Abstract
Background
Ethiopia has adopted the World Health Organization recommendation for TB and HIV collaborative activities since 2004. These collaborative activities have been scaled up in a phased manner and covered large number of health facilities across the nation. However, there is scarcity of information on implementation of these collaborative activities in Ethiopia.
Objective
To assess the status of implementation of TB and HIV collaborative activities in health facility settings of Ethiopia.
Methods
A cross sectional study mainly quantitative supplemented by qualitative methods was undertaken from May 10 to July 10, 2014 in 132 selected health facilities. Statistical analysis was performed using SPSS version 20.
Result
About 81% of the respondents in the selected health facilities reported the screening of People Living with HIV in care for TB at every follow up visit, whereas, only 28.7% of those health facilities reported the screening of PLWHIV for TB at enrolment to HIV chronic care. About half of the public health facilities assessed were not implementing Isoniazid Preventive Therapy and only 18.2% of eligible clients were getting this Preventive Therapy. Among the co-infected patients, 32% were not linked to chronic care services and 45.3% were not getting ART during TB treatment. On the other hand, about two thirds of the co-infected patients are getting the Cotrimoxazole Prophylaxis Therapy.
Conclusion
Most of anti-TB and HIV collaborative activities were not implemented as expected in the health facilities. Thus it needs integration from the ministry to the health facilities level in order to improve the collaborative activities.
Keywords: Tuberculosis screening, Ionized Prophylaxis Therapy, Cotrimexazole Prophylaxis Therapy, people living with HIV
Introduction
Tuberculosis (TB) and Human Immuno deficiency Virus (HIV) are major sources of morbidity and mortality throughout the world (WHO 2015). An estimated 10.4 million new TB cases and 1.8 million deaths, including 0.4 million HIV co-infected people occurred in 2015 (WHO 2016b) and there were an estimated 2.1 million new HIV infections worldwide, adding up to a total of 36.7 million [34.0 million–39.8 million] people living with HIV occurred in 2015 (WHO 2016a). In addition, 1/3 of the worldwide population is latently infected with Mycobacterium tuberculosis (Mtb). Mtb is an intracellular pathogen capable of infecting and surviving within the host’s mononuclear cells, particularly macrophages (Crevel et al 2002). HIV is the strongest risk factor for developing TB in those with latent or new Mtb; increasing the risk of developing TB by 20 to 37 fold in people living with HIV (PLWHIV) compared to those who do not have HIV infection (WHO 2011). In view of the worsening of HIV/AIDS and TB situation in the world and taking into account the close association between the two diseases, WHO has issued a policy recommendation to address the dual epidemic in 2004. The national TB prevention and control program recommended TB screening to be integrated in every services outlet of the health facilities. It is also indicated that all HIV positive clients should be screened for TB symptoms both at the time of enrolment as well as at every follow up visit (FMOH 2014). Since then Ethiopia adopted the TB/HIV collaborative initiative and started to pilot it in nine health facilities. Subsequently, the national program developed a national TB/HIV guideline to standardize the implementation of TB/HIV collaborative activities and expand it nation-wide and all packages of the TB/HIV collaborative initiatives have been implemented in the country.
The implementation status of the national TB prevention and control program in Ethiopia had been reviewed and this recommended assessing the adequate coverage in the National TB strategic plan and wide implementation of the TB/HIV collaborative activities at the service delivery points. There is scarcity of data on the seven core indicators of the TB/HIV collaborative activities and only two of these have been captured and reported by the Health Management Information System (HMIS) until recently. Besides, the quality of TB/HIV information generated by HMIS is a matter of concern. Therefore, this study aimed to assess the status of implementation of TB/HIV collaborative activities and to evaluate the extent of implementation on determinants of Intensified Case Finding (ICF) which is IPT, CPT and ART coverage among People Living With HIV (PLHIV) in Ethiopia.
Materials and Methods
Study design, study setting and study population
A cross sectional study design and multi stage cluster sampling method was used. A total of 132 public health facilities (33 Hospitals and 99 Health centers) from 126 hospitals and 2371 health centers those providing TB Directly Observed Therapy, Short Course (TB DOTS) service were included. Randomly selected records of HIV positive TB patients on chronic care from July 1, 2013 to March 31, 2014, TB and ART focal persons in the health facilities were the study populations.
Sample size Determination
The sample size for patient record review was determined assuming a prevalence of 32% IPT uptake among HIV infected clients, free of active TB, from previous study. With a 95% certainty and a maximum discrepancy of 6% between the sample and the underlying population were considered for all objectives. An additional 10% non-response rate was considered as a contingence and a design effect of 2 was taken for the multistage nature of the sampling. And accordingly, the required minimum sample size for IPT was 511 per reporting domain. The sample size for pastoralist and urban was 511 each, while for the Agrarian was 1,178 with the overall sample size of 2,200 patient records. At the health facility, all patients’ record were listed and numbered consecutively during the enrolment time.
The number of patients was determined by starting randomly and review the records systematically based on the total number listed. Two key informant interviews (KIT) were administered for TB and ART focal persons in the selected facilities. Pretested standard and structured questionnaires for the quantitative data and semi-structured questionnaires for the qualitative (KII) data were used. Data were double entered and analyzed using Census and Survey Processing System (CSPro) and SPSS version 20 respectively. The survey was conducted after getting an approval from the Institutional Review Board of EPHI.
Results
Implementation of ICF
Among the 2,161 HIV infected clients enrolled in the study, 1880 (87%) were screened for TB during their last follow up visit. There was no major variation in screening coverage across the different agro-ecological settings. However, public health centers perform better in terms of TB screening coverage compared to hospitals (87% vs. 83%). About 81% of the respondents in the selected health facilities reported screening of PLHIV in care for TB at every follow up visit; On the other hand, only 28.7% of health facilities reported screening of PLHIV for TB at enrolment to HIV chronic care. Six percent of the respondents in the selected health facilities reported screening of PLHIV only when patient complains of TB symptoms. Fifty three percent of the health facilities were not adhering to the recommended guidance for TB screening among PLHIV. Forty one percent of the health facilities/ART clinics had the current national guideline (Programmatic management of TB, TB/HIV and Multi Drug Resistant TB, 2012) at the time of visit and from those only 19.5% of the facilities adhered to the nationally recommended guideline for investigating TB screening positive PLHIV to confirm/rule out TB.
Isoniazid Preventive Therapy (IPT)
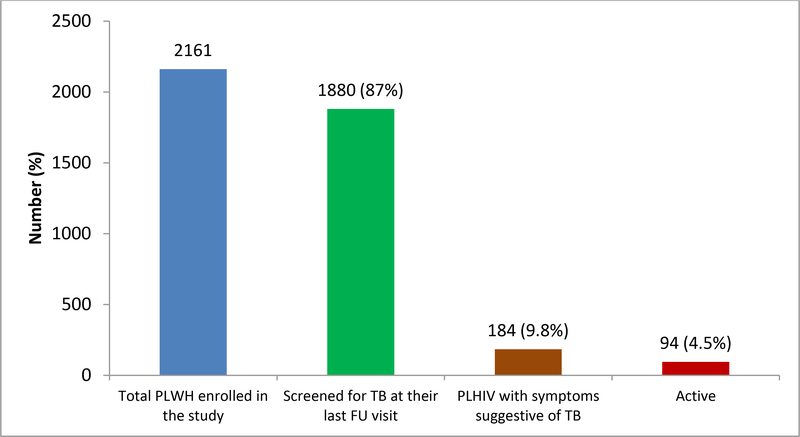
Out of 87 ART health facilities, only 50% of them (11 hospitals and 33 public Health Centers) were providing IPT service. Among 1880 PLHIV screened for TB, 184 (9.8%) were found to have presumptive TB); while the incidence of TB among HIV positive clients enrolled to the study was 4.5% (Figure 1).
Figure 1:
Proportion of PLHIV under chronic care screened for TB symptoms and diagnosed with active TB
Among the 1,696 PLHIV screened and found to be negative for TB, only 308 (18.2%) had started IPT. The implementation of IPT is low in Pastoralist compared to urban setting and there was no significant difference in IPT implementation between Urban and Agrarian settings. Among the PLHIV clients enrolled to IPT, 105 were eligible for evaluation and the IPT completion rate was 64.8% (Table 1). Only two clients who started IPT have developed active TB disease during the course of their treatment.
Table 1:
IPT completion rate in different agro-ecologic setting and health facility type
| Variable | N (%) | IPT completion | ||
|---|---|---|---|---|
| OR (95% CI) | P-value | |||
| Agro ecological setting | Urban | 14 (20.9) | 1 | |
| Agrarian | 41 (61.2) | 0.72 (0.48, 1.07) | 0.106 | |
| Pastoralist | 12 (17.9) | 0.56 (0.32, 0.98) | 0.046 | |
| Health Facility type | Public hospital | 8 (11.8) | 0.36 (0.128, 1.013) | |
| Public health center | 60 (88.2) | 1 | ||
The major barrier for the IPT implementation as reported by respondents from selected health facilities was INH supply interruption 84.2%.
Patient’s residence, sex and having history of previous treatment were found to be the independent predictors of ART initiation among TB patients co-infected with HIV (Table 2).
Table 2:
Socio-demographic and clinical factors associated with ART uptake in TB/HIV co-infected patients
| Parameters | N (%) | AOR (95% C.I.) | P-value | |
|---|---|---|---|---|
| Residence | Urban | 1290 ( 86.3) | ||
| Rural | 204 (13.7) | 0.62 (0.50, 0.76) | <0.001 | |
| Sex | Male | 740 (49.5) | ||
| Female | 754 (50.5) | 1.34 (1.09, 1.65) | 0.006 | |
| Age | 0–14 | 74(5) | ||
| 15–44 | 1194(79.9) | 1.00 (0.50, 2.06) | 0.980 | |
| 45+ | 226(15.1) | 1.86(0.87, 4.00) | 0.110 | |
| Weight | <29 kg | 72 (4.8) | ||
| 30–39 kg | 196(13.1) | 0.74(0.37, 1.51) | 0.411 | |
| 40–54 kg | 879 (58.8) | 0.78 (0.38, 1.57) | 0.480 | |
| 55+kg | 347 (23.2) | 0.60 (0.29, 1.25) | 0.171 | |
| Type of TB | Pulmonary Positive | 327(21.9) | ||
| Pulmonary Negative | 719(48.1) | 1.12(0.86, 1.40) | 0.428 | |
| Extra pulmonary | 448 (30) | 1.12(0.84, 1.49) | 0.443 | |
| Treatment | New | 1279 (85.6) | ||
| History | Retreatment (R,F,D) | 66 (4.4) | 2.46 (1.41,4.31) | 0.002 |
| Others(O) | 92 (6.2) | 1.83 (1.09, 3.08) | 0.022 | |
| Transfer in (T) | 57 (3.8) | 1.52(0.99, 2.31) | 0.055 |
Patient’s residence, sex, and being in the age group of 15–44 years were found to be the independent predictors of CPT uptake among TB patients co-infected with HIV (Table 3)
Table 3:
Factors associated with CPT initiation for TB patients co-infected with HIV
| Parameters | N (%) | AOR (95% C.I.) | P-value | |
|---|---|---|---|---|
| Residence | Urban | 1291(86.4) | 0.621 (0.5, 0.77) | <0.001 |
| Rural | 204 (13.6) | |||
| Sex | Male | 741(49.6) | 0.019 | |
| Female | 754(50.4) | 0.775 (0.626, 0.96) | ||
| Age | 0–14 | 74(4.9) | ||
| 15–44 | 1195(79.9) | 0.295(0.119, 0.731) | 0.008 | |
| 45+ | 226(15.1) | 0.453 (0.175, 1.173) | 0.103 | |
| Weight | <29 kg | 72(4.8) | ||
| 30–39 kg | 197(13.2) | 0.746 (0.327, 1.706) | 0.488 | |
| 40–54 kg | 879(58.8) | 0.626(0.278, 1.412) | 0.260 | |
| 55+kg | 347(23.2) | 0.557 (0.241, 1.287) | 0.171 | |
| Type of TB | Pulmonary Positive | 327(21.9) | ||
| Pulmonary Negative | 720(48.2) | 1.267 (0.977, 1.644) | 0.074 | |
| Extra pulmonary | 448(30.0) | 0.921 (0.69, 1.229) | 0.577 | |
| Treatment | New(N) | 1280(85.6) | ||
| History | Retreatment (R,F,D) | 66(4.4) | 1.264(0.736, 2.171) | 0.397 |
| Others(O) | 92(6.2) | 1.246(0.739, 2.101) | 0.409 | |
| Transfer in (T) | 57(3.8) | 1.223 (0.789, 1.896) | 0.369 |
Discussion
This study assessed the status of implementation of TB/HIV collaborative activities including IPT, CPT and ART coverage among PLHIV in Ethiopia. The finding revealed that only 19.5% of health facilities were strictly adhered to the national guidelines for TB-HIV collaborative activities. This might be due to lack of motivation and knowledge gap of the health workers. TB screening for HIV patients was much lower (28.7%) compared to follow up visits. This could be related to the unavailability and lack of motivation for use of revised national guideline for programmatic management of TB, TB/HIV and Multi Drug Resistant TB (MDR TB). More than 59% of the public health facilities were not used the revised National Guideline at HIV care units and this is in line with external midterm review of the TB program report (unpublished result). The IPT uptake among the eligible PLHTV in this study was higher (18.2%) compared to national TB/HIV sentinel surveillance report of 2013 (Denegetu and Dolamo 2014); while lower compared to the finding conducted in six health facilities (Wesen and Mitike 2012) and study conducted in a teaching hospitals of Addis Ababa (Kassa et al. 2012a) which could be attributed to Isoniazid supply interruption and stock out.
This is in contrast to previous literature, which focuses on the confidence of health workers in prescribing INH prophylaxis for eligible PLHIV (Kassa et al. 2012a). The implementation of IPT was lower (17.9%) in pastoralist areas, which could be due to poor access to health services relatively by pastoralist community. This study showed that a relatively high proportion (64.8%) of patients who commenced treatment for LTBI completed the six month scheduled course compared to other studies (Goswami et al. 2012; Diaz et al. 2010). However, the completion rate is lower compared to the studies conducted in Australia (Dobler and Marks 2012) and Italy (Codecasa et al. 2013).
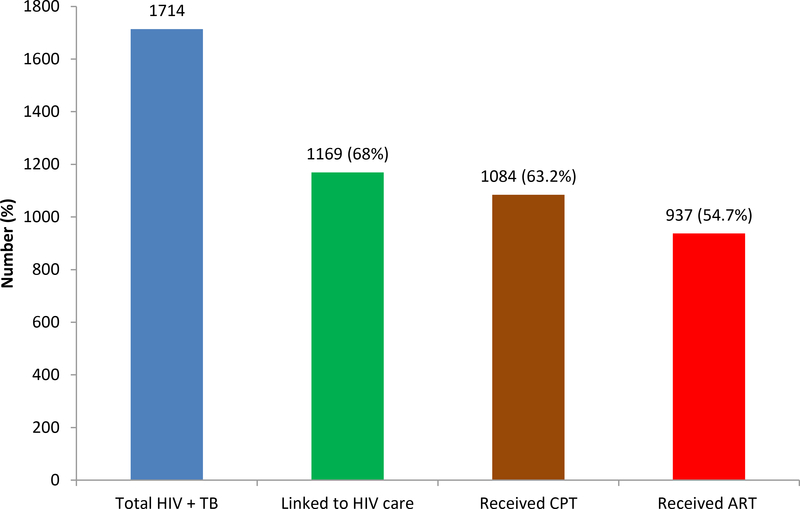
One third of co-infected patients were not linked to the chronic HIV care and treatment service. This might be related to supply interruption, knowledge gap and lack of staff motivation. ART uptake coverage for TB-HIV co-infected patients was lower (54.7%) than the global recommended (FMOH 2014; Schütz et al. 2010; WHO 2010) and other African countries (Pepper et al. 2011; Tayler-Smith et al. 2011) and India (Njozing et al. 2010) but similar to the 2013 national surveillance report in Ethiopia (EPHI 2013) and another study in Cameroon (Njozing et al. 2010). Patients from rural, female and previous treated patients had low proportion of uptake of ART relative to patients from urban settings, males and new TB patients respectively. This is similar with previous report in Cameroon (Njozing et al. 2010), in South Africa (Pepper et al. 2011). The coverage of CPT for TB patients co-infected with HIV in this study were lower (63.2%) compared from global recommended and target (FMOH 2014). However, this finding is higher than the figure reported in a study in Cameroon (Njozing et al. 2010) and Addis Ababa (Denegetu and Dolamo 2014); while much lower than the CPT Coverage reported in a study from India (Dave et al. 2014). In this study, initiation of CPT was affected by Patient’s residence, sex, and age; females, which is similar with previous study in Cameroon (Njozing et al. 2010). Poor documentation of data regarding linkage for care, CPT and ART coverage could be another factor for the low ART coverage.
Conclusion
The practice of TB screening both at enrolment and every follow up visit of HIV care, and the care and treatment coverage of IPT, CPT and ART uptake for TB-HIV patients were very low at both public hospitals and health centers of Ethiopia. Therefore, higher officials should give stress on the major barriers of TB-HIV collaborative activities i.e. accessibility of health service, drug supply interruption and lack of training of health worker.
Supplementary Material
Figure 2:
TB patients co-infected with HIV linked to chronic HIV care service and received ART and CPT
Reference
- Codecasa LR, Murgia N & Ferrarese M (2013). Isoniazid preventive treatment: predictors of adverse events and treatment completion. International Journal of Tuberculosis Lung Disease, 17(7):903–908. [DOI] [PubMed] [Google Scholar]
- Crevel R, Van Ottenhoff THM & Meer D (2002). Innate Immunity to Mycobacterium lowest ART initiation in Somali region and highest being in Dire Dawa City administration and Hareri region (EPHI. 2013 tuberculosis, 15 (2), 94–309. htrtp://doi.org10.l128/CMR.15.2.294. [Google Scholar]
- Dave P, Kapadiya D & Modi B (2014). Impact of TB-HIV collaborative activities on case fatality among HIV-infected Tuberculosis patients patients in Gujarat, India. Journal of Research in Medical and Dental Science, 2:1. [Google Scholar]
- Denegetu AW & Dolamo BL (2014). Tuberculosis case finding and isoniazid preventive therapy among people living with HIV at public health facilities of Addis Ababa, Ethiopia: A cross-sectional facility based study. BMC public health, 14:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denegetu AW & Dolamo BL (2014). HIV Screening among TB Patients and Co-trimoxazole Preventive Therapy for TB/HIV Patients in Addis Ababa: Facility Based Descriptive Study. PLoS ONE, 9(2):e86614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz A, Diez M & Bleda MJ (2010). Eligibility for and outcome of treatment of latent tuberculosis infection in a cohort of HIV- infected people in Spain. BMC Infectious Diseases, 10:267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobler CC & Marks GB (2012). Completion of Treatment for Latent Tuberculosis Infection with Monthly Drug Dispensation Directly through the Tuberculosis Clinic. PLoS ONE, 7(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejeta E, Birhanu T & Wolde T (2014). TB Treatment outcomes among TB/HTV co-infected cases treated under DOTS in Western Ethiopia. Journal of AIDS and HIV Research, 6 (8): 164–71. [Google Scholar]
- EPHI (2013). National TB/HIV Sentinel Surveillance report; Ethiopia. [Google Scholar]
- FMOH (2012). Clinical and Programmatic Management of TB, Leprosy and TB-HIV: National TB Program Guideline; Ethiopia. Addis Ababa. [Google Scholar]
- FMOH (2014). National Guideline for Comprehensive HIV Prevention, Care and Treatment. HIV/AIDS Program; Ethiopia. Addis Ababa. [Google Scholar]
- FMOH (2013). Guidelines for clinical and programmatic management of TB, TB/HIV and Leprosy in Ethiopia; Addis Ababa. [Google Scholar]
- Getahun H, Granich R & Sculier D (2010). Implementation of isoniazid preventive therapy for people living with HIV worldwide: barriers and solutions. AIDS, 24 (Suppl 5):S57–S65. [DOI] [PubMed] [Google Scholar]
- Goswami NDE, Gadkowski LB & Piedrahita C (2012). Predictors of latent tuberculosis treatment Initiation and completion at a US public health clinic: a prospective cohort study. BMC Public Health, 12:468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harries AD, Lawn SD & Getahun H (2012). HIV and tuberculosis-science and implementation to turn the tide and reduce deaths. Journal of the International AIDS Society, 15:17396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassa A, Jerene D & Assefa Y (2012a). Evaluation of collaborative TB/HIV activities in a general hospital in Addis Ababa, Ethiopia. BMC Res Notes, 5:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njozing NB, Miguel SS & Tih PM. (2010). Assessing the accessibility of HIV care package samong tuberculosis patients in the North West Region, Cameroon. BMC Public Health, 10:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunn AJ, Mwaba P & Chintu C (2008). Role of Co- trimoxazole prophylaxis in reducing mortality in HIV infected adults being treated to tuberculosis: randomized clinical trial, British Medical Journal, 337:a257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper DJ, Marais S & Wilkinson RJ (2011). Barriers to initiation of antiretroviral during Antituberculosis therapy in Africa. PLoS ONE, 6:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sculier D, Getahun H & Lienhardt C (2011). Improving the prevention, diagnosis and treatment of TB among people living with HIV: the role of operational research. Journal of the International AIDS Society, 14(Suppl 1):S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutz C, Meintjes G & Almajid F (2010). Clinical management of tuberculosis and HIV-1 co-infection. European Research Journal, 36:1460–1481. [DOI] [PubMed] [Google Scholar]
- Tayler-Smith K, Zachariah R & Manzi M (2011). Antiretroviral treatment uptake and attrition among HTV-Positive patients with tuberculosis in Kibera, Keneya: Short communication. Tropical Medicine and International Health, 16:11. [DOI] [PubMed] [Google Scholar]
- Wesen A & Mitike G (2012). Provision and awareness for isoniazid preventive therapy among PLHIV in Addis Ababa, Ethiopia: BMC International Health and Human Rights, 12:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (2010). The Global Plan to Stop TB, 2011–2015. Geneva. [Google Scholar]
- WHO (2011). Guidelines for intensified tuberculosis case-finding and isoniazid preventive therapy for people living with HIV in resource constrained settings. Geneva. [Google Scholar]
- WHO (2014). Global TB Report: Annex 2 Country Profile. World Health Organization; Geneva. [Google Scholar]
- WHO (2015). Definitions and reporting framework for tuberculosis-2013 revision (updated December 2014) (WHO/HTM/TB/2013.2). Geneva: (pp. 5–130). Retrieved from www.WHO.int/iris/bitstream/10665/79199/1/9789241505345_eng.pdf, accesed 15 August 2015. [Google Scholar]
- WHO (2016a). Global AIDS update. Retrieved from http://www.unaids.org/sites/defauhfiles/media_asset/global-AIDS-update-2016_en.pdf
- WHO (2016b). WHOConsolidated guidelines on the use of ART drugs for treating and preventing HIV infection. WHO. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.