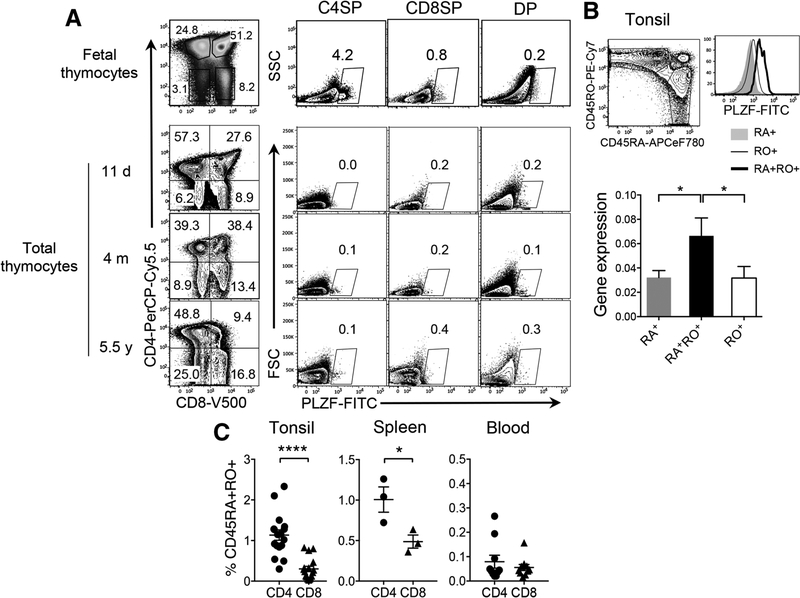
In humans, promyelocytic leukaemia zinc finger (PLZF)+CD161+CD4 T cells develop in the fetal thymus [1] but these cells were not detectable in thymi obtained from children (11 days to 5.5 years) (Fig. 1A). However, we found PLZF+CD4 T cells within CD45RA+RO+CD4 T cells from tonsils and spleens but rarely in blood (Fig. 1B, left panel and 1C; Supporting Information Fig. 1A and B). PLZF expression was also confirmed by mRNA analysis in these cells from tonsils (Fig. 1B, right panel). CD8 T cells had a very low frequency of CD45RA+RO+ cells compared to CD4 T cells and these cells did not express PLZF (Fig. 1B). We also validated that CD45RA+RO+CD4 T cells are not mucosal-associated invariant T cells [2] (data not shown). PLZF-expressing NKT cells were excluded from all our analyses by CD1d-Tetramer staining (Supporting Information Fig. 2)
Figure 1.
PLZF+CD4 T cells express both CD45RA and CD45RO and reside in lymphoid tissues. (A) Total cells from fetal or child thymii samples were stained with surface antibodies and gated based on CD4 and CD8 expression. PLZF+ cells in each subset are shown. PLZF-expressing NKT cells were excluded from our analyses by CD1d-Tetramer staining (Supporting Information Fig. 2). One representative is shown of total 3 fetal thymi, which were examined in independent experiments. Total 9 after-birth thymi were examined in independent experiments of which, three thymi of different ages are shown. d, m, and y indicates days, months, and years, respectively of subject’s age. (B) Left panel, Tonsillar lymphocytes were stained with surface antibodies, followed by intranuclear staining for PLZF. CD4+ T cells were gated into CD45RA+, CD45RO+, and CD45RA+RO+ cells were compared for PLZF expression by flow cytometry (n = 8) from six independent experiments. Right panel, CD45RA+, CD45RO+ and CD45RA+RO+CD4 T cells from tonsils were sorted to prepare RNA and then cDNA. PLZF gene expression was compared by qPCR. Relative expression of PLZF over HPRT is shown. Error bars represent the mean ± SEM from triplicate wells. *p < 0.05. Two independent experiments were performed. (C) CD4 and CD8 T cells from tonsils (n = 17), spleens (n = 3), and blood (n = 10) were analyzed by flowcytometry as in (B) to compare the frequency of CD45RA+RO+CD4 versus CD8 T cells. Error bars represent the mean ± SEM from pooled data from at least three independent experiments. *p < 0.05; ****p < 0.0001 (t-test).
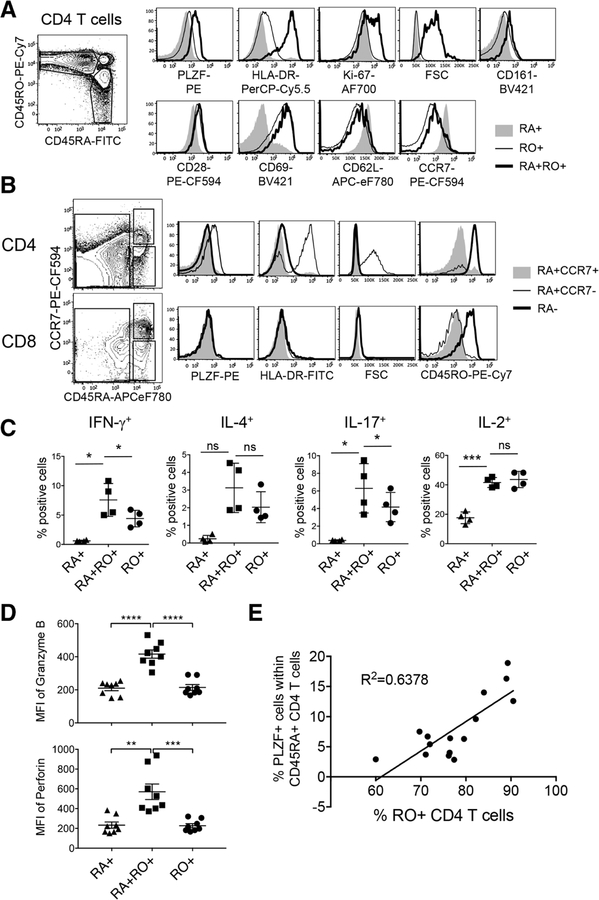
Further analyses revealed that CD45RA+RO+CD4 T cells expressed PLZF, HLA-DR, and Ki-67 and were larger in size indicating that these are activated and proliferating cells (Fig. 2A, top panel). They also expressed higher levels of CD161, similar to fetal PLZF+CD4 T cells [1] (Fig. 2A). Expression of CD28, CD69, CD62L, and CCR7 in CD45RA+RO+CD4 T cells was comparable to CD45RO+CD4 T cells (Fig. 2A, bottom panel). All three subpopulations expressed comparable levels of CD44 and TCRαβ, while Eomes was not expressed by any (Supporting Information Fig. 1C). Together, PLZF+CD45RA+RO+CD4 T cells seem to exhibit an effector/memory phenotype and reside in lymphoid tissues. These cells differ from previously reported CD45RA+RO+CD4 T cells in the blood of patients with recent viral infections, in that these are tissue-resident and express PLZF [3].
Figure 2.
PLZF+CD45RA+RO+CD4 T cells show characteristics of terminally differentiated effector memory cells (A) CD45RA+, CD45RO+, CD45RA+RO+CD4 T cells from tonsils were analyzed for the expression of indicated molecules by flow cytometry after surface and intranuclear staining. Total 16 tonsils were examined and one representative data are shown. (B) Naїve (RA+CCR7+), central and effector memory (RA−) and Temra (RA+CCR7−) CD4 (top) or CD8 (bottom) T cells were assessed for the expression of indicated molecules by flow cytometry. Total 16 tonsils were examined and one representative data are shown. (C) Tonsillar CD4 T cells were stimulated with PMA and Ionomycin for 5 hours in the presence of Monensin, followed by intracellular staining to assess the expression of cytokines. Graphs illustrate the summary of data as mean ± SEM (n = 4). (D) Freshly isolated lymphocytes from tonsils were gated as CD45RA+, CD45RO+, and CD45RA+RO+CD4 T cells and assessed for the expression of Granzyme B and perforin after staining with cocktail of antibodies. Error bars represent the mean ± SEM. ns, not significant. (n = 8) (E)± Tonsillar CD4 T cells were examined by flow cytometry to assess the frequency of PLZF+ cells. The frequency of PLZF+ cells within CD45RA+CD4 T cells was calculated and plotted against the frequency of CD45RO+CD4 T cells (n = 15). ns, not significant; *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001 (t-test). All data are representative of or pooled from at least three independent experiments.
Naїve T cells express lymph node-homing CC-Chemokine receptor 7 (CCR7), whereas memory T cells are subdivided into CD45RA−CCR7+ central memory T cells (Tcm) and CD45RA− CCR7− effector memory T cells (Tem) [4]. In addition, CD45RA+CCR7- terminally differentiated effector memory cells (Temra) have been identified [4, 5]. The expression pattern of CD45RA, CD45RO, and CCR7 on PLZF+CD4 T cells was similar to that shown by Temra cells, raising a question whether PLZF+CD45RA+RO+CD4 T cells are Temra or Temra-like cells. Indeed, in tonsils, PLZF expression was restricted to Temra (CD45RA+CCR7−) CD4 T cells (Fig. 2B), which were larger in size and HLA-DR+. Notably, Temra CD8 T cells did not express PLZF, HLA-DR or CD45RO (Fig. 2B). CD45RA+RO+CD4 T cells from spleen but not blood exhibited similar Temra-like phenotype (Supporting Information Fig. 1B).
Functionally, like Temra CD8 T cells, Temra CD4 T cells possess cytolytic function; express IFN-γ but not IL-2 after activation through their TCR or stimulation with antigens [6, 7]. Therefore, we next assessed the function of PLZF+CD4 T cells. Significantly higher frequencies of IFN-γ+ and IL-17+ cells were present in CD45RA+RO+CD4 T cells compared to CD45RO+CD4 T cells, while IL-2+ or IL-4+ cells were comparable between them. As expected, CD45RA+ naїve CD4 T cells in tonsils did not express any effector cytokines except IL-2 upon stimulation while all cytokines were expressed by CD45RO+ (Fig. 2C) [8]. Moreover, CD45RA+RO+CD4 T cells expressed high levels of granzyme B and perforin (Fig. 2D). Although CD4 Temra cells are not well-characterized due to their low frequency, expression of granzyme B and perforin in PLZF+CD45RA+RO+CD4 T cells align with the distinct characteristics of Temra cells. Lastly, tonsil samples with higher percentages of CD45RO+CD4 T cells had more PLZF+ cells (Fig. 2E) suggesting that PLZF+CD45RA+RO+CD4 T cells arise or accumulate during immune responses.
In summary, we show that PLZF+CD4 T cells are not detectable in the thymus after birth but are present in the peripheral lymphoid organs. They exhibit remarkable similarities with Temra cells in their phenotype and function. The function of PLZF in Temra CD4 T cells is not known. Further investigations are warranted to understand if Temra cell generation requires PLZF, or if PLZF expression is a result of Temra cell differentiation.
Supplementary Material
Acknowledgments:
We acknowledge NIH Tetramer Facility for providing CD1d tetramers. This work was partly supported by NIH Grants R01 AI111899, R01073146, U19 AI096113 (to J.V.G.), and by the NCI Award P30CA046592 by the use of Flow Cytometry and Tissue & Molecular Pathology.
Abbreviations:
- PLZF
promyelocytic leukaemia zinc finger
- Temra
terminally differentiated effector memory cells
Footnotes
Conflict of interest: The authors declare no commercial or financial conflict of interest.
The detailed Materials and methods for Technical comments are available online in the Supporting information
References
- 1.Lee YJ et al. , J. Exp. Med 2010. 207: 237–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin E et al. , PLoS Biol. 2009. 7: e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rentenaar RJ et al. , J. Clin. Invest 2000. 105: 541–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sallusto F et al. , Nature 1999. 401: 708–712. [Google Scholar]
- 5.Sathaliyawala T et al. , Immunity 2013. 38: 187–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farber DL et al. , Nat. Rev. Immunol 2014. 14: 24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thome JJC et al. , Nat. Med 2016. 22: 72–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geginat J et al. , J. Exp. Med 2001. 194: 1711–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.