ABSTRACT
Lymphocyte activation gene 3 (LAG-3) is expressed on activated T cells, natural killer cells or B cells, and functions to negatively regulate homeostasis of these cells. Anti-LAG-3 antibodies might be useful for antitumor immunotherapy. In this study, we characterized a novel anti-LAG-3 antibody, LBL-007, which was isolated from a human antibody phage display library. LBL-007 was found to specifically bind to human LAG-3 antigen, but not to human CD4 or mouse LAG-3. LBL-007 bound activated T cells and promoted interleukin-2 secretion. LBL-007 internalization efficacy by endocytosis into different cells was better than that of another anti-LAG-3 antibody, relatlimab analog. Moreover, LBL-007 was able to block LAG-3 binding to MHC class II molecules and liver sinusoidal endothelial cell lectin, and block LAG-3-induced downstream signaling. In mice transplanted with colorectal cancer cells, treatment with either anti-PD-1 antibody or LBL-007 (10 mg/kg per mouse twice a week for three weeks) resulted in a significant delay in tumor growth compared with control IgG treatment, and their combination was even more effective. Serum LBL-007 levels were highly stable in monkeys after a single intravenous injection of LBL-007 at 3, 10, or 30 mg/kg. This study demonstrated that the combination of LBL-007 with an anti-PD-1 antibody is a promising antitumor regimen for immunotherapy of solid tumors in future that deserves further study.
KEYWORDS: Immunotherapy, LAG-3, anti-LAG-3 antibody, anti-PD-1, solid tumor
Introduction
Lymphocyte-activation gene 3 (LAG-3), a member of the immunoglobulin (Ig) superfamily, is a type I transmembrane protein with four extracellular Ig-like domains. It is usually expressed on activated T cells, natural killer cells or B cells, and functions to negatively regulate homeostasis of these cells.1,2 As an activation marker of CD4+ or CD8 + T cells under physiological conditions, LAG-3 has been identified as a new-generation immune checkpoint protein.3,4 It plays various roles, including inhibition of Th1 cell proliferation, reduced production of interleukin (IL)-2, interferon-γ, and tumor necrosis factor in T cells.5-7 Structurally, LAG-3 (also known as CD223) is similar to CD4, but it has a higher affinity to major histocompatibility complex (MHC) class II molecules than CD4.8 LAG-3 can also bind to liver sinusoidal endothelial cell lectin (LSECtin), a cell-surface lectin and a member of the dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN) family.9,10 LSECtin mediates the recognition of viral and bacterial pathogens by antigen-presenting cells11 and the hepatic natural killer cell response.12 When expressed on melanoma cells, LSECtin promotes tumor progression through inhibition of anti-tumor T-cell responses.13 Therefore, blocking MHC class II molecules and other potential ligands might prove effective for immune activation in cancer cells. During tumorigenesis and cancer progression, LAG-3 enables tumor cells to escape from immune surveillance. Recent review articles indicate that LAG-3 might serve as a cancer immunotherapy target because it negatively regulates T-cell activity, and, in combination with PD-1, can mediate a state of exhaustion.4,14 Nevertheless, the precise molecular mechanisms of LAG-3 downstream signaling and interplay of other inhibitory receptors remain largely unknown.
Numerous anti-LAG-3 agents are currently being tested against solid tumors in clinical trials. Except for IMP321, which is a LAG-3-Ig fusion protein, all the drugs are antibodies. Relatlimab (BMS-986016, Bristol-Myers Squibb, human IgG4) was the first commercially developed anti-LAG-3 antibody to enter clinical trials (in 2013), and it is currently in Phase 2 clinical trials. However, because of the limited effectiveness of immune checkpoint antagonists alone, researchers have tried various combinations of antagonist treatments to enhance treatment efficacy.15,16 For example, nivolumab, an antibody against the immune checkpoint programmed cell death protein 1 (PD-1, also known as CD279) is being studied in combination with relatlimab for immunotherapy of solid tumors (NCT01968109). Blockade of the PD-1 pathway and LAG-3 in mice and humans showed better anti-tumor activity than blockade of either molecule alone.17-19 In this study, we identified and characterized a novel fully human anti-LAG-3 antibody, LBL-007.
Results
Identification of a novel anti-human LAG-3 antibody
We isolated anti-human LAG-3 antibodies from a human phage library. More than 300 phage clones were obtained after three rounds of selection with recombinant human LAG-3 protein, and 16 clones were confirmed to specifically bind to LAG-3 by enzyme-linked immunosorbent assay (ELISA). Sequence alignment analysis revealed that clones 2, 8, 13, and 14 had unique sequences, and their light chain variable regions were all kappa. These single-chain variable fragments (scFv) were converted to full IgG4 molecules for farther assessments. Electrophoresis under reducing and non-reducing conditions revealed that the molecular weights of the anti-LAG-3 monoclonal antibodies were close to that of complete human IgG (data not shown). Clone 2 (LBL-007) exhibited the best binding ability to human LAG-3 antigen. Thus, subsequent experiments focused on the characterization and functional analysis of LBL-007, the sequence of which is showed in supplement material (Supplementary Figure 1).
Binding specificity of LBL-007 to human LAG-3
We first evaluated the binding specificity of LBL-007 to human LAG-3 antigen by ELISA. We observed dose-dependent binding of LBL-007 to human LAG-3 recombinant protein (Figure 1A), similar to that of relatlimab analog (which is prepared in-house, hereafter referred to as relatlimab analog). LBL-007 bound neither to mouse LAG-3 antigen (Figure 1B) nor to CD4, a T cell receptor, structurally similar to LAG-3 (Figure 1C). Next, we evaluated the efficacy of LBL-007 binding to stable hLAG-3-transfected Chinese hamster ovary epithelial cells (CHO-K1) by flow cytometry. LBL-007 dose-dependently bound to CHO-K1 cells with a half maximal effective concentration (EC50) of 1.26 nM as quantified from the mean fluorescence intensity (Figure 1D). We further characterized the LBL-007 binding affinity using the ForteBio assay. LBL-007 had an equilibrium dissociation constant (KD) of 4.39E-10 M to human LAG-3 protein and of 2.58E-9 M to cynomolgus monkey LAG-3 (Table 1). These data demonstrated that this newly identified anti-human LAG-3 antibody was quite specific and possesses a high affinity for human LAG-3.
Figure 1.

Evaluation of LBL-007 binding specificity.
LBL-007 and control antibody were evaluated for their ability to bind human LAG-3 (A) or mouse LAG-3 (B) or CD4 protein (C) by ELISA. (D) Effect of LBL-007 antibody binding to CHO-K1/hLAG-3 cells by flow cytometry. Absorbance data and mean fluorescent intensity are showed as means ± SD on triplicates.
Table 1.
Binding affinities of LBL-007 antibody.
| Antibody | Antigen | ka(M−1S−1) | kd(S−1) | KD(M) |
|---|---|---|---|---|
| LBL-007 | Human LAG-3 | 1.22E+05 | 5.36E-05 | 4.39E-10 |
| Cynomolgus monkey LAG-3 | 2.01E+05 | 5.20E-04 | 2.58E-09 |
The binding affinity was measured by using the Fortebio. ka, Association rate constant [M-1S-1]; kd, Dissociation rate constant[S-1]; KD, Equilibrium dissociation constants [M].
LBL-007 internalization through endocytosis
Receptor-mediated antibody internalization has implications for the efficacy and dosage of therapeutic antibodies.20 The internalization efficacy of LBL-007 needed to be evaluated before its development as a potential therapeutic. We tested internalization of pHAb-dye-labeled LBL-007 in Jurkat-LAG-3 and HEK293-LAG-3 cells. The concentrations of antibodies LBL-007 and relatlimab analog were 1.22 mg/ml and 1.7 mg/ml, respectively (see Material and Methods). The dye labeling ratio of LBL-007 was 5.68, while ratio of relatlimab analog was 6.38. After incubation at 37°C, pHAb-dye derived fluorescence was readily detected by flow cytometry in both cell lines (Figure 2A,B). Dye-labeled relatlimab analog also emitted fluorescence, but not as strongly as LBL-007, indicating that a greater amount of LBL-007 reached acidic cell compartments, such as lysosomes or the lysosomal system. The fluorescence intensity varied after 5 h in Jurkat-LAG-3 cells, while in HEK293-LAG-3 cells varied as of 2 h. Our data showed that the average endocytosis efficiency of LBL-007 was approximately twice as much as that of relatlimab analog, indicating that LBL-007 might inhibit receptor functions and tumor growth more effectively than relatlimab analog.
Figure 2.
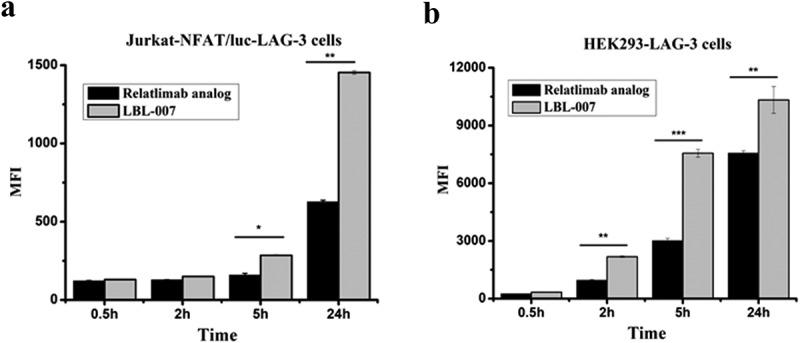
Efficacy of LBL-007 endocytosis into cells.
(A, B) LBL-007 and relatlimab analog were conjugated with pHAb Reactive Dye. When antibody-pHAb conjugates track through the endosome or lysosomal system, pH sensor dye will become fluorescent, which is detected by flow cytometry. Endocytosis of the antibody was determined using Jurkat-NFAT-LAG-3 and HEK293-LAG-3 cells. Mean fluorescent intensity is showed as means ± SD on triplicates. *P < .05, **P < .01, and ***P < .001 by Student’s t-test.
LBL-007 blockage of LAG-3 ligand binding
Both LAG-3 and CD4 can bind to MHC class II molecules to inhibit T-lymphocyte functions. Anti-LAG-3 antibody can block such binding and activate downstream signals for T-lymphocyte activation. We assessed the ability of LBL-007 to inhibit human LAG-3 binding to MHC class II molecules using an in-vitro binding assay. Compared with the control antibody, LBL-007 (IC50, 11.46 nM) was able to specifically inhibit LAG-3 binding to MHC class II molecules on Daudi cells (Figure 3A). Next, we assessed the ability of LBL-007 to inhibit human LAG-3 binding to LSECtin. An ELISA blocking assay revealed that LBL-007 effectively inhibited LAG-3 binding to human LSECtin, whereas relatlimab analog had a limited blocking effect (Figure 3B). The IC50 of LBL-007 is 6.48 nM.
Figure 3.
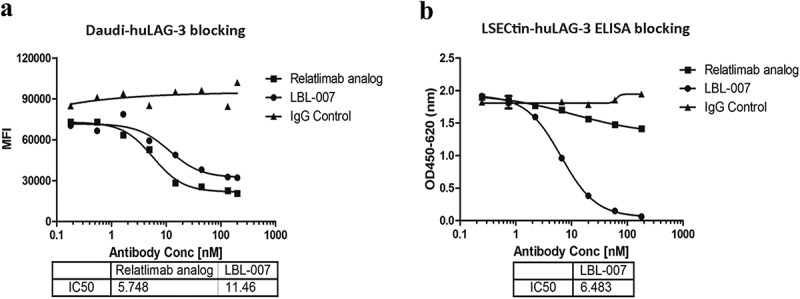
LBL-007 blocks binding of LAG-3 to ligands.
(A) In-vitro binding assay was performed to show LBL-007 inhibition on LAG-3 binding to MHC class II in Daudi-hLAG-3 cells. Data are showed as mean fluorescent intensity and IC50. Each sample was assayed in triplicate. (B) ELISA blocking assay was used to assess the ability of the anti-LAG-3 antibodies to inhibit human LAG-3 binding to human LSECtin. Absorbance data are expressed as means ± SD on triplicates.
LBL-007 binds to activated T cells for cytokine production
LAG-3 is mainly expressed on activated T cells to negatively regulate T cells and cytokine secretion. Reversal of LAG-3 repression was measured as an increase in IL-2 production or nuclear factor of activated T cells (NFAT) activation in response to treatment with an anti-LAG-3 antibody.21 To this end, we performed a reporter assay. In this assay, upon addition of a super-antigen such as staphylococcal enterotoxin D (SED) to Raji cells, LAG-3 on Jurkat-NFAT-LAG-3 cells could bind to MHC class II molecules of Raji cells, thus inhibit luciferase expression. When an anti-LAG-3 antibody is added, this blocking effect is reversed, and consequently, NFAT is activated, fluorescent signal is detected and IL-2 secretion is induced. The NFAT reporter assay revealed that LBL-007 was able to dose-dependently activate fluorescent signal in Jurkat-NFAT-LAG-3 cells (Figure 4A), indicating that LBL-007 was able to bind to LAG-3 to activate downstream signaling in the tumor cells. After 40 h, supernatants were collected. LBL-007 binding to Jurkat cells resulted in a dose-dependent increase in IL-2 production (Figure 4B). Natural T cells were isolated from human peripheral blood mononuclear cells (PBMCs) of healthy donors. After 7 days, binding of LBL-007 to activated human T cells was detected (Figure 4C). The fluorescence increased with increasing antibody concentration. After SEB stimulation, LBL-007 led to strong IL-2 secretion at approximately 80 pg/mL, which was twice as much as the level induced by control antibody (Figure 4D). Together, these data indicated that LBL-007 was able to block the negative regulation of LAG-3 on T cell activation and functions, resulting in elevated production of IL-2.
Figure 4.

Effects of LBL-007 on regulation of activated T cells and increase of IL-2 production.
(A,B) NFAT reporter assay was used to reveal LBL-007 binding to Jurkat-NFAT-LAG-3 cells. The corresponding fluorescence intensity of serial dilutions and EC50 was detected. After activating NFAT, Supernatants were collected and IL-2 levels were measured using the Human IL-2 DuoSet ELISA Kit. Data are expressed as the mean ± SD on triplicates. (C) A series of diluted concentrations of antibodies binding to activated human T cells was assessed by flow cytometry. Data are showed as Mean Fluorescent Intensity and EC50. (D) Different concentrations of LBL-007, relatlimab analog, and IgG4 were incubated with human PBMCs for 3 days, the IL-2 level in the supernatants was measured using ELISA Kit. Data are expressed as the mean ± SD on triplicates. *P < .05 and **P < .01 by Student’s t-test.
In-vivo anti-tumor activity of LBL-007
Simultaneous blockade of LAG-3 and PD-1 can facilitate T cell-mediated immune responses to suppress tumor growth in a mouse model.17 We assessed tumor growth in a transplanted murine colorectal cancer model. Dual anti-LAG-3/anti-PD-1 treatment suppressed tumor growth in all mice (n = 8) (Figure 5A). Treatment with either anti-PD-1 antibody or LBL-007 resulted in a significant delay of tumor growth compared with that of the IgG control. However, the combination treatment was more effective than either of the single therapies (P < .01). Tumor weights in the control group were significantly higher than those in experimental groups (Figure 5B). Together, these data demonstrated that LBL-007 and anti-PD-1 antibody had synergistic antitumor activity in this murine colorectal cancer model.
Figure 5.
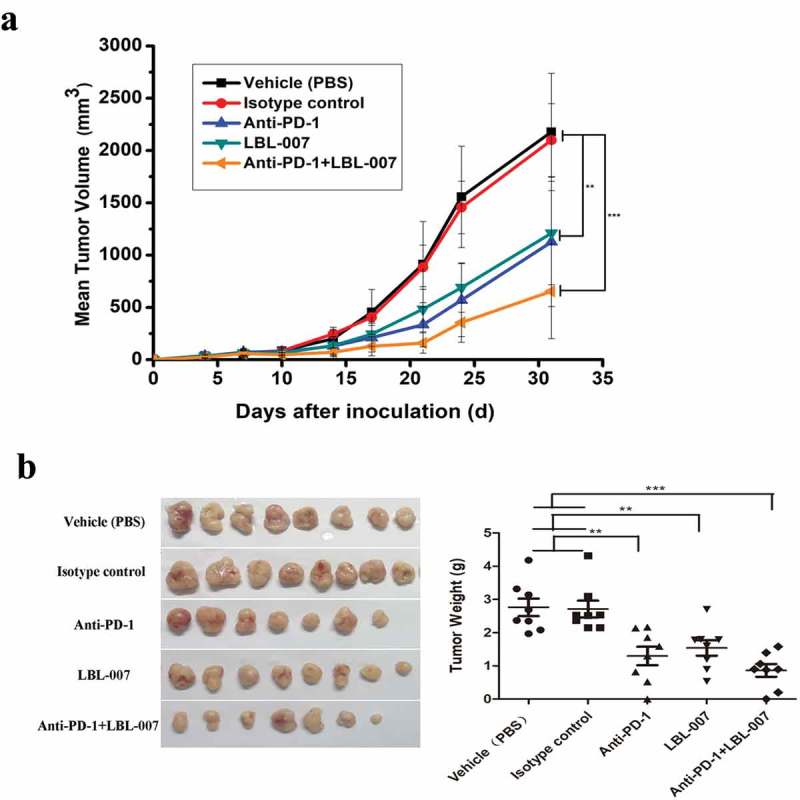
Combined effects of LBL-007 and anti-PD-1 antibody on inhibition of mouse colorectal cancer cell growth in vivo.
C57BL/6-hLAG-3 mice (n = 40) were randomized (n = 8 mice/group) when MC38 colon adenocarcinoma tumor volumes reached approximately 80 mm3. (A) Tumor volumes after treatment with the vehicle (PBS), isotype control, anti-PD-1, LBL-007 or a combination of LBL-007 and anti-PD-1 antibody (10 mg/kg each, twice a week). (B) Tumor tissues were weighed. Data are presented as mean tumor volume ± SEM. **P < .01, and ***P < .001 by one-way ANOVA.
LBL-007 pharmacokinetics in monkeys
Serum LBL-007 concentrations in cynomolgus monkeys were measured by ELISA to calculate the relevant pharmacokinetic parameters of LBL-007 in vivo. Serum LBL-007 levels were below the detection limit (7.813 ng/ml) before LBL-007 administration. The main pharmacokinetic parameters of LBL-007 in monkeys after a single intravenous injection are summarized in Table 2. The data illustrated that an injection dose of 10 mg/kg in monkeys had longer half-life and larger volume of distribution. The area under the concentration (AUC) and the clearance (CL) were proportional to the injection dose. There is a difference in the concentration-time profiles between different injection doses. At 672 hours after injection does of 3 mg/kg, the serum concentration of LBL-007 was still 10 μg/mL, suggesting that LBL-007 was highly stable in vivo (Figure 6).
Table 2.
Pharmacokinetic parameters of LBL-007 antibody levels in monkeys.
| t1/2 |
C5min |
AUClast |
AUC0-∞ |
V |
CL |
MRTlast |
||
|---|---|---|---|---|---|---|---|---|
| dose (mg/kg) |
(h) | (µg/mL) | (h*µg/mL) | (h*µg/mL) | (mL/kg) | (mL/h/kg) | (h) | |
| 3 | Mean | 275.31 | 73.22 | 9013.81 | 11364.48 | 109.27 | 0.27 | 229.32 |
| SD | 13.87 | 17.27 | 2327.64 | 2970.21 | 33.84 | 0.07 | 5.17 | |
| 10 | Mean | 335.31 | 194.4 | 26601.72 | 34144.9 | 148.28 | 0.31 | 224.5 |
| SD | 36.9 | 53.34 | 9867.22 | 12139.57 | 37.13 | 0.11 | 5.52 | |
| 30 | Mean | 193.52 | 616.15 | 63570.08 | 69937.95 | 119.44 | 0.43 | 203.43 |
| SD | 31.95 | 122.23 | 934.48 | 3042.52 | 14.57 | 0.02 | 2.54 |
Pharmacokinetic parameters were calculated using a non-compartmental analysis. T1/2, half-life; C5min, concentration of 5 min; AUC, The area under the concentration versus time curve; V, apparent volume of distribution; CL, clearance; MRT, Mean Residence Time.
Figure 6.
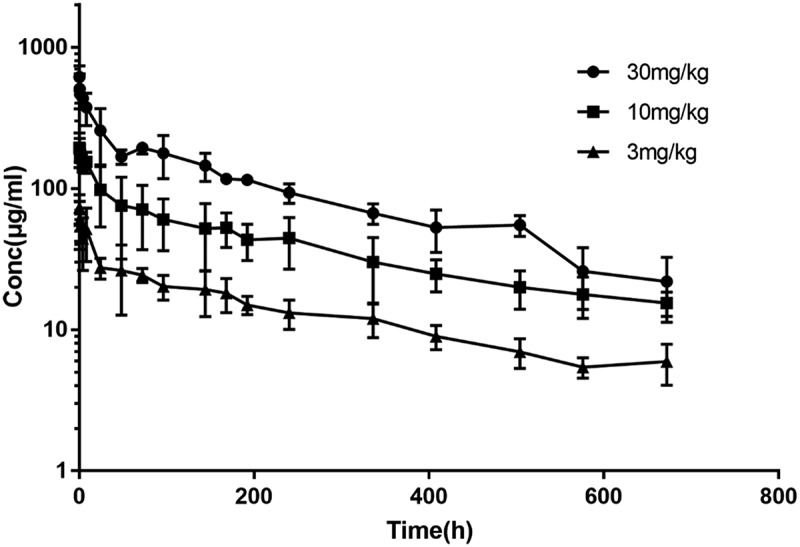
Plasma levels of LBL-007 after intravenous administration into monkeys.
LBL-007 was intravenously injected into cynomolgus monkeys (n = 2/group) at different concentrations, and plasma levels of LBL-007 were assessed at the 18 indicated time points.
Discussion
Cancer immunotherapy has recently made rapid advances, and improved immune checkpoint antagonists for the treatment of various solid tumors, including anti-CTLA-4 ipilimumab, and anti-PD-1 pembrolizumab, have been developed.22-25 However, in addition to drug resistance and side effects,26-28 the response rate to ipilimumab was only 15% in patients, and that to anti-PD-1/PD-L1 antibodies was less than 40%.29 Additional immune checkpoint blockade may increase the response rate to current PD-1/PD-L1 therapy, and has gained increasing attention.30,31 Phage display is widely used in human antibodies screening and structure optimization benefitting from several advantages, such as fast and easy use, and diversity. In a previous study, we used LoxP-Cre system-mediated single-cell recombination to construct a natural phage antibody library,32 which allowed the identification of complex and varied antigens. In this study, we used this natural library to screen anti-human LAG-3 antibodies and successfully identified a novel antibody, LBL-007, which showed high affinity to LAG-3 antigen and blocked the functions and downstream signaling of LAG-3 in cells.
LAG-3 can bind to MHC class II, and even has a higher binding affinity for these molecules than CD4.8 LSECtin can also mediate tumor immune escape.13 It is likely that LAG3-ligand interaction is a critical aspect of its inhibitory function. We found that LBL-007 blocked the binding of LAG-3 and MHC class II molecules, which could activate T cell receptor signal pathway and increase IL-2 production in Jurkat-NFAT-LAG-3 cells. LBL-007 was able to block LAG-3 binding to LSECtin using in vitro experiment. However, the exact pathway by which LAG-3 transmits inhibitory signals is still unclear. Overall, LBL-007 has the ability to block the binding of LAG-3 to two ligands simultaneously, consequently relieving the immunosuppressive function of LAG-3. Blockade of these two LAG-3–ligand interactions might broaden the antitumor effect of LBL-007.
As shown on clinicaltrials.gov, numerous LAG-3-modulating agents have entered clinical trials. For example, IMP321 (Prima BioMed/Immutep) was designed as an antigen-presenting cell activator. Relatlimab (Bristol-Myers Squibb) is a human IgG4 antibody identified from a mouse-source phage library screen, while LAG525 (Novartis) and MK-4280 (Merck) are humanized antibodies. LBL-007 is a novel anti-LAG-3 antibody obtained from a large human phage library. It showed good binding specificity and stable metabolic parameters in monkeys. In this study, LBL-007 showed better uptake into cells than relatlimab analog, suggesting that LBL-007 may have broader applications. Also, it should be noted that relatlimab analog was prepared in house. In view of different expression system or preparation processes, it cannot be inferred the performance of relatlimab analog is identical to the originator’s relatlimab.
Anti-LAG-3 and anti-PD-1 antibodies have shown synergistic antitumor activities in several mouse cancer models.33,34 We used a transgenic mouse model that expressed human LAG-3 and mouse PD-1, which was appropriate to assess the effects of LBL-007 and anti-mouse PD-1 antibody. We found that tumor growth under LBL-007 therapy was significantly lower than under IgG control treatment, indicating that LBL-007 alone possesses a certain degree of anti-tumor activity in vivo. The combination of anti-mouse PD-1 antibody and LBL-007 had an even better inhibitory effect on tumor growth than the monotherapies, which was consistent with previous findings.17,18 LAG-3 is often co-expressed with PD-1 on exhausted T cells, and targeting of both using anti-LAG-3 and anti-PD-1 antibodies was very effective at reinvigorating T cells and eliminating mouse tumors.16,35 Accumulating evidence suggests that multiple immune checkpoint inhibitors can optimize antitumor immunity. However, the mechanism by which PD-1 and LAG-3 pathways work together to inhibit T-cell functions is not known.
In conclusion, we identified and characterized a fully human anti-LAG-3 antibody, LBL-007. LBL-007 could suppress the growth of mouse colorectal cancer cells in vivo either as a monotherapy or in combination with PD-1 antibody. Our data provide support for preclinical-to-clinical development of LBL-007 as a promising combinatorial strategy for cancer immunotherapy. Further studies are needed to uncover the mechanism of LAG-3 signal transduction and the interaction between LAG-3 and PD-1.
Materials and methods
Cell lines and reagents
The human African-American Burkitt’s lymphoma Daudi cell line CCL-213, the Burkitt’s lymphoma Raji cell line CCL-86, and the human acute T cell leukemia Jurkat cell line TIB-152 were obtained from the American Type Culture Collection (ATCC). The cell lines were authenticated by short-tandem repeat fingerprinting by Beijing Microread Genetics Company. HEK293 cells were purchased from National Infrastructure of Cell Line Resource (China). These cells were cultured in Roswell Park Memorial Institute (RPMI)-1640 medium supplemented with 10% fetal bovine serum (FBS) (10099–141, Gibco). Jurkat cells and HEK293 cells were transduced with human LAG-3 cDNA (NM_014143) by electroporation. C57BL/6 murine colon adenocarcinoma MC38 cells were from National Infrastructure of Cell Line Resource and transduced with the ovalbumin (OVA) gene by retroviral transduction. The cells were subsequently cloned by limiting dilution. Clones highly expressing OVA protein were selected using an ELISA kit (CEB459Ge, Cloud Clone Corp.). The MC38-OVA cells were maintained in complete medium with 10% FBS and 4μg/mL puromycin (A11138–03, Gibco). The CHO-K1 cell line CCL-61 (ATCC) was maintained in F-12K medium containing 10% FBS and was transfected with human LAG-3 cDNA by electroporation. This cell line was not authenticated. All cells were cultivated at 37°C in 5% CO2. Anti-mouse PD-1 antibody (BE0146) was purchased from BioXcell. Relatlimab analog, LBL-007, and control IgG were expressed using the Expi293™ Expression System (A14635, Thermo Fisher Scientific).
Animals
Six-week-old female C57BL/6-hLAG-3 transgenic mice were obtained from Shanghai Model Organisms Center, Inc. (Shanghai, China). Cynomolgus monkeys were purchased from Primate Experimental Animal Development Co. (Hainan, China). All animal experiments were conducted in accordance with the guidelines of the Committee on Animals of the Chinese Academy of Sciences.
Phage display library construction and phage selection
We used a non-immunized human scFv phage library,36 which was constructed from mixed PBMCs of 20 donors to achieve a titer of 2 × 1011, for lead-scFv isolation by phage display. LAG-3 (LA3-H5222, Acrobiosystems) at 10 mg/mL in phosphate-buffered saline (PBS) was immobilized onto immunotubes (Nunc) at 4°C overnight. The tube was incubated with the phage antibody library at 37°C for 3 h, and then washed with PBST (PBS containing 0.1% Tween-20) to remove non-specific bindings. LAG-3-binding phages were isolated by three sequential rounds of panning with glycine-hydrochloric acid (0.2 M, pH 2.2). XL1-blue bacteria (200150, Agilent) were added into the mixtures for phage antibody recovery in three rounds, and each selection round comprised a cycle of phage binding to immobilized antigen, elution of the bound phage, and propagation of the enriched phage. Antibody-positive phage clones were assessed by ELISA and all antibody-positive phage clones were sequenced for confirmation.
Production of anti-human LAG-3 antibodies
Anti-human LAG-3 antibodies were produced using a standard protocol. In brief, we constructed the VH and VL regions of the scFv and cloned them into a human IgG4 heavy chain vector and light chain vector, respectively, using a previously reported methodology.37 To construct a relatlimab analog for research, genes encoding the heavy and light chains of relatlimab (published patent US_9505839_B2) were inserted into the expression vector pcDNA3.1. Humanized antibody expression vectors were co-transfected into 293F cells using the Expi293 expression system following the manufacturer’s instructions. We collected the culture supernatants for sandwich ELISA of antibody production. The sandwich ELISA used a goat anti-human IgG (2048–05, Southern Biotechnology) as the capture antibody and goat anti-human kappa-horseradish peroxidase (HRP) (2060–05, Southern Biotechnology) as the detecting antibody. The recombinant antibodies from culture supernatants were purified by protein A affinity chromatography and the antibody concentrations were measured with a spectrophotometer (NanoDrop 8000, Thermo Fisher Scientific) at 280 nm and confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and western blotting.
ELISA
ELISA was used to assess the specificity of binding between LBL-007 and human LAG-3 and the ability of the anti-LAG-3 antibodies to inhibit human LAG-3 binding to human LSECtin. Specifically, human LAG-3 protein or recombinant human CD4 protein (10400-H08H1, Sinobiological) were immobilized onto 96-well plates at 4°C overnight and nonspecific binding sites were blocked by incubation with 1% bovine serum albumin (BSA, 810652, Merck Millipore) in PBS at 37°C for 3 h. After blocking, the plates were washed three times with PBST. The different anti-LAG-3 antibodies and IgG control were serially diluted in binding buffer (PBS containing 0.05% Tween-20 and 0.5% BSA). LSECtin (CLG-H5250, Acrobiosystems) was biotinylated with an EZ-Link™Sulfo-NHS-LC-Biotinylation Kit (21435, Thermo Fisher Scientific) and was mixed with the serially diluted anti-LAG-3 antibodies and human IgG control. The mixtures were added into culture plates, incubated at 37°C for 1 h, and then washed three times with PBST. Then, streptavidin-HRP (DY998, R&D Systems) was added and the plates were incubated at room temperature for 30 min. After three washes with PBST, tetrabenzidine substrate (TMB, CW0050,CWBIO) was added for color development and the reactions were stopped with 1 M H2SO4. The absorbance at 450–620 nm was determined with a spectrophotometer. Measurements were done in triplicate and the experiment was repeated three times independently.
Reporter gene assays
To test the potency of antibodies in vitro, a reporter assay was used. We used Jurkat-hLAG-3 cells as effector cells and a luciferase reporter Luc2P driven by an NFAT response element (NFAT-RE, E848A, Promega). The target cells were MHC class II-expressing Raji cells. For this experiment, Raji cells, SED (DT303, ToxinTech), and Jurkat-NFAT-LAG-3 cells were added into a 96-well white assay plate. Then serial dilutions (highest assay concentration was 50 μg/mL, 3-fold dilution at 8 points) of test antibody and reference samples were added. After 16–18 h of incubation at 37°C, One-GloTM Luciferase Assay reagent (E6110, Promega) was added into the plate and mixed uniformly. Luminescence was read in a TECAN F200 reader. EC50 values were analyzed with the agonist dose-response non-linear regression fit in GraphPad Prism. Relative potency was calculated by the formula: (EC50 reference antibody/EC50 test antibody) ×100%.
Raji cells were preloaded with ppSED (DP303, ToxinTech). Raji cells and Jurkat-NFAT-LAG-3 cells were added into 96-well white assay plates and mixed uniformly. Eight-point serial dilutions of control IgG and LBL-007 were prepared. The plates were incubated at 37°C in a CO2 incubator. Supernatants were collected after 40 h and IL-2 levels were measured using the Human IL-2 DuoSet ELISA Kit (DY202, R&D) following the manufacturer’s instruction.
Binding to activated t cells and cytokine production
Human PBMCs were isolated from healthy donors of our labs. Participants gave informed written consent and the collection of blood samples for research purposes were approved by the Ethics Committee from our institution. PBMCs cells were collected after 7 days with SEB (BT202, ToxinTech) stimulation and co-cultured with antibodies at a series of diluted concentrations from 20 to 0.0091μg/mL. Antibodies that bound to human T cells were detected with phycoerythrin (PE)-goat anti-human IgG (2040–09, Southern Biotechnology) by flow cytometry. Human PBMCs were stimulated with SEB for 48 h. LBL-007, relatlimab analog, and IgG4 were added to the medium. After 3 days of incubation, the IL-2 level in the supernatants was measured using the Human IL-2 DuoSet ELISA Kit.
Flow cytometry
CHO-K1/hLAG-3 cell suspensions (1 × 105 cells/sample) were prepared and were incubated with LBL-007, relatlimab analog, or IgG isotype at 4°C for 40 min and washed twice with PBS by centrifugation. Next, a secondary antibody conjugated with fluorescein was added to cells and the mixtures were incubated at 4°C for 40 min in the dark, washed twice with PBS by centrifugation, and then resuspended in PBS for flow cytometry (BD Accuri C5).
To assess the ability of anti-LAG-3 antibodies to inhibit human LAG-3 binding to MHC class II molecules, an in-vitro binding assay was performed. LAG-3 fusion protein, comprising human LAG-3 extracellular domain fused to mouse Fc (hLAG-3-mFc, 16498-H05H, Sinobiological), reacted with Daudi cells. LBL-007 was serially diluted in PBS with 0.5% BSA, and hLAG-3-mFc fusion protein was added. The mixtures were incubated at room temperature for 20 min. Then, 2 × 105 Daudi cells were added and the plates incubated at 4°C for 30 min. The cells were pelleted (3 min, 400 × g), washed once in PBS buffer with 0.5% BSA, and re-pelleted. Binding of hLAG-3-mIg to the Daudi cells was detected using a R-PE-conjugated AffiniPure Goat Anti-Human IgG, Fcγ Fragment Specific (109–116–098, Jackson ImmunoResearch). Then, the cells were washed twice as described above, resuspended in PBS, and analyzed with the BD Accuri C5 flow cytometer. IC50 values were calculated.
Assessment of anti-LAG-3 antibody affinity
The kinetic binding activity of LBL-007 to human LAG-3 protein and cynomolgus monkey LAG-3 protein (LA3-C52A0, Acrobiosystems) was measured using the Octet RED 96 system (Fortebio). LBL-007 was biotinylated using an EZ-LinkTM Sulfo-NHS-LC-Biotinylation Kit. The biotin-labeled LBL-007 was loaded onto pre-equilibrated streptavidin biosensors. Human and cynomolgus monkey LAG-3 proteins at concentrations ranging from 3.125 nM to 100 nM were bound to the antibody. The binding between a ligand immobilized on the biosensor tip surface and an analyte in solution produces changes the thickness of the biological layer, which are measured in real time. Then, the data were fitted to a 1:1 binding model using Octet software.
Antibody internalization
Endocytosis of antibodies was determined using Jurkat-NFAT-LAG-3 and HEK293-LAG-3 cells. In particular, LBL-007 and relatlimab analog were first conjugated with pHAb Reactive Dye (G9845, Promega) following the manual instructions. The pHAb Reactive Dye is a hydrophilic bright pH-sensor dye that becomes fluorescent at acidic pH, and can be used for high-throughput antibody internalization screening assays. Upon receptor-mediated internalization, antibody-pHAb conjugates track through the endosomal and lysosomal systems, the pH drops, and dyes become highly fluorescent.20,38 Antibody concentration and dye-to-antibody ratio were calculated as follows: antibody concentration (mg/ml) = A280-(A532 × 0.256)/1.4; and the dye-to-antibody ratio = A532 × 150000/Ab concentration×75000. The cells were incubated with the dye-conjugated LBL-007 and relatlimab analog at 10 µg/mL per 1 × 105 cells at 37°C for 0.5, 2, 5, or 24 h. After two washes with PBS, the cells were collected by centrifugation and the fluorescence signal was measured by flow cytometry.
Animal experiments
C57BL/6-hLAG-3 transgenic mice expressing human LAG-3 and mouse PD-1 were housed in alternating 12-h light/dark cycles and received specific pathogen-free mouse chow and sterile drinking water ad libitum in the animal facility. To generate colon adenocarcinoma tumor model, forty 6-week-old female C57BL/6-hLAG-3 transgenic mice were injected subcutaneously with MC38-OVA cells (1 × 106) into the right flank. Tumor formation and growth were monitored twice per week using a caliper and tumor mass in mm3 was calculated [length×(width)2]/2. When the average tumor volume reached ~80 mm3, mice were randomly grouped (n = 8 mice each group), and were intraperitoneally injected with antibodies twice a week. Groups 1 to 5 were administered the vehicle (PBS), isotype human IgG4 control, anti-mouse PD-1, LBL-007, and a combination of anti-mouse PD-1 antibody and LBL-007 at 10 mg/kg body weight, respectively. On day 31, the mice were sacrificed by cervical dislocation after anesthesia. Tumors were collected and photographed.
Pharmacokinetic analysis
Pharmacokinetic experiments were conducted in cynomolgus monkeys in three groups for low, medium, and high doses of LBL-007 of 3, 10, and 30 mg/kg, respectively (n = 2 monkeys per group of each male and female). LBL-007 was administered as a single intravenous injection and thereafter, blood samples were collected at 5 min, 30 min, 1 h, 4 h, 8 h, 24 h, 48 h, 72 h, 96 h, 144 h, 168 h, 192 h, 240 h, 336 h, 408 h, 504 h, 576 h, and 672 h. Serum levels of LBL-007 were determined by the National Chengdu Center for Safety Evaluation of Drugs and the relevant pharmacokinetic parameters were calculated using PhoenixWinNonlin (Pharsight) 6.4 (Certara, USA).
Statistical analysis
The data are expressed as the mean ± SD and were analyzed with unpaired Student’s t-tests or one-way variance (ANOVA). *P < .05, **P < .01 or ***P < .001 was considered statistically significant.
Funding Statement
This work was supported by the National Natural Science Foundation of China [31470897]; Grant of Navy General Hospital of PLA [CXPY201817];
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Triebel F, Jitsukawa S, Baixeras E, Roman-Roman S, Genevee C, Viegas-Pequignot E, Hercend T.. LAG-3, a novel lymphocyte activation gene closely related to CD4. J Exp Med. 1990;171:1393–405. PMID: 2187904. doi: 10.1084/jem.171.5.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baixeras E, Huard B, Miossec C, Jitsukawa S, Martin M, Hercend T, Auffray C, Triebel F, Piatier-Tonneau D.. Characterization of the lymphocyte activation gene 3-encoded protein. A new ligand for human leukocyte antigen class II antigens. J Exp Med. 1992;176:327–37. PMID: 1380059. doi: 10.1084/jem.176.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Workman CJ, Rice DS, Dugger KJ, Kurschner C, Vignali DA. Phenotypic analysis of the murine CD4-related glycoprotein, CD223 (LAG-3). Eur J Immunol. 2002;32:2255–63. PMID: 12209638. doi:. [DOI] [PubMed] [Google Scholar]
- 4.Andrews LP, Marciscano AE, Drake CG, Vignali DA. LAG3 (CD223) as a cancer immunotherapy target. Immunol Rev. 2017;276:80–96. PMID: 28258692. doi: 10.1111/imr.12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huard B, Tournier M, Hercend T, Triebel F, Faure F. Lymphocyte-activation gene 3/major histocompatibility complex class II interaction modulates the antigenic response of CD4+ T lymphocytes. Eur J Immunol. 1994;24:3216–21. PMID: 7805750. doi: 10.1002/eji.1830241246. [DOI] [PubMed] [Google Scholar]
- 6.Prigent P, El Mir S, Dreano M, Triebel F. Lymphocyte activation gene-3 induces tumor regression and antitumor immune responses. Eur J Immunol. 1999;29:3867–76. PMID: 10601994. doi:. [DOI] [PubMed] [Google Scholar]
- 7.Huang CT, Workman CJ, Flies D, Pan X, Marson AL, Zhou G, Hipkiss EL, Ravi S, Kowalski J, Levitsky HI, et al. Role of LAG-3 in regulatory T cells. Immunity. 2004;21:503–13. PMID: 15485628. doi: 10.1016/j.immuni.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 8.Huard B, Prigent P, Tournier M, Bruniquel D, Triebel F. CD4/major histocompatibility complex class II interaction analyzed with CD4- and lymphocyte activation gene-3 (LAG-3)-Ig fusion proteins. Eur J Immunol. 1995;25:2718–21. PMID: 7589152. doi: 10.1002/eji.1830250949. [DOI] [PubMed] [Google Scholar]
- 9.Liu W, Tang L, Zhang G, Wei H, Cui Y, Guo L, Gou Z, Chen X, Jiang D, Zhu Y, et al. Characterization of a novel C-type lectin-like gene, LSECtin: demonstration of carbohydrate binding and expression in sinusoidal endothelial cells of liver and lymph node. J Biol Chem. 2004;279:18748–58. PMID: 14711836. doi: 10.1074/jbc.M311227200. [DOI] [PubMed] [Google Scholar]
- 10.Tang L, Yang J, Liu W, Tang X, Chen J, Zhao D, Wang M, Xu F, Lu Y, Liu B, et al. Liver sinusoidal endothelial cell lectin, LSECtin, negatively regulates hepatic T-cell immune response. Gastroenterology. 2009;137:1498–508e1–5 PMID: 19632227. doi: 10.1053/j.gastro.2009.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang YW, Meng XJ. Identification of a porcine DC-SIGN-related C-type lectin, porcine CLEC4G (LSECtin), and its order of intron removal during splicing: comparative genomic analyses of the cluster of genes CD23/CLEC4G/DC-SIGN among mammalian species. Dev Comp Immunol. 2009;33:747–60. PMID: 19166875. doi: 10.1016/j.dci.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang J, Wang H, Wang M, Liu B, Xu H, Xu F, Zhao D, Hu B, Zhao N, Wang J, et al. Involvement of LSECtin in the hepatic natural killer cell response. Biochem Biophys Res Commun. 2016;476:49–55. PMID: 27184407. doi: 10.1016/j.bbrc.2016.05.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu F, Liu J, Liu D, Liu B, Wang M, Hu Z, Du X, Tang L, He F. LSECtin expressed on melanoma cells promotes tumor progression by inhibiting antitumor T-cell responses. Cancer Res. 2014;74:3418–28. PMID: 24769443. doi: 10.1158/0008-5472.CAN-13-2690. [DOI] [PubMed] [Google Scholar]
- 14.Turnis ME, Andrews LP, Vignali DA. Inhibitory receptors as targets for cancer immunotherapy. Eur J Immunol. 2015;45:1892–905. PMID: 26018646. doi: 10.1002/eji.201344413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anderson AC, Joller N, Kuchroo VK. Lag-3, Tim-3, and TIGIT: co-inhibitory receptors with specialized functions in immune regulation. Immunity. 2016;44:989–1004. PMID: 27192565. doi: 10.1016/j.immuni.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baumeister SH, Freeman GJ, Dranoff G, Sharpe AH. Coinhibitory pathways in immunotherapy for cancer. Annu Rev Immunol. 2016;34:539–73. PMID: 26927206. doi: 10.1146/annurev-immunol-032414-112049. [DOI] [PubMed] [Google Scholar]
- 17.Woo SR, Turnis ME, Goldberg MV, Bankoti J, Selby M, Nirschl CJ, Bettini ML, Gravano DM, Vogel P, Liu CL, et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2012;72:917–27. PMID: 22186141. doi: 10.1158/0008-5472.CAN-11-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang RY, Eppolito C, Lele S, Shrikant P, Matsuzaki J, Odunsi K. LAG3 and PD1 co-inhibitory molecules collaborate to limit CD8+ T cell signaling and dampen antitumor immunity in a murine ovarian cancer model. Oncotarget. 2015;6:27359–77. PMID:26318293. doi: 10.18632/oncotarget.4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang RY, Francois A, McGray AR, Miliotto A, Odunsi K. Compensatory upregulation of PD-1, LAG-3, and CTLA-4 limits the efficacy of single-agent checkpoint blockade in metastatic ovarian cancer. Oncoimmunology. 2017;6:e1249561 PMID: 28197366. doi: 10.1080/2162402X.2016.1249561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nath N, Godat B, Zimprich C, Dwight SJ, Corona C, McDougall M, Urh M. Homogeneous plate based antibody internalization assay using pH sensor fluorescent dye. J Immunol Methods. 2016. April;431:11–21 . PMID: 26851520. doi: 10.1016/j.jim.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 21.Bhagwat B, Cherwinski H, Sathe M, Seghezzi W, McClanahan TK, de Waal Malefyt R, Willingham A. Establishment of engineered cell-based assays mediating LAG3 and PD1 immune suppression enables potency measurement of blocking antibodies and assessment of signal transduction. J Immunol Methods. 2018;456:7–14. PMID: 29427592. doi: 10.1016/j.jim.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 22.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. PMID: 22658127. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gravitz L. Cancer immunotherapy. Nature. 2013;504:S1 PMID: 24352357. doi: 10.1038/504S1a. [DOI] [PubMed] [Google Scholar]
- 24.Drake CG, Lipson EJ, Brahmer JR. Breathing new life into immunotherapy: review of melanoma, lung and kidney cancer. Nat Rev Clin Oncol. 2014;11:24–37. PMID: 24247168. doi: 10.1038/nrclinonc.2013.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patnaik A, Kang SP, Rasco D, Papadopoulos KP, Elassaiss-Schaap J, Beeram M, Drengler R, Chen C, Smith L, Espino G, et al. Phase I study of pembrolizumab (MK-3475; anti-PD-1 monoclonal antibody) in patients with advanced solid tumors. Clin Cancer Res. 2015;21:4286–93. PMID: 25977344. doi: 10.1158/1078-0432.CCR-14-2607. [DOI] [PubMed] [Google Scholar]
- 26.Chang SS. Re: efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma: updated results from a phase 1/2 open-label study. J Urol. 2018;199:1110–12. PMID: 29677898. doi: 10.1016/j.juro.2018.02.033. [DOI] [PubMed] [Google Scholar]
- 27.Eggermont AM, Chiarion-Sileni V, Grob JJ, Dummer R, Wolchok JD, Schmidt H, Hamid O, Robert C, Ascierto PA, Richards JM, et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2015;16:522–30. PMID: 25840693. doi: 10.1016/S1470-2045(15)70122-1. [DOI] [PubMed] [Google Scholar]
- 28.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23–34. PMID: 26398076. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gong J, Chehrazi-Raffle A, Reddi S, Salgia R. Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: a comprehensive review of registration trials and future considerations. J Immunother Cancer. 2018;6:8 PMID: 29357948. doi: 10.1186/s40425-018-0316-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dempke WCM, Fenchel K, Uciechowski P, Dale SP. Second- and third-generation drugs for immuno-oncology treatment-The more the better? Eur J Cancer. 2017;74:55–72. PMID: 28335888. doi: 10.1016/j.ejca.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 31.Garber K. Industry ‘road tests’ new wave of immune checkpoints. Nat Biotechnol. 2017;35:487–88. PMID: 28591107. doi: 10.1038/nbt0617-487. [DOI] [PubMed] [Google Scholar]
- 32.Qiao Y, Zhou L, Wang Y, Liu F, Xie P, Chen Y, Zhang D, Zhao X. Screening for antibodies against intact cancer cells with a naive large phage antibody library. Int J Mol Med. 2012;29:37–46. PMID: 21956702. doi: 10.3892/ijmm.2011.800. [DOI] [PubMed] [Google Scholar]
- 33.Matsuzaki J, Gnjatic S, Mhawech-Fauceglia P, Beck A, Miller A, Tsuji T, Eppolito C, Qian F, Lele S, Shrikant P, et al. Tumor-infiltrating NY-ESO-1-specific CD8+ T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proc Natl Acad Sci U S A. 2010;107:7875–80. PMID: 20385810. doi: 10.1073/pnas.1003345107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grosso JF, Goldberg MV, Getnet D, Bruno TC, Yen HR, Pyle KJ, Hipkiss E, Vignali DAA, Pardoll DM, Drake CG. Functionally distinct LAG-3 and PD-1 subsets on activated and chronically stimulated CD8 T cells. J Immunol. 2009;182:6659–69. PMID: 19454660. doi: 10.4049/jimmunol.0804211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wierz M, Pierson S, Guyonnet L, Viry E, Lequeux A, Oudin A, Niclou SP, Ollert M, Berchem G, Janji B, et al. Dual PD1/LAG3 immune checkpoint blockade limits tumor development in a murine model of chronic lymphocytic leukemia. Blood. 2018;131:1617–21. PMID: 29439955. doi: 10.1182/blood-2017-06-792267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sblattero D, Bradbury A. Exploiting recombination in single bacteria to make large phage antibody libraries. Nat Biotechnol. 2000. January;18(1):75–80. PMID:10625396. doi: 10.1038/71958. [DOI] [PubMed] [Google Scholar]
- 37.Li MZ, Elledge SJ. Harnessing homologous recombination in vitro to generate recombinant DNA via SLIC. Nat Methods. 2007. March;4(3):251–56. PMID: 17293868. doi: 10.1038/nmeth1010. [DOI] [PubMed] [Google Scholar]
- 38.Feng Y, Wang Y, Zhu Z, Li W, Sussman RT, Randall M, Bosse KR, Maris JM, Dimitrov DS. Differential killing of CD56-expressing cells by drug-conjugated human antibodies targeting membrane-distal and membrane-proximal non-overlapping epitopes. MAbs. 2016. May-Jun;8(4):799–810. PMID: 26910291. doi: 10.1080/19420862.2016.1155014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


