Abstract
In this review we examine the effects of both over- and under-production of heme oxygenase-1 (HO-1) and HO activity on a broad spectrum of biological systems and on vascular disease. In a few instances e.g., neonatal jaundice, overproduction of HO-1 and increased HO activity results in elevated levels of bilirubin requiring clinical intervention with inhibitors of HO activity. In contrast HO-1 levels and HO activity are low in obesity and the HO system responds to mitigate the deleterious effects of oxidative stress through increased levels of bilirubin (anti-inflammatory) and CO (anti-apoptotic) and decreased levels of heme (pro-oxidant). Site specific HO-1 overexpression diminishes adipocyte terminal differentiation and lipid accumulation of obesity mediated release of inflammatory molecules. A series of diverse strategies have been implemented that focus on increasing HO-1 and HO activity that are central to reversing the clinical complications associated with diseases including, obesity, metabolic syndrome and vascular disease.
Keywords: obesity, hyperbilirubinemia, bilirubin, adipocyte inflammation, fatty liver, hypertension
1. Introduction
Heme oxygenase (HO) is the rate limiting enzyme in the catabolism of heme, a 2 step enzymatic process that results in the formation of equimolar amounts of biliverdin, iron and carbon monoxide (CO). The biliverdin formed is rapidly converted to bilirubin by biliverdin reductase[56, 57]. The heme catabolic process was first described by Schmid and his colleagues. HO was shown to be inducible by a broad spectrum of chemicals in addition to heme, its natural substrate. HO is present in the reticuloendothelial system, hematopoietic stem cells, bone marrow and in all cells studied [60, 61]. Many of the studies conducted in this period focused on how the activity of the enzyme could be up- or down regulated, the resultant biological responses to perturbations in HO activity and the potential clinical application of controlling the activity of this enzyme in humans. This has resulted in a two-pronged approach that has focused on down-regulation (inhibition) of HO activity e.g., neonatal jaundice; and upregulation of HO activity e.g., obesity, hypertension, metabolic syndrome etc., leading to the development of new therapeutic modalities that could moderate disease progression in humans. This review examines how HO can be manipulated and highlights HO as an enzyme of critical importance for pharmacological development.
1.1. Heme Oxygenase
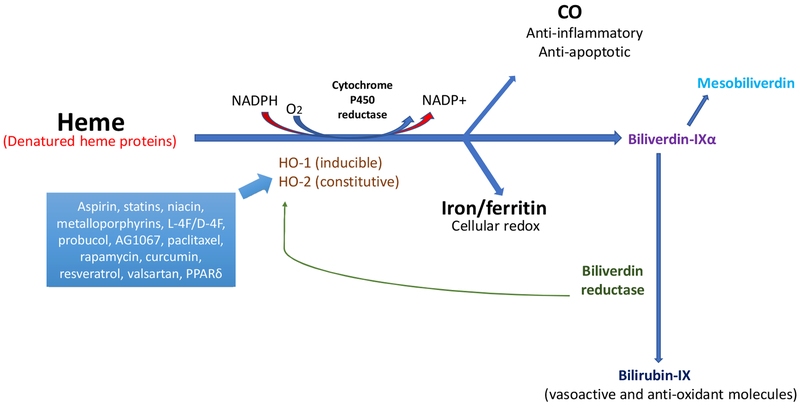
HO exists in two forms, HO-1, the inducible form, and HO-2, the constitutive form. Both isozymes degrade heme in an identical stereospecific manner to biliverdin with the concurrent release of CO and iron [62]. In mammals, biliverdin is rapidly reduced by biliverdin reductase to bilirubin [54, 55]. HO-1 and HO-2 are alike in terms of mechanism, cofactor and substrate requirements, as well as their susceptibility to inhibition by synthetic metalloporphyrins in which the central iron atom is replaced by other elements including tin, zinc, cobalt and chromium (reviewed in [59]). HO-1 is induced by a variety of drugs and chemical agents including statins, aspirin, niacin, certain prostaglandins, eicosanoids such as epoxyeicosatrienoic (EETs) and free and complexed metals [62]. Iron, bilirubin and CO, the three degradation products of heme degradation, have important regulatory functions in cells. Iron is an essential requirement for the synthesis of hemoglobin and ferritin. The constitutive nature of HO-2, however, makes it less attractive as a drug target (Figure 1).
Figure 1:
Drug actions in the heme degradation pathway. HO-1 (inducible) and HO-2 (constitutive) cleave free heme or denatured heme proteins to generate CO, ferritin, and biliverdin, which is subsequently converted to bilirubin by biliverdin reductase. CO has both anti-inflammatory and anti-apoptotic properties [3, 4, 14, 15]. Ferritin is essential for cellular redox reactions [24–26]. Serum bilirubin levels are positively linked with a decreased risk of CVD and protection against diabetes and vascular dysfunction [36, 37]. Drugs focused on the heme degradation pathway predominantly induce HO-1 activity, possibly by interacting with the gene promoter. Biliverdin reductase functions via a direct increase of HO-1 or an increase in bilirubin levels to promote a reduction in oxidative species [54, 55], and mesobiliverdin enhances β-cell function in the pancreas through its antioxidant properties [36]. This pathway provides the basis for multiple pharmaceutical and genetic agents that can protect against CVD by increasing HO-1 expression.
The first therapeutic approach to regulate HO activity began by devising a means to downregulate HO activity by competitive inhibition. This was recognized early [63, 64] and achieved clinical fruition in neonatology when a safe, rapidly acting and effective method for transiently blocking bilirubin production in newborns was developed [64, 65]. The development of inhibitors of HO activity having a pharmacological profile permitting their use in infants provided the first demonstration of the potential clinical usefulness of agents that can downregulate HO. However, when selecting an inducer or an inhibitor of HO activity, one must consider the mechanism of induction/inhibition and duration of response. Rapid response of HO-1 mRNA by inducers such as cobalt protoporphyrin IX dichloride (CoPP) do not reflect an immediate increase in HO activity [66], suggesting that an increase of HO-1 mRNA is meaningless unless HO activity is also determined. Induction of HO-1 by heme or CoPP has a differential effect on the timing of an increase in HO activity, with heme resulting in a maximum increase in HO activity at 16 hr, whereas with CoPP maximal HO activity is attained at 3 days and lasts for extended periods of time up to 14 days after a single administration of the compound [62]. In metabolic syndrome and vascular disease, elevation of inducible nitric oxide synthase (iNOS) and peroxynitrite resulted in inactivation of HO-1 protein [67, 68] and decreased HO activity. These considerations regarding the time frame of both induction and inhibition of HO activity, not just HO-1 expression levels, must be considered in the development of new therapeutic strategies targeting HO-1 for the treatment of obesity-hypertension and metabolic syndrome. As an alternative strategy to drugs, gene therapy has been identified as a long lasting (1 year) and effective way to induce HO-1 expression to prevent CVD [19, 67, 69–73].
Both biliverdin and bilirubin are potent antioxidants and may exert cellular protective effects against injurious stimuli in vivo and in vitro [74, 75]. CO has been identified as a second messenger in the central nervous system (CNS) [76] and suppresses endothelial cell apoptosis through activation of p38 MAPK [14, 77]. CO has a multitude of functions in biology and medicine which are described in series of articles [3, 4, 78] and include acting as a vasodilator via stimulation of soluble guanylate cyclase (sGC).
1.2. Detrimental Effect of High Levels of Heme Degradation; Inhibition of Heme Metabolism
Hyperbilirubinemia occurs in human newborns when the rate of bilirubin production is several fold greater than that of adults. The peak bilirubin levels that newborns achieve are dependent upon gestational age, 3 days after birth in full-term infants and later in preterm infants. The increase in plasma bilirubin occurs due to a rapid degradation of fetal hemoglobin and the immaturity of UDP-glucuronosyltransferase which is responsible for the formation of bilirubin mono- and di-glucuronides prior to excretion in the bile. This can lead to an increase in unconjugated bilirubin which, if severely elevated, can cross the blood brain barrier resulting in a spectrum of neurological insufficiencies ranging from the subtle to the overt, the latter manifest as kernicterus/bilirubin encephalopathy[79]. The levels of bilirubin that are considered sufficient for clinical intervention depend on a number of variables including gestational age, rate of increase in bilirubin and clinical considerations e.g. ABO incompatibility, G6PD deficiency, Rh and mode of treatment [80]. The current method of choice is phototherapy where soluble bilirubin photoisomers are produced and excreted in the bile [81]. The use of phototherapy has been questioned with regard to safety; DNA damage, erythrocyte damage, retinal damage; and also due to prolonged separation of mother and child [82]. In addition, phototherapy only photoisomerized ~15% of total bilirubin in the newborn making it inefficient in treating Crigler-Najjar individuals. Thus, the management of elevated levels of bilirubin by interdicting the formation of bilirubin rather than instituting clinical intervention when bilirubin levels reach a predetermined level by the use of inhibitors of heme oxygenase activity is an attractive proposition to clinicians, newborns and their parents.
1.3. Heme Oxygenase Inhibitors, Clinical Use
In 1980 the Kappas group and Maines reported that metalloporphyrins in which the central metal iron of iron protoporphyrin (heme) had been replaced by tin and zinc respectively were competitive inhibitors of heme oxygenase activity. These studies were extended to chromium in an examination of HO activity inhibitors in the control of hyperbilirubinemia in a series of animal models of jaundice [65, 83]. Tin protoporphyrin (SnPP) was initially regarded as the metalloporphyrin of choice, however, tin mesoporphyrin (SnMP) proved more efficacious and was used in a series of clinical studies. Several other compounds including ZnMP, CrMP and zinc 2,4-bis glyclol deuteroporphyrin are inhibitors of HO activity. Non metalloporphyrins including imidazole dioxolane compounds have been reported to inhibit HO activity as well as specific HO isoenzymes [84].
SnPP and SnMP have been reported to control serum bilirubin and biliary bilirubin levels in normal volunteers [85], liver disease[86] and Crigler-Najjar type 1 syndrome [87, 88]. Clinical trials in newborns with ABO incompatibility showed SnPP was efficacious in controlling hyperbilirubinemia [85]. A randomized, double blinded, placebo controlled dose ranging study in preterm infants demonstrated that the administration of SnMP within 24 hours of birth moderated the development of hyperbilirubinemia and reduced the need for phototherapy by up to 75% in a dose dependent manner when compared to control newborns. A higher incidence of erythema was noted in SnMP-treated infants who received phototherapy compared with control newborns. The erythema was not dose dependent, was transient and resolved without sequelae [89]. A trial in near term and term infants showed that phototherapy was eliminated when SnMP was administered to infants when phototherapy would normally have been initiated [90]. This was confirmed in healthy infants of ≥ 38 and ≤ 41 weeks gestational age following an uncomplicated pregnancy with a PBC of between 15 mg/dL and 18 mg/dl 48–96 hours after birth. In the control group 19 of 86 newborns required phototherapy (initiated at 19.5 mg/dL) compared with 0 of 80 in the SnMP-treated group [91]. GGPD deficiency is a genetic defect the predisposes newborns to severe hyperbilirubinemia. In this trial 31% of the control group required phototherapy due to hyperbilirubinemia while none of the 225 GGPD-deficient infants who received a single dose of SnMP required phototherapy and thus were able to be discharged earlier than the control infants [92]. The use of metalloporphyrins in the management of neonatal hyperbilirubinemia is reviewed in detail [59, 93, 94].
The approach of interdicting bilirubin production in the severely jaundiced newborn for a sufficient period of time to allow the maturation of UDP glucuronyl transferase makes clinical sense rather than waiting for the bilirubin level to rise until it reaches the point of clinical intervention requiring disposal. The efficacy of this approach has been convincingly demonstrated by the SnMP studies described above. In addition to efficacy no short- or long-term adverse events have been noted in newborns administered SnMP. This shortens that time of medical care, does not interrupt parent/infant bonding and reduces cost to the parents, clinicians and hospital. In addition, a single injection is a boon in conditions/countries where phototherapy is unavailable.
1.4. Porphyria
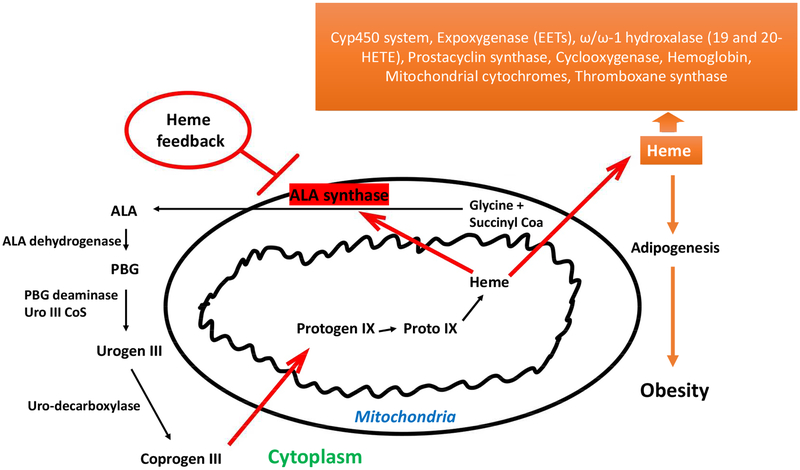
Porphyria refers to a group of disorders that result from an accumulation of porphyrins produced in the heme biosynthetic pathway and are classified as either acute, which largely affects the nervous system, and cutaneous, which mainly affects the skin. Porphyria is usually inherited as a result of deficiency in one of the eight enzymes in the heme biosynthetic pathway (Figure 2). In addition to hereditary forms, some porphyrias e.g., porphyria cutanea tarda can be acquired as a result of liver disease, excessive alcohol use and estrogen medications. Inhibitors of HO have been used to treat porphyria.
Figure 2:
Heme synthetic pathway. The rate-limiting synthetic enzymes are believed to be ALA synthase and, in part, porphobilinogen deaminase (PBGD). Both enzymes exist in adipocytes and in erythroid and non-erythroid forms. In non-erythroid cells such as liver, kidney, heart, ALA synthase essentially plays a housekeeping role, maintaining intracellular heme levels. High levels of heme thus repress the synthesis of ALA synthase while stimulating heme degradation through the induction of HO-1[31, 32]. In the origin of hematopoietic-derived cells such as adipocyte and erythroid cells, heme is essential for cellular proliferation and differentiation, and increase ALA synthase mRNA levels and enzyme activity. Further, excess heme enhances the synthesis of globin mRNA [59]. An iron-binding element has been located on the 5′ untranslated region of the erythroid type cells ALA synthase, so it is possible that the enzyme is actually regulated by intracellular levels of iron. Thus, an increase in heme may induce HO-1, increasing the levels of free iron which in turn stimulate the formation of adipocyte ALA synthase mRNA and adipocyte during differentiation.
Metalloporphyrins that inhibit HO activity and decrease bilirubin, CO and iron production increase the biliary excretion of unmetabolized heme [95, 96] but have no effect on the metabolic disposition of preformed bilirubin [85, 97]. SnPP/SnMP led to a prolonged increase in heme saturation of tryptophan pyrrolase indicating an increase in the “heme pool” related to tryptophan pyrrolase [96, 98] and SnMP also suppressed chemically induced hepatic porphyria. In addition, SnMP, when administered to bile duct cannulated rats, caused a prompt and sustained decrease in the levels of bilirubin in the bile and an enhancement of biliary heme excretion in these animals.
SnPP/SnMP were examined for efficacy in decreasing the excretion of heme pathway precursors in patients with intermittent and variegate porphyria. Both metalloporphyrins reduced the excretion of ALA, PBG and porphyrins indicating that the rate limiting enzyme of heme biosynthesis, ALA-synthase was inhibited by the metalloporphyrins [99, 100]. The mode of action remains unclear. In an animal model of porphyria both SnPP and SnMP reduced the rapid increase in ALA synthase. It may be that the metalloporphyrins act directly in a manner similar to heme, due to structural similarities, the normal feedback control mechanism of heme biosynthesis in liver. In contrast SnPP and SnMP potently inhibit HO activity which may result in increased intrahepatic concentrations of endogenous heme. This hypothesis is supported by the rapid transient increase in heme saturation and activity of the heme dependent hepatic enzyme tryptophan pyrrolase [96, 98] that occurs after SnPP/SnMP administration. Cutaneous photosensitivity was the only side effect noted in the patients. The photosensitivity was self-limiting. Further studies are necessary to determine whether these compounds may be therapeutically useful in porphyria patients.
1.5. Obesity, Ischemia Reperfusion Injury
Increased adipose tissue macrophages contribute to obesity induced metabolic syndrome. A high fat diet (HFD) fed to C57BL/6J mice increased HO-1 levels in visceral adipose tissue. After mice were irradiated and reconstituted with wild type or HO-1 +/− bone marrow they were fed a HFD for 24 weeks. HO-1 +/− chimeras were protected from insulin resistance induced by a HFD. There was a concomitant reduction in adipose macrophage infiltration and angiogenesis suggesting that HO-1 affects myeloid cell migration towards adipose tissue during obesity. CO and bilirubin enhanced macrophage migration by increased phosphorylation of p38 and FAK respectively. This highlights the novel role of hematopoietic cell HO-1 in promoting adipose macrophage infiltration and the development of insulin resistance during obesity. Myeloid HO-1 modulates macrophage polarization and protects against ischemia-reperfusion injury [101].
Hepatic ischemia-reperfusion injury (IRI) is a risk factor for acute and chronic rejection in liver transplantation. Decreased HO-1 levels in human post reperfusion liver transplant biopsies correlated with a decrease in liver function and patient survival. Macrophages are the main sources of HO-1 in human and mouse IR stressed livers. In a murine model of hepatic warm IRI, myeloid specific deletion lacked SIRT1/p53, exacerbated liver inflammation and IR hepatocellular death. In contrast SIRT1 activation restored p53 signaling and rescued livers from IR damage suggesting a class of macrophages activated through a HO-1-SIRT1-p53 axis to protect against hepatic inflammation. This axis offers a portal to new therapeutic strategies to benefit liver transplant recipients [102]. Recently, H202 protects against ischemia-reperfusion injury in a mouse fatty liver model via regulation of HO-1 and Sirt [103], while increase of HO-1 levels by EETs prevented fatty liver fibrosis and lipid uptake [104].
1.6. Detrimental Effects of Low Levels of Heme Degradation; Beneficial Effects of Overexpression of HO-1 and Increased HO Activity
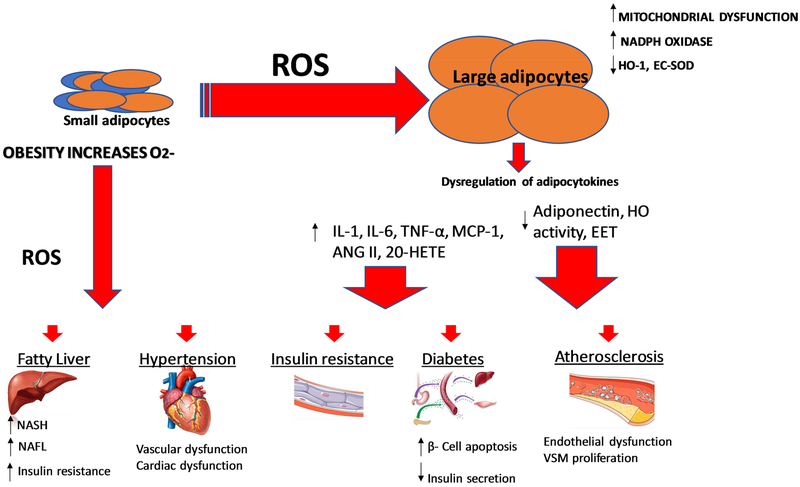
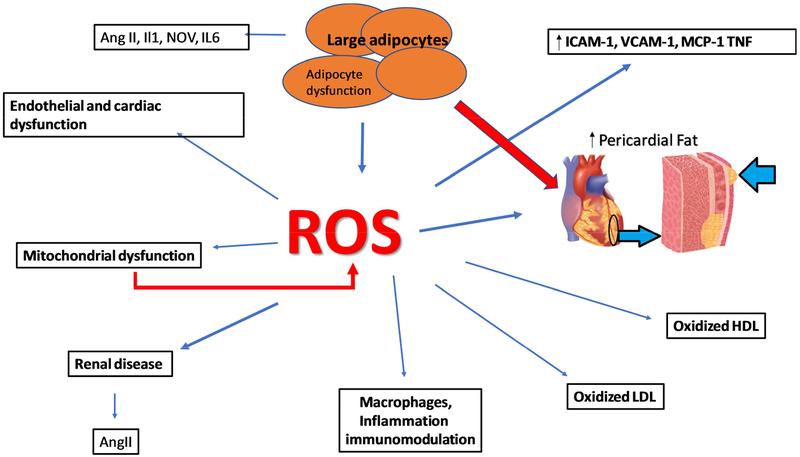
Obesity and metabolic syndrome are on the increase in the US and in West Virginia and Mississippi specifically resulting in increased demands on these individuals and their families, healthcare professionals and on healthcare costs. Obesity is the major risk factor in vascular dysfunction, insulin resistance and vascular disease e.g., diabetes and hypertension [105–107]. This is manifest by decreased levels of HO-1 and increased levels of inflammatory cytokines and insulin resistance [108, 109]. The resultant consequences of obesity derived adipocyte dysfunction are significant as perturbations in adipocyte-derived paracrine factors impact the function of numerous organs including the vasculature [110–112]. Increased levels of ROS and heme enhance pre-adipocyte differentiation and adipogenesis [31, 38, 59, 113]. It should be emphasized that increased levels of ROS do not increase HO-1 expression exacerbating the progression of obesity and metabolic syndrome [113] (Figure 3). Activation of the angiotensin II system and NADPH oxidase occurs in obesity with the resultant development of CVD, hypertension and diabetes, in part, as an impairment of adipocyte function [105, 114, 115]. Low levels of HO-1 are a direct result of the development of obesity mediated hypertension [116–118] and the inactivation of HO-1 by peroxynitrite [39, 67, 68] increases cellular heme content [39, 67, 68].
Figure 3:
Obesity increases risk for cardiovascular disease. Obesity leads to an increase in ROS within adipocytes, accomplished by increasing NADPH oxidase activity, mitochondrial ROS production, and heme levels while repressing antioxidative enzymes such as HO-1 and SOD. This increase in adipocyte ROS and heme leads to increased adipocyte differentiation, maturation, resulting in increased production of proinflammatory compounds such as cytokines and decreased production of antioxidative compounds and compounds preventing adipocyte growth and differentiation. The consequences of obesity-mediated adipocyte dysfunction may lead to vascular dysfunction which is a prelude to vascular disease and hypertension.
Heme with increased levels of ROS, produces both vascular, adipocyte dysfunction [59, 104, 119, 120] (Figure 3).
Local adipose tissue renin-angiotensin system and its activation of the systemic and adipose in rodents with diet-induced obesity and hypertension are well described [114, 115]. Additionally, Sodhi, et al. recently showed that increased levels of Ang II contribute toward adipose tissue dysregulation, which is abated by PPARδ-mediated upregulation and activation of the heme-HO system. Angiotensin II activates cellular oxidases and precipitates redox imbalance. Increased Ang II levels in adipocytes induce oxidative stress and attenuate adiponectin release. Additionally, upregulation of the antioxidant enzyme system, that is, the heme–heme oxygenase system (HO) has also been shown to reduce Ang II-induced oxidative stress, with abatement of associated cardiovascular complications. Induction of the HO-1 gene, both in vivo and in cell cultures, reduces adipocyte hypertrophy with an increase in adiponectin levels and the number of small adipocytes, which are regarded as ‘healthy’ insulin-sensitive adipocytes. Recent reports have indicated that inhibitors of the RAAS attenuate oxidative stress and improve the metabolic profile in various animal models. PPARδ-agonist-mediated HO-1 activation prevents oxidative stress and associated dysfunctional adipogenesis in animals with an overactive renin–angiotensin system. AngII-dependent cardio-vascular pathologies in the 2k1clip model of hypertension are rescued in animals with increased levels of HO-1 is evidence of the ability of this antioxidant system to reverse the ROS-dependent effects of AngII [121].
We will examine, in this review, the role of HO-1, HO activity and the heme degradation products biliverdin/bilirubin and CO in mitigating obesity, oxidative stress and improving cell survival. This review will also focus on the role of basic research on the heme degradation pathway in the development of therapeutic approaches to prevent the onset of obesity, hypertension and diabetes and the adverse events that are manifest in the development and progression of atherosclerotic disease, [122, 123].
1.7. Impact of Heme on Adipocyte Differentiation-Adipogenesis and Obesity
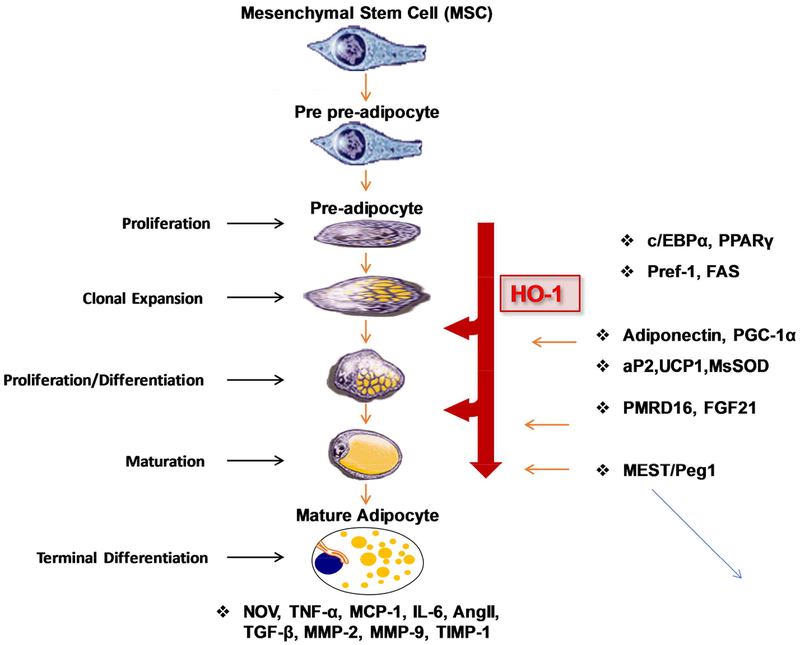
Heme is essential for the increase in pre-adipocyte differentiation, adipogenesis [31] and synthesis and an increase in heme is associated with recruitment of REV-ERB ligands and an increase in adipogenesis [113]. More recently, the heme-mediated increase of 3T3-cell differentiation was found to be dependent on suppression of sirtuin 1 (SIRT1) [124]. The effect of heme on cell differentiation was not limited to 3T3 cells as heme is indispensable for hematopoietic stem cells differentiation to myeloid and erythroid cell linages [125, 126]. While the decrease of cellular heme levels by an increase in heme degradation decreases adipogenesis, it increases osteoblast differentiation [127, 128]. Similar to HO-1 overexpression, EET increased osteoblast differentiation but decreased adipocyte differentiation [128–131]. This finding was strengthened by administration of CoPP which perturbed adipocyte differentiation in adult rats and resulted in a prompt weight loss, without a decrease in food consumption[132]. This beneficial effect of CoPP of reducing adiposity is shared by other pharmacological agents including hemin, Apo-A1 mimetic peptide L-4F and D-4F, EET and peroxisome proliferators-activated receptors alpha. HO-1 expression is also transcriptionally regulated by PPARα and PPARγ, indicating a mechanism of anti-inflammatory and antiproliferative action of PPAR ligands involved in the upregulation of HO-1 [133]. Hemin, EET, and L-4F are also associated with a decrease in visceral subcutaneous fat and an increase in insulin sensitivity [20, 123, 133–135], as well as a decrease in the number of large adipocytes (differentiated adipocytes) and an increase in the number of smaller “healthy” adipocytes [19, 130, 131, 136]. HO-1 derived CO and bilirubin attenuate obesity presumably via regulation of adiogenesis that maintains pre-adipocytes in an early differentiation stage, i.e., smaller adipocytes that are regarded as healthy, insulin adipocytes and are capable of producing adiponectin [135, 137, 138]. In contrast decreased hypertrophy resulted in large unflamed adipocytes and enhanced TNFα levels (Figure 4). Compounds which increase HO-1 levels and HO activity may have cardiovascular benefit as induction of HO-1 attenuates inflammatory markers and hypertension [139], reduces glomerular injury [140] and obstructive nephropathy [141]. In addition, CoPP induction of HO-1 protects skeletal muscle and ameliorates high fat diet induced liver injury [142–144].
Figure 4:
Schematic representation of the pathway from mesenchymal stem cell to mature adipocyte and where perturbations in the levels of HO-1 may affect this pathway. CO and bilirubin maintain pre-adipocytes in an early differentiation stage through regulation of adipogenesis.
The protective role of HO-1 in fatty liver [120] and in ischemia-reperfusion injury has recently been reviewed [145]. Fructose-mediated non-alcoholic fatty liver is attenuated by HO-1-Sirt1 [143] presumably by PPAR γ binding to HO-1 and prevent adipocyte dysfunction [121]. 5-aminolevulinic acid/sodium ferrous sulphate decreased body weight, fat weight, hepatic lipid deposits and improved levels of blood glucose while suppressing glomerular tuft area in mice fed a HFD. These effects were a consequence of increased HO-1 expression [146]. Quercetin protected TNFα induced muscle atrophy under obese conditions through Nrf2 mediated HO-1 induction accompanied by the inactivation of NF-Kb [147]. Oxidative stress, inflammation, complement inactivation and lipid metabolites in the retina are linked to obesity in ob/ob mice suggesting a therapeutic target for HO-1 [148]. Similarly, lutein and zeaxanthin modulated oxidative stress and increased HO-1 gene in retinal tissue [149].
It should be emphasized that HO-1 does not directly increase adiponectin per se; the HO-1-mediated antioxidant mechanism and decrease in heme are associated with an increase in thiol and superoxide dismutase levels and decreased levels of ROS resulting in increased levels of adiponectin [20, 38, 123, 134, 150, 151].
1.8. HO-1, Inflammation and Cardiovascular Disease
The protective role of HO-1 and CO on inflammation occurs in many different disease models including ethanol-induced liver cell death [152] and obesity induced liver fibrosis and lipid uptake increase, oxHDL and endothelial dysfunction in women with obesity [104, 119, 120]. Resveratrol both in vitro and in vivo upregulates HO-1 expression, NAD(P)H; quinone oxidoreductase 1, gamma glutamylcysteine synthetase via activation of nuclear factor (erythroid-derived)-like 2 (Nrf2) genes. The beneficial effects of resveratrol were attenuated in Nrf(−/−) mice fed a HFD, indicating that the endothelial protective function of resveratrol is mediated by the activation of Nrf2 [153]. Resveratrol as well as other natural HO-1 inducers are reported to prevent CVD [154]. Furthermore, loss of RIIP3 reduced ROS, inflammatory response and lipid deposition in PAL-stimulated cells. This was associated with activation of Nrf2 and HO-1 and that HFD induced hepatic steatosis was regulated by RIP3 through TLR-4/NF-kB and Nrf-2/HO-1 pathways [155]. The beneficial effect of HO-1 induction along with a subsequent increase in adiponectin and EET production is not limited to obesity. The HO-1-TTP signaling pathway has been shown to be effective in: 1) treatment of inflammatory diseases [156]; 2) induction of mitochondrial biogenesis [157]; 3) preservation of cardiac function [158]; 4) down regulation of inflammatory response to osteoarthritis [159]; 5) decreased LPS-induced vascular inflammation triggered by bacterial infection; 6) suppression of macrophage migration [160] 7) decreased contact hypersensitivity [161] and regulators of pregnancy and preeclampsia, highlighting a role as a valuable therapeutic tool in the management of a broad spectrum of health problems [162]. The absence of HO-1 exacerbated ventricle dilation in hypoxia [163], atherosclerosis and vascular remodeling [163]. CoPP-mediated upregulation of HO-1 inducing vascular and cardioprotection is well described [125,126] and includes HO-1 mediated breakdown of heme to bilirubin and CO, as well as direct signaling of HO-1 to nuclear transcription factors (Figure 5). CO releases molecules that offer cardioprotection [127] and both bilirubin and CO are potent antioxidants, antiapoptosis respectively, (Figure 5) that relieve oxidative stress, reduce pathological remodeling of the heart [164] and obesity, and increases left ventricular ejection fraction in humans and mice [165, 166]. Furthermore, HO-1 induction increases transcription of PGC-1, a key moderator of energy metabolism. PGC-1 targets SIRT3, a mitochondrial deacetylase, and promotes mitochondrial biogenesis, (Figure 6) suppression of ROS and improves mitochondrial FA oxidation [120] (Figure 6). Additionally, HO-1’s role in cardioprotection is amplified through reducing the proliferative response to vascular injury, and an increase in HO-1 inhibited lesion formation [128] in HO-1 deficient mice. Recently, Peterson et al. showed that HO-1 gene targeting of endothelial cells yields positive effects on adiposity and vascular dysfunction [167]. These findings all highlight HO-1’s protective properties against obesity and cardiovascular dysfunction.
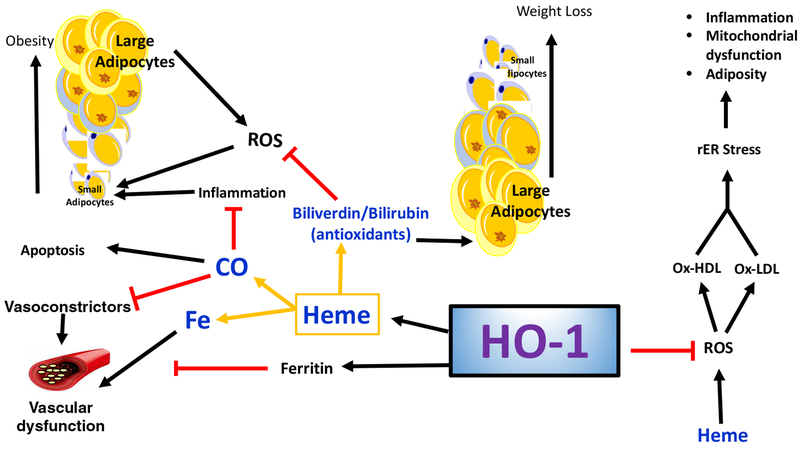
Figure 5:
Schematic representation of HO-1 pathways. HO-1 catalyzes the breakdown of stress inducing heme into its byproducts biliverdin/bilirubin, CO and free iron. Biliverdin/bilirubin attenuates adiposity via remodeling of hypertrophic adipocytes and inhibition of ROS-mediated adipogenesis. CO has anti-inflammatory and anti-apoptosis properties and offers cardioprotection via inhibition of vasoconstrictors. HO-1 also protects vascular function from deleterious free iron.
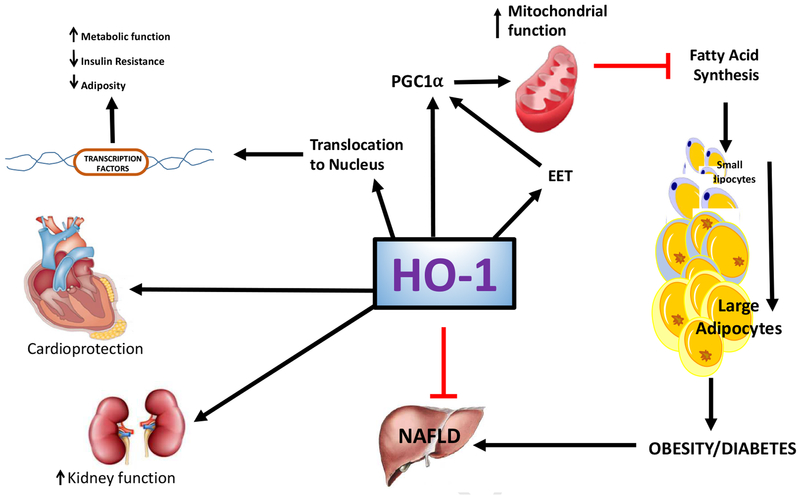
Figure 6:
HO-1 upregulates EET and PGC1α to increase mitochondrial biogenesis and integrity, which inhibits FAS and adiposity. Additionally, HO-1 has been shown to translocate to the nucleus and upregulate transcription factors to reduce insulin resistance and adiposity and increase metabolic function, although the exact mechanisms of this pathway have yet to be fully elucidated. HO-1 also promotes cardioprotective properties, shows improved kidney function and attenuates NAFLD and obesity.
Furthermore, CoPP improved both cardiac function and coronary flow by reducing oxidative stress, restoring eNOS/iNOS balance and increasing HO-1 levels thereby improving both endothelial function and insulin sensitivity in an animal model of diabetes [19, 50, 168]. Endothelial progenitor cell function inversely correlates with the long term glucose control in diabetic patients which is associated with a decrease in HO-1 and adiponectin levels[169]. These results demonstrate that HO-1 levels determine atherosclerotic lesion progression [170] and that the induction of the HO-1 pathway provides an important adaptive mechanism to reduce the severity of vascular dysfunction, thus representing a potential therapeutic target for vascular diseases.
1.9. HO-1 /HO-2 on Hypertension
The biological action of HO-1 and HO-2 gene expression suggests a capacity to participate in the regulation of renal function and blood pressure [8, 171–175]. HO-2 deficiency contributes to a diabetes-mediated increase in superoxide anion and renal dysfunction [176]. Salt-sensitive hypertension in Dahl salt-sensitive rats is exacerbated by inhibition of HO activity via inhibition of the pressure-natriuretic response [177]. Inhibition of HO activity blunts pressure-natriuresis via two mechanisms; the first being a decrease in renal blood flow, implying that the renal HO system supports renal circulation via formation of CO [178–180]. This hypothesis is supported by the upregulation of HO-1 expression by both CoPP [181] and SnCl2 which increases mesenteric artery relaxation in spontaneously hypertensive rats (SHR) and decreases the CYP4A-mediated generation of vasoconstrictors by 20-HETE, and that HO-1-derived CO counterbalances 20-HETE mediated vasoconstriction [9]. Secondly, HO-1 can regulate renal tubular function by regulating ROS production in renal tubules and by regulation of renal sodium transporters such as the NKCC2 channel of the thick ascending loop of Henle [182].
1.10. HO-1 and Regulation of Lipid-mediators Signaling in Hypertension and Obesity
The cytochrome P450 (CYP) monooxygenases/epoxygenases family is responsible for formation of 20-HETE and EETs [183, 184]. Upon formation, EETs are subjected to rapid hydrolysis by epoxide hydrolases (EHs) and ROS (preventable by HO-1 induction) to their respective dihydroxyepoxytrienoic acids (DHETs), as well as to esterification primarily to glycerophospholipids. Vasodilatory, anti-inflammatory and anti-apoptotic actions of EETs well established as is the fact that sEH inhibition increases cellular and circulating EET [185, 186]. EET and HO-1 appear to act symbiotically to form a module that serves as a molecular “switch” to genetically reprogram the adipocyte phenotype to express lower levels of MEST and prevent hypoadiponectinemia [130, 131, 136]. EETs are the first lipid-mediator derived from lipid metabolism via the CYP system known to regulate insulin sensitivity and abate obesity associated adipose tissue and vascular dysfunction; an effect which is reversed by inhibition of HO activity [12].
The mechanism by which EET increases HO-1 could be related to an EET-mediated decrease in Bach1, a known suppressor of HO-1 gene expression or increase of PGC1α, that is associated with increase of HO-1 [187, 188]. It is also possible that EETs act as a transcriptional regulator of the HO-1 promoter through glucocorticoid and AP-1 binding sites which are present on the human promoter [189]. These binding sites can activate HO-1 gene expression [189] and subsequently increase HO activity. Recently, a novel EET isomer, 11,12-EET, promoted hematopoietic stem cell transplant by activating a unique activation protein 1 and of P13K the pathway [190]. Thus EET activation of HO-1 may lead to the clinical application of EETs in drug development. EET increases PGC1α and subsequently increases HO-1 [187, 191, 192]. Recently, upregulation of HO-1 increases EETs, that resulted in expression of PGC1α (Figure 7).
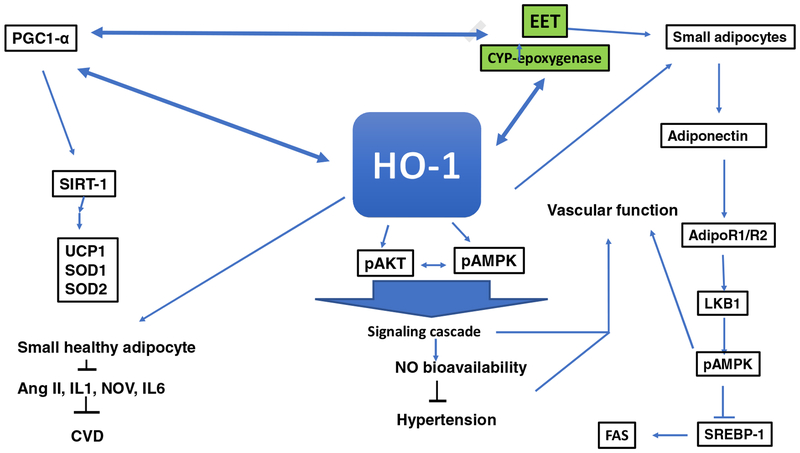
Figure 7:
Schematic representation of the potential mechanism of HO-1 signaling pathways. HO-1 signaling pathways act to improve vascular function and attenuate adiposity adipocyte differentiation. Some of these signaling targets are insulin receptors, adiponectin, via an increase in small adipocytes, EET, SIRT-1, Wnt10b, and β-catenin. The decrease in ROS as a result of an increase of HO-1 and HO-1 derived biliverdin/bilirubin provides stability to EET, leading to an enhancement of insulin sensitivity and an increase in vascular function. HO-1 also translocates into the mitochondria, increasing mitochondrial biogenesis and transport carriers and decreasing mitochondrial ROS [40–42].
It is clear that the pleiotropic effect of the HO system and its subsequent signalling mechanisms lead to increases in EETs, adiponectin and NO bioavailability. Activation of EETs can also increase HO activity. The antioxidant action of HO metabolites is associated with expansion of small adipocytes which are associated with increased adiponectin and downstream signals that include phosphorylated liver kinase B1 (pLKB1), pAMPK, phosphorylated endothelial nitric oxide synthase (peNOS) and an increase in NO bioavailability [12, 20, 129, 131, 193]. Upregulation of these pathways is associated with improved vascular function and attenuation of hypertension. It is evident that the pleiotropic effect of the HO system and signalling mechanism [12, 19, 39, 194, 195] and increase in biliverdin leads to increases in the protection of EET from degradation by ROS and adiponectin. Recently, ablation of soluble epoxide hydrolase increases EETs, and reprogram white fat to beige like fat through an increase of HO-1 [196]. These results establish the interdependence of five protective pathways, namely HO, EETs, bilirubin, carbon monoxide and adiponectin, all of which are affected by perturbations in HO activity and result in the prevention of obesity, hypertension and insulin resistance. Activation of these pathways also protects the vasculature from injury which is known to increase organ dysfunction and vascular diseases (Figure 7).
1.11. HO-1/HO-2 and Health Impact in Obesity
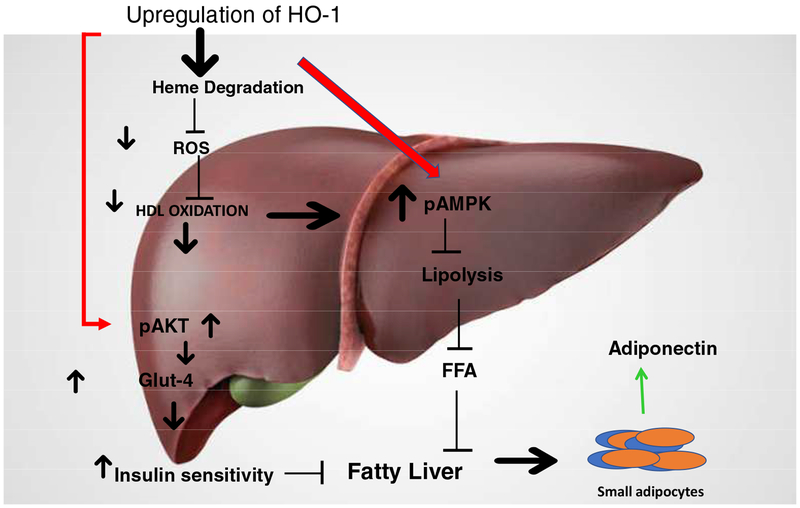
Abdominal obesity is associated with insulin resistance and the pathogenesis of T2DM and hypertension, contributes to high serum levels of LDL and triglycerides but low serum levels of HDL, and leads to the development of atherosclerotic CVD. HO-1-mediated decreases of ROS and LDL occurred in a number of diabetes models [20, 50]. Upregulation of hepatic HO-1 decreased heme and ROS in liver resulting in lowered levels of oxHDL and oxLDL. The subsequent increase in pAKT AND Glut4 increased insulin sensitivity. In addition, pAMPK was increased resulting in decreased lipolysis and FFA levels and the attenuation of fatty liver. Leptin-deficient mice and mice fed a HFD exhibit a metabolic syndrome-like phenotype which includes an increase in LDL which is amenable to rescue by increases in HO-1, HO activity and adiponectin [20]. Chronic HO-1 induction increased oxygen consumption, and lowered body weight in obese melanocortin-4 receptor deficient mice with an improvement in vascular function [197, 198]. Induction of HO-1 by inducers by gene transfer increased the number of “healthy” adipocytes, increased plasma adiponectin levels, improved insulin sensitivity and decreased inflammatory adipokine levels and blood pressure. (Figure 8). This beneficial effect of HO-1 induction on adipocyte morphology was confirmed in Zucker diabetic rats [199] and in ob/ob diabetic mice, where increased levels of HO-1 and HO activity prevented weight gain and decreased visceral and subcutaneous fat levels. Upregulation of HO-1 decreased adipogenesis in mesenchymal stem cells (MSCs) and increased adiponectin levels in culture media, these positive effects of increased HO-1 levels are reversed by inhibition of HO activity [108, 134]. These studies affirm the presence of an HO-1-adiponectin-EET regulatory module that is crucial in the development of therapeutic approaches to ameliorate the deleterious effects of obesity, diabetes and metabolic syndrome. Chronic HO-1 induction also increases oxygen consumption [104, 200]. This effect on oxygen consumption is independent of the melanocortin-4 system as chronic treatment of obese melanocortin-4 receptor deficient mice results in the attenuation of obesity and type II diabetes [197, 198]. The effect of HO-1 induction of oxygen consumption is likely mediated through increased levels of CO since chronic CORM treatment increased oxygen consumption and attenuated obesity in mice fed a high fat diet [201].
Figure 8:
Fatty acids (FAs) are metabolized in the mitochondria by β-oxidation. In the insulin resistance state, FAs increase in circulation and insulin resistance increases. FAs are transported into the cell by a membrane protein, activated by binding with coenzyme A and transported into the mitochondria by binding with carnitine. Excessive FAs cause mitochondrial dysfunction, TG accumulation, increased inflammation and LDL oxidation. Increased levels of HO-1 decrease oxHDL and oxLDL, increase pAMPK, stimulate lipolysis and decrease FFAs concentration.
While induction of HO-1 improved insulin sensitivity, downregulation of the peripheral endocannabinoid system, and a reduction in adipose tissue volume and adipose tissue remodeling, some sex dependent differences occur [20, 135]. Adipocyte HO-1 induction by CoPP, L-4F and VECAD promoter HO-1 attenuated metabolic syndrome in both obese male and female mice although the rate of weight gain was slowed only in obese male animals. [18, 20, 134]. These results emphasize that gender differences are critical in the development of therapeutic approaches targeting induction of HO-1 for the treatment of obesity and diabetes [20].
Another approach which may be beneficial for the treatment of obesity is the adipose-specific induction of HO-1. Induction of HO-1 in adipocytes reversed the detrimental metabolic consequences of obesity, including insulin resistance and dyslipidemia as well as decreasing blood pressure in a mouse model of obesity [20, 202]. These studies further highlight the protective cardiovascular role of the HO-1-adiponectin axis in hypertensive animals [50].
While adipose specific targeting of the HO-1 gene was successful in attenuating adiposity, vascular dysfunction and hypertension in mice fed a HFD, one report indicated that HO-1 overexpression in adipocytes does not protect against HFD induced obesity and the development of insulin resistance [203]. These differences in phenotypes reported between these two studies are not clear, although the specific activity of HO in the adipose tissue of the transgenic model was not reported. However, elevated levels of HO-1 derived bilirubin as seen in humans and mice with hyperbilirubinemia due to Gilbert’s Syndrome are resistant to hepatic steatosis [204]. Further, Serum bilirubin levels are negatively associated with abdominal obesity [205]. HO-1 derived bilirubin via biliverdin reductase A reduces hepatic steatosis [206]. In agreement with the concept that bilirubin plays an important role, obesity patients with Gilbert’s Syndrome and elevated levels of bilirubin are resistant to hepatic steatosis by decreased phosphorylation of PPARα [207]. More importantly serum bilirubin levels are negatively associated with abdominal obesity and hypertriglyceridemia [208].
In addition, HO-1-derived bilirubin attenuates obesity, as does HO-1 derived CO. Chronic treatment with a CO-releasing molecule reverses dietary induced obesity in mice [209]. Repetitive administration of CO donors such as CORM 401 produce transient uncoupling activity of CO resulting in a lower weight gain and increased insulin sensitivity in obese mice fed a HFD [210].
HO-1 overexpression ameliorated the development of non-alcoholic fatty liver disease in obese leptin deficient mice via a decrease in hepatic heme [211]. In addition, increased levels of HO-1 decreased hepatic lipid droplet size, fatty acid synthase levels, PPARα and glucose transporter 1; these beneficial effects were reversed by inhibition of HO activity indicating that low levels of HO-1 and HO activity exacerbate the development of obesity induced fatty liver [198, 211]. As in 3T3 adipocyte cells, an increase in heme increases adipogenesis [31, 113] without upregulation of HO-1 [113]; however, chronic induction of HO-1 decreases adipocyte heme which in turn decreases adipogenesis [131]. This decrease in adipogenesis is also associated with an increase in the levels of CYP-epoxygenase-derived EETs and adiponectin [212].
In a human study, increasing BMI in patients undergoing CABG correlated with increased levels of ROS and increased expression of p47 phox and xanthine oxidase and decreased levels of HO-1, eNOS and mitochondrial aldehyde dehydrogenase. In addition, VCAM-1 was elevated the right atrial myocardial tissue and with CCL5/RANTES in serum [213]. In an animal model of metabolic syndrome an EET agonist (AUDA) improved mitochondrial function and increased levels of PGC1α and HO-1 expression and normalized levels of inflammatory cytokines. Raspberry ketone increased the expression of HO-1 with an accompanying increase in brown like adipocyte formation. This effect was reversed by inhibition of HO activity [214]. Similarly, CoPP inhibited the development of type 2 diabetes in mice [215]. The use of natural occurring polyphenolic derivates to stimulate the HO system in metabolic dysfunction with a focus on the clinical role of HO-1 and HO activity to restore the balance between pro- and antioxidant systems has been recently reviewed [216].
A number of other clinical studies have examined the relationship between HO-1 and obesity. CD163 expression was upregulated in human adipose tissue and soluble CD163 concentration was elevated in obese (BMI > 40 Kg m−2) compared to lean subjects (BMI < 30 Kg M−2). The HO-1 gene was upregulated in adipose tissue and expressed predominantly in macrophages [217, 218] and in fat tissue [212]. Similarly, diminished upregulation of visceral adipose HO-1 correlates with waist-to-hip ratio and insulin resistance [219]. Visceral adipose tissue expression of HMOX1 negatively correlated with insulin resistance [219]. Morbid obesity is associated with thrombophilia. Adipocytes obtained from obese patients exhibited increased HO activity as nonsmoking bariatric patients increased COHb concentrations, indicative of HO-1 upregulation [220]. Assessment of HO activity by measuring CO production may yield conflicting results unless adequate steps are taken to differentiate between HO-dependent and HO-independent CO generation [221–223]. Increased CO formation in an HO-independent manner due to photo-oxidation was observed via the peroxidation of lipids along with the auto-oxidation of organic molecules such as phenols and flavonoids as a result of severe stress [221]. These differences in HO dependent versus HO independent CO generation must be considered when interpreting the results of studies in which CO production is measured as an index of HO activity.
1.12. Therapeutic Potential of HO-1 and Signaling Pathways
Induction of HO-1 restores six mitochondrial carriers, i.e., carnitine, citrate, phosphate, deoxynucleotide, ATP and dicarboxylate in diabetes [40]. An increase in AKT phosphorylation is also critical to cell survival in diabetes [224]. The alteration in mitochondrial function both in vitro and in vivo correlated with the levels of activation of AKT and the BcL-2 family of proteins [225, 226]. A decrease in BcL-2 family members contributed to apoptosis and the translocation of cytochrome c from the mitochondria to cytosol [224, 225, 227]. Activation of AKT augmented ATP synthesis [228] and promoted the association of hexokinase with the voltage-dependent anion channel (VDAC) channel and, in so doing, resulted in VDAC closure which blocked release of cytochrome c. The lack of HO-1/HO-2 resulting in decreased HO activity increased apoptotic cell death [118, 229, 230]. While these results suggest that increases in mitochondrial HO-1 may favorably modulate the balance between pro-and anti-apoptotic mechanisms, the clinical applicability of targeting either HO-1 or its metabolites, bilirubin and CO, specifically to the mitochondria has not been tested as therapeutic approach for the treatment of diabetes, although pre-clinical results support such a clinical application [16].
Obesity is a major cardiovascular risk factor and is manifest by increasing BMI in individuals undergoing CABG who have increased levels of ROS, p47 phox and xanthine oxidase, decreased levels of HO-1, eNOS and mitochondrial aldehyde dehydrogenase and elevated levels of inflammatory markers [213]. Improved endogenous epoxyeicosatrienoic acid levels improves heart function in metabolic syndrome (Figure 9). This effect is blocked by SnMP [231]. Brown like adipose tissue is considered “healthy” adipose tissue and promoting white adipose tissue to acquire brown like characteristics is a therapeutic approach to treat obesity. Raspberry ketone increased HO-1 and p62 while decreasing Atg12 in rats. This effect was blocked by inhibition of HO activity [214]. CoPP activated the Nrf2/HO-1 pathway potentiating the antinociceptive action of CB2R in type 2 diabetic mice [215]. Irisin regulates HO-1/adiponectin levels in perivascular adipose tissue with a resultant improvement in endothelial function in obese mice fed a HFD [232]. The ability of naturally occurring phenols to upregulate the HO system to improve metabolic syndrome in obesity and obesity related disease has recently been reviewed [216]. CYP450 epoxygenase derived EET reversed heart failure in obesity-induced diabetic cardiomyopathy through PGC-1α activation. An EET agonist decreased pericardial adipose expression of NOV, normalized fraction shortening, increased PGC-1α and HO-1 levels, insulin receptor phosphorylation and improved mitochondrial function. Deletion of PGC-1α reversed these effects in an obese mouse model of the metabolic syndrome [233]. HO-1 induction improved insulin sensitivity, down regulated the peripheral endocannabinoid system, reduced adipose tissue volume and resulted in adipose tissue remodeling in an obesity diabetic animal model [135]. These results suggest that HO-1 is a therapeutic target to improve obesity and its associated health risks and complications.
Figure 9:
Diminished HO-1 levels increased ROS resulting in increased inflammation [1–7] and vasoconstriction [8, 9] and decreased vasodilation [10–12], endothelial progenitor cells [13] and endothelial and cardiac cell function [16–23]. As seen in the scheme, ROS increase pro-inflammatory molecules [7, 27–30], angiotensin II [18, 29, 30, 33–35], free radicals [38, 39], VSM proliferation [43–49], LDL [20, 50], endothelin [51–53], and IL-18 [58]. The overall effect is the worsening of cardiovascular disease and the development of the disease state.
HO-1 expression is transcriptionally regulated by PPARα in human vascular endothelial and smooth muscle cells. This is indicative of both anti- inflammatory and anti-proliferative action of PPAR by upregulation of HO [133]. The discovery that downregulation of PGC-1α prevented the beneficial effect of EET-mediated increase of HO-1 and mitochondrial integrity and metabolic function in an animal model of obesity [187]. Ablation of adipose tissue HO-1 expression increased levels of white fat when compared to beige fat and decreased levels of PGC-1α in female mice [234]. CYP-450 epoxygenase derived epoxyeicosatrienoic acid reverses heart failure in an animal model of obesity induced cardiomyopathy through increased levels of PGC-1α [235]. More recently, beneficial effects of increased levels of the HO-1 and PGC-1α module include enhanced antioxidant activity and improved LV function are linked. HO-1-PGC-1α levels in epicardial fat promoted antioxidant formation with an increase in thermogenic gene levels essential for attenuating cardiometabolic dysfunction. Beta cell destruction, a result of elevated intracellular levels of ROS, comprising superoxide radicals, hydrogen peroxide and nitric oxide, is a process that occurs through both apoptotic and necrotic mechanisms [236]. T cell-mediated infiltration of the pancreas led to ROS generation and increased levels of proinflammatory cytokines. The HO system regulates T cell proliferation and immune response [237, 238]. The lack of HO-1 modulates T cell proliferation and maturation while CoPP increases HO-1 levels in CD4+ T cells [46, 239]. An increase in HO activity decreased infiltrated CD11c+ dendritic cells suggesting that increased HO activity can prevent the development and/or moderate the diabetic state [59]. HO-1 upregulation provided cytoprotection to pancreatic beta cells in vivo [240, 241]. Increased HO-1 levels have a salutary effect, modulating the pancreas phenotype and making beta cells resistant to oxidative stress and, thus, preventing the development of type 1 diabetes. A protective effect is also seen in diabetes where insulin increased HO-1 levels through the pI3K/Akt pathway and Nrf2 in renal cells [242]. HO-2 deficiency in diabetic HO-2 knockout mice caused major renal morphological injury and impaired renal function that was rescued by upregulation of HO-1 in the STZ animal model of diabetes [176].
The significant role that upregulation of HO-1 plays in obesity/diabetes stems from the presence of binding sites for several transcriptional factors including CRE B, OKT1, STATS and glucocorticoid-response elements that are expressed on the human HO-1 promoter [189, 243]. Targeting HO-1 and the products of the degradation of heme stems from the finding that induction of HO-1 increases oxygen consumption, heat production and lowers body weight [197]. Upregulation of HO-1 reduces body weight in obese animals, while also decreasing adipokines including TNF, IL-6, MCP-1 and increasing adipocyte secretion of adiponectin (Figure 7).
1.13. HO-1 Genetic Polymorphism and its Impact on Metabolic Diseases
The existence of genetic polymorphism in the HO-1 gene indicates the potential importance of HO-1 in the pathogenesis of cardiovascular and pulmonary diseases [244]. The larger the size of the (GT)n repeats in the HO-1 gene promoter, the greater the chance of reducing HO-1 indelibility by ROS in cigarette smoke and reducing bilirubin, resulting in the development of emphysema [244]. Patients with short (<25 GT) dinucleotide repeats in the HO-1 gene promoter on either allele had less restenosis than patients with longer (≥25 GT) dinucleotide repeats [245]. Diabetic patients who have Gilbert syndrome, have a lower rate of vascular complications, compared to individuals with normal bilirubin levels and diabetes [246]. Individuals with shorter (GT)n repeats, when compared to individuals with longer (GT)n repeats, have a higher transcriptional activity and thus higher HO-1 levels. In an Asian population, with type-2 diabetes and carrying longer (≥32) (GT)n repeats had higher oxidative stress and increased susceptibility to the development of coronary artery disease and atherosclerosis [247]. Individuals with significant risk factors (hyperlipidemia, diabetes, and smoking) for coronary artery disease and who possessed shorter (<27) (GT)n repeats were associated with less disease [248]. A cohort study found that patients with short (<25 GT) dinucleotide repeats in the HO-1 gene promoter on either allele had restenosis significantly less often that patients with longer (≥25 GT) dinucleotide repeats [249]. These data imply that up-regulation of HO-1, associated with shorter dinucleotide repeats, may be protective after balloon angioplasty. However, not all studies support the clinical effect of genetic polymorphism, for example, in a study of 1807 patients with coronary artery disease, no clinically relevant association of a HO-promoter polymorphism and ischemic events after coronary stenting was reported [250]. In support of this finding, no evidence of a protective effect for short alleles, i.e., low (GT)n repeat, for graft or recipient survival in clinical renal transplant was seen [251]. In addition to (GT)n dinucleotide-length polymorphism, a single nucleotide polymorphism in the HO-1 promoter, T(−413)A, correlated with a reduced incidence of ischemic heart disease [252]. In a study of 3,104 patients with vascular disease, restenosis after percutaneous coronary intervention was associated with angiotensin II-type l receptor 116 A/C polymorphism but was not associated with polymorphism of HO-1 [253]. These studies both advocate and/or contradict the role of the HO-1 gene in genetic polymorphism and atherosclerotic processes. In Japanese obese male subjects, higher numbers of individuals with a BMI < 25 kg/m2 had sparse dermis. The number of individuals with the long allele of the HMOX-1 promoter was higher in the obese sparse dermis group [254]. In more than 800 patients aged between 45–84 years [255], there was an association between the HO-1 variable number tandem repeat polymorphism and CVD confined to subjects with a high number of repeats on both HO-1 alleles, providing evidence of atherogenesis and decreased antioxidant defense system in vascular high risk subjects. These studies support an important role of HO-1 gene regulation in the atherosclerotic disease processes.
Recent research has focused on obesity and the human state. In human mammary epithelial cells (HMEC) estrogen-receptor positive MCF-7 cells and triple negative MDA-MB-231 cells leptin induced ROS production to a different degree in the 3 cell lines, most noticeably in HMEC where HO-1 levels increased[256]. Markers of obesity and growth in preeclamptic and normotensive pregnant women and HO-1 was higher in maternal and cord blood of preeclamptic women compared to normotensive pregnant women [257]. A potential role for statins as an inducer of the HO system in the treatment of preeclampsia has recently been advocated [258]. HO-1 induction attenuates fructose-induced hepatic lipid deposition, prevents hepatic fibrosis development and abates NAFLD vascular dysfunction. These effects are mediated by activation of SIRT1 gene expression [143]. Bariatric surgery decreases HO-1 levels in morbidly obese individuals with severe obstructive sleep apnea and is associated with decreases in insulin resistance and inflammation [259].
A new approach would examine whether increasing HO-1 expression via either pharmaceutical or genetic agents has the potential to correct for the GT repeat leading to low expression, or whether introducing anti-oxidative agents may correct for the increased oxidative stress caused by low HO-1 expression. Genetic testing may also have a role in identifying patients with HO-1 polymorphisms for which HO-1 based therapies could have a corrective effect.
1.14. Concluding Remarks
The wide spectrum of inducers of HO-1 highlights the pivotal role this enzyme plays in providing protection against metabolic insults in humans. This offers an obvious target for designing compounds with clinical application in multiple human disease states. This is in contrast to inhibitors of heme oxygenase activity where the successful use of inhibitors of HO activity has been widely described.
2. ACKNOWLEDGEMENTS
This work was supported by NIH grants HL55601 (NGA). We thank Mrs. Jennifer Brown for her outstanding assistance in preparing the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST:
None.
4. REFERENCES
- [1].Willis D, Moore AR, Frederick R, Willoughby DA, Heme oxygenase: a novel target for the modulation of the inflammatory response., Nat Med 2. 2 (1996) 87–90. [DOI] [PubMed] [Google Scholar]
- [2].Brusko TM, Wasserfall CH, Agarwal A, Kapturczak MH, Atkinson MA, An integral role for heme oxygenase-1 and carbon monoxide in maintaining peripheral tolerance by CD4+CD25+ regulatory T cells, J Immunol 174(9) (2005) 5181–5186. [DOI] [PubMed] [Google Scholar]
- [3].Ryter SW, Alam J, Choi AM, Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications, Physiol Rev 86(2) (2006) 583–650. [DOI] [PubMed] [Google Scholar]
- [4].Otterbein LE, Soares MP, Yamashita K, Bach FH, Heme oxygenase-1: unleashing the protective properties of heme, Trends Immunol 24(8) (2003) 449–455. [DOI] [PubMed] [Google Scholar]
- [5].George JF, Braun A, Brusko TM, Joseph R, Bolisetty S, Wasserfall CH, Atkinson MA, Agarwal A, Kapturczak MH, Suppression by CD4+CD25+ regulatory T cells is dependent on expression of heme oxygenase-1 in antigen-presenting cells, Am. J Pathol 173(1) (2008) 154–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Minamino T, Christou H, Hsieh CM, Liu Y, Dhawan V, Abraham NG, Perrella MA, Mitsialis SA, Kourembanas S, Targeted expression of heme oxygenase-1 prevents the pulmonary inflammatory and vascular responses to hypoxia, Proc. Natl. Acad. Sci. U. S. A 98(15) (2001) 8798–8803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Soares MP, Seldon MP, Gregoire IP, Vassilevskaia T, Berberat PO, Yu J, Tsui TY, Bach FH, Heme oxygenase-1 modulates the expression of adhesion molecules associated with endothelial cell activation, J. Immunol 172(6) (2004) 3553–3563. [DOI] [PubMed] [Google Scholar]
- [8].Kaide J-I, Zhang F, Wei Y, Jiang H, Yu C, Wang WH, Balazy M, Abraham NG, Nasjletti A, Carbon monoxide of vascular origin attenuates the sensitivity of renal arterial vessels to vasoconstrictors, J. Clin. Invest 107(9) (2001) 1163–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kaide J, Zhang F, Wei Y, Wang W, Gopal VR, Falck JR, Laniado-Schwartzman M, Nasjletti A, Vascular CO counterbalances the sensitizing influence of 20-HETE on agonist-induced vasoconstriction, Hypertension 44(2) (2004) 210–216. [DOI] [PubMed] [Google Scholar]
- [10].Sacerdoti D, Bolognesi M, Di PM, Gatta A, McGiff JC, Schwartzman ML, Abraham NG, Rat mesenteric arterial dilator response to 11,12-epoxyeicosatrienoic acid is mediated by activating heme oxygenase, Am. J Physiol Heart Circ. Physiol 291(4) (2006) H1999–H2002. [DOI] [PubMed] [Google Scholar]
- [11].Abraham NG, Quan S, Mieyal PA, Yang L, Burke-Wolin T, Mingone CJ, Goodman AI, Nasjletti A, Wolin MS, Modulation of cGMP by human HO-1 retrovirus gene transfer in pulmonary microvessel endothelial cells, Am. J Physiol Lung Cell Mol. Physiol 283(5) (2002) L1117–L1124. [DOI] [PubMed] [Google Scholar]
- [12].Abraham NG, Sodhi K, Silvis AM, Vanella L, Favero G, Rezzani R, Lee C, Zeldin DC, Schwartzman ML, CYP2J2 targeting to endothelial cells attenuates adiposity and vascular dysfunction in mice fed a high-fat diet by reprogramming adipocyte phenotype, Hypertension 64(6) (2014) 1352–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sambuceti G, Morbelli S, Vanella L, Kusmic C, Marini C, Massollo M, Augeri C, Corselli M, Ghersi C, Chiavarina B, Rodella LF, L’Abbate A, Drummond G, Abraham NG, Frassoni F, Diabetes Impairs the Vascular Recruitment of Normal Stem Cells by Oxidant Damage; Reversed by Increases in pAMPK, Heme Oxygenase-1 and Adiponectin, Stem Cells 27(2) (2009) 399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Otterbein LE, Choi AM, Heme oxygenase: colors of defense against cellular stress, Am. J. Physiol Lung Cell Mol. Physiol 279(6) (2000) L1029–L1037. [DOI] [PubMed] [Google Scholar]
- [15].Mishra M, Ndisang JF , A critical and comprehensive insight on heme oxygenase and related products including carbon monoxide, bilirubin, biliverdin and ferritin in type-1 and type-2 diabetes, Curr. Pharm. Des 20(9) (2014) 1370–1391. [DOI] [PubMed] [Google Scholar]
- [16].Dulak J, Deshane J, Jozkowicz A, Agarwal A, Heme oxygenase-1 and carbon monoxide in vascular pathobiology: focus on angiogenesis, Circulation 117(2) (2008) 231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Cao J, Drummond G, Inoue K, Sodhi K, Li XY, Omura S, Upregulation of Heme Oxygenase-1 Combined with Increased Adiponectin Lowers Blood Pressure in Diabetic Spontaneously Hypertensive Rats through a Reduction in Endothelial Cell Dysfunction, Apoptosis and Oxidative Stress, Int. J. Mol. Sci 9(12) (2008) 2388–2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Cao J, Sodhi K, Inoue K, Quilley J, Rezzani R, Rodella L, Vanella L, Germinario L, Stec DE, Abraham NG, Kappas A, Lentiviral-human heme oxygenase targeting endothelium improved vascular function in angiotensin II animal model of hypertension, Hum. Gene Ther 22(3) (2011) 271–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Cao J, Peterson SJ, Sodhi K, Vanella L, Barbagallo I, Rodella LF, Schwartzman ML, Abraham NG, Kappas A, Heme oxygenase gene targeting to adipocytes attenuates adiposity and vascular dysfunction in mice fed a high-fat diet, Hypertension 60(2) (2012) 467–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Burgess A, Li M, Vanella L, Kim DH, Rezzani R, Rodella L, Sodhi K, Canestraro M, Martasek P, Peterson SJ, Kappas A, Abraham NG, Adipocyte heme oxygenase-1 induction attenuates metabolic syndrome in both male and female obese mice, Hypertension 56(6) (2010) 1124–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kruger AL, Peterson S, Turkseven S, Kaminski PM, Zhang FF, Quan S, Wolin MS, Abraham NG, D-4F induces heme oxygenase-1 and extracellular superoxide dismutase, decreases endothelial cell sloughing, and improves vascular reactivity in rat model of diabetes, Circulation 111(23) (2005) 3126–3134. [DOI] [PubMed] [Google Scholar]
- [22].Cai C, Teng L, Vu D, He JQ, Guo Y, Li Q, Tang XL, Rokosh G, Bhatnagar A, Bolli R, The heme oxygenase 1 inducer (CoPP) protects human cardiac stem cells against apoptosis through activation of the extracellular signal-regulated kinase (ERK)/NRF2 signaling pathway and cytokine release, J. Biol. Chem 287(40) (2012) 33720–33732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Liu X, Pachori AS, Ward CA, Davis JP, Gnecchi M, Kong D, Zhang L, Murduck J, Yet SF, Perrella MA, Pratt RE, Dzau VJ, Melo LG, Heme oxygenase-1 (HO-1) inhibits postmyocardial infarct remodeling and restores ventricular function, FASEB J 20(2) (2006) 207–216. [DOI] [PubMed] [Google Scholar]
- [24].Balla G, Jacob HS, Balla J, Rosenberg M, Nath K, Apple F, Easton JW, Vercellotti GM, Ferritin: a cytoprotective antioxidant strategm of endothelium, J. Biol. Chem 267 (1992) 18148–18153. [PubMed] [Google Scholar]
- [25].Yunoki K, Inoue T, Sugioka K, Nakagawa M, Inaba M, Wada S, Ohsawa M, Komatsu R, Itoh A, Haze K, Yoshiyama M, Becker AE, Ueda M, Naruko T, Association between hemoglobin scavenger receptor and heme oxygenase-1-related anti-inflammatory mediators in human coronary stable and unstable plaques, Hum. Pathol 44(10) (2013) 2256–2265. [DOI] [PubMed] [Google Scholar]
- [26].Gozzelino R, Soares MP, Coupling heme and iron metabolism via ferritin H chain, Antioxid. Redox. Signal 20(11) (2014) 1754–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kushida T, LiVolti G, Goodman AI, Abraham NG, TNF-alpha-mediated cell death is attenuated by retrovirus delivery of human heme oxygenase-1 gene into human microvessel endothelial cells, Transplant. Proc 34(7) (2002) 2973–2978. [DOI] [PubMed] [Google Scholar]
- [28].Basuroy S, Bhattacharya S, Tcheranova D, Qu Y, Regan RF, Leffler CW, Parfenova H, HO-2 provides endogenous protection against oxidative stress and apoptosis caused by TNF-alpha in cerebral vascular endothelial cells, Am. J. Physiol Cell Physiol 291(5) (2006) C897–C908. [DOI] [PubMed] [Google Scholar]
- [29].Agarwal R, Proinflammatory effects of oxidative stress in chronic kidney disease: role of additional angiotensin II blockade, Am. J Physiol Renal Physiol 284(4) (2003) F863–F869. [DOI] [PubMed] [Google Scholar]
- [30].Mazza F, Goodman A, Lombardo G, Vanella A, Abraham NG, Heme oxygenase-1 gene expression attenuates angiotensin II-mediated DNA damage in endothelial cells, Exp. Biol. Med. (Maywood.) 228(5) (2003) 576–583. [DOI] [PubMed] [Google Scholar]
- [31].Chen JJ, London IM, Hemin enhances the differentiation of mouse 3T3 cells to adipocytes, Cell 26(1 Pt 1) (1981) 117–122. [DOI] [PubMed] [Google Scholar]
- [32].Abraham NG, Molecular regulation--biological role of heme in hematopoiesis, Blood Rev 5(1) (1991) 19–28. [DOI] [PubMed] [Google Scholar]
- [33].Abraham NG, Heme oxygenase attenuated angiotensin II-mediated increase in cyclooxygenase activity and decreased isoprostane F2alpha in endothelial cells, Thromb. Res 110(5–6) (2003) 305–309. [DOI] [PubMed] [Google Scholar]
- [34].Quan S, Yang L, Shenouda S, Schwartzman ML, Nasjletti A, Goodman AI, Abraham NG, Expression of human heme oxygenase-1 in the thick ascending limb attenuates angiotensin II-mediated increase in oxidative injury, Kidney Int 65(5) (2004) 1628–1639. [DOI] [PubMed] [Google Scholar]
- [35].Yang L, Quan S, Nasjletti A, Laniado-Schwartzman M, Abraham NG, Heme oxygenase-1 gene expression modulates angiotensin II-induced increase in blood pressure, Hypertension 43(6) (2004) 1221–1226. [DOI] [PubMed] [Google Scholar]
- [36].Ito T, Chen D, Chang CW, Kenmochi T, Saito T, Suzuki S, Takemoto JY, Mesobiliverdin IXalpha Enhances Rat Pancreatic Islet Yield and Function, Front Pharmacol 4 (2013) 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Dong H, Huang H, Yun X, Kim DS, Yue Y, Wu H, Sutter A, Chavin KD, Otterbein LE, Adams DB, Kim YB, Wang H, Bilirubin increases insulin sensitivity in leptin-receptor deficient and diet-induced obese mice through suppression of ER stress and chronic inflammation, Endocrinology 155(3) (2014) 818–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Abraham NG, Kushida T, McClung J, Weiss M, Quan S, Lafaro R, Darzynkiewicz Z, Wolin M, Heme oxygenase-1 attenuates glucose-mediated cell growth arrest and apoptosis in human microvessel endothelial cells, Circ. Res 93(6) (2003) 507–514. [DOI] [PubMed] [Google Scholar]
- [39].Abraham NG, Rezzani R, Rodella L, Kruger A, Taller D, Li VG, Goodman AI, Kappas A, Overexpression of human heme oxygenase-1 attenuates endothelial cell sloughing in experimental diabetes, Am. J Physiol Heart Circ. Physiol 287(6) (2004) H2468–H2477. [DOI] [PubMed] [Google Scholar]
- [40].Di Noia MA, Van DS, Palmieri F, Yang LM, Quan S, Goodman AI, Abraham NG, Heme oxygenase-1 enhances renal mitochondrial transport carriers and cytochrome C oxidase activity in experimental diabetes, J Biol. Chem 281(23) (2006) 15687–15693. [DOI] [PubMed] [Google Scholar]
- [41].Converso DP, Taille C, Carreras MC, Jaitovich A, Poderoso JJ, Boczkowski J, HO-1 is located in liver mitochondria and modulates mitochondrial heme content and metabolism, FASEB J (2006). [DOI] [PubMed] [Google Scholar]
- [42].Kim J, Zarjou A, Traylor AM, Bolisetty S, Jaimes EA, Hull TD, George JF, Mikhail FM, Agarwal A, In vivo regulation of the heme oxygenase-1 gene in humanized transgenic mice, Kidney Int 82(3) (2012) 278–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Duckers HJ, Boehm M, True AL, Yet SF, San H, Park JL, Clinton WR, Lee ME, Nabel GJ, Nabel EG, Heme oxygenase-1 protects against vascular constriction and proliferation, Nat. Med 7(6) (2001) 693–698. [DOI] [PubMed] [Google Scholar]
- [44].Durante W, Kroll MH, Christodoulides N, Peyton KJ, Schafer AI, Nitric oxide induces heme oxygenase-1 gene expression and carbon monoxide production in vascular smooth cells, Circ. Res 80 (1997) 557–564. [DOI] [PubMed] [Google Scholar]
- [45].Li Volti G, Wang J, Traganos F, Kappas A, Abraham NG, Differential effect of heme oxygenase-1 in endothelial and smooth muscle cell cycle progression, Biochem. Biophys Res Commun 296(5) (2002) 1077–1082. [DOI] [PubMed] [Google Scholar]
- [46].Chen S, Kapturczak MH, Wasserfall C, Glushakova OY, Campbell-Thompson M, Deshane JS, Joseph R, Cruz PE, Hauswirth WW, Madsen KM, Croker BP, Berns KI, Atkinson MA, Flotte TR, Tisher CC, Agarwal A, Interleukin 10 attenuates neointimal proliferation and inflammation in aortic allografts by a heme oxygenase-dependent pathway, Proc. Natl. Acad. Sci. U. S. A 102(20) (2005) 7251–7256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Liu XM, Chapman GB, Wang H, Durante W, Adenovirus-mediated heme oxygenase-1 gene expression stimulates apoptosis in vascular smooth muscle cells, Circulation 105(1) (2002) 79–84. [DOI] [PubMed] [Google Scholar]
- [48].Liu XM, Chapman GB, Peyton KJ, Schafer AI, Durante W, Carbon monoxide inhibits apoptosis in vascular smooth muscle cells, Cardiovasc. Res 55(2) (2002) 396–405. [DOI] [PubMed] [Google Scholar]
- [49].Morita T, Mitsialis SA, Hoike H, Liu Y, Kourembanas S, Carbon monoxide controls the proliferation of hypoxic smooth muscle cells, J. Biol. Chem 272 (1997) 32804–32809. [DOI] [PubMed] [Google Scholar]
- [50].Cao J, Inoue K, Sodhi K, Puri N, Peterson SJ, Rezzani R, Abraham NG, High-fat diet exacerbates renal dysfunction in SHR: reversal by induction of HO-1-adiponectin axis, Obesity. (Silver. Spring) 20(5) (2012) 945–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Govindaraju V, Teoh H, Hamid Q, Cernacek P, Ward ME, Interaction between endothelial heme oxygenase-2 and endothelin-1 in altered aortic reactivity after hypoxia in rats, Am. J. Physiol Heart Circ. Physiol 288(2) (2005) H962–H970. [DOI] [PubMed] [Google Scholar]
- [52].Stanford SJ, Walters MJ, Mitchell JA, Carbon monoxide inhibits endothelin-1 release by human pulmonary artery smooth muscle cells, Eur. J Pharmacol 486(3) (2004) 349–352. [DOI] [PubMed] [Google Scholar]
- [53].Zhang F, Kaide JI, Yang L, Jiang H, Quan S, Kemp R, Gong W, Balazy M, Abraham NG, Nasjletti A, CO modulates pulmonary vascular response to acute hypoxia: relation to endothelin, Am. J Physiol Heart Circ. Physiol 286(1) (2004) H137–H144. [DOI] [PubMed] [Google Scholar]
- [54].Kapitulnik J, Maines MD, Pleiotropic functions of biliverdin reductase: cellular signaling and generation of cytoprotective and cytotoxic bilirubin, Trends Pharmacol. Sci 30(3) (2009) 129–137. [DOI] [PubMed] [Google Scholar]
- [55].Ahmad Z, Salim M, Maines MD, Human biliverdin reductase is a leucine zipper-like DNA-binding protein and functions in transcriptional activation of heme oxygenase-1 by oxidative stress, J. Biol. Chem 277(11) (2002) 9226–9232. [DOI] [PubMed] [Google Scholar]
- [56].Estabrook RW, Cooper DY, Rosenthal O, The light reversible carbon monoxide inhibition of the steroid C21-hydroxylase system of the adrenal cortex, Biochem. Z 338 (1963) 741–755. [PubMed] [Google Scholar]
- [57].Tenhunen R, Marver H, Pimstone NR, Trager WF, Cooper DY, Schmid R, Enzymatic degradation of heme. Oxygenative cleavage requiring cytochrome P-450, Biochemistry 11(9) (1972) 1716–1720. [DOI] [PubMed] [Google Scholar]
- [58].Zabalgoitia M, Colston JT, Reddy SV, Holt JW, Regan RF, Stec DE, Rimoldi JM, Valente AJ, Chandrasekar B, Carbon monoxide donors or heme oxygenase-1 (HO-1) overexpression blocks interleukin-18-mediated NF-kappaB-PTEN-dependent human cardiac endothelial cell death, Free Radic. Biol. Med 44(3) (2008) 284–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Abraham NG, Kappas A, Pharmacological and clinical aspects of heme oxygenase, Pharmacol. Rev 60 (2008) 79–127. [DOI] [PubMed] [Google Scholar]
- [60].Abraham NG, Lutton JD, Levere RD, Erythroid colony development as a function of age: the role of marrow cellular heme, J. Gerontol 38(1) (1983) 13–18. [DOI] [PubMed] [Google Scholar]
- [61].Abraham NG, Feldman E, Falck JR, Lutton JD, Schwartzman ML, Modulation of erythropoiesis by novel human bone marrow cytochrome P450-dependent metabolites of arachidonic acid, Blood 78(6) (1991) 1461–1466. [PubMed] [Google Scholar]
- [62].Abraham NG, Drummond GS, Lutton JD, Kappas A, The biological significance and physiological role of heme oxygenase, Cell. Physiol. Biochem 6 (1996) 129–168. [Google Scholar]
- [63].Kappas A, Drummond G, Control of heme metabolism with synthetic metalloporphyrins, J. Clin. Invest 77 (1986) 335–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Kappas A, A method for interdicting the development of severe jaundice in newborns by inhibiting the production of bilirubin, Pediatrics 113(1 Pt 1) (2004) 119–123. [DOI] [PubMed] [Google Scholar]
- [65].Drummond GS, Kappas A, Chemoprevention of neonatal jaundice: potency of tin-protoporphyrin in an animal model, Science 217(4566) (1982) 1250–1252. [DOI] [PubMed] [Google Scholar]
- [66].Lin JH, Villalon P, Martasek P, Abraham NG, Regulation of heme oxygenase gene expression by cobalt in rat liver and kidney, Eur. J Biochem 192(3) (1990) 577–582. [DOI] [PubMed] [Google Scholar]
- [67].Kruger AL, Peterson SJ, Schwartzman ML, Fusco H, McClung JA, Weiss M, Shenouda S, Goodman AI, Goligorsky MS, Kappas A, Abraham NG, Up-regulation of heme oxygenase provides vascular protection in an animal model of diabetes through its antioxidant and antiapoptotic effects, J Pharmacol. Exp. Ther 319(3) (2006) 1144–1152. [DOI] [PubMed] [Google Scholar]
- [68].Kinobe R, Ji Y, Nakatsu K, Peroxynitrite-mediated inactivation of heme oxygenases, BMC. Pharmacol 4(1) (2004) 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Jazwa A, Stoszko M, Tomczyk M, Bukowska-Strakova K, Pichon C, Jozkowicz A, Dulak J, HIF-regulated HO-1 gene transfer improves the post-ischemic limb recovery and diminishes TLR-triggered immune responses - Effects modified by concomitant VEGF overexpression, Vascul. Pharmacol 71 (2015) 127–138. [DOI] [PubMed] [Google Scholar]
- [70].Li Q, Guo Y, Ou Q, Wu WJ, Chen N, Zhu X, Tan W, Yuan F, Dawn B, Luo L, Hunt GN, Bolli R, Gene transfer as a strategy to achieve permanent cardioprotection II: rAAV-mediated gene therapy with heme oxygenase-1 limits infarct size 1 year later without adverse functional consequences, Basic Res. Cardiol 106(6) (2011) 1367–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Du D, Chang S, Chen B, Zhou H, Chen ZK, Adenovirus-mediated heme oxygenase transfer inhibits graft arteriosclerosis in rat aortic transplants, Transplant. Proc 39(10) (2007) 3446–3448. [DOI] [PubMed] [Google Scholar]
- [72].Liu X, Simpson JA, Brunt KR, Ward CA, Hall SR, Kinobe RT, Barrette V, Tse MY, Pang SC, Pachori AS, Dzau VJ, Ogunyankin KO, Melo LG, Preemptive heme oxygenase-1 gene delivery reveals reduced mortality and preservation of left ventricular function 1 yr after acute myocardial infarction, Am. J. Physiol Heart Circ. Physiol 293(1) (2007) H48–H59. [DOI] [PubMed] [Google Scholar]
- [73].Chen W, Yang S, Ping W, Fu X, Xu Q, Wang J, CYP2J2 and EETs protect against lung ischemia/reperfusion injury via anti-inflammatory effects in vivo and in vitro, Cell Physiol Biochem 35(5) (2015) 2043–2054. [DOI] [PubMed] [Google Scholar]
- [74].Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN, Bilirubin is an antioxidant of possible physiological importance, Science 235(4792) (1987) 1043–1046. [DOI] [PubMed] [Google Scholar]
- [75].Hopkins PN, Wu LL, Hunt SC, James BC, Vincent GM, Williams RR, Higher serum bilirubin is associated with decreased risk for early familial coronary artery disease, Arterioscler. Thromb. Vasc. Biol 16(2) (1996) 250–255. [DOI] [PubMed] [Google Scholar]
- [76].Verma A, Hirsch DJ, Glatt CE, Ronnett GV, Snyder SH, Carbon monoxide: a putative neural messenger, Science 259 (1993) 381–384. [DOI] [PubMed] [Google Scholar]
- [77].Zhan Y, Kim S, Izumi Y, Izumiya Y, Nakao T, Miyazaki H, Iwao H, Role of JNK, p38, and ERK in platelet-derived growth factor-induced vascular proliferation, migration, and gene expression, Arterioscler. Thromb. Vasc. Biol 23(5) (2003) 795–801. [DOI] [PubMed] [Google Scholar]
- [78].Kim HP, Ryter SW, Choi AM, CO as a cellular signaling molecule, Annu. Rev. Pharmacol. Toxicol 46 (2006) 411–449. [DOI] [PubMed] [Google Scholar]
- [79].Cashore WJ, Oh W, Unbound bilirubin and kernicterus in low-birth-weight infants, Pediatrics 69(4) (1982) 481–485. [PubMed] [Google Scholar]
- [80].Newman TB, Maisels MJ, Evaluation and treatment of jaundice in the term newborn: a kinder, gentler approach, Pediatrics 89(5 Pt 1) (1992) 809–818. [PubMed] [Google Scholar]
- [81].McDonagh AF, Palma LA, Trull FR, Phototherapy for neonatal jaundice: configurational isomers of bilirubin, J. Am. Chem. Soc 104 (1982) 6865–6867. [Google Scholar]
- [82].Abdy NA, Martinez R, Chea I, Boczar B, Nuno T, Woolridge D, A pilot study demonstrating the efficacy of transcutaneous bilirubin meters to quantitatively differentiate contusions from Congenital Dermal Melanocytosis, Child Abuse Negl 80 (2018) 108–112. [DOI] [PubMed] [Google Scholar]
- [83].Drummond GS, Kappas A, Sn-protoporphyrin inhibition of fetal and neonatal brain heme oxygenase. Transplacental passage of the metalloporphyrin and prenatal suppression of hyperbilirubinemia in the newborn animal, J. Clin. Invest 77(3) (1986) 971–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Vlahakis JZ, Kinobe RT, Bowers RJ, Brien JF, Nakatsu K, Szarek WA, Imidazole-dioxolane compounds as isozyme-selective heme oxygenase inhibitors, J. Med. Chem 49(14) (2006) 4437–4441. [DOI] [PubMed] [Google Scholar]
- [85].Berglund L, Angelin B, Blomstrand R, Drummond G, Kappas A, Sn-protoporphyrin lowers serum bilirubin levels, decreases biliary bilirubin output, enhances biliary heme excretion and potently inhibits hepatic heme oxygenase activity in normal human subjects, Hepatology 8(3) (1988) 625–631. [DOI] [PubMed] [Google Scholar]
- [86].Berglund L, Angelin B, Hultcrantz R, Einarsson K, Emtestam L, Drummond G, Kappas A, Studies with the haeme oxygenase inhibitor Sn-protoporphyrin in patients with primary biliary cirrhosis and idiopathic haemochromatosis, Gut 31(8) (1990) 899–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Galbraith RA, Drummond GS, Kappas A, Suppression of bilirubin production in the Crigler-Najjar type I syndrome: studies with the heme oxygenase inhibitor tin-mesoporphyrin [see comments], Pediatrics 89(2) (1992) 175–182. [PubMed] [Google Scholar]
- [88].Rubaltelli FF, Guerrini P, Reddi E, Jori G, Tin-protoporphyrin in the management of children with Crigler-Najjar disease, Pediatrics 84(4) (1989) 728–31. [PubMed] [Google Scholar]
- [89].Valaes TS, Petmezaki S, Henschke GS, Drummond S, Kappas A, Control of jaundice in preterm newborns by an inhibitor of bilirubin production: studies with tin-mesoporphyrin, Pediatrics 93 (1994) 1–11. [PubMed] [Google Scholar]
- [90].Kappas A, Drummond GS, Henschke C, Valaes T, Direct comparison of Sn-mesoporphyrin, an inhibitor of bilirubin production, and phototherapy in controlling hyperbilirubinemia in term and near-term newborns, Pediatrics 95(4) (1995) 468–474. [PubMed] [Google Scholar]
- [91].Martinez JC, Garcia HO, Otheguy LE, Drummond GS, Kappas A, Treatment of hyperbilirubinemia pharmacologic approach SnMP(tin-mesoporphyrin), J Perinatol 21 Suppl 1 (2001) S101–3; discussion S104–7. [DOI] [PubMed] [Google Scholar]
- [92].Valaes T, Drummond GS, Kappas A, Control of hyperbilirubinemia in glucose-6-phosphate dehydrogenase-deficient newborns using an inhibitor of bilirubin production, Sn-mesoporphyrin, Pediatrics 101(5) (1998) E1. [DOI] [PubMed] [Google Scholar]
- [93].Stevenson DK, Wong RJ, Metalloporphyrins in the management of neonatal hyperbilirubinemia, Semin Fetal Neonatal Med 15(3) (2010) 164–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Schulz S, Wong RJ, Vreman HJ, Stevenson DK, Metalloporphyrins - an update, Front Pharmacol 3 (2012) 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Kappas A, Simionatto CS, Drummond GS, Sassa S, Anderson KE, The liver excretes large amounts of heme into bile when heme oxygenase is inhibited competitively by Sn-protoporphyrin, Proc. Natl. Acad. Sci. U. S. A 82(3) (1985) 896–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Drummond GS, Galbraith RA, Sardana MK, Kappas A, Reduction of the C2 and C4 vinyl groups of Sn-protoporphyrin to form Sn- mesoporphyrin markedly enhances the ability of the metalloporphyrin to inhibit in vivo heme catabolism, Arch. Biochem. Biophys 255(1) (1987) 64–74. [DOI] [PubMed] [Google Scholar]
- [97].Simionatto CS, Anderson KE, Drummond GS, Kappas A, Studies on the mechanism of Sn-protoporphyrin suppression of hyperbilirubinemia. Inhibition of heme oxidation and bilirubin production, J Clin Invest 75(2) (1985) 513–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Sardana MK, Kappas A, Dual control mechanism for heme oxygenase: tin(IV)-protoporphyrin potently inhibits enzyme activity while markedly increasing content of enzyme protein in liver, Proc. Natl. Acad. Sci. U. S. A 84(8) (1987) 2464–2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Galbraith RA, Kappas A, Regulation of food intake and body weight by cobalt porphyrins in animals, Proc. Natl. Acad. Sci. U. S. A 86(19) (1989) 7653–7657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Dover SB, Graham A, Fitzsimons E, Moore MR, McColl KE, Haem-arginate plus tin-protoporphyrin for acute hepatic porphyria, Lancet 338(8761) (1991) 263. [DOI] [PubMed] [Google Scholar]
- [101].Zhang M, Nakamura K, Kageyama S, Lawal AO, Gong KW, Bhetraratana M, Fujii T, Sulaiman D, Hirao H, Bolisetty S, Kupiec-Weglinski JW, Araujo JA, Myeloid HO-1 modulates macrophage polarization and protects against ischemia-reperfusion injury, JCI. Insight 3(19) (2018) e120596–s158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Nakamura KZ; Kageyama S; Ke B; Fujii T; Sosa RA; Reed EF; Datta N; Zarrinpar A; Busuttil RW; Araujo JA; Kupiec-Weglinski JW;, Macrophage heme oxygenase-1-SIRT1-p53 axis regulates sterile inflammation in liver ischemia-reperfusion injury, J Hepatol 67(6) (2017) 1232–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Li S, Fujino M, Ichimaru N, Kurokawa R, Hirano S, Mou L, Takahara S, Takahara T, Li XK, Molecular hydrogen protects against ischemia-reperfusion injury in a mouse fatty liver model via regulating HO-1 and Sirt1 expression, Sci Rep 8(1) (2018) 14019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Raffaele M, Bellner L, Singh SP, Favero G, Rezzani R, Rodella LF, Falck JR, Abraham NG, Vanella L, Epoxyeicosatrienoic intervention improves NAFLD in leptin receptor deficient mice by an increase in PGC1alpha-HO-1-PGC1alpha-mitochondrial signaling, Experimental cell research 380(2) (2019) 180–187. [DOI] [PubMed] [Google Scholar]
- [105].Hall JE, The kidney, hypertension, and obesity, Hypertension 41(3 Pt 2) (2003) 625–633. [DOI] [PubMed] [Google Scholar]
- [106].Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM, Prevalence of overweight and obesity in the United States, 1999–2004, JAMA 295(13) (2006) 1549–1555. [DOI] [PubMed] [Google Scholar]
- [107].Fontana L, Eagon JC, Trujillo ME, Scherer PE, Klein S, Visceral fat adipokine secretion is associated with systemic inflammation in obese humans, Diabetes 56(4) (2007) 1010–1013. [DOI] [PubMed] [Google Scholar]
- [108].Li M, Kim DH, Tsenovoy PL, Peterson SJ, Rezzani R, Rodella LF, Aronow WS, Ikehara S, Abraham NG, Treatment of obese diabetic mice with a heme oxygenase inducer reduces visceral and subcutaneous adiposity, increases adiponectin levels, and improves insulin sensitivity and glucose tolerance, Diabetes 57(6) (2008) 1526–1535. [DOI] [PubMed] [Google Scholar]
- [109].Briggs DB, Jones CM, Mashalidis EH, Nunez M, Hausrath AC, Wysocki VH, Tsao TS, Disulfide-dependent self-assembly of adiponectin octadecamers from trimers and presence of stable octadecameric adiponectin lacking disulfide bonds in vitro, Biochemistry 48(51) (2009) 12345–12357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Wang ZV, Scherer PE, Adiponectin, cardiovascular function, and hypertension, Hypertension 51(1) (2008) 8–14. [DOI] [PubMed] [Google Scholar]
- [111].Elmarakby AA, Imig JD, Obesity is the major contributor to vascular dysfunction and inflammation in high fat diet hypertensive rats, Clin. Sci. (Lond) 118 (2010) 291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Wang MH, Smith A, Zhou Y, Chang HH, Lin S, Zhao X, Imig JD, Dorrance AM, Downregulation of renal CYP-derived eicosanoid synthesis in rats with diet-induced hypertension, Hypertension 42(4) (2003) 594–599. [DOI] [PubMed] [Google Scholar]
- [113].Kumar N, Solt LA, Wang Y, Rogers PM, Bhattacharyya G, Kamenecka TM, Stayrook KR, Crumbley C, Floyd ZE, Gimble JM, Griffin PR, Burris TP, Regulation of adipogenesis by natural and synthetic REV-ERB ligands, Endocrinology 151(7) (2010) 3015–3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Cassis LA, Police SB, Yiannikouris F, Thatcher SE, Local adipose tissue renin-angiotensin system, Curr. Hypertens. Rep 10(2) (2008) 93–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Boustany CM, Bharadwaj K, Daugherty A, Brown DR, Randall DC, Cassis LA, Activation of the systemic and adipose renin-angiotensin system in rats with diet-induced obesity and hypertension, Am. J Physiol Regul. Integr. Comp Physiol 287(4) (2004) R943–R949. [DOI] [PubMed] [Google Scholar]
- [116].Chang SH, Barbosa-Tessmann I, Chen C, Kilberg MS, Agarwal A, Glucose deprivation induces heme oxygenase-1 gene expression by a pathway independent of the unfolded protein response, J. Biol. Chem 277(3) (2002) 1933–1940. [DOI] [PubMed] [Google Scholar]
- [117].Chang SH, Garcia J, Melendez JA, Kilberg MS, Agarwal A, Haem oxygenase 1 gene induction by glucose deprivation is mediated by reactive oxygen species via the mitochondrial electron-transport chain, Biochem. J 371(Pt 3) (2003) 877–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Quan S, Kaminski PM, Yang L, Morita T, Inaba M, Ikehara S, Goodman AI, Wolin MS, Abraham NG, Heme oxygenase-1 prevents superoxide anion-associated endothelial cell sloughing in diabetic rats, Biochem. Biophys. Res. Commun 315(2) (2004) 509–516. [DOI] [PubMed] [Google Scholar]
- [119].Peterson SJ, Shapiro JI, Thompson E, Singh S, Liu L, Weingarten JA, O’Hanlon K, Bialczak A, Bhesania SR, Abraham NG, Oxidized HDL, Adipokines, and Endothelial Dysfunction: A Potential Biomarker Profile for Cardiovascular Risk in Women with Obesity, Obesity (Silver Spring) 27(1) (2019) 87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Sacerdoti D, Singh SP, Schragenheim J, Bellner L, Vanella L, Raffaele M, Meissner A, Grant I, Favero G, Rezzani R, Rodella LF, Bamshad D, Lebovics E, Abraham NG, Development of NASH in Obese Mice is Confounded by Adipose Tissue Increase in Inflammatory NOV and Oxidative Stress, Int J Hepatol 2018 (2018) 3484107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Sodhi K, Puri N, Hyun KD, Hinds TD Jr., Stechschulte LA, Favero G, Rodella L, Shapiro JI, Jude D, Abraham NG, PPAR-delta binding to heme oxygenase 1 promoter prevents angiotensin II induced adipocyte dysfunction in goldblatt hypertensive rats, Int. J Obes. (Lond) (2013). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [122].Abraham NG, Tsenovoy PL, McClung J, Drummond GS, Heme oxygenase: a target gene for anti-diabetic and obesity, Curr. Pharm. Des 14(5) (2008) 412–421. [DOI] [PubMed] [Google Scholar]
- [123].Ndisang JF, Role of heme oxygenase in inflammation, insulin-signalling, diabetes and obesity, Mediators. Inflamm 2010 (2010) 359732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Puri N, Sodhi K, Haarstad M, Kim DH, Bohinc S, Foglio E, Favero G, Abraham NG, Heme induced oxidative stress attenuates sirtuin1 and enhances adipogenesis in mesenchymal stem cells and mouse pre-adipocytes, J. Cell Biochem 113(6) (2012) 1926–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Chertkov JL, Jiang S, Lutton JD, Levere RD, Abraham NG, Hemin stimulation of hemopoiesis in murine long-term bone marrow culture, Exp. Hematol 19(9) (1991) 905–909. [PubMed] [Google Scholar]
- [126].Lutton JD, Chertkov JL, Jiang S, Kappas A, Levere RD, Abraham NG, Synergistic effect of heme and IL-1 on hematopoietic stromal regeneration after radiation, Am. J Hematol 44(3) (1993) 172–178. [DOI] [PubMed] [Google Scholar]
- [127].Barbagallo I, Vanella A, Peterson S, Kim DH, Tibullo D, Giallongo C, Vanella L, Parrinello N, Palumbo G, Di Raimondo F, NG A, Asprinio D, Overexpression of heme oxygenase-1 increases human osteoblast stem cell differentiation, J Bone Miner Metab 28(3) (2010) 276–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Vanella L, Kim DH, Asprinio D, Peterson SJ, Barbagallo I, Vanella A, Goldstein D, Ikehara S, Kappas A, Abraham NG, HO-1 expression increases mesenchymal stem cell-derived osteoblasts but decreases adipocyte lineage, Bone 46(1) (2010) 236–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Burgess AP, Vanella L, Bellner L, Gotlinger K, Falck JR, Abraham NG, Schwartzman ML, Kappas A, Heme oxygenase (HO-1) rescue of adipocyte dysfunction in HO-2 deficient mice via recruitment of epoxyeicosatrienoic acids (EETs) and adiponectin, Cell Physiol Biochem 29(1–2) (2012) 99–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Kim DH, Vanella L, Inoue K, Burgess A, Gotlinger K, Manthati VL, Koduru SR, Zeldin DC, Falck JR, Schwartzman ML, Abraham NG, Epoxyeicosatrienoic acid agonist regulates human mesenchymal stem cell-derived adipocytes through activation of HO-1-pAKT signaling and a decrease in PPARgamma, Stem Cells Dev 19(12) (2010) 1863–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Vanella L, Kim DH, Sodhi K, Barbagallo I, Burgess AP, Falck JR, Schwartzman ML, Abraham NG, Crosstalk between EET and HO-1 downregulates Bach1 and adipogenic marker expression in mesenchymal stem cell derived adipocytes, Prostaglandins Other Lipid Mediat 96(1–4) (2011) 54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Galbraith RA, Kappas A, Regulation of food intake and body weight in rats by the synthetic heme analogue cobalt protoporphyrin, Am J Physiol 261(6 Pt 2) (1991) R1388–R1394. [DOI] [PubMed] [Google Scholar]
- [133].Kronke G, Kadl A, Ikonomu E, Bluml S, Furnkranz A, Sarembock IJ, Bochkov VN, Exner M, Binder BR, Leitinger N, Expression of heme oxygenase-1 in human vascular cells is regulated by peroxisome proliferator-activated receptors, Arterioscler. Thromb. Vasc. Biol 27(6) (2007) 1276–1282. [DOI] [PubMed] [Google Scholar]
- [134].Peterson SJ, Drummond G, Kim DH, Li M, Kruger AL, Ikehara S, Abraham NG, L-4F treatment reduces adiposity, increases adiponectin levels, and improves insulin sensitivity in obese mice, J. Lipid Res 49(8) (2008) 1658–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Nicolai A, Li M, Kim DH, Peterson SJ, Vanella L, Positano V, Gastaldelli A, Rezzani R, Rodella LF, Drummond G, Kusmic C, L’Abbate A, Kappas A, Abraham NG, Heme oxygenase-1 induction remodels adipose tissue and improves insulin sensitivity in obesity-induced diabetic rats, Hypertension 53(3) (2009) 508–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Vanella L, Sodhi K, Kim DH, Puri N, Maheshwari M, Hinds TD Jr., Bellner L, Goldstein D, Peterson SJ, Shapiro JI, Abraham NG, Increased heme-oxygenase 1 expression decreases adipocyte differentiation and lipid accumulation in mesenchymal stem cells via upregulation of the canonical Wnt signaling cascade, Stem Cell Res. Ther 4(2) (2013) 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Vanella L, Sodhi K, Kim DH, Puri N, Maheshwari M, Hinds TD, Bellner L, Goldstein D, Peterson SJ, Shapiro JI, Abraham NG, Increased heme-oxygenase 1 expression in mesenchymal stem cell-derived adipocytes decreases differentiation and lipid accumulation via upregulation of the canonical Wnt signaling cascade, Stem Cell Res. Ther 4(2) (2013) 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Peterson SJ, Vanella L, Gotlinger K, Jiang H, Bialczak A, Singh SP, Sodhi K, Maher E, O’Hanlon K, Shapiro JI, Abraham NG, Oxidized HDL is a Potent Inducer of Adipogenesis and Causes Activation of the Ang-II and 20-HETE Systems in Human Obese Females, Prostaglandins Other Lipid Mediat (2016). [DOI] [PubMed] [Google Scholar]
- [139].Elmarakby AA, Faulkner J, Baban B, Sullivan JC, Induction of hemeoxygenase-1 reduces renal oxidative stress and inflammation in diabetic spontaneously hypertensive rats, Int. J. Hypertens 2012 (2012) 957235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Elmarakby AA, Faulkner J, Baban B, Saleh MA, Sullivan JC, Induction of hemeoxygenase-1 reduces glomerular injury and apoptosis in diabetic spontaneously hypertensive rats, Am. J. Physiol Renal Physiol 302(7) (2012) F791–F800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Iwai T, Kitamoto K, Teramoto K, Machida Y, Tamada S, Yukimura T, Iwao H, Nakatani T, Miura K, Cobalt protoporphyrin attenuates rat obstructive nephropathy: role of cellular infiltration, Urology 72(2) (2008) 432–438. [DOI] [PubMed] [Google Scholar]
- [142].Shamloul R, Wang R, Increased intracavernosal pressure response in hypertensive rats after chronic hemin treatment, J. Sex Med 3(4) (2006) 619–627. [DOI] [PubMed] [Google Scholar]
- [143].Sodhi K, Puri N, Favero G, Stevens S, Meadows C, Abraham NG, Rezzani R, Ansinelli H, Lebovics E, Shapiro JI, Fructose Mediated Non-Alcoholic Fatty Liver Is Attenuated by HO-1-SIRT1 Module in Murine Hepatocytes and Mice Fed a High Fructose Diet, PLoS. One 10(6) (2015) e0128648. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [144].Sodhi K, Puri N, Inoue K, Falck JR, Schwartzman ML, Abraham NG, EET agonist prevents adiposity and vascular dysfunction in rats fed a high fat diet via a decrease in Bach 1 and an increase in HO-1 levels, Prost. Other Lipid Mediat 98(3–4) (2012) 133–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Li S, Fujino M, Takahara T, Li XK, Protective role of heme oxygenase-1 in fatty liver ischemia-reperfusion injury, Med Mol Morphol (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Kamiya A, Hara T, Tsuda M, Tsuru E, Kuroda Y, Ota U, Karashima T, Fukuhara H, Inoue K, Ishizuka M, Nakajima M, Tanaka T, 5-Aminolevulinic acid with ferrous iron improves early renal damage and hepatic steatosis in high fat diet-induced obese mice, J Clin Biochem Nutr 64(1) (2019) 59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Kim CS, Kwon Y, Choe SY, Hong SM, Yoo H, Goto T, Kawada T, Choi HS, Joe Y, Chung HT, Yu R, Quercetin reduces obesity-induced hepatosteatosis by enhancing mitochondrial oxidative metabolism via heme oxygenase-1, Nutr. Metab (Lond) 12 (2015) 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Natoli R, Fernando N, Dahlenburg T, Jiao H, Aggio-Bruce R, Barnett NL, Chao de la Barca JM, Tcherkez G, Reynier P, Fang J, Chu-Tan JA, Valter K, Provis J, Rutar M, Obesity-induced metabolic disturbance drives oxidative stress and complement activation in the retinal environment, Mol Vis 24 (2018) 201–217. [PMC free article] [PubMed] [Google Scholar]
- [149].Tuzcu M, Orhan C, Muz OE, Sahin N, Juturu V, Sahin K, Lutein and zeaxanthin isomers modulates lipid metabolism and the inflammatory state of retina in obesity-induced high-fat diet rodent model, BMC Ophthalmol 17(1) (2017) 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Konrad FM, Braun S, Ngamsri KC, Vollmer I, Reutershan J, Heme oxygenase-1 attenuates acute pulmonary inflammation by decreasing the release of segmented neutrophils from the bone marrow, Am. J. Physiol Lung Cell Mol. Physiol 307(9) (2014) L707–L717. [DOI] [PubMed] [Google Scholar]
- [151].Turkseven S, Kruger A, Mingone CJ, Kaminski P, Inaba M, Rodella LF, Ikehara S, Wolin MS, Abraham NG, Antioxidant mechanism of heme oxygenase-1 involves an increase in superoxide dismutase and catalase in experimental diabetes, Am. J Physiol Heart Circ. Physiol 289(2) (2005) H701–H707. [DOI] [PubMed] [Google Scholar]
- [152].Bakhautdin B, Das D, Mandal P, Roychowdhury S, Danner J, Bush K, Pollard K, Kaspar JW, Li W, Salomon RG, McMullen MR, Nagy LE, Protective role of HO-1 and carbon monoxide in ethanol-induced hepatocyte cell death and liver injury in mice, J. Hepatol 61(5) (2014) 1029–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Ungvari Z, Bagi Z, Feher A, Recchia FA, Sonntag WE, Pearson K, de CR, Csiszar A, Resveratrol confers endothelial protection via activation of the antioxidant transcription factor Nrf2, Am. J. Physiol Heart Circ. Physiol 299(1) (2010) H18–H24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Barbagallo I, Galvano F, Frigiola A, Cappello F, Riccioni G, Murabito P, D’Orazio N, Torella M, Gazzolo D, Li VG, Potential therapeutic effects of natural heme oxygenase-1 inducers in cardiovascular diseases, Antioxid. Redox. Signal 18(5) (2013) 507–521. [DOI] [PubMed] [Google Scholar]
- [155].Chenxu G, Minxuan X, Yuting Q, Tingting G, Jing F, Jinxiao L, Sujun W, Yongjie M, Deshuai L, Qiang L, Linfeng H, Xuyuan N, Mingxing W, Ping H, Jun T, Loss of RIP3 initiates annihilation of high-fat diet initialized nonalcoholic hepatosteatosis: A mechanism involving Toll-like receptor 4 and oxidative stress, Free Radic Biol Med 134 (2019) 23–41. [DOI] [PubMed] [Google Scholar]
- [156].Jamal UM, Joe Y, Zheng M, Blackshear PJ, Ryter SW, Park JW, Chung HT, A functional link between heme oxygenase-1 and tristetraprolin in the anti-inflammatory effects of nicotine, Free Radic. Biol. Med 65 (2013) 1331–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Kim SK, Joe Y, Zheng M, Kim HJ, Yu JK, Cho GJ, Chang KC, Kim HK, Han J, Ryter SW, Chung HT, Resveratrol induces hepatic mitochondrial biogenesis through the sequential activation of nitric oxide and carbon monoxide production, Antioxid. Redox. Signal 20(16) (2014) 2589–2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Chen TM, Li J, Liu L, Fan L, Li XY, Wang YT, Abraham NG, Cao J, Effects of heme oxygenase-1 upregulation on blood pressure and cardiac function in an animal model of hypertensive myocardial infarction, Int. J. Mol. Sci 14(2) (2013) 2684–2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Clerigues V, Guillen MI, Gomar F, Alcaraz MJ, Haem oxygenase-1 counteracts the effects of interleukin-1beta on inflammatory and senescence markers in cartilage-subchondral bone explants from osteoarthritic patients, Clin. Sci. (Lond) 122(5) (2012) 239–250. [DOI] [PubMed] [Google Scholar]
- [160].Yin H, Li X, Gong Q, Jin X, Gu H, Yuan B, Zhang B, Zheng F, Gong F, Zhu J, Heme oxygenase-1 upregulation improves lipopolysaccharide-induced acute lung injury involving suppression of macrophage migration inhibitory factor, Mol. Immunol 47(15) (2010) 2443–2449. [DOI] [PubMed] [Google Scholar]
- [161].Pae HO, Ae HY, Chai KY, Chung HT, Heme oxygenase-1 attenuates contact hypersensitivity induced by 2,4-dinitrofluorobenzene in mice, Immunopharmacol. Immunotoxicol 30(2) (2008) 207–216. [DOI] [PubMed] [Google Scholar]
- [162].George EM, Warrington JP, Spradley FT, Palei AC, Granger JP, The heme oxygenases: important regulators of pregnancy and preeclampsia, Am. J. Physiol Regul. Integr. Comp Physiol 307(7) (2014) R769–R777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Yet SF, Perrella MA, Layne MD, Hsieh CM, Maemura K, Kobzik L, Wiesel P, Christou H, Kourembanas S, Lee ME, Hypoxia induces severe right ventricular dilatation and infarction in heme oxygenase-1 null mice, J. Clin. Invest 103(8) (1999) R23–R29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Cao J, Singh SP, McClung J, Joseph G, Vanella L, Barbagallo I, Jiang H, Falck JR, Arad M, Shapiro JI, NG A, EET Intervention on Wnt1, NOV and HO-1 Signaling Prevents Obesity-Induced Cardiomyopathy in Obese Mice, Am. J. Physiol Heart Circ. Physiol 313(2) (2017) H368–H380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Bhattacharya I, Ullrich A, Endothelin-1 inhibits adipogenesis: role of phosphorylation of Akt and ERK1/2, FEBS Lett 580(24) (2006) 5765–5771. [DOI] [PubMed] [Google Scholar]
- [166].Singh S, McClung J, Thompson E, Glick Y, Greenberg M, Acosta-Baez G, Edris B, Shapiro J, Abraham NG, Cardioprotective heme oxygenase-1-PGC-1α signaling in epicardial fat attenuates cardiovascular risk in humans as in obese mice, Obesity (epub (June 13, 2019)). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Peterson SJ, Rubinstein R, Faroqui M, Raza A, Boumaza I, Zhang Y, Stec D, Abraham NG, Positive Effects of Heme Oxygenase Upregulation on Adiposity and Vascular Dysfunction: Gene Targeting vs. Pharmacologic Therapy, Int J Mol Sci 20(10) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Cao J, Puri N, Sodhi K, Bellner L, Abraham NG, Kappas A, Apo A1 Mimetic Rescues the Diabetic Phenotype of HO-2 Knockout Mice via an Increase in HO-1 Adiponectin and LKBI Signaling Pathway, Int. J Hypertens 2012 (2012) 628147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Issan Y, Hochhauser E, Kornowski R, Leshem-Lev D, Lev E, Sharoni R, Vanella L, Puri N, Laniado-Schwartzman M, Abraham NG, Porat E, Endothelial progenitor cell function inversely correlates with long-term glucose control in diabetic patients: association with the attenuation of the heme oxygenase-adiponectin axis, Can. J Cardiol 28(6) (2012) 728–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Cheng C, Noordeloos AM, Jeney V, Soares MP, Moll F, Pasterkamp G, Serruys PW, Duckers HJ, Heme oxygenase 1 determines atherosclerotic lesion progression into a vulnerable plaque, Circulation 119(23) (2009) 3017–3027. [DOI] [PubMed] [Google Scholar]
- [171].Kang L, Hillestad ML, Grande JP, Croatt AJ, Barry MA, Farrugia G, Katusic ZS, Nath KA, Induction and functional significance of the heme oxygenase system in pathological shear stress in vivo, Am. J. Physiol Heart Circ. Physiol 308(11) (2015) H1402–H1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Nath KA, Heme oxygenase-1: a provenance for cytoprotective pathways in the kidney and other tissues, Kidney Int 70(3) (2006) 432–443. [DOI] [PubMed] [Google Scholar]
- [173].Agarwal A, Nick HS, Renal response to tissue injury: lessons from heme oxygenase-1 GeneAblation and expression, J. Am. Soc. Nephrol 11(5) (2000) 965–973. [DOI] [PubMed] [Google Scholar]
- [174].Deshane J, Wright M, Agarwal A, Heme oxygenase-1 expression in disease states, Acta Biochim. Pol 52(2) (2005) 273–284. [PubMed] [Google Scholar]
- [175].Sabaawy HE, Zhang F, Nguyen X, Elhosseiny A, Nasjletti A, Schwartzman M, Dennery P, Kappas A, Abraham NG, Human heme oxygenase-1 gene transfer lowers blood pressure and promotes growth in spontaneously hypertensive rats, Hypertension 38(2) (2001) 210–215. [DOI] [PubMed] [Google Scholar]
- [176].Goodman AI, Chander PN, Rezzani R, Schwartzman ML, Regan RF, Rodella L, Turkseven S, Lianos EA, Dennery PA, Abraham NG, Heme oxygenase-2 deficiency contributes to diabetes-mediated increase in superoxide anion and renal dysfunction, J. Am. Soc. Nephrol 17(4) (2006) 1073–1081. [DOI] [PubMed] [Google Scholar]
- [177].Li N, Yi F, dos Santos EA, Donley DK, Li PL, Role of renal medullary heme oxygenase in the regulation of pressure natriuresis and arterial blood pressure, Hypertension 49(1) (2007) 148–154. [DOI] [PubMed] [Google Scholar]
- [178].Rodriguez F, Kemp R, Balazy M, Nasjletti A, Effects of exogenous heme on renal function. Role of heme oxygenase and cyclooxygenase, Hypertension 42 (2003) 680–684. [DOI] [PubMed] [Google Scholar]
- [179].Rodriguez F, Lamon BD, Gong W, Kemp R, Nasjletti A, Nitric oxide synthesis inhibition promotes renal production of carbon monoxide, Hypertension 43(2) (2004) 347–351. [DOI] [PubMed] [Google Scholar]
- [180].Johnson RA, Lavesa M, DeSeyn K, Scholer MJ, Nasjletti A, Heme oxygenase substrates acutely lower blood pressure in hypertensive rats, Am. J. Physiol 271(3 Pt 2) (1996) H1132–H1138. [DOI] [PubMed] [Google Scholar]
- [181].Li Z, Wang Y, Man RY, Vanhoutte PM, Upregulation of heme oxygenase-1 potentiates EDH-type relaxations in the mesenteric artery of the spontaneously hypertensive rat, Am. J. Physiol Heart Circ. Physiol 305(10) (2013) H1471–H1483. [DOI] [PubMed] [Google Scholar]
- [182].Stec DE, Drummond HA, Gousette MU, Storm MV, Abraham NG, Csongradi E, Expression of heme oxygenase-1 in thick ascending loop of henle attenuates angiotensin II-dependent hypertension, J Am. Soc. Nephrol 23(5) (2012) 834–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Zeldin DC, Epoxygenase pathways of arachidonic acid metabolism, J. Biol. Chem 276(39) (2001) 36059–36062. [DOI] [PubMed] [Google Scholar]
- [184].Capdevila JH, Falck JR, Harris RC, Cytochrome P450 and arachidonic acid bioactivation. Molecular and functional properties of the arachidonate monooxygenase, J. Lipid Res 41(2) (2000) 163–181. [PubMed] [Google Scholar]
- [185].Node K, Huo Y, Ruan X, Yang B, Spiecker M, Ley K, Zeldin DC, Liao JK, Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids, Science 285(5431) (1999) 1276–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Spiecker M, Liao JK, Vascular protective effects of cytochrome p450 epoxygenase-derived eicosanoids, Arch. Biochem. Biophys 433(2) (2005) 413–420. [DOI] [PubMed] [Google Scholar]
- [187].Singh SP, Bellner L, Vanella L, Cao J, Falck JR, Kappas A, Abraham NG, Downregulation of PGC-1alpha Prevents the Beneficial Effect of EET-Heme Oxygenase-1 on Mitochondrial Integrity and Associated Metabolic Function in Obese Mice, J. Nutr. Metab 2016 (2016) Article ID 9039754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Grieve DJ, Byrne JA, Cave AC, Shah AM, Role of oxidative stress in cardiac remodelling after myocardial infarction, Heart Lung Circ 13(2) (2004) 132–138. [DOI] [PubMed] [Google Scholar]
- [189].Lavrovsky Y, Schwartzman ML, Levere RD, Kappas A, Abraham NG, Identification of binding sites for transcription factors NF-kappa B and AP-2 in the promoter region of the human heme oxygenase 1 gene, Proc. Natl. Acad. Sci. U. S. A 91(13) (1994) 5987–5991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Li P, Lahvic JL, Binder V, Pugach EK, Riley EB, Tamplin OJ, Panigrahy D, Bowman TV, Barrett FG, Heffner GC, McKinney-Freeman S, Schlaeger TM, Daley GQ, Zeldin DC, Zon LI, Epoxyeicosatrienoic acids enhance embryonic haematopoiesis and adult marrow engraftment, Nature 523(7561) (2015) 468–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Singh SP, Schragenheim J, Cao J, Falck JR, Abraham NG, Bellner L, PGC-1 alpha regulates HO-1 expression, mitochondrial dynamics and biogenesis: Role of epoxyeicosatrienoic acid, Prostaglandins Other Lipid Mediat 125 (2016) 8–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Waldman M, Bellner L, Vanella L, Schragenheim J, Sodhi K, Singh SP, Lin D, Lakhkar A, Li J, Hochhauser E, Arad M, Darzynkiewicz Z, Kappas A, Abraham NG, Epoxyeicosatrienoic Acids Regulate Adipocyte Differentiation of Mouse 3T3 Cells, Via PGC-1alpha Activation, Which Is Required for HO-1 Expression and Increased Mitochondrial Function, Stem Cells Dev 25(14) (2016) 1084–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Kusmic C, L’Abbate A, Sambuceti G, Drummond G, Barsanti C, Matteucci M, Cao J, Piccolomini F, Cheng J, Abraham NG, Improved myocardial perfusion in chronic diabetic mice by the up-regulation of pLKB1 and AMPK signaling, J. Cell Biochem 109(5) (2010) 1033–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Abraham NG, Kappas A, Heme oxygenase and the cardiovascular-renal system, Free Radic. Biol. Med 39(1) (2005) 1–25. [DOI] [PubMed] [Google Scholar]
- [195].Otterbein LE, Kolls JK, Mantell LL, Cook JL, Alam J, Choi AM, Exogenous administration of heme oxygenase-1 by gene transfer provides protection against hyperoxia-induced lung injury, J. Clin. Invest 103(7) (1999) 1047–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Liu L, Puri N, Raffaele M, Schragenheim J, Singh SP, Bradbury JA, Bellner L, Vanella L, Zeldin DC, Cao J, Abraham NG, Ablation of soluble epoxide hydrolase reprogram white fat to beige-like fat through an increase in mitochondrial integrity, HO-1-adiponectin in vitro and in vivo, Prostaglandins Other Lipid Mediat 138 (2018) 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Csongradi E, Docarmo JM, Dubinion JH, Vera T, Stec DE, Chronic HO-1 induction with cobalt protoporphyrin (CoPP) treatment increases oxygen consumption, activity, heat production and lowers body weight in obese melanocortin-4 receptor-deficient mice, Int. J. Obes. (Lond) 36(2) (2012) 244–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Hosick PA, Stec DE, Heme oxygenase, a novel target for the treatment of hypertension and obesity?, Am. J. Physiol Regul. Integr. Comp Physiol 302(2) (2012) R207–R214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Ndisang JF, Jadhav A, Mishra M, The heme oxygenase system suppresses perirenal visceral adiposity, abates renal inflammation and ameliorates diabetic nephropathy in Zucker diabetic fatty rats, PLoS. One 9(1) (2014) e87936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Schragenheim J, Bellner L, Cao J, Singh SP, Bamshad D, McClung JA, Maayan O, Meissner A, Grant I, Stier CT Jr., Abraham NG, EET enhances renal function in obese mice resulting in restoration of HO-1-Mfn1/2 signaling, and decrease in hypertension through inhibition of sodium chloride co-transporter, Prostaglandins Other Lipid Mediat 137 (2018) 30–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Hosick PA, AlAmodi AA, Storm MV, Gousset MU, Pruett BE, Gray W III, Stout J, Stec DE, Chronic carbon monoxide treatment attenuates development of obesity and remodels adipocytes in mice fed a high-fat diet, Int. J. Obes. (Lond) 38(1) (2014) 132–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Abraham NG, Junge JM, Drummond GS, Translational Significance of Heme Oxygenase in Obesity and Metabolic Syndrome, Trends Pharmacol. Sci 37(1) (2016) 17–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Huang JY, Chiang MT, Chau LY, Adipose overexpression of heme oxygenase-1 does not protect against high fat diet-induced insulin resistance in mice, PLoS. One 8(2) (2013) e55369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Hinds TD Jr., Hosick PA, Chen S, Tukey RH, Hankins MW, Nestor-Kalinoski A, Stec DE, Mice with hyperbilirubinemia due to Gilbert’s syndrome polymorphism are resistant to hepatic steatosis by decreased serine 73 phosphorylation of PPARalpha, Am J Physiol Endocrinol Metab 312(4) (2017) E244–E252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Call for scientific submissions 2016 ACC RAC, J Chiropr Educ 29(1) (2015) 68–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Weaver L, Hamoud AR, Stec DE, Hinds TD Jr., Biliverdin reductase and bilirubin in hepatic disease, Am J Physiol Gastrointest Liver Physiol 314(6) (2018) G668–G676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Allen NJ, Eroglu C, Cell Biology of Astrocyte-Synapse Interactions, Neuron 96(3) (2017) 697–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].O’Brien L, Hosick PA, John K, Stec DE, Hinds TD Jr., Biliverdin reductase isozymes in metabolism, Trends Endocrinol. Metab 26(4) (2015) 212–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Hosick PA, AlAmodi AA, Hankins M, Stec D, Chronic treatment with a carbon monoxide releasing molecular revereses dietary induced obesity in mice, Adipocyte 5(2) (2016) 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Braud L, Pini M, Muchova L, Manin S, Kitagishi H, Sawaki D, Czibik G, Ternacle J, Derumeaux G, Foresti R, Motterlini R, Carbon monoxide-induced metabolic switch in adipocytes improves insulin resistance in obese mice, JCI Insight 3(22) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Hinds TD Jr., Sodhi K, Meadows C, Fedorova L, Puri N, Kim DH, Peterson SJ, Shapiro J, Abraham NG, Kappas A, Increased HO-1 levels ameliorate fatty liver development through a reduction of heme and recruitment of FGF21, Obesity. (Silver. Spring) 22(3) (2014) 705–712. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [212].Burgess A, Vanella L, Bellner L, Schwartzman ML, Abraham NG, Epoxyeicosatrienoic acids and heme oxygenase-1 interaction attenuates diabetes and metabolic syndrome complications, Prostaglandins Other Lipid Mediat 97(1–2) (2012) 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Gramlich Y, Daiber A, Buschmann K, Oelze M, Vahl CF, Munzel T, Hink U, Oxidative Stress in Cardiac Tissue of Patients Undergoing Coronary Artery Bypass Graft Surgery: The Effects of Overweight and Obesity, Oxid Med Cell Longev 2018 (2018) 6598326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Leu SY, Tsai YC, Chen WC, Hsu CH, Lee YM, Cheng PY, McDonnell C, Leanez S, Pol O, Raspberry ketone induces brown-like adipocyte formation through suppression of autophagy in adipocytes and adipose tissue J Nutr Biochem 56(11) (2018) 116–125. [DOI] [PubMed] [Google Scholar]
- [215].McDonnell CL; Pol O;, The Inhibitory Effects of Cobalt Protoporphyrin IX and Cannabinoid 2 Receptor Agonists in Type 2 Diabetic Mice, Int J Mol Sci 18((11)) (2017) pii: E2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Pittala V, Vanella L, Salerno L, Romeo G, Marrazzo A, Di Giacomo C, Sorrenti V, Effects of Polyphenolic Derivatives on Heme Oxygenase-System in Metabolic Dysfunctions, Curr Med Chem 25(13) (2018) 1577–1595. [DOI] [PubMed] [Google Scholar]
- [217].Abraham NG, Drummond G, CD163-Mediated hemoglobin-heme uptake activates macrophage HO-1, providing an antiinflammatory function, Circ. Res 99(9) (2006) 911–914. [DOI] [PubMed] [Google Scholar]
- [218].Philippidis P, Mason JC, Evans BJ, Nadra I, Taylor KM, Haskard DO, Landis RC, Hemoglobin scavenger receptor CD163 mediates interleukin-10 release and heme oxygenase-1 synthesis: antiinflammatory monocyte-macrophage responses in vitro, in resolving skin blisters in vivo, and after cardiopulmonary bypass surgery, Circ. Res 94(1) (2004) 119–126. [DOI] [PubMed] [Google Scholar]
- [219].Shakeri-Manesch S, Zeyda M, Huber J, Ludvik B, Prager G, Stulnig TM, Diminished upregulation of visceral adipose heme oxygenase-1 correlates with waist-to-hip ratio and insulin resistance, Int. J. Obes. (Lond) 33(11) (2009) 1257–1264. [DOI] [PubMed] [Google Scholar]
- [220].Nielsen VG, Galvani CA, Boyle PK, Steinbrenner EB, Matika RW, Bariatric patients have plasmatic hypercoagulability and systemic upregulation of heme oxygenase activity, Blood Coagul. Fibrinolysis 26(2) (2015) 200–204. [DOI] [PubMed] [Google Scholar]
- [221].Rodgers PA, Vreman HJ, Dennery PA, Stevenson DK, Sources of carbon monoxide (CO) in biological systems and applications of CO detection technologies, Semin. Perinatol 18(1) (1994) 2–10. [PubMed] [Google Scholar]
- [222].Nagababu E, Rifkind JM, Heme degradation by reactive oxygen species, Antioxid. Redox. Signal 6(6) (2004) 967–978. [DOI] [PubMed] [Google Scholar]
- [223].Wu L, Wang R, Carbon monoxide: endogenous production, physiological functions, and pharmacological applications, Pharmacol. Rev 57(4) (2005) 585–630. [DOI] [PubMed] [Google Scholar]
- [224].Varma S, Lal BK, Zheng R, Breslin JW, Saito S, Pappas PJ, Hobson Ii RW, Duran WN, Hyperglycemia Alters PI3k and Akt Signaling and Leads to Endothelial Cell Proliferative Dysfunction, Am. J. Physiol Heart Circ. Physiol 289(4) (2005) H1744–H1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Bojunga J, Nowak D, Mitrou PS, Hoelzer D, Zeuzem S, Chow KU, Antioxidative treatment prevents activation of death-receptor- and mitochondrion-dependent apoptosis in the hearts of diabetic rats, Diabetologia 47(12) (2004) 2072–2080. [DOI] [PubMed] [Google Scholar]
- [226].Chen W, Salojin KV, Mi QS, Grattan M, Meagher TC, Zucker P, Delovitch TL, Insulin-like growth factor (IGF)-I/IGF-binding protein-3 complex: therapeutic efficacy and mechanism of protection against type 1 diabetes, Endocrinology 145(2) (2004) 627–638. [DOI] [PubMed] [Google Scholar]
- [227].Srinivasan S, Stevens M, Wiley JW, Diabetic peripheral neuropathy: evidence for apoptosis and associated mitochondrial dysfunction, Diabetes 49(11) (2000) 1932–1938. [DOI] [PubMed] [Google Scholar]
- [228].Huang TJ, Verkhratsky A, Fernyhough P, Insulin enhances mitochondrial inner membrane potential and increases ATP levels through phosphoinositide 3-kinase in adult sensory neurons, Mol. Cell Neurosci 28(1) (2005) 42–54. [DOI] [PubMed] [Google Scholar]
- [229].Nascimento AL, Luscher P, Tyrrell RM, Ultraviolet A (320–380 nm) radiation causes an alteration in the binding of a specific protein/protein complex to a short region of the promoter of the human heme oxygenase 1 gene, Nucleic Acids Res 21(5) (1993) 1103–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [230].Dennery PA, Spitz DR, Yang G, Tatarov A, Lee CS, Shegog ML, Poss KD, Oxygen toxicity and iron accumulation in the lungs of mice lacking heme oxygenase-2, J. Clin. Invest 101(5) (1998) 1001–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [231].Liu L, Huang X, Gao J, Guo Y, Di Y, Sun S, Deng X, Cao J, Improved endogenous epoxyeicosatrienoic acid production mends heart function via increased PGC 1alpha-mitochondrial functions in metabolic syndrome, J Pharmacol Sci 138(2) (2018) 138–145. [DOI] [PubMed] [Google Scholar]
- [232].Hou N, Du G, Han F, Zhang J, Jiao X, Sun X, Irisin Regulates Heme Oxygenase-1/Adiponectin Axis in Perivascular Adipose Tissue and Improves Endothelial Dysfunction in Diet-Induced Obese Mice, Cell Physiol Biochem 42(2) (2017) 603–614. [DOI] [PubMed] [Google Scholar]
- [233].McClung JA, Kruger AL, Ferraris A, Vanella L, Tsenovoy P, Weiss MB, Abraham NG, Usefulness of clopidogrel to protect against diabetes-induced vascular damage, Am. J Cardiol 105(7) (2010) 1014–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [234].Singh S, Grant I, Meissner A, Kappas A, Abraham N, Ablation of adipose-HO-1 expression increases white fat over beige fat through inhibition of mitochondrial fusion and of PGC1alpha in female mice, Horm Mol Biol Clin Investig 31(1) (2017). [DOI] [PubMed] [Google Scholar]
- [235].Singh SP, McClung JA, Bellner L, Cao J, Waldman M, Schragenheim J, Arad M, Hochhauser E, Falck JR, Weingarten JA, Peterson SJ, Abraham NG, CYP-450 Epoxygenase Derived Epoxyeicosatrienoic Acid Contribute To Reversal of Heart Failure in Obesity-Induced Diabetic Cardiomyopathy via PGC-1 alpha Activation., Cardiovascular Pharmacology (Open Access) (2329–6607 (Print)) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [236].Rabinovitch A, Suarez WL, Thomas PD, Strynadka K, Simpson I, Cytotoxic effects of cytokines on rat islets: evidence for involvement of free radicals and lipid peroxidation, Diabetologia 35(5) (1992) 409–413. [DOI] [PubMed] [Google Scholar]
- [237].Choi BM, Pae HO, Jeong YR, Kim YM, Chung HT, Critical role of heme oxygenase-1 in Foxp3-mediated immune suppression, Biochem. Biophys. Res. Commun 327(4) (2005) 1066–1071. [DOI] [PubMed] [Google Scholar]
- [238].Pae HO, Oh GS, Choi BM, Chae SC, Kim YM, Chung KR, Chung HT, Carbon monoxide produced by heme oxygenase-1 suppresses T cell proliferation via inhibition of IL-2 production, J. Immunol 172(8) (2004) 4744–4751. [DOI] [PubMed] [Google Scholar]
- [239].Chauveau C, Remy S, Royer PJ, Hill M, Tanguy-Royer S, Hubert FX, Tesson L, Brion R, Beriou G, Gregoire M, Josien R, Cuturi MC, Anegon I, Heme oxygenase-1 expression inhibits dendritic cell maturation and proinflammatory function but conserves IL-10 expression, Blood 106(5) (2005) 1694–1702. [DOI] [PubMed] [Google Scholar]
- [240].Choudhary AK, Rennie J, Cairns C, Borthwick G, Hughes J, Morton NM, Kluth D, Conway BR, Administration of heme arginate ameliorates murine type 2 diabetes independently of heme oxygenase activity, PLoS. One 8(10) (2013) e78209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [241].Pileggi A, Molano RD, Berney T, Cattan P, Vizzardelli C, Oliver R, Fraker C, Ricordi C, Pastori RL, Bach FH, Inverardi L, Heme oxygenase-1 induction in islet cells results in protection from apoptosis and improved in vivo function after transplantation, Diabetes 50(9) (2001) 1983–1991. [DOI] [PubMed] [Google Scholar]
- [242].Harrison EM, McNally SJ, Devey L, Garden OJ, Ross JA, Wigmore SJ, Insulin induces heme oxygenase-1 through the phosphatidylinositol 3-kinase/Akt pathway and the Nrf2 transcription factor in renal cells, FEBS J 273(11) (2006) 2345–2356. [DOI] [PubMed] [Google Scholar]
- [243].Lavrovsky Y, Drummond GS, Abraham NG, Downregulation of the human heme oxygenase gene by glucocorticoids and identification of 56b regulatory elements, Biochem. Biophys. Res. Commun 218(3) (1996) 759–765. [DOI] [PubMed] [Google Scholar]
- [244].Yamada N, Yamaya M, Okinaga S, Nakayama K, Sekizawa K, Shibahara S, Sasaki H, Microsatellite polymorphism in the heme oxygenase-1 gene promoter is associated with susceptibility to emphysema, Am. J. Hum. Genet 66(1) (2000) 187–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [245].Exner M, Schillinger M, Minar E, Mlekusch W, Schlerka G, Haumer M, Mannhalter C, Wagner O, Heme oxygenase-1 gene promoter microsatellite polymorphism is associated with restenosis after percutaneous transluminal angioplasty, J. Endovasc. Ther 8(5) (2001) 433–440. [DOI] [PubMed] [Google Scholar]
- [246].Inoguchi T, Sasaki S, Kobayashi K, Takayanagi R, Yamada T, Relationship between Gilbert syndrome and prevalence of vascular complications in patients with diabetes, JAMA 298(12) (2007) 1398–1400. [DOI] [PubMed] [Google Scholar]
- [247].Chen YH, Lin SJ, Lin MW, Tsai HL, Kuo SS, Chen JW, Charng MJ, Wu TC, Chen LC, Ding YA, Pan WH, Jou YS, Chau LY, Microsatellite polymorphism in promoter of heme oxygenase-1 gene is associated with susceptibility to coronary artery disease in type 2 diabetic patients, Hum. Genet 111(1) (2002) 1–8. [DOI] [PubMed] [Google Scholar]
- [248].Kaneda H, Ohno M, Taguchi J, Togo M, Hashimoto H, Ogasawara K, Aizawa T, Ishizaka N, Nagai R, Heme oxygenase-1 gene promoter polymorphism is associated with coronary artery disease in Japanese patients with coronary risk factors, Arterioscler. Thromb. Vasc. Biol 22(10) (2002) 1680–1685. [DOI] [PubMed] [Google Scholar]
- [249].Endler G, Exner M, Schillinger M, Marculescu R, Sunder-Plassmann R, Raith M, Jordanova N, Wojta J, Mannhalter C, Wagner OF, Huber K, A microsatellite polymorphism in the heme oxygenase-1 gene promoter is associated with increased bilirubin and HDL levels but not with coronary artery disease, Thromb. Haemost 91(1) (2004) 155–161. [DOI] [PubMed] [Google Scholar]
- [250].Tiroch K, Koch W, von BN, Kastrati A, Schomig A, Heme oxygenase-1 gene promoter polymorphism and restenosis following coronary stenting, Eur. Heart J 28(8) (2007) 968–973. [DOI] [PubMed] [Google Scholar]
- [251].Courtney AE, McNamee PT, Middleton D, Heggarty S, Patterson CC, Maxwell AP, Association of functional heme oxygenase-1 gene promoter polymorphism with renal transplantation outcomes, Am. J Transplant 7(4) (2007) 908–913. [DOI] [PubMed] [Google Scholar]
- [252].Ono K, Goto Y, Takagi S, Baba S, Tago N, Nonogi H, Iwai N, A promoter variant of the heme oxygenase-1 gene may reduce the incidence of ischemic heart disease in Japanese, Atherosclerosis 173(2) (2004) 315–319. [DOI] [PubMed] [Google Scholar]
- [253].Wijpkema JS, van Haelst PL, Monraats PS, Bruinenberg M, Zwinderman AH, Zijlstra F, van der SG, de Winter RJ, Doevendans PA, Waltenberger J, Jukema JW, Tio RA, Restenosis after percutaneous coronary intervention is associated with the angiotensin-II type-1 receptor 1166A/C polymorphism but not with polymorphisms of angiotensin-converting enzyme, angiotensin-II receptor, angiotensinogen or heme oxygenase-1, Pharmacogenet. Genomics 16(5) (2006) 331–337. [DOI] [PubMed] [Google Scholar]
- [254].Ibuki A, Minematsu T, Yoshida M, Iizaka S, Matsumoto M, Sugama J, Sanada H, Microsatellite polymorphism in the Heme oxygenase-1 gene promoter is associated with dermal collagen density in Japanese obese male subjects, PLoS One 13(7) (2018) e0199994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [255].Pechlaner R, Willeit P, Summerer M, Santer P, Egger G, Kronenberg F, Demetz E, Weiss G, Tsimikas S, Witztum JL, Willeit K, Iglseder B, Paulweber B, Kedenko L, Haun M, Meisinger C, Gieger C, Muller-Nurasyid M, Peters A, Willeit J, Kiechl S, Heme oxygenase-1 gene promoter microsatellite polymorphism is associated with progressive atherosclerosis and incident cardiovascular disease, Arterioscler. Thromb. Vasc. Biol 35(1) (2015) 229–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [256].Mahbouli S, Talvas J, der Vartanian A, Ortega S, Rouge S, Vasson MP, Rossary A, Activation of antioxidant defences of human mammary epithelial cells under leptin depend on neoplastic state, BMC Cancer 18(1) (2018) 1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [257].Kharb S, Bala J, Nanda S, Markers of obesity and growth in preeclamptic and normotensive pregnant women, J Obstet Gynaecol 37(5) (2017) 610–615. [DOI] [PubMed] [Google Scholar]
- [258].Putra RA, Effendi JS, Permadi W, Bandiara R, Fauziah PN, Kharb S, Bala J, Nanda S, Role of statin as inducer of Hmox-1 system in treatment of preeclampsia, Cell Mol Biol (Noisy-le-grand) 64(10) (2018) 1–4. [PubMed] [Google Scholar]
- [259].Tirado R, Masdeu MJ, Vigil L, Rigla M, Luna A, Rebasa P, Pareja R, Hurtado M, Caixas A, Impact of Bariatric Surgery on Heme Oxygenase-1, Inflammation, and Insulin Resistance in Morbid Obesity with Obstructive Sleep Apnea, Obes Surg 27(9) (2017) 2338–2346. [DOI] [PubMed] [Google Scholar]