Abstract
Background:
Persons with HIV have elevated risk for cardiovascular disease, but little is known about the risk of ventricular ectopy and ventricular tachycardia (VE/VT) for HIV-infected (HIV+) persons.
Methods:
We evaluated the presence and anatomic origin of VE/VT for HIV+ persons and controls by screening a cohort using International Classification of Diseases codes and adjudicating positive screens by chart review. We sought to evaluate (1) presence of VE/VT and (2) likely anatomic origin of the VE/VT based on electrocardiogram.
Results:
There was no significant difference in the prevalence of VE/VT for HIV+ or uninfected persons. Among HIV+ persons, worse HIV control was associated with significantly greater odds of VE/VT. Exploratory analyses suggested that HIV+ persons may have a greater likelihood of VE/VT originating from the left ventricle.
Conclusion:
Although worse HIV control was associated with higher odds of VE/VT among persons with HIV, odds of VE/VT were not higher for persons with HIV than uninfected persons.
Keywords: HIV, AIDS, ventricular arrhythmia, ventricular ectopy, sudden cardiac death
What Do We Already Know About This Topic?
Persons living with HIV have higher burdens of cardiovascular disease than the general population, including sudden cardiac death.
How Does This Research Contribute to the Field?
Ventricular arrhythmia is the most common cause of sudden cardiac death, however little is known regarding rates and type of ventricular arrhythmia in persons living with HIV compared to the general population.
What Are the Implications for Theory, Practice, or Policy?
Differences in rates or types of ventricular arrythmia in persons living with HIV could prompt more aggressive detection and prevention of ventricular arrhythmia in the HIV population, new avenues of research exploring possible factors contributing to ventricular arrhythmia in this population, and efforts to increase access to adequate cardiovascular care.
Introduction
With widespread use of effective antiretroviral therapy (ART), HIV has transitioned from being fatal within a few years of contraction to a chronic disease marked by high rates of noncommunicable disease complications.1 Cardiovascular disease (CVD) is now a leading cause of morbidity and mortality in the HIV-infected (HIV+) population.1-3 Persons living with HIV are at elevated risk of various manifestations of CVD, including myocardial infarction, pulmonary hypertension, congestive heart failure, and atrial arrhythmias.2,4-10 Furthermore, HIV+ persons appear to have a several-fold greater risk of sudden cardiac death (SCD) than uninfected persons.7 Ventricular arrhythmia is the most common cause of SCD in adults, but little is known about the prevalence and origin of ventricular ectopy and ventricular tachycardia (VE/VT) in HIV+ persons. Potential contributors to VE/VT in HIV may include direct viral effects on cardiomyocytes, an increased inflammatory state in HIV+ persons, differences in exposure to QTC-prolonging drugs, and/or changes in the myocardial substrate due to differences in myocardial scar formation observed in previous studies.11 Although prior studies have evaluated electrocardiogram (ECG) findings in people with HIV on ART as well as SCD in HIV, none to our knowledge has investigated VE/VT and related morphology.7,12,13
The purpose of this study was to evaluate the presence, anatomical origin, and clinical factors associated with VE/VT for HIV+ persons and uninfected controls. We used a large cohort of HIV+ and uninfected persons receiving care at a large, urban medical center to screen for possible VE/VT and subsequently performed physician adjudication of presence and morphology of VE/VT. Our central hypotheses were (1) VE/VT is more prevalent for HIV+ persons than uninfected controls and (2) lower nadir CD4 count and higher peak HIV viral load are associated with greater odds of VE/VT among HIV+ persons.
Methods
Study Population
We studied a nested cohort of the HIV Electronic Comprehensive Cohort of CVD Complications (HIVE-4CVD), which consists of HIV+ and uninfected persons receiving care at Northwestern Medicine (Chicago, Illinois) frequency matched on age, sex, race/ethnicity, and Zip code of residence.11,14,15 Clinical data within HIVE-4CVD were obtained during the course of clinical care between January 1, 2000, and July 12, 2016, and available for analysis for all study participants. Participants were excluded from analysis for this study if they had no visits with body weight recorded in the medical record, as the absence of this data point suggests a lack of meaningful clinical visits and interaction in the health system. This left an analyzable population of 4656 HIV+ and 5002 uninfected persons for this study.
Screening and Adjudication of Ventricular Ectopy and Ventricular Tachycardia
HIVE-4CVD participants were screened for VE/VT using administrative codes (International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) 427.1, 427.4, 427.41, 427.42, 427.5, 427.69; ICD-10-CM I46, I49.01, I49.02). Two physicians then independently reviewed charts of persons with administrative codes suggesting VE/VT to confirm VE/VT diagnosis based on (1) physician note confirming diagnosis of ventricular ectopy or arrhythmia and/or (2) ECG or other rhythm monitor data demonstrating VE/VT. Ventricular ectopy/VT was defined as any premature ventricular contractions, nonsustained ventricular tachycardia (NSVT), or ventricular tachycardia (VT). Patient charts were then reviewed for the presence of VE/VT in any of the following: (1) ECG, (2) non-ECG electrocardiographic studies including Holter monitors, event monitors, and cardiac stress testing results, and (3) prior physician documentation of VE/VT. If ECG data were available, these data were used preferentially over non-ECG studies to determine the presence and morphologic characteristics of the VE/VT. If no electrocardiographic studies were available, physician notes were evaluated for physician diagnosis of VE/VT. The most recent progress note, cardiology note, and discharge summary were evaluated for mention of VE/VT. If none of these contained mention of VE/VT, the chart review was stopped and the patient was determined not to have confirmed VE/VT.
Characterization of the Anatomical Origin of Ventricular Ectopy and Ventricular Tachycardia
For each patient with VE/VT confirmed electrocardiographically, the earliest ECG or electrocardiographic study with evidence of VE/VT was used to determine morphology of the VE/VT. Lead V1 was examined to determine left or right bundle branch block morphology. Leads II, III, and aVF were examined to determine inferior or superior axis. Ventricular ectopy/VT with a left bundle branch morphology and inferior axis was considered outflow tract origin, VE/VT with a right bundle branch morphology and superior axis was considered to be left ventricular (LV) in origin, and other combinations of morphology were not classified as either outflow tract or LV origin. This method of classifying ventricular arrhythmia is in accordance with current standard electrophysiologic practices.16 If the first study did not have the proper leads to make a judgement on the anatomical origin, or if the morphology of the VE/VT was indeterminate, no determination of morphology was made or recorded. If no electrocardiographic data were available but prior physician documentation described VE/VT, then the presence of VE/VT was noted but no anatomic origin-related characteristics were recorded. Concordance rates of presence and origin of VE/VT were ≥95% for the 2 adjudicating physicians; disputes were resolved by consensus.
Exposures and Covariates
The primary exposure variables of interest were presence or absence of HIV infection and, for analyses among HIV+ persons, nadir CD4+ lymphocyte count (cells/mm3) and peak HIV viral load (copies/mL). HIV diagnosis was defined by validated criteria we have previously described which consisted of (1) positive HIV-1 antibody, antigen, or serology, (2) HIV viral load greater than the lower limit of detection, and/or (3) concurrent orders of CD4 count and HIV viral load on at least 2 dates.15,17 Covariates included age, sex, race/ethnicity (white non-Hispanic, black non-Hispanic, Hispanic, or other/unknown), diabetes, and hypertension. Diabetes was defined based on administrative codes and either a hemoglobin A1c value >6.5% or prescription of any diabetic medication.15 Hypertension was defined by administrative codes because of the potential for systematic differences in blood pressure values for participants with different frequencies of inpatient versus outpatient visits.15 Additional descriptive covariates included a history of myocardial infarction (MI) based on diagnosis codes that have demonstrated adequate levels of agreement with chart review (ICD-9-CM 410-412, ICD-10-CM 121-123)18-20 and heart failure (HF) hospitalization (defined as at least one inpatient administrative code of the following: ICD-10 398.91, 402.01, 402.11, 402.91, 404.01, 404.03, 404.11, 404.13, 404.91, 404.93, 425, 428, 429; or ICD-10 I42, I43, I50).21,22
Statistical Analysis
The primary outcome was the presence of VE/VT, which was compared (1) for HIV+ versus uninfected persons and (2) by nadir CD4 count and peak HIV viral load among HIV+ persons. An exploratory analysis evaluated anatomical origins of VE/VT for HIV+ persons versus uninfected controls. We used nonconditional logistic regression to evaluate the odds of VE/VT for HIV+ versus uninfected persons in sequentially adjusted models. For comparison of odds of VE by HIV serostatus, we used 3 models, which were (1) unadjusted, (2) adjusted for age, sex, and race, and (3) adjusted for age, sex, race, hypertension diabetes, and body mass index (BMI). Model 3 was chosen as the primary model on which to report for this comparison as it accounted for the highest number of factors that could confound the relationship between HIV status and VE/VT. For analyses of VE/VT among HIV+ persons, we used nonconditional logistic regression to compare odds of VE/VT per each increase in nadir CD4 count of 100 cells/mm3 and each log10 copies/mL increase in peak HIV viral load. These analyses were also done using models that were (1) unadjusted, (2) adjusted for age, sex, and race, (3) adjusted for age, sex, race, hypertension, diabetes, and BMI, and (4) adjusted for age, sex, race, hypertension, diabetes, BMI, use of ART, and use of protease inhibitors. Model 4 was chosen as the primary model on which to report for this comparison as it accounted for the highest number of factors that could confound associations of CD4 count and HIV viral load with VE/VT. For the exploratory analysis, we compared VE/VT anatomical origin (outflow tract and LV origin) for HIV+ versus uninfected persons with VE/VT for whom morphology data were available.
Results
Of 4656 HIV+ and 5002 uninfected persons included for analysis, 138 HIV+ persons and 160 uninfected controls had confirmed diagnoses of VE/VT. Table 1 displays demographic and clinical characteristics of the 4656 HIV+ and 5002 uninfected persons analyzed overall. Similar proportions of HIV+ and uninfected persons were male and hypertensive; there were small but statistically significant differences in age and the prevalence of diabetes between groups. Persons with HIV had significantly lower BMI than uninfected persons and were more likely to have a history of HF hospitalization and MI. These differences between HIV+ and uninfected persons largely remained when we compared characteristics among those with adjudicated VE/VT (Table 2). For both sets of analyses, the vast majority of HIV+ persons had been on ART at some point, which is reflective of contemporary HIV care. When we analyzed the prevalence of VE/VT for HIV+ versus uninfected persons, there was no significant difference in the odds of VE/VT between groups (Table 3).
Table 1.
Demographic and Clinical Characteristics (N = 9658).
| HIV+ (n = 4656) | Uninfected Controls (n = 5002) | P Value | |
|---|---|---|---|
| Age (mean ± SD) | 49.3 ± 11.4 | 48.3 ± 11.4 | <.001 |
| White (n, %) | 1638, 35.5 | 2100, 43.6 | <.001 |
| Black (n, %) | 1525, 31.7 | 1510, 32.8 | |
| Hispanic (n, %) | 206, 4.3 | 166, 3.6 | |
| Unknown or other race/ethnicity (n, %) | 985, 20.5 | 1297, 28.1 | |
| Male sex (n, %) | 3978, 83.0 | 4217, 81.7 | .09 |
| Hypertension (n, %) | 1657, 34.6 | 1720, 33.3 | .19 |
| Diabetes (n, %) | 456, 9.5 | 560, 10.9 | .03 |
| BMI (mean ± SD) | 25.9 ± 5.6 | 28.7 ± 6.5 | <.001 |
| History of HF hospitalization (n, %) | 542 (11.3%) | 429 (8.3%) | <.001 |
| History of MI (n, %) | 569 (12.2%) | 464 (9.3%) | <.001 |
| Ever on ART (n, %) | 4205, 87.7 | N/a | N/a |
| Ever on PI (n, %) | 2336, 48.7 | N/a | N/a |
Abbreviations: ART, antiretroviral therapy; BMI, body-mass index; MI, myocardial infarction; PI, protease inhibitor; SD, standard deviation; HF, heart failure.
Table 2.
Demographic and Clinical Characteristics of Participants with Adjudicated VE/VT.
| HIV+ (n = 138) | Uninfected Controls (n = 160) | P Value | |
|---|---|---|---|
| Age (mean ± SD) | 58.6 ± 11.7 | 56.6 ± 10.0 | .11 |
| Male sex (n, %) | 115, 83.3 | 136, 85 | .15 |
| White (n,%) | 51, 37.8 | 70, 46.7 | .32 |
| Black (n, %) | 60, 44.4 | 62, 42.3 | |
| Hispanic (n,%) | 6, 4.4 | 3, 2 | |
| Other or unknown race/ethnicity (n, %) | 18, 13.3 | 15, 10 | |
| Hypertension (n, %) | 118, 85.5 | 117, 73.1 | <.05 |
| Diabetes (n, %) | 44, 31.9 | 48, 30.0 | .73 |
| BMI (mean ± SD) | 26.6 ± 7.7 | 29.4 ± 7.0 | <.001 |
| History of HF hospitalization (n, %) | 81, 58.7 | 86, 53.8 | .39 |
| History of MI (n, %) | 80, 58.0 | 88, 55.0 | .61 |
| Ever on ART (n, %) | 126, 91.3 | N/a | N/a |
| Ever on protease inhibitor (n, %) | 79, 57.3 | N/a | N/a |
Abbreviations: ART, antiretroviral therapy; BMI, body-mass index; MI, myocardial infarction; SD, standard deviation; VE, ventricular ectopy; VT, ventricular tachycardia.
Table 3.
Odds Ratio of VE/VT by HIV Status in Sequentially Adjusted Models.
| Model 1 | P | Model 2 | P | Model 3 | P | |
|---|---|---|---|---|---|---|
| HIV+ (versus uninfected) | 0.93 (0.74-1.17) | .52 | 1.02 (0.80-1.29) | .89 | 0.95 (0.74-1.23) | .71 |
| Age | 1.07 (1.06-1.08) | <.001 | 1.04 (1.03-1.06) | <.001 | ||
| Male sex | 0.95 (0.68-1.32) | .75 | 0.95 (0.67-1.35) | .78 | ||
| White | Referent | Referent | ||||
| Black | 1.56 (1.19-2.04) | .001 | 1.18 (0.89-1.57) | .24 | ||
| Hispanic | 1.08 (0.540-2.16) | .83 | 0.83 (0.39-1.74) | .61 | ||
| HTN | 4.76 (3.43-6.61) | <.001 | ||||
| DM | 1.76 (1.33-2.32) | <.001 | ||||
| BMI | 1.00 (0.98-1.02) | .89 |
Abbreviations: BMI, body-mass index; DM, diabetes mellitus; HTN, hypertension; VE, ventricular ectopy; VT, ventricular tachycardia.
Among HIV+ persons, higher nadir CD4 count (less immune suppression) was associated with a significantly lower odds of VE/VT (Table 4). This pattern was demonstrated in unadjusted analyses (odds ratio 0.83, 95% confidence interval [CI]: 0.75-0.91 per 100 cells/mm3 greater CD4 count) and was not substantially attenuated after adjustment for age, sex, and race, after additional adjustment for diabetes and hypertension, and after further adjustment for protease inhibitor and ART use. Similarly, worse HIV viral control was associated with a greater odds of VE/VT (Table 5), with each log10 increase in peak HIV viral load associated with a 1.24 greater odds (95% CI: 1.06-1.45) of VE/VT after adjustment for age, sex, race, diabetes, hypertension, protease inhibitor use, and ART use.
Table 4.
Odds Ratios of VE/VT as CD4 Counts Decrease among HIV+ Persons.
| Model 1 | P | Model 2 | P | Model 3 | P | Model 4 | P | |
|---|---|---|---|---|---|---|---|---|
| Nadir CD4 counta | 0.83 (0.75-0.91) | <.001 | 0.89 (0.81-0.98) | .020 | 0.87 (0.78-0.96) | .008 | 0.87 (0.78-0.97) | .010 |
| Age | 1.09 (1.07-1.11) | <.001 | 1.06 (1.04-1.08) | <.001 | 1.06 (1.04-1.08) | <.001 | ||
| Male sex | 1.07 (0.66-1.73) | .78 | 1.07 (0.64-1.79) | .79 | 1.07 (0.64-1.79) | .80 | ||
| White | Referent | Referent | Referent | |||||
| Black | 1.48 (0.99-2.24) | .05 | 1.17 (0.76-1.81) | .47 | 1.17 (0.76-1.81) | .48 | ||
| Hispanic | 1.68 (0.69-4.09) | .25 | 1.15 (0.42-3.15) | .78 | 1.15 (0.42-3.15) | .79 | ||
| HTN | 7.69 (4.35-13.62) | <.001 | 7.70 (4.34-13.7) | <.001 | ||||
| DM | 1.76 (1.17-2.64) | .006 | 1.76 (1.17-2.64) | .007 | ||||
| BMI | 1.01 (0.98-1.05) | .36 | 1.01 (0.98-1.05) | .36 | ||||
| Ever on ART | 0.96 (0.45-1.57) | .92 | ||||||
| Ever on PI | 1.04 (0.69-1.57) | .84 | ||||||
Abbreviations: ART, antiretroviral therapy; BMI, body-mass index; DM, diabetes mellitus; HTN, hypertension; PI, protease inhibitor; VE, ventricular ectopy; VT, ventricular tachycardia.
aOdds ratios for VE/VT displayed for every 100 cells/mm3 increase in nadir CD4 counts.
Table 5.
Odds Ratio of VE/VT by Peak HIV Viral Load among HIV+ Persons.
| Model 1 | P | Model 2 | P | Model 3 | P | Model 4 | P | |
|---|---|---|---|---|---|---|---|---|
| Peak HIV viral loada | 1.10 (0.95-1.27) | .21 | 1.21 (1.04-1.40) | .01 | 1.24 (1.06-1.45) | .007 | 1.24 (1.06-1.45) | .007 |
| Age | 1.09 (1.07-1.11) | <.001 | 1.06 (1.04-1.08) | <.001 | 1.06 (1.04-1.08) | <.001 | ||
| Male sex | 1.24 (0.74-2.06) | .41 | 1.25 (0.72-2.15) | .43 | 1.25 (0.72-2.17) | .42 | ||
| White | Referent | Referent | Referent | |||||
| Black | 1.47 (0.939-2.31) | .09 | 1.07 (0.66-1.74) | .79 | 1.08 (0.67-1.76) | .75 | ||
| Hispanic | 1.68 (0.63-4.45) | .30 | 1.02 (0.33-3.13) | .97 | 1.02 (0.33-3.14) | .97 | ||
| Diagnosis of hypertension | 9.14 (4.73-17.7) | <.001 | 9.00 (4.65-17.4) | <.001 | ||||
| Diagnosis of diabetes mellitus | 1.92 (1.23-3.01) | .004 | 1.92 (1.22-3.01) | .004 | ||||
| Mean BMI | 1.00 (0.97-1.04) | .86 | 1.00 (0.97-1.04) | .88 | ||||
| Ever on ART | 1.24 (0.52-2.96) | .63 | ||||||
| Ever on protease inhibitor | 1.07 (0.68-1.69) | .77 | ||||||
Abbreviations: ART, antiretroviral therapy; BMI, body-mass index; VE, ventricular ectopy; VT, ventricular tachycardia.
aOdds ratios are for each log10 increase in peak HIV viral load.
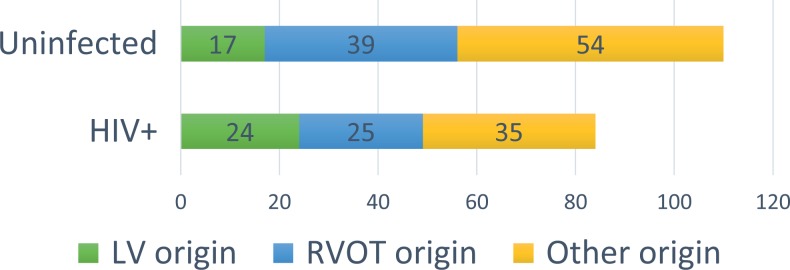
In exploratory analyses of the VE/VT anatomical origin (outflow tract, LV, or other), anatomical origin was able to be classified for 84 HIV+ and 110 uninfected persons (Figure 1). Of these, 24 (28.6%) HIV+ versus 17 (15.5%) uninfected persons had LV origin VE/VT. Meanwhile, 25 (29.8%) HIV+ and 39 (35.5%) uninfected persons had outflow tract VE/VT (P = .40).
Figure 1.
Anatomic origin by electrocardiographic characteristics for HIV+ individuals compared to matched, uninfected controls. Ectopy or arrythmia was classified as high right ventricle morphology, left ventricular outflow tract morphology, or unable to be classified. LVOT indicates left ventricular outflow tract; RV, right ventricle.
Discussion
In this study, we found that markers of HIV severity (higher peak viral load, worse immunosuppression) were associated with greater odds of VE/VT, but there was no significant difference in the presence of VE/VT for HIV+ versus uninfected persons overall. Exploratory analyses suggested that differences in the anatomical origins of VE/VT may exist for HIV+ versus uninfected persons, with HIV+ persons being more likely to have VE/VT originating from the LV.
This is the first study to our knowledge comparing prevalence and electrocardiographic morphology of VE/VT for HIV+ persons versus uninfected controls. Based on previous studies finding elevated rates of SCD among HIV+ persons7 and the fact that ventricular arrhythmia is commonly associated with an increased risk of SCD in the general population,23,24 we hypothesized that HIV+ persons would have a significantly greater prevalence of VE/VT than uninfected persons. Although we did not find a difference in the prevalence of VE/VT for HIV+ versus uninfected persons in this study, our exploratory analysis suggested different patterns of VE/VT anatomical origin for HIV+ versus uninfected persons, whereby HIV+ persons had somewhat more VE/VT originating in the LV. This finding, taken together with (1) prior studies demonstrating more myocardial fibrosis, greater rates of MI, and more extensive post-MI myocardial scarring for HIV+ versus uninfected persons5,11,25-27 and (2) the poor prognosis of ventricular arrhythmias in the setting of structural heart disease,28 raises the possibility that malignant ventricular arrhythmia is implicated in higher SCD rates among HIV+ persons. This determination is outside the scope of the present analysis but underscores the importance of future, larger studies evaluating ventricular arrhythmias and potential mechanisms of SCD in HIV.
We also found that, among HIV+ persons, worsening markers of disease severity—lower CD4 counts and higher HIV viral loads—were associated with significantly greater odds of VE/VT. While not altogether surprising, these differences are potentially meaningful; based on our findings, for instance, an HIV+ person with a nadir CD4 count of 700 cells/mm3 would have approximately one-third the odds of VE/VT compared with someone with nadir CD4 count 200 cells/mm3. Likewise, with peak HIV viral loads ranging considerably and with greater than single log10 differences common between HIV+ persons,29 the finding that a 2-4 log10 difference in peak HIV viral load is associated with a 1.5- to 2-fold greater odds of VE/VT may be clinically meaningful.
Despite these novel findings, there are several limitations of this study to acknowledge. The primary end point in this study, VE/VT, is not clinical and has inherent heterogeneity—a single ventricular ectopic beat certainly has different clinical implications than sustained monomorphic VT. We were unable to evaluate VT alone in this study because there were insufficient adjudicated VT events for sufficiently powered analyses. This is likely due to the low overall prevalence of symptomatic, clinically captured VT as well as our rigorous VE/VT adjudication criteria, which used administrative codes for screening but required physician diagnosis and/or electrocardiographic evidence for diagnosis. Although diagnosis codes alone may be adequate for identifying fatal arrhythmias, their performance is less consistent when applied to other arrhythmias (including ventricular ectopic beats and NSVT).30,31 Furthermore, physician chart review enabled us to identify the morphology and likely anatomic origins of VE/VT in this study.
Because this is a single-center observational study that is retrospective in nature, our ability to make causal inferences is limited. The findings of this study, which was performed in a single large urban medical center in the United States, may not be generalizable to other care settings. This was unavoidable given the nature of our cohort, which offered the unique opportunity to comprehensively evaluate VE/VT for HIV+ persons in clinical care. We judged these limitations to be acceptable given the novel focus of this study in the context of emerging data on HIV-associated cardiovascular risks.
Conclusions
There was no significant difference in the presence of ventricular arrhythmias for HIV+ versus uninfected persons. Among HIV+ persons, worse viral and immunologic status, as manifested by higher HIV viral load and lower CD4 count, is associated with significantly greater odds of ventricular arrhythmias. The anatomical origins of ventricular arrhythmias may differ for HIV+ versus uninfected persons; future studies should investigate ventricular arrhythmia origins among HIV+ persons.
Footnotes
Authors’ Note: Our study was approved by the Northwestern University IRB (approval number STU00200433). All patients provided written informed consent prior to enrollment in the study.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the American Heart Association Fellow-to-Faculty Award [16FTF31200010; PI: Feinstein].
ORCID iD: Alexander J. Meyer  https://orcid.org/0000-0003-0484-4465
https://orcid.org/0000-0003-0484-4465
References
- 1. Palella FJ, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV outpatient study investigators. N Engl J Med. 1998;338(13):853–860. doi:10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 2. Feinstein MJ, Bahiru E, Achenbach C, et al. Patterns of cardiovascular mortality for HIV-infected adults in the United States: 1999 to 2013. Am J Cardiol. 2016;117(2):214–220. doi:10.1016/j.amjcard.2015.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Trickey A, May MT, Vehreschild J, et al. Cause-specific mortality in HIV-positive patients who survived ten years after starting antiretroviral therapy. PLoS One. 2016;11(8):e0160460 doi:10.1371/journal.pone.0160460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Butt AA, Chang C-C, Kuller L, et al. Risk of heart failure with human immunodeficiency virus in the absence of prior diagnosis of coronary heart disease. Arch Intern Med. 2011;171(8):737–743. doi:10.1001/archinternmed.2011.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Anne-Lise P, Chang C-CH, So-Armah KA, et al. Human immunodeficiency virus infection, cardiovascular risk factor profile and risk for acute myocardial infarction. J Acquir Immune Defic Syndr. 2015;68(2):209–216. doi:10.1097/QAI.0000000000000419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hsu JC, Li Y, Marcus GM, et al. Atrial fibrillation and atrial flutter in human immunodeficiency virus-infected persons: incidence, risk factors, and association with markers of HIV disease severity. J Am Coll Cardiol. 2013;61(22):2288–2295. doi:10.1016/j.jacc.2013.03.022. [DOI] [PubMed] [Google Scholar]
- 7. Tseng ZH, Secemsky EA, Dowdy D, et al. Sudden cardiac death in patients with human immunodeficiency virus infection. J Am Coll Cardiol. 2012;59(21):1891–1896. doi:10.1016/j.jacc.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Barnett CF, Hsue PY. Human immunodeficiency virus–associated pulmonary arterial hypertension. Clin Chest Med. 2013;34(2):283–292. doi:10.1016/j.ccm.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Friis-Møller N, Ryom L, Smith C, et al. An updated prediction model of the global risk of cardiovascular disease in HIV-positive persons: the data-collection on adverse effects of anti-HIV drugs (D: A: D) study. Eur J Prev Cardiol. 2015;23(2):214–223. doi:10.1177/2047487315579291. [DOI] [PubMed] [Google Scholar]
- 10. Idris NS, Grobbee DE, Burgner D, et al. Cardiovascular manifestations of HIV infection in children. Eur J Prev Cardiol. 2014;22(11):1452–1461. doi:10.1177/2047487314560086. [DOI] [PubMed] [Google Scholar]
- 11. Feinstein MJ, Mitter SS, Yadlapati A, et al. HIV-Related myocardial vulnerability to infarction and coronary artery disease. J Am Coll Cardiol. 2016;68(18):2026–2027. doi:10.1016/j.jacc.2016.07.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Soliman EZ, Lundgren JD, Roediger MP, et al. Boosted protease inhibitors and the electrocardiographic measures of QT and PR durations. AIDS. 2011;25(3):367–377. doi:10.1097/QAD.0b013e328341dcc0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dawood FZ, Roediger MP, Grandits G, et al. Determinants of developing widened spatial QRS-T angle in HIV-infected individuals: results from the Strategies for Management of Antiretroviral Therapy [SMART] study. J Electrocardiol. 2014;47(2):264–271. doi:10.1016/j.jelectrocard.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sanders JM, Steverson AB, Pawlowski AE, et al. Atrial arrhythmia prevalence and characteristics for human immunodeficiency virus-infected persons and matched uninfected controls. PLoS One. 2018;13(3):1–11. doi:10.1371/journal.pone.0194754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Steverson AB, Pawlowski AE, Schneider D, et al. Clinical characteristics of HIV-infected patients with adjudicated heart failure. Eur J Prev Cardiol. 2017;24(16):1746–1758. doi:10.1177/2047487317732432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Al-khatib SM, Ackerman MJ, Gillis AM, et al. 2017 AHA/ACC/hrs guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. 2017. doi:10.1161/CIR.0000000000000549.
- 17. Felsen UR, Bellin EY, Cunningham CO, Zingman BS. Development of an electronic medical record-based algorithm to identify patients with unknown HIV status. AIDS Care. 2014;26(10):1318–1325. doi:10.1080/09540121.2014.911813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Quan H, Li B, Saunders LD, et al. Assessing validity of ICD-9-CM and ICD-10 administrative data in recording clinical conditions in a unique dually coded database. 2007:1424–1441. doi:10.1111/j.1475-6773.2007.00822.x. [DOI] [PMC free article] [PubMed]
- 19. Mccormick N, Lacaille D, Bhole V, Avina-zubieta JA. Validity of myocardial infarction diagnoses in administrative databases: a systematic review. 2014;9(3). doi:10.1371/journal.pone.0092286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Patel AB, Quan H, Welsh RC, et al. Validity and utility of ICD-10 administrative health data for identifying ST- and non-ST-elevation myocardial infarction based on physician chart review. CMAJ Open. 2015;3(4):E413–E418. doi:10.9778/cmajo.20150060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Iribarren C, Tolstykh I V, Miller MK, Sobel E, Eisner MD. Adult asthma and risk of coronary heart disease, cerebrovascular disease, and heart failure: a prospective study of 2 matched cohorts. Am J Epidemiol. 2012;176(11):1014–1024. doi:10.1093/aje/kws181. [DOI] [PubMed] [Google Scholar]
- 22. Saczynski JS, Andrade SE, Harrold LR, et al. Mini-sentinel systematic evaluation of health outcome of interest definitions for studies using administrative and claims data: heart failure. Pharmacoepidemiol Drug Saf. 2012;21(0 1):10.1002/pds.2313. doi:0.1002/pds.2313. [Google Scholar]
- 23. Bikkina M, MG L, Levy D. Prognostic implications of asymptomatic ventricular arrhythmias: the Framingham Heart Study. Ann Intern Med. 1992;117(12):990–996. doi:10.7326/0003-4819-117-12-990. [DOI] [PubMed] [Google Scholar]
- 24. Ataklte F, Erqou S, Laukkanen J, Kaptoge S. Meta-analysis of ventricular premature complexes and their relation to cardiac mortality in general populations. Am J Cardiol. 2013;112(8):1263–1270. doi:10.1016/j.amjcard.2013.05.065. [DOI] [PubMed] [Google Scholar]
- 25. Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92(7):2506–2512. doi:10.1210/jc.2006-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Post WS, Budoff M, Kingsley L, et al. Associations between HIV infection and subclinical coronary atherosclerosis: the multicenter AIDS cohort study (MACS). Ann Intern Med. 2014;160(7):458–467. doi:10.7326/M13-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Holloway CJ, Ntusi N, Suttie J, et al. Comprehensive cardiac magnetic resonance imaging and spectroscopy reveal a high burden of myocardial disease in HIV patients. Circulation. 2013. 128(8):814–822. http://circ.ahajournals.org/content/128/8/814.abstract. Accessed January 27, 2019. [DOI] [PubMed] [Google Scholar]
- 28. Anderson KP, Decamilla J, Moss AJ. Clinical significance of ventricular tachycardia (3 beats or longer) detected during ambulatory monitoring after myocardial infarction. Circulation. 1978;57(5):890–897 [DOI] [PubMed] [Google Scholar]
- 29. Davenport MP, Zhang L, Shiver JW, Casmiro DR, Ribeiro RM, Perelson AS. Influence of peak viral load on the extent of CD4+ T-cell depletion in simian HIV infection. J Acquir Immune Defic Syndr. 2006;41(3):259–265. doi:10.1097/01.qai.0000199232.31340.d3. [DOI] [PubMed] [Google Scholar]
- 30. Hennessy S, Leonard CE, Newcomb C, Kimmel SE, Bilker WB. Cisapride and ventricular arrhythmia. Br J Clin Pharmacol. 2008;66(3):375–385. doi:10.1111/j.1365-2125.2008.03249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McDonald KM, Hlatky MA, Saynina O, Geppert J, Garber AM, McClellan MB. Trends in hospital treatment of ventricular arrhythmias among Medicare beneficiaries, 1985 to 1995. Am Heart J. 2018;144(3):413–421. doi:10.1067/mhj.2002.125498. [DOI] [PubMed] [Google Scholar]