Abstract
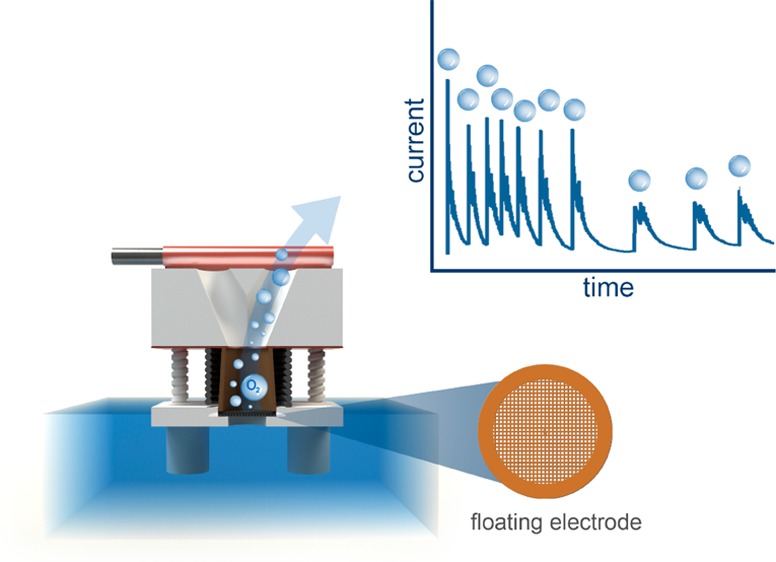
The future significance of energy conversion has stimulated intense investigation of various electrocatalytic materials. Hence electrocatalysts have become the subject of electrochemical characterization on a daily basis. In certain cases of interest, when measuring electrochemical reactions beyond the onset potentials, however, appropriateness of existing electroanalytical methods may be questioned and alternative approaches need to be developed. The present study highlights some shortcomings in the electrochemical investigation of gas evolving reactions. The oxygen evolution reaction (OER) is selected as a case example with a specific focus on the electrochemical stability of a nanoparticulate iridium catalyst. When conventional electrochemical methods, such as thin film rotating disc electrodes are employed to study the materials’ stability, the intrinsic degradation is masked by oxygen bubbles, which are inherently being formed during the reaction, especially when high current densities are used. In this Letter, we present a solution to this issue, the so-called floating electrode arrangement. Its elegant usage enables fast and reliable electrochemical characterization of oxygen evolution electrocatalysts.
One of the pillars of the new energy system is the use of hydrogen as an energy carrier, easily produced under electrolysis of clean water and using renewable solar or wind energy. While alkaline water electrolysis strongly prevailed over the second half of the previous century, the last couple of decades have seen a rapid development of proton exchange membranes (PEM), due to their ability to sustain high current densities and effectiveness in handling fast and large current alternations. Indeed, the PEM technology has recently been recognized as a competitor for alkaline water electrolysis (AWE), especially when it comes to large scale deployment as it promises lower costs. However, PEM technology is still underdeveloped in terms of reaching its ultimate performance.1,2 At the moment, efficient water electrolyzers rely on rare platinum group metal (PGM) catalysts. This is likely to cause problems if electrolysis systems have to be produced on a larger scale due to their limited availability and high cost. Enormous space for improvement is available by optimizing the anodic counterpart of water electrolysis, namely, the oxygen evolution reaction (OER). The most widely used electrocatalyst for OER are Ir and Ru-based materials with the latter being more reactive but not stable enough in the usual operating window.3,4 In comparison to other PGMs, Ir is even more scarce, with only 0.001 ppm in earth’s crust. Therefore, an optimal OER catalyst design should at the same time pursue an increase of the available electrochemically active surface area (ECSA) and its stability enhancement.5−7
In order to reliably differentiate between numerous proposed catalysts, a prerequisite for efficient progress is an appropriate and reliable electrochemical characterization of potential materials. In most cases, in the initial stage, new catalysts are tested in conventional electrochemical cells where rotating disc electrodes (RDE) are employed as working electrodes. Usually glassy carbon, boron-doped diamond (BDD), or gold discs are used as substrates on which a catalyst is deposited. However, these electrodes suffer from several pitfalls. For example, GC electrodes, and to some extent BDD, tend to oxidize under harsh conditions of OER. This leads to passivation of the electrode surface and subsequent inaccurate estimation of kinetic data.8 Additionally, when characterizing gas-evolving reactions such as OER, the effect of gas bubbles on the active surface area should be taken into account, as discussed in several recent publications.9−12 More specifically, Zeradjanin et al. showed that under OER conditions there are regions on the electrode surface where the reaction interface does not contain a sufficient amount of solvent to form a solvation sphere around each generated molecule of oxygen.11 Consequently, oxygen bubbles nucleate in the nanoscale region and eventually grow, causing passivation of the active surface.10,13,14 Gas bubbles tend to be removed more effectively with imposing convection induced by rotation of the electrode. However, as thoroughly discussed by Zeradjanin et al., even at a rotation of 10 000 rpm, the size of the diffusion layer is insufficient to remove nanobubbles and even microbubbles in the case of RDE.11 Therefore, immediate attention should be given to the effect of oxygen bubbles, especially since the state-of-the-art research within the topic of OER catalysis is focusing on the development of stable high surface area composites. In the course of a stability test where high potentials (up to 1.8 V vs reversible hydrogen electrode (RHE)) are usually imposed, the catalysts are even more prone to the formation of bubbles. The present Letter directly addresses this issue. For this purpose, electrochemical performance of an OER catalyst based on Ir nanoparticles is comparatively investigated using a conventional RDE and a newly developed floating electrode design.
Electrochemical Characterization
For the purpose of this study, a novel three electrode system has been developed, where the working electrode operates in the so-called floating mode. This means that the electrode is not dipped in the electrolyte solution but is rather placed on its surface. The working electrode compartment is made of two polyether ether ketone (PEEK) holders which are connected via PEEK screws. In the present setup (Figure 1a,b), the upper part of the PEEK holder was coated with a copper foil tape (thickness 0.06 mm, Goldpart International Co., Ltd). Between these elements, a transmission electron microscopy (TEM) gold grid (Ted Pella, outer diameter 3.05 mm, 200 mesh grid) was inserted and served as the working electrode (see Figure 1). Between the grid and the upper part of the PEEK holder, a metallic spring, a metallic cone, and a gas diffusion layer (GDL) were inserted. A GDL of 280 μm thickness and 40% Teflon weight wet proofing (Toray Carbon Paper 090, Fuel CellStore) was used. It serves as a spacer to separate the working electrode and its electric contact (metallic cone). We note that Teflon gives the carbon material a hydrophobic property, which prevents the electrolyte to penetrate to the metallic cone or spring hence causing their corrosion. Additionally, the acidic vapors could still pass through GDL and could damage the spring or cause other down stream corrosion. In order to prevent this, a rubber tube was inserted through the cone to the GDL. In this way, vapors cannot reach metallic parts. GDL, together with the metallic cone served as an electric contact for the TEM grid (working electrode, see Scheme S-1b). A vacuum pump (Ted Pella, for details see the Supporting Information section Vacuum suction methodology) was connected to one of the holes of the peek housing via rubber tubing. Its role was to assist in the removal of the oxygen bubbles, which are formed in the course of OER (unless stated otherwise vacuum strength level was always 1 in terms of relative units, see the Supporting Information section Vacuum suction methodology). The investigated electrocatalyst (homemade Ir nanoparticles, see the Supporting Information sections Synthesis of high surface area iridium-based nanoparticles and Transmission electron microscopy characterization) was deposited on the grid by drop-casting a water suspension of the catalyst on the TEM grid. The grid was placed on a Teflon holder that was connected to a vacuum pump (Ted Pella, see the Supporting Information section Vacuum suction methodology). Once the suspension was dropcasted, the vacuum was turned on. This led to the formation of uniform catalyst film coverage of the grid (see the Supporting Information section Scanning electron microscopy characterization). A platinum rod was used as a counter electrode and Ag/AgCl as a reference electrode. HClO4 (0.1 M, 70%, 99.999% trace metals basis, Merck) was used as a working electrolyte. Analogous electrochemical experiments (as in the case of the floating electrode study) were performed in the typical RDE configuration under 1600 rpm. A boron doped diamond disk electrode (BDD, d = 5 mm, NeoCoat) was used as a working RDE electrode. Chronoamperometry was performed on each of the cell configurations, either under ambient or vacuum suction conditions. In the latter case, a vacuum pump was connected to the nonelectrolyte side of the TEM grid (Figure 1). Ohmic drop compensation (95%) was performed during electrochemical experiments.
Figure 1.
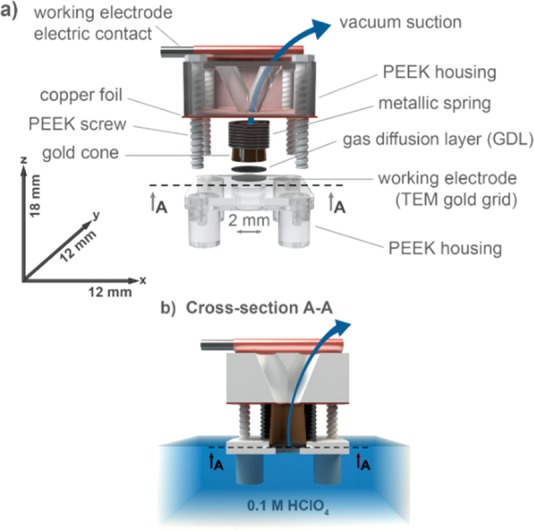
Schematic representation of the floating electrode setup in an exploded view (a) and the cross-section (b) along the line A–A of part a. The dimensions of the entire assembly are shown on the coordinate system in part a. The working electrode is placed on the surface of the electrolyte solution so that it is in contact with the electrolyte on the bottom and the air on the top.
Results and Discussion
The concept of a floating electrode has been known since the 1960s when it was introduced in the field of phosphoric acid fuel cells.15,16 After that, however, its development has been almost nonexistent. Only recently the concept has been revived by Kuchernak’s group17 followed by a few other studies that have employed a similar concept to study mass transport-limited reactions such as oxygen reduction (ORR) and hydrogen oxidation (HOR) reactions.18,19 In the present study, however, the floating electrode is not used primarily in order to enhance the transport of gas to the surface of the catalyst. Rather than that, we employ such an electrode, for the first time, as an oxygen evolution reaction (OER) diagnostic tool. Since OER is a gas evolving reaction, in contrast to HOR and ORR, high mass transfer of reactants is not an issue. By contrast, the main issue of OER is the removal of the products, namely, oxygen gas. The floating electrode configuration might, however, effectively circumvent the “passivation” of the electrode surface with oxygen bubbles, which presents a serious issue in the RDE setups. Such circumvention could be provided due to the special positioning of the catalyst layer which is in direct contact with the gas phase. In order to inspect the feasibility of the floating electrode for OER characterization, an in-house electrochemical system was developed (Figure 1). The electrochemical activity for OER was evaluated under linear sweep voltammetry (LSV) conditions. The electrochemical performance of two distinctly different electrode loadings (1 and 5 μg) was initially tested in order to test the basic feasibility of the floating electrode method (Figure S-6). As demonstrated, both electrode loadings gave more or less the same OER performance (in terms of mass normalized current densities). This is a direct confirmation that the catalyst layer was completely utilized in both cases.
In continuation, the ability of the floating electrode to efficiently remove oxygen bubbles was tested during a potentiostatic treatment. A potential hold was performed at 2.0 V (Figure S-7). A vacuum pump was connected to the working electrode housing (see Figure 1a,b). We note that such potential is outside the scope of oxygen evolution reaction investigation. We have used such vigorous conditions in order to intentionally induce the formation of oxygen bubbles. It can be seen that the presence of vacuum suction during electrochemical treatment has a clear effect on the current response (Figure S-7a,b). In particular, vacuum enhances the occurrence of repetitive current oscillations, whereas without vacuum suction oscillations stop, which indicates the onset of passivation of the electrode with bubbles. Thus, the oscillations seen under vacuum occur due to the efficient transient removal of the continuously growing oxygen bubbles through the holes of the Au grid. Once the bubbles have been removed, the electrochemical surface area is revived and again available for OER to proceed. By gradually increasing the level of vacuum, the current response increases as well (Figure S-7a,b). Overall, the electrochemical comparison of vacuum and ambient (nonvacuum) treatments clearly indicated the potential benefits of the former mode.
In order to demonstrate the potential advantages of the floating electrode in a more quantitative way, the results obtained with this method were compared to the results of a typical conventional electrochemical methodology for characterization of electrocatalysts, the thin film rotating disc electrode (TF-RDE) method (Figure 2, and Figure S-9). The experimental protocol consisted of OER activity estimation via linear sweep voltammetry (LSV) treatment (20 mV s–1), followed by a chronoamperometric protocol (1.8 or 2.0 V vs RHE, 2000 s) and an additional activity LSV estimation (20 mV s–1) to estimate the activity decay. Performance comparison of the methods is demonstrated in Figure 2. The same total catalyst loadings were used in both cases. Fascinatingly, under chronoamperometric conditions, the OER activity of the two electrode types is remarkably different, that is, the mass normalized current densities are in general higher in the case of the floating electrode. This is due to the better utilization of the catalyst layer in the former. The reason for such better utilization is the continual removal of gas bubbles, which is not present in the case of RDE (see Figure S-10). As seen from the chronoamperometric response, the repetitive removal of gas bubbles results in oscillations described before. More importantly, however, the average current reaches a steady-state level much faster and at significantly higher values. Very likely, this value is much closer to the actual intrinsic activity of the catalyst. After potentiostatic treatment, the LSV protocol was performed again in order to estimate the decay of OER activity (Figure 2b) during potentiostatic treatment. The OER activity is lower in the case of RDE configuration. This trend is in line with the fact that oxygen bubbles stay in the catalyst film and passivate the electrode surface in the RDE configuration (see Figure S-10). Hence the effective catalyst surface is lower in this case. In any case, the comparison of floating and RDE configuration highlights a serious pitfall of conventional RDE methodology in the case of OER diagnostics. Namely, RDE passivation of the electrode via oxygen bubbles obviously cannot be avoided, which means that the activity readings are affected in an uncontrolled way. This directly impacts the optimal development of novel electrocatalysts. For example, by using RDE methodology solely, the electrochemical performance of different electrocatalysts is difficult to compare, which may result in misleading trends. However, it has to be noted that further optimization of the floating electrode architecture is necessary in order to maximize the removal of the oxygen bubbles.
Figure 2.
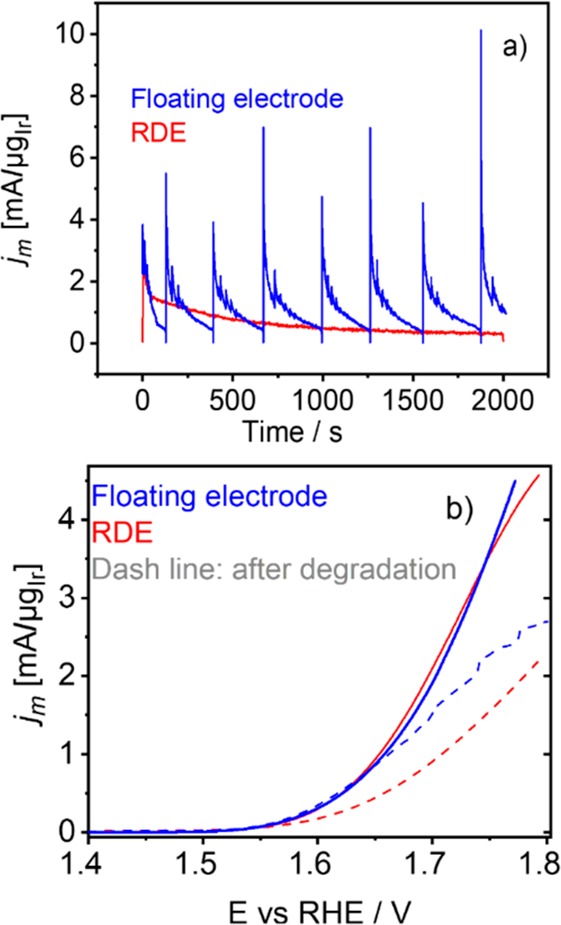
Comparison of electrochemical performance of the floating electrode and RDE. (a) Chronoamperometric treatment at 1.8 V vs RHE (2000 s) and (b) OER activity during LSV polarization before (solid lines) and after potentiostatic treatment (dashed lines).
Conclusion
The present study shows that the use of conventional electrochemical methods for investigating gas-evolving reactions, such as oxygen evolution reactions, suffers from the unavoidable blockage of the active surface by the formed gas bubbles. In the case of high surface area electrocatalysts, the evolution of bubbles passivates the electrode surface; hence, their intrinsic characteristics (activity, stability) are difficult to monitor. This issue was demonstrated with the rotating disc electrode method and a high surface area nanoparticulate iridium catalyst. As an alternative to RDE, the floating electrode concept was introduced, where a TEM grid was used as a working electrode. In general, this approach hugely reduces the passivation of the electrode with gas bubbles and hence allows performance measurements of the catalyst that are much closer to the real intrinsic values. This approach offers a new dimension toward a more efficient development of gas evolving electrocatalysts.
Acknowledgments
P.J. and N.H. kindly acknowledge Grant Nos. Z1-9165 and Z2-8161 from the Slovenian Research Agency. G.K.P acknowledges the Slovenian Research Agency for funding.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.analchem.9b01317.
SEM and TEM characterization and additional electrochemical analysis (PDF)
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Danilovic N.; Ayers K. E.; Capuano C.; Renner J. N.; Wiles L.; Pertoso M. (Plenary) Challenges in Going from Laboratory to Megawatt Scale PEM Electrolysis. ECS Trans. 2016, 75, 395–402. 10.1149/07514.0395ecst. [DOI] [Google Scholar]
- Babic U.; Suermann M.; Büchi F. N.; Gubler L.; Schmidt T. J. Critical Review—Identifying Critical Gaps for Polymer Electrolyte Water Electrolysis Development. J. Electrochem. Soc. 2017, 164, F387–F399. 10.1149/2.1441704jes. [DOI] [Google Scholar]
- Danilovic N.; Subbaraman R.; Chang K.-C.; Chang S. H.; Kang Y. J.; Snyder J.; Paulikas A. P.; Strmcnik D.; Kim Y.; Myers D.; Stamenkovic V. R.; Markovic N. M. Activity–Stability Trends for the Oxygen Evolution Reaction on Monometallic Oxides in Acidic Environments. J. Phys. Chem. Lett. 2014, 5, 2474–2478. 10.1021/jz501061n. [DOI] [PubMed] [Google Scholar]
- Fabbri E.; Habereder A.; Waltar K.; Kötz R.; Schmidt T. J. Developments and Perspectives of Oxide-Based Catalysts for the Oxygen Evolution Reaction. Catal. Sci. Technol. 2014, 4, 3800–3821. 10.1039/C4CY00669K. [DOI] [Google Scholar]
- Siracusano S.; Hodnik N.; Jovanovic P.; Ruiz-Zepeda F.; Šala M.; Baglio V.; Aricò A. S. New Insights into the Stability of a High Performance Nanostructured Catalyst for Sustainable Water Electrolysis. Nano Energy 2017, 40, 618. 10.1016/j.nanoen.2017.09.014. [DOI] [Google Scholar]
- Yu H.; Danilovic N.; Wang Y.; Willis W.; Poozhikunnath A.; Bonville L.; Capuano C.; Ayers K.; Maric R. Nano-Size IrOx Catalyst of High Activity and Stability in PEM Water Electrolyzer with Ultra-Low Iridium Loading. Appl. Catal., B 2018, 239, 133–146. 10.1016/j.apcatb.2018.07.064. [DOI] [Google Scholar]
- Alia S. M.; Rasimick B.; Ngo C.; Neyerlin K. C.; Kocha S. S.; Pylypenko S.; Xu H.; Pivovar B. S. Activity and Durability of Iridium Nanoparticles in the Oxygen Evolution Reaction. J. Electrochem. Soc. 2016, 163, F3105–F3112. 10.1149/2.0151611jes. [DOI] [Google Scholar]
- Geiger S.; Kasian O.; Mingers A. M.; Nicley S. S.; Haenen K.; Mayrhofer K. J. J.; Cherevko S. Catalyst Stability Benchmarking for the Oxygen Evolution Reaction: The Importance of Backing Electrode Material and Dissolution in Accelerated Aging Studies. ChemSusChem 2017, 10, 4140–4143. 10.1002/cssc.201701523. [DOI] [PubMed] [Google Scholar]
- Zeradjanin A. R.; Ventosa E.; Bondarenko A. S.; Schuhmann W. Evaluation of the Catalytic Performance of Gas-Evolving Electrodes Using Local Electrochemical Noise Measurements. ChemSusChem 2012, 5, 1905–1911. 10.1002/cssc.201200262. [DOI] [PubMed] [Google Scholar]
- Wang R.; Jiang W.; Xia D.; Liu T.; Gan L. Improving the Wettability of Thin-Film Rotating Disk Electrodes for Reliable Activity Evaluation of Oxygen Electrocatalysts by Triggering Oxygen Reduction at the Catalyst-Electrolyte-Bubble Triple Phase Boundaries. J. Electrochem. Soc. 2018, 165, F436–F440. 10.1149/2.0371807jes. [DOI] [Google Scholar]
- Zeradjanin A. R. Frequent Pitfalls in the Characterization of Electrodes Designed for Electrochemical Energy Conversion and Storage. ChemSusChem 2018, 11, 1278–1284. 10.1002/cssc.201702287. [DOI] [PubMed] [Google Scholar]
- Garcia A. C.; Koper M. T. M. Effect of Saturating the Electrolyte with Oxygen on the Activity for the Oxygen Evolution Reaction. ACS Catal. 2018, 8, 9359–9363. 10.1021/acscatal.8b01447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- German S. R.; Edwards M. A.; Chen Q.; Liu Y.; Luo L.; White H. S. Electrochemistry of Single Nanobubbles. Estimating the Critical Size of Bubble-Forming Nuclei for Gas-Evolving Electrode Reactions. Faraday Discuss. 2016, 193, 223–240. 10.1039/C6FD00099A. [DOI] [PubMed] [Google Scholar]
- Soto Á. M.; German S. R.; Ren H.; Van Der Meer D.; Lohse D.; Edwards M. A.; White H. S. The Nucleation Rate of Single O2Nanobubbles at Pt Nanoelectrodes. Langmuir 2018, 34, 7309–7318. 10.1021/acs.langmuir.8b01372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giner J.; Parry J. M.; Smith S.; Turchan M. Methods for Characterizing the Structure and Electrochemical Behavior of Teflon-Bonded Pt Electrodes. J. Electrochem. Soc. 1969, 116, 1692. 10.1149/1.2411664. [DOI] [Google Scholar]
- Kunz H. R. The Catalytic Activity of Platinum Supported on Carbon for Electrochemical Oxygen Reduction in Phosphoric Acid. J. Electrochem. Soc. 1975, 122, 1279. 10.1149/1.2134000. [DOI] [Google Scholar]
- Zalitis C. M.; Kramer D.; Kucernak A. R. Electrocatalytic Performance of Fuel Cell Reactions at Low Catalyst Loading and High Mass Transport. Phys. Chem. Chem. Phys. 2013, 15, 4329–4340. 10.1039/c3cp44431g. [DOI] [PubMed] [Google Scholar]
- Martens S.; Asen L.; Ercolano G.; Dionigi F.; Zalitis C.; Hawkins A.; Martinez Bonastre A.; Seidl L.; Knoll A. C.; Sharman J.; Strasser P.; Jones D.; Schneider O. A Comparison of Rotating Disc Electrode, Floating Electrode Technique and Membrane Electrode Assembly Measurements for Catalyst Testing. J. Power Sources 2018, 392, 274–284. 10.1016/j.jpowsour.2018.04.084. [DOI] [Google Scholar]
- Long Z.; Li Y.; Deng G.; Liu C.; Ge J.; Ma S.; Xing W. Micro-Membrane Electrode Assembly Design to Precisely Measure the in Situ Activity of Oxygen Reduction Reaction Electrocatalysts for PEMFC. Anal. Chem. 2017, 89, 6309–6313. 10.1021/acs.analchem.7b01507. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


