves with that next, seemingly impossible goal, like moving from “treat all” to “immediately treat all,” we will never end AIDS.
Footnotes
Authors’ Note: Y.Z. prepared the initial draft of manuscript. All authors contributed to several revisions of the manuscript. The views and opinions expressed herein belong to the authors alone, and do not represent the official policy, or endorsement of their affiliated institution.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the China National AIDS Program and the National Science and Technology Major Project on Prevention and Treatment of Major Infectious Diseases including AIDS and Viral Hepatitis via National Health Commission of the People’s Republic of China (Grant 2012ZX10001-007; 2018ZX10721102).
ORCID iD: Zunyou Wu  https://orcid.org/0000-0002-0839-4548
https://orcid.org/0000-0002-0839-4548
References
- 1. World Health Organization. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection—Recommendations for a Public Health Approach. 2nd ed 2016. http://www.who.int/hiv/pub/arv/arv-2016/en/. Accessed February 8, 2019. [PubMed]
- 2. Joint United Nations Programme on HIV/AIDS. 90-90-90—An Ambitious Treatment Target to Help End the AIDS Epidemic. http://www.unaids.org/en/resources/documents/2017/90-90-90. Accessed February 8, 2019.
- 3. Joint United Nations Programme on HIV/AIDS. Ending AIDS: Progress Towards the 90-90-90 Targets. http://www.unaids.org/en/resources/documents/2017/20170720_Global_AIDS_update_2017. Accessed February 8, 2019.
- 4. Joint United Nations Programme on HIV/AIDS. Fact Sheet—Latest Global and Regional Statistics on the Status of the HIV Epidemic. http://www.unaids.org/en/resources/fact-sheet. Accessed February 8, 2019.
- 5. Ma Y, Dou Z, Guo W, et al. The human immunodeficiency virus care continuum in China: 1985-2015. Clin Infect Dis. 2018;66(6):833–839. [DOI] [PubMed] [Google Scholar]
- 6. Gu D, Mao Y, Tang Z, et al. Loss to follow-up from HIV screening to ART initiation in rural China. PLoS One. 2016;11(10):e0164346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mao Y, Wu Z, McGoogan JM, et al. Care cascade structural intervention versus standard of care in the diagnosis and treatment of HIV in China: a cluster-randomized controlled trial protocol. BMC Health Serv Res. 2017;17(1):397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ministry of Health Working Group on Clinical AIDS Treatment. China Free Antiretroviral Therapy Manual [in Chinese]. 3rd ed Beijing, China: People’s Medical Publishing House; 2012. [Google Scholar]
- 9. Zhao Y, Wu Z, McGoogan JM, et al. Immediate antiretroviral therapy decreases mortality among patients with high CD4 counts in China: a nationwide, retrospective cohort study. Clin Infect Dis. 2018;66(5):727–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhao Y, Wu Z, McGoogan JM, et al. Nationwide cohort study of antiretroviral therapy timing: treatment dropout and virological failure in China, 2011-2015. Clin Infect Dis. 2019;68(1):43–50. doi:10.1093/cid/ciy400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wu Z, Zhao Y, Ge X, et al. Simplified HIV testing and treatment in China: analysis of mortality rates before and after a structural intervention. PLoS Med. 2015;12(9):e1001874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wu Z, Tang Z, Mao Y, et al. Testing and linkage to HIV care in China: a cluster-randomised trial. Lancet HIV. 2017;4(12):e555–e565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zang X, Tang H, Min JE, et al. Cost-effectiveness of the ‘One4All’ HIV linkage intervention in Guangxi Zhuang Autonomous Region, China. PLoS One. 2016;11(11):e0167308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rosen S, Maskew M, Fox MP, et al. Initiating antiretroviral therapy for HIV at a patient’s first clinic visit: the RapIT randomized controlled trial. PLoS Med. 2016;13(5):e1002015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mburu G, Paing AZ, Myint NN, et al. Retention and mortality outcomes from a community-supported public-private HIV treatment programme in Myanmar. J Int AIDS Soc. 2016;19(1):20926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brown LB, Havlir DV, Ayieko J, et al. High levels of retention in care with streamlined care and universal test and treat in East Africa. AIDS. 2016;30(18):2855–2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kroon EDMB, Phanuphak N, Shattock AJ, et al. Acute infection detection and immediate treatment estimated to reduce transmission by 89% among men who have sex with men in Bangkok. J Int AIDS Soc. 2017;20(1):21708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kwarisiima D, Kamya MR, Owaraganise A, et al. High rates of viral suppression in adults and children with high CD4+ counts using a streamlined ART delivery model in the SEARCH trial in rural Uganda and Kenya. J Int AIDS Soc. 2017;20(suppl 4):21673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Song A, Liu X, Huang X, et al. From CD4-based initiation to treating all HIV-infected adults immediately: an evidence-based meta-analysis. Front Immunol. 2018;9:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rajasuriar R, Wright E, Lewin SR. Impact of antiretroviral therapy (ART) timing on chronic immune activation/inflammation and end-organ damage. Curr Opin HIV/AIDS. 2015;10(1):35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Molina JM, Grund B, Gordin F, et al. Which HIV-infected adults with high CD4 T-cell counts benefit most from immediate initiation of antiretroviral therapy? A post-hoc subgroup analysis of the START trial. Lancet HIV. 2018;5(4):e170–e180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tymejczyk O, Brazier E, Yiannoutsos C, et al. HIV treatment eligibility expansion and timely antiretroviral treatment initiation following enrollment in HIV care: a metaregression analysis of programmatic data from 22 countries. PLoS Med. 2018;15(3):e1002534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wu Z, Zhao Y, McGoogan JM. Prioritisation of subgroups for immediate antiretroviral therapy. Lancet HIV. 2018;5(5):e206. [DOI] [PubMed] [Google Scholar]
- 24. MacCarthy S, Hoffmann M, Ferguson L, et al. The HIV care cascade: models, measures and moving forward. J Int AIDS Soc. 2015;18:19395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ndawinz JD, Anglaret X, Delaporte E, et al. New indications for delay in initiation of antiretroviral treatment: estimates for Cameroon. Bull World Health Organ. 2015;93(8):521–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Supervie V, Marty L, Lacombe JM, Dray-Spira R, Costagliola D. Looking beyond the cascade of HIV care to the end of the AIDS epidemic: estimation of the time interval from HIV infection to viral suppression. J Acquir Immune Defic Syndr. 2016;73(3):348–355. [DOI] [PubMed] [Google Scholar]