Abstract
Systemic drug delivery methods such as oral or parenteral administration of free drugs possess relatively low treatment efficiency and marked adverse side effects. The use of nanoparticles for drug delivery in most cases substantially enhances drug efficacy, improves pharmacokinetics and drug release and limits their side effects. However, further enhancement in drug efficacy and significant limitation of adverse side effects can be achieved by specific targeting of nanocarrier-based delivery systems especially in combination with local administration. The present review describes major advantages and limitations of organic and inorganic nanocarriers or living cell-based drug and nucleic acid delivery systems. Among these, different nanoparticles, supramolecular gels, therapeutic cells as living drug carriers etc. have emerged as a new frontier in modern medicine.
Keywords: Drug, siRNA and nucleotide delivery, drug targeting, organic and inorganic nanoparticles, supramolecular gel, cell-based carriers
1. Introduction
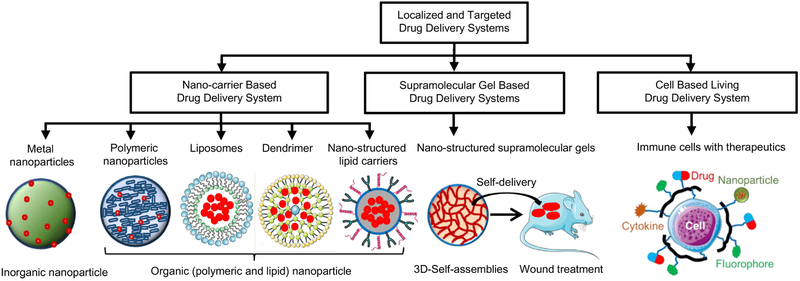
It is well known that the main goal of drug delivery is effective and safe delivery of a drug to its target tissues, organs and cells. However, transport of a drug to its specific targets represents one of the major challenges for an effective drug delivery [1]. Conventional enteral or parenteral drug delivery methods of systemic drug administration include the formulation of a drug into suitable dosage forms for oral/gastrointestinal administration (e. g. tablets, capsules, solutions, suspensions, syrups, etc.) or sterile drug preparations (solutions, suspensions, emulsions, or reconstituted lyophilized powders) suitable for administration by injection (intravenous, epidural, intramuscular, subcutaneous, etc.) [2, 3]. Nevertheless, these systemic drug delivery methods are associated with several limitations such as relatively low site-specific bioavailability of administered drugs, unfavorable distribution of drugs throughout the body and, in most cases, low accumulation in the target site, adverse side effects, etc. [4]. Besides, these drug delivery systems can’t fulfill the ever-growing need of the modern medicine such as personalized medicine and targeted therapies. Therefore, there was an urgent need to develop novel drug delivery systems for localized and targeted delivery of therapeutics. Over the decades, researchers have extensively studied development of carrier-based drug delivery systems with truly targeted features towards specific disease as well as to prevent drug degradation, resistance, drug side effects and also improve the potential of individualized medicine [5, 6]. Various surface markers and cytokines such as integrins, folate, growth factors etc., which are known to express on disease cells and their microenvironments, were explored for developing counter marker functionalized drug carrier to recognize the target disease cells [7]. Carrier-based targeted and localized drug delivery systems, e.g. supramolecular gels [8, 9], nanocarriers such as different types of nanoparticles [10-14], liposomes [15-19], nanostructures lipid carriers [20, 21], dendrimers [22-28], quantum dots [29], live cells as living drug carriers [30], micelles [31-33], polymeric nanoparticles [34, 35] emulsions [36] and microemulsions [37, 38], etc. were widely explored in the past few years. Such targeted drug delivery systems not only lead to accumulation of drug in the specific organs, tissues or cells resulting in increased treatment efficacy, but also reduce the drug availability into other organs thereby decreasing adverse side effects. To keep a distinctive perspective, contributions describing drug delivery through polymeric system [39], micelles [31], emulsions [36] have been already described in corresponding reviews and are out of the scope of this review. This review will present the advances of various carrier based targeted drug delivery systems and provide their perspectives on future directions. Mainly, we will summarize major research findings on the development of various nanocarriers, nanostructured supramolecular gels, and live cell-based targeted drug delivery approaches as outlined in Fig. 1.
Fig. 1.
Various carrier-based systems for localized and targeted delivery of drugs and other therapeutics. Nanocarrier-based systems such as nanoparticles, liposomes, dendrimers, nanostructured lipid carriers are shown with drug/therapeutic (marked as red dot) encapsulated for their site-specific controlled delivery applications. Formation of nanostructured supramolecular topical gels and their in vivo application are shown as self-delivery system. Other drug carriers such as therapeutic living cells decorated with drug (red color), nanoparticle (olive color), cytokine (orange color), fluorophore (green color) are shown as living delivery systems for disease specific drug targeting. Part of this figure was created using Servier Medical Art images, which are licensed under a Creative Commons Attribution 3.0 Unported License (https://smart.servier.com).
2. Targeted drug delivery
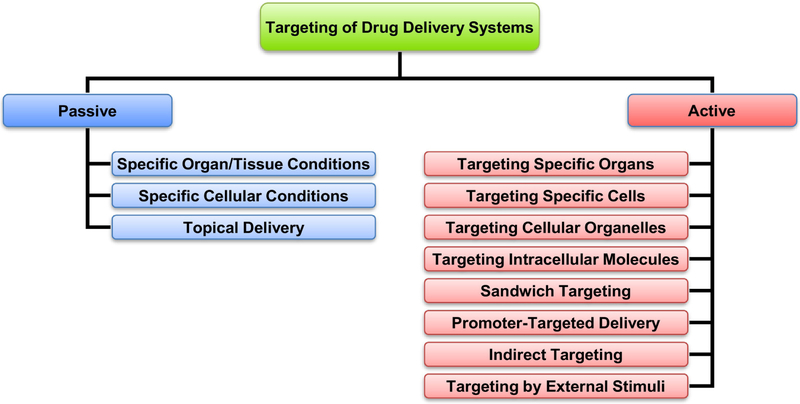
Targeting a drug delivery system to the specific organs, cells or cell organelles increases the accumulation of the delivered active components(s) in the desired site of action and limits their accumulation in the healthy organs, tissues and cells. This in turn enhances the efficacy of the treatment and prevents severe adverse side effects upon the healthy organs and tissues. Previously, we proposed to subdivide all possible types of drug targeting on two major categories: passive and active targeting (Fig. 2) [40]. So-called passive targeting relies on specific conditions in an entire organ or tissue which led to the accumulation of a drug delivery system with a specific size, molecular mass, charge, etc. In the case of cancer, it was found that high molecular weight drugs are preferentially accumulated in the solid tumors. Such situation was termed by Maeda and co-workers as enhanced permeability and retention (EPR) effect and results from the increased permeability of tumor vasculature in combination with limited lymphatic drainage [41, 42]. However, such passive targeting is effective only when the diseased cells are localized in a relatively compact structure (e.g. solid tumor in case of cancer) and will not work if the affected cells are spread over the entire body (e.g. cancer metastases). In addition, passive targeting approach may utilize specific conditions in the diseased tissues or cells (e.g. low pH) or the local delivery of active components specifically to the affected organ or tissue (e.g. topical delivery to skin or inhalation for lung delivery). In contrast, active targeting allows for a precise hunting for diseased cells whether they are localized in a certain part or spread over the body. The active targeting in most cases is achieved by adding to the surface of a carrier a targeting moiety – a ligand specific to a certain substances overexpressed on the surface of targeted cells (for targeting specific cells) or inside cells (for targeting intracellular organelles). For these purposes, antibody or their fragments [43-45], peptides, proteins or other ligands for the receptors overexpressed in targeted cells [46-50] or some exotic targeting mechanisms (sandwich targeting [51-53], promoter-targeted delivery [54-56], indirect targeting [57-59] or targeting by external stimuli [60-65]) are mainly used. In the present review, we briefly describe the use of targeting ligands to the receptors overexpressed in targeted cells, specifically in cancer cells. For more details related to other methods of active targeting the reader is referred to the corresponding reviews [40, 66-70].
Fig. 2.
Different types of targeted delivery systems. Modified from [40].
2.1. Targeting ligands for cancer cells
Probably, the one of the first and most widely used targets for the delivery of drugs, nucleic acids and other therapeutics specifically to cancer cells are folate [71-73]. Folate receptors are overexpressed in most types of cancer cells. Consequently, conjugation of a ligand to a folate receptor with an anticancer drug or, most efficiently, to a drug delivery system, will provide for an active targeting of a payload explicitly to cancer cells. Folic acid and anti-folate receptor antibodies are the most widely used ligands to folate binding proteins on the surface of cancer cells [74]. Antibodies to specific cancer cells or their fragments are also being widely used directly as therapeutic agents against various cancers or as targeting moieties for drug delivery systems [75-79]. However, the lack of specificity of folate-based ligands and possible adverse side effects of antibodies as xenobiotics limit their clinical use for cancer targeting of drugs or nucleic acids [80-82]. Peptide receptors represent another promising object for active targeting of drug delivery to cancer cells. Many peptide ligands (somatostatin analogs, vasoactive intestinal peptide, gastrin-releasing peptide, cholecystokinin/gastrin, neurotensin, substance P, neuropeptide Y, luteinizing hormone-releasing hormone - LHRH, bombesin, etc.) for these receptors have been used as targeting moieties for a specific delivery of drugs and nucleic acids to cancer cells [12, 13, 16, 21, 83-87]. Our laboratory pioneered in the use of LHRH peptide for targeting of its receptors in different cancer cells [12, 13, 16, 21]. We found that receptors specific to LHRH peptide overexpressed in many types of cancer cells and do not express in cells from visceral organs [16, 18, 88]. One interesting finding was revealed in our laboratory related to the use of LHRH peptide for targeting different nanoparticles to cancer. As expected, we found differences in organ distribution of non-targeted nanoparticles. However, the addition of LHRH peptide as a targeting moiety to all studied and protein-based nanoparticles, etc.; inorganic – gold, mesoporous silica nanoparticles leveled off these differences. All different nanocarriers (polymers, dendrimers, liposomes) targeted with LHRH peptide were equally efficient for tumor-specific treatment and imaging [16].
3. Nanoscale-based localized and targeted delivery systems
Nanoscale drug delivery [89] is an integral part of the drug delivery systems. This type of system encapsulates drug or other therapeutically active components and carries them to its target site. Recently, nanoscale-based drug delivery has attracted considerable attention due to their wide applications in the targeted delivery, controlled release of drugs, as carriers for DNA, RNA in gene therapy etc. [90]. To date, a large number of nanoscale drug delivery systems (organic and inorganic) have been developed and investigated. Organic and inorganic nanoparticles represent materials of multiple dimensions that demonstrate unique physical and chemical properties dependent on size, shape and composition. Organic and inorganic nanocarriers include but are not limited to: organic - dendrimers, polymeric micelles, liposomes, solid lipid and protein-based nanoparticles, etc.; inorganic – gold, mesoporous silica, superparamagnetic iron oxide and paramagnetic nanoparticles, quantum dots, etc. [91, 92]. This includes the first Food and Drug Administration (FDA) approved liposomal nanoparticles “Doxil”, which was known for encapsulating doxorubicin and its use in treatment of various cancers [93]. In past few years, researchers paid significant attention to develop various types of nanocarrier-based systems for targeted drug delivery application. Here, we will summarize the advances and limitations of such nanocarrier-based organic and inorganic systems which are known to encapsulate, protect, and target therapeutics towards the disease site.
3.1. Polymeric nanoparticles and dendrimers
Biodegradable polymeric nanoparticles are versatile in delivering small molecule drugs, nucleic acids, proteins etc. Over the decades, both synthetic and natural polymer-based nanoparticles such as polylactic acid-co-glycolic acid (PLGA), poly (alkyl cyanoacrylate) (PACA), polycaprolactone (PCL), polyanhydrides, polyethyleneimine (PEI), chitosan, and gelatin were investigated in therapeutic delivery applications (Fig. 3) [94, 95]. Among these, PLGA is an FDA approved nanoparticle platform for delivery of leuprolide for prostate cancer [96, 97].
Fig. 3.
Schematic illustration of nanoparticles used in drug delivery application Reproduced with permission from [95].
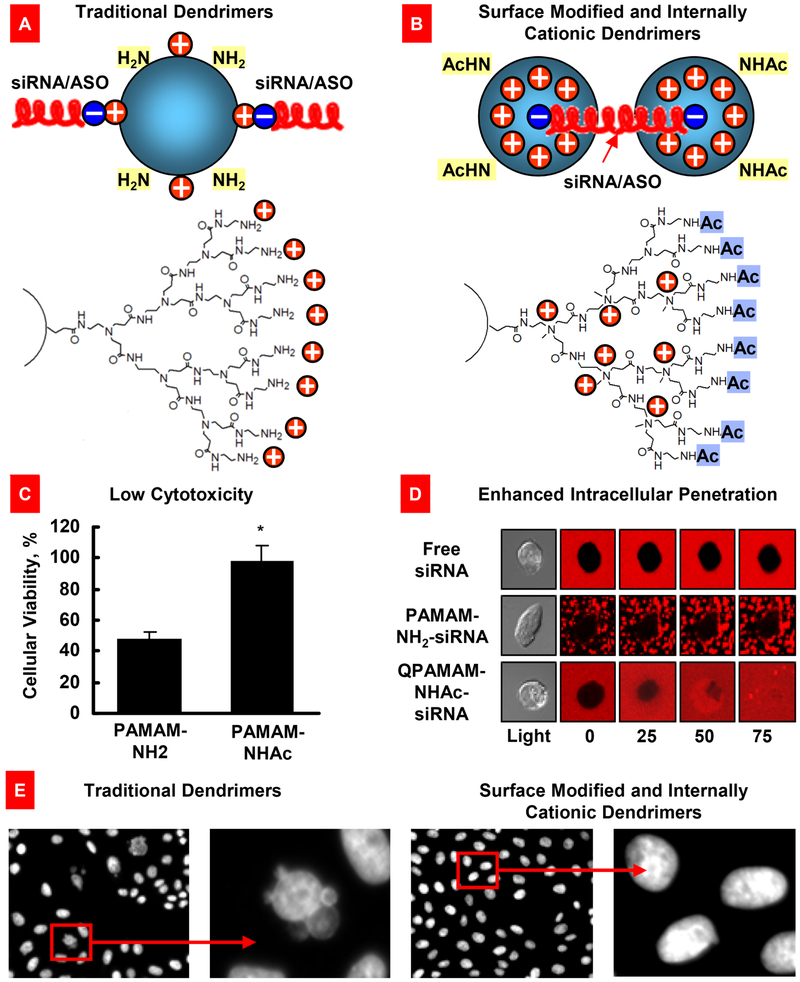
Number of reports revealed that PEG-PCL nanoparticles are safe, biocompatible and provided efficient delivery of hydrophobic payloads (e.g., anticancer drugs, imaging agents etc.) to various cancer tumors [34, 35, 98-100]. PCL based nanoparticles were also explored for the controlled release of many anticancer drugs. Due to slow degradation of the PCL polymer, it can be used as a long term-controlled delivery device of therapeutics [101]. PEI is a cationic polymer and it can non-covalently interact with nucleic acids to produce nanoparticles, which were explored for the delivery of siRNA or DNA for gene therapy [102]. However, due to cationic property, PEI nanoparticles without bound nucleic acid were found to be toxic in live cells. Wu et al. modified PEI with a neutral polymer PEG to decrease its toxicity [103]. Other types of modifications were proposed to make PPI and polyamidoamine (PAMAM) dendrimer complexes with nucleic acids, chemotherapeutic drugs and phototherapeutic agents, practically non cyto- and genotoxic to cells and animals and enhance their cellular internalization (Fig. 4) [23, 24, 26-28, 104, 105].
Fig. 4.
Surface modified and internally cationic quaternized PAMAM dendrimers for efficient nucleic acid intracellular delivery. (A) Traditional dendrimers with non-modified surface and external positive charges for siRNA or antisense oligonucleotides (ASO) binding. (B) Modified dendrimers with acetylated surface and internal positive charges for siRNA binding. (C) Cytotoxicity of a traditional (PAMAM-NH2) and surface-modified quaternized (QPAMAM-NHAc) dendrimers. Means ± SD are shown. *P < 0.05 when compared with PAMAM-NH2. (D) Cellular internalization of free siRNA, and dendrimer–siRNA complexes with a traditional (PAMAM-NH2) and internally cationic and surface-modified (QPAMAM-NHAc) dendrimers. siRNA were labeled with a red fluorescence dye, conjugated with different dendrimers, and added to the incubation medium of living cancer cells. Real-time fluorescence was registered using a fluorescent microscope. Free siRNA and traditional dendrimer–siRNA complexes are poorly internalized by cancer cells, while modified dendrimers provided for an efficient intracellular delivery of siRNA. (E) Genotoxicity (formation of micronuclei) of traditional and modified PAMAM dendrimers. Representative fluorescence microscopy images of CHO-K1 cells incubated within 24 hours with non-modified and modified dendrimers. Modified from [6, 23, 104].
Dendrimers have been widely used as delivery vehicles for many anticancer drugs such as cisplatin, paclitaxel, doxorubicin etc. For examples, Kirkpatrick et al. [106] developed various polyamidoamine dendrimers for active carriers for cisplatin. The authors observed that both drug loading and release were dependent on the size of the dendrimer and with an increase in the size of dendrimer, both loading and release of cisplatin increased. The anticancer activity of cisplatin conjugated dendrimer was assessed against various ovarian cancer cell lines such as A2780, A2780cis and A2780cp and in animal studies against A2780 xenografts. Ooya et al. [107] prepared polyglycerol dendrimers of paclitaxel (poorly water-soluble hydrophobic drug) and observed improved water solubility of the drug even at a very low dendrimer concentration. Khandare et al. [22] prepared dendrimer by conjugating paclitaxel with a linear bis-PEG and polyamidoamine. The authors observed a higher anticancer activity of the dendrimer conjugate against A2780 human ovarian cancer cells. Doxorubicin is another well-known anticancer drug for the treatment of many types of cancer. Kojima et al. [108] designed DOX-dendrimer with surface decorated by PEG and collagen peptides. This dendrimer-DOX prodrug was more active against highly invasive MDA-MB-231 cells when compared with free non-bound drug.
3.2. Solid lipid nanoparticles
Solid lipid nanoparticles (SLNs) produced from natural lipids or synthetic lipids are non-toxic and versatile nanocarriers for drug delivery application since they are made without any organic solvent and can carry both the lipophilic or hydrophilic drugs [109]. SLNs have large surface area and high drug loading ability and offer controlled release and protection of drugs [110].
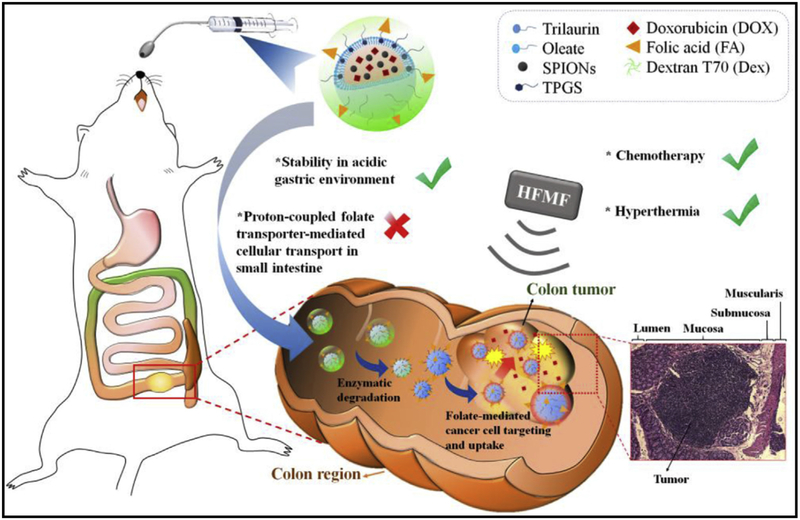
In a recent study, Shen et al. demonstrated that dual targeted SLNs composed of both doxorubicin (DOX) and superparamagnetic iron oxide nanoparticles (SPIONs) for chemo/magneto-thermal combination therapy against colon cancer. The surface of these SLNs was coated with hierarchical targeting of folate (FA) and dextran in a sequential layer-by-layer manner. The authors observed enhanced accumulation of the DOX/SPIONs-loaded SLNs at colon tumor sites leading to effective inhibition of the orthotopic colon tumor in mice (Fig. 5). These results demonstrated the prominent therapeutic efficacy of dual targeting SLNs for local treatment by oral administration [111].
Fig. 5.
Schematic illustration of the hierarchically targetable solid lipid nanoparticles for the local chemo/thermo combination therapy against colon cancer by oral administration. Reproduced with permission from [111].
3.3. Liposomes
Liposomes are a type of small nanosized vesicles that are composed of self-assembled phospholipids into bilayers with spherical shape [112, 113]. Due to the polar nature of the liposomal core, it can encapsulate hydrophilic drugs within the vesicles as well as hydrophobic therapeutics can be easily incorporated within the phospholipid bilayers. Neutral conventional and PEGylated liposomes are also nontoxic and can be modified with various ligands or functionalities for site specific drug targeting [16, 104, 112, 113]. Because of these features, liposomes are widely used for drug delivery applications. Recently, an important new finding was reported: liposomal formulation of paclitaxel prevented paclitaxel induced neuropathy [114]. Cationic liposomes were successfully used for gene and nucleotide delivery [18, 115-117]. In fact, liposome-based anticancer therapeutics such as DaunoXome, Myocet, VincaXome, DepoCyt, and Caelyx are currently available in the market for clinical use and many new liposomal formulations have been explored for clinical trials (Table 1) [118, 119].
Table 1.
Liposome-based anticancer therapeutics currently available in the market for clinical use. Modified from [118, 119].
| Product name |
Administration route |
Active drug or therapeutic |
Lipid components* |
Application | References |
|---|---|---|---|---|---|
| Abelcet | Intravenous | Amphotericin B | DMPC and DMPG | Fungal infections | [254] |
| Ambisome | Intravenous | Amphotericin B | DSPG, HSPC, Cholesterol and amphotericin B | Fungal infections | [255] |
| DepoDur | Epidural | Morphone sulfate | DOPC, DPPG, Cholesterol and triolein | Pain treatment | [256] |
| DepoCyt | Spinal | Cytarabine | DOPC, DPPG, Cholesterol and triolein | Neoplastic meningitis | [257] |
| Dauno-Xome | Intravenous | Daunorubicin | DSPC and cholesterol | Blood cancer | [258] |
| Doxil | Intravenous | Doxorubicin | HSPC, PEG and Cholesterol, | Ovarian/Breast cancer, AIDS-related Kaposi's sarcoma | [259] |
| Epaxal | Intramuscular | Inactivated hepatitis A virus | DOPC and DOPE | Hepatitis A | [260] |
| Lipodox | Intravenous | Doxorubicin | HSPC, DSPE PEG and Cholesterol, | Ovarian/Breast cancer | [261] |
| Marqibo | Intravenous | Vincristine | Sphingomyelin and Cholesterol | Acute lymphoblastic leukemia | [262] |
| Lipoplatin | Intravenous | Cisplatin | DPPG, SPC, MPEG-2000-DSPE and Cholesterol, | Pancreatic cancer, breast and lung cancer | [263] |
DSPG: 1,2-Distearoyl-sn-glycero-3-phosphoglycerol; HSPC: Hydrogenated Soy l-α-phosphatidylcholine; DOPC: 1,2-Dioleoyl-sn-glycero-3-phosphocholine; DPPG: 1,2-Dipalmitoyl-sn-glycero-3[Phospho-rac-1-glycerol; DSPC: 1,2-Distearoyl-sn-glycero-3-phosphocholine; DOPE: 1,2-Dioleoyl-sn-glycero-3-phosphoethanolamine; DMPC: 1,2-dimyristoyl-sn-glycero-3-phosphocholine; DMPG: 1,2-Dimyristoyl-sn-glycero-3-phosphoglycerol; DSPE: 1,2-Distearoyl-phosphatidyl ethanolamine.
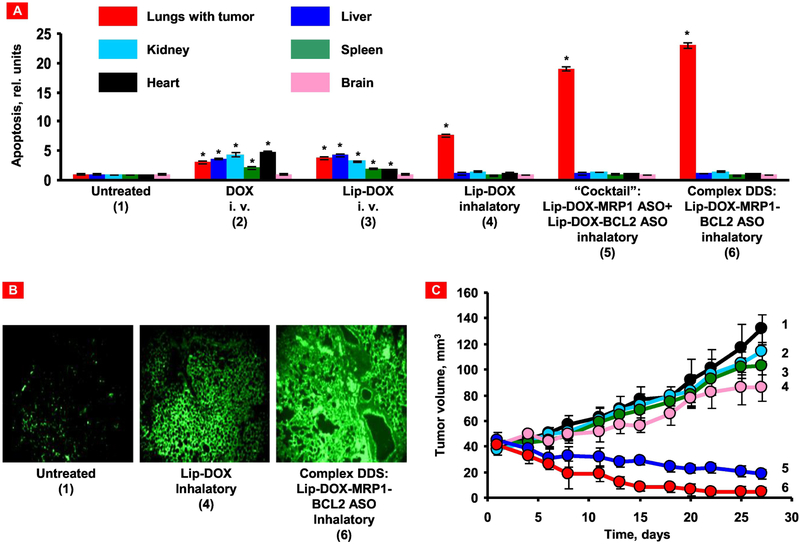
Liposomal delivery system composed of an anticancer drug doxorubicin, an antisense oligonucleotides targeted to MRP1 mRNA as a suppressor of pump resistance and BCL2 mRNA as a suppressor of nonpump resistance was investigated using an orthotopic murine model of human lung carcinoma [120]. High anticancer efficiency and low adverse side effects on healthy organs of this nanoscale drug delivery system were observed when compared with the individual components of the system used for treatment separately (Fig. 6). This was a successful demonstration of a combinatorial local inhalation delivery of drugs and suppressors of pump and nonpump cellular resistance for the treatment of lung cancer.
Fig. 6.
Local inhalation codelivery of anticancer drug and suppressors of pump and nonpump cellular resistance enhances apoptosis induction in the lung tumor, decreases tumor size, and prevents adverse side effects of treatment on healthy organs. An orthotopic mouse model of lung cancer was created by intratracheal instillation of human A549 lung cancer cells into nude mice. Untreated mice (1), mice treated by i.v injection of DOX (2), by i.v. injection of liposomal DOX (3), by inhalation with liposomal DOX (4), by inhalation with a mixture of the two systems (liposomal DOX + ASO targeted to MRP1 mixed with liposomal DOX + ASO targeted to BCL2 mRNA) (5), and by inhalation with one complex liposomal delivery system containing DOX, ASO targeted to MRP1, and BCL2 mRNA (6). (A) Apoptosis induction in the lungs with tumor and other organs 4 weeks after beginning of treatment. Enrichment of histone-associated DNA fragments (mono- and oligonucleosomes) per gram tissue in the tumor and organs of control animals was set to unit 1, and the degree of apoptosis was expressed in relative units. Apoptosis measurements were performed 24 h after last treatment. (B) Typical fluorescent microscopy images of tumor tissue slides labeled by TUNEL 24 h after last treatment. (C) Changes in tumor volume after beginning of treatment. Mice were treated on days 0, 3, 7, 11, 14, 17, 21, and 24. Mean ± SD are shown. *P < 0.05 when compared with untreated animals. Reproduced with permission from [120].
Another work demonstrated that liposomal formulation for the local pulmonary delivery of prostaglandin E2 (PGE2) in combination with siRNA – suppressors of signaling pathways of idiopathic pulmonary fibrosis (IPF) was successfully used to treat IPF [115, 121]. The results obtained on mice with bleomycin-induced IPF revealed that treatment with PGE2 and selected siRNAs delivered by liposomes prevented the disturbances in the expression of genes associated with the development of IPF whereas restricted inflammation and fibrotic injury in the lung tissues as well as limited hydroxyproline accumulation in the lungs.
3.4. Nanostructured lipid carriers
Lipid nanoparticles commonly known as nanostructured lipid carriers (NLCs) have gained recent attention for drug delivery applications. NLCs are usually prepared from biodegradable and biocompatible lipid materials and offer advantages such as enhanced drug loading capacity, prevention of drug expulsion, etc. as compared to that of the nanoemulsions, polymeric nanoparticles etc. carriers [122, 123]. Researchers have developed NLCs to deliver the drugs by different administration routes (intravenous injection, topical skin, ocular, pulmonary delivery, etc.). Because of these unique advantages, NLCs has become a versatile platform for both drug and gene delivery applications [21, 124, 125].
Earlier studies revealed that NLCs formulation of both camptothecin and topotecan showed better cytotoxicity and cellular uptake properties against melanoma and leukemia tumor cells [126]. Since, PEG is not recognized by the cells of reticuloendothelial system (RES), various NLCs decorated with PEG polymers were developed to achieve both long time stability and bioavailability in circulation [127]. Liu and co-workers prepared docetaxel-containing NLCs made up of biocompatible components such as stearic acid, glyceryl monostearate, soy lecithin, etc. and investigated their anticancer activity in lung cancer cells. The authors observed stronger cytotoxicity effect of the NLC-drug in A549 lung cancer cells when compared with treatment by drug duopafei only. The inhibition rate of the tumor growth on melanoma-bearing mice was 43%, 63%, and 90% for the duopafei, NLCs (10 mg/kg), and NLCs (20 mg/kg) treatment respectively.
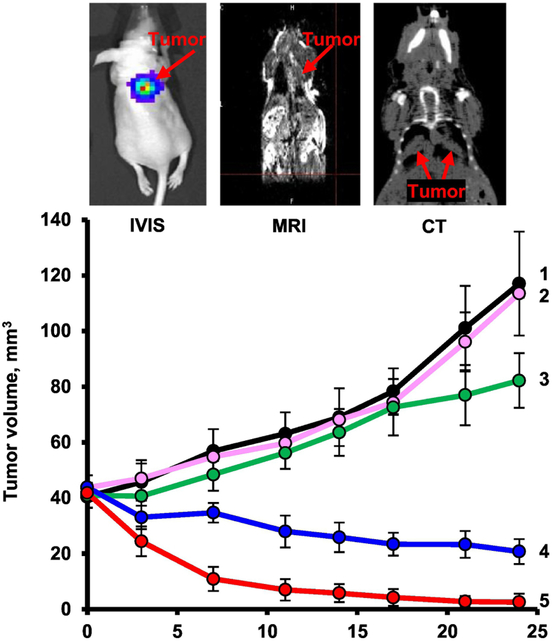
The multifunctional nanocarrier-based system containing nanostructured lipid carriers, anticancer drug (doxorubicin or paclitaxel), siRNAs targeted to MRP1 and BCL2 mRNAs (suppressors of pump and non-pump cellular resistance) and a synthetic analog of luteinizing hormone-releasing hormone (LHRH) as a targeting moiety specific to the receptors that are overexpressed in the plasma membrane of lung cancer cells have been used for treatment by inhalation of mice with orthotropic model of human lung cancer. The results demonstrated high efficiency of multifunctional NLCs for tumor-targeted local delivery by inhalation of anticancer drugs and mixture of siRNAs (Fig. 7) [21]. Recently, NLCs were used for the inhalation co-delivery of lumacaftor and ivacaftor for treatment of lung manifestations of cystic fibrosis [125].
Fig. 7.
Changes in lung tumor volume after beginning of treatment. An orthotopic mouse model of lung cancer was created by intratracheal instillation of human A549 lung cancer cells into nude mice. Lung tumor was evaluated by bioluminescence optical imaging (IVIS), magnetic resonance imaging (MRI) and computed tomography (CT). Mice were treated on days 0, 3, 7, 11, 14, 17, 21, and 24. 1 – Untreated mice; 2 – LHRH-NLC (inhalation); 3 – TAX (i.v.); 4 – LHRH-NLC-TAX (inhalation); 5 – LHRH-NLC-TAX-siRNAs targeted to MRP1 and BCL2 mRNAs (inhalation). Means ± SD are shown. Modified from [21, 135].
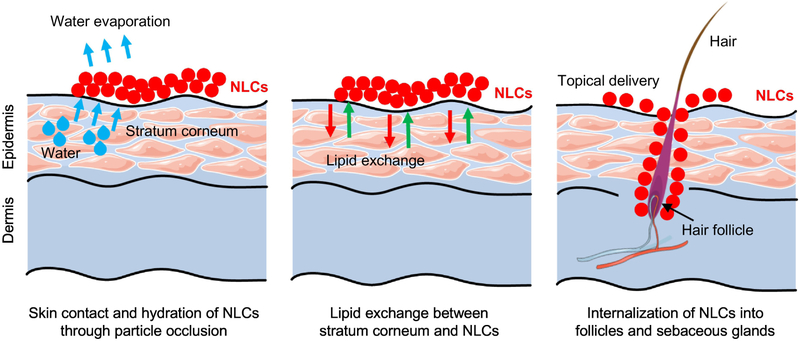
NLCs have a potential of delivering drugs via the follicles in skin. Follicle is composed of sebaceous glands, which release sebum (a mixture of triglycerides, squalene and waxes). This lipid like environment in follicles is beneficial for entrapping NLCs since the presence of glyceride lipids in NLCs make it easier to entrap into the follicles/sebaceous glands in skin (Fig. 8) [128]. NLCs are generally formulated with bio-compatible and bio-degradable lipids such as Precirol, PEG-containing triglycerides with some emulsifiers, which are most common ingredients in the topical cosmetics. Since the drug is encapsulated into NLCs, the administration of NLCs may reduce irritation and other side effects of the drug due to avoidance of direct contact with the skin tissues [129].
Fig. 8.
Possible mechanisms of skin permeation by nanostructured lipid carriers (NLCs). Part of this figure was created using Servier Medical Art images, which are licensed under a Creative Commons Attribution 3.0 Unported License (https://smart.servier.com).
Recently, researchers exploited NLC based formulations of various anti-inflammatory agents for treating inflammation-related skin diseases. Han and co-workers prepared NLC formulation of flurbiprofen (a NSAID widely used for gout and arthritis treatment) and investigated its stability as well as skin permeation through rat skin [130]. The authors reported that the flurbiprofen contained NLCs were stable over a long period of time. Also, the permeation of flurbiprofen after 12 h was 4.5 times higher when delivered by NLCs when compared with free drug treatment in PBS. González-Mira et al. also prepared flurbiprofen based NLCs using stearic acid or Compritol as solid lipids, and a mixture of medium chain triglycerides and castor oil as liquid lipids [131]. The authors observed an improved flurbiprofen delivery when the drug was trapped in Compritol NLCs when compared with the other control treatments. The authors investigated in vitro and in vivo irritancy and tolerability by the Skintex and Draize tests, and the results revealed that the NLCs are non-irritants. Doktorovová et al. developed and tested NLCs for topical delivery of fluticasone, which is mostly used for the treatment of skin disorders caused by atopic dermatitis and psoriasis [132]. The authors observed high entrapment efficacy of fluticasone and storage stability of NLCs. Similar results were reported by other group that studied NLC-based valdecoxib, celecoxib anti-inflammatory formulations [133, 134]. Thus, these studies concur that NLCs have been found to show promising treatment efficiency due to their controlled release over time, enhanced permeability into the skin, minimal skin irritation, etc.
3.4.1. Recent advances in application of nanomaterials for inhalation delivery of siRNA and antisense oligonucleotide
Over the past decade, numerous reports revealed that nanoparticles can be employed as efficient vehicles for local delivery of therapeutic agents (drugs and nucleic acids) to the lungs via inhalation [13, 14, 21, 115, 116, 120, 121, 125, 135-139]. It was confirmed experimentally that by changing the delivery route from parenteral to pulmonary, some nanoparticles predominantly localized and retained in the lungs while their accumulation in other organs was limited [13, 14, 116, 120, 121, 135, 140, 141]. By analyzing various nanomaterials (e.g., micelles, liposomes, lipid nanoparticles, etc.), extensive research conducted in our laboratory showed that lipid-based nanoparticles demonstrate superior accumulation in the lungs in comparison to non-lipid-based carriers and they are most appropriate for effective delivery of therapeutic agents via the inhalation route [116]. Among various therapeutic agents, siRNA and/or antisense oligonucleotides have a significant potential for the treatment of lung diseases either alone or in combination with conventional drugs [21, 115, 120, 135, 138, 139]. To avoid nucleolytic degradation and improve cellular internalization of unmodified nucleic acids, nanoparticles were extensively explored as vehicles for pulmonary delivery of siRNA and/or oligonucleotides molecules to various disease sites [21, 115, 120, 138, 139, 141-146]. Although there are numerous reports focused on the development of nanocarriers for siRNA via the inhalation route, not all of them evaluated the prepared formulations in animal studies. In this review, we summarize recent reports that validated the potential of nanoparticles for siRNA or antisense oligonucleotide delivery to the lungs via inhalation route in suitable animal models.
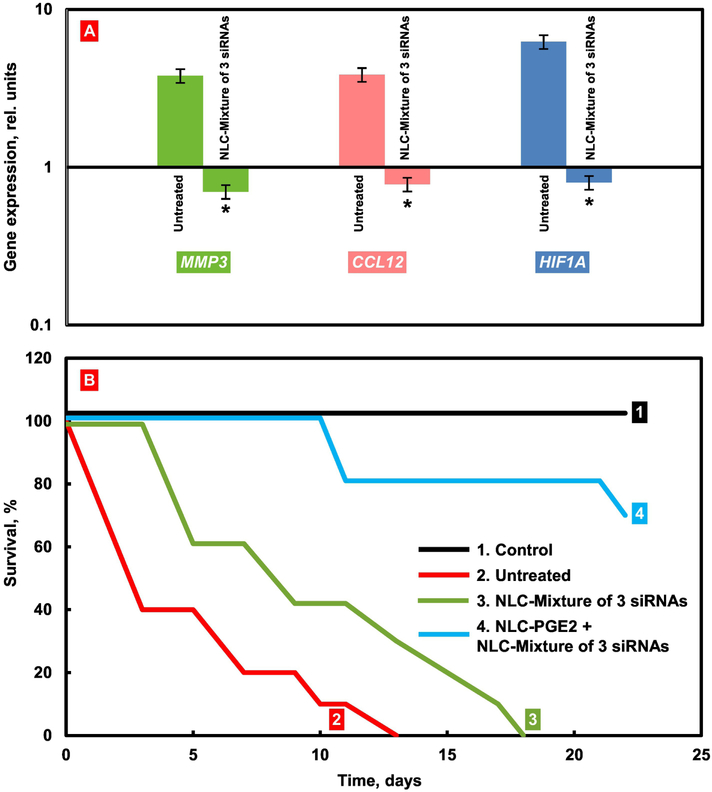
Most of the systems employed for the delivery of nucleic acids were prepared by complexation of negatively charged siRNA with positively charged lipids or polymers resulting in nanoparticles with a size in the range of 80 – 420 nm. It was shown that siRNA delivered by both lipid- and polymer-based nanoparticles via inhalation results in significant suppression of the mRNA in the targeted diseased cells [21, 115, 121, 135, 141-146]. For instance, it was shown that three types of mRNAs - MMP3, CCL12, and HIF1Alpha were highly overexpressed in idiopathic pulmonary fibrosis - IPF (Fig. 9). Inhalation delivery by lipid-based nanoparticles of the mixture of three siRNAs targeted to MMP3, CCL12, and HIF1Alpha mRNAs substantially suppressed all of these mRNAs (Fig. 9A). However, the suppression of targeted mRNAs led to only limited improvement in mice survival (Fig. 9B, plot 3). In contrast, the delivery of these siRNAs in combination with a therapeutic agent (prostaglandin E2) substantially improved the survival of mice with IPF (Fig. 9B, plot 4) [115]. In addition, the delivered siRNA molecules in combination with conventional drugs demonstrate the significant therapeutic potential for treating lung diseases such as cancer and pulmonary or cystic fibrosis [21, 115, 120, 125, 141-146]. Our research group significantly contributed to this field by developing nanostructured lipid carriers (NLCs), and demonstrating their efficiency to concurrently deliver multiple siRNA sequences and small drug molecules to different disease cells in the lungs [21, 115]. For example, NLC as a vehicle was employed for the mentioned simultaneous delivery of prostaglandin and three different siRNAs to the lungs [115]. In a separate study, Taratula et al. [21] further validated the NLC efficiency to concurrently deliver multiple siRNAs and chemotherapeutic agents via the inhalation to the lungs of mice with lung cancer.
Fig. 9.
Treatment of mice with Idiopathic Pulmonary Fibrosis (IPF). IPF was induced by intratracheal instillation of 1.5 U/kg of bleomycin. Mice were treated by inhalation with substances indicated twice a week for three weeks starting next day after the bleomycin administration. (A) Expression of targeted genes in lung tissues of mice with IPF. At the end of treatment, lungs were harvested and homogenized. The expression of MMP3 (A), CCL12 (B) and HIF1A (C) genes was measured by the QPCR and presented as fold change relative to the healthy animals. Means ± SD are shown. *P < 0.05 when compared with untreated mice with pulmonary fibrosis. (B) Survival of mice (Kaplan-Meier survival plot) with IPF. Modified from [115].
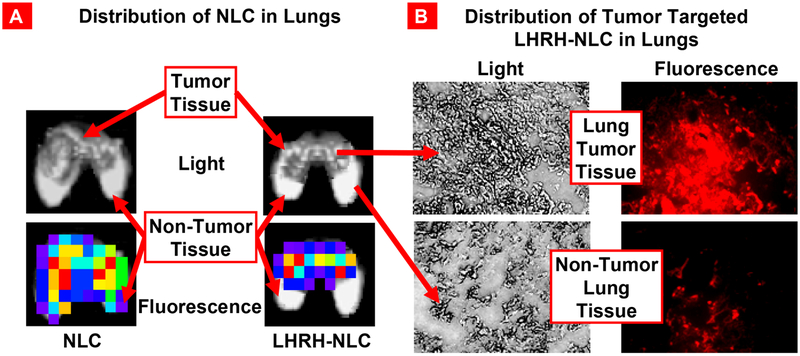
In addition to the favorable organ distribution of therapeutics locally delivered to the lungs by inhalation, the efficacy of the treatment and further limitation of adverse side effects on healthy lung tissues can be achieved by targeting delivery systems specifically to diseased cells. Previously we showed that luteinizing hormone-releasing hormone (LHRH) receptors are highly expressed in many cancer cells including lung cancer [16, 18, 88]. Consequently, it was hypothesized that conjugation of LHRH peptide to a nanocarrier would enhance its accumulation predominately in lung cancer cells mostly avoiding healthy lung tissues. The hypothesis was supported by several studies. In particular, it was found that targeting of lung cancer cells by LHRH peptide significantly influences NLCs distribution in the lungs following inhalation. It was revealed that PEGylated NLCs labeled with a fluorescent dye (Cy5.5) were uniformly distributed throughout the lungs after inhalation [21]. The same NLCs equipped with LHRH peptide accumulated in tumors while avoiding healthy lung tissues (Fig. 10) [21]. Hence, the NLC-mediated tumor-targeted delivery of siRNAs and chemotherapeutic agents resulted in efficient inhibition of cancer growth and prevention of adverse side effects on healthy organs.
Fig. 10.
Distribution of NLCs in the lungs. (A) Distribution of fluorescently labeled (Cy5.5) non-targeted and LHRH-tumor targeted NLCs in mouse lungs bearing human lung cancer. (B) Distribution of fluorescently labeled (Cy5.5) LHRH-tumor - targeted (NLC-LHRH) in mouse lungs bearing human lung tumor cells (tumor and non-tumor tissues; bright field and fluorescence microscope images; red color represents distribution of NLC-LHRH in tumor and non-tumor lung tissues). Modified from [21].
In addition to the NLCs, mesoporous silica nanoparticles and several polymers such as polyesters, polyethylenimine, and chitosan derivatives were successfully employed for the preparation of siRNA-loaded nanoparticles for inhalation therapies [141-143, 145, 147]. For example, Yan et al. developed novel amino thiols, -ene functional polyesters for complexation of siRNA into nanoparticles and demonstrated that these polyester-based nanoparticles specifically deliver siRNA to the mouse lungs after inhalation and result in ~65% knockdown of a targeted luciferase gene in A549-Luc orthotopic lung tumors [141, 147]. Xu et al. also used poly(ester amine) polymer as a nanocarrier for Akt1 siRNA to urethane-induced lung cancer tumors in mice through a nose-only inhalation system. The delivered siRNA dramatically suppress the expression of the targeted Akt1 protein in the lungs (>80%) but did not affect its expression in other organs. As a result, the Akt1 siRNA delivered to mice twice a week for four weeks substantially inhibit lung tumor growth [145].
Luo et al. [143] demonstrated that guanidinylated chitosan-based nanoparticles can be considered as promising carriers for siRNA delivery to the lungs and specifically to the bronchial epithelial cells. It was also validated that a targeting ligand, salbutamol, enhanced specificity of these nanoparticles to lung cells harbored with the β2-adrenergic receptor and substantially increase the siRNA-mediated gene silencing in vivo, resulting in ~40% knockdown of the targeted gene. Capel et al. [142] reported that piperazine-substituted chitosans can efficiently complex siRNA into nanoparticles and deliver siRNA to the lungs following intratracheal aerolisation. The obtained results, however, suggested that the delivered siRNA was not homogeneously distributed through the lungs and the accumulation pattern varied from mouse to mouse.
Finally, polyethyleneimine (PEI)-based nanoparticles represent another type of siRNA vehicle for inhalation delivery. Xu et al. [144] designed a conjugate of 25 kDa PEI and doxorubicin for electrostatic complexation of siRNA molecules into nanoparticles. Pulmonary administration of the designed nanoparticles to the mice with metastatic lung cancers provided a preferential accumulation of both DOX and siRNA in the lungs and resulted in significant anticancer effect. However, histopathological analysis of the lungs treated with the PEI-based nanoparticles revealed thickening of the alveolar wall due to the inflammatory response.
In summary, by using various murine models, it was verified that specifically designed lipid- and polymer-based nanoparticles can be used as efficient and safe carriers for pulmonary delivery of siRNA. The novel combinatorial therapies based on these nanoplatforms can potentially shift the current paradigm for the treatment of lung diseases. However, only a limited number of reports have been published on the inhalation delivery of siRNA. These investigations can be considered only as a proof-of-concept for the idea of inhalation treatment of lung disease by the combination delivery of siRNA and other therapeutic agents. A further validation of these platforms in suitable large animal models is essential for advancing the designed therapies to clinical trials.
3.5. Mesoporous silica nanoparticles
Mesoporous silica nanoparticles (MSNs) are a type of nonbiodegradable nanoparticles which were also exploited for various drug delivery applications including peptides and nucleic acids [13, 148-150]. By modulating the additives used in the preparation of MSNs, their properties such as pore size, mesoporous nature, load efficiency etc. can be altered to facilitate their drug delivery application. Hom and co-workers developed modified MSNs by decorating its surface by covalent conjugation or non-covalent entrapment of PEG molecules or targeting ligands for selective drug delivery applications [151]. They also modified the surface of MSNs with the cationic polymer such as PEI which electrostatically attract anionic nuclei acid molecules and facilitate their delivery.
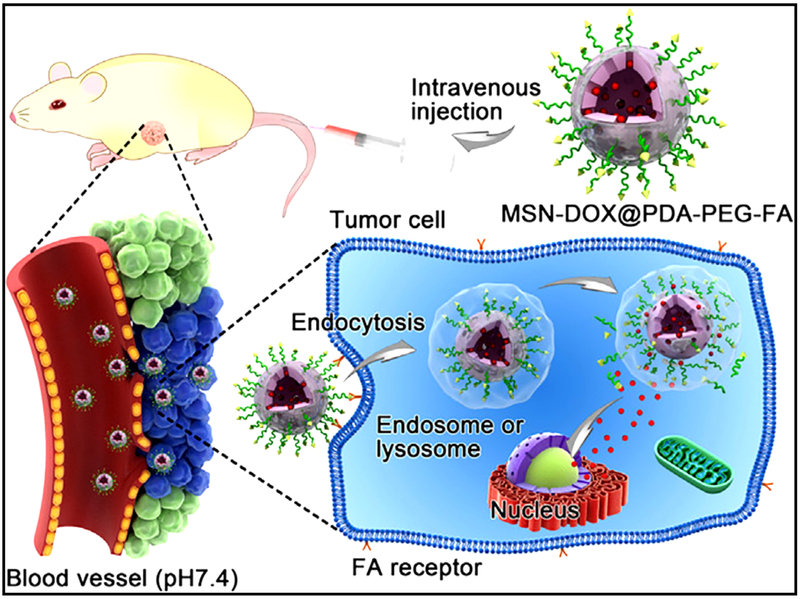
Cheng et al. developed a poly (ethylene glycol)-folic acid-functionalized and poly dopamine-modified multifunctional MSNs (namely, MSNs@PDA-PEG-FA) for site specific controlled delivery of doxorubicin [152]. Doxorubicin was released from the MSNs@PDA-PEG-FA system when these MSNs were dispersed in acidic conditions by pH stimulation. Treatment using the MSNs system exhibited better antitumor effects (Fig. 11).
Fig. 11.
Schematic illustration of DOX-loaded MSNs@PDA–PEG–FA. Reproduced with permission from [152].
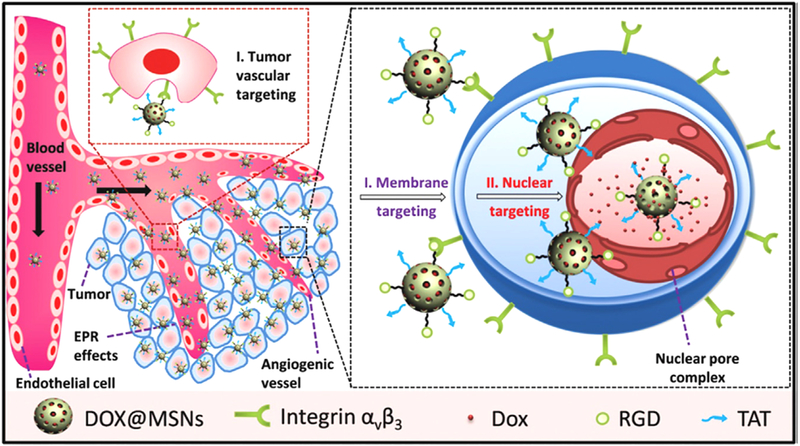
Recently, Pan et al. modified MSNs with trans-activating transcriptor (TAT) peptide for nuclear-targeted anticancer drug delivery application [153]. This TAT peptide-modified MSNs (MSNs-TAT) system showed intra-nuclear localization in cancer cells and released the loaded drug inside the nucleus. Later, the authors decorated the TAT peptide-modified MSNs with RGD peptide. This dual targeted MSNs (MSNs-RGD/TAT) were found to first bind to the tumor vasculature and then to the cell membrane and finally to the nucleus leading to its nuclear uptake. This sequential dual targeting system enhanced the therapeutic efficacy (Fig. 12) [153].
Fig. 12.
Sequential targeting drug delivery based on RGD and TAT peptides co-conjugated MSNs for effective cancer therapy. Reproduced with permission from [153].
3.6. Gold and iron-oxide nanoparticles
Gold nanoparticles have unique physicochemical and optical properties which enable their extensive use in image based diagnostic and drug delivery applications [154]. Because of very small size in nature, gold nanoparticles can easily penetrate cells and deliver drugs, genes, and imaging agents. Gold NPs can be easily modified by various functional groups, targeting ligands and PEG-containing linkers to conjugate therapeutics or nucleic acids for cell specific payload delivery. Gold nanoparticles were also found to be less toxic due to inert nature of metallic gold and have higher efficiency in cellular uptake as well as stability in the circulation. Hence, gold nanoparticles have been widely used not only for drug or gene delivery, but also for imaging applications [11, 155].
Iron oxide nanoparticles, which are also known as super paramagnetic nanoparticles, have received significant attention to both researchers and clinicians because of their wide scope of applications [12, 156, 157]. Due to magnetic nature, iron oxide nanoparticles can be visualized by Magnetic Resonance imaging (MRI) and it can be used for MRI based imaging applications [158, 159]. In addition, magnetic iron oxide nanoparticles generate heat after exposure to an alternating magnetic field and therefore, they are employed for magnetic hyperthermia and temperature-triggered drug release [98, 160]. Finally, iron oxide nanoparticles can be attracted to the tumor cells to deliver the drugs through an external magnetic field. This strategy can prevent release of the loaded drug or therapeutics in healthy tissue thereby reducing the systematic side effects. These nanoparticles also have potential for in vivo applications due to their easy biodegradable feature and the degraded iron can be absorbed by hemoglobin in body. Some of the commercial iron oxide nanoparticles such as ferridex I.V., ferumoxytol, and combidex were used or continued currently to be used for therapeutic or imaging applications [161].
3.7. Supramolecular gels
Topical drug delivery for skin diseases via the transdermal route is more convenient and safer than the other drug delivery routes - since administering drugs locally rather than via systemic routes decreases side effects and drug toxicity as well as increase treatment’s efficiency [162]. Recently, nanostructured supramolecular gels have been utilized to design formulations as topical and localized systems for the delivery of drugs [163].
Supramolecular gels are viscoelastic liquid like or solid like material, made up of a liquid phase immobilized in a solid three-dimensional matrix. The formation of this solid 3-D network is a result of self-assembly process of gelator molecules through various non-covalent bonding such as hydrogen bonding, π–π stacking, van der Waals interactions etc. [164]. Small molecular weight (MW ≤ 3000) substances which can solidify different organic and aqueous solvents are termed as low molecular weight gelators (LMWGs) [165]. Based on the type of solvent (organic or aqueous), the subsequent gel is named as organogel or hydrogel, respectively [166]. Gels derived from LMWGs are known as “Supramolecular Gels” [167].
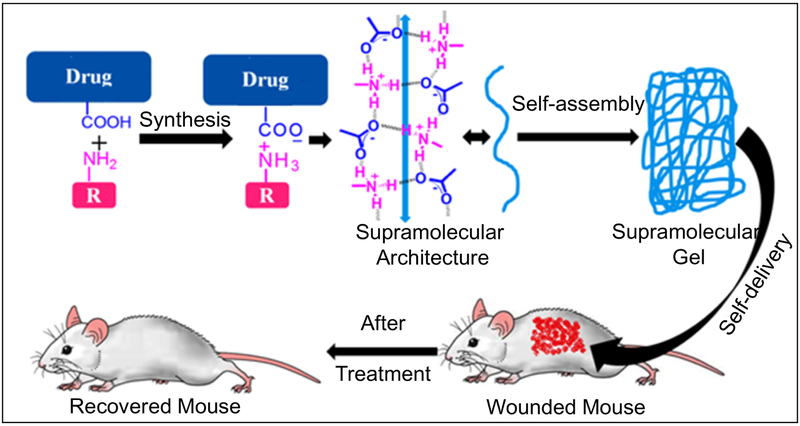
Over the years, LMWGs have been used in a number of plausible applications (e.g. in sensors [168], dye adsorbing agents [169], MRI contrast agents [170], oil spill recovery [171] etc.). Researchers also explored supramolecular gels for different biomedical applications (e.g. tissue engineering [172], biomineralization [173], wound treatment [174], medical diagnostics [175], enzyme mimics [176], inhibitors of cancer cell growth [177, 178], 3D cell cultures [179] etc.). Recently, supramolecular gel has also become an emerging carrier in controlled drug delivery devices as outlined in Fig. 13 [180, 181]. In topical formulations, a drug molecule is loaded into a vehicle molecule such as a polymer. However, major concerns related to such drug delivery systems include physical or chemical loading of the drug into the transporter polymer and its release, synthetic access of the polymeric vehicle molecule, biocompatibility, biodegradability and cytotoxicity of the polymeric vehicle molecule, etc. To overcome these restrictions, researchers developed an alternative approach named the self-delivery [182], where a vehicle molecule is not needed. In a self-delivery system, the drug is directly transformed to supramolecular gelator after non-covalent or covalent synthesis. These types of gel may be used for the delivery of the gelator drugs to the affected site by a local or systemic administration.
Fig. 13.
Preparation and testing of supramolecular gels for topical drug delivery application Low molecular weight gelator molecule (derived from salt formation between the carboxylic acid functionalized drug and primary amine) first self-assemble to form 1D network which further self-assemble to form 3D cross-linked network - under which the solvent molecules are trapped to produce supramolecular gels. Drug based supramolecular topical gel is shown as self-delivery systems for treating skin diseases in mouse.
3.7.1. Supramolecular hydrogels
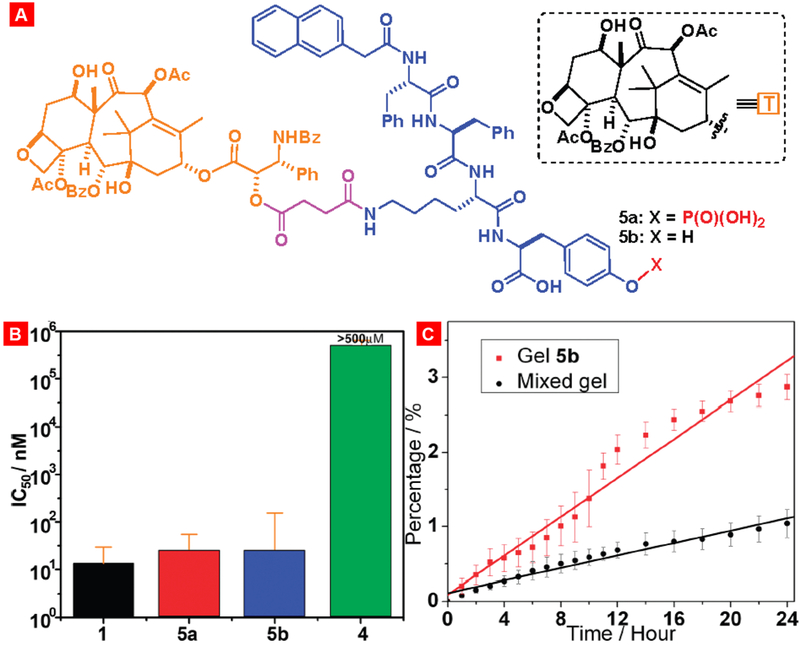
Supramolecular hydrogels possess high degree of physical stability, chemical tunability, loading capacity, temperature-sensitive functionality etc. properties which make these materials suitable for drug delivery application. Xu and his team were one of the pioneers in developing supramolecular gel-based drug delivery application [183]. They demonstrated that a taxol-tetrapeptide conjugate formed hydrogels upon enzymatic cleavage of a phosphate group. MTT assay of this conjugate in HeLa cells showed similar IC50 values like the free taxol. Also, the hydrogel formulated from this conjugate showed slow and sustained release profile which was comparable to other taxol based gel formulations [184-186]. Gao et al. reported another hydrogelator based on the well-established antineoplastic agent paclitaxel. The IC50 value of this hydrogelator in HeLa cells was found to be 25 nM, which was comparable to the parenterally administered drug paclitaxel (Fig. 14) [184].
Fig. 14.
Toxicity and drug release profile of different taxol-loaded gels. (A) Structures of the gelators 5a and 5b. (B) Cytotoxicity of taxol (1) and gelators 5a, 5b, and 4 after incubated with HeLa cells for 48 h. (C) Accumulative drug release profile of two types of taxol gels in 100 mM PBS buffers. Reproduced with permission from [184].
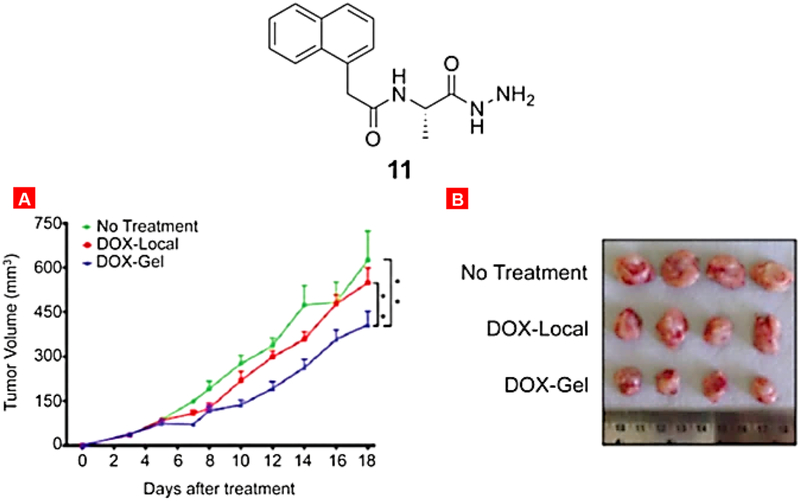
Bajaj and his co-workers developed amino acid based injectable hydrogel for the entrapment and controlled delivery of a well-known anticancer drug doxorubicin [187]. The results of the experiments showed controlled release of DOX from the hydrogel. Mice bearing colon and breast tumors were treated with hydrogel containing DOX (DOX-gel) and the results revealed that subcutaneously injected DOX-gel reduced the tumor volume better as compared to the untreated control and hydrogel gel without any DOX as well as direct intravenous injection of DOX solution (Fig. 15). Yang and his group designed and synthesized a gelator precursor by decorating paclitaxel with a folic acid - a targeting ligand for folate receptor overexpressed in cancer and tyrosine phosphate - an precursor known to promote self-assembly [188]. This enzymatic precursor molecule produces a transparent hydrogel in PBS buffer upon dephosphorylation by phosphatases. This hydrogel was capable of releasing the parent drug upon the enzymatic cleavage of the ester bond by esterases [189].
Fig. 15.
Structure of the gelator molecule 11. Mice were treated with DOX-local administration and with DOX-gel (made with gelator 11). (A) Tumor volume. Means ± SD are shown. *P < 0.005. (B) representative pictures of excised tumors. Reproduced with permission from [187].
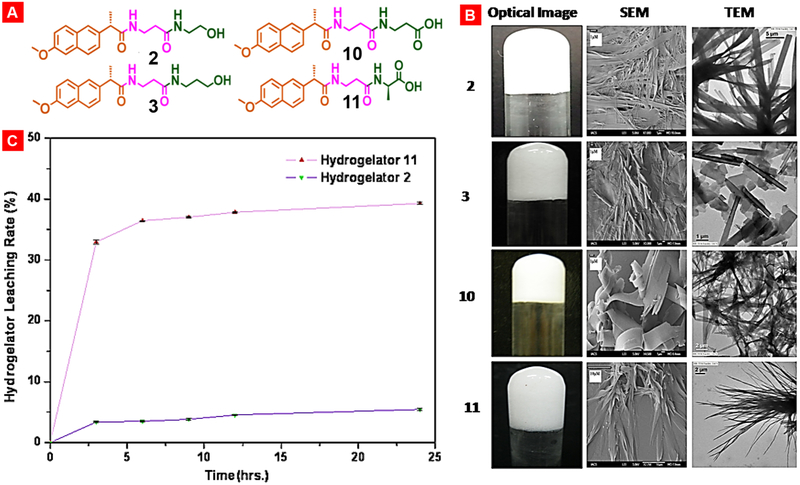
Dastidar and his group made a significant contribution to development of supramolecular gel based topical drug delivery systems to treat diseases such as skin inflammation, allergy etc. In a separate investigation of this group, researchers altered naproxen with amino acids/alcohols to generate several hydrogelator molecules to produce opaque hydrogels which demonstrated tape and/or sheet like morphologies revealed by scanning or transmission electron microscopes. All there hydrogels were non-toxic and showed the level of anti-inflammatory properties similar to the free parent drug (Fig. 16). Among these, a pair of hydrogels demonstrated a continuous persistent drug release indicating their possible application as self-delivery system [190].
Fig. 16.
Modifications of nonsteroidal anti-inflammatory drug naproxen by decorating the parent drug with β-amino acid/amino alcohols/L-amino acids results in potent hydrogelators namely 2, 3, 10 and 11. (A) Structures of NSAID-naproxen derived various supramolecular gelators. (B) Opaque hydrogels and the corresponding gel-network morphologies. (C) Sustained release of hydrogelator 2 and 11 in PBS (pH 7.4). Reproduced with permission from [190].
Barthelemy and co-authors developed a lipid-based hydrogel for the slow and sustained delivery of nucleic acid to the targeted cells. By combining a monosaccharide, a lipid chain, and a nucleoside together they designed a gelator which was capable of gelling water above 2.5 wt %. Their studies indicated that this hydrogelator was non-toxic in Huh7 cells after 5 days of treatment. The authors also observed an improved uptake of oligonucleotides by the cells [191]. Wang and co-workers described the gelation of water using a bioactive butanoic acid and hydrogelation was probed by hydrogen bonding interaction. When this hydrogel was immersed in a 37 °C water bath, gel dissociated and released the bio-active drug molecules [192]. Saez et al. described a new class of hydrogelators derived from model drugs such as benzylamine and phenethylamine. These model drug-based hydrogels showed nanofiber morphology in TEM images. The authors observed that the amide bond of the gelator scaffold undergo hydrolysis in presence of trypsin to release the model drug [193].
Patil et al. developed an amphiphile-based hydrogelator derived from essential biomolecule riboflavin. This amphiphile molecule forms hydrogels both at low and neutral pH. The hydrogel was found to bio-compatible and capable of delivering siRNA efficiently into human cells [194]. Lin with co-authors developed and synthesized amphiphile peptide based hydrogelators obtained from the anticancer drug paclitaxel. This peptide amphiphile self-assembles to form 3D SAFINs resulting in gelation in PBS. This hydrogel suppressed the proliferation of various cancer cells including breast, lung and prostate cancer. The authors found that the use of GSH induced the discharge of paclitaxel from this hydrogel [195].
Dastidar and co-authors delivered another NSAID namely indomethacin using supramolecular topical gel [196]. The authors reported a new type of peptide based indomethacin conjugates, most of which displayed excellent gelation ability in water, 0.9 % NaCl and a few organic solvents. All these gelators were biocompatible as assessed in MTT assay and two of these gelators showed anti-inflammatory effect similar to indomethacin as revealed in PGE2 assay. Leaching experiment demonstrated that these hydrogels have potential to release the gelator drug in localized and controlled way.
3.7.2. Supramolecular organogels
Supramolecular organogels produced from vegetable and synthetic oils and pharmaceutical solvents such as methyl salicylate have been recently exploited for various biomedical applications [197]. Though their use is tempered mostly because of non-biocompatible nature of components of the organogels, still some recent attempts were made on the design of biocompatible organogels for drug delivery application. Leroux with co-workers described a series of amphiphilic organogelators based on L-alanine [198]. These gelators were able to produce thermoreversible gels in various vegetable and synthetic oils. The organogels were injected subcutaneously in rat and the tissue samples were investigated by histopathological analysis. These results revealed a decent biocompatibility of the organogels within the 8 weeks of treatment and represented a platform for the slow and prolonged release of payload. In another work, Leroux et al. developed tyrosine based organogelator, which was capable of producing injectable organogel in safflower oil [199]. An acetylcholinesterase (AChE) inhibitor rivastigmine, was loaded in this organogel and injected in rat for the treatment of Alzheimer’s disease. The authors analyzed the rat-hippocampus tissue sample and observed that this organogel treatment inhibitited the AChE in the brain. These results suggested that tyrosine based organogels may be useful for the sustained delivery of cholinesterase inhibitors.
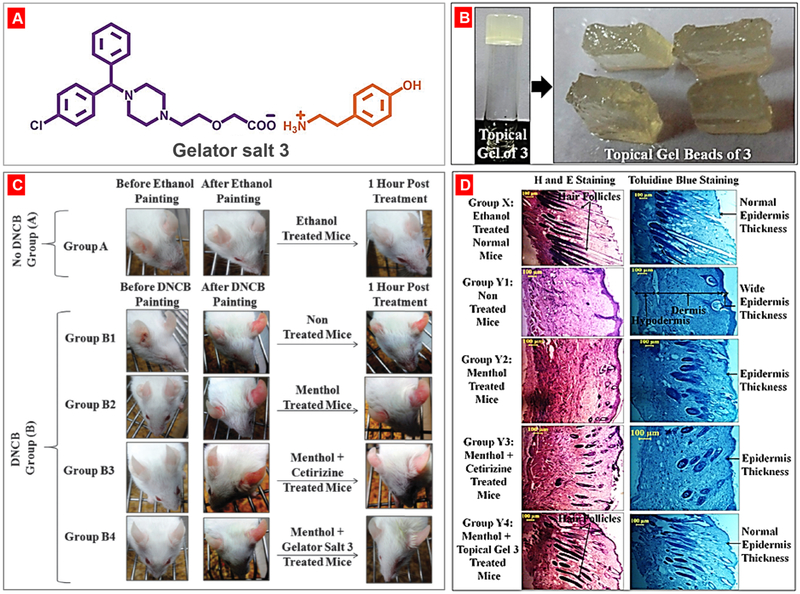
By exploiting supramolecular synthon approach [200, 201], Dastidar and his group discovered a new set of simple salt adducts of the well-known drug naproxen. One of this salt-adduct prepared from amino acid serinol, showed gelation ability in methyl salicylate in presence of 1 % menthol. This naproxen-serinol gelator salt was non-toxic in RAW 264.7 cells and retained its anti-inflammatory property like its parent drug. The methyl salicylate/menthol topical gel was capable to treat imiquimod (IMQ)-induced skin rash, redness and other pathological conditions in mice [202]. Majumder et al. modified an anti-allergic drug (cetirizine) to its simple salt based supramolecular gelator. This gelator produced biocompatible and nontoxic supramolecular topical gel in ethylsalicylate/menthol solvent mixture [203]. This supramolecular gel was topically applied to treat allergic ear and skin redness conditions in mice caused by dinitrochlorobenzene (DNCB). In the histology studies, the authors observed both reduced epidermis skin thikness and increased growth of hair folicles in mice with topical gel treatment when compared with non-treated controls (Fig. 17).
Fig. 17.
Topical cetirizine gel application for treatment of dinitrochlorobenzene (DNCB)-induced allergic conditions. (A) Chemical structures of gelator salt 3; (B) Methylsalycylate/menthol topical gel and gel beads of salt 3; (C) Treatment of DNCB-induced allergic ears redness of BALB/c male mice with the anti-allergic gelator salt 3. (D) Histological features of the dorsal skin tissues of mice. (Group X) no skin allergic induction by DNCB as the normal mice; (Group Y1) no treatment after skin allergy induction by DNCB; (Group Y2) menthol solution application after skin allergy induction by DNCB; (Group Y3) mother drug cetirizine solution application after skin allergy induction by DNCB; and (Group Y4) methylsalycylate/menthol topical gel of salt 3 application after skin allergic induction by DNCB. Reproduced with permission from [203].
Following this simple salt formation strategy, Roy et al. developed a multi-drug containing supramolecular topical gel system derived from a NSAID and an anti-viral agent [201]. The authors designed and synthesized several primary ammonium monocarboxylate salts and different NSAIDs and an antiviral drug. Most of these salt adducts were capable of forming supramolecular gels in various organic solvents. One of these gelator salts obtained from diclofenac showed anti-inflammatory response and can be used for combination therapy.
3.7.3. Supramolecular metallogels
Metal based therapeutics have been known from the discovery of cisplatin as one of the widely used chemotherapeutic drugs for various cancers [204-206]. Recently, researchers also focused on the development of metal or metal ion based nano-structured supramolecular gels for biomedical applications. Supramolecular gel containing a metal or metal ion as a part of its gel forming self-assembled network is referred as metallogel [207-209]. Like supramolecular gel, metallogel formation is also controlled by week non-covalent bonding and interactions including hydrogen bonding [210, 211], π–π interactions [212, 213], metal coordination [214] and host–guest inclusion [215] of the gelator molecules. Metallogels have highly tunable and stimuli responsive properties, which makes them suitable for different material applications as well as drug delivery [216].
Zhang and co-authors introduced the first supramolecular metallo-hydrogel derived from a tripeptide gelling scaffold and a ruthenium complex. The sol-gel transition phenomenon of this metallo-hydrogel was dependent on the redox state of the metal ion. This metallohydrogel showed low affinity to nucleic acids suggesting its cell compatibility even at high concentration and it can be used as a hydrogelator for biomedical applications [217]. Micklitsch and co-workers reported a metal-responsive hydrogel system, which was formed when β-hairpin peptide scaffold was reacted with zinc complex [218]. The quantity of zinc ion present in the metallohydrogel was controlled by the quantity of the gelator scaffold (β-hairpin). This metallohydrogel showed controlled delivery of Zn2+ ions via. a wound bed to promote the healing of bacterial infection. Xu et al. also observed that zinc ion could trigger the self-assembling of a naphthyl-based peptide molecule into hydrogels [219]. The morphology and the mechanical properties of this metallohydrogel were dependent on the quantity of zinc present in the gel system. The authors investigated the growth of E. coli culture using this hydrogel and found that the addition of zinc improved anti-bacterial activity of a non-metal containing gel.
By using salt metathesis technique, Roy et al. developed a metallohydrogels composed of Zn(II) and a NSAID-based peptide scaffold for drug delivery application [220]. The authors observed salt metathesis of various NSAIDs and their amide derivatives in presence of Zn(NO3)2 and the resulted Zn(II)-NSAID complex displayed metallohydrogel formation. One such metallohydrogel produced from DIF.TRIS.Zn complex was non-toxic and it showed both anti-inflammatory and antibacterial activities.
Thus, the suprasmolecular gel based systems have been reported to show promising results in delivery of drugs without the use of an additional vehicle such as various polymers usually used in commercial topical gel formulation thereby making this system more feasible and attractive than traditional polymeric gel system in biomedical application. However, the practical use of supramolecular gels in drug delivery is still in its infancy. Nevertheless, there are still many opportunities for further improvements of supramolecular gels in various biomedical applications.
3.8. Live cell-based drug delivery systems
Cell-based drug delivery systems have received growing interest in the past decade since they revolutionized the current diagnostic and therapeutic delivery techniques [221-225]. Therapeutic cells such as circulating erythrocytes, stem cells and immune cells have innate disease sensing property which enables these cells to function as living carriers for disease targeting [226, 227]. Besides, such therapeutics cells are mobile in nature and can travel through blood without showing any immunogenicity as well as these cells have the ability to pass through the blood brain barrier to access disease cells in the brain [228]. Because of these unique features, therapeutic cells have received significant attention to both researcher and clinicians to explore for targeted drug delivery applications [229, 230]. Cell based drug delivery system is prepared by covalent or non-covalent conjugation of drugs or drug-loaded particles such as therapeutic nanoparticles on the surface of live cells. Over the decade, various chemo selective methods have been exploited to conjugate cargo molecules/therapeutic agents on the surface of live cells [231-233]. Researchers also exploited nanoparticle mediated conjugation of therapeutics, proteins and biologics on the surface of live cells [234]. This review highlights the recent advances in developing cell-based living drug delivery systems. We will mainly focus on the methods of drug and/or therapeutic nanoparticle conjugation on the surface of live cells and their applications.
3.8.1. Drug/Therapeutic conjugated live cell-based drug delivery system
Live cell membrane is abundant with several functional groups including primary amines and thiols which have been widely used for bioconjugation of small molecules and therapeutics on live cell surface for biomedical applications. For example, Muzykantov with co-authors conjugated a thrombolytic agent on the surface of red blood cells (RBCs) through avidin-biotin bridges [235, 236]. These surface modified RBCs were used for prophylactic fibrinolysis. In early studies, researchers used carrier erythrocytes to encapsulate or conjugate small–molecule drugs, nucleic acids, proteins, and therapeutic nanoparticles employed for different applications and treating various diseases [237]. Due to high biocompatibility, these systems showed better therapeutic benefits. Many types of stem cells (SCs) such as mesenchymal stem cells (MSCs) and neural stem cells (NSCs) were also explored as carriers for tumor-specific drug delivery research due to their intrinsic ability to migrate towards tumor microenvironment [238]. Genetically modified stem cells are able to secret various therapeutic cytokines such as interferon-β (IFN-β) [239, 240], IL12/18 [241-243] and inhibit the growth of the tumor.
In a recent work, Takayama et al. developed a method for long-term modification of a stable reporter protein (Nluc) as a model drug on the surface of murine mesenchymal stem cells C3H10T1/2 cells through avidin-biotin complex method [244]. This cell surface modification lasted for at least 14 days in vitro without major effects on the cell viability, migration and differentiation ability while this modification lasted in vivo for at least 7 days. These results suggested that this method could be useful for long-term surface modification of drugs for effective MSC-based therapy.
3.8.2. Nanoparticle conjugated live cell-based drug delivery system
In order to increase the accumulation of nanoparticles in tumor tissue, researchers developed cell based drug delivery system where therapeutic nanoparticles were conjugated on the surface of live steam cells [245-247]. It was shown that DOX loaded anti-CD73 antibody linked silica nanoparticles were anchored on the surface of steam cells [248, 249]. Treatment with these nanoparticle modified steam cells increased cell apoptosis and inhibited the metastasis of U251 glioma tumor. Phagocytic cells were also explored to load and deliver various nanomaterials such as nanoparticles, liposomes etc. For example, Mooney et al. developed steam cell based system by phagocytosis of therapeutic gold nanoparticles [250]. This gold nanoparticle encapsulated steam cells displayed better distribution of gold nanoparticles in tumor tissue and potentially high photothermal therapeutic efficiency when compared with free gold nanoparticles. In another work, Mooney et al. decorated the surface of neural stem cells (NSCs) with docetaxel (DTX)-loaded pH sensitive nanoparticles via biotin-avidin linker [245]. The authors observed that drug-loaded nanoparticles can be conjugated to NSCs without impairing NSC viability and the distribution of the nanoparticles was better in intratumoral region.
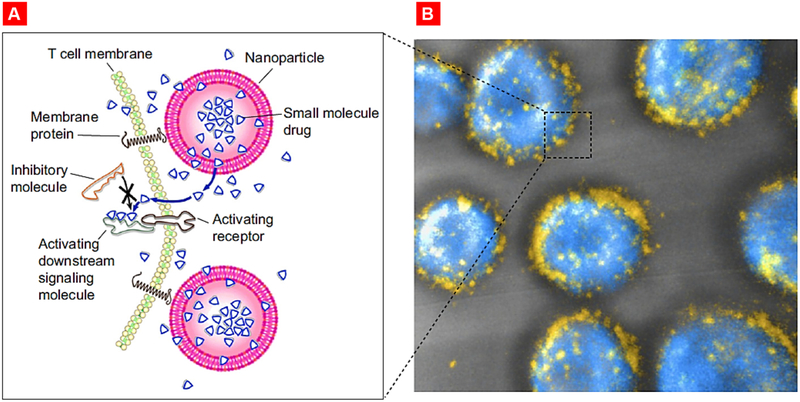
Stephan et al. functionalized maleimide-modified nanoparticles on the surface thiol groups for the T-cell synapse specific delivery of therapeutics (Fig. 19) [251]. These surface- conjugated nanoparticles rapidly polarized toward the nascent immunological synapse. The authors loaded a dual inhibitor of Shp1 and Shp2 namely NSC-87877 (which downregulate T-cell receptor activation in the synapse) within a nanoparticle followed by its conjugation with the surface of tumor-specific T cells. Adoptive transfer of NSC-87877 loaded nanoparticle conjugated T cells into mice with advanced prostate cancer resulted in better survival of treated animals.
Fig. 19.
Targeted release of cell membrane-permeable, immunomodulatory compounds from T-cell-linked nanoparticles. (A) Schematic view of strategy to modulate T-cell responses via nanoparticle conjugation to membrane proteins: Surface-conjugated drug-loaded nanoparticles slowly release their cargo compounds, which locally permeate the plasma membrane and block molecules in the cytosol that dampen T-cell activation. (B) 3D reconstruction of confocal microscopy images showing CD8+ effector T-cells (CFSE stain shown in blue) immediately after conjugation with fluorescent multilamellar lipid vesicles (yellow). Reproduced with permission from [251].
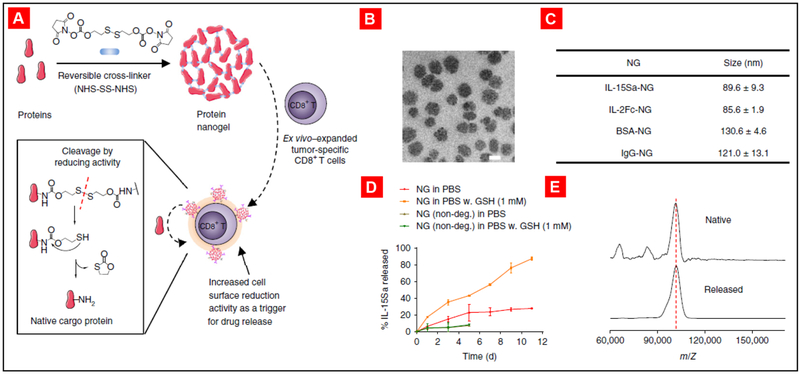
Irvine and co-authors proposed a strategy to enhance the outcomes of cell therapy via the conjugation of adjuvant drug-loaded synthetic nanoparticles on the surfaces of live therapeutic T-cells [252]. The authors observed that the nanoparticle conjugation was stable on cell surface and it did not affect the key cellular functions. These results also demonstrated that conjugation of drug-loaded particles directly to the donor cells increases their therapeutic impact. In another recent work from the same group, Tang et al. reported delivery of a protein drug by live T cells [253]. Large quantities of supporting protein drugs were conjugated on the surface of T cells forming protein nanogels (NGs) that selectively release the therapeutic protein in response to T cell receptor activation in tumor microenvironment. It was shown that the usage of the cytokine interleukin-15 nanogel increased the T cell expansion, allowed for the delivery of much higher doses of cytokine without notable cytotoxicity and enhanced the efficacy of tumor clearance by mice (Fig. 18).
Fig. 18.
Synthesis and characterization of T cell receptor (TCR) signaling-responsive protein nanogels. (A) Scheme for protein nanogel (NG) synthesis and release of protein in response to reducing activity in the local microenvironment. (B) Representative transmission electron microscopy image of NGs prepared from a human IL-15 superagonist (IL-15Sa). Scale bar, 50 nm. (C) Hydrodynamic sizes of different NGs, as determined by dynamic light scattering. Means ± SD are shown. (D) Release kinetics of cytokines from redox-responsive or nondegradable IL-15Sa-NGs in PBS with or without added glutathione (GSH) as a reducing agent. Means ± SD are shown. (E) Representative MALDI mass spectrometric analysis of released and native cytokines. Reproduced with permission from [253].
Thus, the past decade has witnessed a remarkable progress in the development of live cell-based targeted drug delivery systems and their clinical applications leading to a significant improvement in diagnostic and therapeutic outcomes. The major therapeutic benefit of cell-based drug delivery systems includes their disease specific delivery of therapeutics as well as the ability to cure the hard to treat diseases.
4. Conclusion
Delivery of therapeutics by nanocarriers demonstrates clear advantages over free non-bound drugs in terms of pharmacokinetics, body distribution, limitation of adverse side effects and therapeutic efficacy. Moreover, targeting of drug carriers specifically to diseased organs, tissues and cells further enhances their organ distribution and therapeutic potential while limiting adverse side effects upon healthy tissues. Furthermore, it was found that such targeting practically nivellates differences in the body distribution and therapeutic efficacy between these nanoparticles. In addition to characteristics of nanoparticles and their targeting, the type of route of administration plays an important role in the efficacy and adverse side effects of treatment. While systemic delivery of non-targeted systems in most cases is accompanied by substantial adverse side effects, local delivery limits such effects whereas improves the treatment. Likewise, local delivery of targeted therapeutics (e.g. local pulmonary delivery of nanoparticles for treatment of diseases) combines the advantages of both approaches and further enhances the efficacy of the treatment and limits its adverse side effects. Nevertheless, some issues in formulation, toxicity, targeting, safety, efficacy and storage stability of nanoparticles need to be addressed in further investigations.
Acknowledgements:
Funding: This work was supported in part by the National Institutes of Health [grant numbers CA238871, CA208818, HL118312, EB020351 and CA234006].
Abbreviations
- 3D SAFINs
3-dimensional (3D) self-assembled fibrillar networks
- AChE
acetylcholinesterase
- Cy5.5
cyanine5.5 NHS ester
- DMPC
1,2-dimyristoyl-sn-glycero-3-phosphocholine
- DMPG
1,2-Dimyristoyl-sn-glycero-3-phosphoglycerol
- DNCB
dinitrochlorobenzene
- DOPC
1,2-Dioleoyl-sn-glycero-3-phosphocholine
- DOPE
1,2-Dioleoyl-sn-glycero-3-phosphoethanolamine
- DOX
doxorubicin
- DPPG
1,2-Dipalmitoyl-sn-glycero-3[Phospho-rac-1-glycerol
- DSPC
1,2-Distearoyl-sn-glycero-3-phosphocholine
- DSPE
1,2-Distearoyl-phosphatidyl ethanolamine
- DSPG
1,2-Distearoyl-sn-glycero-3-phosphoglycerol
- DTX
docetaxel
- EPR
enhanced permeability and retention
- FA
folic acid1
- GSH
glutathione
- HSPC
hydrogenated Soy l-α-phosphatidylcholine
- IC50
50% inhibitory concentration; here – a drug concentration that kills 50% of cells
- IFN-β
interferon-β
- IMQ
imiquimod
- IPF
idiopathic pulmonary fibrosis
- LHRH
luteinizing hormone-releasing hormone
- LMWGs
low molecular weight gelators
- MRI
magnetic resonance imaging
- MSCs
mesenchymal stem cells
- MSNs
mesoporous silica nanoparticles
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NGs
nanogels
- NLCs
nanostructured lipid carriers
- NSAID
nonsteroidal anti-inflammatory drug
- NSCs
neural stem cells
- PACA
poly (alkyl cyanoacrylate)
- PAMAM
polyamidoamine
- PCL
polycaprolactone
- PDA
polydiacetylene
- PEG
poly(ethylene glycol)
- PEI
polyethyleneimine
- PGE2
prostaglandin E2
- PLGA
polylactic acid-co-glycolic acid
- PPI
poly(propylene imine)
- RBCs
red blood cells
- RES
reticuloendothelial system
- RGD
arginylglycylaspartic acid
- SCs
stem cells
- SEM
scanning electron microscope
- SLNs
solid lipid nanoparticles
- SPIONs
superparamagnetic iron oxide nanoparticles
- TAT
trans-activating transcriptor
- TEM
transmission electron microscope
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare no conflict of interest in the publication of this work.
References
- [1].Mainardes RM, Silva LP, Drug delivery systems: past, present, and future, Curr Drug Targets, 5 (2004) 449–455. [DOI] [PubMed] [Google Scholar]
- [2].Robinson DH, Mauger JW, Drug delivery systems, Am J Hosp Pharm, 48 (1991) S14–23. [PubMed] [Google Scholar]
- [3].Viswanathan P, Muralidaran Y, Ragavan G, Chapter 7 - Challenges in oral drug delivery: a nano-based strategy to overcome, in: Andronescu E, Grumezescu AM (Eds.) Nanostructures for Oral Medicine, Elsevier, 2017, pp. 173–201. [Google Scholar]
- [4].Bardal SK, Waechter JE, Martin DS, Chapter 2 - Pharmacokinetics, in: Bardal SK, Waechter JE, Martin DS (Eds.) Applied Pharmacology, Philadelphia, 2011, pp. 17–34. [Google Scholar]
- [5].Koushik OS, Rao YV, Kumar P, Karthikeyan R, Nano Drug Delivery Systems to Overcome Cancer Drug Resistance - A Review, J Nanomed Nanotechnol, 7 (2016) 378–387. [Google Scholar]
- [6].Minko T, Rodriguez-Rodriguez L, Pozharov V, Nanotechnology approaches for personalized treatment of multidrug resistant cancers, Adv Drug Deliv Rev, 65 (2013) 1880–1895. [DOI] [PubMed] [Google Scholar]
- [7].Yu B, Tai HC, Xue W, Lee LJ, Lee RJ, Receptor-targeted nanocarriers for therapeutic delivery to cancer, Mol Membr Biol, 27 (2010) 286–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Mejia Oneto JM, Khan I, Seebald L, Royzen M, In Vivo Bioorthogonal Chemistry Enables Local Hydrogel and Systemic Pro-Drug To Treat Soft Tissue Sarcoma, ACS Cent Sci, 2 (2016) 476–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Dastidar P, Roy R, Parveen R, Sarkar K, Supramolecular Synthon Approach in Designing Molecular Gels for Advanced Therapeutics, Adv Therap, 2 (2019) 1800061. [Google Scholar]
- [10].Tran S, DeGiovanni PJ, Piel B, Rai P, Cancer nanomedicine: a review of recent success in drug delivery, Clin Transl Med, 6 (2017) 44–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Chen AM, Taratula O, Wei D, Yen HI, Thomas T, Thomas TJ, Minko T, He H, Labile catalytic packaging of DNA/siRNA: control of gold nanoparticles "out" of DNA/siRNA complexes, ACS Nano, 4 (2010) 3679–3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Taratula O, Garbuzenko O, Savla R, Wang YA, He H, Minko T, Multifunctional nanomedicine platform for cancer specific delivery of siRNA by superparamagnetic iron oxide nanoparticles-dendrimer complexes, Curr Drug Deliv, 8 (2011) 59–69. [DOI] [PubMed] [Google Scholar]
- [13].Taratula O, Garbuzenko OB, Chen AM, Minko T, Innovative strategy for treatment of lung cancer: targeted nanotechnology-based inhalation co-delivery of anticancer drugs and siRNA, J Drug Target, 19 (2011) 900–914. [DOI] [PubMed] [Google Scholar]
- [14].Garbuzenko OB, Winkler J, Tomassone MS, Minko T, Biodegradable Janus nanoparticles for local pulmonary delivery of hydrophilic and hydrophobic molecules to the lungs, Langmuir, 30 (2014) 12941–12949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zylberberg C, Matosevic S, Pharmaceutical liposomal drug delivery: a review of new delivery systems and a look at the regulatory landscape, Drug Deliv, 23 (2016) 3319–3329. [DOI] [PubMed] [Google Scholar]
- [16].Saad M, Garbuzenko OB, Ber E, Chandna P, Khandare JJ, Pozharov VP, Minko T, Receptor targeted polymers, dendrimers, liposomes: which nanocarrier is the most efficient for tumor-specific treatment and imaging?, J Control Release, 130 (2008) 107–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Minko T, Pakunlu RI, Wang Y, Khandare JJ, Saad M, New generation of liposomal drugs for cancer, Anticancer Agents Med Chem, 6 (2006) 537–552. [DOI] [PubMed] [Google Scholar]
- [18].Zhang M, Garbuzenko OB, Reuhl KR, Rodriguez-Rodriguez L, Minko T, Two-in-one: combined targeted chemo and gene therapy for tumor suppression and prevention of metastases, Nanomedicine (Lond), 7 (2012) 185–197. [DOI] [PubMed] [Google Scholar]
- [19].Mendes LP, Sarisozen C, Luther E, Pan J, Torchilin VP, Surface-engineered polyethyleneimine-modified liposomes as novel carrier of siRNA and chemotherapeutics for combination treatment of drug-resistant cancers, Drug Deliv, 26 (2019) 443–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Gomes MJ, Martins S, Ferreira D, Segundo MA, Reis S, Lipid nanoparticles for topical and transdermal application for alopecia treatment: development, physicochemical characterization, and in vitro release and penetration studies, Int J Nanomedicine, 9 (2014) 1231–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Taratula O, Kuzmov A, Shah M, Garbuzenko OB, Minko T, Nanostructured lipid carriers as multifunctional nanomedicine platform for pulmonary co-delivery of anticancer drugs and siRNA, J Control Release, 171 (2013) 349–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Khandare JJ, Jayant S, Singh A, Chandna P, Wang Y, Vorsa N, Minko T, Dendrimer versus linear conjugate: Influence of polymeric architecture on the delivery and anticancer effect of paclitaxel, Bioconjug Chem, 17 (2006) 1464–1472. [DOI] [PubMed] [Google Scholar]
- [23].Patil ML, Zhang M, Betigeri S, Taratula O, He H, Minko T, Surface-modified and internally cationic polyamidoamine dendrimers for efficient siRNA delivery, Bioconjug Chem, 19 (2008) 1396–1403. [DOI] [PubMed] [Google Scholar]
- [24].Taratula O, Garbuzenko OB, Kirkpatrick P, Pandya I, Savla R, Pozharov VP, He H, Minko T, Surface-engineered targeted PPI dendrimer for efficient intracellular and intratumoral siRNA delivery, J Control Release, 140 (2009) 284–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Shah V, Taratula O, Garbuzenko OB, Taratula OR, Rodriguez-Rodriguez L, Minko T, Targeted nanomedicine for suppression of CD44 and simultaneous cell death induction in ovarian cancer: an optimal delivery of siRNA and anticancer drug, Clin. Cancer Res, 19 (2013) 6193–6204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Schumann C, Chan S, Millar JA, Bortnyak Y, Carey K, Fedchyk A, Wong L, Korzun T, Moses AS, Lorenz A, Shea D, Taratula O, Khalimonchuk O, Taratula O, Intraperitoneal nanotherapy for metastatic ovarian cancer based on siRNA-mediated suppression of DJ-1 protein combined with a low dose of cisplatin, Nanomedicine, 14 (2018) 1395–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Schumann C, Taratula O, Khalimonchuk O, Palmer AL, Cronk LM, Jones CV, Escalante CA, Taratula O, ROS-induced nanotherapeutic approach for ovarian cancer treatment based on the combinatorial effect of photodynamic therapy and DJ-1 gene suppression, Nanomedicine, 11 (2015) 1961–1970. [DOI] [PubMed] [Google Scholar]
- [28].Taratula O, Savla R, Minko T, Poly(propyleneimine) dendrimers as potential siRNA delivery nanocarrier: from structure to function, International Journal of Nanotechnology, 8 (2011) 36–52. [Google Scholar]
- [29].Savla R, Taratula O, Garbuzenko O, Minko T, Tumor targeted quantum dotmucin 1 aptamer-doxorubicin conjugate for imaging and treatment of cancer, J Control Release, 153 (2011) 16–22. [DOI] [PubMed] [Google Scholar]
- [30].Fliervoet LAL, Mastrobattista E, Drug delivery with living cells, Adv Drug Deliv Rev, 106 (2016) 63–72. [DOI] [PubMed] [Google Scholar]
- [31].Torchilin VP, Micellar nanocarriers: pharmaceutical perspectives, Pharm Res, 24 (2007) 1–16. [DOI] [PubMed] [Google Scholar]
- [32].Oberoi HS, Nukolova NV, Kabanov AV, Bronich TK, Nanocarriers for delivery of platinum anticancer drugs, Adv Drug Deliv Rev, 65 (2013) 1667–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Wan X, Beaudoin JJ, Vinod N, Min Y, Makita N, Bludau H, Jordan R, Wang A, Sokolsky M, Kabanov AV, Co-delivery of paclitaxel and cisplatin in poly(2-oxazoline) polymeric micelles: Implications for drug loading, release, pharmacokinetics and outcome of ovarian and breast cancer treatments, Biomaterials, 192 (2019) 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Duong T, Li X, Yang B, Schumann C, Albarqi HA, Taratula O, Taratula O, Phototheranostic nanoplatform based on a single cyanine dye for image-guided combinatorial phototherapy, Nanomedicine, 13 (2017) 955–963. [DOI] [PubMed] [Google Scholar]
- [35].Li X, Schumann C, Albarqi HA, Lee CJ, Alani AWG, Bracha S, Milovancev M, Taratula O, Taratula O, A Tumor-Activatable Theranostic Nanomedicine Platform for NIR Fluorescence-Guided Surgery and Combinatorial Phototherapy, Theranostics, 8 (2018) 767–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Shrestha H, Bala R, Arora S, Lipid-Based Drug Delivery Systems, J Pharm (Cairo), 2014 (2014) 801820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Sahle FF, Metz H, Wohlrab J, Neubert RH, Lecithin-based microemulsions for targeted delivery of ceramide AP into the stratum corneum: formulation, characterizations, and in vitro release and penetration studies, Pharm Res, 30 (2013) 538–551. [DOI] [PubMed] [Google Scholar]
- [38].Rehman FU, Shah KU, Shah SU, Khan IU, Khan GM, Khan A, From nanoemulsions to self-nanoemulsions, with recent advances in self-nanoemulsifying drug delivery systems (SNEDDS), Expert Opin Drug Deliv, 14 (2017) 1325–1340. [DOI] [PubMed] [Google Scholar]
- [39].Jaimes-Aguirre L, Gibbens-Bandala BV, Morales-Avila E, Ocampo-Garcia BE, Seyedeh-Fatemeh M, Amirhosein A, Polymer-Based Drug Delivery Systems, Development and Pre-Clinical Status, Curr Pharm Des, 22 (2016) 2886–2903. [DOI] [PubMed] [Google Scholar]
- [40].Minko T, Dharap SS, Pakunlu RI, Wang Y, Molecular targeting of drug delivery systems to cancer, Curr Drug Targets, 5 (2004) 389–406. [DOI] [PubMed] [Google Scholar]
- [41].Maeda H, The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting, Adv Enzyme Regul, 41 (2001) 189–207. [DOI] [PubMed] [Google Scholar]
- [42].Maeda H, Polymer therapeutics and the EPR effect, J Drug Target, 25 (2017) 781–785. [DOI] [PubMed] [Google Scholar]
- [43].Richards DA, Exploring alternative antibody scaffolds: Antibody fragments and antibody mimics for targeted drug delivery, Drug Discov Today Technol, 30 (2018) 35–46. [DOI] [PubMed] [Google Scholar]
- [44].Fercher C, Keshvari S, McGuckin MA, Barnard RT, Evolution of the magic bullet: Single chain antibody fragments for the targeted delivery of immunomodulatory proteins, Exp Biol Med, 243 (2018) 166–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Awwad S, Angkawinitwong U, Overview of Antibody Drug Delivery, Pharmaceutics, 10 (2018) 83–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Jiang Z, Guan J, Qian J, Zhan C, Peptide ligand-mediated targeted drug delivery of nanomedicines, Biomater Sci, 7 (2019) 461–471. [DOI] [PubMed] [Google Scholar]
- [47].Li Y, Du L, Wu C, Yu B, Zhang H, An F, Peptide Sequence-Dominated Enzyme-Responsive Nanoplatform for Anticancer Drug Delivery, Curr Top Med Chem, 19 (2019) 74–97. [DOI] [PubMed] [Google Scholar]
- [48].Tesauro D, Accardo A, Diaferia C, Milano V, Guillon J, Ronga L, Rossi F, Peptide-Based Drug-Delivery Systems in Biotechnological Applications: Recent Advances and Perspectives, Molecules (Basel, Switzerland), 24 (2019) 351–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Dharap SS, Wang Y, Chandna P, Khandare JJ, Qiu B, Gunaseelan S, Sinko PJ, Stein S, Farmanfarmaian A, Minko T, Tumor-specific targeting of an anticancer drug delivery system by LHRH peptide, Proc Natl Acad Sci U S A, 102 (2005) 12962–12967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Li X, Taratula O, Taratula O, Schumann C, Minko T, LHRH-Targeted Drug Delivery Systems for Cancer Therapy, Mini Rev Med Chem, 17 (2017) 258–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Hoya K, Guterman LR, Miskolczi L, Hopkins LN, A novel intravascular drug delivery method using endothelial biotinylation and avidin-biotin binding, Drug Deliv, 8 (2001) 215–222. [DOI] [PubMed] [Google Scholar]
- [52].Lewis MR, Wang M, Axworthy DB, Theodore LJ, Mallet RW, Fritzberg AR, Welch MJ, Anderson CJ, In vivo evaluation of pretargeted 64Cu for tumor imaging and therapy, J Nucl Med, 44 (2003) 1284–1292. [PubMed] [Google Scholar]
- [53].Lu Y, Low PS, Targeted immunotherapy of cancer: development of antibody-induced cellular immunity, J Pharm Pharmacol, 55 (2003) 163–167. [DOI] [PubMed] [Google Scholar]
- [54].Takeda T, Inaba H, Yamazaki M, Kyo S, Miyamoto T, Suzuki S, Ehara T, Kakizawa T, Hara M, DeGroot LJ, Hashizume K, Tumor-specific gene therapy for undifferentiated thyroid carcinoma utilizing the telomerase reverse transcriptase promoter, J Clin Endocrinol Metab, 88 (2003) 3531–3538. [DOI] [PubMed] [Google Scholar]
- [55].Godbey WT, Atala A, Directed apoptosis in Cox-2-overexpressing cancer cells through expression-targeted gene delivery, Gene Ther, 10 (2003) 1519–1527. [DOI] [PubMed] [Google Scholar]
- [56].De Palma M, Venneri MA, Naldini L, In vivo targeting of tumor endothelial cells by systemic delivery of lentiviral vectors, Hum Gene Ther, 14 (2003) 1193–1206. [DOI] [PubMed] [Google Scholar]
- [57].Mei Y, Wang R, Jiang W, Bo Y, Zhang T, Yu J, Cheng M, Wu Y, Cheng J, Ma W, Recent progress in nanomaterials for nucleic acid delivery in cancer immunotherapy, Biomater Sci, 7 (2019) 2640–2651. [DOI] [PubMed] [Google Scholar]
- [58].Wei Y, Du Q, Jiang X, Li L, Li T, Li M, Fan X, Li Y, Kariminia S, Li Q, Efficacy and safety of combination immunotherapy for malignant solid tumors: A systematic review and meta-analysis, Crit Rev Oncol Hematol, 138 (2019) 178–189. [DOI] [PubMed] [Google Scholar]
- [59].Yi LI, Weifan Y, Huan Y, Chimeric antigen receptor-engineered regulatory T lymphocytes: promise for immunotherapy of autoimmune disease, Cytotherapy, 2019. May 16 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- [60].Gourevich D, Gerold B, Arditti F, Xu D, Liu D, Volovick A, Wang L, Medan Y, Gnaim J, Prentice P, Cochran S, Melzer A, Ultrasound activated nano-encapsulated targeted drug delivery and tumour cell poration, Adv Exp Med Biol, 733 (2012) 135–144. [DOI] [PubMed] [Google Scholar]
- [61].Horsley H, Owen J, Browning R, Carugo D, Malone-Lee J, Stride E, Rohn JL, Ultrasound-activated microbubbles as a novel intracellular drug delivery system for urinary tract infection, J Control Release, 301 (2019) 166–175. [DOI] [PubMed] [Google Scholar]
- [62].Albinali KE, Zagho MM, Deng Y, Elzatahry AA, A perspective on magnetic core-shell carriers for responsive and targeted drug delivery systems, Int J Nanomedicine, 14 (2019) 1707–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Lambricht L, Lopes A, Kos S, Sersa G, Preat V, Vandermeulen G, Clinical potential of electroporation for gene therapy and DNA vaccine delivery, Expert Opin Drug Deliv, 13 (2016) 295–310. [DOI] [PubMed] [Google Scholar]
- [64].Luft C, Ketteler R, Electroporation Knows No Boundaries: The Use of Electrostimulation for siRNA Delivery in Cells and Tissues, J Biomol Screen, 20 (2015) 932–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Shi J, Ma Y, Zhu J, Chen Y, Sun Y, Yao Y, Yang Z, Xie J, A Review on Electroporation-Based Intracellular Delivery, Molecules (Basel, Switzerland), 23 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Shreya AB, Raut SY, Managuli RS, Udupa N, Mutalik S, Active Targeting of Drugs and Bioactive Molecules via Oral Administration by Ligand-Conjugated Lipidic Nanocarriers: Recent Advances, AAPS PharmSciTech, 20 (2018) 15. [DOI] [PubMed] [Google Scholar]
- [67].Yoo J, Park C, Yi G, Lee D, Koo H, Active Targeting Strategies Using Biological Ligands for Nanoparticle Drug Delivery Systems, Cancers, 11 (2019) 640–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Alavi M, Hamidi M, Passive and active targeting in cancer therapy by liposomes and lipid nanoparticles, Drug Metab Pers Ther, 34 (2019) 1–8. [DOI] [PubMed] [Google Scholar]
- [69].Alhajj N, Chee CF, Wong TW, Rahman NA, Abu Kasim NH, Colombo P, Lung cancer: active therapeutic targeting and inhalational nanoproduct design, Expert Opin Drug Deliv, 15 (2018) 1223–1247. [DOI] [PubMed] [Google Scholar]
- [70].Attia MF, Anton N, Wallyn J, Omran Z, Vandamme TF, An overview of active and passive targeting strategies to improve the nanocarriers efficiency to tumour sites, J Pharm Pharmacol, 2019. May 3 [Epub Ahead of Print]. [DOI] [PubMed] [Google Scholar]
- [71].Dhawan D, Ramos-Vara JA, Naughton JF, Cheng L, Low PS, Rothenbuhler R, Leamon CP, Parker N, Klein PJ, Vlahov IR, Reddy JA, Koch M, Murphy L, Fourez LM, Stewart JC, Knapp DW, Targeting folate receptors to treat invasive urinary bladder cancer, Cancer Res., 73 (2013) 875–884. [DOI] [PubMed] [Google Scholar]
- [72].Frigerio B, Bizzoni C, Jansen G, Leamon CP, Peters GJ, Low PS, Matherly LH, Figini M, Folate receptors and transporters: biological role and diagnostic/therapeutic targets in cancer and other diseases, J Exp Clin Cancer Res, 38 (2019) 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Zhou Y, Unno K, Hyjek E, Liu H, Zimmerman T, Karmakar S, Putt KS, Shen J, Low PS, Wickrema A, Expression of functional folate receptors in multiple myeloma, Leuk. Lymphoma, 59 (2018) 2982–2989. [DOI] [PubMed] [Google Scholar]
- [74].Zhao X, Li H, Lee RJ, Targeted drug delivery via folate receptors, Expert Opin Drug Deliv, 5 (2008) 309–319. [DOI] [PubMed] [Google Scholar]
- [75].Chu DT, Bac ND, Nguyen KH, Tien NLB, Thanh VV, Nga VT, Ngoc VTN, Anh Dao DT, Hoan LN, Hung NP, Trung Thu NT, Pham VH, Vu LN, Pham TAV, Thimiri Govinda Raj DB, An Update on Anti-CD137 Antibodies in Immunotherapies for Cancer, Int J Mol Sci, 20 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Alley SC, Okeley NM, Senter PD, Antibody-drug conjugates: targeted drug delivery for cancer, Curr Opin Chem Biol, 14 (2010) 529–537. [DOI] [PubMed] [Google Scholar]
- [77].Nakada T, Sugihara K, Jikoh T, Abe Y, Agatsuma T, The Latest Research and Development into the Antibody-Drug Conjugate, [fam-] Trastuzumab Deruxtecan (DS-8201a), for HER2 Cancer Therapy, Chem Pharm Bull (Tokyo), 67 (2019) 173–185. [DOI] [PubMed] [Google Scholar]
- [78].Alibakhshi A, Abarghooi Kahaki F, Ahangarzadeh S, Yaghoobi H, Yarian F, Arezumand R, Ranjbari J, Mokhtarzadeh A, de la Guardia M, Targeted cancer therapy through antibody fragments-decorated nanomedicines, J Control Release, 268 (2017) 323–334. [DOI] [PubMed] [Google Scholar]
- [79].Kunath K, Kopeckova P, Minko T, Kopecek J, HPMA copolymer-anticancer drug-OV-TL16 antibody conjugates. 3. The effect of free and polymer-bound adriamycin on the expression of some genes in the OVCAR-3 human ovarian carcinoma cell line, Eur J Pharm Biopharm, 49 (2000) 11–15. [DOI] [PubMed] [Google Scholar]
- [80].Marcucci F, Caserta CA, Romeo E, Rumio C, Antibody-Drug Conjugates (ADC) Against Cancer Stem-Like Cells (CSC)-Is There Still Room for Optimism?, Front Oncol, 9 (2019) 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Hong D, Sloane DE, Hypersensitivity to Monoclonal Antibodies used for Cancer and Inflammatory/Connective Tissue Diseases, Ann Allergy Asthma Immunol, (2019). [DOI] [PubMed] [Google Scholar]
- [82].Cruz E, Kayser V, Monoclonal antibody therapy of solid tumors: clinical limitations and novel strategies to enhance treatment efficacy, Biologics, 13 (2019) 33–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Moody TW, Peptide receptors as cancer drug targets, Ann N Y Acad Sci, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Engel JB, Tinneberg HR, Rick FG, Berkes E, Schally AV, Targeting of Peptide Cytotoxins to LHRH Receptors For Treatment of Cancer, Curr Drug Targets, 17 (2016) 488–494. [DOI] [PubMed] [Google Scholar]
- [85].Moody TW, Nuche-Berenguer B, Nakamura T, Jensen RT, EGFR Transactivation by Peptide G Protein-Coupled Receptors in Cancer, Curr Drug Targets, 17 (2016) 520–528. [DOI] [PubMed] [Google Scholar]
- [86].Moody TW, Nuche-Berenguer B, Jensen RT, Vasoactive intestinal peptide/pituitary adenylate cyclase activating polypeptide, and their receptors and cancer, Curr Opin Endocrinol Diabetes Obes, 23 (2016) 38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Baratto L, Jadvar H, Iagaru A, Prostate Cancer Theranostics Targeting Gastrin-Releasing Peptide Receptors, Mol Imaging Biol, 20 (2018) 501–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Minko T, Nanotechnology and drug resistance, Adv Drug Deliv Rev, 65 (2013) 1665–1666. [DOI] [PubMed] [Google Scholar]
- [89].Waite CL, Roth CM, Nanoscale drug delivery systems for enhanced drug penetration into solid tumors: current progress and opportunities, Crit Rev Biomed Eng, 40 (2012) 21–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Panyam J, Labhasetwar V, Biodegradable nanoparticles for drug and gene delivery to cells and tissue, Adv Drug Deliv Rev, 55 (2003) 329–347. [DOI] [PubMed] [Google Scholar]
- [91].Anselmo AC, Mitragotri S, A Review of Clinical Translation of Inorganic Nanoparticles, Aaps j, 17 (2015) 1041–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Mitragotri S, Stayton P, Organic nanoparticles for drug delivery and imaging, MRS Bulletin, 39 (2014)219–223. [Google Scholar]
- [93].Niu G, Cogburn B, Hughes J, Preparation and characterization of doxorubicin liposomes, Methods Mol Biol, 624 (2010) 211–219. [DOI] [PubMed] [Google Scholar]
- [94].Zhang J, Saltzman M, Engineering biodegradable nanoparticles for drug and gene delivery, Chem Eng Prog, 109 (2013) 25–30. [PMC free article] [PubMed] [Google Scholar]
- [95].Ding M, Song N, He X, Li J, Zhou L, Tan H, Fu Q, Gu Q, Toward the next-generation nanomedicines: design of multifunctional multiblock polyurethanes for effective cancer treatment, ACS Nano, 7 (2013) 1918–1928. [DOI] [PubMed] [Google Scholar]
- [96].Danhier F, Ansorena E, Silva JM, Coco R, Le Breton A, Preat V, PLGA-based nanoparticles: an overview of biomedical applications, J Control Release, 161 (2012) 505–522. [DOI] [PubMed] [Google Scholar]
- [97].Acharya S, Sahoo SK, PLGA nanoparticles containing various anticancer agents and tumour delivery by EPR effect, Adv Drug Deliv Rev, 63 (2011) 170–183. [DOI] [PubMed] [Google Scholar]
- [98].Albarqi HA, Wong LH, Schumann C, Sabei FY, Korzun T, Li X, Hansen MN, Dhagat P, Moses AS, Taratula OR, Taratula O, Biocompatible nanoclusters with high heating efficiency for systemically delivered magnetic hyperthermia., ACS Nano, [Epub ahead of print] (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Grossen P, Witzigmann D, Sieber S, Huwyler J, PEG-PCL-based nanomedicines: A biodegradable drug delivery system and its application, J Control Release, 260 (2017)46–60. [DOI] [PubMed] [Google Scholar]
- [100].Taratula O, Doddapaneni BS, Schumann C, Li X, Bracha S, Milovancev M, Alani A, Taratula O, Naphthalocyanine-based biodegradable polymeric nanoparticles for image-guided combinatorial phototherapy, Chem Mater, 27 (2015)6155–6165. [Google Scholar]
- [101].Danafar H, MPEG-PCL copolymeric nanoparticles in drug delivery systems, Cogent Med, 3 (2016) 1142411. [DOI] [PubMed] [Google Scholar]
- [102].Lungwitz U, Breunig M, Blunk T, Gopferich A, Polyethylenimine-based non-viral gene delivery systems, Eur J Pharm Biopharm, 60 (2005) 247–266. [DOI] [PubMed] [Google Scholar]
- [103].Wu Y, Wang W, Chen Y, Huang K, Shuai X, Chen Q, Li X, Lian G, The investigation of polymer-siRNA nanoparticle for gene therapy of gastric cancer in vitro, Int J Nanomedicine, 5 (2010) 129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Shah V, Taratula O, Garbuzenko OB, Patil ML, Savla R, Zhang M, Minko T, Genotoxicity of different nanocarriers: possible modifications for the delivery of nucleic acids, Curr Drug Discov Technol, 10 (2013) 8–15. [PMC free article] [PubMed] [Google Scholar]
- [105].Schumann C, Chan S, Khalimonchuk O, Khal S, Moskal V, Shah V, Alani AW, Taratula O, Taratula O, Mechanistic Nanotherapeutic Approach Based on siRNA-Mediated DJ-1 Protein Suppression for Platinum-Resistant Ovarian Cancer, Mol Pharm, 13 (2016) 2070–2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Kirkpatrick GJ, Plumb JA, Sutcliffe OB, Flint DJ, Wheate NJ, Evaluation of anionic half generation 3.5-6.5 poly(amidoamine) dendrimers as delivery vehicles for the active component of the anticancer drug cisplatin, J Inorg Biochem, 105 (2011) 1115–1122. [DOI] [PubMed] [Google Scholar]
- [107].Ooya T, Lee J, Park K, Hydrotropic dendrimers of generations 4 and 5: synthesis, characterization, and hydrotropic solubilization of paclitaxel, Bioconjug Chem, 15 (2004) 1221–1229. [DOI] [PubMed] [Google Scholar]
- [108].Kojima C, Nishisaka E, Suehiro T, Watanabe K, Harada A, Goto T, Magata Y, Kono K, The synthesis and evaluation of polymer prodrug/collagen hybrid gels for delivery into metastatic cancer cells, Nanomedicine, 9 (2013) 767–775. [DOI] [PubMed] [Google Scholar]
- [109].Muller RH, Mader K, Gohla S, Solid lipid nanoparticles (SLN) for controlled drug delivery - a review of the state of the art, Eur J Pharm Biopharm, 50 (2000) 161–177. [DOI] [PubMed] [Google Scholar]
- [110].Han Y, Zhang P, Chen Y, Sun J, Kong F, Co-delivery of plasmid DNA and doxorubicin by solid lipid nanoparticles for lung cancer therapy, Int J Mol Med, 34 (2014) 191–196. [DOI] [PubMed] [Google Scholar]
- [111].Shen MY, Liu TI, Yu TW, Kv R, Chiang WH, Tsai YC, Chen HH, Lin SC, Chiu HC, Hierarchically targetable polysaccharide-coated solid lipid nanoparticles as an oral chemo/thermotherapy delivery system for local treatment of colon cancer, Biomaterials, 197 (2019) 86–100. [DOI] [PubMed] [Google Scholar]
- [112].Lamichhane N, Udayakumar TS, D'Souza WD, Simone CB 2nd, Raghavan SR, Polf J, Mahmood J, Liposomes: Clinical Applications and Potential for Image-Guided Drug Delivery, Molecules (Basel, Switzerland), 23 (2018) 288–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Deshpande PP, Biswas S, Torchilin VP, Current trends in the use of liposomes for tumor targeting, Nanomedicine (Lond), 8 (2013) 1509–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Zang X, Lee JB, Deshpande K, Garbuzenko OB, Minko T, Kagan L, Prevention of paclitaxel-induced neuropathy by formulation approach, J Control Release, 303 (2019) 109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Garbuzenko OB, Ivanova V, Kholodovych V, Reimer DC, Reuhl KR, Yurkow E, Adler D, Minko T, Combinatorial treatment of idiopathic pulmonary fibrosis using nanoparticles with prostaglandin E and siRNA(s), Nanomedicine, 13 (2017) 1983–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Garbuzenko OB, Mainelis G, Taratula O, Minko T, Inhalation treatment of lung cancer: the influence of composition, size and shape of nanocarriers on their lung accumulation and retention, Cancer Biol Med, 11 (2014) 44–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Saad M, Garbuzenko OB, Minko T, Co-delivery of siRNA and an anticancer drug for treatment of multidrug-resistant cancer, Nanomedicine, 3 (2008) 761–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Chang HI, Yeh MK, Clinical development of liposome-based drugs: formulation, characterization, and therapeutic efficacy, Int J Nanomedicine, 7 (2012) 49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Bulbake U, Doppalapudi S, Kommineni N, Khan W, Liposomal Formulations in Clinical Use: An Updated Review, Pharmaceutics, 9 (2017) 12–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Garbuzenko OB, Saad M, Pozharov VP, Reuhl KR, Mainelis G, Minko T, Inhibition of lung tumor growth by complex pulmonary delivery of drugs with oligonucleotides as suppressors of cellular resistance, Proc Natl Acad Sci U S A, 107 (2010) 10737–10742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Ivanova V, Garbuzenko OB, Reuhl KR, Reimer DC, Pozharov VP, Minko T, Inhalation treatment of pulmonary fibrosis by liposomal prostaglandin E2, Eur J Pharm Biopharm, 84 (2013) 335–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Khosa A, Reddi S, Saha RN, Nanostructured lipid carriers for site-specific drug delivery, Biomed Pharmacother, 103 (2018) 598–613. [DOI] [PubMed] [Google Scholar]
- [123].Gaba B, Fazil M, Ali A, Baboota S, Sahni JK, Ali J, Nanostructured lipid (NLCs) carriers as a bioavailability enhancement tool for oral administration, Drug Deliv, 22 (2015) 691–700. [DOI] [PubMed] [Google Scholar]
- [124].Fang CL, Al-Suwayeh SA, Fang JY, Nanostructured lipid carriers (NLCs) for drug delivery and targeting, Recent Pat Nanotechnol, 7 (2013) 41–55. [PubMed] [Google Scholar]
- [125].Garbuzenko OB, Kbah N, Kuzmov A, Pogrebnyak N, Pozharov V, Minko T, Inhalation treatment of cystic fibrosis with lumacaftor and ivacaftor co-delivered by nanostructured lipid carriers, J Control Release, 296 (2019) 225–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Zhao X, Zhao Y, Geng L, Li X, Wang X, Liu Z, Wang D, Bi K, Chen X, Pharmacokinetics and tissue distribution of docetaxel by liquid chromatography-mass spectrometry: evaluation of folate receptor-targeting amphiphilic copolymer modified nanostructured lipid carrier, J Chromatogr B Analyt Technol Biomed Life Sci, 879 (2011) 3721–3727. [DOI] [PubMed] [Google Scholar]
- [127].Montero A, Fossella F, Hortobagyi G, Valero V, Docetaxel for treatment of solid tumours: a systematic review of clinical data, Lancet Oncol, 6 (2005) 229–239. [DOI] [PubMed] [Google Scholar]
- [128].Chia-Lang F, Saleh AA-S, Jia-You F, Nanostructured Lipid Carriers (NLCs) for Drug Delivery and Targeting, Recent Pat Nanotechnol, 7 (2013) 41–55. [PubMed] [Google Scholar]
- [129].Beloqui A, Solinis MA, Rodriguez-Gascon A, Almeida AJ, Preat V, Nanostructured lipid carriers: Promising drug delivery systems for future clinics, Nanomedicine, 12 (2016) 143–161. [DOI] [PubMed] [Google Scholar]
- [130].Han F, Yin R, Che X, Yuan J, Cui Y, Yin H, Li S, Nanostructured lipid carriers (NLC) based topical gel of flurbiprofen: design, characterization and in vivo evaluation, Int J Pharm, 439 (2012) 349–357. [DOI] [PubMed] [Google Scholar]
- [131].Gonzalez-Mira E, Nikolic S, Garcia ML, Egea MA, Souto EB, Calpena AC, Potential use of nanostructured lipid carriers for topical delivery of flurbiprofen, J Pharm Sci, 100 (2011) 242–251. [DOI] [PubMed] [Google Scholar]
- [132].Doktorovova S, Araujo J, Garcia ML, Rakovsky E, Souto EB, Formulating fluticasone propionate in novel PEG-containing nanostructured lipid carriers (PEG-NLC), Colloids and surfaces. B, Biointerfaces, 75 (2010) 538–542. [DOI] [PubMed] [Google Scholar]
- [133].Patlolla RR, Chougule M, Patel AR, Jackson T, Tata PN, Singh M, Formulation, characterization and pulmonary deposition of nebulized celecoxib encapsulated nanostructured lipid carriers, J Control Release, 144 (2010) 233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Han F, Li S, Yin R, Shi X, Jia Q, Investigation of nanostructured lipid carriers for transdermal delivery of flurbiprofen, Drug Dev Ind Pharm, 34 (2008) 453–458. [DOI] [PubMed] [Google Scholar]
- [135].Kuzmov A, Minko T, Nanotechnology approaches for inhalation treatment of lung diseases, J Control Release, 219 (2015) 500–518. [DOI] [PubMed] [Google Scholar]
- [136].Mainelis G, Seshadri S, Garbuzenko OB, Han T, Wang Z, Minko T, Characterization and application of a nose-only exposure chamber for inhalation delivery of liposomal drugs and nucleic acids to mice, J Aerosol Med Pulm Drug Deliv, 26 (2013) 345–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Savla R, Minko T, Nanotechnology approaches for inhalation treatment of fibrosis, J Drug Target, 21 (2013) 914–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Merkel OM, Rubinstein I, Kissel T, siRNA delivery to the lung: what's new?, Adv Drug Deliv Rev, 75 (2014) 112–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Ruigrok MJR, Frijlink HW, Hinrichs WLJ, Pulmonary administration of small interfering RNA: The route to go?, J Control Release, 235 (2016) 14–23. [DOI] [PubMed] [Google Scholar]
- [140].Garbuzenko OB, Saad M, Betigeri S, Zhang M, Vetcher AA, Soldatenkov VA, Reimer DC, Pozharov VP, Minko T, Intratracheal versus intravenous liposomal delivery of siRNA, antisense oligonucleotides and anticancer drug, Pharm Res, 26 (2009) 382–394. [DOI] [PubMed] [Google Scholar]
- [141].Yan Y, Zhou K, Xiong H, Miller JB, Motea EA, Boothman DA, Liu L, Siegwart DJ, Aerosol delivery of stabilized polyester-siRNA nanoparticles to silence gene expression in orthotopic lung tumors, Biomaterials, 118 (2017) 84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Capel V, Vllasaliu D, Watts P, Clarke PA, Luxton D, Grabowska AM, Mantovani G, Stolnik S, Water-soluble substituted chitosan derivatives as technology platform for inhalation delivery of siRNA, Drug Deliv, 25 (2018) 644–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Luo Y, Zhai X, Ma C, Sun P, Fu Z, Liu W, Xu J, An inhalable beta(2)-adrenoceptor ligand-directed guanidinylated chitosan carrier for targeted delivery of siRNA to lung, J Control Release, 162 (2012) 28–36. [DOI] [PubMed] [Google Scholar]
- [144].Xu C, Tian H, Wang P, Wang Y, Chen X, The suppression of metastatic lung cancer by pulmonary administration of polymer nanoparticles for co-delivery of doxorubicin and Survivin siRNA, Biomater Sci, 4 (2016) 1646–1654. [DOI] [PubMed] [Google Scholar]
- [145].Xu CX, Jere D, Jin H, Chang SH, Chung YS, Shin JY, Kim JE, Park SJ, Lee YH, Chae CH, Lee KH, Beck GR Jr., Cho CS, Cho MH, Poly(ester amine)-mediated, aerosol-delivered Akt1 small interfering RNA suppresses lung tumorigenesis, Am J Respir Crit Care Med, 178 (2008) 60–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Zamora-Avila DE, Zapata-Benavides P, Franco-Molina MA, Saavedra-Alonso S, Trejo-Avila LM, Resendez-Perez D, Mendez-Vazquez JL, Isaias-Badillo J, Rodriguez-Padilla C, WT1 gene silencing by aerosol delivery of PEI-RNAi complexes inhibits B16-F10 lung metastases growth, Cancer Gene Ther, 16 (2009) 892–899. [DOI] [PubMed] [Google Scholar]
- [147].Yan Y, Liu L, Xiong H, Miller JB, Zhou K, Kos P, Huffman KE, Elkassih S, Norman JW, Carstens R, Kim J, Minna JD, Siegwart DJ, Functional polyesters enable selective siRNA delivery to lung cancer over matched normal cells, Proc Natl Acad Sci U S A, 113 (2016) E5702–5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Bharti C, Nagaich U, Pal AK, Gulati N, Mesoporous silica nanoparticles in target drug delivery system: A review, Int J Pharm Investig, 5 (2015) 124–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Chen AM, Zhang M, Wei D, Stueber D, Taratula O, Minko T, He H, Co-delivery of doxorubicin and Bcl-2 siRNA by mesoporous silica nanoparticles enhances the efficacy of chemotherapy in multidrug-resistant cancer cells, Small, 5 (2009) 2673–2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Huang X, Tao Z, Praskavich JC Jr., Goswami A, Al-Sharab JF, Minko T, Polshettiwar V, Asefa T, Dendritic silica nanomaterials (KCC-1) with fibrous pore structure possess high DNA adsorption capacity and effectively deliver genes in vitro, Langmuir, 30 (2014) 10886–10898. [DOI] [PubMed] [Google Scholar]
- [151].Hom C, Lu J, Liong M, Luo H, Li Z, Zink JI, Tamanoi F, Mesoporous silica nanoparticles facilitate delivery of siRNA to shutdown signaling pathways in mammalian cells, Small, 6 (2010) 1185–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Cheng W, Nie J, Xu L, Liang C, Peng Y, Liu G, Wang T, Mei L, Huang L, Zeng X, pH-Sensitive Delivery Vehicle Based on Folic Acid-Conjugated Polydopamine-Modified Mesoporous Silica Nanoparticles for Targeted Cancer Therapy, ACS Appl Mater Interfaces, 9 (2017) 18462–18473. [DOI] [PubMed] [Google Scholar]
- [153].Pan L, Liu J, He Q, Shi J, MSN-mediated sequential vascular-to-cell nuclear-targeted drug delivery for efficient tumor regression, Adv Mater, 26 (2014) 6742–6748. [DOI] [PubMed] [Google Scholar]
- [154].Cai W, Gao T, Hong H, Sun J, Applications of gold nanoparticles in cancer nanotechnology, Nanotechnol Sci Appl, 1 (2008) 17–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Mendes R, Fernandes AR, Baptista PV, Gold Nanoparticle Approach to the Selective Delivery of Gene Silencing in Cancer-The Case for Combined Delivery?, Genes, 8 (2017) 94–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Jiang S, Eltoukhy AA, Love KT, Langer R, Anderson DG, Lipidoid-coated iron oxide nanoparticles for efficient DNA and siRNA delivery, Nano Lett, 13 (2013) 1059–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Wang Z, Qiao R, Tang N, Lu Z, Wang H, Zhang Z, Xue X, Huang Z, Zhang S, Zhang G, Li Y, Active targeting theranostic iron oxide nanoparticles for MRI and magnetic resonance-guided focused ultrasound ablation of lung cancer, Biomaterials, 127 (2017) 25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Medarova Z, Balcioglu M, Yigit MV, Controlling RNA Expression in Cancer Using Iron Oxide Nanoparticles Detectable by MRI and In Vivo Optical Imaging, Methods Mol Biol, 1372 (2016) 163–179. [DOI] [PubMed] [Google Scholar]
- [159].Savla R, Garbuzenko OB, Chen S, Rodriguez-Rodriguez L, Minko T, Tumor-targeted responsive nanoparticle-based systems for magnetic resonance imaging and therapy, Pharm Res, 31 (2014) 3487–3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Dani RK, Schumann C, Taratula O, Taratula O, Temperature-tunable iron oxide nanoparticles for remote-controlled drug release, AAPS PharmSciTech, 15 (2014) 963–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Wang YX, Idee JM, A comprehensive literatures update of clinical researches of superparamagnetic resonance iron oxide nanoparticles for magnetic resonance imaging, Quant Imaging Med Surg, 7 (2017) 88–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Keleb E, Sharma RK, Mosa EB, Aljahwi AAZ, Transdermal Drug Delivery System-Design and Evaluation, Int J Adv Pharm Sci, 1 (2010) 201–211. [Google Scholar]
- [163].Parveen R, Dastidar P, Supramolecular Gels by Design: Towards the Development of Topical Gels for Self-Delivery Application, Chemistry, 22 (2016) 9257–9266. [DOI] [PubMed] [Google Scholar]
- [164].George M, Weiss RG, Molecular organogels. Soft matter comprised of low-molecular-mass organic gelators and organic liquids, Acc Chem Res, 39 (2006) 489–497. [DOI] [PubMed] [Google Scholar]
- [165].Terech P, Weiss RG, Low Molecular Mass Gelators of Organic Liquids and the Properties of Their Gels, Chem Rev, 97 (1997) 3133–3160. [DOI] [PubMed] [Google Scholar]
- [166].Estroff LA, Hamilton AD, Water gelation by small organic molecules, Chem Rev, 104 (2004) 1201–1218. [DOI] [PubMed] [Google Scholar]
- [167].Sangeetha NM, Maitra U, Supramolecular gels: functions and uses, Chem Soc Rev, 34 (2005)821–836. [DOI] [PubMed] [Google Scholar]
- [168].Chen J, Wu W, McNeil AJ, Detecting a peroxide-based explosive via molecular gelation, Chem. Commun. (Camb.), 48(2012) 7310–7312. [DOI] [PubMed] [Google Scholar]
- [169].Ray S, Das AK, Banerjee A, pH-Responsive, Bolaamphiphile-Based Smart Metallo-Hydrogels as Potential Dye-Adsorbing Agents, Water Purifier, and Vitamin B12 Carrier, Chem Mater, 19 (2007) 1633–1639. [Google Scholar]
- [170].Weerasekare M, Taraban MB, Shi X, Jeong EK, Trewhella J, Yu YB, Sol and gel states in peptide hydrogels visualized by Gd(III)-enhanced magnetic resonance imaging, Biopolymers, 96 (2011) 734–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Konda M, Maity I, Rasale DB, Das AK, A New Class of Phase-Selective Synthetic β-Amino Acid Based Peptide Gelator: From Mechanistic Aspects to Oil Spill Recovery, ChemPlusChem, 79 (2014) 1482–1488. [Google Scholar]
- [172].Lee KY, Mooney DJ, Hydrogels for tissue engineering, Chem Rev, 101 (2001) 1869–1879. [DOI] [PubMed] [Google Scholar]
- [173].Schnepp ZAC, Gonzalez-McQuire R, Mann S, Hybrid Biocomposites Based on Calcium Phosphate Mineralization of Self-Assembled Supramolecular Hydrogels, Adv Mater, 18(2006) 1869–1872. [Google Scholar]
- [174].Yang Z, Liang G, Ma M, Abbah AS, Lu WW, Xu B, d-Glucosamine-based supramolecular hydrogels to improve wound healing, Chem Commun (Camb), (2007) 843–845. [DOI] [PubMed] [Google Scholar]
- [175].Kiyonaka S, Sada K, Yoshimura I, Shinkai S, Kato N, Hamachi I, Semi-wet peptide/protein array using supramolecular hydrogel, Nat Mater, 3 (2004) 58–64. [DOI] [PubMed] [Google Scholar]
- [176].Wang Q, Yang Z, Zhang X, Xiao X, Chang CK, Xu B, A supramolecular-hydrogel-encapsulated hemin as an artificial enzyme to mimic peroxidase, Angew Chem Int Ed Engl, 46 (2007) 4285–4289. [DOI] [PubMed] [Google Scholar]
- [177].Standley SM, Toft DJ, Cheng H, Soukasene S, Chen J, Raja SM, Band V, Band H, Cryns VL, Stupp SI, Induction of cancer cell death by self-assembling nanostructures incorporating a cytotoxic peptide, Cancer Res., 70 (2010) 3020–3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Deb J, Majumder J, Bhattacharyya S, Jana SS, A novel naproxen derivative capable of displaying anti-cancer and anti-migratory properties against human breast cancer cells, BMC cancer, 14 (2014) 567–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Jayawarna V, Ali M, Jowitt T, Miller A, Saiani A, Gough J, Ulijn RV, Nanostructured Hydrogels for Three-Dimensional Cell Culture Through Self-Assembly of Fluorenylmethoxycarbonyl–Dipeptide, Adv Mater, 18 (2006) 611–614. [Google Scholar]
- [180].Feng Z, Zhang T, Wang H, Xu B, Supramolecular catalysis and dynamic assemblies for medicine, Chem Soc Rev, 46 (2017) 6470–6479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Mayr J, Saldias C, Diaz Diaz D, Release of small bioactive molecules from physical gels, Chem Soc Rev, 47 (2018) 1484–1515. [DOI] [PubMed] [Google Scholar]
- [182].Wang H, Yang Z, Molecular hydrogels of hydrophobic compounds: a novel self-delivery system for anti-cancer drugs, Soft Matter, 8 (2012) 2344–2347. [Google Scholar]
- [183].Du X, Zhou J, Shi J, Xu B, Supramolecular Hydrogelators and Hydrogels: From Soft Matter to Molecular Biomaterials, Chem Rev, 115 (2015) 13165–13307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Gao Y, Kuang Y, Guo ZF, Guo Z, Krauss IJ, Xu B, Enzyme-instructed molecular self-assembly confers nanofibers and a supramolecular hydrogel of taxol derivative, J Am Chem Soc, 131 (2009) 13576–13577. [DOI] [PubMed] [Google Scholar]
- [185].Mao L, Wang H, Tan M, Ou L, Kong D, Yang Z, Conjugation of two complementary anti-cancer drugs confers molecular hydrogels as a co-delivery system, Chem Commun (Camb), 48 (2012) 395–397. [DOI] [PubMed] [Google Scholar]
- [186].Wang H, Lv L, Xu G, Yang C, Sun J, Yang Z, Molecular hydrogelators consist of Taxol and short peptides/amino acids, J Mater Chem, 22 (2012) 16933–16938. [Google Scholar]
- [187].Singh M, Kundu S, Reddy MA, Sreekanth V, Motiani RK, Sengupta S, Srivastava A, Bajaj A, Injectable small molecule hydrogel as a potential nanocarrier for localized and sustained in vivo delivery of doxorubicin, Nanoscale, 6 (2014) 12849–12855. [DOI] [PubMed] [Google Scholar]
- [188].Wang H, Wei J, Yang C, Zhao H, Li D, Yin Z, Yang Z, The inhibition of tumor growth and metastasis by self-assembled nanofibers of taxol, Biomaterials, 33 (2012) 5848–5853. [DOI] [PubMed] [Google Scholar]
- [189].Wang H, Yang C, Wang L, Kong D, Zhang Y, Yang Z, Self-assembled nanospheres as a novel delivery system for taxol: a molecular hydrogel with nanosphere morphology, Chem Commun (Camb), 47 (2011) 4439–4441. [DOI] [PubMed] [Google Scholar]
- [190].Majumder J, Das MR, Deb J, Jana SS, Dastidar P, beta-Amino acid and amino-alcohol conjugation of a nonsteroidal anti-inflammatory drug (NSAID) imparts hydrogelation displaying remarkable biostability, biocompatibility, and anti-inflammatory properties, Langmuir, 29 (2013) 10254–10263. [DOI] [PubMed] [Google Scholar]
- [191].Godeau G, Bernard J, Staedel C, Barthelemy P, Glycosyl-nucleoside-lipid based supramolecular assembly as a nanostructured material with nucleic acid delivery capabilities, Chem Commun (Camb), (2009) 5127–5129. [DOI] [PubMed] [Google Scholar]
- [192].Jiang Wang Y, Yan L, Tang L, Yu J, Assembling and releasing performance of supramolecular hydrogels formed from simple drug molecule as the hydrogelator, Chin Chem Lett, 18 (2007) 1009–1012. [Google Scholar]
- [193].Sáez JA, Escuder B, Miravet JF, Supramolecular hydrogels for enzymatically triggered self-immolative drug delivery, Tetrahedron, 66 (2010) 2614–2618. [Google Scholar]
- [194].Patil SP, Kim SH, Jadhav JR, Lee JH, Jeon EM, Kim KT, Kim BH, Cancer-specific gene silencing through therapeutic siRNA delivery with B vitamin-based nanoassembled low-molecular-weight hydrogelators, Bioconjug Chem, 25 (2014) 1517–1525. [DOI] [PubMed] [Google Scholar]
- [195].Lin R, Cheetham AG, Zhang P, Lin YA, Cui H, Supramolecular filaments containing a fixed 41% paclitaxel loading, Chem Commun (Camb), 49 (2013) 4968–4970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Roy R, Deb J, Jana SS, Dastidar P, Peptide conjugates of a nonsteroidal anti-inflammatory drug as supramolecular gelators: synthesis, characterization, and biological studies, Chem Asian J, 9 (2014) 3196–3206. [DOI] [PubMed] [Google Scholar]
- [197].Zhang L, Jiao T, Ma K, Xing R, Liu Y, Xiao Y, Zhou J, Zhang Q, Peng Q, Self-Assembly and Drug Release Capacities of Organogels via Some Amide Compounds with Aromatic Substituent Headgroups, Materials (Basel, Switzerland), 9 (2016) 551–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Motulsky A, Lafleur M, Couffin-Hoarau AC, Hoarau D, Boury F, Benoit JP, Leroux JC, Characterization and biocompatibility of organogels based on L-alanine for parenteral drug delivery implants, Biomaterials, 26 (2005) 6242–6253. [DOI] [PubMed] [Google Scholar]
- [199].Bastiat G, Plourde F, Motulsky A, Furtos A, Dumont Y, Quirion R, Fuhrmann G, Leroux JC, Tyrosine-based rivastigmine-loaded organogels in the treatment of Alzheimer's disease, Biomaterials, 31 (2010) 6031–6038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Dastidar P, Supramolecular gelling agents: can they be designed?, Chem Soc Rev, 37 (2008) 2699–2715. [DOI] [PubMed] [Google Scholar]
- [201].Roy R, Deb J, Jana SS, Dastidar P, Exploiting supramolecular synthons in designing gelators derived from multiple drugs, Chemistry, 20 (2014) 15320–15324. [DOI] [PubMed] [Google Scholar]
- [202].Majumder J, Deb J, Das MR, Jana SS, Dastidar P, Designing a simple organic salt-based supramolecular topical gel capable of displaying in vivo self-delivery application, Chem Commun (Camb), 50 (2014) 1671–1674. [DOI] [PubMed] [Google Scholar]
- [203].Majumder J, Deb J, Husain A, Jana SS, Dastidar P, Cetirizine derived supramolecular topical gel in action: rational design, characterization and in vivo self-delivery application in treating skin allergy in mice, J Mater Chem, 3 (2015) 6634–6644. [DOI] [PubMed] [Google Scholar]
- [204].Rosenberg B, Vancamp L, Krigas T, Inhibition of Cell Division in Escherichia Coli by Electrolysis Products from a Platinum Electrode, Nature, 205 (1965) 698–699. [DOI] [PubMed] [Google Scholar]
- [205].Rosenberg B, VanCamp L, Trosko JE, Mansour VH, Platinum compounds: a new class of potent antitumour agents, Nature, 222 (1969) 385–386. [DOI] [PubMed] [Google Scholar]
- [206].Medici S, Peana M, Nurchi VM, Lachowicz JI, Crisponi G, Zoroddu MA, Noble metals in medicine: Latest advances, Coord Chem Rev, 284 (2015) 329–350. [Google Scholar]
- [207].Piepenbrock MO, Lloyd GO, Clarke N, Steed JW, Metal- and anion-binding supramolecular gels, Chem Rev, 110 (2010) 1960–2004. [DOI] [PubMed] [Google Scholar]
- [208].Steed JW, Anion-tuned supramolecular gels: a natural evolution from urea supramolecular chemistry, Chem Soc Rev, 39 (2010) 3686–3699. [DOI] [PubMed] [Google Scholar]
- [209].Xing B, Choi MF, Xu B, A stable metal coordination polymer gel based on a calix[4]arene and its 'uptake' of non-ionic organic molecules from the aqueous phase, Chem Commun (Camb), (2002) 362–363. [DOI] [PubMed] [Google Scholar]
- [210].Li B, Tang L, Qiang L, Chen K, Novel polymer nanowires with triple hydrogen-bonding sites fabricated by metallogel template polymerization and their adsorption of thymidine, Soft Matter, 7 (2011) 963–969. [Google Scholar]
- [211].Wu D, Jiang R, Luo L, He Z, You J, Bromide anion-triggered visible responsive metallogels based on squaramide complexes, Inorg Chem Front, 3 (2016) 1597–1603. [Google Scholar]
- [212].Tam AY-Y, Wong KM-C, Wang G, Yam VW-W, Luminescent metallogels of platinum(ii) terpyridyl complexes: interplay of metal…metal, τ–π and hydrophobic–hydrophobic interactions on gel formation, Chem Commun (Camb), (2007) 2028–2030. [DOI] [PubMed] [Google Scholar]
- [213].Tam AY, Wong KM, Yam VW, Influence of counteranion on the chiral supramolecular assembly of alkynylplatinum(II) terpyridyl metallogels that are stabilised by Pt…Pt and pi-pi interactions, Chemistry, 15 (2009) 4775–4778. [DOI] [PubMed] [Google Scholar]
- [214].Dukh M, Šaman D, Kroulík J, černý I, Pouzar V, Král V, Drašar P, Metal coordination as a tool for controlling the self-assembling and gelation properties of novel type cholic amide–phenanthroline gelating agent, Tetrahedron, 59(2003)4069–4076. [Google Scholar]
- [215].Yan X, Cook TR, Pollock JB, Wei P, Zhang Y, Yu Y, Huang F, Stang PJ, Responsive supramolecular polymer metallogel constructed by orthogonal coordination-driven self-assembly and host/guest interactions, J Am Chem Soc, 136 (2014) 4460–4463. [DOI] [PubMed] [Google Scholar]
- [216].Jung JH, Lee JH, Silverman JR, John G, Coordination polymer gels with important environmental and biological applications, Chem Soc Rev, 42 (2013) 924–936. [DOI] [PubMed] [Google Scholar]
- [217].Zhang Y, Zhang B, Kuang Y, Gao Y, Shi J, Zhang XX, Xu B, A redox responsive, fluorescent supramolecular metallohydrogel consists of nanofibers with single-molecule width, J Am Chem Soc, 135 (2013) 5008–5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Micklitsch CM, Knerr Pj Fau - Branco MC, Branco Mc Fau - Nagarkar R, Nagarkar R Fau - Pochan DJ, Pochan Dj Fau - Schneider JP, Schneider JP, Zinc-triggered hydrogelation of a self-assembling β-hairpin peptide, Angew Chem Int Ed Engl, 50 (2011) 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Xu C, Cai Y, Ren C, Gao J, Hao J, Zinc-Triggered Hydrogelation of Self-assembled Small Molecules to Inhibit Bacterial Growth, Sci Rep, 5 (2015) 7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Roy R, Bhagyalalitha M, Choudhury P, Dastidar P, Salt metathesis for developing injectable supramolecular metallohydrogelators as a multi-drug-self-delivery system, Chem Commun (Camb), 52 (2016) 13811–13814. [DOI] [PubMed] [Google Scholar]
- [221].Gutierrez Millan C, Colino Gandarillas CI, Sayalero Marinero ML, Lanao JM, Cell-based drug-delivery platforms, Ther Deliv, 3 (2012) 25–41. [DOI] [PubMed] [Google Scholar]
- [222].Li T, Dong H, Zhang C, Mo R, Cell-based drug delivery systems for biomedical applications, Nano Research, 11 (2018) 5240–5257. [Google Scholar]
- [223].Yoo JW, Irvine DJ, Discher DE, Mitragotri S, Bio-inspired, bioengineered and biomimetic drug delivery carriers, Nat Rev Drug Discov, 10 (2011) 521–535. [DOI] [PubMed] [Google Scholar]
- [224].van Dommelen SM, Vader P, Lakhal S, Kooijmans SA, van Solinge WW, Wood MJ, Schiffelers RM, Microvesicles and exosomes: opportunities for cell-derived membrane vesicles in drug delivery, J Control Release, 161 (2012) 635–644. [DOI] [PubMed] [Google Scholar]
- [225].Batrakova EV, Gendelman HE, Kabanov AV, Cell-mediated drug delivery, Expert Opin Drug Deliv, 8 (2011) 415–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [226].Akao Y, Iio A, Itoh T, Noguchi S, Itoh Y, Ohtsuki Y, Naoe T, Microvesicle-mediated RNA molecule delivery system using monocytes/macrophages, Mol Ther, 19 (2011) 395–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Hu CM, Fang RH, Zhang L, Erythrocyte-inspired delivery systems, Adv Healthc Mater, 1 (2012) 537–547. [DOI] [PubMed] [Google Scholar]
- [228].Batrakova EV, Kabanov AV, Cell-mediated drug delivery to the brain, J Drug Deliv Sci Technol, 23 (2013) 419–433. [Google Scholar]
- [229].Choi MR, Stanton-Maxey KJ, Stanley JK, Levin CS, Bardhan R, Akin D, Badve S, Sturgis J, Robinson JP, Bashir R, Halas NJ, Clare SE, A cellular Trojan Horse for delivery of therapeutic nanoparticles into tumors, Nano Lett, 7 (2007) 3759–3765. [DOI] [PubMed] [Google Scholar]
- [230].Alizadeh D, Zhang L, Hwang J, Schluep T, Badie B, Tumor-associated macrophages are predominant carriers of cyclodextrin-based nanoparticles into gliomas, Nanomedicine, 6 (2010) 382–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [231].Rabuka D, Forstner MB, Groves JT, Bertozzi CR, Noncovalent cell surface engineering: incorporation of bioactive synthetic glycopolymers into cellular membranes, J Am Chem Soc, 130 (2008) 5947–5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [232].Stabler CL, Sun XL, Cui W, Wilson JT, Haller CA, Chaikof EL, Surface re-engineering of pancreatic islets with recombinant azido-thrombomodulin, Bioconjug Chem, 18 (2007) 1713–1715. [DOI] [PubMed] [Google Scholar]
- [233].Park J, Andrade B, Seo Y, Kim MJ, Zimmerman SC, Kong H, Engineering the Surface of Therapeutic "Living" Cells, Chem Rev, 118 (2018) 1664–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [234].Stephan MT, Irvine DJ, Enhancing Cell therapies from the Outside In: Cell Surface Engineering Using Synthetic Nanomaterials, Nano today, 6 (2011) 309–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [235].Murciano JC, Medinilla S, Eslin D, Atochina E, Cines DB, Muzykantov VR, Prophylactic fibrinolysis through selective dissolution of nascent clots by tPA-carrying erythrocytes, Nat Biotechnol, 21 (2003) 891–896. [DOI] [PubMed] [Google Scholar]
- [236].Murciano JC, Higazi AA, Cines DB, Muzykantov VR, Soluble urokinase receptor conjugated to carrier red blood cells binds latent pro-urokinase and alters its functional profile, J Control Release, 139 (2009) 190–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [237].Hamidi M, Zarrin A, Foroozesh M, Mohammadi-Samani S, Applications of carrier erythrocytes in delivery of biopharmaceuticals, J Control Release, 118 (2007) 145–160. [DOI] [PubMed] [Google Scholar]
- [238].Stuckey DW, Shah K, Stem cell-based therapies for cancer treatment: separating hope from hype, Nat Rev Cancer, 14 (2014) 683–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [239].Ito S, Natsume A, Shimato S, Ohno M, Kato T, Chansakul P, Wakabayashi T, Kim SU, Human neural stem cells transduced with IFN-beta and cytosine deaminase genes intensify bystander effect in experimental glioma, Cancer Gene Ther, 17 (2010) 299–306. [DOI] [PubMed] [Google Scholar]
- [240].Dembinski JL, Wilson SM, Spaeth EL, Studeny M, Zompetta C, Samudio I, Roby K, Andreeff M, Marini FC, Tumor stroma engraftment of gene-modified mesenchymal stem cells as anti-tumor therapy against ovarian cancer, Cytotherapy, 15 (2013) 20–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [241].Seo SH, Kim KS, Park SH, Suh YS, Kim SJ, Jeun SS, Sung YC, The effects of mesenchymal stem cells injected via different routes on modified IL-12-mediated antitumor activity, Gene Ther, 18 (2011) 488–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [242].Ryu CH, Park SH, Park SA, Kim SM, Lim JY, Jeong CH, Yoon WS, Oh WI, Sung YC, Jeun SS, Gene therapy of intracranial glioma using interleukin 12-secreting human umbilical cord blood-derived mesenchymal stem cells, Hum Gene Ther, 22 (2011) 733–743. [DOI] [PubMed] [Google Scholar]
- [243].Xu G, Jiang XD, Xu Y, Zhang J, Huang FH, Chen ZZ, Zhou DX, Shang JH, Zou YX, Cai YQ, Kou SB, Chen YZ, Xu RX, Zeng YJ, Adenoviral-mediated interleukin-18 expression in mesenchymal stem cells effectively suppresses the growth of glioma in rats, Cell Biol Int, 33 (2009) 466–474. [DOI] [PubMed] [Google Scholar]
- [244].Takayama Y, Kusamori K, Hayashi M, Tanabe N, Matsuura S, Tsujimura M, Katsumi H, Sakane T, Nishikawa M, Yamamoto A, Long-term drug modification to the surface of mesenchymal stem cells by the avidin-biotin complex method, Sci Rep, 7 (2017) 16953–16963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [245].Mooney R, Weng Y, Garcia E, Bhojane S, Smith-Powell L, Kim SU, Annala AJ, Aboody KS, Berlin JM, Conjugation of pH-responsive nanoparticles to neural stem cells improves intratumoral therapy, J Control Release, 191 (2014) 82–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [246].Cao B, Yang M, Zhu Y, Qu X, Mao C, Stem cells loaded with nanoparticles as a drug carrier for in vivo breast cancer therapy, Adv Mater, 26 (2014) 4627–4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [247].Huang X, Zhang F, Wang H, Niu G, Choi KY, Swierczewska M, Zhang G, Gao H, Wang Z, Zhu L, Choi HS, Lee S, Chen X, Mesenchymal stem cell-based cell engineering with multifunctional mesoporous silica nanoparticles for tumor delivery, Biomaterials, 34 (2013) 1772–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [248].Li L, Guan Y, Liu H, Hao N, Liu T, Meng X, Fu C, Li Y, Qu Q, Zhang Y, Ji S, Chen L, Chen D, Tang F, Silica nanorattle-doxorubicin-anchored mesenchymal stem cells for tumor-tropic therapy, ACS Nano, 5 (2011) 7462–7470. [DOI] [PubMed] [Google Scholar]
- [249].Schnarr K, Mooney R, Weng Y, Zhao D, Garcia E, Armstrong B, Annala AJ, Kim SU, Aboody KS, Berlin JM, Gold nanoparticle-loaded neural stem cells for photothermal ablation of cancer, Adv Healthc Mater, 2 (2013) 976–982. [DOI] [PubMed] [Google Scholar]
- [250].Mooney R, Roma L, Zhao D, Van Haute D, Garcia E, Kim SU, Annala AJ, Aboody KS, Berlin JM, Neural stem cell-mediated intratumoral delivery of gold nanorods improves photothermal therapy, ACS Nano, 8 (2014) 12450–12460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [251].Stephan MT, Stephan SB, Bak P, Chen J, Irvine DJ, Synapse-directed delivery of immunomodulators using T-cell-conjugated nanoparticles, Biomaterials, 33 (2012) 5776–5787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [252].Stephan MT, Moon JJ, Um SH, Bershteyn A, Irvine DJ, Therapeutic cell engineering with surface-conjugated synthetic nanoparticles, Nat Med, 16 (2010) 1035–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [253].Tang L, Zheng Y, Melo MB, Mabardi L, Castano AP, Xie YQ, Li N, Kudchodkar SB, Wong HC, Jeng EK, Maus MV, Irvine DJ, Enhancing T cell therapy through TCR-signaling-responsive nanoparticle drug delivery, Nat Biotechnol, 36 (2018) 707–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [254].Adedoyin A, Swenson CE, Bolcsak LE, Hellmann A, Radowska D, Horwith G, Janoff AS, Branch RA, A pharmacokinetic study of amphotericin B lipid complex injection (Abelcet) in patients with definite or probable systemic fungal infections, Antimicrob. Agents Chemother, 44 (2000) 2900–2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [255].Meunier F, Prentice HG, Ringden O, Liposomal amphotericin B (AmBisome): safety data from a phase II/III clinical trial, J Antimicrob Chemother, 28 Suppl B (1991) 83–91. [DOI] [PubMed] [Google Scholar]
- [256].Alam M, Hartrick CT, Extended-release epidural morphine (DepoDur): an old drug with a new profile, Pain Pract, 5 (2005) 349–353. [DOI] [PubMed] [Google Scholar]
- [257].Veronese FM, Harris JM, Introduction and overview of peptide and protein pegylation, Adv Drug Deliv Rev, 54 (2002) 453–456. [DOI] [PubMed] [Google Scholar]
- [258].Petre CE, Dittmer DP, Liposomal daunorubicin as treatment for Kaposi's sarcoma, Int J Nanomedicine, 2 (2007) 277–288. [PMC free article] [PubMed] [Google Scholar]
- [259].Green AE, Rose PG, Pegylated liposomal doxorubicin in ovarian cancer, Int J Nanomedicine, 1 (2006) 229–239. [PMC free article] [PubMed] [Google Scholar]
- [260].Usonis V, Bakasenas V, Valentelis R, Katiliene G, Vidzeniene D, Herzog C, Antibody titres after primary and booster vaccination of infants and young children with a virosomal hepatitis A vaccine (Epaxal), Vaccine, 21 (2003) 4588–4592. [DOI] [PubMed] [Google Scholar]
- [261].Burade V, Bhowmick S, Maiti K, Zalawadia R, Ruan H, Thennati R, Lipodox(R) (generic doxorubicin hydrochloride liposome injection): in vivo efficacy and bioequivalence versus Caelyx(R) (doxorubicin hydrochloride liposome injection) in human mammary carcinoma (MX-1) xenograft and syngeneic fibrosarcoma (WEHI 164) mouse models, BMC cancer, 17 (2017) 405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [262].Rodriguez MA, Pytlik R, Kozak T, Chhanabhai M, Gascoyne R, Lu B, Deitcher SR, Winter JN, Marqibo I, Vincristine sulfate liposomes injection (Marqibo) in heavily pretreated patients with refractory aggressive non-Hodgkin lymphoma: report of the pivotal phase 2 study, Cancer, 115 (2009) 3475–3482. [DOI] [PubMed] [Google Scholar]
- [263].Stathopoulos GP, Boulikas T, Lipoplatin formulation review article, J Drug Deliv, 2012 (2012) 581363. [DOI] [PMC free article] [PubMed] [Google Scholar]