ABSTRACT
The microbiome in the gut is a diverse environment, housing the majority of our bacterial microbes. This microecosystem has a symbiotic relationship with the surrounding multicellular organism, and a balance and diversity of specific phyla of bacteria support general health. When gut bacteria diversity diminishes, there are systemic consequences, such as gastrointestinal and psychological distress. This pathway of communication is known as the microbiome–gut–brain axis. Interventions such as probiotic supplementation that influence microbiome also improve both gut and brain disorders. Recent evidence suggests that aerobic exercise improves the diversity and abundance of genera from the Firmcutes phylum, which may be the link between the positive effects of exercise on the gut and brain. The purpose of this review is to explain the complex communication pathway of the microbiome–gut–brain axis and further examine the role of exercise on influencing this communication highway.
Keywords: Exercise, gut, brain, probiotics, microbiome–gut–brain axis
Introduction
The microbiome in the gut is a diverse environment, housing the majority of our bacterial microbes. Consisting of over 1,100 genera among different phyla, the gut epithelium harbors nearly 39 trillion microbes which is a 1:1 ratio of microbes to eukaryotic cells in the human body.1,2 The gut microbiome promotes digestion and food absorption for energy production, performs a fundamental role in the function of the immune system,3-5 produces and communicates with hormonal products as an endocrine-like organ,6 and appears to have an impact on brain function.7-12 The diverse roles of the microbiome are mediated through production and release of various molecules. For example, microbes are capable of producing short-chain fatty acids (SCFAs) which are used as nutrients for colonocytes and brain microglia cells, support cholesterol metabolism, and regulate various hormones involved in appetite.13,14 Gut bacteria are also capable of producing enzymes that regulate inflammatory pathways and amino acids that have both free radical generating and scavenging properties.15,16 The gut microbiome is not only comprised of bacteria, but houses other prokaryotes (i.e., Archaea) along with viruses and fungi. The current review will specifically emphasize the bacterial component of the gut.
In human adults, approximately 90% of the gut bacteria belong to the Bacteroidetes and Firmicutes phyla, whereas the minority bacteria belongs to Proteobacteria, Actinobacteria, Fusobacteria, and Verrucomicrobia phyla.17,18 The Firmicutes phylum contains over 250 genera of bacteria, including Lactobacillus and Clostridium, while Bacteroidetes phylum includes around 20 genera, the most abundant being Bacteroides.18 The gut bacterial community is maintained through the symbiotic relationship between both pathogenic and nonpathogenic bacteria and can be demonstrated by balanced ratio of the Bacteroidetes or Firmicutes phyla and other nondominant phyla.19,20 Substantial shifts to the microbial communities in response to dietary changes, antibiotics, or invasions of pathogens may cause a shift to a nonequilibrium, or inflammatory state, and has important health implications.21 Gut microbiome disruption is thought to play a key role in the development of several diseases including type 2 diabetes,8,22,23 irritable bowel syndrome (IBS),24 cardiovascular diseases,23 allergies, mood disorders,7,9,11, and many others via intestinal inflammation.3,6
The gut has long been overlooked when it comes to human health and disease prevention. Emerging research has proved that an imbalanced diet of highly saturated fats, high sugar, and low fiber intake has a large influence on the composition of the microbiome.8 The alterations induced by poor dietary habits contribute to gastrointestinal (GI) dysfunction that may further lead to the development of inflammatory diseases.25 Interestingly, a prebiotic intervention that promoted changes in the microbiota of diabetic rats lowered inflammation while improving glucose intolerance.26,27 Moreover, individuals diagnosed with inflammatory conditions such as IBS and obesity have been found to have a comorbidity of lower cognitive function28,29 and higher instances of stress-related psychiatric symptoms, such as anxiety.30-32 In accordance, interventions that specifically treat neural disorders such as selective serotonin uptake inhibitors (SSRIs) have been shown to improve GI function.33 These data have sparked curiosity among investigators, which has led to a growth of research into understanding the connection between the GI tract and the brain.7,9,11,30,34 Further interest has grown in discovering the role of the microbiome in mediating the connection between the gut and brain. Altering the gut microbiome through probiotic supplementation has improved symptoms of both psychological disorders (e.g., depression and anxiety) and cognition and positively supports GI function.35-39 This demonstrates the complexity of the relationship between the gut microbiome and the brain.
Regular aerobic exercise has been shown to prevent age-related global brain atrophy and increases brain volume in the frontal lobes and left superior temporal lobe, which are important for cognition and control of attention and memory.40 Moderate-intensity aerobic exercise training has also promoted improvement (in older adults aged 60–79 years) in functional activation in the brain which allows for increased efficiency when completing tasks, as well as regulating behavior and mood.41 More recently, aerobic exercise has been shown to impact the gut by increasing microbiome diversity and functional metabolism in both humans and mice. Altering the bacterial profiles and influencing the by-products produced from gut bacteria through exercise may have the potential to reverse the conditions associated with obesity, metabolic diseases, poor diet, along with neural and behavioral disorders.42-45 To date, the impact of exercise on the relationship between the gut and the brain is unknown. Therefore, the purposes of this review are to briefly introduce the complex interaction between the microbiome, gut, and brain and to further explain how exercise impacts these relationships. For this review, we have chosen to state this relationship as the microbiome–gut–brain axis where the composition of the gut microbiota influences both the GI and central nervous systems (CNSs).34,46 More specifically, alterations to the microbiome may impact (both positively and negatively) GI (e.g., secretion, motility, and integrity) and higher brain function (e.g., neurotransmission, neurogenesis, and behavior), and these influences may be bidirectional.34
Microbiome–gut–brain axis pathways of communication
The relationship between the gut and the brain begins in utero as the CNS and the enteric nervous system (ENS) are derived from the same tissues during fetal development.7 The communication between the gut and the brain is a bidirectional pathway that is mediated through the autonomic nervous system (ANS) efferent and afferent signals via the vagus nerve; neuroendocrine signaling through the hypothalamic–pituitary–adrenal axis (HPA axis), and serotonin (5-HT) regulation.47-50 It has now been established that alterations of gut microbiome enact some influence on the communication between the gut and brain through these pathways.
The vagus nerve serves a critical role in communication between the gut microbes and the brain, as it connects the CNS to the ENS.51,52 The vagus nerve is able to communicate with gut microbiota in the ENS and transfer vital information to the CNS where it is deciphered, to then generate a response based on the information received. For example, if the information received via the vagus nerve from the gut indicates an imbalance in gut microbiome bacteria, the CNS will then decipher if an inflammatory response is necessary. Decreased vagus nerve activity has been associated with conditions such as IBD,53 IBS,54 and depression55-57 and is attributed to dysfunction of gastric motility and gastric emptying.58 Specifically targeted probiotic supplements that alter the microbiome have also been shown to improve brain function, and this positive neural benefit is abolished when a vagotomy procedure is performed among rodents.59 More specifically, mice induced with colitis demonstrated lower anxiety-like behavior after 21 days of being fed a probiotic supplement containing Bifidobacterium longum. The anxiolytic effect of Bifidobacterium longum was lost when vagotomized mice were fed the supplement. The authors concluded that the behavior benefits of the Bifidobacterium longum is vagally mediated.59 This provides evidence that vagal communication between the gut and brain is influenced by the release of molecules from the gut microbiota.
The HPA axis regulates an organism’s response to a multitude of stressors (e.g., physical or mental).60,61 The HPA is influenced by the GI system through a complex neural-immunoregulatory mechanism. Afferent feedback from the gut via the vagus nerve acts on the hypothalamus and hippocampus regions of the brain, resulting in activation of the HPA.62 It also appears that disruption to the gut mucosal layer via lipopolysaccharide (LPS) insult promotes the release of pro-inflammatory cytokines, which are capable of exaggerating HPA activation.47,63 HPA axis hyperresponsiveness and disturbances in the gut microbiome are found in those suffering from both IBS and psychological disorders.61 This demonstrates potential roles for the neuroendocrine system and the gut microbiome in the regulation of both the gut and brain. Rodents raised in a germ-free environment (no-microorganism exposure and no potential for gut microbiota colonization) from birth are found to have an inflated release of adrenocorticotropic hormone (ACTH) and corticosterone levels in response to physical restraint stress exposure when compared to free-living control rodents.6,34,48,64-66 The germ-free rodents also displayed lower expression of brain-derived neurotrophic factor (BDNF) in the hippocampal and cortex regions of the brain. BDNF promotes neurogenesis and is vital for CNS growth and health.67 BDNF has also been shown to be an important regulator of GI tight junction protein expression and regulation.68 Treatment with Bifidobacterium ameliorated the exaggerated HPA response in the germ-free rodents and restored BDNF levels in the brain.64 It has become clear that the gut microbiome is important in the adequate development of both the HPA axis and the CNS during early life.64,69 While extremely complex, when a diverse and healthy microbiome communicates with the CNS, tighter HPA control occurs, which may further promote both neural and GI growth through BDNF regulation.70,71
Serotonin (5-HT) is a crucial neurotransmitter and hormone and is often known as one of the primary mood and cognition regulators.72 Lower platelet serotonin receptor function is associated with higher levels of anxiety and depression, while higher platelet serotonin is associated with improved mood.73 It is also a major contributor to the modulation of intestinal secretion and motility,74,75 as well as a key signaling molecule within the microbiome–gut–brain axis.30,65 Recently, it has been demonstrated that indigenous bacteria in the gut regulate serotonin synthesis and release.76 In addition, germ-free animals have demonstrated higher levels of serotonin in the hippocampal region of the brain while showing lower expression in the colon.76,77 This demonstrates that the microbiome plays a role in the regulation of serotonin and may influence both brain and gut function. It appears that various gut microbe strains synthesize serotonin and may be the reason why germ-free rodents have lower expression of 5-HT in the gut. Other strains control tryptophan metabolism, the precursor to 5-HT synthesis, and have been shown to influence both tryptophan and serotonin levels.78,79 For example, the Bacteroides fragilis bacterium is a known consumer of tryptophan, and supplementation has been linked to improving intestinal wall stability, and also protection against CNS demyelinating disease.65,80,81 Autism spectrum disorder is a developmental disorder that affects behavior and is commonly associated with GI symptoms. Autistic like mice demonstrated improved GI function and also began to resemble normal behaving animals after supplementation with Bacteroides fragilis.81
In summary, the microbiome is capable of influencing both brain and gut function by modulating vagus nerve afferent feedback; influencing the hyperresponsiveness of the HPA axis; and altering the regulation of the tryptophan and serotonin synthesis pathways. However, it is important to understand that other communication channels in the gut–brain axis such as GABA-glutamate and catecholamines have been shown to be influenced by specific microbiota.82,83
Exercise effect on the microbiota–gut–brain axis
The bulk of previous exercise research on GI physiology has shown a negative effect. For example, in several studies from our group and others, intense exercise leads to an increase in intestinal permeability, GI damage, and mild endotoxemia.84-86 However, targeted exercise therapies have been developed to treat those suffering from IBS with reported improvements in symptoms of the disorder.87 Exercise also promotes cognition and improved symptoms of both mood and psychological disorders among humans. Cognitive improvements have been observed among stroke patients, while symptoms of depression and schizophrenia have also been improved after aerobic exercise training.88-90 Interestingly, the improvements observed among IBS patients after exercise were linked to changes in mental health and emotion.87 Evidence suggests that the bacterial diversity of the gut contributes to the disordered state of GI disorders by negatively impacting GI function and the psychological state.91,92 Altering the microbiome through probiotic supplements improves both GI integrity and mood among IBS patients.92,93 Probiotic supplements also reduced stress-induced GI symptoms (abdominal pain and nausea) among participants with a history of elevated stress.94 Exciting research within the last 5 years has demonstrated that exercise promotes a diverse community of microbes that contribute to the biodiversity of the bacteria in the gut.42-44,66 Exercise appears to be a potential external influence on the capacity to alter the gut biodiversity in both quantitative and qualitative ways.6 Exercise alterations to the gut microbiome may provide a link to the exercise-related benefits on GI function, mood, and higher brain centers. Figure 1 highlights the impact of exercise on the microbiome, and how alterations may mediate improvements in both the gut and brain.
Figure 1.
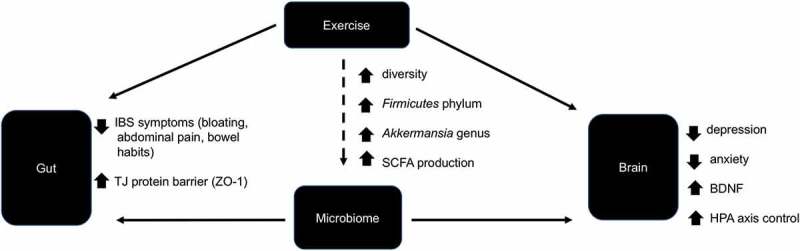
The role of exercise in the microbiome–gut–brain axis. Exercise and the gut microbiome have been independently shown to improve symptoms of IBS and stabilize the TJ barrier and have also been linked to decrease psychological disorders (e.g. depression, anxiety), promote neurogenesis (through BDNF), and improve HPA axis control. It has been demonstrated that aerobic exercise caused greater diversity in the microbiome along with increasing genera of the Firmicutes phylum, which produce short-chain fatty acids. In addition, athletes have demonstrated higher levels of the Akkermansia genus, which has been linked to metabolic and neural diseases. The extent to which the effect of exercise on the gut and brain is mediated through alterations in the microbiome is unknown. The dotted line represents a proposed mechanism. Solid lines represent known mechanisms. BDNF – brain-derived neurotropic factor, SCFA – short-chain fatty acids, ZO-1 – zona occluden-1 protein, HPA axis – hypothalamic–pituitary–adrenal axis, TJ – tight junction.
Research conducted among volunteers from the American Gut Project95 concluded that those who exercised at a higher frequency demonstrated diversity in the Firmcutes phylum (specifically, Faecalibacterium prausnitzii and species from the genus Oscillospira, Lachnospira, and Coprococcus). Athletes have been shown to exhibit higher diversity of gut microorganisms compared to nonathletes. More specifically, in a group of professional rugby players, 22 phyla were detected, while only 11 phyla were identified in age-matched low BMI control and 9 phyla in an age-matched high BMI control. The distinct phyla among the athletes correlated with both dietary protein intake and creatine kinase, suggesting that diet and exercise facilitate diversity in the gut. The researchers identified higher proportions of the Firmicutes phyla and lower Bacteroidetes phyla in the athlete group compared to the high BMI control.42 While the athlete group had greater diversity than the low BMI control, it was difficult for the researchers to identify specific bacteria that help to understand the role of extreme exercise on the microbiome in this study. Interestingly, both the athletes and low BMI group demonstrated a higher proportion of the Akkermansia genus (Verrucomicrobia phylum), which has been negatively associated with metabolic dysfunction in humans and rodents.42 The administration of Akkermansia has been shown to reversed diet-induced metabolic disorders in obese mice.96 The Akkermansia genus has also been linked to cognitive impairment as a result of poor dietary habits.97 Mice demonstrated a 5.4-fold reduction in Akkermansia and associated behavioral decline in response to the Western diet (high fat and sugar intake).98 While the link between exercise changes to the gut microbiome and resulting changes in brain function is unknown, the alterations among athletes appear to be a result of exercise and dietary behaviors, which may provide protection against both metabolic and cognitive decline.
In a follow-up study, the same group of researchers confirmed the microbial diversity among elite rugby athletes compared to nonactive, age-matched control and again demonstrated higher levels of Akkermansia.99 The researchers also performed metabolomic phenotyping of the microbiome to further understand the differences between athletes and controls at the functional metabolic level. The athletes reported higher activity of several pathways, including amino acid synthesis, and carbohydrate metabolism. As a result, athletes demonstrated an enriched profile of SCFAs, indicating greater production and host absorption rates. Identified SFCAs in the athletes were acetate, propionate, and butyrate, which are produced by gut microbes from protein, fibers, and nondigestible starches.100 The researchers attributed the differences between athletes and controls to heavy physical activity and the associated diet. They highlighted the impact of the high protein and fiber diet among the athletic group and the resulting metabolism by gut microbes. In support of these findings, Estaki et al.101 distinctly identified an association between cardiorespiratory fitness and microbiome function rather than diversity of specific bacterial taxa. A strong relationship was observed between aerobic fitness (VO2max) and fecal SCFAs, indicating that more aerobically fit humans produce higher rates of SFCAs.101 Total dietary protein intake was also a significant contributor to the diversity and functional changes (SFCA production) in the gut, further highlighting the role of diet. Higher SFCA production is attributed to many health benefits, including protection against inflammation and atherosclerosis.102 SCFAs produced from the microbiome also appear to provide nutrients to brain microglia to support healthy maturation and function.103 In summary, a link has been established between the functional by-products (i.e., SFCA) of the gut microbiome and healthy behaviors (exercise and diet).99
The bulk of research examining the role of exercise interventions on changes in the gut microbiome has been performed in rodent models. Kang et al.104 analyzed changes in the gut bacteria among mice after a 16-week exercise program (forced wheel running 5 days/week) compared to a high-fat diet group.104 Exercise appeared to increase the bacteria of phylum Firmicutes and lower Bacteroidetes, which was surprisingly similar to the changes observed in the high-fat diet group. Conversely, Evans et al.105 reported opposite findings with lower Firmicutes and higher Bacteroidetes after 12 weeks of voluntary wheel running. The group also demonstrated exercise prevention of high-fat diet-induced obesity and suggested a microbial altering mechanism of exercise on averting high-fat diet-related obesity.105 The differential results between the Kang and Evans groups may be attributed to the forced vs. voluntary wheel running protocols utilized in the studies, respectively (discussed in next section). A link has been made between low gut Bacteriodetes and obesity and further supported by increased Bacteriodetes in response to weight loss among obese individuals.106 The richness and diversity of the gut microbiome has been correlated with various metabolic markers where low richness corresponds with higher adiposity, insulin resistance, and dyslipidemia.107 Exercise is an established treatment for metabolic disorders, and these results may indicate that exercise-related changes in the gut microbiome may support metabolic health.
In a short voluntary wheel running exercise protocol (6 days), Queipo-Ortuno et al.108 reported an increase in genera Lactobacillus and Blautia coccoides-Eubacterium rectale from the Firmicutes phylum, along with genus Bifidobacterium from the Actinobaceria phylum. All three genera are capable of producing SCFAs,109 which may be considered a functional change in the gut microbiome as a result of exercise. Queipo-Ortuno et al.108 also posted a positive association between Lactobacillus and Bifidobacterium and serum leptin levels in the animals. Leptin is a hormone secreted from adipose tissue and helps to control appetite and eating behavior.110 These results demonstrate that exercise alterations to the gut microbiome are related to dietary food intake and may indicate a connection between exercise, the gut, and behavioral change.
Several recent exercise intervention studies have been conducted among human subjects. While the primary aim of this review is to assess the impact of exercise training on the gut microbiome. Zhao et al.111 analyzed fecal samples before and after a half-marathon event among human participants. An increase in the richness of the Actinobacteria phylum was reported with a notable correlation between the Coriobacteriaceae family and several metabolites. Coriobacteriaceae has been associated with higher levels of high-density lipoprotein (HDL) and metabolic improvements, and the researchers identified the change in bacteria as a potential mechanism for exercise-induced health benefits.112,113 These results indicate that an acute bout of exercise, while extreme (i.e., half-marathon) has a profound impact on the gut microbiome.
In the first known human exercise study, Allen et al.114 compared microbiota changes between lean and obese humans before and after a 6-week aerobic exercise program and further evaluated changes after a period of de-training. Exercise caused changes in gut microbiome diversity and microbial production of SCFAs among both the lean and obese subjects. The authors aimed to identify groups of bacteria that responded to exercise training and identified the Faecalibacterium, Lachnospira, Clostridia, and Roseburia genera, which are all part of the Firmicutes phylum. These genera increased in abundance in response to exercise training and returned to baseline upon cessation. A notable finding was that changes in the gut were associated with changes in lean mass and reduction in fat mass, meaning that shifts in microbial content were more substantial among those subjects that demonstrate these changes. These findings suggest that aerobic exercise affects the content and diversity of the gut microbiome in humans and may additionally raise the question about how gut microbes influence adaptations to exercise.
The identified “exercise” genera by Allen et al.114 were all producers of SCFAs and more specifically butyrate. Butyrate and other SFCAs are known energy substrates for colonocytes, with butyrate being the primary energy source.115,116 This may explain why exercise improves symptoms and reduces the risk for IBS and colon cancer.117 Further, butyrate has been shown to stimulate neural proliferation in the dentate gyrus brain regions of mice and has also been used to induce neurogenesis after ischemic brain insult in adult rodents.118,119 These results may provide a link between exercise-induced microbial SCFA production and improvements in both brain and GI function. However, it is unknown if the positive benefits of exercise on gut and brain are mediated through adjustments to the microbiome (see Figure 1).
The limited amount of research explaining how exercise influences the microbiome has indicated that genera from the Firmicutes phylum appear to be the most responsive to exercise-induced changes. Given that the Firmicutes phylum consists of over 250 genera, it is difficult to understand which genera lead to systemic inflammation and which lead to a healthy gut environment. The work from Allen et al.114 has implied that genera that produce SCFA appear to respond to exercise; however, this is the only known study among humans to evaluate changes after an aerobic exercise intervention, and then upon cessation of exercise. In the only other human exercise study, Cronin et al.120 reported minimal change in gut bacterial diversity among healthy male and females who participated in an 8-week combined aerobic and resistance exercise program. The conflicting results may be due to the exercise regimen where the aerobic training program in the Allen et al.114 study appeared to be more vigorous, and 100% compliance was reported. Importantly, Cronin et al.120 implemented a nonexercise control to accurately assess and compare sedentary and exercise behavior. They concluded that while the gut microbiome is adaptable, 8 weeks of exercise may not be enough stimulus to enact change. These studies have advanced the field and future work is needed to fully characterize how the microbiome adapts to exercise, and other various forms of physical activity.
Exercise influences the microbiome–gut–brain axis pathways of communication
As mentioned previously, the vagus nerve is at the interface of the microbiome–gut–brain axis. Altered vagus nerve activation is commonly found in patients with both GI (IBS, IBD) and psychological disorders (depression).121,122 Various forms of exercise, including yoga and aerobic exercise training, have been shown to improve parasympathetic tone.123 Meta-analytical studies have identified yoga as a proven mode of exercise to improve symptoms of IBS and depression,124,125 which may be partially mediated by autonomic influences. As previously discussed, alterations in the gut microbiome affect vagal communication between the gut and brain.126 An intact vagus nerve is critical for the benefits of probiotics supplements containing strains of Bifidobacterium and Lactobacillus to be effective.55 Further, any mediating effect of probiotics on the HPA axis and depression-related symptoms are abolished through the vagotomy procedure.127 Therefore, it is logical to suggest that any alterations that exercise may have on the diversity and expression of gut flora would potentially be manifested through influence on vagal communication between the gut and brain. Unfortunately, empirical evidence currently does not exist to verify this conclusion.
Improvements in brain structure and function have been documented as an adaptation to repetitive aerobic exercise.40,128,129 Regular moderate-intensity aerobic exercise prevents age-related brain volume loss and increased brain volume in areas responsible for cognition and control of attention and memory.40 The positive benefits of exercise on neural function appear to be mediated through regulation of BDNF. BDNF is central in the growth and the survival of striatal neurons in the brain, and it also plays a critical role in regulating mood disorders, along with learning and memory.34,130,131 According to Linnarsson et al.,132 BDNF is known for its protective role in the adult brain, because the genetic deletion of this protein results in apoptosis (death of cells) in mice. Decreased levels of BDNF in the hippocampus have also been associated with anxiety and depression and is often comorbid with gut inflammatory diseases, such as IBS, and or inflammatory-bowel disease (IBD).8,133 Oral supplementation with Bifidobacterium has been shown to increase BDNF expression in the brains of rodents, while aerobic exercise in piglets has also been shown to increase Bifidobacterium in the gut.64,134 Antibiotic treatments that killed gut bacteria also delayed the growth of brain cells in the hippocampus regions of mice.135 Both probiotics and aerobic exercise individually rescued the decline in neurogenesis and cognitive function among mice treated with the antibiotic.135 However, it is unknown if the exercise effects on brain neurogenesis and cognition are mediated through Bifidobacterium.136
Elevated HPA axis activation and gut–microbiome disruption is seen when an exercising individual exceeds 60% of maximum oxygen uptake (VO2max) or during prolonged exercise (>90 min). Psychological stress, such as the kind experienced by athletes pre-competition, has also led to an increased HPA axis activation and greater gut disruption.10 Likewise, research in mice has found that moderate forced treadmill running (8–12 m/min at a 5% grade for 40 min, 5 times per week) exacerbated colitis symptoms when compared to voluntary exercise, which attenuated symptoms.137 The researchers concluded that the forced exercise may have been perceived as a psychological stressor by the mice, whereas the voluntary wheel running is a conscious decision. Hyperresponsive HPA axis appears to be influenced by the diversity of the gut microbiome where the lack of, or absence of bacteria, caused robust HPA activation in response to psychological stress.64 Supplementation with Bifidobacterium species reversed HPA overactivation and alleviated symptoms of anxiety and distress among rodents and humans, respectively.35 While previously noted, the Bifidobacterium strains are influenced by aerobic exercise, where animals with free access to wheel running showed increased numbers of Bifidobacterium.108 Furthermore, force wheel running resulted in reduced levels of Lactobacillus, which is important for protection against disease-causing microorganisms.138 It is important to mention that microbiome-altering effect of voluntary wheel running has only been shown in male mice, whereas null findings were reported in a combined cohort of male and female mice.138,139 However, both voluntary and forced exercise appear to influence the average operational taxonomic units (OTUs) of several microbial species. For example, force running increased Ruminococcus, Butyrivibrio, and Oscillospira, which are all within Firmicutes phylum. Of note, many of these various species are being considered as psychobiotics due to their role in neuropsychiatric disorders.140 In summary, it appears that the psychological state of exercise influences the microbiome, and these changes may further impact the HPA axis regulation and mood disorder symptoms.
Aerobic exercise has been shown to also influence serotonin regulation. In rats, 5-HT synthesis and metabolism increased in both the brain stem and the hippocampus in response to exercise,141 and this increase in 5-HT resulted in the reduction of depressive and anxiety symptoms.142 Wipfili et. al.142 described the effects of exercise as similar to the those of SSRIs used to treat depression and anxiety. The following bacteria in the gut microbiome have been found to produce serotonin: Lactococcus lactis, Lactobacillus plantarum, Streptococcus thermophile,143 Morganella morganii, and Klebsiella pneumonia.144 Unfortunately, there are no previous studies that thoroughly examined if exercise has any influence on these specific strains. Because some of these serotonin-producing strains stem from the lactobacilis genera (which is a component of Firmicutes phyla), exercise may support the increased presence of these strains through the natural diversification of gut microbiota that occurs as a result of exercise. Therefore, the production of serotonin through an exercise-adapted gut microbiome might possibly be an explanation as to why subjects exhibit lower stress-related symptoms, such as anxiety and depression, after participating in physical activity. These statements, however, are purely assumptions and more research is needed to confirm this suggestion.
Conclusion
Although the exact causal relationship is unknown, current evidence allows for the assumption that exercise may mediate a bidirectional relationship between the gut and brain through alterations in the microbiome. This relationship may explain why exercise can be a therapeutic factor and strategy for both psychological and GI disorders. The main identified phyla that respond to exercise are Firmicutes and Actinobacteria, which contain the Lactobacillus and Bifidobacterium genera, respectively. In addition, the SCFA-producing genera from the Firmicutes phylum also appear to increase in response to exercise. Future work should target underlying mechanisms of how specifically exercise influences the microbiome, and the mediators involved in the gut–brain axis. This may lead to the development of various treatment combinations (exercise + probiotics) to ameliorate (or improve) specific disease states.
Funding Statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contributions
AD, CM, and MZ all participated in manuscript development, design, and drafting.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Sender R, Fuchs S, Milo R.. Are we really vastly outnumbered? Revisiting the ratio of bacterial to host cells in humans. Cell. 2016;164(3):337–340. doi: 10.1016/j.cell.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 2.Chen J, He X, Huang J. Diet effects in gut microbiome and obesity. J Food Sci. 2014;79:4. doi: 10.1111/1750-3841.12397. [DOI] [PubMed] [Google Scholar]
- 3.Peters H, De Vries W, Vanberge-Henegouwen G, Akkermans L. Potential benefits and hazards of physical activity and exercise on the gastrointestinal tract. Gut. 2001;48:435–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mach N, Fuster-Botella D. Endurance exercise and gut microbiota: A review. J Sport Health Sci. 2017;6(2):179–197. doi: 10.1016/j.jshs.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cronin O, Molloy MG, Shanahan F. Exercise, fitness, and the gut. Curr Opin Gastroenterol. 2016;32(2):67–73. doi: 10.1097/MOG.0000000000000240. [DOI] [PubMed] [Google Scholar]
- 6.Bermon S, Petriz B, Kajeniene A, Prestes J, Castell L, Franco OL. The microbiota: an exercise immunology perspective. Exerc Immunol Rev. 2015;21:9. [PubMed] [Google Scholar]
- 7.Sharon G, Sampson TR, Geschwind DH, Mazmanian SK. The central nervous system and the gut microbiome. Cell. 2016;167(4):915–932. doi: 10.1016/j.cell.2016.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Proctor C, Thiennimitr P, Chattipakorn N, Chattipakorn SC. Diet, gut microbiota and cognition. Metab Brain Dis. 2017;32(1):1–17. doi: 10.1007/s11011-016-9917-8. [DOI] [PubMed] [Google Scholar]
- 9.Foster JA, Rinaman L, Cryan JF. Stress & the gut-brain axis: regulation by the microbiome. Neurobiol Stress. 2017;7:124–136. doi: 10.1016/j.ynstr.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clark A, Mach N. Exercise-induced stress behavior, gut-microbiota-brain axis and diet: a systematic review for athletes. J Int Soc Sports Nutr. 2016;13:43. doi: 10.1186/s12970-016-0155-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burokas A, Arboleya S, Moloney RD, Peterson VL, Murphy K, Clarke G, Stanton C, Dinan TG, Cryan JF. Targeting the microbiota-gut-brain axis: prebiotics have anxiolytic and antidepressant-like effects and reverse the impact of chronic stress in mice. Biol Psychiatry. 2017;82(7):472–487. doi: 10.1016/j.biopsych.2016.12.031. [DOI] [PubMed] [Google Scholar]
- 12.Braniste V, Al-Asmakh M, Kowal C, Anuar F, Abbaspour A, Tóth M, Korecka A, Bakocevic N, Ng LG, Guan NL, et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med. 2014;6(263):263ra158–263ra158. doi: 10.1126/scitranslmed.3009759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wong JM, de Souza R, Kendall CW, Emam A, Jenkins DJ. Colonic health: fermentation and short chain fatty acids. J Clin Gastroenterol. 2006;40:235–243. [DOI] [PubMed] [Google Scholar]
- 14.Lin HV, Frassetto A, Kowalik EJ Jr, Nawrocki AR, Lu MM, Kosinski JR, Hubert JA, Szeto D, Yao X, Forrest G, et al. Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PLoS One. 2012;7(4):e35240. doi: 10.1371/journal.pone.0035240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang WW, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368(17):1575–1584. doi: 10.1056/NEJMoa1109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelly D, Campbell JI, King TP, Grant G, Jansson EA, Coutts AG, Pettersson S, Conway S. Commensal anaerobic gut bacteria attenuate inflammation by regulating nuclear-cytoplasmic shuttling of PPAR-γ and RelA. Nat Immunol. 2004;5(1):104. doi: 10.1038/ni1018. [DOI] [PubMed] [Google Scholar]
- 17.Goodrich JK, Waters JL, Poole AC, Sutter JL, Koren O, Blekhman R, Beaumont M, Van Treuren W, Knight R, Bell JT, et al. Human genetics shape the gut microbiome. Cell. 2014;159(4):789–799. doi: 10.1016/j.cell.2014.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mariat D, Firmesse O, Levenez F, Guimarăes V, Sokol H, Doré J, Corthier G, Furet J-P. The Firmicutes/Bacteroidetes ratio of the human microbiota changes with age. BMC Microbiol. 2009;9(1):123. doi: 10.1186/1471-2180-9-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Conlan S, Kong HH, Segre JA. Species-level analysis of DNA sequence data from the NIH human microbiome project. PLoS One. 2012;7(10):e47075. doi: 10.1371/journal.pone.0047075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Group NHW, Peterson J, Garges S, Giovanni M, McInnes P, Wang L, Schloss JA, Bonazzi V, McEwen JE, Wetterstrand KA, et al. The NIH human microbiome project. Genome Res. 2009;19(12):2317–2323. doi: 10.1101/gr.096651.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489(7415):220. doi: 10.1038/nature11468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hartstra AV, Bouter KE, Bäckhed F, Nieuwdorp M. Insights into the role of the microbiome in obesity and type 2 diabetes. Diabetes Care. 2015;38(1):159–165. doi: 10.2337/dc14-0769. [DOI] [PubMed] [Google Scholar]
- 23.Li D, Kirsop J, Tang WW. Listening to our gut: contribution of gut microbiota and cardiovascular risk in diabetes pathogenesis. Curr Diab Rep. 2015;15(9):63. doi: 10.1007/s11892-015-0634-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dinan TG, Cryan JF. Melancholic microbes: a link between gut microbiota and depression? Neurogastroenterol Motil. 2013;25(9):713–719. doi: 10.1111/nmo.12198. [DOI] [PubMed] [Google Scholar]
- 25.Cani PD, Delzenne NM. Involvement of the gut microbiota in the development of low grade inflammation associated with obesity: focus on this neglected partner. Acta Gastroenterol Belg. 2010;73:267–269. [PubMed] [Google Scholar]
- 26.Cani PD, Possemiers S, Van de Wiele T, Guiot Y, Everard A, Rottier O, Geurts L, Naslain D, Neyrinck A, Lambert DM, et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut. 2009;58(8):1091–1103. doi: 10.1136/gut.2008.165886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Everard A, Lazarevic V, Derrien M, Girard M, Muccioli GG, Neyrinck AM, Neyrinck AM, Possemiers S, Van Holle A, François P, et al. Responses of gut microbiota and glucose and lipid metabolism to prebiotics in genetic obese and diet-induced leptin-resistant mice. Diabetes. 2011;60(11):2775–2786. doi: 10.2337/db11-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mayer EA. Gut feelings: the emerging biology of gut–brain communication. Nat Rev Neurosci. 2011;12(8):453. doi: 10.1038/nrn3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kennedy P, Clarke G, O‘Neill A, Groeger J, Quigley E, Shanahan F, Cryan JF, Dinan TG. Cognitive performance in irritable bowel syndrome: evidence of a stress-related impairment in visuospatial memory. Psychol Med. 2014;44(7):1553–1566. doi: 10.1017/S0033291713002171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kennedy PJ, Cryan JF, Dinan TG, Clarke G. Irritable bowel syndrome: a microbiome-gut-brain axis disorder? World J Gastroenterol. 2014;20(39):14105. doi: 10.3748/wjg.v20.i39.14105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Castanon N, Lasselin J, Capuron L. Neuropsychiatric comorbidity in obesity: role of inflammatory processes. Front Endocrinol (Lausanne). 2014;5:74. doi: 10.3389/fendo.2014.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lackner JM, Ma CX, Keefer L, Brenner DM, Gudleski GD, Satchidanand N, Firth R, Sitrin MD, Katz L, Krasner SS, et al. Type, rather than number, of mental and physical comorbidities increases the severity of symptoms in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2013;11(9):1147–1157. doi: 10.1016/j.cgh.2013.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thorkelson G, Bielefeldt K, Szigethy E. Empirically supported use of psychiatric medications in adolescents and adults with IBD. Inflamm Bowel Dis. 2016;22(6):1509–1522. doi: 10.1097/MIB.0000000000000734. [DOI] [PubMed] [Google Scholar]
- 34.Cryan JF, O’mahony S. The microbiome‐gut‐brain axis: from bowel to behavior. Neurogastroenterol Motil. 2011;23(3):187–192. doi: 10.1111/j.1365-2982.2010.01664.x. [DOI] [PubMed] [Google Scholar]
- 35.Messaoudi M, Lalonde R, Violle N, Javelot H, Desor D, Nejdi A, Bisson J-F, Rougeot C, Pichelin M, Cazaubiel M, et al. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br J Nutr. 2011;105(5):755–764. doi: 10.1017/S0007114510004319. [DOI] [PubMed] [Google Scholar]
- 36.Benton D, Williams C, Brown A. Impact of consuming a milk drink containing a probiotic on mood and cognition. Eur J Clin Nutr. 2007;61(3):355. doi: 10.1038/sj.ejcn.1602546. [DOI] [PubMed] [Google Scholar]
- 37.Desbonnet L, Garrett L, Clarke G, Bienenstock J, Dinan TG. The probiotic Bifidobacteria infantis: an assessment of potential antidepressant properties in the rat. J Psychiatr Res. 2008;43(2):164–174. doi: 10.1016/j.jpsychires.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 38.Hoveyda N, Heneghan C, Mahtani KR, Perera R, Roberts N, Glasziou P. A systematic review and meta-analysis: probiotics in the treatment of irritable bowel syndrome. BMC Gastroenterol. 2009;9(1):15. doi: 10.1186/1471-230X-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ringel Y, Quigley EM, Lin HC. Using probiotics in gastrointestinal disorders. Am J Gastroenterol Suppl. 2012;1(1):34. doi: 10.1038/ajgsup.2012.7. [DOI] [Google Scholar]
- 40.Colcombe SJ, Erickson KI, Scalf PE, Kim JS, Prakash R, McAuley E, Elavsky S, Marquez DX, Hu L, Kramer AF. Aerobic exercise training increases brain volume in aging humans. J Gerontol A Biol Sci Med Sci. 2006;61(11):1166–1170. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- 41.Gomez‐Pinilla F, Hillman C. The influence of exercise on cognitive abilities Compr Physiol. 2013;3(1):403–428. doi: 10.1002/cphy/c110063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clarke SF, Murphy EF, O’sullivan O, Lucey AJ, Humphreys M, Hogan A. Hayes P, O'Reilly M, Jeffery IB, Wood-Martin R, et al. Exercise and associated dietary extremes impact on gut microbial diversity. Gut. 2014;63(12):1913–1920. doi: 10.1136/gutjnl2013-306541. [DOI] [PubMed] [Google Scholar]
- 43.Mika A, Van Treuren W, González A, Herrera JJ, Knight R, Fleshner M. Exercise is more effective at altering gut microbial composition and producing stable changes in lean mass in juvenile versus adult male F344 rats. PLoS One. 2015;10(5):e0125889. doi: 10.1371/journal.pone.0125889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Welly RJ, Liu T-W, Zidon TM, Rowles JL III, Park Y-M, Smith TN, Swanson KS, Padilla J, Vieira-Potter VJ. Comparison of diet vs. exercise on metabolic function & gut microbiota in obese rats. Med Sci Sports Exerc. 2016;48(9):1688. doi: 10.1249/MSS.0000000000000964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Monda V, Villano I, Messina A, Valenzano A, Esposito T, Moscatelli F, Viggiano A, Cibelli G, Chieffi S, Monda M, et al. Exercise modifies the gut microbiota with positive health effects. Oxid Med Cell Longev. 2017;2017. doi: 10.1155/2017/3831972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Clarke G, Dinan T, Cryan J. Microbiome–gut–brain Axis In: Highlander SK, Rodriguez-Valera F, White BA, editors. Encyclopedia of metagenomics: environmental metagenomics. Boston (MA): Springer US; 2015. p. 425–437. [Google Scholar]
- 47.Grenham S, Clarke G, Cryan JF, Dinan TG. Brain–gut–microbe communication in health and disease. Front Physiol. 2011;2:94. doi: 10.3389/fphys.2011.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13(10):701. doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- 49.Forsythe P, Kunze W, Bienenstock J. Moody microbes or fecal phrenology: what do we know about the microbiota-gut-brain axis? BMC Med. 2016;14(1):58. doi: 10.1186/s12916-016-0604-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brookes SJ, Spencer NJ, Costa M, Zagorodnyuk VP. Extrinsic primary afferent signalling in the gut. Nat Rev Gastroenterol Hepatol. 2013;10(5):286. doi: 10.1038/nrgastro.2013.29. [DOI] [PubMed] [Google Scholar]
- 51.Forsythe P, Kunze WA, Bienenstock J. On communication between gut microbes and the brain. Curr Opin Gastroenterol. 2012;28(6):557–562. doi: 10.1097/MOG.0b013e3283572ffa. [DOI] [PubMed] [Google Scholar]
- 52.Forsythe P, Bienenstock J, Kunze WA. Vagal pathways for microbiome-brain-gut axis communication In: Microbial endocrinology: the microbiota-gut-brain axis in health and disease. Adv Exp Med Biol. 2014;817:115–133. doi: 10.1007/978-1-4939-0897-4. [DOI] [PubMed] [Google Scholar]
- 53.Pellissier S, Dantzer C, Mondillon L, Trocme C, Gauchez A-S, Ducros V, Mathieu N, Toussaint B, Fournier A, Canini F, et al. Relationship between vagal tone, cortisol, TNF-alpha, epinephrine and negative affects in Crohn’s disease and irritable bowel syndrome. PLoS One. 2014;9(9):e105328. doi: 10.1371/journal.pone.0105328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu Q, Wang EM, Yan XJ, Chen SL. Autonomic functioning in irritable bowel syndrome measured by heart rate variability: A meta‐analysis. J Dig Dis. 2013;14(12):638–646. doi: 10.1111/1751-2980.12092. [DOI] [PubMed] [Google Scholar]
- 55.Malick M, Gilbert K, Daniel J, Arseneault‐Breard J, Tompkins T, Godbout R, Rousseau G. Vagotomy prevents the effect of probiotics on caspase activity in a model of postmyocardial infarction depression. Neurogastroenterol Motil. 2015;27(5):663–671. doi: 10.1111/nmo.12540. [DOI] [PubMed] [Google Scholar]
- 56.Li S, Zhai X, Rong P, McCabe MF, Wang X, Zhao J, Ben H, Wang S, Slattery DA. Therapeutic effect of vagus nerve stimulation on depressive-like behavior, hyperglycemia and insulin receptor expression in Zucker fatty rats. PLoS One. 2014;9(11):e112066. doi: 10.1371/journal.pone.0112066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grimonprez A, Raedt R, Baeken C, Boon P, Vonck K. The antidepressant mechanism of action of vagus nerve stimulation: evidence from preclinical studies. Neurosci Biobehav Rev. 2015;56:26–34. doi: 10.1016/j.neubiorev.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 58.Kheder SH, Heller J, Bär J, Wutzler A, Menge B, Juckel G. Autonomic dysfunction of gastric motility in major depression. J Affect Disord. 2018;226:196–202. doi: 10.1016/j.jad.2017.09.049. [DOI] [PubMed] [Google Scholar]
- 59.Bercik P, Park A, Sinclair D, Khoshdel A, Lu J, Huang X, Deng Y, Blennerhassett PA, Fahnestock M, Moine D, et al. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut–brain communication. Neurogastroenterol Motil. 2011;23(12):1132–1139. doi: 10.1111/j.1365-2982.2011.01796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tsigos C, Chrousos GP. Hypothalamic–pituitary–adrenal axis, neuroendocrine factors and stress. J Psychosom Res. 2002;53:865–871. [DOI] [PubMed] [Google Scholar]
- 61.Farzi A, Fröhlich EE, Holzer P. Gut microbiota and the neuroendocrine system. Neurotherapeutics. 2018;15(1):1–18. doi: 10.1007/s13311-017-0600-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hosoi T, Okuma Y, Matsuda T, Nomura Y. Novel pathway for LPS-induced afferent vagus nerve activation: possible role of nodose ganglion. Auton Neurosci: Basic Clin. 2005;120(1):104–107. doi: 10.1016/j.autneu.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 63.Dinan TG, Quigley EM, Ahmed SM, Scully P, O’Brien S, O’Mahony L, O’Mahony S, Shanahan F, Keeling PWN. Hypothalamic-pituitary-gut axis dysregulation in irritable bowel syndrome: plasma cytokines as a potential biomarker? Gastroenterology. 2006;130(2):304–311. doi: 10.1053/j.gastro.2005.11.033. [DOI] [PubMed] [Google Scholar]
- 64.Sudo N, Chida Y, Aiba Y, Sonoda J, Oyama N, Yu XN, Kubo C, Koga Y. Postnatal microbial colonization programs the hypothalamic–pituitary–adrenal system for stress response in mice. J Physiol. 2004;558(1):263–275. doi: 10.1113/jphysiol.2004.063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.O’Mahony S, Clarke G, Borre Y, Dinan T, Cryan J. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav Brain Res. 2015;277:32–48. doi: 10.1016/j.bbr.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 66.Bressa C, Bailén-Andrino M, Pérez-Santiago J, González-Soltero R, Pérez M, Montalvo-Lominchar MG, Maté-Muñoz JL, Domínguez R, Moreno D, Larrosa M, et al. Differences in gut microbiota profile between women with active lifestyle and sedentary women. PLoS One. 2017;12(2):e0171352. doi: 10.1371/journal.pone.0171352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schecterson LC, Bothwell M. Novel roles for neurotrophins are suggested by BDNF and NT-3 mRNA expression in developing neurons. Neuron. 1992;9:449–463. [DOI] [PubMed] [Google Scholar]
- 68.Li C, Cai -Y-Y, Yan Z-X. Brain-derived neurotrophic factor preserves intestinal mucosal barrier function and alters gut microbiota in mice. Kaohsiung J Med Sci. 2018;34(3):134–141. doi: 10.1016/j.kjms.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bravo JA, Julio-Pieper M, Forsythe P, Kunze W, Dinan TG, Bienenstock J, Cryan JF. Communication between gastrointestinal bacteria and the nervous system. Curr Opin Pharmacol. 2012;12(6):667–672. doi: 10.1016/j.coph.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 70.von Boyen GB, Reinshagen M, Steinkamp M, Adler G, Kirsch J. Enteric nervous plasticity and development: dependence on neurotrophic factors. J Gastroenterol. 2002;37(8):583–588. doi: 10.1007/s005350200093. [DOI] [PubMed] [Google Scholar]
- 71.Heijtz RD, Wang S, Anuar F, Qian Y, Björkholm B, Samuelsson A, Hibberd ML, Forssberg H, Pettersson S. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci. 2011;108(7):3047–3052. doi: 10.1073/pnas.1010529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jenkins TA, Nguyen JC, Polglaze KE, Bertrand PP. Influence of tryptophan and serotonin on mood and cognition with a possible role of the gut-brain axis. Nutrients. 2016;8(1):56. doi: 10.3390/nu8010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Young SN. How to increase serotonin in the human brain without drugs. J Psychol Psychiatr Neuroscil. 2007;32:394. [PMC free article] [PubMed] [Google Scholar]
- 74.Gershon MD, Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology. 2007;132(1):397–414. doi: 10.1053/j.gastro.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 75.Mawe GM, Hoffman JM. Serotonin signalling in the gut—functions, dysfunctions and therapeutic targets. Nat Rev Gastroenterol Hepatol. 2013;10(8):473. doi: 10.1038/nrgastro.2013.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, Ma L, Nagler CR, Ismagilov RF, Mazmanian SK, Hsiao EY. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161(2):264–276. doi: 10.1016/j.cell.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Clarke G, Grenham S, Scully P, Fitzgerald P, Moloney R, Shanahan F, Dinan TG, Cryan JF. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol Psychiatry. 2013;18(6):666. doi: 10.1038/mp.2012.77. [DOI] [PubMed] [Google Scholar]
- 78.Yanofsky C. RNA-based regulation of genes of tryptophan synthesis and degradation, in bacteria. Rna. 2007;13(8):1141–1154. doi: 10.1261/rna.620507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Strasser B, Gostner JM, Fuchs D. Mood, food, and cognition: role of tryptophan and serotonin. Curr Opin Clin Nutr Metab Care. 2016;19(1):55–61. doi: 10.1097/MCO.0000000000000237. [DOI] [PubMed] [Google Scholar]
- 80.Ochoa-Reparaz J, Mielcarz D, Wang Y, Begum-Haque S, Dasgupta S, Kasper D, Kasper L. A polysaccharide from the human commensal bacteroides fragilis protects against CNS demyelinating disease. Mucosal Immunol. 2010;3(5):487. doi: 10.1038/mi.2009.138. [DOI] [PubMed] [Google Scholar]
- 81.Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T, Codelli JA, Chow J, Reisman SE, Petrosino JF, et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155(7):1451–1463. doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yunes R, Poluektova E, Dyachkova M, Klimina K, Kovtun A, Averina O, Orlova VS, Danilenko VN. GABA production and structure of gadB/gadC genes in lactobacillus and bifidobacterium strains from human microbiota. Anaerobe. 2016;42:197–204. doi: 10.1016/j.anaerobe.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 83.Asano Y, Hiramoto T, Nishino R, Aiba Y, Kimura T, Yoshihara K, Koga Y, Sudo N. Critical role of gut microbiota in the production of biologically active, free catecholamines in the gut lumen of mice. Am J Physiol Gastrointest Liver Physiol. 2012;303(11):G1288–G1295. doi: 10.1152/ajpgi.00341.2012. [DOI] [PubMed] [Google Scholar]
- 84.Zuhl M, Dokladny K, Mermier C, Schneider S, Salgado R, Moseley P. The effects of acute oral glutamine supplementation on exercise-induced gastrointestinal permeability and heat shock protein expression in peripheral blood mononuclear cells. Cell Stress Chaperones. 2015;20(1):85–93. doi: 10.1007/s12192-014-0528-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lambert GP. Intestinal barrier dysfunction, endotoxemia, and gastrointestinal symptoms: the ‘canary in the coal mine’during exercise-heat stress? In: Thermoregulation and human performance. Med Sport Sci; 2008;53:61–73. doi: 10.1159/000151550. [DOI] [PubMed] [Google Scholar]
- 86.Dokladny K, Zuhl MN, Moseley PL. Intestinal epithelial barrier function and tight junction proteins with heat and exercise. J Appl Physiol. 2015;120(6):692–701. doi: 10.1152/japplphysiol.00536.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Johannesson E, Simrén M, Strid H, Bajor A, Sadik R. Physical activity improves symptoms in irritable bowel syndrome: a randomized controlled trial. Am J Gastroenterol. 2011;106(5):915. doi: 10.1038/ajg.2010.480. [DOI] [PubMed] [Google Scholar]
- 88.Wu MH, Lee CP, Hsu SC, Chang CM, Chen CY. Effectiveness of high-intensity interval training on the mental and physical health of people with chronic schizophrenia. Neuropsychiatr Dis Treat. 2015;11:1255. doi: 10.2147/NDT.S81482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.McNeil JK, LeBlanc EM, Joyner M. The effect of exercise on depressive symptoms in the moderately depressed elderly. Psychol Aging. 1991;6(3):487. doi: 10.1037/0882-7974.6.3.487. [DOI] [PubMed] [Google Scholar]
- 90.Quaney BM, Boyd LA, McDowd JM, Zahner LH, He J, Mayo MS, Macko RF. Aerobic exercise improves cognition and motor function poststroke. Neurorehab Neural Repar. 2009;23(9):879–885. doi: 10.1177/1545968309338193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jeffery IB, O’toole PW, Öhman L, Claesson MJ, Deane J, Quigley EM, Simrén M. An irritable bowel syndrome subtype defined by species-specific alterations in faecal microbiota. Gut. 2012;61(7):997–1006. doi: 10.1136/gutjnl-2011-301501. [DOI] [PubMed] [Google Scholar]
- 92.Kajander K, Myllyluoma E, Rajilić‐Stojanović M, Kyrönpalo S, Rasmussen M, Järvenpää S, Zoetendal EG, de Vos WM, Vapaatalo H, Korpela R. Clinical trial: multispecies probiotic supplementation alleviates the symptoms of irritable bowel syndrome and stabilizes intestinal microbiota. Aliment Pharmacol Ther. 2008;27(1):48–57. doi: 10.1111/j.1365-2036.2007.03542.x. [DOI] [PubMed] [Google Scholar]
- 93.Choi CH, Jo SY, Park HJ, Chang SK, Byeon J-S, Myung S-J. A randomized, double-blind, placebo-controlled multicenter trial of Saccharomyces boulardii in irritable bowel syndrome: effect on quality of life. J Clin Gastroenterol. 2011;45(8):679–683. doi: 10.1097/MCG.0b013e318204593e. [DOI] [PubMed] [Google Scholar]
- 94.Diop L, Guillou S, Durand H. Probiotic food supplement reduces stress-induced gastrointestinal symptoms in volunteers: a double-blind, placebo-controlled, randomized trial. Nutr Res. 2008;28(1):1–5. doi: 10.1016/j.nutres.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 95.McFadzean R. Exercise can help modulate human gut microbiota. CU Scholar. 2014. Undergraduate honors thesis 155. [Google Scholar]
- 96.Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, Guiot Y, Derrien M, Muccioli GG, Delzenne NM, et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci USA. 2013;110(22):9066–9071. doi: 10.1073/pnas.1219451110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Noble EE, Hsu TM, Kanoski SE. Gut to brain dysbiosis: mechanisms linking western diet consumption, the microbiome, and cognitive impairment. Front Behav Neurosci. 2017;11:9. doi: 10.3389/fnbeh.2017.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bruce-Keller AJ, Salbaum JM, Luo M, Blanchard E IV, Taylor CM, Welsh DA, Berthoud H-R. Obese-type gut microbiota induce neurobehavioral changes in the absence of obesity. Biol Psychiatry. 2015;77(7):607–615. doi: 10.1016/j.biopsych.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Barton W, Penney NC, Cronin O, Garcia-Perez I, Molloy MG, Holmes E, Shanahan F, Cotter PD, O’Sullivan O. The microbiome of professional athletes differs from that of more sedentary subjects in composition and particularly at the functional metabolic level. Gut. 2018;67(4):625–633. doi: 10.1136/gutjnl-2016-313627. [DOI] [PubMed] [Google Scholar]
- 100.Macfarlane GT, Macfarlane S. Bacteria, colonic fermentation, and gastrointestinal health. J AOAC Int. 2012;95:50–60. [DOI] [PubMed] [Google Scholar]
- 101.Estaki M, Pither J, Baumeister P, Little JP, Gill SK, Ghosh S, Ahmadi-Vand Z, Marsden KR, Gibson DL. Cardiorespiratory fitness as a predictor of intestinal microbial diversity and distinct metagenomic functions. Microbiome. 2016;4(1):42. doi: 10.1186/s40168-016-0189-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ohira H, Tsutsui W, Fujioka Y. Are short chain fatty acids in gut microbiota defensive players for inflammation and atherosclerosis? J Atheroscler Thromb. 2017;24(7):660–672. doi: 10.5551/jat.RV17006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Erny D, Hrabe de Angelis AL, Jaitin D, Wieghofer P, Staszewski O, David E, Keren-Shaul H, Mahlakoiv T, Jakobshagen K, Buch T, et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci. 2015;18(7):965–977. doi: 10.1038/nn.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kang SS, Jeraldo PR, Kurti A, Miller MEB, Cook MD, Whitlock K, Goldenfeld N, Woods JA, White BA, Chia N, et al. Diet and exercise orthogonally alter the gut microbiome and reveal independent associations with anxiety and cognition. Mol Neurodegener. 2014;9(1):36. doi: 10.1186/1750-1326-9-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Evans CC, LePard KJ, Kwak JW, Stancukas MC, Laskowski S, Dougherty J, Moulton L, Glawe A, Wang Y, Leone V, et al. Exercise prevents weight gain and alters the gut microbiota in a mouse model of high fat diet-induced obesity. PLoS One. 2014;9(3):e92193. doi: 10.1371/journal.pone.0092193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444(7122):1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 107.Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G, Almeida M, Arumugam M, Batto J-M, Kennedy S, et al. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500(7464):541–546. doi: 10.1038/nature12506. [DOI] [PubMed] [Google Scholar]
- 108.Queipo-Ortuño MI, Seoane LM, Murri M, Pardo M, Gomez-Zumaquero JM, Cardona F, Casanueva F, Tinahones FJ, Sanz Y. Gut microbiota composition in male rat models under different nutritional status and physical activity and its association with serum leptin and ghrelin levels. PLoS One. 2013;8(5):e65465. doi: 10.1371/journal.pone.0065465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Barcenilla A, Pryde SE, Martin JC, Duncan SH, Stewart CS, Henderson C, Flint HJ. Phylogenetic relationships of butyrate-producing bacteria from the human gut. Appl Environ Microbiol. 2000;66:1654–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Boakes RA. Self-starvation in the rat: running versus eating. Span J Psychol. 2007;10:251–257. [DOI] [PubMed] [Google Scholar]
- 111.Zhao X, Zhang Z, Hu B, Huang W, Yuan C, Zou L. Response of gut microbiota to metabolite changes induced by endurance exercise. Front Microbiol. 2018;9:765. doi: 10.3389/fmicb.2018.00765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Martinez I, Wallace G, Zhang C, Legge R, Benson AK, Carr TP, Moriyama EN, Walter J. Diet-induced metabolic improvements in a hamster model of hypercholesterolemia are strongly linked to alterations of the gut microbiota. Appl Environ Microbiol. 2009;75(12):4175–4184. doi: 10.1128/AEM.00380-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liu H, Zhang H, Wang X, Yu X, Hu C, Zhang X. The family Coriobacteriaceae is a potential contributor to the beneficial effects of Roux-en-Y gastric bypass on type 2 diabetes. Surg Obes Relat Dis. 2018;14(5):584–593. doi: 10.1016/j.soard.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 114.Allen JM, Mailing LJ, Niemiro GM, Moore R, Cook MD, White BA, Holscher HD, Woods JA. Exercise alters gut microbiota composition and function in lean and obese humans. Med Sci Sports Exerc. 2018;50:747–757. doi: 10.1249/MSS.0000000000001495. [DOI] [PubMed] [Google Scholar]
- 115.Donohoe DR, Garge N, Zhang X, Sun W, O’Connell TM, Bunger MK, Bultman SJ. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 2011;13(5):517–526. doi: 10.1016/j.cmet.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.M Astbury S, Corfe BM. Uptake and metabolism of the short-chain fatty acid butyrate, a critical review of the literature. Curr Drug Metab. 2012;13:815–821. [DOI] [PubMed] [Google Scholar]
- 117.Wolin KY, Yan Y, Colditz GA, Lee I. Physical activity and colon cancer prevention: a meta-analysis. Br J Cancer. 2009;100(4):611. doi: 10.1038/sj.bjc.6604917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yoo DY, Kim W, Nam SM, Kim DW, Chung JY, Choi SY, Yoon YS, Won MH, Hwang IK. Synergistic effects of sodium butyrate, a histone deacetylase inhibitor, on increase of neurogenesis induced by pyridoxine and increase of neural proliferation in the mouse dentate gyrus. Neurochem Res. 2011;36(10):1850. doi: 10.1007/s11064-011-0501-7. [DOI] [PubMed] [Google Scholar]
- 119.Kim HJ, Leeds P, Chuang DM. The HDAC inhibitor, sodium butyrate, stimulates neurogenesis in the ischemic brain. J Neurochem. 2009;110(4):1226–1240. doi: 10.1111/j.1471-4159.2009.06212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cronin O, Barton W, Skuse P, Penney NC, Garcia-Perez I, Murphy EF, Woods T, Nugent H, Fanning A, Melgar S, et al. A prospective metagenomic and metabolomic analysis of the impact of exercise and/or whey protein supplementation on the gut microbiome of sedentary adults. mSystems. 2018;3(3):e00044–18. doi: 10.1128/mSystems.00044-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rottenberg J. Cardiac vagal control in depression: a critical analysis. Biol Psychol. 2007;74(2):200–211. doi: 10.1016/j.biopsycho.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 122.Gershon MD. Nerves, reflexes, and the enteric nervous system: pathogenesis of the irritable bowel syndrome. J Clin Gastroenterol. 2005;39:S184–S193. [DOI] [PubMed] [Google Scholar]
- 123.Vempati R, Telles S. Yoga-based guided relaxation reduces sympathetic activity judged from baseline levels. Psychol Rep. 2002;90(2):487–494. doi: 10.2466/pr0.2002.90.2.487. [DOI] [PubMed] [Google Scholar]
- 124.Schumann D, Anheyer D, Lauche R, Dobos G, Langhorst J, Cramer H. Effect of yoga in the therapy of irritable bowel syndrome: a systematic review. Clin Gastroenterol Hepatol. 2016;14(12):1720–1731. doi: 10.1016/j.cgh.2016.04.026. [DOI] [PubMed] [Google Scholar]
- 125.Cramer H, Lauche R, Langhorst J, Dobos G. Yoga for depression: A systematic review and meta‐analysis. Depress Anxiety. 2013;30(11):1068–1083. doi: 10.1002/da.22166. [DOI] [PubMed] [Google Scholar]
- 126.Perez-Burgos A, Wang B, Mao Y-K, Mistry B, Neufeld K-AM, Bienenstock J, Kunze W. Psychoactive bacteria Lactobacillus rhamnosus (JB-1) elicits rapid frequency facilitation in vagal afferents. Am J Physiol Gastrointest Liver Physiol. 2012;304(2):G211–G220. doi: 10.1152/ajpgi.00128.2012. [DOI] [PubMed] [Google Scholar]
- 127.Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, Bienenstock J, Cryan JF. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci. 2011;108(38):16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci. 2003;14(2):125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- 129.Kramer AF, Colcombe SJ, McAuley E, Scalf PE, Erickson KI. Fitness, aging and neurocognitive function. Neurobiol Aging. 2005;26(1):124–127. doi: 10.1016/j.neurobiolaging.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 130.Rasmussen P, Brassard P, Adser H, Pedersen MV, Leick L, Hart E, Secher NH, Pedersen BK, Pilegaard H. Evidence for a release of brain‐derived neurotrophic factor from the brain during exercise. Exp Physiol. 2009;94(10):1062–1069. doi: 10.1113/expphysiol.2009.048512. [DOI] [PubMed] [Google Scholar]
- 131.Molteni R, Wu A, Vaynman S, Ying Z, Barnard R, Gomez-Pinilla F. Exercise reverses the harmful effects of consumption of a high-fat diet on synaptic and behavioral plasticity associated to the action of brain-derived neurotrophic factor. Neuroscience. 2004;123:429–440. [DOI] [PubMed] [Google Scholar]
- 132.Linnarsson S, Björklund A, Ernfors P. Learning deficit in BDNF mutant mice. Eur J Neurosci. 1997;9:2581–2587. [DOI] [PubMed] [Google Scholar]
- 133.Sarkar A, Lehto SM, Harty S, Dinan TG, Cryan JF, Burnet PW. Psychobiotics and the manipulation of bacteria–gut–brain signals. Trends Neurosci. 2016;39(11):763–781. doi: 10.1016/j.tins.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Smith F, Clark JE, Overman BL, Tozel CC, Huang JH, Rivier JE, Blikslager AT, Moeser AJ. Early weaning stress impairs development of mucosal barrier function in the porcine intestine. Am J Physiol Gastrointest Liver Physiol. 2009;298(3):G352–G363. doi: 10.1152/ajpgi.00081.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Möhle L, Mattei D, Heimesaat MM, Bereswill S, Fischer A, Alutis M, French T, Hambardzumyan D, Matzinger P, Dunay IR, et al. Ly6Chi monocytes provide a link between antibiotic-induced changes in gut microbiota and adult hippocampal neurogenesis. Cell Rep. 2016;15(9):1945–1956. doi: 10.1016/j.celrep.2016.04.074. [DOI] [PubMed] [Google Scholar]
- 136.Cerdá B, Pérez M, Pérez-Santiago JD, Tornero-Aguilera JF, González-Soltero R, Larrosa M. Gut microbiota modification: another piece in the puzzle of the benefits of physical exercise in health? Front Physiol. 2016;7:51. doi: 10.3389/fphys.2016.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cook MD, Martin SA, Williams C, Whitlock K, Wallig MA, Pence BD, Woods JA. Forced treadmill exercise training exacerbates inflammation and causes mortality while voluntary wheel training is protective in a mouse model of colitis. Brain Behav Immun. 2013;33:46–56. doi: 10.1016/j.bbi.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lamoureux EV, Grandy SA, Langille MGI. Moderate exercise has limited but distinguishable effects on the mouse microbiome. mSystems. 2017;2:4. doi: 10.1128/mSystems.00006-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Allen JM, Berg Miller ME, Pence BD, Whitlock K, Nehra V, Gaskins HR, White BA, Fryer JD, Woods JA. Voluntary and forced exercise differentially alters the gut microbiome in C57BL/6J mice. J Appl Physiol. 2015;118(8):1059–1066. doi: 10.1152/japplphysiol.01077.2014. [DOI] [PubMed] [Google Scholar]
- 140.Lou De Santis G, Kavvadia M, Alwardat N Abd Almajeed Abbaas, Bigioni G, Zeppieri C, Cascapera S, De Lorenzo A. Psychobiotics as integrative therapy for neuropsychiatric disorders with special emphasis on the microbiota-gut-brain axis. Biomed Prev. 2017;2:111. [Google Scholar]
- 141.Dey S, Singh R, Dey P. Exercise training: significance of regional alterations in serotonin metabolism of rat brain in relation to antidepressant effect of exercise. Physiol Behav. 1992;52:1095–1099. [DOI] [PubMed] [Google Scholar]
- 142.Wipfli B, Landers D, Nagoshi C, Ringenbach S. An examination of serotonin and psychological variables in the relationship between exercise and mental health. Scand J Med Sci Sports. 2011;21(3):474–481. doi: 10.1111/j.1600-0838.2009.01049.x. [DOI] [PubMed] [Google Scholar]
- 143.Özoğul F, Kuley E, Özoğul Y, Özoğul İ. The function of lactic acid bacteria on biogenic amines production by food-borne pathogens in arginine decarboxylase broth. Food Sci Technol Res. 2012;18(6):795–804. doi: 10.3136/fstr.18.795. [DOI] [Google Scholar]
- 144.Özoğul F. Production of biogenic amines by Morganella morganii, Klebsiella pneumoniae and Hafnia alvei using a rapid HPLC method. Eur Food Res Technol. 2004;219(5):465–469. doi: 10.1007/s00217-004-0988-0. [DOI] [Google Scholar]


