Abstract
Beliefs that it is harmful to mix medications with alcohol (ie, interactive toxicity beliefs) are a known source of intentional antiretroviral therapy (ART) nonadherence. This study examined a serial process model of alcohol-ART interactive toxicity beliefs, alcohol-ART avoidance behaviors, and ART adherence in the association between alcohol use and HIV viral load. Participants were 198 patients receiving ART from a community clinic in the southeastern United States; 125 reported current alcohol use. Results showed that current alcohol use was associated with detectable HIV viral load, partially accounted for by alcohol-ART interactive toxicity beliefs, alcohol-ART avoidance behaviors, and ART adherence. There was a significant indirect effect of the serial chain of interactive toxicity beliefs—avoidance behaviors—adherence, indicating the 3 intermediating variables partially accounted for the relationship between alcohol use and HIV viral load. Addressing alcohol use as a barrier to ART adherence requires multipronged approaches that address intentional nonadherence.
Keywords: alcohol use, intentional nonadherence, HIV treatment
What Do We Already Know about This Topic?
Alcohol is a robust barrier to HIV treatment adherence, and beliefs about mixing alcohol with medications further diminish adherence.
How Does Your Research Contribute to the Field?
Beliefs that it is hazardous to mix alcohol with antiretroviral medications are directly associated with behaviors aimed to avoid mixing alcohol and medications, leading to intentional nonadherence.
What Are Your Research’s Implications toward Theory, Practice, or Policy?
Interventions targeting alcohol use as a barrier to antiretroviral medication adherence should directly address alcohol-medication beliefs and behaviors to reduce intentional nonadherence.
Introduction
Antiretroviral therapy (ART) has transformed HIV infection from a nearly universally fatal disease to a manageable chronic illness. By suppressing HIV replication, ART dramatically slows the course of HIV infection and prevents the onset of AIDS. In addition, early treatment with ART preserves the immune system and reduces HIV infectiousness.1 Advances in ART have lowered pill burden, reduced drug toxicities, and offer regimens that are more forgiving of nonadherence.2 However, lower pill burden and effective drug dosing with even once-daily ART regimens have not always resulted in marked improvements in viral load relative to twice-daily regimens.3,4 Previous estimates in what has become known as the HIV treatment cascade were that 1 in 5 people with HIV who are treated with ART have detectable HIV viral loads.5 Recent research shows that these concerning estimates are actually overly optimistic, with more than 1 in 3 people (38%) receiving ART in clinical care not demonstrating durable control of HIV replication.6-8 Among the reasons for these missed opportunities are the challenges that many people living with HIV face in achieving and sustaining treatment retention and ART adherence.9,10 Alcohol and other drug use are among the most common factors at the core of disparate HIV-related health outcomes.11
Alcohol use, for example, effects memory, planning, organizational, and other cognitive functions that can result in missed medical appointments, prescription lapses, and missed medication doses.12 Studies show temporal and dose–response relationships between alcohol consumption and missed ART, with drinkers missing more doses than nondrinkers and binge drinkers missing more doses than nonbinge drinkers.13 The dose–response relationship between alcohol and ART adherence further shows that even limited or occasional use of alcohol impedes ART adherence.14,15 Alcohol use itself can accelerate HIV disease progression both directly by decreasing CD4 cells16 and indirectly by interfering with ART adherence.13 More concerning than sporadic missed doses are prolonged treatment interruptions that can occur with drinking.17 Among people living with HIV who drink alcohol, as many as half are nonadherent to ART and treatment interruptions when drinking are closely associated with overall nonadherence.18 And while alcohol use can impede adherence by causing missed doses and unintentional lapses in medication taking, there is growing concern that beliefs regarding the hazards of mixing alcohol with ART can lead to intentional nonadherence.18
Medication beliefs are potent determinants of adherence to medicines.19,20 Specific beliefs about the risks for toxicity from mixing ART with alcohol, known as interactive toxicity beliefs, such as believing that alcohol and ART should never be mixed and that alcohol interferes with the effects of ART, have been shown to adversely impact ART adherence.18 In the absence of co-occurring liver disease, there are no risks associated with moderate drinking and taking ART, and even heavy drinking poses limited risks with only certain classes of ART.21,22 The association between mortality and alcohol use among people living with HIV who do not have liver complications is therefore attributable to the independent health effects of alcohol abuse and the impact of alcohol use on ART adherence.23 Alcohol interactive toxicity beliefs are common among people living with HIV, including persons who continue drinking while taking ART.24 Alcohol interactive toxicity beliefs may motivate some people to stop drinking when initiating ART.18 However, among individuals who continue using alcohol after initiating ART, half report skipping their medications when drinking.25 Furthermore, these associations are observed at the day level. Specifically, in studies that have monitored daily drinking and ART, results show that interactive toxicity beliefs predict nonadherence on days when drinking coincides with medications.25 One study that monitored daily ART adherence and daily alcohol use over the course of 2565 patient-days found that alcohol interactive toxicity beliefs predicted ART nonadherence on days when drinking and not drinking, with interactive toxicity beliefs accounting for more variance in nonadherence than frequency of alcohol use, depression, and general medication concern beliefs.26 And while interactive toxicity beliefs are found across cultures with similar impacts on ART nonadherence,27-30 the mechanisms by which interactive toxicity beliefs impede adherence have not yet been reported.
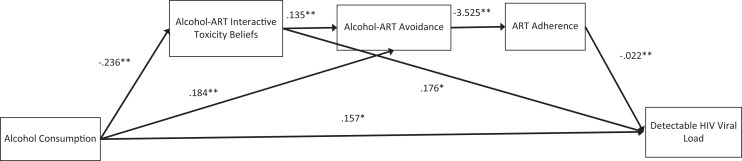
The current study was undertaken to examine interactive toxicity beliefs and associated behaviors as mechanisms in the association between alcohol use and poor clinical outcomes among people living with HIV who are receiving ART. We specifically tested 3 hypotheses in the context of a serial process model (see Figure 1) where the effects of alcohol use on HIV viral load (undetectable/detectable) would be partially accounted for by 3 serially positioned variables: interactive toxicity beliefs—alcohol-ART avoidance behaviors—ART adherence. First, we hypothesized there are direct effects of alcohol use on HIV load; greater alcohol consumption would be directly associated with greater likelihood of detectable HIV viral load. Second, we hypothesized serial direct effects of greater alcohol use on interactive toxicity beliefs, which would predict behaviors intended to avoid mixing alcohol and ART, and which in turn would predict poorer adherence, and ultimately detectable HIV viral load. Our third hypothesis concerned the indirect effects of alcohol use on HIV viral load through the serially related intermediating factors (beliefs-avoidance behaviors-nonadherence); alcohol’s indirect effect on viral load would be through the chain of all 3 intermediating factors.
Figure 1.
Serial process model predicting HIV viral load from alcohol use through interactive toxicity beliefs, alcohol-ART avoidance behaviors, and ART adherence. Note: *P < .05, **P < .01.
Methods
Setting and Participants
Patients were actively recruited from a publicly funded HIV clinic in central Georgia serving a small city and surrounding rural areas. More than 65% of people living with HIV in rural areas of the United States reside in southern states and more than half of people living with HIV in Georgia are in areas outside of major metropolitan areas.31 The 13 counties served by the clinic have residents living in poverty at more than double the national rate.32 A total of 124 men and 74 women completed the study measures between February and April 2016. Participants were receiving HIV treatment at the clinic site. None of the participants in this study had been diagnosed with significant liver disease.
Audio Computer-Assisted Self-Interviews
Demographic and Health Characteristics
We collected participant demographic characteristics (ie, gender, age, years of education, race, employment status, etc), history of incarceration, mental health treatment, and substance use services.
Alcohol and Other Drug Use
To assess alcohol use, we administered the Alcohol Use Disorders Identification Test (AUDIT), a 10-item scale designed to measure alcohol consumption and identify risks for alcohol abuse and dependence.33 The first 3 items of the AUDIT represent quantity and frequency of alcohol use and the remaining 7 items concern problems incurred from drinking alcohol. The first 3 items of the scale were used to index quantity and frequency of alcohol use in the previous month, composing the AUDIT–Consumption (AUDIT-C) subscale. Scores on the AUDIT-C range from 0 to 12 with acceptable internal consistency34; current study internal consistency, α = .90. We also asked participants whether they had used other drugs in the past month, including marijuana, cocaine/crack, inhalants (eg, poppers), and amphetamines.
Alcohol-ART Interactive Toxicity Beliefs
Participants completed 3 indicators of diverging beliefs about drinking alcohol and taking ART adapted from previous research.18 These items represented beliefs and perceptions that it is hazardous to take HIV medications when drinking alcohol with reference to other persons and not asking about personal actions. The specific items were, “Alcohol and HIV medications should never be mixed,” “Alcohol breaks down HIV medications so they will not work right,” and “A person should stop taking their HIV medications if they are going to be drinking” responded to on 4-point ratings, strongly disagree = 1 to strongly agree = 4, and summed to create an overall index (α =.68).
Alcohol-ART Avoidance Behaviors
Participants completed 6 items to assess behaviors that explicitly refer to not taking ART when drinking alcohol. These behaviors were adapted from previous research on substance use–related intentional nonadherence.35,36 The beliefs are shown in the Results section and were responded to regarding whether participants had personally performed each of the 6 actions, not practiced = 0, practiced = 1. We summed the number of behaviors endorsed to create the index of alcohol-related intentional nonadherence (α = .93).
Medication Adherence
We used a rating scale to assess ART adherence over the previous month. The adherence rating scale, often described as a visual analog scale, asks individuals to indicate the point along a continuum showing how much of their ART they have taken in the past month. For computerized administration, we adapted the response format by using a 100-point slide bar tool anchored by 0%, 50%, and 100%. The standard instructions are designed to counter socially desirable response biases by acknowledging that it can be difficult to take ART.37 The instructions read, “We would be surprised if most people take 100% of their medications. Below, 0% means you have taken none of their HIV medications this past month, 50% means you have taken half of your HIV medications this past month, and 100% means you have taken every single dose this past month. What percent of your HIV medications did you take?” Participants indicated the percentage of medications taken by clicking their mouse anywhere on the 100-point slide bar continuum. The adherence rating scale used in this study has been found reliable and valid,38 including significantly associated with HIV load.39,40
HIV Viral Load and CD4 Count
Lab reports of blood plasma HIV viral load and absolute CD4 counts most proximal and within 3 months of the survey completion were abstracted from electronic medical records. In accordance with HIV treatment guidelines,41 we define detectable viral load as >200 copies/mL—a threshold that nearly eliminates most cases of apparent viremia caused by viral load blips or assay variability. We used the clinically meaningful determination of detectable/undetectable viral load as the end point in our analytic models. We also collected absolute CD4 counts to describe participant health status; CD4 counts under 500 indicate immune system impairment and values under 200 cells/mm3 are diagnostic for AIDS.
Procedures
Participants were recruited through targeted convenience sampling. During a scheduled office visit, clinic patients were invited to participate in the study. A total of 257 patients were invited to complete the survey while waiting for their clinical appointment and 198 agreed, yielding a 77% response rate. Following informed consent, participants completed the audio computer-assisted self-interview and provided permission for the researchers to retrieve their electronic medical records. Participants were compensated for their time to complete the study measures with a US$15 cash (ATM) card. The university institutional review boards approved all procedures.
Data Analyses
For the purpose of descriptive analyses, we examined the demographic and health characteristics of the entire sample, with 124 persons reporting any current alcohol use and 74 reporting no current alcohol use. Descriptive analyses compared participants who were currently using alcohol as reported on the AUDIT-C to those not using alcohol. Comparisons used contingency table χ2 tests for categorical variables and independent t tests for continuous variables. We also conducted descriptive analyses to identify potential correlates of viral load (undetectable/detectable), using contingency table χ2 tests and independent t tests. In addition, we examined the frequencies of behavioral practices for avoiding mixing alcohol with medications for those participants reporting any alcohol use on the AUDIT-C. Descriptive correlational analyses were also performed with respect to the relationships among variables for all participants. Bivariate associations among continuous variables were examined with Pearson correlation coefficients and point biserial correlations for dichotomous viral load in relation to continuous measures.
Our main analyses tested the serial process model in Figure 1 that specifies multiple associations between alcohol use (AUDIT-C score) → Viral load, accounted for by Interactive Toxicity Beliefs → Avoiding ART when Drinking → ART Adherence. We used the SPSS IBM Process version 3.1 macro to test multiple factors using bootstrap statistical techniques.42 Multiple intermediating variable models are appropriately analyzed using regression when data are cross-sectional.43 The PROCESS macro estimates all paths designated in the model. Specifically, we used the model 6 template for multiple (3) variables in an x–y relationship.42
PROCESS detects binary outcome variables, such as HIV viral load in this study, and estimates the direct and indirect effects, as well as the paths from the intermediating variables to the outcome using logistic regression. Thus, coefficients predicting the intermediating factors are estimated using ordinary least squares regression, and paths for the dichotomous outcome are estimated with maximum-likelihood based logistic regression. To control for duration of HIV diagnosis, years since testing HIV positive was included as a covariate. This model tests the effects of the predictor variable (alcohol use) on 3 intermediating variables (M1 = alcohol interactive toxicity beliefs, M2 = avoiding taking ART when drinking, and M3 = adherence; a paths), the direct effects of the intermediating variables on the outcome (viral load, b paths), and the effects of the predictor variable on the outcome (c path). We computed 95% confidence intervals (CI) for the indirect effects of alcohol use on viral load through alcohol-ART interactive toxicity beliefs, alcohol-ART avoidance behaviors, and adherence that were estimated from 5000 bootstrap resamples. All analyses used P < .05 as the criterion for statistical significance.
Results
Table 1 shows the demographic and health characteristics of the current sample partitioned by current alcohol use. Participants who drank alcohol were significantly more likely to report other drug use, were older, and were diagnosed with HIV infection fewer years. In terms of current health status, 12% of the sample showed evidence of advanced HIV disease with CD4 counts under 200 and 27% had detectable HIV viral loads, with drinkers more likely to have detectable HIV. Table 2 shows the descriptive analyses for comparisons of participants with undetectable and detectable viral loads. Results showed expected differences in ART adherence and CD4 counts, as well as age and a trend for years since testing HIV positive.
Table 1.
Demographic and Health Characteristics of Participants Who Did and Did Report Current Alcohol Use.
| Characteristic | Current Alcohol Use, n = 124 | No Current Alcohol Use, n = 74 | χ2 | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Gender | 0.2 | ||||
| Men | 73 | 58 | 45 | 61 | |
| Women | 46 | 37 | 26 | 35 | |
| Transgender | 5 | 4 | 3 | 4 | |
| Race | 3.2 | ||||
| African American | 110 | 88 | 58 | 78 | |
| White | 11 | 10 | 13 | 18 | |
| Other | 3 | 2 | 3 | 4 | |
| Employment status | 5.0 | ||||
| Disabled | 45 | 36 | 34 | 46 | |
| Unemployed | 46 | 37 | 29 | 39 | |
| History of incarceration | 64 | 51 | 37 | 50 | 0.1 |
| Mental health history | 50 | 40 | 30 | 41 | 0.1 |
| Substance use treatment history | 31 | 25 | 21 | 28 | 0.3 |
| Cannabis use in past month | 47 | 37 | 9 | 12 | 15.1a |
| Cocaine use in past month | 11 | 9 | 0 | 0 | N/A |
| Any drug use in past month | 56 | 45 | 11 | 15 | 18.6a |
| ART adherence | |||||
| <80% | 30 | 24 | 12 | 17 | 1.6 |
| <85% | 32 | 26 | 13 | 19 | 1.9 |
| <90% | 34 | 27 | 13 | 19 | 2.35b |
| HIV > 200 copies/mL | 40 | 32 | 14 | 20 | 4.3b |
| CD4 count <200 cells/mm3 | 16 | 13 | 9 | 12 | 0.1 |
| M | SD | M | SD | t | |
| Age (years) | 43.7 | 11.5 | 40.0 | 12.1 | 3.0a |
| Years of education | 12.6 | 1.9 | 12.4 | 1.6 | 0.8 |
| Years since testing HIV positive | 11.8 | 8.3 | 16.1 | 8.6 | 3.4a |
| AUDIT–Consumption score | 3.5 | 2.3 | |||
| CD4 count (cells/mm3) | 587.0 | 339.6 | 564.7 | 295.7 | 0.5 |
Abbreviations: ART, antiretroviral therapy; AUDIT, Alcohol Use Disorders Identification Test; N/A comparison invalid due to empty cells; M, mean; SD, standard deviation.
a P < .01.
b P < .05.
Table 2.
Demographic and Health Characteristics of Participants with Undetectable and Detectable HIV Viral Load.
| Undetectable Viral Load (HIV < 200 copies/mL), n = 144 | Detectable Viral Load (HIV ≥ 200 copies/mL), n = 54 | ||||
|---|---|---|---|---|---|
| n | % | n | % | χ2 | |
| Characteristic | |||||
| Gender | 0.1 | ||||
| Men | 87 | 60 | 35 | 65 | |
| Women | 52 | 36 | 18 | 33 | |
| Transgender | 6 | 4 | 3 | 2 | 0.7 |
| Race | 3.9 | ||||
| African American | 113 | 78 | 49 | 91 | |
| White | 20 | 14 | 5 | 9 | |
| Other | 7 | 5 | 0 | ||
| Employment status | 3.0 | ||||
| Disabled | 59 | 41 | 18 | 33 | |
| Unemployed | 55 | 38 | 20 | 37 | |
| History of incarceration | 72 | 50 | 28 | 52 | 0.1 |
| Mental health history | 56 | 39 | 23 | 43 | 0.2 |
| Substance use treatment history | 37 | 26 | 15 | 28 | 0.1 |
| Cannabis use in past month | 35 | 24 | 20 | 37 | 2.5 |
| Cocaine use in past month | 7 | 4 | 4 | 2 | 0.4 |
| Any drug use in past month | 44 | 31 | 22 | 41 | |
| ART adherence | |||||
| <80% | 21 | 15 | 21 | 39 | 14.3a |
| <85% | 22 | 16 | 22 | 41 | 15.2a |
| <90% | 22 | 16 | 24 | 44 | 19.3a |
| CD4 count <200 cells/mm3 | 8 | 6 | 15 | 28 | 20.6a |
| M | SD | M | SD | t | |
| Age (years) | 47.4 | 11.5 | 41.6 | 12.3 | 3.0a |
| Years of education | 12.5 | 1.9 | 12.7 | 1.5 | 0.6 |
| Years since testing HIV positive | 14.2 | 8.4 | 11.6 | 9.0 | 1.8c |
| AUDIT–Consumption score | 1.9 | 2.4 | 2.7 | 2.6 | 2.0b |
| CD4 count (cells/mm3) | 641.3 | 271.4 | 417.6 | 304.4 | 0.5 |
Abbreviations: ART, antiretroviral; AUDIT, Alcohol Use Disorders Identification Test; M, mean; SD, standard deviation.
a p < .01.
b p < .05.
c p < .10.
Alcohol-ART Avoidance Behaviors
Table 3 shows the number and proportions of the subgroup of participants who drink alcohol as indexed on the AUDIT-C reporting intentional nonadherence behavior when drinking. The most frequently occurring behaviors were waiting to take ART after drinking (55%) and not mixing alcohol and HIV medications because it is not safe (40%). In addition, nearly 1 in 3 participants indicated that they wait to drink if they have taken their ART and 1 in 5 indicated that they stop taking ART when drinking alcohol.
Table 3.
Alcohol-ART Avoidance Behaviors among HIV-Positive Drinkers.a
| Avoidance Behavior | n | % |
|---|---|---|
| I skip taking my HIV medications if I will be drinking alcohol. | 13 | 10 |
| I do not mix alcohol and HIV medications because it is not safe. | 49 | 39 |
| If I were to drink alcohol, I would stop taking my HIV medications because I would not want to mix them. | 26 | 21 |
| I wait to drink alcohol until I am not taking HIV medications. | 38 | 30 |
| If I know I am going to be drinking alcohol, I won’t take my medications that day. | 19 | 15 |
| I wait at least a couple of hours after I take my medicine to drink alcohol. | 67 | 54 |
aN = 124.
Bivariate Correlations among Model Variables
Alcohol Use Disorders Identification Test–Consumption-C scores were significantly inversely related to interactive toxicity beliefs, positively related to alcohol-ART avoidance behaviors, and positively related to detectable viral load (see Table 4). Interactive toxicity beliefs were also associated with alcohol-ART avoidance behaviors in the expected direction; greater interactive toxicity beliefs were associated with engaging in avoidance behaviors. In addition, alcohol-ART avoidance behaviors were significantly inversely related to ART adherence and adherence was inversely related to detectable viral load. This pattern of associations was consistent with the proposed process model.
Table 4.
Bivariate Correlations among Model Variables.a
| Alcohol Consumption | Interactive Toxicity Beliefs | Alcohol-ART Interaction Avoidance | ART Adherence | |
|---|---|---|---|---|
| Interactive toxicity beliefs | −.19b | |||
| Alcohol-ART interaction avoidance | .25b | .13c | ||
| ART adherence | −.06 | .06 | −.18b | |
| HIV viral load | .14d | .12 | .12 | −.30b |
Abbreviation: ART, antiretroviral.
aN = 198.
b P < .01.
c P < .10.
d P < .05.
Alcohol Use and HIV Viral Load Serial Process Model
We tested the serial process model using multiple regression analyses that included alcohol use (independent variable), HIV viral load (dichotomous undetectable/detectable, dependent variable), and alcohol interactive toxicity beliefs, alcohol-ART avoidance behaviors, and ART adherence (intermediate variables). Results of the serial process model are shown in Figure 1. Years since testing HIV positive was included in the model and was not significant in any paths. The full model was significant in predicting HIV viral load, model χ2 = 23.64, P < .001, using McFadden estimated R 2, the model accounted for 11.1% of the variance in viral load.
For tests of direct effects, results confirmed the first hypothesis showing that AUDIT-C scores significantly predicted detectable HIV viral load, b = .157 (se = .073), P < .05, 95% CI: .014 to .300. In the test of the second hypothesis, the direct effect of alcohol use predicting alcohol interactive toxicity beliefs was significant, b = −.236, t = 3.09, P < .01, and alcohol-ART avoidance behaviors, b = .184, t = 4.11, P < .01, but not ART adherence, b = .179, t = −.21, P > .1. Alcohol interactive toxicity beliefs were directly related to HIV viral load, b = .176, z = 2.34, P < .01. Alcohol interactive toxicity beliefs significantly predicted alcohol-ART avoidance behaviors, b = .135, t = 3.16, P < .01, and alcohol-ART avoidance behaviors in turn predicted ART adherence, b = −3.52, t = −2.57, P < .01, and ART adherence predicted HIV viral load, b = −.022, z = −3.60, P < .01.
In the test of our third hypothesis, the indirect effects of the 3 serially positioned factors were tested using 5000 bootstrap resamples. The indirect effect of alcohol use on HIV viral load through the 3 serially aligned factors (Interactive Toxicity Beliefs → Avoiding ART when Drinking → ART Adherence) was significant, b = −.002, se = .002, 95% CI: −.0009 to −.0003. The indirect effect of alcohol use on viral load through interactive toxicity beliefs was also significant, b = −.0041, se = .026, 95% CI: −.1241 to −.0085. All other possible paths were included in the model and none of the other indirect effects were significant.
Discussion
The current study found that 62% of people receiving ART reported current alcohol use. We found that alcohol drinkers were more likely to report other drug use, were older, and were diagnosed with HIV a shorter time than nondrinkers. Current alcohol use was also related to detectable HIV viral load. Among participants who reported current alcohol use, more than half endorsed at least one behavior associated with intentionally avoiding taking ART when drinking. Thirty-nine percent of participants who reported drinking alcohol indicated that they do not mix alcohol and ART because it is not safe and 21% stated that they would stop taking their ART when drinking. We also found that alcohol interactive toxicity beliefs were associated with drinking less and level of drinking was positively related to engaging in alcohol-ART avoidance behaviors. These findings are consistent with past research on alcohol interactive toxicity beliefs24,25,27 and affirm the current model of underlying mechanisms in the association between alcohol consumption and HIV viral load.
We found support for the 3 study hypotheses that link alcohol interactive toxicity beliefs, alcohol-ART avoidance, and ART adherence in the association between alcohol use and detectable HIV viral load. Alcohol use demonstrated direct effects on viral load and direct effects on alcohol-ART avoidance behaviors, while alcohol-ART interactive toxicity beliefs had a direct effect on viral load. We also found that the indirect effect of the 3 serially aligned variables, interactive toxicity beliefs—avoidance behaviors—adherence, was also significant. These findings show that interactive toxicity beliefs and associated behaviors indicative of intentional nonadherence partially account for the association between alcohol use and viral load. Additionally, the indirect effect of alcohol use on unsuppressed HIV through alcohol interactive toxicity beliefs suggests a unique role of interactive toxicity beliefs on HIV treatment outcomes beyond any specific avoidance and adherence behaviors. It should be noted that our model only accounted for 11% of the variance in HIV viral load, suggesting that additional factors, including unmeasured beliefs and unintentional nonadherence, should be examined in future research. Alcohol interactive toxicity beliefs may impact treatment outcomes as part of a broader spectrum of medication concerns that are known to impede medication adherence.44,45
Results of the current study should be interpreted in light of its methodological limitations. We sampled participants receiving services from a publicly funded HIV care provider and our sample was one of convenience. Thus, our participants cannot be considered representative of people living with HIV. In addition, the study was conducted in just one state in the southeastern United States and is therefore also geographically constrained. The limitations of self-reported drinking are well-known and likely underestimated alcohol use. We also relied on self-reported measures for all of the other social and behavioral variables in this study. Although we used state-of-the-science measures delivered by computerized interviews, the results are still subject to reporting biases. For example, we did not observe an expected association between alcohol use and ART adherence, and this lack of association may have resulted from the restricted range of drinking reported on the AUDIT-C. Finally, the study design was cross-sectional and therefore does not allow for directional or causal inferences. With these limitations in mind, we believe that the current findings add to the existing literature and have implications for designing interventions aimed to address intentional nonadherence among people receiving ART who drink alcohol.
Interventions to improve ART adherence have typically concentrated on implementing reminders and memory aids, improving medication adherence skills, managing structural barriers to adherence, and resolving other sources of unintentional nonadherence.46-48 With respect to alcohol use and adherence, most approaches have either tried to reduce drinking or used strategies for remembering to take medications when drinking. Although sources of alcohol-related unintentional nonadherence remain important targets for interventions, fully addressing the influence of alcohol on ART adherence will require attending to intentional nonadherence as well. Interventions should aim to both reduce missed doses of ART that result from unintentional nonadherence and address interactive toxicity beliefs associated with intentional nonadherence. Clinical assessments of alcohol use should directly assess patient beliefs about drinking and taking ART. It is also important to note that for patients who are diagnosed with liver disease, drinking is highly hazardous, and concerns about mixing alcohol and ART are well-founded.49,50 Any reassurance that mixing alcohol and ART is safe, therefore, does not apply to patients with co-occurring liver disease.
Believing that it is better to not take ART than mix ART with alcohol should be addressed directly with corrective information. However, disputing erroneous interactive toxicity beliefs should take care not to inadvertently increase alcohol use because reducing alcohol consumption remains an important goal for improving the health of people living with HIV and maximizing HIV treatment outcomes. We therefore suggest taking a broader health literacy approach to correcting interactive toxicity beliefs as opposed to challenging any one given belief.51 Clear messages with accurate information should be delivered in routine clinical care with the aim of replacing false assumptions and folklore regarding alcohol use and medications.
Footnotes
Authors’ Note: The study was approved by the University of Connecticut Institutional Review Board Protocol H14-184GDPH and all participants gave written informed consent.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This project was supported by National Institute of Alcohol Abuse and Alcoholism Grant R01-AA023727.
References
- 1. Cohen MS, McCauley M, Gamble TR. HIV treatment as prevention and HPTN 052. Curr Opin HIV AIDS. 2012;7(2):99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gordon LL, Gharibian D, Chong K, Chun H. Comparison of HIV virologic failure rates between patients with variable adherence to three antiretroviral regimen types. AIDS Patient Care STDS. 2015;29(7):384–388. [DOI] [PubMed] [Google Scholar]
- 3. Nachega JB, Parienti JJ, Uthman OA, et al. Lower pill burden and once-daily antiretroviral treatment regimens for HIV infection: a meta-analysis of randomized controlled trials. Clin Infect Dis. 2014;58(9):1297–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen Y, Chen K, Kalichman SC. Barriers to HIV medication adherence as a function of regimen simplification. Ann Behav Med. 2017;51(1):67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gardner EM, McLees MP, Steiner JF, Del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis. 2011;52(6):793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Crepaz N, Tang T, Marks G, Mugavero MJ, Espinoza L, Hall HI. Durable viral suppression and transmission risk potential among persons with diagnosed HIV infection: United States, 2012-2013. Clin Infect Dis. 2016;63(7):976–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Marks G, Patel U, Stirratt MJ, et al. Single viral load measurements overestimate stable viral suppression among HIV patients in care: clinical and public health implications. J Acquir Immune Defic Syndr. 2016;73(2):205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Marks G, Gardner LI, Rose CE, et al. Time above 1500 copies: a viral load measure for assessing transmission risk of HIV-positive patients in care. AIDS. 2015;29(8):947–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Walcott M, Kempf MC, Merlin JS, Turan JM. Structural community factors and sub-optimal engagement in HIV care among low-income women in the Deep South of the USA. Cult Health Sex. 2016;18(6):682–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kalichman S, Kalichman MO, Cherry C. Medication beliefs and structural barriers to treatment adherence among people living with HIV infection. Psychol Health. 2016;31(4):383–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Williams EC, Hahn JA, Saitz R, Bryant K, Lira MC, Samet JH. Alcohol use and human immunodeficiency virus (HIV) infection: current knowledge, implications, and future directions. Alcohol Clin Exp Res. 2016;40(10):2056–2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hendershot CS, Stoner SA, Pantalone DW, Simoni JM. Alcohol use and antiretroviral adherence: review and meta-analysis. J Acquir Immune Defic Syndr. 2009;52(2):180–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Braithwaite RS, McGinnis KA, Conigliaro J, et al. A temporal and dose-response association between alcohol consumption and medication adherence among veterans in care. Alcohol Clin Exp Res. 2005;29(7):1190–1197. [DOI] [PubMed] [Google Scholar]
- 14. Parsons JT, Rosof E, Mustanski B. The temporal relationship between alcohol consumption and HIV-medication adherence: a multilevel model of direct and moderating effects. Health Psychol. 2008;27(5):628–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mayston R, Kinyanda E, Chishinga N, Prince M, Patel V. Mental disorder and the outcome of HIV/AIDS in low-income and middle-income countries: a systematic review. AIDS. 2012;26(suppl 2):S117–S135. [DOI] [PubMed] [Google Scholar]
- 16. Barve SS, Kelkar SV, Gobejishvilli L, Joshi-Barve S, McClain CJ. Mechanisms of alcohol-mediated CD4+ T lymphocyte death: relevance to HIV and HCV pathogenesis. Front Biosci. 2002;7:d1689–d1696. [DOI] [PubMed] [Google Scholar]
- 17. Parienti JJ, Das-Douglas M, Massari V, et al. Not all missed doses are the same: sustained NNRTI treatment interruptions predict HIV rebound at low-to-moderate adherence levels. PLoS One. 2008;3(7):e2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kalichman SC, Amaral CM, White D, et al. Alcohol and adherence to antiretroviral medications: interactive toxicity beliefs among people living with HIV. J Assoc Nurses AIDS Care. 2012;23(6):511–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Horne R, Cooper V, Gellaitry G, Date HL, Fisher M. Patients’ perceptions of highly active antiretroviral therapy in relation to treatment uptake and adherence: the utility of the necessity-concerns framework. J Acquir Immune Defic Syndr. 2007;45(3):334–341. [DOI] [PubMed] [Google Scholar]
- 20. Horne R, Buick D, Fisher M, Leake H, Cooper V, Weinman J. Doubts about necessity and concerns about adverse effects: identifying the types of beliefs that are associated with non-adherence to HAART. Int J STD AIDS. 2004;15(1):38–44. [DOI] [PubMed] [Google Scholar]
- 21. Bilal U, Lau B, Lazo M, et al. Interaction between alcohol consumption patterns, antiretroviral therapy type, and liver fibrosis in persons living with HIV. AIDS Patient Care STDS. 2016;30(5):200–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Staltari O, Leporini C, Caroleo B, et al. Drug-drug interactions: antiretroviral drugs and recreational drugs. Recent Pat CNS Drug Discov. 2014;9(3):153–163. [DOI] [PubMed] [Google Scholar]
- 23. Marshall BDL, Tate JP, McGinnis KA, et al. Long-term alcohol use patterns and HIV disease severity. AIDS. 2017;31(9):1313–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kalichman SC, Amaral CM, White D, et al. Prevalence and clinical implications of interactive toxicity beliefs regarding mixing alcohol and antiretroviral therapies among people living with HIV/AIDS. AIDS Patient Care STDS. 2009;23(6):449–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kalichman SC, Grebler T, Amaral CM, et al. Intentional non-adherence to medications among HIV positive alcohol drinkers: prospective study of interactive toxicity beliefs. J Gen Intern Med. 2013;28(3):399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pellowski JA, Kalichman SC, Kalichman MO, Cherry C. Alcohol-antiretroviral therapy interactive toxicity beliefs and daily medication adherence and alcohol use among people living with HIV. AIDS Care. 2016;28(8):963–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Conroy AA, McKenna SA, Leddy A, et al. “If she is drunk, I don’t want her to take it”: partner beliefs and influence on use of alcohol and antiretroviral therapy in South African couples. AIDS Behav. 2017;21(7):1885–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kekwaletswe CT, Morojele NK. Patterns and predictors of antiretroviral therapy use among alcohol drinkers at HIV clinics in Tshwane, South Africa. AIDS Care. 2014;26(suppl 1):S78–S82. [DOI] [PubMed] [Google Scholar]
- 29. Hahn JA, Emenyonu NI, Fatch R, et al. Declining and rebounding unhealthy alcohol consumption during the first year of HIV care in rural Uganda, using phosphatidylethanol to augment self-report. Addiction. 2016;111(2):272–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Santos GM, Emenyonu NI, Bajunirwe F, et al. Self-reported alcohol abstinence associated with ART initiation among HIV-infected persons in rural Uganda. Drug Alcohol Depend. 2014;134:151–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Centers for Disease Control and Prevention. HIV/AIDS statistics and surveillance. 2015. http://www.cdc.gov/hiv/topics/surveillance/basic.htm#hivaidsexposure. Accessed June 6, 2018.
- 32. Boston D. Poverty rates in Georgia. 2008. http://poverty.suite101.com/article.cfm/poverty_in_georgia. Accessed June 6, 2018.
- 33. Saunders JB, Aasland OG, Babor TF, DeLaFuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption II. Addictions. 1993;88(6):791–804. [DOI] [PubMed] [Google Scholar]
- 34. Maisto SA, Conigliaro J, McNeil M, Kraemer K, Kelley ME. An empirical investigation of the factor structure of the AUDIT. Psychol Assess. 2000;12(3):346–353. [DOI] [PubMed] [Google Scholar]
- 35. Altice F, Mostashari F, Friedland G. Trust and the acceptance of and adherence to antiretroviral therapy. JAIDS. 2001;28(1):47–58. [DOI] [PubMed] [Google Scholar]
- 36. Sankar A, Wunderlich T, Neufeld S, Luborsky M. Sero-positive African Americans’ beliefs about alcohol and their impact on anti-retroviral adherence. AIDS Behavior. 2007;11(2):195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Simoni J, Kurth AE, Pearson C, Pantalone DW, Merrill J, Frick P. Self-report measures of antiretroviral therapy adherence: a review with recommendations for HIV Research and Clinical Management. AIDS Behavior. 2006;10(3):227–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Finitsis DJ, Pellowski JA, Huedo-Medina TB, Fox MC, Kalichman SC. Visual analogue scale (VAS) measurement of antiretroviral adherence in people living with HIV (PLWH): a meta-analysis. J Behav Med. 2016;39(6):1043–1055. [DOI] [PubMed] [Google Scholar]
- 39. Bangsberg DR, Hecht FM, Charlebois ED, Chesney M, Moss A. Comparing objective measures of adherence to HIV antiretroviral therapy: electronic medication monitors and unannounced pill counts. AIDS and Behavior. 2001;5(3):275–281. [Google Scholar]
- 40. Giordano TP, Guzman D, Clark R, Charlebois ED, Bangsberg D. Measuring adherence to antiretroviral therapy in a diverse population using a visual analogue scale. HIV Clinical Trials. 2004;5(2):74–79. [DOI] [PubMed] [Google Scholar]
- 41. International Advisory Panel on HIV Care Continuum Optimization. IAPAC guidelines for optimizing the HIV care continuum for adults and adolescents. J Int Assoc Provid AIDS Care. 2015;14(suppl 1):S3–S34. [DOI] [PubMed] [Google Scholar]
- 42. Hayes AF. Model templates for PROCESS for SPSS and SAS. New York: Guilford Press. 2013.
- 43. Judd CM, Kenny DA. Process analysis: estimating mediation in treatment evaluations. Evaluation Rev. 1981;5(5):602–619. [Google Scholar]
- 44. Cooper V, Horne R, Gellaitry G, et al. The impact of once-nightly versus twice-daily dosing and baseline beliefs about HAART on adherence to efavirenz-based HAART over 48 weeks: the NOCTE study. J Acquir Immune Defic Syndr. 2010;53(3):369–377. [DOI] [PubMed] [Google Scholar]
- 45. Cooper V, Moyle GJ, Fisher M, et al. Beliefs about antiretroviral therapy, treatment adherence and quality of life in a 48-week randomised study of continuation of zidovudine/lamivudine or switch to tenofovir DF/emtricitabine, each with efavirenz. AIDS Care. 2011;23(6):705–713. [DOI] [PubMed] [Google Scholar]
- 46. Amico KR, Harman JJ, Johnson BT. Efficacy of antiretroviral therapy adherence interventions: a research synthesis of trials, 1996 to 2004. J Acquir Immune Defic Syndr. 2006;41(3):285–297. [DOI] [PubMed] [Google Scholar]
- 47. Finitsis DJ, Pellowski JA, Johnson BT. Text message intervention designs to promote adherence to antiretroviral therapy (ART): a meta-analysis of randomized controlled trials. PLoS One. 2014;9(2):e88166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Simoni JM, Amico KR, Smith L, Nelson K. Antiretroviral adherence interventions: translating research findings to the real world clinic. Curr HIV/AIDS Rep. 2010;7(1):44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Conigliaro J, Madenwald T, Bryant K, et al. The veterans aging cohort study: observational studies of alcohol use, abuse, and outcomes among human immunodeficiency virus-infected veterans. Alcohol Clin Exp Res. 2004;28(2):313–321. [DOI] [PubMed] [Google Scholar]
- 50. Samet JH, Horton NJ, Meli S, Freedberg KA, Palepu A. Alcohol consumption and antiretroviral adherence among HIV-infected persons with alcohol problems. Alcohol Clin Exp Res. 2004;28(4):572–577. [DOI] [PubMed] [Google Scholar]
- 51. Jacobs RJ, Caballero J, Ownby RL, Kane MN. Development of a culturally appropriate computer-delivered tailored Internet-based health literacy intervention for Spanish-dominant Hispanics living with HIV. BMC Med Inform Decis Mak. 2014;14:103. [DOI] [PMC free article] [PubMed] [Google Scholar]