Abstract
Purpose:
PROTECT, a phase III randomized placebo-controlled study, evaluated pazopanib efficacy and safety in the adjuvant RCC setting. The relationship between pazopanib exposure (Ctrough) and efficacy and safety was evaluated.
Experimental design:
Evaluable steady-state blood trough concentrations were collected from 311 patients at week 3 or 5 (early Ctrough), and 250 patients at week 16 or 20 (late Ctrough). Pazopanib pharmacokinetic (PK) data was analyzed via a population model approach. Relationship between Ctrough or dose intensity and disease-free survival (DFS) was explored via Kaplan-Meier and multivariate analysis. Adverse events (AEs) and AE-related treatment discontinuation proportions were summarized by Ctrough quartiles.
Results:
Most (>90%) patients with early or late Ctrough data started on 600 mg. Mean early and late Ctrough overlapped across dose levels. Patients with higher early Ctrough quartiles achieved longer DFS (adjusted hazard ratio [HR], 0.58; 95% CI, 0.42–0.82; P = 0.002). Patients achieving early or late Ctrough >20.5 µg/mL had significantly longer DFS, not estimable (NE) vs 29.5 months, P = 0.006, and NE vs 29.9 months, P = 0.008, respectively. Dose intensity up to week 8 did not correlate with DFS, consistent with population PK model-based simulations showing overlapping pazopanib exposure with 600 mg and 800 mg doses. The proportion of AE-related treatment discontinuation and grade 3/4 AEs, with the exception of hypertension, was not correlated to Ctrough.
Conclusions:
In the adjuvant setting, higher pazopanib Ctrough was associated with improved DFS, and did not increase treatment discontinuations or grade 3/4 AEs with the exception of hypertension.
Keywords: kidney cancer, pharmacokinetics, exposure–response
Statement of translational relevance:
Currently no adjuvant treatments are approved for locally advanced renal cell carcinoma (RCC) to reduce the risk of disease recurrence following surgical resection of the primary tumor. In the PROTECT study, adjuvant pazopanib at 600 mg daily following nephrectomy did not meet the primary endpoint of improved disease-free survival (DFS) compared with placebo. The current analyses of pazopanib exposure (Ctrough) showed that higher pazopanib exposure was associated with improved DFS, without an increase in grade 3/4 adverse events with the exception of hypertension. Pharmacokinetic simulations showed overlapping pazopanib exposure with 600 mg and 800 mg doses. The results suggest that patients achieving higher pazopanib Ctrough derived more clinical benefit from adjuvant pazopanib therapy.
Introduction
Currently no adjuvant treatments are approved for locally advanced renal cell carcinoma (RCC) to reduce the risk of disease recurrence following surgical resection of the primary tumor. Up to 40% of these patients experience recurrence (1). Agents targeting the vascular endothelial growth factor receptor (VEGFR) are effective for patients with advanced RCC, and have recently been investigated in phase III trials in the adjuvant setting (2–4).
Pazopanib is a VEGFR tyrosine kinase inhibitor (TKI) approved for the treatment of advanced RCC at the daily dose of 800 mg (5, 6). The randomized, placebo-controlled phase III PROTECT study () evaluated pazopanib as an adjuvant treatment for locally advanced RCC following nephrectomy (4). The starting dose was changed from 800 mg daily to 600 mg daily due to a higher-than-expected study treatment discontinuation rate based on a blinded safety monitoring and the assumption that all these discontinuations occurred in the pazopanib arm. This suggested a minimum of 20% discontinuation rate in the pazopanib arm at the time of the safety review (4). At the time the decision to reduce the starting dose was made (based on a blinded safety aggregate), the discontinuation rate was considered high as compared to that observed with pazopanib in the advanced/metastatic setting. The results of the primary endpoint of disease-free survival (DFS) in ITT600 showed no benefit over placebo in the adjuvant setting. Patients in ITT800 had a 31% reduced risk of recurrence or death (secondary endpoint of the study).
In advanced or metastatic RCC, the relationship between exposure and efficacy endpoints have been established for several other approved VEGFR-TKIs. Model-predicted sunitinib steady-state AUC correlated with longer time to tumor progression and overall survival (OS), and was significantly associated with the probability of an objective response (7). Axitinib exposure-response analyses showed that AUC at the end of 4 weeks of study treatment was significantly associated with clinical responses (8, 9). Higher axitinib exposure was associated with a higher probability of response, longer progression-free survival (PFS) and OS, and was an independent predictor of survival (9). Additionally, in patients undergoing axitinib dose-titration, higher AUC was associated with a higher probability of response (9). Pazopanib has also demonstrated a relationship between exposure and efficacy in the advanced RCC setting (10). In several studies, the pazopanib efficacy Ctrough threshold associated with significant increases in PFS and tumor shrinkage in advanced RCC was established as 20.5 µg/mL.
In the current analysis, we aimed to characterize the relationship between pazopanib trough concentrations (Ctrough) and key efficacy endpoint (DFS) and safety endpoints (adverse events [AEs] and AE-related treatment discontinuation).
Patients and Methods
Study design
Detailed description of the PROTECT study design has been previously reported (4). In summary, 1538 patients with resected nonmetastatic clear cell RCC that was pT2G3–4N0, pT3-T4 GanyN0, or pTanyGany, N1 (as per tumor, node, metastasis [TNM] classification [AJCC 2010 version] and Fuhrman nuclear grades), were randomly assigned 1:1 to pazopanib or matching placebo for one year. The study was initiated with a starting dose of 800 mg per day, and 198 and 205 patients were assigned to the pazopanib and placebo groups, respectively (ITT800). Due to a higher-than-expected treatment discontinuation rate, a protocol amendment reduced the starting dose to 600 mg per day, and 571 and 564 patients were assigned to pazopanib and placebo, respectively (ITT600). The primary endpoint was amended to investigator-assessed DFS in the ITT600 population; DFS was assessed in the ITT800 population as a secondary endpoint. Safety assessments were conducted on patients randomly assigned to a starting dose of 600 mg or 800 mg who received ≥1 dose of study treatment. Patients in ITT600 could be maintained at this dose or escalated to 800 mg after 8–12 weeks based on safety and tolerability. Dose reductions in ITT600 and ITT800 were allowed for management of treatment-related toxicity.
Local institutional review boards approved the study. The study was conducted in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki, and all patients provided written informed consent.
Pazopanib exposure in the PROTECT study
Following the introduction of the 600 mg starting dose, predose blood sampling was performed to determine steady-state pazopanib Ctrough, collected at week 3 or 5 (early Ctrough) and week 16 or 20 (late Ctrough). In addition to blood sampling for trough concentrations, serial pharmacokinetic (PK) sampling (3 additional time points) was conducted in 68 pazopanib-treated subjects during weeks 3 or 5 at 1–2 hours, 3–4 hours, and 6–8 hours after administration of study treatment. Pazopanib plasma concentrations were determined via a validated liquid chromatography tandem mass spectrometry (LC-MS/MS) method. Briefly, pazopanib and the internal standard (IS) were extracted from samples using protein precipitation in human plasma. The calibration curves were linear over the range of 100 (lower limit of quantification [LLOQ]) to 50000 (upper limit of quantification [ULOQ]) ng/mL in human plasma.
Patients were evaluable for PK analysis if they received ≥1 dose of pazopanib and had a corresponding PK sample. Predose samples must have been collected before the next pazopanib dose and 20–28 hours after the last dose administration; steady-state samples required at least 10 consecutive daily pazopanib doses without a dose change or interruption. Evaluable steady-state Ctrough was collected from 311 patients at week 3 or 5 and from 250 patients at week 16 or 20.
Population PK analysis
The pazopanib population PK model was previously developed with historical PK data including 451 subjects (healthy volunteers and cancer patients) and 4011 PK samples (rich/sparse sampling) collected after a single intravenous infusion of 5 mg (7 subjects) and daily oral doses ranging from 50 to 2000 mg (Supplementary Table S1). Starting doses of 800, 600, and 400 mg represented 66%, 2.2%, and 13% of historical PK samples, respectively. The pazopanib historical population PK model was a two-compartment disposition model with delayed first-order absorption and first-order elimination including an oral bioavailability decreasing with dose and time (11).
The historical population PK model was used to compute empirical Bayes estimates (EBEs) of pazopanib plasma concentrations for each patient having evaluable PK samples in the PROTECT study given their actual dosing/sampling time and covariate data. The predicted and observed pazopanib plasma concentrations for the PROTECT study were compared by visual inspection of diagnostic plots (prediction-corrected visual predictive check, normalized prediction distribution errors, observed vs predicted concentrations). If the historical population PK model was inadequate to describe PK data in the PROTECT study (suggesting different PK properties in the adjuvant population), then model parameters were re-estimated using a dataset containing both historical and PROTECT PK data, which included a covariate effect for the PROTECT population to quantify any deviation from historical data. The final population PK model was used to simulate steady-state exposure metrics (area under the curve from 0 to 24 hours [AUC0–24h,ss], maximum concentration [Cmax,ss], and Ctrough,ss) for continuous daily oral doses of 200, 400, 600 or 800 mg. Model parameters were estimated by a nonlinear mixed effect modeling approach using Monolix 4.3.2.
Exposure/dose-efficacy analyses
The relationship between Ctrough and DFS was evaluated using Kaplan-Meier curves of DFS by early and late Ctrough quartiles. Furthermore, a multivariate Cox proportional hazard regression analysis for DFS using TNM staging, Fuhrman nuclear grade, and both early and late Ctrough as covariates was performed. A similar Cox proportional hazard regression analysis for DFS using TNM staging, Fuhrman nuclear grade, and either early or late Ctrough was also performed. DFS was compared in patients with early and late Ctrough ≤20.5 or >20.5 μg/mL, which is the threshold for pazopanib Ctrough associated with longer PFS and higher tumor shrinkage in advanced RCC (10). Kaplan-Meier curves were used to plot DFS by > or ≤ median dose intensity up to week 8 to investigate the potential effect of the starting dose on DFS.
Exposure-safety analyses
Exposure-safety relationships were investigated between early Ctrough and the proportion of AEs of interest based on pazopanib’s known safety profile that was reported within the first 12 weeks of treatment, and between late Ctrough and the proportion of AEs occurring later than 12 weeks up to the date of last dose (+28 days). The AEs analyzed included increased alanine aminotransferase (ALT), diarrhea, hypertension, hand-foot syndrome, stomatitis, and cytopenia (thrombocytopenia and leukopenia/neutropenia). The effect of pazopanib exposure on AE-related treatment discontinuations was investigated by comparing the percentage of patients with AE-related treatment discontinuations across Ctrough quartiles.
Results
Pazopanib PK exposure in the PROTECT study
A total of 311 and 250 patients had evaluable samples for early and late Ctrough measurements, respectively. Most patients with available Ctrough samples (>90%) were in the ITT600 group. Among patients in ITT600, 118 (21%) had a protocol-defined dose escalation by week 12. Table 1 summarizes early and late Ctrough by pazopanib steady-state dose. Ctrough values were assigned to a steady-state dose if 10 consecutive doses without dose modifications or interruptions were administered prior to sampling. The geometric mean (geometric coefficient of variation [CV%]) of the early Ctrough at 600 mg was 31.4 µg/mL (56.7%); few early Ctrough samples were collected at steady-state doses of 800 mg or 400 mg given that most patients were on the starting dose of 600 mg at the time of PK sample collection, i.e. Week 3 or 5. Early Ctrough overlapped across dose levels. Because dose escalations (from 600 mg to 800 mg after 8 to 12 weeks based on subject’s tolerability) and dose reductions (from 600 mg to 400 mg to manage treatment-related toxicity) were allowed, a greater proportion of late Ctrough samples were obtained during treatment with 800 mg and 400 mg compared with early Ctrough. The geometric mean late Ctrough overlapped across dose levels, and pazopanib exposure was variable, ranging from 23.2 µg/mL to 28.6 µg/mL, with geometric mean CV% for Ctrough ranging from 56.7% to 70.6% across dose levels (Table 1). The quartiles for pazopanib early and late Ctrough are presented in Supplementary Table S2.
Table 1.
Ctrough by dose level in PROTECTa
| Scheduled sampling time point | Statistic | Pazopanib actual dose | ||
|---|---|---|---|---|
| 400 mg | 600 mg | 800 mg | ||
| Week 3 or 5 (early Ctrough) |
Total number of samples | 20 | 288 | 7 |
| Evaluable samples | 20 | 285 | 6 | |
| Mean, μg/mL (SD) | 40.8 (15.6) | 34.8 (15.4) | 34.5 (22.2) | |
| CV% | 38.2 | 44.3 | 64.4 | |
| Geometric mean (CV%) | 36.4 (61.0) | 31.4 (56.7) | 35.3 (70.6) | |
| Median, μg/mL (range) | 46.6 (6.6, 67.8) | 34.3 (0.0, 8.6) | 37.0 (0.0, 62.6) | |
| Week 16 or 20 (late Ctrough) |
Total number of samples | 73 | 94 | 88 |
| Evaluable samples | 71 | 93 | 86 | |
| Mean, μg/mL (SD) | 27.9 (13.0) | 28.9 (14.2) | 31.8 (15.9) | |
| CV% | 46.7 | 49.0 | 50.0 | |
| Geometric mean (CV%) | 23.2 (116.1) | 25.3 (69.9) | 28.6 (62.6) | |
| Median, μg/mL (range) | 28.2 (0.0, 55.9) | 28.8 (0.0, 75.6) | 29.7 (0.0, 83.2) | |
A PK sample was assigned to a dose level if 10 consecutive doses without dose modification or interruption were administered prior to sampling. Most early Ctrough samples were collected during treatment with 600 mg, but because dose escalations (from 600 mg to 800 mg after 8–12 weeks) and dose reductions were allowed, a greater proportion of late Ctrough samples were obtained at the 400 mg and 800 mg doses compared with early Ctrough.
Abbreviations: CV, coefficient of variation; PK, pharmacokinetic; SD, standard deviation.
Exposure-efficacy analyses
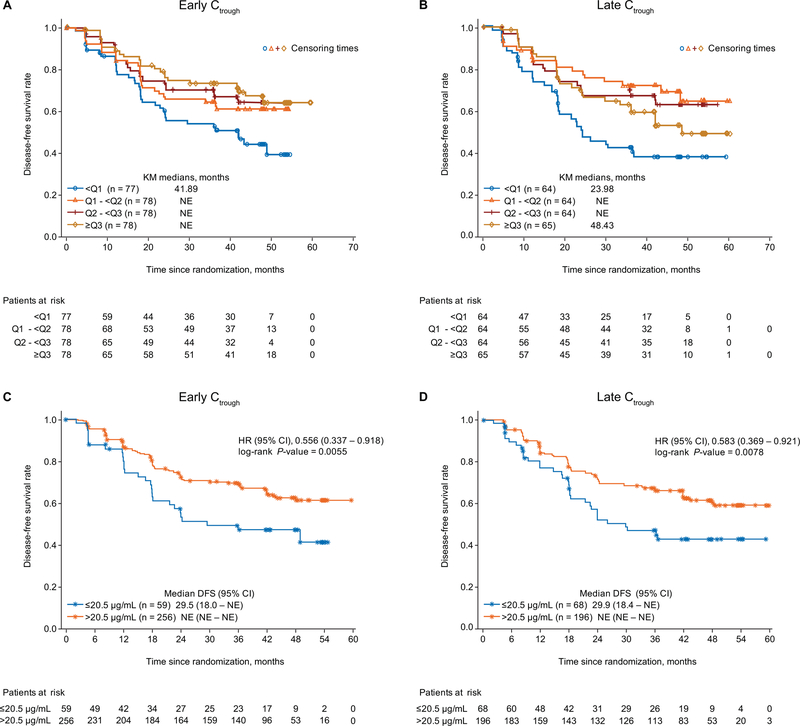
Longer DFS was observed in patients achieving higher early Ctrough quartiles (Figure 1). For late Ctrough, patients in the upper 3 quartiles (Q2 to Q4) trended towards longer DFS compared with the lowest quartile (Q1). In the multivariate Cox regression analysis with both early and late Ctrough, early Ctrough was a significant covariate for DFS (hazard ratio [HR], 0.58; 95% CI, 0.42–0.82; P = 0.002), indicating a correlation between early Ctrough and pazopanib efficacy. To evaluate the independent association of early and late PK exposure with DFS and given the correlation between early Ctrough and late Ctrough (Pearson correlation coefficient = 0.45), two separate Cox regression models were evaluated. The multivariate analysis between DFS and either early or late Ctrough showed both exposure metrics to be significant covariates for DFS (P = 0.000758 and 0.000496 for early and late Ctrough, respectively).
Figure 1.
Relationship between pazopanib Ctrough and DFS is plotted by early (A) and late (B) Ctrough quartiles, and using the 20.5 μg/mL cut-off for early (C) and late (D) Ctrough
Abbreviations: CI, confidence interval; Ctrough, trough concentration; DFS, disease-free survival; HR, hazard ratio; NE, not estimable.
The threshold Ctrough associated with increased PFS and tumor shrinkage in advanced RCC patients, >20.5 µg/mL (10), was achieved by 82% and 75% of patients for early and late Ctrough, respectively. A significantly longer DFS was observed in patients achieving early or late Ctrough >20.5 µg/mL (Figure 1).
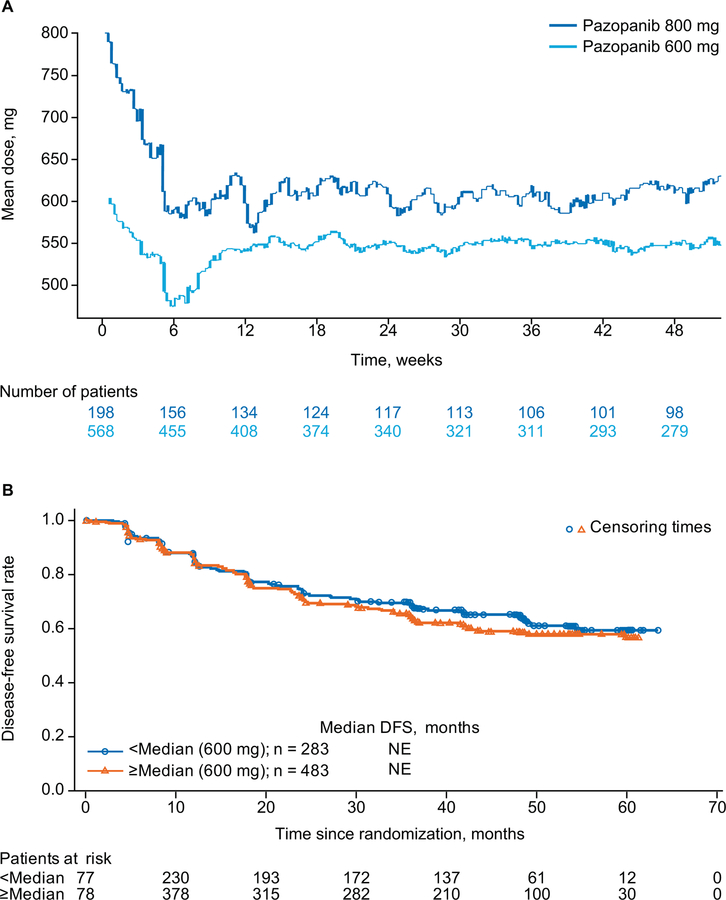
Dose-efficacy analyses
The median dose intensity across the entire treatment period was 593.7 mg in the 600 mg and 648.4 mg in the 800 mg dose cohorts. The mean pazopanib daily dose (dose intensity) decreased in both cohorts during the first ~8 weeks of treatment. Dose intensity increased between weeks 8 and 12 in the 600 mg dose cohort, and from week 12 remained ~550 mg in the 600 mg dose cohort and ~600 mg in the 800 mg dose cohort (Figure 2). The main difference in dose intensity between the two dose cohorts appeared during the initial phase of treatment (in the first 8 to 12 weeks) (Figure 2). Thus, the median dose intensity up to week 8, determined to be 600 mg, was used as a cutoff to explore the relationship between DFS and pazopanib starting dose. No relationship was observed between DFS and dose intensity up to week 8 when using this median cut-off (Figure 2).
Figure 2.
Mean pazopanib daily dose (dose intensity) over time (A) and dose intensity by DFS up to week 8 (B).
Abbreviations: DFS, disease-free survival; NE, not estimable.
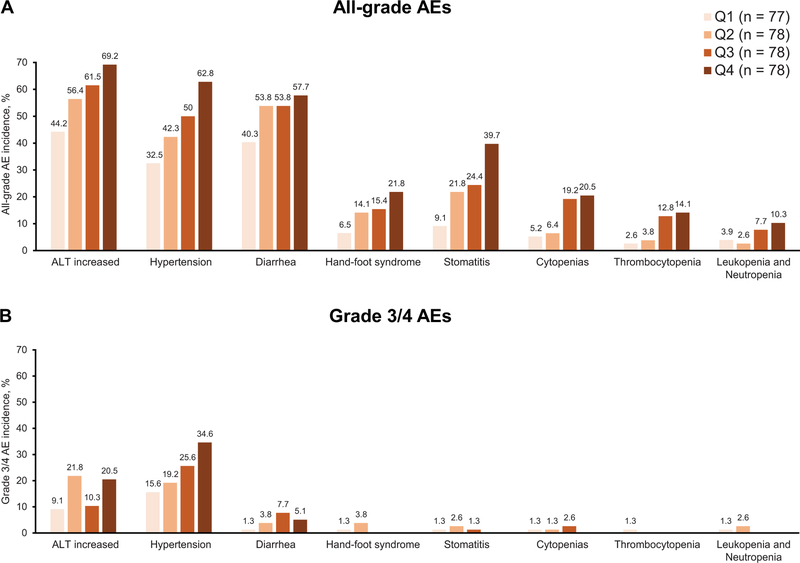
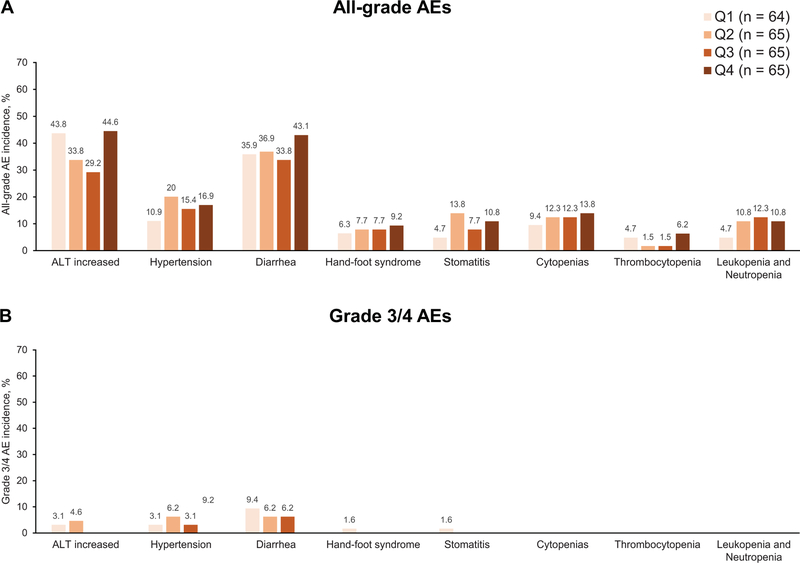
Exposure-safety analyses
The proportion of patients with AE-related discontinuations was similar across early Ctrough quartiles (Table 2). There was a concentration-dependent relationship between pazopanib early Ctrough and the proportion of all-grade AEs during the first 12 weeks of treatment (Figure 3A). No clear relationship was observed between early Ctrough and the proportion of grade 3/4 AEs during the first 12 weeks of treatment, with the exception of hypertension (Figure 3B). There was no apparent relationship between late Ctrough and the proportion of all-grade or grade 3/4 AEs occurring later than 12 weeks up to the date of last dose (+28 days) (Figure 4).
Table 2.
AE-related treatment discontinuations by early Ctrough quartiles
| Quartile | AE-related treatment discontinuations, % |
|---|---|
| Q1 | 26.0 |
| Q2 | 26.9 |
| Q3 | 34.6 |
| Q4 | 28.6 |
Abbreviations: AE, adverse event; Q, quartile.
Figure 3.
Percentage of all-grade (A) and grade 3/4 (B) adverse events of interest based on pazopanib’s known safety profile during the first 12 weeks of treatment by pazopanib early Ctrough quartiles. The number of patients within each quartile are shown in the figure; each patient had one evaluable Ctrough measurement.
Abbreviation: AEs, adverse events; Ctrough, trough concentrations; Q, quartile.
Figure 4.
Percentage of selected all-grade (A) and grade 3/4 (B) adverse events after week 12 up to last date of dose (+28 days) by pazopanib late Ctrough quartiles. The number of patients within each quartile are shown in the figure; each patient had one evaluable Ctrough measurement.
Abbreviation: AEs, adverse events; Ctrough, trough concentration; Q, quartile.
Population PK analysis
The PROTECT population PK dataset comprised 537 patients and 1079 PK samples collected at 600 mg (69%), 400 mg (16%), 800 mg (13%) and 200 mg (2%). Pazopanib exposure in the PROTECT study was not properly described by the historical population PK model. Ctrough and Cmax were under-predicted by about 30%, suggesting a higher oral bioavailability in PROTECT patients despite comparable patient characteristics for most of the explored covariates between PROTECT and historical populations (Supplementary Table S3). Nevertheless, a greater proportion of historical patients had a lower Eastern Cooperative Oncology Group performance status compared with PROTECT patients (36.6% vs. 5.40%), which would be expected in the advanced/metastatic disease setting.
Therefore, model parameters were re-estimated using a combined dataset of historical and PROTECT data. Including a covariate effect on oral bioavailability for the PROTECT population led to adequate description of pazopanib Ctrough and Cmax when considering a 30% higher exposure compared with the historical population. Inspection of prediction-corrected visual predictive check plots revealed good agreement between median observed Ctrough and the 90% prediction interval of simulated median Ctrough, confirming the final model was appropriate to describe pazopanib PKs in the PROTECT study (Supplementary Figure S1). Final parameter estimates are shown in Supplementary Table S4. All model parameters were estimated with good precision (% relative standard error < 12%). Inter-subject variability was high on absorption parameters (73–92%) and less pronounced on clearance (~ 50–60%). Absolute oral bioavailability was estimated at 21% after repeated administration of 800 mg, to be 40% higher at 400 mg than 800 mg, and 30% lower at steady state than after single administration. Co-administration with food was estimated to decrease the absorption rate constant by 62% and to increase oral bioavailability by 2.3- and 2.9-fold after a single administration of 400 and 800 mg, respectively. Co-administration of gastric pH-elevating agents led to a 12% decrease of pazopanib oral bioavailability and was considered clinically not relevant.
Because of the limited early Ctrough samples available at the 800 mg dose (n=7), a population PK model-based simulation was used to explore the impact of dose and patient population (historical vs. PROTECT) on pazopanib PKs by deriving steady-state exposure metrics (AUC0–24h,ss; Cmax,ss; Ctrough,ss) under the fasted state. The comparison of simulated steady-state exposure metrics by week 4 across dose levels (200 mg to 800 mg) and patient population (historical vs. PROTECT) is shown in Supplementary Figure S2. Simulated mean values of exposure metrics at steady state obtained after 800 mg were less than 2.5 fold higher compared with values after 200 mg, reflecting the dose-dependent exposure of pazopanib driven by solubility-limited absorption. Simulated mean values of steady-state exposure metrics were on average 25% higher after 600 mg and 30% higher after 800 mg in PROTECT compared with historical pazopanib trials. In the PROTECT population, the simulated values of steady-state exposure metrics were on average only 17% higher at 800 mg compared with 600 mg.
Discussion
The exposure response analysis presented in the current report from the PROTECT study, primarily based on data from patients starting pazopanib at 600 mg, indicated a correlation between higher early Ctrough and longer DFS. Patients who achieved early or late Ctrough >20.5 μg/mL, the efficacy threshold identified for advanced RCC (10), had longer DFS than patients with Ctrough ≤20.5 μg/mL. Dose intensity in the first 8 weeks in PROTECT was not correlated to DFS, suggesting that a higher pazopanib starting dose does not explain the favorable reduction in the relative risk of relapse achieved in ITT800. Therefore, the current exposure-response analysis suggests that some patients achieve higher pazopanib Ctrough, which is associated with improved DFS, regardless of whether the starting dose was 600 mg or 800 mg, and regardless of subsequent dose reduction. This finding is consistent with the exposure-response relationships for pazopanib, sunitinib, and axitinib in the advanced/metastatic RCC setting where clinical outcome was correlated to higher PK exposure (7–10). Further clinical testing could be warranted to identify and confirm specific target thresholds for VEGF-TKIs, which may help guide PK-guided dosing vs standard VEGF-TKIs treatment. It is important to highlight that at this point, the available data does not support VEGF-TKIs treatment discontinuation for patients with a Ctrough below a certain threshold if they are still achieving clinical benefit from treatment. The minor role of pazopanib starting dose on clinical outcome is supported by the solubility-limited absorption of pazopanib, the high inter-individual variability in pazopanib exposure (~60–70%), the overlapping pazopanib exposure between 600 and 800 mg as predicted by the population PK model simulations as well as the similar DFS rates at yearly time points between pazopanib-treated patients in ITT800 and ITT600 (4). Our finding that pazopanib Ctrough exposure, but not pazopanib prescribing dose, is crucial for improved DFS in the adjuvant setting in RCC patients, could be the primary driver of discrepancies in outcomes of the recent adjuvant VEGF--TKI trials (2–4).
Two other phase III studies evaluating the efficacy and safety of VEGFR-TKIs in the adjuvant RCC setting have recently been reported (2, 3). Adjuvant sunitinib in the S-TRAC study demonstrated a statistically significant improvement in DFS over placebo (P = 0.03) (3). The starting dose in this study was maintained at the same schedule approved in advanced RCC (50 mg daily on a 4-week on/2-week off schedule). In the ASSURE study, neither sunitinib nor sorafenib improved DFS compared to placebo (2). However, in contrast to S-TRAC and similar to PROTECT, a reduced starting dose was implemented partway through the ASSURE study to reduce treatment-related discontinuations (2). This pattern could be mistakenly perceived as if the higher starting dose explains a primary cause for the disparate outcome between adjuvant trials. However, sub-analyses of ASSURE suggest an absence of treatment effect in both patients starting at the full and reduced doses (12). Consistent with findings from PROTECT, quartiles of dose intensity per cycle did not correlate with DFS for either sunitinib or sorafenib in a subanalysis of high-risk clear cell RCC patients in ASSURE (12). These results suggest that a higher starting dose may not be the primary driver for the disparate outcome across adjuvant RCC trials. However, given the high inter-patient variability in VEGF-TKIs exposure and the overlapping exposure expected from the lower and higher doses tested (for example, only 17% higher pazopanib exposure from the 800 mg vs 600 mg dose), the disparate outcomes could be simply due to different exposure levels in different trials. For example, pazopanib exposure was 25%−30% higher in PROTECT compared with historical pazopanib trials despite comparable patient characteristics for most of the explored covariates in both PROTECT and historical populations. The underlying cause for higher pazopanib exposure in PROTECT is not clearly understood. To our knowledge, neither S-TRAC nor ASSURE trials collected PK data and therefore, the exposure levels in these trials are unknown. Further clinical testing could be warranted to understand sunitinib exposure levels in the adjuvant setting.
In PROTECT, higher early Ctrough was associated with a greater proportion of all-grade AEs during the first 12 weeks. Mild adverse events (grade 1 and 2) usually do not meet the protocol-defined discontinuation criteria, and therefore are unlikely to result in a difference in discontinuation rate. Despite the relationship observed between Ctrough and the proportion of all-grade AEs, the proportion of on-treatment mild toxicities (grade 1/2) was similar overall in the 2 dose groups (33% and 38% in the 800 mg and 600 mg groups, respectively), suggesting that the higher dose is not associated with an increase in the proportion of mild adverse events. The higher-dose group was associated with slightly higher grade 3/4 toxicity (66% vs 59% in the 800 mg group and the 600 mg, respectively). The difference in grade 3/4 toxicity did not translate into a higher discontinuation rate (39% vs 35% in the 800 mg group and the 600 mg, respectively) (4). Furthermore, patients in both dose groups had a similar time on study drug—52% by month 9 and 49 % by month 12. The similar proportion of all-grade AEs, grade 3/4 safety profile, and time on study treatment in the two dose groups is consistent with the overlapping exposure expected due to the solubility-limited bioavailability of pazopanib (steady-state exposure only 17% higher at 800 mg compared with 600 mg).
However, there was no correlation between Ctrough and the proportion of grade 3/4 AEs or AE-related treatment discontinuations, with the exception of grade 3/4 hypertension. The increased proportion of grade 3/4 hypertension with increasing Ctrough observed in PROTECT is consistent with results from a phase II study in advanced RCC (10). Hypertension is a common on-target AE with VEGFR-TKIs (13,14), and is well managed by dose reduction/interruption and/or treatment with anti-hypertensive agents (15). Only 3% of patients permanently discontinued pazopanib due to hypertension in the 600 and 800 mg cohorts in PROTECT (4), suggesting that hypertension was clinically well managed in this study.
At the time of the blinded aggregate safety review, ALT elevation was the most common single AE leading to withdrawal of study treatment and was one of the main drivers in reducing the starting dose during the study from 800 mg to 600 mg. The exposure-safety analysis did not reveal a clear relationship between pazopanib exposure and ALT elevation. This is in line with the similar percentage of all-grade ALT increased (35% and 33%) and treatment discontinuation due to ALT elevation (16% and 18%) in the Safety600mg and Safety800mg populations, respectively (4). A phase II study in advanced RCC similarly found no relationship between grade 3/4 increased ALT and pazopanib exposure, as suggested by the same percentage (4%) of increased ALT in both upper and lower Ctrough quartiles in this study (10).
The PKs of pazopanib in PROTECT patients was adequately described by a two-compartment disposition model with delayed first order absorption and first-order elimination, including an oral bioavailability decreasing with dose and time. The current population PK analysis revealed dose-dependent PKs for pazopanib, which is in agreement with the dose-escalation study [VEG10003] reporting an increase of Cmax and AUC0–24h in a less than dose-proportional fashion over the range of 50 mg to 2000 mg, reaching a plateau for doses above 800 mg (16). This suggests absorption is limited by pazopanib’s low solubility. Population PK model-based simulations showed that steady-state exposure metrics were on average only 17% higher at 800 mg compared with 600 mg.
The current population PK analysis suggests time-dependent PKs of pazopanib. Indeed, the oral bioavailability was estimated to be 30% lower at steady state than after single administration. There is no strong evidence supporting this result, but pazopanib is a substrate of efflux transporters (P-gp, BCRP) and mainly metabolized by CYP3A4 enzymes. Therefore, it can be speculated that auto-induction of those transporters and/or CYP3A4 enzymes may decrease over time the fraction of dose reaching the systemic circulation (i.e., reducing the fraction absorbed and/or increasing the first pass effect).
The observed early Ctrough in PROTECT was under-predicted by ~30% compared with the historical population PK model, suggesting higher pazopanib exposure in PROTECT despite comparable patient characteristics for most of the explored covariates in both PROTECT and historical populations. Inclusion of a covariate effect on pazopanib oral bioavailability for the PROTECT population led to adequate description of pazopanib exposure confirming a 30% higher exposure in the PROTECT study compared with historical PK data. Nevertheless, the underlying cause for higher pazopanib exposure in PROTECT is not clearly understood. Future clinical studies aiming to identify genotypes or other biomarkers associated with improved pazopanib exposure may allow the identification of patient subgroups more likely to benefit from adjuvant pazopanib therapy.
Conclusions
This exposure-response analysis of patients primarily treated with the starting dose of 600 mg daily pazopanib found that higher exposure (Ctrough) was associated with improved DFS in the adjuvant RCC setting. Patients with Ctrough (early or late) >20.5 μg/mL achieved longer DFS. This suggests that patients achieving higher pazopanib Ctrough derived more clinical benefit from adjuvant pazopanib therapy. This implies that potential benefit to adjuvant therapy is driven by a pharmacodynamic benefit rather than by dose. Furthermore, pazopanib exposure was not associated with dose, which is unsurprising given the non-linear PK of pazopanib and pronounced inter-subject variability in pazopanib exposure. Grade 3/4 AEs and AE-related discontinuations did not correlate with pazopanib exposure, except for grade 3/4 hypertension, which was clinically well managed.
Supplementary Material
Acknowledgements
Editorial assistance was provided by Chris Ontiveros, PhD (ApotheCom, New York, NY), and Julia Burke, PhD (ApotheCom, Auckland, New Zealand), and was funded by Novartis Pharmaceuticals Corporation.
Patients treated at Memorial Sloan Kettering Cancer Center were supported in part by Memorial Sloan Kettering Cancer Center Support Grant/Core Grant (P30 CA008748)
Grant Support
The analyses and studies described in this report were funded by Novartis Oncology (and initiated by GlaxoSmithKline).
Financial Support: This study was sponsored by Novartis initiated by GlaxoSmithKline; pazopanib is an asset of Novartis AG as of March 2, 2015
C. N. Sternberg reports receiving honoraria from Novartis, Pfizer and IPSEN, and is a consultant for Eisai and Bristol-Myers Squibb. F. Donskov reports receiving research grants from Novartis and Pfizer. C. Doehn reports receiving honoraria from Amgen, Bayer Healthcare, Bristol-Myers Squibb, GlaxoSmithKline, Novartis and Pfizer, has stock ownership in Astra Zeneca, Bayer Healthcare, and Bristol-Myers Squibb, and serves on advisory boards for Amgen, Bayer Healthcare, Bristol-Myers Squibb, Eisai, GlaxoSmithKline, Ipsen, Novartis and Pfizer. M. Elmeliegy, G. Baneyx, H. Banerjee and P. Aimone are employees of Novartis. R.J. Motzer reports consulting for Novartis, Pfizer, Eisai, Exelixis and Merck, and obtaining research funding to Memorial Sloan Kettering Cancer Center for Bristol-Myers Squibb, Pfizer, Genentech Roche, and Eisai.
Footnotes
Disclosure of Potential Conflicts of Interest
The product in this study was the property of GlaxoSmithKline during the development of the study. The product was transferred to Novartis on March 2, 2015. GlaxoSmithKline financial spending for the US authors listed below (during the period of 2010–2014) was obtained from: www.fortherecordpayments.us.gsk.com (January 1–December 31, 2014) and https://openpaymentsdata.cms.gov (starting August 1, 2013) and is shown in the table below. Novartis does not have access to any other payments that may have been made on behalf of GlaxoSmithKline.
Novartis Pharma AG, an affiliate of NPC, and/or other NPC affiliates may have provided additional compensation to 1 or more of the authors of this publication for investigator, consulting, and/or other activities. NPC, during the period of October 2010–September 2015, provided financial compensation and/or value to the following authors of this publication as listed in the table below.
No potential conflicts of interest were disclosed by the other authors.
Clinicaltrials.gov Identifier:
REFERENCES
- 1.Janzen NK, Kim HL, Figlin RA, Belldegrun AS. Surveillance after radical or partial nephrectomy for localized renal cell carcinoma and management of recurrent disease. Urol Clin North Am 2003;30:843–52. [DOI] [PubMed] [Google Scholar]
- 2.Haas NB, Manola J, Uzzo RG, Flaherty KT, Wood CG, Kane C, et al. Adjuvant sunitinib or sorafenib for high-risk, non-metastatic renal-cell carcinoma (ECOG-ACRIN E2805): a double-blind, placebo-controlled, randomised, phase 3 trial. Lancet 2016;387:2008–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ravaud A, Motzer RJ, Pandha HS, George DJ, Pantuck AJ, Patel A, et al. Adjuvant sunitinib in high-risk renal-cell carcinoma after nephrectomy. N Engl J Med 2016;375:2246–54. [DOI] [PubMed] [Google Scholar]
- 4.Motzer RJ, Haas NB, Donskov F, et al. Randomized phase III trial of adjuvant pazopanib versus placebo after nephrectomy in patients with localized or locally advanced renal cell carcinoma. J Clin Oncol 2017; September 13:JCO2017735324. doi: 10.1200/JCO.2017.73.5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sternberg CN, Hawkins RE, Wagstaff J, Salman P, Mardiak J, Barrios CH, et al. A randomised, double-blind phase III study of pazopanib in patients with advanced and/or metastatic renal cell carcinoma: final overall survival results and safety update. Eur J Cancer 2013;49:1287–96. [DOI] [PubMed] [Google Scholar]
- 6.Hutson TE, Davis ID, Machiels JP, De Souza PL, Rottey S, Hong BF, et al. Efficacy and safety of pazopanib in patients with metastatic renal cell carcinoma. J Clin Oncol 2010;28:475–80. [DOI] [PubMed] [Google Scholar]
- 7.Houk BE, Bello CL, Poland B, Rosen LS, Demetri GD, Motzer RJ. Relationship between exposure to sunitinib and efficacy and tolerability endpoints in patients with cancer: results of a pharmacokinetic/pharmacodynamic meta-analysis. Cancer Chemother Pharmacol 2010;66:357–71. [DOI] [PubMed] [Google Scholar]
- 8.Rini BI, Garrett M, Poland B, Dutcher JP, Rixe O, Wilding G, et al. Axitinib in metastatic renal cell carcinoma: results of a pharmacokinetic and pharmacodynamic analysis. J Clin Pharmacol 2013;53:491–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rini BI, Melichar B, Fishman MN, Oya M, Pithavala YK, Chen Y, et al. Axitinib dose titration: analyses of exposure, blood pressure and clinical response from a randomized phase II study in metastatic renal cell carcinoma. Ann Oncol 2015;26:1372–7. [DOI] [PubMed] [Google Scholar]
- 10.Suttle AB, Ball HA, Molimard M, Hutson TE, Carpenter C, Rajagopalan D, et al. Relationships between pazopanib exposure and clinical safety and efficacy in patients with advanced renal cell carcinoma. Br J Cancer 2014;111:1909–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baneyx G, Combs FP, Huang PH, Elmeliegy M, editors. Population pharmacokinetic modeling of pazopanib in healthy volunteers and patients with advanced renal cell carcinoma. Proceedings of the 26th Annual Meeting of the Population Approach Group in Europe. Budapest, Hungary; June 6–9, 2017. [Google Scholar]
- 12.Haas NB, Manola J, Dutcher JP, Flaherty KT, Uzzo RG, Atkins MB, et al. Adjuvant treatment for high-risk clear cell renal cancer: updated results of a high-risk subset of the ASSURE randomized trial. JAMA Oncol 2017. [DOI] [PMC free article] [PubMed]
- 13.Liu B, Ding F, Liu Y, Xiong G, Lin T, He D, et al. Incidence and risk of hypertension associated with vascular endothelial growth factor receptor tyrosine kinase inhibitors in cancer patients: a comprehensive network meta-analysis of 72 randomized controlled trials involving 30013 patients. Oncotarget 2016;7:67661–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donskov F, Michaelson MD, Puzanov I, Davis MP, Bjarnason GA, Motzer RJ, et al. Sunitinib-associated hypertension and neutropenia as efficacy biomarkers in metastatic renal cell carcinoma patients. Br J Cancer 2015;113:1571–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maitland ML, Bakris GL, Black HR, Chen HX, Durand JB, Elliott WJ, et al. Initial assessment, surveillance, and management of blood pressure in patients receiving vascular endothelial growth factor signaling pathway inhibitors. J Natl Cancer Inst 2010;102:596–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hurwitz HI, Dowlati A, Saini S, Savage S, Suttle AB, Gibson DM, et al. Phase I trial of pazopanib in patients with advanced cancer. Clin Cancer Res 2009;15:4220–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.