Abstract
Objectives:
The study aimed to determine the prevalence of otitis media (OM)-associated bacterial flora of asymptomatic people living with HIV (PLH) on antiretroviral therapy (ART) and assess antibiotic resistance profiles of the bacteria.
Methodology:
Ear secretion specimens were collected by syringe or cotton swabs. Isolated bacteria were subjected to antibiotic sensitivity tests.
Results:
Of 290 recruited PLH, 81.7% were females and 18.3% males; their CD4+ counts ranged from 0 to 1770. Staphylococcus aureus, Klebsiella pneumonia, and Escherichia coli were the predominant bacteria. High antibiotic resistance was detected on Streptococcus pyogenes and Streptococcus pneumoniae. The prevalence rate of OM-associated bacteria (OAB) was 93.4%, and majority of the bacteria were resistant to multiple antibiotics. Linear association between the prevalence of OAB with both duration of ART and CD4+ counts was observed.
Conclusion:
High prevalence rates of OAB and antibiotic resistance were observed. Negative correlation between CD4+ counts and prevalence of OAB was revealed.
Keywords: antibiotic resistance, otitis media–associated bacteria, HIV/AIDS
Introduction
Human immunodeficiency virus (HIV) infection continues to be a vexing problem among people living with HIV (PLH), particularly children, and it is associated with a diversity of health problems in the head and neck regions including otitis media (OM).1,2 Otitis media is a set of inflammatory diseases of the middle-ear. The most important conditions are acute OM without perforation, acute OM with perforation, OM with effusion, and chronic suppurative OM.3-5 The disease is a common entity in both immunocompetent and immunosuppressed individuals.5-8 However, recurrent microbial infections and complications secondary to OM are more common in PLH.4,6,8-10
Streptococcus pneumoniae and Moraxella catarrhalis were previously regarded as the most common bacteria attributed to OM in all age groups.11-13 A notable change in the microbiology of OM occurred with the spread of β-lactamase-producing strains of Haemophilus influenza and M catarrhalis,13-15 which emerged simultaneously with HIV infection. Since HIV/AIDS is also accompanied by a number of opportunistic infections (OIs), PLH are at risk for a wide spectrum of diseases both common and exotic.16-18 As the HIV infection progresses to AIDS as a consequence of decreased CD4+ T cells, PLH become ever more at risk of the atypical OIs12,19, since patients who have responded to highly active antiretroviral therapy (HAART) may have had CD4+ counts that were low in the past but have increased as a result of the therapy. Such CD4+ T-cell counts probably mask causal immune deficiency.20,21 An individual’s risk of OIs ought to be based on their previous lowest CD4 counts, not on their most recent CD4 counts.22
Worldwide, OM continues to be an important public health problem among PLH.9,12,18 The most common OM pathogen, S pneumoniae, has been implicated as part of the current antibiotic resistance crisis.23,24 Multidrug-resistant pathogens have also been isolated from patients with invasive infections, from immunosuppressed individuals, and from carriers.25,26 Globalization (easy travel around the world) has further disseminated the antimicrobial-resistant microorganisms; hence, antimicrobial-resistant infections have become a global calamity.27,28
Published data on chronic suppurative OM are difficult to find in developing countries and for that reason the optimal management of OM is still an unsolved problem.5,29 Firstly, because there is no consensus on diagnostic criteria,30 and secondly, a majority of the previous studies have focused on clinical diagnosis with little or no report on the microbiological etiology of OM.31,32 There exists a dearth of knowledge on studies conducted on prevalence rates of OM in PLH in relation to microbiological examination. Thus, this study intended to assess the prevalence of OM-associated bacterial flora of asymptomatic PLH and antibiotic resistance profiling of the bacteria. Because PLH are constantly exposed to both antiretroviral (ARV) medications and antimicrobial agents for prophylaxis of OIs, any observed changes in antibiotic susceptibility may have crucial therapeutic implications.31
Materials and Methods
Study Design and Sampling Procedures
Nonprobability (convenience sampling) technique was employed to recruit all PLH (with and without OM) attending the center for treatment and care (CTC) services at Morogoro Regional Referral Hospital willing to participate in the study. The lower age limit of eligibility was 2 months of age.33 Acute OM was diagnosed on the basis of otoscopic findings of either middle-ear effusion or purulent otorrhea with duration of less than 24 hours and/or patients’ complaints. HIV-infected pregnant women in their second or third trimester and patients below 18 years of age were excluded from the study unless parents’ or guardians’ verbal or written consents were obtained.33
Data Collection
Following a written informed consent, a standardized clinical history and physical examinations were conducted. This was preceded with collection of ear secretion specimens. A structured questionnaire was used to collect data on antibiotics usage prior to registration or attendance at CTC. Emphasis was on whether PLH might have been using antibiotics for prophylaxis of OIs, which could be associated with current observations on antibiotic resistance. Patient’s personal particulars on age, sex, weight, and other information relevant to the study were also sought through the guided interviews. The selection of 7 tested antibiotics for this study emanated from the information offered by the PLH via the interviews. Participants were then requested to return to the clinics on the 1st, 4th, and 24th weeks after the baseline visit. At each visit, ear secretion specimens were collected and the standardized clinical history and physical examination were repeated.
Microbiological Laboratory Procedures
Middle-ear secretion specimens were collected on sterile swabs or cotton wools. Initial isolation procedures for microorganisms were performed on blood and chocolate agar plates and aerobically incubated at 37°C, while chocolate agar plates were incubated in the presence of 5% to 10% carbon dioxide. All isolated bacteria were subcultured onto transport nutrient agar slant and sent to Muhimbili University of Health and Allied Sciences (MUHAS), at Pharmaceutical Microbiology Laboratory for further identification and in vitro susceptibility testing. Antimicrobial susceptibility testing was performed on Mueller-Hinton agar plates.
The identified bacterial isolates were subjected to susceptibility testing against 7 widely used and commercially available antibiotic sensitivity discs, namely ampicillin (30 µg), erythromycin (15 µg), amoxicillin/clavulanic acid (Augmentin; 30 µg), ciprofloxacin (5 µg), amikacin (30 µg), gentamicin (10 µg), and co-trimoxazole (25 µg); Oxoid Limited, Basingstoke, United Kingdom), using the Kirby-Bauer disk diffusion method.34 Antibiograms were validated using standardized control strains of Escherichia coli (ATCC 25922), Klebsiella pneumoniae (ATCC 700603), and Staphylococcus aureus (ATCC25923) and Pseudomonas aeruginosa (ATCC27853). Bacterial isolates interpretive criteria were used to evaluate inhibition zone (IZ) as per Clinical Standards Laboratory Institute guidelines.35
Data Analysis
Data were analyzed using the IBM SPSS Statistics 20 computer package (SPSS for Windows 20.0; SPSS Inc, Chicago, Illinois) software. Logistic regression technique was used to test for statistical independence of CD4+ T-cell counts, age, body weights, and duration of antiretroviral therapy (ART) in relation to the prevalence of OM and antibiotic-resistance rates among isolated bacteria. Where the null hypotheses of independence were rejected, subsequent analyses were conducted at the appropriate level of detail for further comparisons. Analysis of variance was employed to compare means of IZs of the isolated bacteria across the population. The Dunnett test (2 sided) was used to compare the level of significant differences in antibiotic susceptibility/IZ between each bacterium with its respective control/reference strain. Differences in IZ among the isolated bacteria and other tested variables were considered significant at P < .05.
Ethical Consideration
Following approval of this study by the MUHAS ethical committees and permission to conduct the study from the hospital authorities, all participants were clearly informed of the objectives of the study and were provided with both verbal and written informed consent. For PLH under 18 years old, apart from their parents/guardians’ consents, a personal informed verbal consent was sought from each of them. They were also vividly explained that all information gathered and laboratory results will be used for academic purpose and betterment of their health care at large. In order to maintain confidentiality, data were coded and then entered into the computer database for analysis and interpretation. Neither patients’ names nor any other personal details were disclosed.
Results
Demographic Characteristics of the Study Population in Relation to OM
A total of 290 PLH with asymptomatic OM attending the CTC services at Morogoro Regional Referral Hospital from July 2015 to September 2017 were recruited in this study. Of 290 PLH, 237 (81.7%) and 53 (18.3%) were females and males, respectively. The ages of the PLH were between 2 months and 81 years, with the median age of 43 years and the modal age of between 36 and 53 years. Slightly more than half of the participants were of age ranging from 36 to 54 (56%). Their body weights ranged from 8 to 172 kg, with the median weight of 58 kg. Significant differences were observed with regard to the prevalence OAB among patients’ age groups and CD4+ T-cell counts (P < .05). As per patient clinic records, the attendance at the CTC was dated back from 2003 to 2017. The immunological status (CD4+ T-cell counts per milliliter) ranged from 0 to 1770 CD4+ cells/milliliter cubic (mm3), with the median of 455 (350-700) CD4+ T cells/mL (Table 1).
Table 1.
Demographics of the study population in relation to prevalence of OAB.
| Variables | Otitis Media (%) | P Value | |
|---|---|---|---|
| None | Present | ||
| Sex | |||
| Females | 14 (4.8) | 223 (76.9) | .103 |
| Males | 5 (1.6) | 48 (16.7) | |
| CD4+ T-cell countsa | |||
| 0-350 | 6 (2.1) | 93 (33.0) | .001 |
| 351-700 | 8 (2.8) | 122 (43.0) | |
| 701-1050 | 2 (0.7) | 34 (12.0) | |
| 1051-1450 | 1 (0.35) | 14 (5.0) | |
| 1451-1800 | 1 (0.35) | 2 (0.7) | |
| Age group | |||
| 0.2-17 | 1 (0.3) | 9 (3.1) | .001 |
| 18-35 | 5 (1.7) | 66 (22.7) | |
| 36-53 | 9 (3.1) | 149 (51.3) | |
| 54-71 | 3 (1.0) | 46 (15.9) | |
| 72-89 | 1 (0.3) | 1 (0.3) | |
| Body weight | |||
| 8-45 | 2 (0.7) | 27 (9.6) | .527 |
| 46-65 | 9 (3.1) | 154 (53.4) | |
| 66-85 | 7 (2.4) | 67 (23.1) | |
| 86-105 | 1 (0.3) | 20 (7.0) | |
| 106-125 | 0 (0) | 2 (0.7) | |
| >125 | 0 (0) | 1 (0.3) | |
aEight children’s status expressed in terms of viral load is excluded.
Bacteria Isolation and Prevalence of OAB With Respect to HIV Status
From 290 PLH, a total of 10 different species of bacteria were isolated and identified (also classified as significant bacterial growth [SBG]), in which 271 (93.65%) patients had the presence of bacterial flora associated with ear infection/OM, while 19 (6.4%) patients had no ear infection (no bacterial growth). Majority of the isolated bacteria was comprised of S aureus (48.3%; n = 129 ± 21) (‘1’- stands for the superscript ‘a’) and the least was Streptococcus pyogenes (0.7%; n = 2), as shown in Tables 2 to 4.
Table 2.
Otitis media-associated bacterial flora isolated from PLH in relation to patients’ CD4+ T cell counts.
| Identified Microbes | CD4+ T-Cell Counts | Total | ||||
|---|---|---|---|---|---|---|
| 0-350 | 351-700 | 701-1050 | 1051-1450 | Over 1450 | ||
| NBG (none) | 6 (2.0) | 8 (3.0) | 2 (0.7) | 1 (0.3) | 1 (0.3) | 18 (6.4)a |
| Pseudomonas aeruginosa | 5 (2.2) | 8 (3.0) | 1 (0.3) | 1 (0.3) | 0 (0.0) | 15 (5.3) |
| Escherichia coli | 10 (4.0) | 16 (6.0) | 8 (3.0) | 2 (0.7) | 0 (0.0) | 36 (12.8) |
| Klebsiella oxytoca | 8 (2.8) | 4 (1.4) | 0 (0.0) | 2 (0.7) | 0 (0.0) | 14 (5.0) |
| Klebsiella pneumoniae | 16 (5.7) | 12 (4.3) | 3 (1.1) | 4 (1.4) | 0 (0.0) | 35 (12.4) |
| Staphylococcus aureus | 48 (17.0) | 63 (22.3) | 15 (5.3) | 3 (1.1) | 0 (0.0) | 129 (45.7)a |
| Moraxella catarrhalis | 2 (0.7) | 3 (1.1) | 4 (1.4) | 0 (0.0) | 0 (0.0) | 9 (3.2) |
| Proteus mirabilis | 0 (0.0) | 2 (0.7) | 1 (0.3) | 0 (0.0) | 0 (0.0) | 3 (1.1) |
| Staphylococcus epidermidis | 3 (1.1) | 7 (2.5) | 2 (0.7) | 0 (0.0) | 2 (0.7) | 14 (5.0) |
| Streptococcus pneumonia | 0 (0.0) | 5 (2.0) | 0 (0.0) | 2 (0.7) | 0 (0.0) | 7 (2.5) |
| Streptococcus pyogenes | 0 (0.0) | 0 (0.0) | 2 (0.7) | 0 (0.0) | 0 (0.0) | 2 (0.7) |
| Total | 98 (34.7) | 128 (45.4) | 38 (13.5) | 15 (5.3) | 3 (1.1) | 282 (100.0)a |
Abbreviation: NBG, no bacterial growth.
a-cEight children status expressed in terms of viral load is excluded.
Table 3.
Resistance profiles of OAB against commonly used antibiotics.a
| Isolated Bacteria | Antibiotic Resistance Rates (n [%]) | Total (N = 271) | ||||||
|---|---|---|---|---|---|---|---|---|
| CIP-5 | AMI-30 | AUG-30 | AMP-10 | ERY-15 | CXT -23/75 | GEN-10 | ||
| P aeruginosa | (0.0) | 3 (20.0) | 8 (53.3) | 11 (73.3) | 6 (40.0) | 3 (20.0) | 1 (6.7) | n = 15 |
| E coli | 4 (10.8) | 1 (2.7) | 6 (16.2) | 19 (51.4) | 17 (45.9) | 19 (51.4) | 4 (10.8) | n = 37 |
| K oxytoca | 1 (6.3) | 7 (4.8) | 5 (31.3) | 7 (43.8) | 10 (62.5) | 8 (50.0) | 3 (18.8) | n = 16 |
| K pneumoniae | 2 (5.4) | 5 (13.5) | 18 (48.6) | 19 (51.2) | 13 (35.1) | 6 (16.2) | 1 (2.7) | n = 37 |
| S aureus | 16 (12.2) | 4 (3.0) | 14 (10.7) | 61 (46.5) | 49 (37.4) | 56 (42.7) | 26 (19.8) | n = 131 |
| M catarrhalis | 3 (33.3) | 5 (55.6) | 1 (11.1) | 5 (55.6) | 0 (0.0) | 5 (55.6) | 0 (0.0) | n = 9 |
| P mirabilis | 0 (0.0) | 0 (0.0) | 2 (66.7) | 2 (66.7) | 3 (100.0) | 0 (0.0) | 1 (33.3) | n = 3 |
| S epidermidis | 4 (28.6) | 3 (21.4) | 4 (28.6) | 7 (50.0) | 9 (64.3) | 5 (55.5) | 0 (0.0) | n = 14 |
| S pneumoniae | 2 (28.6) | 0 (0.0) | 1 (14.3) | 1 (14.3) | 5 (71.4) | 3 (42.9) | 1 (14.3) | n = 7 |
| S pyogenes | 2 (100.0) | 2 (100.0) | 2 (100.0) | 0 (0.0) | 2 (100.0) | 2 (100.0) | 0 (0.0) | n = 2 |
| Resistance rates per drug | 34 (12.5) | 30 (11.1) | 61 (22.5) | 132 (48.7) | 114 (42.1) | 107 (39.5) | 37 (13.6) | |
aTotal number of isolated bacteria is 271; of these, 139 exhibited resistances to the antibiotics.
Table 4.
Overall antibiotic susceptibility profiles of isolated OAB.
| Identified Bacteria | NBG | Antibiotic Susceptibility (%) | Resistance Rates (%) | Total (%) | ||
|---|---|---|---|---|---|---|
| Sensitive | Intermediate | Resistant | ||||
| NBG (none) | 19 (6.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | - | 19 (6.5) |
| P aeruginosa | - | 6 (2.1) | 1 (0.34) | 8 (2.8) | 53.3 | 15 (5.2) |
| E coli | - | 15 (5.2) | 2 (0.7) | 20 (6.9) | 54.1 | 37 (12.8) |
| K oxytoca | - | 6 (2.1) | 0 (0.0) | 10 (3.4) | 62.5 | 16 (5.5) |
| K pneumoniae | - | 16 (5.5) | 2 (0.7) | 19 (6.6) | 51.4 | 37 (12.7) |
| S aureus | - | 59 (20.3) | 9 (3.1) | 63 (22.1) | 48.1 | 131 (45.3) |
| M catarrhalis | - | 2 (0.7) | 2 (0.7) | 5 (1.7) | 55.6 | 9 (3.1) |
| P mirabilis | - | 0 (0.00) | 1 (0.34) | 2 (0.7) | 67.0 | 3 (1.0) |
| S epidermidis | - | 4 (1.4) | 1 (0.34) | 9 (3.1) | 64.3 | 14 (4.8) |
| S pneumoniae | - | 2 (0.7) | 0 (0.0) | 5 (1.7) | 71.4 | 7 (2.4) |
| S pyogenes | - | (0.0) | 0 (0.0) | 2 (0.7) | 100.0 | 2 (0.7) |
| Total | 19 (6.5) | 110 (38.0) | 18 (6.2) | 143 (49.3)a | 52.8a | 290 (100.0) |
Abbreviation: NBG, no bacterial growth.
aOf 271 isolated bacteria, 143 exhibited resistance to the antibiotics, which is equivalent to 52.8%.
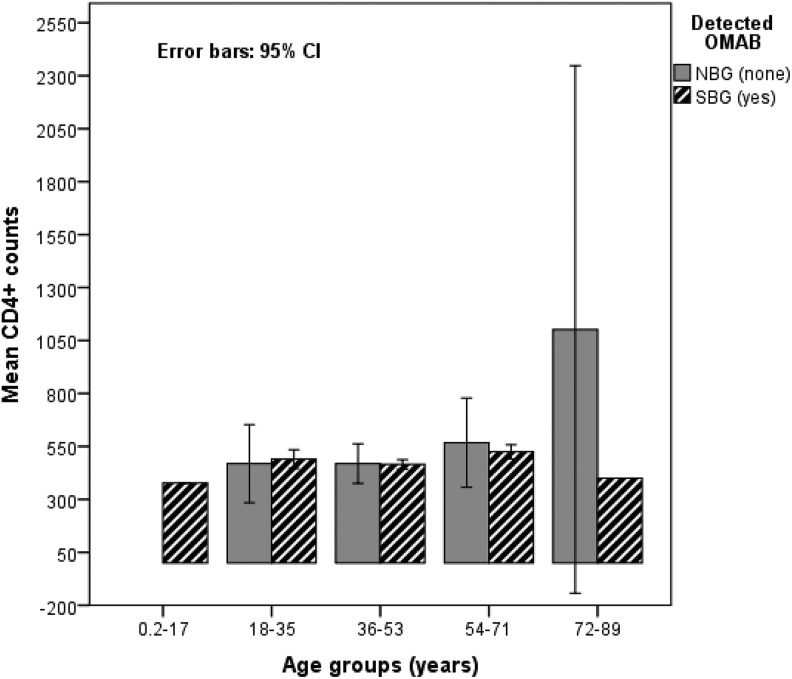
About 80.1% (n = 226) of OAB cases were observed among patients with low CD4+ counts (0-700 CD4+ counts/mm3) as compared to their counterparts with higher CD4+ counts (χ2 = 304.89; df = 40; P < .01), and a statistical significant association between the CD4+ counts and the prevalence of OAB was evident (ϕ = 0.578; P < .01). Only 2 (11.1%) PLH with over 1050 CD4+ counts had no SBG/OAB (Table 2). Over 94.0% of PLH aged between 36 and 53 years with CD4+ counts less than 500 cells/mm3 had OAB, as shown in Table 1 and Figure 1.
Figure 1.
Patients’ immunological status/ CD4+ T cell counts in relation to ages and occurrence of OAB.
Prevalence Rates of Antibiotic-Resistant OM-Associated Pathogens
A total of 7 commonly used antibiotics, namely ciprofloxacin (CIP-5), amikacin (AMI-30), ampicillin (AMP-30), Augmentin (AUG-30), erythromycin (ERY-15), co-trimoxazole (CXT-25), and gentamicin (GEN-10), were employed for susceptibility testing of the isolated bacteria. Of 271 isolated bacteria, 143 (52.8%) were resistant to the tested antibiotics and showed statistically significant differences of IZ with respect to the reference strains (P < .05), as shown in Tables 3 and 4.
Half of the isolated bacterial species (n = 5) exerted resistance of varying magnitudes to all 7 tested antibiotics. All isolates of Proteus mirabilis and S pyogenes were resistant to erythromycin (Table 3).
Over 70% (n = 5) isolates of S pneumoniae and 100% (n = 3) of P mirabilis were resistant to erythromycin. At least 2 isolates of S aureus were resistant to 2 or more of the antibiotics (Table 3). The highest antibiotic resistance rates were exerted against erythromycin by 2 gram-positive bacteria, S pyogenes (100%) and S pneumoniae (71.4%), as depicted in Table 4. Over 48% of isolated bacteria (n = 132) showed resistance against ampicillin, and only 30 (11.1%) of the bacterial isolates were resistant to amikacin. More than 66% of the isolated P mirabilis were resistant to both ampicillin and amoxicillin/clavulanic acid (Table 3). Slightly less than half (48%; n = 63) of S aureus isolates were resistant to all tested antibiotics (Table 4).
Statistical Analysis of Associations among Other Investigated Variables
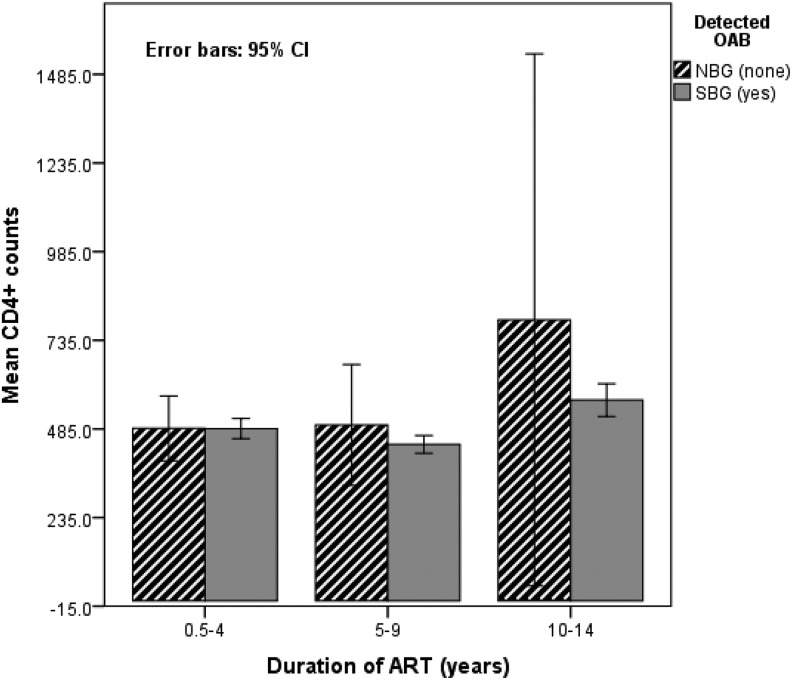
Pearson analysis (2 tailed) showed a significant correlation of patients’ ages with their body weight (Pearson R = .302; P = .001) and between CD4+ counts and body weights (R = .145; P = .01). A positive correlation (R = .062; P = .031; 1 tailed) was also evident between CD4+ T-cell counts and duration of ART (Figure 2), patients’ body weight and CD4+ T-cell counts (R = .137; P = .001), and patients’ ages and body weights (R = .0303; P = .001).
Figure 2.
Patients’ immunological status in relation to duration of ART and OAB.
Patients in the age groups of 18 to 35, 36 to 54, and 55 to 71 (ages ranging from 18 to 71) were over 20 times more likely to have OAB as compared to either older or younger cohorts (P = .01). Sex of participants did not have a statistical significant effect on the prevalence of OM, although males were less likely to have OAB (odds ratio [OR] = 0.696; P > .05). Controlling for patients’ age groups, associations existed between a number of years a patient was on ART or duration of ART with respect to both CD4+ counts (F = 1233.89; df = 167; P = .001) and body weight combined (F = 526; df = 56; P < .01). Weighted least squares regression (weighted by CD4+ counts) showed significant differences among the patients’ age groups with respect to the prevalence of OAB (F = 7.054; df = 4; P < .01).
Patients who were attending CTC for more than 10 years had relatively higher CD4+ T-cell counts with higher mean body weights (P = .001; χ2 = 1153.075) compared to the counterparts. Figure 2 shows that the longer the exposure time to ART, the patient had the less chances of contacting OAB (OR = 0.233; df = 2; P < .01). A statistically significant (F = 10.375; df = 177; P = < .001) association existed between the prevalence of OAB and CD4+ T-cell counts, controlling for the patients’ weights. Nonetheless, a statistically not significant negative correlation between the prevalence of OAB and CD4+ T-cell counts was evident (R = −.020; P = .504, 2 sided).
Discussion
HIV infection affects the immune system and causes OIs in immunocompromised host in a progressive manner. The worldwide spread of HIV infection that is accompanied with several OIs and complications compels PLH to seek extra medical services, including otolaryngological care. However, there is no attestation to show that the immunodeficient state predisposes patients to the development of otologic problems such as acute OM or its complications.8,36,37 Bacteria responsible for OM are among the most common otologic problems reported in HIV-infected patients.12,38 About 94% of PLH with asymptomatic OM were reported in this study as compared to 60% to 80% previously documented in other countries.8,9,39 Although the middle-ear pathogens frequently isolated, such as S pneumoniae, S pyogenes, H influenzae, and M catarrhalis, are similar for both the HIV-infected and the non-HIV-infected patient, Staphylococcus and Pseudomonas are more common in the former.40 Rare microorganisms, pathogens related frequently with HIV infection, are usually isolated in later stages of HIV infection.41 However, the management of OM is similar to that in immunocompetent patients.10,42,43
Improvement in immunologic status using HAART is a primary goal of diminishing the severity of OIs,33 and possible complications were associated with OM among PLH. Administration of prophylactic antibiotics to PLH reduces the frequency of acute and chronic infections such as OM, but treatment should be done cautiously.18,43 Our findings showed that patients under the HAART therapy with higher CD4+ T-lymphocyte counts had relatively lower prevalence of OAB, which coincides with that of Jafari et al.9 Our study also showed that patients’ CD4+ counts improved with increased duration of ART, and an increase in duration of ART led to a decrease in the prevalence of OAB. Besides, positive correlations between patients’ body weights with both CD4+ counts and the prevalence of OM were observed, although the relationship among these variables is unclear.6
Body weights appear to affect immune cell counts over the course of infection.20,44,45 During the introduction of HAART, being either underweight or obese was associated with smaller increases in several important immune cell levels, including the CD4/CD8 ratio.45 It has been shown that higher levels of leptin (a hormone controlling appetite and weight) and lower levels of lipopolysaccharides (LPS) are associated with overweight/obesity and higher amounts of body fat mass percentage. Higher leptin and lower LPS levels had significant association with higher HIV viral load, an indicator of HIV disease progression.46 These controversies on the role of adipose (fat mass) in PLH have not only implications for HIV/AIDS management but also for public health perspective.45 Some researchers suggest that the relation between higher fat mass/body weight and higher CD4+ counts “should be explored further in translational studies to understand the mechanisms and potential therapeutic implications.”43 The prevalence of OAB in PLH might be multifactorial as both environmental and genetic factors as well as functional and/or anatomical characteristics of the eustachian tube seem to play a major role.10,14 Other studies have ascribed the regulatory role played by CD4+ T cells and interleukins 2 and 4 to the OM pathogenesis, which is the conversion of acute to chronic stages of OM, especially in cases of suppurative OM.6,47 Regardless of the local mucosa immunity that plays an important role in the microecology of the nasopharynx normal flora, PLH may also have severe local immune disorders.17
Published data on OM are scarce in developing countries; hence, optimal management of OM is widely debated.4,7,48 The selection of antibiotic therapy is usually empiric because of the difficulty of obtaining cultures.23 Although researchers are in agreement that S pneumoniae and nontypeable H influenzae are the most common bacterial pathogens probably because of their coexistence in biofilm,10,17 the prevalence of resistance is not yet well-documented.48 Our study showed that S aureus and E coli are presumably the emerging OM-associated pathogens in PLH and have acquired antibiotic resistance of significant magnitude. Dissimilar to a report published 2 decades ago,11 this study revealed relatively low prevalence of M catarrhalis and S pneumoniae, which can be due to changes in human normal flora composition as a result of HIV/AIDS pandemic and widespread use of antimicrobial agents.10,12 Concomitant use of ARV medication with antibiotics in the management of HIV/AIDS and OIs seems to have had contributed to the high prevalence of OM among PLH, presumably as result of drugs’ adverse effects. Such effects might not be directly associated with OM-causing pathogens. Both persistent use of antimicrobial agents and exposure of microorganisms to the agents could have significantly ascribed to the observed high antibiotic resistance rates.49
The present study shows high rates of antibiotic resistance, ranging from 48% to 100%. Potentially pathogenic bacteria, such as E coli, S aureus, and P aeruginosa, exhibited high antibiotic resistance rate, which are also opportunistic pathogens, attributed to health-care facility–associated infections.50 Several antibiotics of choice for most bacterial infections such as ciprofloxacin, amoxicillin/clavulanic acid, ampicillin, and co-trimoxazole proved to be obsolete to many OM-associated bacteria. Streptococcus pyogenes was resistant to majority (70%) of the tested antibiotics, while 51% of E coli isolates were resistant to co-trimoxazole, which is an important antibiotic for prophylaxis of OIs common among PLH. In Tanzania, co-trimoxazole has been broadly used for treatment of diarrheal-related diseases,42 though high resistance to the antibiotic had already been reported.49 As oppose to a study conducted in India,31 our study showed that isolates of S aureus were relatively more susceptible to erythromycin and co-trimoxazole. Streptococcus pneumoniae is an opportunistic pathogen that was resistant to erythromycin, thus coinciding with previous studies from Argentina24 and Thailand.51 Erythromycin is one of the antibiotics of choice for upper respiratory infections, pneumonia, and OM in PLH and children42; such resistance may impede management of the infections leading to severe invasive diseases including bacteraemia and meningitis.52
Generally, antibiotics are indicated for the treatment of patients with OM and in selected patients with OM with effusion.7 Other studies elsewhere have reported that most otologic problems are caused by routine organisms that respond to standard therapy or may be as a result of direct consequence of virus or secondary to the pharmacological treatment.39 Hence, some practitioners advocate the restriction of using antimicrobial agents for the treatment of acute OM in certain age groups, especially children under 2 years of age, as there are no shreds of evidence on the effectiveness of antimicrobial therapy in the management of OM in such patients.32 For proper treatment of OM, the selection of antibiotics should be based on specific disease-causing pathogens. Only 38% of the OM-associated bacteria were sensitive to the locally available antibiotics. In Tanzania, like in other resource-constraint countries, prescribers hardly have wide choice for antibiotics for their patients; the reported resistance rate (62%) might be suicidal to patients.
Conclusion
High prevalence of OAB and high rate of antibiotic resistance among the OM-associated bacterial flora of asymptomatic PLH were observed. Staphylococcus aureus, K pneumonia, and E coli were the most frequently OM-associated bacteria. Both S pneumoniae and S pyogenes exhibited the highest rates of antibiotic resistance. Majority of the bacterial isolates were resistant to multiple antibiotics. Inverse relationships between CD4+ counts and prevalence of OM and between duration of ART and OM were eminent. The high prevalence of OAB observed in PLH calls for further investigations, because several bacteria that inhabit the ear canal can be converted into secondary colonizers and travel from the pharynx through the eustachian tube to tympanum cavity. Future studies should also compare the prevalence rates of bacterial flora of asymptomatic PLH to that of immunocompetent individuals. Irrational use of antibiotics may augment morbidity and mortality among the PLH through the development and spread of multiple antibiotic-resistant bacteria.
Acknowledgments
The authors acknowledge the financial support from Sida/Sarec through Muhimbili University of Health and Allied Sciences and extend special appreciation to the Morogoro Regional Referral Hospital management for granting permission to conduct the study at the CTC. The authors also are grateful to the ear, nose, and throat Specialists, research assistants, and other medical personnel for their invaluable assistance during specimen collection.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Financially supported by Sida/Sarec through Muhimbili University of Health and Allied Sciences.
References
- 1. Hrapcak S, Kuper H, Bartlett P, et al. Hearing loss in HIV-infected children in Lilongwe, Malawi. PloS One. 2016;11(8):e0161421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Luzuriaga K, Mofenson LM. Challenges in the elimination of pediatric HIV-1 infection. N Engl J Med. 2016;374(8):761–770. [DOI] [PubMed] [Google Scholar]
- 3. Qureishi A, Lee Y, Belfield K, Birchall JP, Daniel M. Update on otitis media—prevention and treatment. Infect Drug Resist. 2014;7:15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Smith AF, Ianacone DC, Ensink RJ, Melaku A, Casselbrant ML, Isaacson G. Prevalence of hearing-loss among HAART-treated children in the Horn of Africa. Int J Pediatr Otorhinolaryngol. 2017;98:166–170. [DOI] [PubMed] [Google Scholar]
- 5. Zahid T, Ahmed Z, Aqeel Z. Microbiology of chronic suppurative otitis media (CSOM) in tertiary care setup, Civil Hospital Karachi, Pakistan. Pak J Surg, 2016;32(3):176–180. [Google Scholar]
- 6. Weber R, Pinheiro Neto CD, Miziara ID, Araújo Filho BC. HAART impact on prevalence of chronic OM in Brazilian HIV-infected children. Rev Brasil Otorrinolaringol. 2006;72(4):509–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dhingra R, Monga S, Kaur G, Kaur M, Aggarwal V, Singh G. A study to establish the relation of antibiotics and steroids in fungal growth occurring in CSOM patients. Int J Adv Med. 2017;2(2):104–109. [Google Scholar]
- 8. Prasad HK, Bhojwani KM, Shenoy V, Prasad SC. HIV manifestations in otolaryngology. Am J Otolaryngol. 2006;27(3):179–185. [DOI] [PubMed] [Google Scholar]
- 9. Jafari S, Razmpa E, Saeedinejad Z, Sadrhosseini M, Paydary K. Otolaryngological manifestations in HIV infected patients, Tehran Iran. J AIDS Clinic Res. 2012;3(6):160. [Google Scholar]
- 10. Jensen RG, Johansen HK, Bjarnsholt T, Eickhardt-Sørensen SR, Homøe P. Recurrent otorrhea in chronic suppurative otitis media: is biofilm the missing link? Eur Arch Otorhinolaryngol. 2017;274(7):2741–2747. [DOI] [PubMed] [Google Scholar]
- 11. Bluestone CD, Stephenson JS, Martin LM. Ten—year review of OM pathogens. Pediatr Infect Dis J. 1992;11(8 suppl):S7–S11. [DOI] [PubMed] [Google Scholar]
- 12. Casey JR, Pichichero ME. Changes in frequency and pathogens causing acute otitis media in 1995–2003. Pediatr Infect Dis J. 2004;23(9):824–828. [DOI] [PubMed] [Google Scholar]
- 13. Lynch JP III, Zhanel GG. Streptococcus pneumoniae: epidemiology and risk factors, evolution of antimicrobial resistance, and impact of vaccines. Curr Opin Pulm Med. 2010;16(3):217–225. [DOI] [PubMed] [Google Scholar]
- 14. Aliyu IA, Kumurya AS, Bala JA, John OC. Bacteriology of otitis media and its host-environmental-infection factors. APEOHJ. 2017. 18;3(1):20–27. [Google Scholar]
- 15. Broides A, Dagan R, Greenberg D, Givon-Lavi N, Leibovitz E. Acute otitis media caused by Moraxella catarrhalis: epidemiologic and clinical characteristics. Clin Infect Dis. 2009;49(11):1641–1647. [DOI] [PubMed] [Google Scholar]
- 16. Almand EA, Moore MD, Jaykus LA. Virus-bacteria interactions: an emerging topic in human infection. Viruses. 2017;9(3):E58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. de Steenhuijsen Piters WA, Sanders EA, Bogaert D. The role of the local microbial ecosystem in respiratory health and disease. Phil Trans R Soc B. 2015;370(1675):20140294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nesheim SR, Hardnett F, Wheeling JT, et al. Incidence of opportunistic illness before and after initiation of highly active antiretroviral therapy in children. Pediatr Infect Dis J. 2013;32(10):1089–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Okoye AA, Picker LJ. CD4+ T-cell depletion in HIV infection: mechanisms of immunological failure. Immunol Rev. 2013;254(1):54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hunt PW, Brenchley J, Sinclair E, et al. Relationship between T cell activation and CD4+ T cell count in HIV-seropositive individuals with undetectable plasma HIV RNA levels in the absence of therapy. J Infect Dis. 2008;197(1):126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Davis AC, Watson G, Pourat N, Kominski GF, Roby DH. Disparities in CD4+ T-lymphocyte monitoring among human immunodeficiency virus-positive Medicaid beneficiaries: evidence of differential treatment at the point of care In: Open Forum Infectious Diseases. 2014. 1(2). Oxford, United Kingdom, Oxford University Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Joram SL, Paul G, Moses K, et al. Misdiagnosis of HIV treatment failure based on clinical and immunological criteria in Eastern and Central Kenya. BMC Infect Dis. 2017;17(1):383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Medina E, Pieper DH. Tackling threats and future problems of multidrug-resistant bacteria. How to overcome the antibiotic crisis In: Stadler M, Dersch P, eds. How to Overcome the Antibiotic Crisis. Current Topics in Microbiology and Immunology. Vol. 398 Cham, Switzerland: Springer; 2016:3–33. [DOI] [PubMed] [Google Scholar]
- 24. Reijtman V, Gagetti P, Faccone D, et al. Macrolide resistance in Streptococcus pneumoniae isolated from Argentinian pediatric patients suffering from acute otitis media. Rev Argent Microbiol. 2013;45(4):262–266. [DOI] [PubMed] [Google Scholar]
- 25. Rapoport B, Klastersky J, Raftopoulos H, et al. The emerging problem of bacterial resistance in cancer patients; proceedings of a workshop held by MASCC “Neutropenia, Infection and Myelosuppression” Study Group during the MASCC annual meeting held in Berlin on 27–29 June 2013. Support Care Cancer. 2016;24(7):2819–2826. [DOI] [PubMed] [Google Scholar]
- 26. Opintan JA, Newman MJ. Prevalence of antimicrobial resistant pathogens from blood cultures: results from a laboratory based nationwide surveillance in Ghana. Antimicrob Resist Infect Control. 2017;6(1):64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Orji FT, Ukaegbe O, Alex-Okoro J, Ofoegbu VC, Okorafor IJ. The changing epidemiological and complications profile of chronic suppurative otitis media in a developing country after two decades. Eur Arch Otorhinolaryngol. 2016;273(9):2461–2466. [DOI] [PubMed] [Google Scholar]
- 28. Hawkey PM. Multidrug-resistant Gram-negative bacteria: a product of globalization. J Hosp Infect. 2015;89(4):241–247. [DOI] [PubMed] [Google Scholar]
- 29. Akter S, Shamsuzzaman SM, Nehar N, Siddiqui I, Jahan F, Islam S. Bacterial isolates and drug susceptibility patterns of ear discharge from patients with ear infection at Shaheed Monsur Ali Medical College. Bangladesh J Med Microbiol. 2015;9(2):20–23. [Google Scholar]
- 30. Wahid FI, Khan A, Khan IA. Complications of chronic suppurative otitis media: challenge for a developing country. Kulak Burun Bogaz Ihtis Derg. 2014;24(5):265–270. [DOI] [PubMed] [Google Scholar]
- 31. Moorthy PN, Lingaiah J, Katari S, Nakirakanti A. Clinical application of a microbiological study on chronic suppurative otitis media. Int J Otorhinolaryngol Head Neck Surg. 2013;2(6):290. [Google Scholar]
- 32. Coker TR, Chan LS, Newberry SJ, et al. Diagnosis, microbial epidemiology, and antibiotic treatment of acute OM in children: a systematic review. JAMA. 2010;304(19):2161–2169. [DOI] [PubMed] [Google Scholar]
- 33. Masur H, Brooks JT, Benson CA, Holmes KK, Pau AK, Kaplan JE; National Institutes of Health; Centres for Disease Control and Prevention; HIV Medical Association of the Infectious Diseases Society of America. Prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: Updated Guidelines from the Centers for Disease Control and Prevention, National Institutes of Health, and HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis. 2014;58(9):1308–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cheesbrough M. District Laboratory Practice in Tropical Countries. Cambridge, United Kingdom, Cambridge University Press; 2006. [Google Scholar]
- 35. Clinical Laboratories Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing. Wayne, PA: Sixteenth Informational Supplement, Clinical Laboratories Standards Institute; 2006. [Google Scholar]
- 36. Hong SN, Lee WH, Lee SH, Rhee CS, Lee CH, Kim JW. Chronic rhinosinusitis with nasal polyps is associated with chronic otitis media in the elderly. Eur Arch Otorhinolaryngol. 2017;274(3):1463–1470. [DOI] [PubMed] [Google Scholar]
- 37. Dhanasekaran SV, Nair JS, Raja K, Gopalakrishnapillai GK, Chandran AK, Radhakrishnan S. A clinical study on the influence of sinusitis in chronic suppurative otitis media. Bengal J Otolaryngol Head Neck Surg. 2016;24(2):49–53. [Google Scholar]
- 38. Smith A, Gutteridge I, Elliott D, Cronin M. Acute otitis media associated bilateral sudden hearing loss: case report and literature review. J Laryngol Otol 2017;131(S2):S57–S61. [DOI] [PubMed] [Google Scholar]
- 39. Sulyman AB, Kazeem SA, Abdulrahman A, et al. Otolaryngologic manifestations among HIV/AIDS patients in a Nigerian tertiary health institution: an update. Intl Arch Otorhinolaryngol. 2010;14(4):398–403. [Google Scholar]
- 40. Wessman M, Bjarnsholt T, Eickhardt-Sørensen SR, Johansen HK, Homøe P. Mucosal biofilm detection in chronic otitis media: a study of middle ear biopsies from Greenlandic patients. Eur Arch Otorhinolaryngol. 2015;272(5):1079–1085. [DOI] [PubMed] [Google Scholar]
- 41. Rzewnicki I, Olszewska E, Rogowska-Szadkowska D. HIV infections in otolaryngology. Med Sci Monit. 2012;18(3):RA17–RA21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. The Standard Treatment Guidelines and the National Essential Medicine List for Tanzania (NEMLIT). Dar es Salaam, Ministry of Health and Social Welfare, Tanzania: 2013. http://www.who.int/selection_medicines/country_lists/Tanzania_STG_052013.pdf. [Google Scholar]
- 43. Koethe JR, Jenkins CA, Lau B, et al. Higher time-updated body mass index: association with improved CD4+ cell recovery on HIV treatment. J Acquir Immune Defic Syndr. 2016;73(2):197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Martinez SS. Overweight/obesity And HIV disease progression In Hiv+ adults In Botswana. Electronic Theses and Dissertations Florida International University, University Graduate School; 2015: 1826 http://digitalcommons.fiu.edu/etd/1826. [Google Scholar]
- 45. Flegal KM, Graubard BI, Williamson DF, Gail MH. Excess deaths associated with underweight, overweight, and obesity. JAMA. 2005;293(15):1861–1867. [DOI] [PubMed] [Google Scholar]
- 46. Crum-Cianflone NF, Roediger M, Eberly LE, et al. ; Infectious Disease Clinical Research Program HIV Working Group. Obesity among HIV-infected persons: impact of weight on CD4 cell count. AIDS. 2010;24(7):1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mittal R, Robalino G, Gerring R, et al. Immunity genes and susceptibility to otitis media: a comprehensive review. J Genet Genomics. 2014;41(11):567–581. [DOI] [PubMed] [Google Scholar]
- 48. Van Eldere J, Slack MP, Ladhani S, Cripps AW. Non-typeable Haemophilus influenzae, an under-recognised pathogen. Lancet Inf Dis. 2014;14(12):1281–1292. [DOI] [PubMed] [Google Scholar]
- 49. Mwambete KD, Kamuhabwa AA. Resistance of commensal intestinal Escherichia coli and other enterics to co-trimoxazole and commonly used antibiotics in HIV/AIDS patients. J. Clin. Microbiol. 2013;3(3):1–6. doi:10.4172/2327-5073.1000134. [Google Scholar]
- 50. Kizny Gordon AE, Mathers AJ, Cheong EY, et al. The hospital water environment as a reservoir for carbapenem-resistant organisms causing hospital-acquired infections—a systematic review of the literature. Clin Infect Dis. 2017;64(10):1435–1444. [DOI] [PubMed] [Google Scholar]
- 51. Thummeepak R, Leerach N, Kunthalert D, Tangchaisuriya U, Thanwisai A, Sitthisak S. High prevalence of multi-drug resistant Streptococcus pneumoniae among healthy children in Thailand. J Infect Public Health. 2015;8(3):274–281. [DOI] [PubMed] [Google Scholar]
- 52. Schroeder MR, Stephens DS. Macrolide resistance in Streptococcus pneumoniae . Front Cell Infect Microbiol. 2016;6(98):1–9. doi: 10.3389/fcimb.2016.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]