Abstract
Despite advances in coverage and quality of prevention of mother-to-child transmission (PMTCT) programs, infant protection from postnatal HIV infection remains an issue in high HIV-burdened countries. We designed a quality improvement (QI) intervention—the Partnership for HIV-Free Survival (PHFS)—to improve infant survival. PHFS convened leaders in 6 sub-Saharan African nations to discover together the best strategies for implementing and scaling up existing PMTCT protocols to ensure optimal health of mother–baby pairs and HIV-free infant survival. We used 3 core technical components—rapid adaptive design, collaborative learning, and scale-up/sustainability designs—to test strategies for accelerating effective PMTCT programming in complex, resource-poor settings. Learning generated included the need for increased ownership and codesign of improvement initiatives with Ministries of Health, better integration of initiatives into existing programs, and the need to sustain QI capability throughout the system. PHFS can serve as a design prototype for future global networks aiming to accelerate improvement, learning, and results.
Keywords: quality improvement, collaboration, PMTCT, HIV, nutrition
What Do We Already Know about This Topic?
In contrast to success of PMTCT during pregnancy, PMTCT for HIV-I infected breast-feeding mothers remains problematic.
How Does Your Research Contribute to the Field?
This is the first report of effectiveness of using QI in a large scale multi-country effort to reduce post-natal MTCT in breast feeding HIV-positive mothers.
What Are Your Research’s Implications toward Theory, Practice, or Policy?
More rapid improvement and greater country uptake may have been possible with earlier engagement of Ministries of Health.
Background
Countries with the highest burden of HIV in Africa have shown significant improvement in coverage rates for antenatal prevention of mother-to-child transmission (PMTCT) treatment using quality improvement (QI) approaches together with policy and protocol changes.1-4 Despite these advances, the protection of infants from postnatal HIV infection through breast milk and nutritional management of these infants remains a major issue in all high-burden countries, significantly impacting their survival.5,6
The World Health Organization (WHO) provides health system planners and implementers with updated clinical content to help combat the HIV/AIDS epidemic. In 2010, the WHO incorporated into its guidelines a breakthrough understanding that it was relatively safe for infants of HIV-infected mothers to breastfeed so long as their mothers or the infants themselves were taking antiretroviral (ARV) drugs,7 recommending the use of short-term ARV prophylaxis options (option A and option B) for the duration of breastfeeding. In 2012, the WHO8 went a step further, providing in its guidelines the option of full triple ARV drug protection to all HIV-infected pregnant women, for life from the time of diagnosis (option B+).
The Food by Prescription (FBP) program, implemented initially in Kenya, focused on assessment and treatment of severe and moderate acute malnutrition in conjunction with ARV treatment in people living with HIV.9 Based on the FBP experience, the US President’s Emergency Plan for AIDS Relief (PEPFAR) established guidance for nutrition assessment, counseling, and support (NACS) within care and treatment, as well as PMTCT and Orphans and Vulnerable Children programming.10,11
In order to bridge the “know-do gap” between evidence-based knowledge and the actual delivery of care, countries themselves need to adopt implementation methods that take account of local context.12,13 As countries try to achieve reliable application of HIV/AIDS guidelines in pursuit of the high global targets set for HIV processes of care—the “90-90-90” goals14—system implementers need to apply methods that foster continuous learning about what works and what does not in delivering effective care and closing this know-do gap. Using a combination of QI methods and policy and protocol changes, South Africa showed that it was possible to achieve very high rates of effective antenatal PMTCT coverage and rapid lowering of incidence of HIV transmission around the time of birth.2,3
Country success in rapidly applying and scaling up evidence-based protocols and guidelines can be accelerated by their ability to learn from efforts to implement care within their own country setting as well as from other countries that are undertaking similar implementation efforts.15 In response to advances in clinical practice and the increasing recognition of QI as an effective method to implement PMTCT and other primary health-care programs,2 we designed an implementation intervention—the Partnership for HIV-Free Survival (PHFS)—that coordinated a 6-country effort to improve the continuum of care and infant survival in high HIV-burdened countries.
Methods
Context
The 6 countries that participated in PHFS formed a geographically contiguous group of nations in eastern and southern Africa that included most of the highest burden countries with HIV in the sub-Saharan region. Four of the 6 countries (Uganda, Mozambique, Lesotho, and Tanzania) were recipients of PEPFAR NACS Acceleration Funds through the US Agency for International Development (USAID), and the other 2 countries (South Africa and Kenya) were included because of the availability of country-level PEPFAR funds, their high burden of HIV, geographic proximity, and their linkage to support from the 2 primary PHFS technical partners: Institute for Healthcare Improvement (IHI) and the USAID Applying Science to Strengthen and Improve Systems (ASSIST) Project.
Each country had a well-articulated national PMTCT program primarily focused on the antenatal and perinatal care pathway. By the time of the PHFS initiative’s launch in March 2013, all 6 countries were in the process of adopting either WHO option B (triple ARV for the duration of pregnancy and breastfeeding) or option B+ (triple ARV for life from the time of diagnosis in pregnancy) for pregnant women who were HIV positive.
Conceptualization and Partnership Structure and Management
The PHFS was conceptualized in 2012 in response to the Global Plan toward the Elimination of New HIV Infections among Children by 2015 and Keeping their Mothers Alive (eMTCT).16 The PHFS aim was to support national efforts in 6 high HIV-burdened countries to reduce HIV transmission and promote improved nutrition to reduce deaths due to malnutrition, diarrhea, and pneumonia.
Program oversight and management
The PHFS was undertaken as a joint collaboration among USAID (with PEPFAR funding), WHO (Maternal, Newborn, Child and Adolescent Health), and UNICEF (HIV/AIDS Section) under the sponsorship of the Inter-Agency Task Team Working Group on Child Survival and Infant Feeding, and key global technical partners (IHI, USAID ASSIST, HEALTHQUAL, and the USAID-funded Food and Nutrition Technical Assistance III Project, implemented by FHI 360). The PHFS Global Steering Committee convened initially monthly and then quarterly, and each country formed a Steering Committee led by the Ministry of Health (MoH), technical partners, and other stakeholders, which was convened and chaired monthly by senior MoH officials from PMTCT and nutrition departments. Each country Steering Committee selected 1 or more districts and specific intervention facilities in each district for PHFS implementation. The technical partners supported the district management team to convene district or subdistrict multifacility collaborative meetings to learn QI methods, analyze performance, and design improvements and provided QI training to the MoH and local nongovernmental organizations (NGOs).
Theory of change
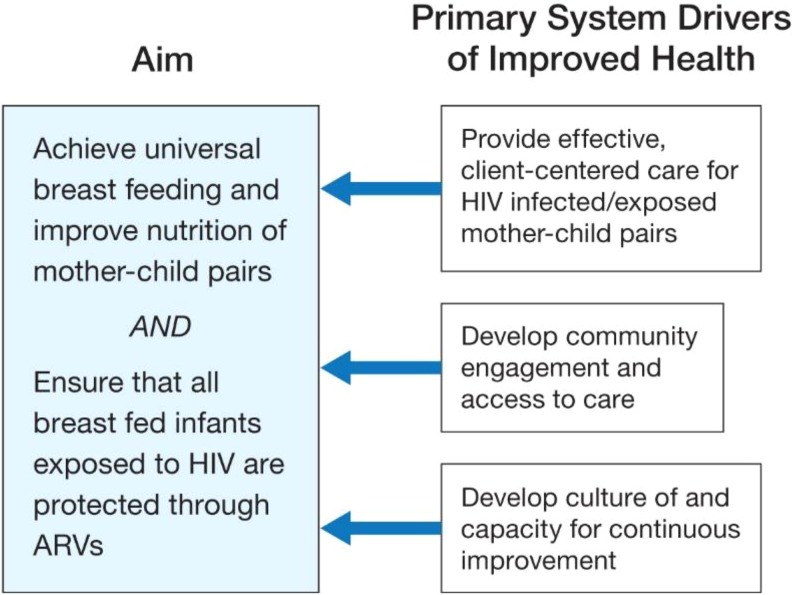
In the design phase of the PHFS, several paths to achieving the goal of HIV-free survival were proposed. At the time of its launch in 2013, the key components of the theory of change (aims, drivers of change, measurement strategy, and next steps for implementation) were codesigned with country teams (MoH leaders, technical experts from those countries, and global experts). The overarching aim had 2 components:1 achieve universal breastfeeding and improve nutrition of mother–child pairs and2 ensure that all breastfed infants exposed to HIV are protected through ARV. Three primary drivers were proposed to achieve these aims:1 effective patient-centered HIV and nutritional care,2 improve community engagement and access to care, and3 develop a culture of and capacity for continuous improvement (at facility, management, and leadership levels; Figure 1). Each of the 6 country teams created a set of actions, measured by indicators similar to those outlined in Table 1 to support those primary drivers of change, and shared those actions with other country teams who were then able to incorporate ideas for change into their own plans. The implementation knowledge generated in this phase of the project was then used in planning the scale-up phase of the PHFS.
Figure 1.
PHFS theory of change. PHFS indicates Partnership for HIV-Free Survival.
Table 1.
PMTCT and Nutrition Process Indicators Chosen for Use in the PHFS.
| Definition | |
|---|---|
| PHFS PMTCT indicators | |
| 1. Clinic attendees | Number of pregnant women, number of infants attending clinic |
| 2. HIV status known of pregnant women and mothers | The HIV status known of each pregnant women or mother attending ANC or postnatal clinics on any given day |
| 3. HIV status of (HIV-exposed) children | The HIV status of each child whose mother is known to be HIV-infected attending an MCH clinic on any given day |
| 4. ARV status of HIV-positive pregnant women and mothers | The number of HIV-positive pregnant women or mothers who attend ANC or postnatal clinics on any given day who are receiving triple ARV |
| 5. ARV status (prophylaxis) of HIV-exposed infants in the first 6 weeks | The number of HIV-exposed infants attending an MCH clinic on any given day who are receiving ARV as prophylaxis in the first 6 weeks of life |
| 6. ARV protection of HIV-exposed breastfeeding children | The number of HIV-exposed children ≤24 months of age who attend an MCH clinic on any given day who is still breastfeeding and is protected from HIV transmission by the mother taking ARV |
| 7. HIV status of exposed infants/children by age | Infants 6 weeks of age, and children 18 months of age born to HIV-infected mothers and who attend the clinic on a given day |
| 8. HIV-infected infants/children on ART | The number of HIV-infected infants and children who attend the clinic on a given day and who are on ART |
| PHFS nutrition indicators | |
| 9. Breastfeeding practices | The number of children ≤24 months of age attending an MCH clinic on any given day who are still receiving any breast milk |
| 10. Nutrition counseling, including BF of HIV-infected pregnant women and mothers | The number of HIV-infected pregnant women and mothers who attend either ANC clinics or MCH clinics (reported separately or together) on a given day and who are counseled on nutrition including breastfeeding |
| 11. Nutrition assessment of HIV-infected pregnant women and mothers | The number of HIV-infected pregnant women and mothers who attend either ANC clinics or MCH clinics (reported separately or together) on a given day and who receive a nutrition assessment |
| 12. Nutrition assessment of HIV-exposed infants and children | The number of HIV-exposed infants and children who attend a clinic on a given day and who receive a nutrition assessment |
| 13. Undernourished HIV-infected pregnant women and mothers | The number of HIV-infected pregnant women and mothers who are undernourished |
| 14. Undernourished HIV-exposed infants and children | The number of HIV-exposed infants and children who attend a clinic on a given day and who are found to be undernourished |
Abbreviations: ANC, antenatal care; ART, antiretroviral treatment; ARV, antiretroviral; BF, breastfeeding; MCH, maternal and child health; PHFS, Partnership for HIV-Free Survival; PMTCT, prevention of mother-to-child transmission.
Technical approach
The PHFS was delivered using QI implementation approaches that could be “grafted” on to existing country PMTCT, maternal, newborn, and child health (MNCH), and nutrition programs and allowed for differences in maturity of country PMTCT programs, choice of PMTCT approach, and variation in country health system designs. At the time of the introduction of the PHFS, evidence-based 20107 and 201317 WHO PMTCT guidelines had been codified by MoHs of each country into clinical protocols that reflected that country’s treatment choice (option B or option B+). The NACS program provided additional training to clinical staff in nutritional assessment, counseling, and infant feeding. The PHFS focused primarily on helping countries to learn how to implement the existing clinical pathways and protocols and then to scale up those learnings. The technical approach had 3 core components: rapid adaptive design, collaborative learning, and scale-up and sustainability designs.
Rapid adaptive design
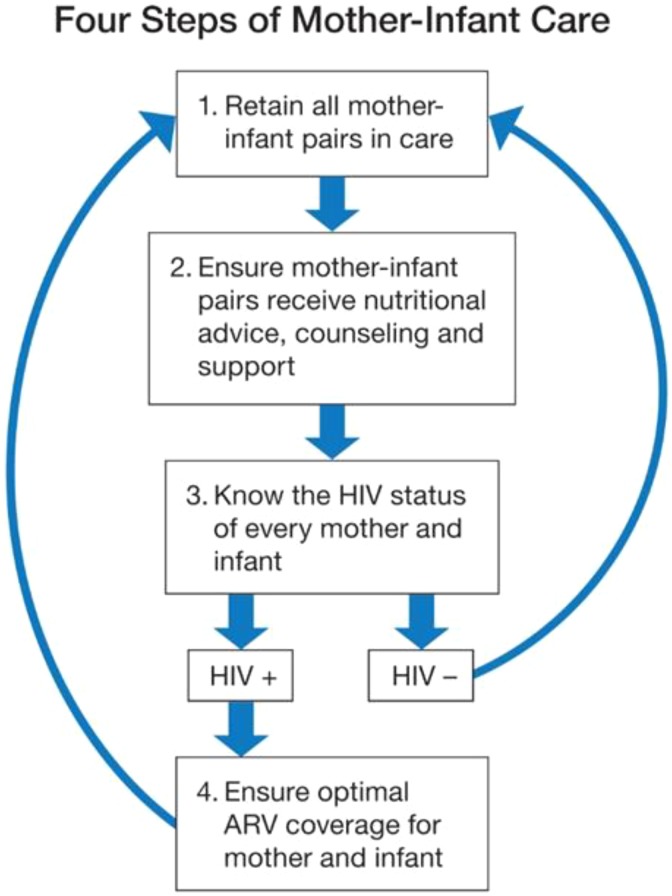
The purpose of the QI intervention was to ensure that the clinical and therapeutic interventions (screening, counseling, testing, ARV prophylaxis or treatment, nutritional support) were reliably administered to mother–child pairs through the continuum of care from antenatal visits, through labor and delivery and the postnatal breastfeeding period. The measured gap in expected performance can be closed by simplifying the system of care processes and then applying a learning approach to improve performance of those processes. To establish a common, simplified view of the system of care for the mother–baby pair, we mapped the key clinical processes to ensure a combined focus on HIV care and nutrition (Figure 2). This process map provided a common reference for teams from each country to plan improvement interventions, measure progress, and share information about the successes and challenges in the care of the mother–baby unit. To achieve better performance for a specific process of care, the QI method starts with setting an aim for expected performance, measuring the gap in actual versus expected performance, and eliciting local ideas for closing that gap, which are then iteratively tested and ultimately implemented and shared for scaling up if found to be successful.18 The main tool for discovery and learning is the Plan-Do-Study-Act cycle19—a rapid-cycle testing system that encourages facility managers and health-care providers to use local data to measure performance and stimulate ideas to adapt and improve interventions their specific context. This approach requires accurate, real-time data.
Figure 2.
Clinical pathway for HIV-free survival.
Collaborative learning
The PHFS used team-based learning at facilities, multiteam collaboration at the district level, and multistakeholder meetings at the national level to foster more rapid learning that could be fed back into local efforts to improve performance and the PHFS design. The PHFS promoted the networking of primary care facilities in health districts using a systematic process of joint knowledge production and sharing—the Breakthrough Series (BTS) collaborative20—developed by IHI. The collaborative design brings together, at regular intervals (3-6 months), facility teams who are taught to use system thinking, reflect on real-time data, and undertake rapid-cycle tests of local change ideas. In between these network meetings, QI mentors and supervisors from district management teams, supported by local and global NGOs, visited facility teams regularly (∼monthly) to coach the QI teams.
The PHFS coordinated peer-to-peer learning between countries through face-to-face as well as virtual engagements. Multicountry meetings brought teams from different countries together to share progress, refocus their aims, and plan strategies for scale-up. In bi-country exchange visits, teams from one country visited and learned from another. A 6-country meeting launched the initiative in March 2013 in Pretoria, South Africa, 2 follow-up regional meetings were held in October 2013, and then 1 other all-country learning meeting was held in Dar es Salaam, Tanzania, in February 2016. Cross-country knowledge exchange visits included a visit by the Lesotho team to Uganda in February 2015 and a visit by the Kenyan team to Tanzania in July 2015.
A range of virtual knowledge-sharing opportunities were created as described (see article “Using a multi-country learning network to harvest and rapidly spread implementation knowledge across programs aimed to reduce mother-to-child transmission of HIV and improve nutrition: perspectives and lessons learned for similar large-scale initiatives” in this supplement). All of these opportunities—face-to-face and virtual—contributed to the development and exchange of information that was intended to accelerate the pace of progress in the effective implementation of PMTCT, MNCH, and nutrition programming for mothers and infants. Over the course of the initiative, we undertook 3 types of activities to assemble field-tested effective implementation knowledge and share this across the system: At a country level, phase 1 teams from each of the 3 East African countries held meetings to systematically harvest implementation knowledge for processes along the continuum of care. This knowledge was then shared with the rest of the PHFS through webinars and reports posted on the PHFS website. At the initial all-country and subsequent regional country meetings, time was assigned for country teams to share success and challenges along the continuum. In the wrap-up phase of the initiative (February 2016), a group of key stakeholders (MoH representatives, country-support NGOs, and the PHFS global steering committee) met in Dar es Salaam, Tanzania, to harvest and document the challenges and successes of the initiative.
Implementing the intervention: Phased scale-up design
The PHFS set an ambitious goal to scale up the learnings and successful changes from the early phase of the intervention to reach all HIV-infected mothers and their infants of all the participating countries through the national PMTCT programs. The phased implementation approach, starting with a small set of districts, was designed to allow the countries to understand the implementation challenges, test ideas for change, learn what works, and develop local capability for supporting the QI method and the design. The phased approach included a set-up phase, where the concept of PHFS was explained and socialized through a series of in-person meetings between the PHFS leadership and stakeholders within each of the countries, a prototype testing phase where the ideas for improvement were tested in a limited number of districts (each of which included a BTS collaborative of facilities), followed by scale-up to a larger number of districts.
At the launch meeting and in subsequent national meetings, we encouraged country teams to identify a set of districts or similar administrative units that could be developed as model systems that could be replicated in the scale-up phase. The phased approach allowed for demonstration and communication of results—a key factor in building will and understanding required for rapid scale-up. Because the intervention was informed by cumulative learning from the previous phases, the country planners could theoretically expand the program more rapidly as the program evolved.
The first 24 months of the initiative was designed to achieve results in the first phase (subdistrict facilities) and build capacity in MoH district teams to scale up the initiative across the participating districts in the second phase. At the end of this phase of work, the ground work for national scale-up would be laid: proof of concept through a set of plausible results, assembly of change packages, stronger data systems, QI capability within district management teams and national MoH teams, less reliance on QI technical partners, and planning for national scale-up underway. In the second phase, well-tested change ideas and tools for implementing postnatal PMTCT from past experience could then be adopted or adapted to new contexts. The NGO partners provided key technical support at all levels of the country health systems—national steering committee, district management teams, and facility QI teams.
Measurement strategy
The PHFS QI intervention relied heavily on the timely availability of data that reflect performance of processes along the continuum of care. Most of the countries had a basic set of PMTCT, MNCH, and nutrition process measures, reported through the District Health Information Systems (DHIS or country equivalents) and HIV-specific data reporting systems. At the launch meeting in March 2013, country participants codeveloped a draft set of indicators to track process performance of PMTCT (8 indicators) and nutrition (6 indicators) processes along the continuum (Table 1).
A survey of countries conducted at the initial launch meeting revealed that very few of the proposed indicators were being collected and routinely reported through the DHIS or other systems. This presented a major challenge to our goal of working within and strengthening the existing data systems and placed initial heavy reliance on supplemental data collection systems that were managed by the technical partners supporting the work in different countries. A working group of data specialists from each country MoH and technical partners was established to see how existing country indicators could provide proxy measures for tracking progress and how to influence the existing data sets collected through the DHIS. During the first year of implementation, a working group was established by USAID to recommend indicators to track the progress of the initiative.
QI capacity building
With an eye to sustainability of the intervention and the national scale-up phase, the initial PHFS design called for significant building of QI skills across government institutions responsible for national, subnational, and district leadership and management of the PHFS initiative. Due to funding constraints, there were no formal QI skill-building trainings offered to the national, subnational, or district management teams. Each supporting partner took responsibility for training the district supervisors in QI methods and exposing district management teams to enabling approaches to change and improvement and use of data for decision-making. In addition, technical partners worked with data managers at facility and district levels to try to improve the accuracy, completeness, and timeliness of data reporting and encouraged the feedback of data to frontline staff and managers.
Materials for program review
In February 2016, we invited the country teams (MoH representatives, country technical partners, and the global steering committee members) to a 2-day meeting in Dar es Salaam, Tanzania, to review the progress of the PHFS. The intensive debriefing sessions reviewed progress from each country along the continuum of care, reflections from teams on what worked and what did not, advice to others initiating similar initiatives, reflections on efforts to embed QI within country health systems, and lessons on efforts to scale up and sustain the activities of PHFS within countries. The composition of the meeting attendees from each of the countries represented the degree of MoH engagement—of 43 attendees, 11 were MoH personnel, 24 were from in country technical partners, 7 from the global steering committee, and 1 USAID observer.
Funding
The initial phase of the PHFS was supported by supplemental PEPFAR funds (NACS Acceleration Fund) to 4 of the target countries—Uganda, Tanzania, Mozambique, and Lesotho—in coordination with country-level PEPFAR support for PMTCT programming in all 6 countries, including Kenya and South Africa. The NACS Acceleration Funds through USAID also supported technical assistance from the key technical partners and supported the regional and all-country face-to-face meetings.
Ethical Approval and Informed Consent
Ethics approval was not sought as this was a QI initiative to improve the uptake of existing government approved, evidence-based clinical interventions. Given that no new clinical interventions were being introduced and no intervention was conducted that could cause harm, we did not seek institutional review board approval or individual informed consent. Only de-identified, aggregate data that were collected were used, and data were used only for the purposes of tracking and improving processes of care, and for learning about interventions that improved care.
Results
Initial Uptake of PHFS
All countries had existing PMTCT, MNCH, and nutrition programs that PHFS attempted to build on. It was clear from the outset that different countries were not at the same level of technical readiness to participate in the PHFS collaborative design, particularly with respect to familiarity with QI methods and their incorporation into the governance and implementation of programs in these countries. Also, there were differences in the level of enthusiasm and engagement of ministries in the PHFS initiative. These 2 factors resulted in varying levels of engagement of the different governments and uptake of the PHFS approaches.
Uganda
The Ministry of Health has for a number of years been using a variety of QI and quality assurance approaches to improve the performance of the health system. The MoH PMTCT and nutrition leads were highly engaged in PHFS and chaired the PHFS steering committee. The country has developed a National Quality Strategy and has deployed QI coaches for each district. Partnership for HIV-Free Survival activities were initiated in 22 facilities in 6 districts.
Tanzania
The Ministry of Health has several years of exposure to QI through the activities of a number of NGOs in the country. The MoH PMTCT and nutrition leads independently co-chaired the PHFS steering committee, coordinated partner interactions, and organized 2 national learning platform events to harvest changes from districts. Partnership for HIV-Free Survival was initiated in 30 facilities across 3 districts, and district coaches were supported by an external NGO.
Kenya
Although the district team leadership was very involved and active in leading the PHFS activities at a local level, the national-level MOH was less engaged, and the PHFS steering committee met only intermittently. The PHFS activities were initiated in 16 facilities in 1 county. District QI coaches were supported by external technical partners. The local district QI teams contributed several innovations to the PHFS that were spread in the learning network, including the development of a mother–infant registry.
South Africa
South Africa had a strong ante-/perinatal PMTCT program that had been scaled up using QI principles, which contributed to the design of the PHFS approach.2,3 Despite this experience, there was lack of enthusiasm at the national and provincial levels for the introduction of another externally driven PMTCT/MNCH/nutrition program, resulting in limited Department of Health support for PHFS. Two South African NGOs funded through other PEPFAR mechanisms used PHFS-designed content, participated in the PHFS learning network, and applied QI approaches to support improvements in PMTCT, MNCH, and nutrition in 25 facilities across 4 districts.
Mozambique
In Mozambique, the MoH assigned districts in 2 provinces to the intervention. However, it was initially difficult for PHFS to influence the implementation of PMTCT, MNCH, and nutrition services since there was an existing QI initiative funded by another US government agency (Health Resources and Services Administration), using a different, standards-based approach. Efforts were made to blend the 2 initiatives. The USAID ASSIST Project also carried out a pilot activity in rural Mozambique to provide support for improving community engagement in access to antenatal, PMTCT, and nutrition care for mother–baby pairs (see article “Community-based improvements to increase identification of pregnant women and promote linkages to antenatal and HIV care in Mozambique” in this supplement).
Lesotho
Lesotho had previously used QI methods for health programming only on a limited scale and there was limited national MoH engagement in the initiative from the outset. Technical support NGOs worked primarily with district management teams to support PHFS activities with 12 facility teams in 3 districts and helped the MoH to institute a system of district QI coaches.
Implementation and Scale-up
All countries had phase 1 demonstration districts that included a number of facilities taking care of HIV-infected pregnant women and mother–baby pairs. Most countries either had or introduced QI coaches over the course of PHFS implementation. These coaches were usually part of the district health management team and incorporated QI coaching into their supervisory activities. These QI coaches received ongoing mentoring from external technical partners.
After 3 years of PHFS implementation, there was wide variation among the countries in the number of facilities that were engaged with the PHFS, degree of engagement with the MoH, and plans for how the initiative would be scaled up and sustained. Tanzania and Uganda had active participation and leadership from the MoH, and this leadership was reflected in a large number of facilities engaged over 3 years and in ambitious scale-up plans. Tanzania expanded the number of facilities involved in PHFS-related activities from 30 to 90, with plans to expand to a further 60 facilities. In Uganda, PHFS partners supported 34 facilities, and the Ugandan MoH had advanced plans to expand PHFS activities to a total of 117 sites in Northern Uganda. Kenya engaged 16 facilities in 1 county but without clear engagement from the national government or plans for scale-up. In Mozambique, there were no specific plans to scale up PHFS-related activities or tools. In Lesotho, technical assistance partners engaged 12 facilities in 3 districts, but this work was done with limited engagement of the national government or scale-up plans in the PEPFAR priority districts. In South Africa, 2 partners supported 25 facilities across 4 districts, but the PHFS design was not scaled up by the national or provincial governments. Uganda and Tanzania took advantage of PHFS resources to capacitate district management teams in QI methods so that they could spread coverage to all facilities in their districts.
Learning
The PHFS design envisioned a learning system that would promote the flow of data and implementation know-how, not only among the facilities that were organized into BTS networks but also among countries that were grappling with and innovating around the same PMTCT/MNCH/nutrition implementation challenges. Despite the considerable difficulties in sharing quantitative data, the PHFS was able to extract and share considerable learning among countries through the face-to-face mechanisms (all-country and regional meetings, exchange visits between countries, harvesting from local BTS networks), as well as active dissemination through virtual methods (webinars, newsletter, social media). In these instances, country ministries were pleased (and proud) to share data that illustrated successful testing of new implementation ideas at specific sites. An additional barrier to analyzing and comparing data was the lack of progress in using the common set of process and outcome indicators that had been developed and proposed during the PHFS set-up phase (see Table 1).
Discussion
Partnership for HIV-Free Survival was an important test of an effort to establish a focused multicountry learning system to support and accelerate implementation of a specific advance in clinical programming.7,17 The PHFS succeeded in bringing together 6 nations and their technical partners around a common aim, using the same content theory and implementation strategy across all countries. The primary achievements of PHFS were to generate new implementation knowledge that can be used to decrease postnatal PMTCT transmission and improve nutrition care in country demonstration sites, to spread new implementation knowledge across the 6 countries, and to influence and increase the pace of deployment of new PMTCT programming, using QI methods, in some of the countries. The cross-cutting papers in this supplement detail the above achievements (see articles “Applying quality improvement approaches to reduce mother-to-child HIV transmission and improve health and nutrition care in five countries: lessons from the Partnership for HIV-Free Survival” and “Using a multi-country learning network to harvest and rapidly spread implementation knowledge across programs aimed to reduce mother-to-child transmission of HIV and improve nutrition: perspectives and lessons learned for similar large-scale initiatives” in this supplement).
We were able to show significantly improved performance of the processes that decreased mother-to-child transmission rates in some sites that PHFS was tracking (see article “Reducing mother-to-child transmission of HIV using quality improvement approaches” in this supplement). Although the PHFS sites are currently only a fraction of the total number of PMTCT sites in each country, the experience and results of the PHFS are informing the national PMTCT programs in some of the countries.
A key accomplishment of PHFS was the establishment of a learning system that was able to move new implementation knowledge more rapidly within and across the PHFS countries. The learning system design meant that new implementation knowledge being generated in the multiple country sites was made available not only to QI teams at facilities but also to district health management teams and to country planners. Local change ideas as well as the more formal collection of successful implementation ideas (change packages) were and made available across the 6 countries through the virtual learning system (see article “Using a multi-country learning network to harvest and rapidly spread implementation knowledge across programs aimed to reduce mother-to-child transmission of HIV and improve nutrition: perspectives and lessons learned for similar large-scale initiatives” in this supplement).
There was significant variation in the uptake of PHFS design and methods across the 6 countries. While PHFS succeeded in some countries (Tanzania, Uganda, and Kenya) in influencing the thinking and actions of the MOH in adopting aspects of PHFS scale-up design and methods to implement the 2010 and 2013 WHO PMTCT guidelines, the other countries either continued to rely primarily on their existing approaches and structures for PMTCT, MNCH, and nutrition programming or have had limited engagement to date in the PHFS process at the ministry level. Countries that had strong and long-standing existing relationships with PHFS lead partners embraced the thinking and incorporated PHFS into their existing country plans. In other countries, the PHFS coincided with an emerging interest in QI methods, and the initiative provided a strong influence in how QI methods were applied.
Another effect of the PHFS was its impact on the pace and performance of existing country efforts around the PMTCT, MNCH, and nutrition continuum. In each of these countries, there was a direct effect of the PHFS that was seen primarily in the facilities and districts that were supported by technical partners (see articles “Increasing HIV-Free Survival of Infants: Reorganizing Care Using Quality Improvement for the Optimal Health and Nutrition of HIV-Positive Women and their Exposed Infants in Uganda”, “Engagement of national stakeholders and communities on healthcare quality improvement: experience from the implementation of the Partnership for HIV-Free Survival (PHFS) in Tanzania”, “Applying quality improvement strategies to health services for HIV-affected mother-baby pairs in rural Kenya”, and “Comparison of the effects of quality improvement strategies on prevention of mother-to-child HIV transmission in a public and a private hospital in Lesotho”). In the East African countries, PHFS seemed to galvanize country PMTCT leadership and accelerate the scale-up of effective postnatal PMTCT, MNCH, and nutrition programs and further institutionalize QI methods. The Tanzania MoH convened 2 national learning platforms to harvest and spread the learnings of PHFS to other facilities. In Uganda, national policy was changed to mandate a mother–baby care point at every facility across the country, a direct result of the benefits shown by PHFS phase 1 work. The MoH of Kenya adopted the mother–baby pair register that was developed by PHFS phase 1 facilities.
The difference in responses of these countries provides important lessons. For countries that did not have strong existing, centrally led QI programs, the value of PHFS was not as self-evident. Some countries were more receptive to PHFS because of existing relationships with technical partners upon which the initiative could be built. The central lesson is that PHFS, as an externally designed initiative, as with other “competing” external initiatives, needed to be fully integrated within and supportive of existing government programs. Where ministries were engaged very early on in the PHFS, greater progress was made in ministries taking on a significant leadership role and increasingly “owning” the activities. In retrospect, a more intensive effort to engage the ministries before the PHFS might have resulted in more uniform buy-in and country leadership.
The open sharing of data beyond the boundaries of each country proved to be a challenge. Ministries of Health are understandably cautious about data being used to judge country programs, and since country data are owned by the ministries, there were attendant restrictions on dissemination of PHFS data. As a result, countries and their NGO partners were hesitant to share routine process and outcome data from PHFS-supported facilities without formal government approval. Since data review and sharing is a core requirement of the QI process, this restriction presented a formidable challenge to real-time, as well as summative cross-country learning.
Conclusion
Partnership for HIV-Free Survival convened 6 sub-Saharan African nations to discover together the best strategies for implementing and scaling up implementation of existing clinical pathways and protocols to ensure HIV-free survival of mother–baby pairs. The 3 core technical components of the initiative—rapid adaptive design, collaborative learning, and scale-up and sustainability designs—provided a dynamic platform to test and share strategies that work in accelerating PMTCT in complex, resource-poor settings. Learning generated through the experience of implementing the PHFS can serve to bolster future initiatives with similar designs, including increased ownership and codesign with MoHs, further integration into existing government programs, and sustainability of QI capability throughout the health system. The PHFS can serve as a prototype for future global networks considering designs for accelerating improvement, learning, and results.
Acknowledgments
Ministries of Health from all 6 countries were leading participants in this large, complex, multicountry initiative. We would like to acknowledge the role of these Ministry of Health leaders and managers as well as the hundreds of district managers, supervisors, frontline staff, and the technical support partner teams on the ground. In particular, we would like to acknowledge Angelina Sassi from IHI who provided key research and writing assistance for this report.
Authors’ Note: This contents in this document are those of the authors and do not necessarily represent the views of PEPFAR, USAID, or the US government.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: USAID and PEPFAR.
References
- 1. World Health Organisation (WHO). Towards Universal Access: Scaling Up Priority HIV/AIDS Interventions in the Health Sector. Geneva: World Health Organisation; 2010. http://www.who.int/hiv/mediacentre/universal_access_progress_report_en.pdf. [Google Scholar]
- 2. Barker P, Barron P, Bhardwaj S, Pillay Y. The role of quality improvement in achieving effective large-scale prevention of mother-to-child transmission of HIV in South Africa. AIDS. 2015;29(suppl 2):S137–S143. [DOI] [PubMed] [Google Scholar]
- 3. Doherty T, Chopra M, Nsibande D, Mngoma D. Improving the coverage of the PMTCT programme through a participatory quality improvement intervention in South Africa. BMC Public Health. 2009;9:406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Osibo B, Oronsaye F, Alo OD, et al. Using small tests of change to improve PMTCT services in northern Nigeria: experiences from implementation of a continuous quality improvement and breakthrough series program. J Acquir Immune Defic Syndr. 1999 2017;75(suppl 2):S165–S172. [DOI] [PubMed] [Google Scholar]
- 5. John-Stewart G, Mbori-Ngacha D, Ekpini R, et al. Breast-feeding and transmission of HIV-1. J Acquir Immune Defic Syndr. 2004;35(2):196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bispo S, Chikhungu L, Rollins N, Siegfried N, Newell ML. Postnatal HIV transmission in breastfed infants of HIV-infected women on ART: a systematic review and meta-analysis [Internet] J Int AIDS Soc. 2017;20(1). http://www.jiasociety.org/index.php/jias/article/view/21251. Accessed August 7, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. WHO. Archived: Guidelines on HIV and Infant Feeding 2010 [Internet]. WHO; http://www.who.int/maternal_child_adolescent/documents/9789241599535/en/. Accessed August 4, 2017. [Google Scholar]
- 8. WHO. HIV and Infant Feeding 2010: An Updated Framework for Priority Action [Internet]. WHO; http://www.who.int/maternal_child_adolescent/documents/9241590777/en/. Accessed August 4, 2017. [Google Scholar]
- 9. Review of Kenya’s Food by Prescription Program. Food and Nutrition Technical Assistance III Project (FANTA) [Internet]. https://www.fantaproject.org/focus-areas/infectious-diseases/kenya-food-by-prescription. Accessed August 4, 2017.
- 10. The U.S. President’s Emergency Plan for AIDS Relief (PEPFAR). Policy Guidance on the Use of Emergency Plan Funds to Address Food and Nutrition Needs. PEPFAR [Internet]. September 2006 https://2009-2017.pepfar.gov/documents/organization/84583.pdf. Accessed August 9, 2017.
- 11. The U.S. President’s Emergency Plan for AIDS Relief (PEPFAR). Orphans and Other Vulnerable Children Programming Guidance for United States Government In-Country Staff and Implementing Partners. PEPFAR [Internet]. July 2006. https://2009-2017.pepfar.gov/documents/organization/83298.pdf. Accessed August 9, 2017.
- 12. Harrison MB, Légaré F, Graham ID, Fervers B. Adapting clinical practice guidelines to local context and assessing barriers to their use. CMAJ Can Med Assoc J. 2010;182(2):E78–E84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dixon-Woods M, Baker R, Charles K, et al. Culture and behaviour in the English National Health Service: overview of lessons from a large multimethod study. BMJ Qual Saf. 2014;23(2):106–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. UNAIDS. 90–90–90 – An Ambitious Treatment Target to Help End the AIDS Epidemic | UNAIDS [Internet]. http://www.unaids.org/en/resources/documents/2017/90-90-90. Accessed August 4, 2017.
- 15. Wenger E. Communities of practice and social learning systems. Organization. 2000;7(2):225–246. [Google Scholar]
- 16. UNAIDS. Global Plan Towards the Elimination of New HIV Infections among Children by 2015 and Keeping Their Mothers Alive. UNAIDS [Internet]. 2011. http://www.unaids.org/en/resources/documents/2011/20110609_JC2137_Global-Plan-Elimination-HIV-Children_en.pdf. Accessed August 4, 2017.
- 17. WHO. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection [Internet]. WHO; http://www.who.int/hiv/pub/guidelines/arv2013/download/en/. Accessed August 7, 2017. [Google Scholar]
- 18. Berwick DM. A primer on leading the improvement of systems. BMJ. 1996;312(7031):619–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. PDSA cycle [Internet]. https://deming.org/management-system/pdsacycle. Accessed August 7, 2017.
- 20. Institute for Healthcare Improvement. The Breakthrough Series: IHI’s Collaborative Model for Achieving Breakthrough Improvement [Internet]. http://www.ihi.org/resources/Pages/IHIWhitePapers/TheBreakthroughSeriesIHIsCollaborativeModelforAchievingBreakthroughImprovement.aspx. Accessed August 7, 2017.