Abstract
Background:
Current HIV treatment guidelines recommend antiretroviral treatment (ART) initiation for all HIV-infected individuals regardless of CD4 count. This study evaluates the immunological and virological status and the clinical characteristics of patients who have started ART in the last 8 years in the Northwest of Spain.
Methods:
All HIV-infected patients who have started ART between January 2009 and December 2016 at a reference hospital in the Northwest of Spain were included in this retrospective observational study. Epidemiological, clinical, and immunovirological features and antiretroviral drugs used for initiation were recorded. A statistical analysis was performed using SPSS version 19 software. Categorical and continuous variables were compared by the specific statistical tests, and a logistic regression model was used to identify time associated with Center for Disease Control and Prevention (CDC) categories change.
Results:
A high proportion of HIV-infected patients (66.7%) had initiated ART with CD4 counts <350 cells/mm3 in the last 8 years. From these, most of them (68.3%) had <350 CD4 counts at first contact with HIV specialist medical team, 12.2% had no indications for ART initiation in the last clinic visit before ART initiation according to the national guidelines at that moment, 11.0% were lost to follow-up because of lack of compliance with scheduled visits and 8.5% of patients refused treatment. A logistic regression model showed that a delay of one month since the first contact with HIV specialist medical team to ART initiation involves a risk of worsening in the CDC clinical category (odds ratio: 1.02 [95% confidence interval: 1.012-1.029]; P < .001). A trend towards an earlier start of ART was observed during 2015 and 2016, likely influenced by the last treatment guidelines recommendations.
Conclusion:
High proportion of HIV-infected patients (66.7%) had initiated ART with CD4 counts <350 cells/mm3 in the last 8 years. The main reasons for this problem were analyzed and an important rate of late diagnosis was identified. However, a trend towards an earlier start of ART was observed during 2015 and 2016, likely influenced by the last treatment guidelines recommendations. These findings highlight the need to promote and facilitate HIV testing to reduce the late diagnosis as well as counseling on HIV prevention, treatment, and linkage care.
Keywords: HIV, antiretroviral therapy, CD4 count, late diagnosis
What Do We Already Know about This Topic?
It was estimated that in Europe, almost 30% of HIV-infected persons remain undiagnosed with an overall incidence of late presentation around 50% of all HIV cases. Late presentation of HIV infection is associated with shorter survival and poor response to treatment, higher frequency of ART toxicity, increased healthcare costs, and rates of HIV transmission. Consequences can be even worse if we delay antiretroviral treatment initiation.
How Does Your Research Contribute to the Field?
This study evaluates the immunological and virological status and the clinical characteristics of patients who have started antiretroviral treatment in the last 8 years in the Northwest of Spain. The results obtained would help to get a deep knowledge of the characteristics of our HIV population to establish the best strategies for HIV diagnosis, prevention, and treatment.
What Are Your Research’s Implications toward Theory, Practice, or Policy?
The research’s implications in real clinical practice are that we now know the main reasons for antiretroviral treatment delay and we will be able to avoid loss of follow-up. Moreover, we identified an important rate of late diagnosis that we didn’t know and still need to improve.
Introduction
The Department of Health and Human Services and European AIDS Clinical Society treatment guidelines for the initiation of antiretroviral therapy (ART) in HIV-infected patients have been updated in the last years based on the available evidence and the improvement in the safety and convenience of the treatments.1,2 From 2009 to 2014 only those patients with CD4 counts below 350 or 500 cells/mm3 with different considerations and levels of evidence, and the presence of, or high risk for, developing various types of (comorbid) conditions were the objective for treatment initiation. In 2014 and 2015, the national HIV treatment guidelines recommend treating all HIV-infected patients regardless of their immunological status, based on experts’ opinions.3 This recommendation has achieved a higher level of evidence in last year,4 due to the last findings from the 2 large randomized controlled trials START and TEMPRANO, showing the benefits reducing the morbidity and mortality associated with the HIV infection of an earlier ART initiation.5,6
This study evaluates the immunological and virological status and the clinical characteristics of patients who have started ART in the last 8 years in the Northwest of Spain. The results obtained would help to know and understand the current characteristics for treatment initiation in the last years in our HIV population to establish the best treatment strategies to improve the clinical follow-up of these patients.
Methods
This is a retrospective observational study including HIV positive patients older than 18 years who have started ART at the University Hospital of A Coruña, a reference hospital in the Northwest of Spain, between January 2009 and December 2016.
The HIV-infection status of patients who have started ART in the last 8 years was retrospectively evaluated. Epidemiological, clinical, and immunovirological features of HIV-infected patients and antiretroviral drugs used for ART initiation were recorded. Patients who only received post-exposure prophylaxis or who started ART in other centers or under clinical trials were excluded from the analysis. Late diagnosis was defined as CD4 count <350 cells/mm3 at the time of HIV diagnosis. In addition, it has been taken into account if patients started ART with CD4 counts <350, 350 to 500, or >500 cells/mm3. The statistical analysis was performed using the Statistical Packages for the Social Sciences software (SPSS 19.0). Categorical variables are presented as number of cases or percentage and were compared by the χ2 test or Fisher exact test, when appropriate. Continuous variables are expressed as mean (standard deviation) and compared by Student t test, Mann-Whitney, and Kruskal-Wallis test, when appropriate. A logistic regression model was used to identify time associated with Center for Disease Control and Prevention (CDC) categories change. A P value of <.05 was considered statistically significant.
Results
Baseline Characteristics of the Study Population
A total of 492 HIV-infected patients who have started ART in the study period were recorded (Table 1). The overall mean nadir CD4 count was 267.1 ± 202.8 cells/mm3 (years 2009: 176.9 cells/mm3; 2016: 393.3 cells/mm3; P < .001). At first contact with our HIV Specialist Medical Team (FirstSMT), the mean CD4 count was 403.8 ± 303.7 cells/mm3 and 45.5% of them had CD4 count <350 cells/mm3. Among the last years, an increase has been observed in the average of CD4 counts at FirstSMT from 383.1 ± 285.9 in 2009 and 2010 to 479.3 ± 341.1 in 2015 to 2016, P = .017. Moreover, the rate of patients with CD4 counts below 350 cells/mm3 at FirstSMT has significantly fallen from 51% during 2009 to 2010 to 36.6% during 2015 to 2016 (P = .021). Of note, the mean age was higher for patients with CD4 counts in the FirstSMT <350 cells/mm3 compared to those ranging from 350 to 500 cells/mm3 or above 500 cells/mm3 (41.2 versus 36.0 versus 37.4, respectively; P < .001). Conversely, CD4 counts levels below 350 cells/mm3 have not been associated with gender (male versus female; P = .351). The overall rate of mortality at the time of completion of the study was 3.7%.
Table 1.
Baseline Characteristics of the Study Population.
| Variables | N = 492 |
|---|---|
| Demographic-epidemiological | |
| Male (%) | 83.7 |
| Age, years (SD) | 38.9 (9.9) |
| Routes of HIV transmission | |
| MSM (%) | 50.2 |
| Heterosexual (%) | 32.9 |
| IDU (%) | 12.8 |
| Unknown (%) | 4.1 |
| Race | |
| Caucasian (%) | 88.2 |
| Latin (%) | 9.6 |
| Black (%) | 2.2 |
| Coinfections | |
| Anti-HCV positive (%) | 13.4 |
| HBsAg positive (%) | 3.7 |
Abbreviations: HBsAg, hepatitis B surface antigen; HCV, hepatitis C virus; IDU, intravenous drug use; MSM, men who have sex with men.
Time Since HIV Diagnosis to ART Initiation
The overall mean time since HIV diagnosis to FirstSMT was 4.5 ± 11.2 months and since FirstSMT until ART initiation (FirstART) 19.4 ± 32.3 months. These figures were maintained during the study period. Of note, the time between FirstSMT and FirstART was >180 days in 42.1% of patients. Analyzing those patients who have delayed the ART initiation more than 180 days, those who required treatment according to guidelines in force at the time (54.1%) delayed it due to patients’ refusal of ART initiation (22.2%), no indication of treatment in the previous follow-up (17.4%), and lack of compliance with the scheduled visits (14.5%). During the time between FirstSMT and FirstART, mean RNA-HIV increased (4.9 ± 0.9 versus 5.0 ± 0.8 log copies/mL, respectively; P < .001), and mean CD4 count decreased (403.8 ± 303.7 vs 287.2 ± 229.5 cells/mm3, respectively; P < .001). The distribution of the current CDC staging system varies regarding the year of FirstART: 57.9% A, 3.5% B, 38.6% C during 2009; and 82.9% A, 3.9% B, 13.2% C in 2016 (P = .002). Of note, 0.8% patients at CDC clinical category A in FirstSMT moved to category B and 5.0% to category C at FirstART. None of the patients at category B in FirstSMT moved to C at FirstART. During the time elapsed between the first follow-up and the treatment prescription, there was a worsening in the CDC clinical stage of 9 patients treated in the first 2 years of the study, whereas this occurred only in 2 patients among those treated during 2015 and 2016 (P = .041).
A logistic regression model was used to assess the impact of lapsed time between FirstSMT and FirstART and the risk of worsening in the CDC clinical category (category A to B or A to C and category B to C). The results showed that a delay of one month since the FirstSMT to FirstART involve a risk of worsening in the CDC clinical stage (odds ratio: 1.02 [95% confidence interval: 1.012-1.029]; P < .001).
Evolution of Mean CD4 Count at ART Initiation in the Last 8 Years
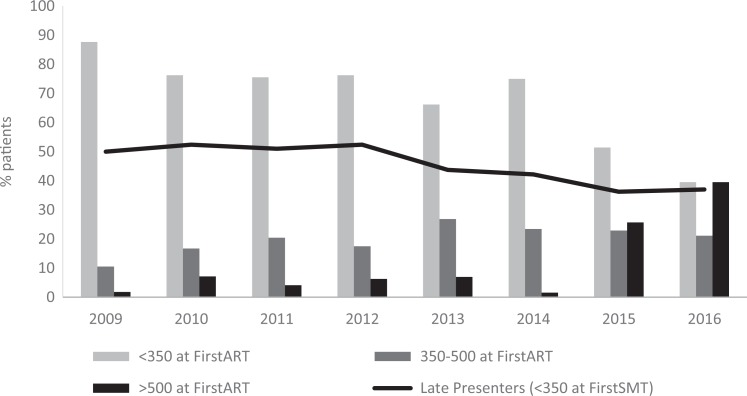
Mean CD4 count at FirstART was maintained during the study period, but a significant increase was observed during 2015 to 2016 (Figure 1): 219.5 ± 154.2 (2009-2010) versus 405.8 ± 306.8 (2015-2016) cells/mm3 (p < 0.001). Overall, 66.7% of patients started ART with CD4 counts <350 cells/mm3 (Table 2). From these patients who started treatment with CD4 counts below 350 cells/mm3, most of them (68.3%) had <350 cells/mm3 at FirstSMT, 12.2% had no indications for ART initiation in the last clinic visit before ART initiation considering the indications on national clinical guidelines in each year, 11% were lost of follow-up because of lack of compliance with the scheduled visit, and 8.5% of patients refused treatment in one or more follow-up visits. The rest of the patients (33.3%) started ART with CD4 counts between 350 and 500 (20.3%) and >500 cells/mm3 (13.0%). Although in 2009 to 2010 only 6.5% of patients started ART with CD4 >500 cells/mm3, during 2015 to 2016 this figure increased to 32.6% (P < .001).
Figure 1.
Evolution of CD4 count levels at antiretroviral treatment (ART) initiation during 2009 to 2016 in the HIV-infected population and late presenters in each year.
Table 2.
HIV Status of Patients at Antiretroviral Treatment Prescription by Years.
| Variables | 2009-2010 (n = 99) | 2011-2012 (n = 112) | 2013-2014 (n = 135) | 2015-2016 (n = 146) |
|---|---|---|---|---|
| Mean CD4, cells/mm3, (SD) | 219.5 (154.2) | 237.6 (169.4) | 249.6 (166.6) | 405.8 (306.8) |
| CD4 count <350 cells/mm3 (%) | 78.3 | 77.5 | 75.0 | 45.2 |
| Nadir CD4, cells/mm3, (SD) | 205.1 (141.6) | 223.4 (152.9) | 237.6 (152.4) | 371.2 (266.6) |
| Mean RNA-HIV, log copies/mL, (SD) | 5.0 (0.7) | 5.2 (0.7) | 5.0 (0.8) | 4.8 (0.8) |
Antiretroviral Treatment Combinations at Treatment Initiation
Regarding the antiretroviral drugs used as FirstART, main NRTI backbone prescribed was TDF/FTC and combinations were based on PI in 40.7% of patients and NNRTI in 33.7% (Table 3). Patients with mean CD4 count at FirstART <350 cells/mm3 received mainly an PI-based therapy (49.6%), in patients with CD4 counts ranging from 350 to 500, NNRTI-based therapies appear to have been predominantly prescribed (51.4%), and in patients with CD4 >500 cells/mm3 an INSTI-based treatment (60%; P < .001). During 2015 and 2016, 71.1% of patients initiated ART with an INSTI-based therapy compared to a less than 8.0% in the previous years (P < .001).
Table 3.
Antiretroviral Treatment Prescription in Naïve Patients by Years.
| Variables (%) | 2009-2010 (n = 99) | 2011-2012 (n = 112) | 2013-2014 (n = 135) | 2015-2016 (n = 146) |
|---|---|---|---|---|
| Backbone | ||||
| TDF/FTC | 86.9 | 92.0 | 82.2 | 62.8 |
| ABC/3TC | 5.1 | 6.2 | 17.1 | 17.9 |
| 3TC/AZT | 7.1 | 1.8 | 0.7 | 0.0 |
| TAF/FTC | 0.0 | 0.0 | 0.0 | 19.3 |
| PI | 60.8 | 42.7 | 53.6 | 16.3 |
| NNRTI | 37.0 | 49.4 | 39.3 | 12.6 |
| INSTI | 2.2 | 7.9 | 7.1 | 71.1 |
Abbreviations: ABC/3TC, abacavir/lamivudine; INSTI, integrase strand transfer inhibitor; NNRTI, non-nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; TAF/FTC, tenofovir alafenamide fumarate/emtricitabine; TDF/FTC, tenofovir disoproxil fumarate/emtricitabine; 3TC/AZT, lamivudine/zidovudine.
Discussion
Evidence that all HIV-infected individuals should be treated regardless of CD4 counts has been provided in different clinical trials and recommended by different treatment guidelines.1–4 This recommendation aims to prevent further damage to the immune system, decreasing AIDS-associated morbidity and mortality, normalizing immune function, and reducing the risk of transmitting HIV infection to other individuals.1–9 Furthermore, the advancements of ART over the last 20 years, with easier to take regimens and fewer side effects, should help for its widespread establishment to all HIV positive patients. However, decision-making on treatment initiation remains a critical phase in the care of patients living with HIV and not all HIV-infected patients are under ART.
This study retrospectively evaluates the characteristics of a large cohort of HIV positive patients in Northwest of Spain who have started ART in the last 8 years. They presented a mean CD4 count of 403.8 ± 303.7 cells/mm3 at FirstSMT and a high rate (45.5%) of late presenters (CD4 count <350 cells/mm3). Overall, these data agree to those reported in our country in recent studies and from European Cohorts.10–14
Reducing late HIV diagnosis and AIDS events are considered a public health priority. In Spain, HIV testing is generally performed in case of risk behavior or clinical suspicion, but is not incorporated as a routine screening. Missed HIV testing opportunities contribute to the increase in late presenters.11,15 It is essential to promote appropriate strategies to detect HIV infections earlier and facilitate access to HIV test.
Moreover, 42.1% of patients postponed longer than 6 months treatment initiation and 54.1% of them required ART according to the national guidelines at that moment. Of them, half delayed it due to patients’ refusal of ART initiation (22.2%), even 2 or more times, and the other due to no indication of treatment in the previous follow-up (17.4%) and lack of compliance with the scheduled visits (14.5%). In a survey conducted in 9 countries in Europe and Australia to evaluate the reasons why patients were not under ART, the most common reasons were a refusal for treatment initiation and the perception of being asymptomatic and therefore not feel the need to be treated.16
The study was conducted in a health area providing coverage to 547 328 individuals in an extension of 2754 km2. This scenario might explain in part the lack of compliance with the scheduled visits, missed or multiple late appointments, because several patients live in rural zones, far from the high-level health center, and sometimes with limited resources to reach the hospital. Other potential barriers to ART access could be low social connectedness, incarceration, and psychiatric comorbidities and substance abuse that can complicate retention-in-care and thus may not benefit from ART.17
It is important to highlight that between FirstSMT and FirstART, mean CD4 count significantly decreased and 5.0% patients in CDC clinical category A moves to C category. These figures are in agreement with others related to a high-income setting.18
As national treatment guidelines recommend treating all HIV-infected patients regardless of CD4 count from 2014 leaves less opportunity to delay therapy. According to recent studies, there are different reasons that might explain rejection of ART initiation. Therefore, in the future, healthcare providers should try to identify individual barriers, and be more proactive given information regard the great benefits of ART, weighing up together with the patient the pros and cons of treatment to make them feel comfortable about their decision to start ART.
The main limitations of the study were small sample size, single-center study, and the retrospective observational design that might introduced uncontrolled bias.
In conclusion, these findings highlight the need to promote and facilitate HIV testing to reduce late diagnosis, promote early counseling interventions on HIV prevention, linkage to care and treatment.
Footnotes
Authors’ Note: The research protocol was approved by the regional ethic committee “Comité de Ética de la Investigación de A Coruña-Ferrol” (register code 2014/564). All participants provided written informed consent prior to enrolment in the study. Only those patients who had signed the informed consent were included.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services, 2016. http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. Accessed December 21, 2018.
- 2. Panel Members of European AIDS Clinical Society. European Guidelines for treatment of HIV-positive adults in Europe version 8.0. EACS, 2015. http://www.eacsociety.org/files/guidelines_8.0-english.pdf. Accessed December 21, 2018.
- 3. Panel de expertos de GeSIDA y Plan Nacional sobre el Sida. Documentos de consenso de Gesida/Plan Nacional sobre el Sida respecto al tratamiento antirretroviral en adultos infectados por el virus de la inmunodeficiencia humana. SEIMC, historical guidelines from 2011 to 2015. http://gesida-seimc.org/category/guias-clinicas/antirretroviral-historial/. Accessed December 21, 2018. [DOI] [PubMed]
- 4. Panel de expertos de GeSIDA y Plan Nacional sobre el Sida. Documento de consenso de GesiDa/Plan nacional sobre el sida respecto al tratamiento antirretroviral en adultos infectados por el virus de la inmunodeficiencia humana (Actualización enero 2017). SEIMC, 2017. http://gesida-seimc.org/wp-content/uploads/2017/02/gesida-guiasclinicas-2017-TAR.pdf. Accessed December 21, 2018. [DOI] [PubMed]
- 5. INSIGHT START Study Group; Lundgren JD, Babiker AG, Gordin F, et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med. 2015;373(9):795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. TEMPRANO ANRS 12136 Study Group; Danel C, Moh R, Gabillard D, et al. A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med. 2015;373(9):808–822. [DOI] [PubMed] [Google Scholar]
- 7. Grinsztejn B, Hosseinipour MC, Ribaudo HJ, et al. ; HPTN 052-ACTG Study Team. Effects of early versus delayed initiation of antiretroviral treatment on clinical outcomes of HIV-1 infection: results from the phase 3 HPTN 052 randomised controlled trial. Lancet Infect Dis. 2014;14(4):281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cohen MS, Chen YQ, McCauley M, et al. ; HPTN 052 Study Team. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. World Health Organization. Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. WHO, 2015. http://apps.who.int/iris/bitstream/10665/186275/1/9789241509565_eng.pdf?ua=1. Accessed May 29, 2017. [PubMed]
- 10. Área de Vigilancia de VIH y Comportamientos de Riesgo. Vigilancia Epidemiológica del VIH y sida en España: Sistema de Información sobre Nuevos Diagnósticos de VIH y Registro Nacional de Casos de Sida. Plan Nacional sobre el Sida - S.G. de Promoción de la Salud y Epidemiología/Centro Nacional de Epidemiología – ISCIII, 2015. http://www.msssi.gob.es/ciudadanos/enfLesiones/enfTransmisibles/sida/vigilancia/InformeVIH_SIDA_2015.pdf. Accessed May 29, 2017.
- 11. Gullón A, Verdejo J, de Miguel R, Gómez A, Sanz J. Factors associated with late diagnosis of HIV infection and missed opportunities for earlier testing. AIDS Care. 2016;28(10):1296–1300. [DOI] [PubMed] [Google Scholar]
- 12. Pernas B, Mena A, Cañizares A, et al. Trends on epidemiological, virological, and clinical features among newly diagnosed HIV-1 persons in Northwest Spain over the last 10 years. J Med Virol. 2015;87(8):1319–1326. [DOI] [PubMed] [Google Scholar]
- 13. Sobrino-Vegas P, García-San Miguel L, Caro-Murillo AM, et al. Delayed diagnosis of HIV infection in a multicenter cohort: prevalence, risk factors, response to HAART and impact on mortality. Curr HIV Res. 2009;7(2):224–230. [DOI] [PubMed] [Google Scholar]
- 14. Late Presenters Working Group in COHERE Study in EuroCoord; Mocroft A, Lundgren J, Antinori A, et al. Late presentation for HIV care across Europe: update from the collaboration of observational HIV epidemiological research Europe (COHERE) study, 2010 to 2013. Euro Surveill. 2015;20(47). [DOI] [PubMed] [Google Scholar]
- 15. Brännström J, Svedhem VJ, Marrone G, et al. Deficiencies in the health care system contribute to a high rate of late HIV diagnosis in Sweden. HIV Med. 2016;17(6):425–435. [DOI] [PubMed] [Google Scholar]
- 16. Fehr J, Nicca D, Goffard JC, et al. Reasons for not starting antiretroviral therapy in HIV-1-infectec individuals: a changing landscape. Infection. 2016;44(4):521–529. [DOI] [PubMed] [Google Scholar]
- 17. Gardner L, Marks G, Shahani L, et al. Assessing efficacy of a retention-in-care intervention among HIV patients with depression, anxiety, heavy alcohol consumption and illicit drug use. AIDS. 2016;30(7):1111–1119. [DOI] [PubMed] [Google Scholar]
- 18. Dragovic G, Smith CJ, Jevtovic D, et al. Choice of first-line antiretroviral therapy regimen and treatment outcomes for HIV in a middle income compared to a high income country: a cohort study. BMC Infect Dis. 2016;16:106. [DOI] [PMC free article] [PubMed] [Google Scholar]