Abstract
The syntheses and O2 reactivities of active-site models of cobalt-substituted ring-cleaving dioxygenases are presented. The pentacoordinate cobalt(II)-aminophenolate complex, [Co(TpMe2)(tBu2APH)], gives rise to two distinct dioxygen adducts at reduced temperatures. The first is a paramagnetic (S = 1/2) cobalt(III)-superoxo species that was characterized with spectroscopic and computational techniques. The identity of the second Co/O2 adduct was elucidated by X-ray crystallography, which revealed an unprecedented cobalt(III)-alkylperoxo structure generated by O2 addition to the metal ion and ligand. These results provide synthetic precedents for proposed intermediates in the catalytic cycles of O2-activating cobalt enzymes.
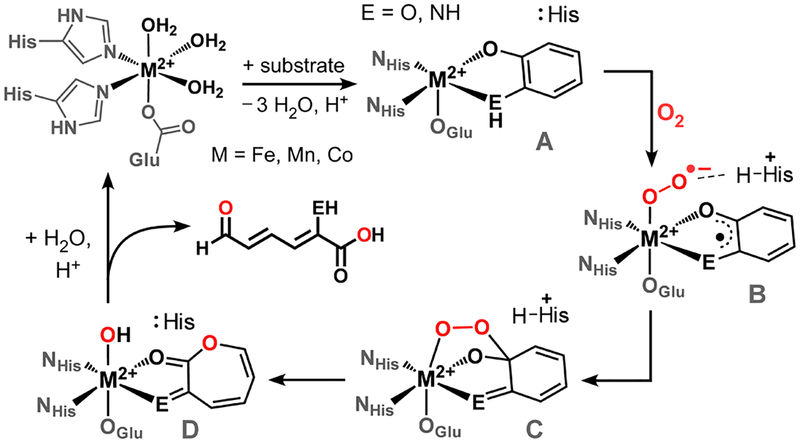
The bacterial breakdown of organic compounds, including human-generated pollutants, often requires dioxygenase enzymes that oxidatively cleave aromatic carbon−carbon bonds using O2.1 Substrates of these ring-cleaving dioxygenases include substituted catechols, o-aminophenols, 1,4-hydro-quinones, and salicylates.2 The active sites of most ring-cleaving dioxygenases feature a mononuclear nonheme iron center bound facially to one Glu (or Asp) and two His residues.3 However, recent studies revealed that an extradiol catechol dioxygenase (CatD), homoprotocatechuate-2,3-dioxygenase (HPCD), exhibits equal or greater activity with Mn or Co in the active site.4 The “promiscuity” of HPCD supports the mechanistic proposal that O2 activation by ring-cleaving dioxygenases does not necessitate a change in metal oxidation state. Instead, the metal facilitates the transfer of one electron from the coordinated substrate to O2, thereby yielding a M(II)-superoxo species with an (imino)semiquinone radical (B in Scheme 1).5 Formation of a substrate-based radical encourages attack by the superoxide ligand to generate a putative alkylperoxo species (C), which undergoes rearrangement to insert an O atom into the substrate ring (D).6 Analogous mechanisms are likely employed by o-aminophenol and 1,4-hydroquinone dioxygenases.7
Scheme 1.
Proposed Mechanism of Ring-Cleaving Dioxygenases
The surprising activity of metal-substituted HPCD has stimulated the synthesis of extradiol CatD models featuring Co and Mn. Yet complexes that replicate the monoanionic, bidentate coordination of the catecholate ligand in the enzyme active site are still lacking. Recently, the Riordan and Hikichi groups reported Co and Mn complexes, respectively, that feature a monoanionic catecholate ligand bound in a monodentate manner.8,9 Exposure of these complexes to O2 results in formation of the corresponding M(II)-semiquinonate (SQ) species via loss of an electron and proton (i.e., net H atom transfer, HAT). Thus, the CatD models fail to replicate the initial O2 binding step of the enzymatic mechanism. In some cases, further reaction of the Co(II)-SQ complexes with O2 affords the intradiol ring-cleavage products in low yield.8
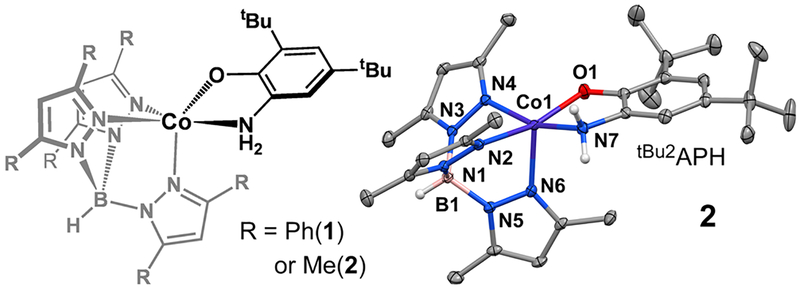
To avoid the shortcomings of the cobalt-catecholate complexes, we decided to pursue cobalt(II) dioxygenase models that contain an aminophenolate ligand instead. Aminophenol dioxygenases (APDOs) are closely related to extradiol CatDs both structurally and mechanistically,2 and although a cobalt-substituted APDO has not been generated to date, it is reasonable to expect such an enzyme to display activity. More importantly, we reckoned that the less acidic −NH2 donor would deter formation of a Co(II)-iminosemiquinone (ISQ) species and provide access to biologically relevant O2 reaction pathways. This hypothesis proved correct, and herein we describe the synthesis of two mononuclear Co(II) complexes (1 and 2 in Figure 1) that feature a monoanionic, bidentate aminophenolate ligand. The 2-histidine-1-carboxylate facial triad of the enzymatic active site is modeled with the TpR2 ligand (R = Ph (1) or Me (2); TpR2 = hydrotris(pyrazolyl-1-yl)borate substituted with R-groups at the 3- and 5-positions). Although HAT reactivity is observed for 1 and 2 under certain conditions, the latter complex gives rise to O2-derived intermediates not observed for the analogous catecholate complexes, including a cobalt-superoxo species that resembles intermediate B. Furthermore, we report the first X-ray structure of a cobalt-alkylperoxo complex with a structure akin to C in the proposed ring-cleaving mechanism.
Figure 1.
Left: Schematic drawing of [Co(TpR2)(tBu2APH)] (1 and 2). Right: X-ray crystal structure of 2.
The crystal structures of complexes 1 and 2 each revealed a five-coordinate Co(II) center in which the monoanionic tBu2APH ligand binds in a bidentate fashion (Figure 1). The TpR2 ligands coordinate facially with average Co−NTp bond distances of 2.11 Å. The Co−N/O bond distances (Table S1) are characteristic of pentacoordinate, high-spin Co(II) complexes. X-band EPR spectra of 1 and 2 (Figure S3) exhibit features arising from the ms = ±3/2 doublet of the S = 3/2 manifold (D < 0). In both spectra, hyperfine splitting from the 59Co nucleus is evident in the low-field resonance near g ~ 7 (ACo = 85 G for 2).
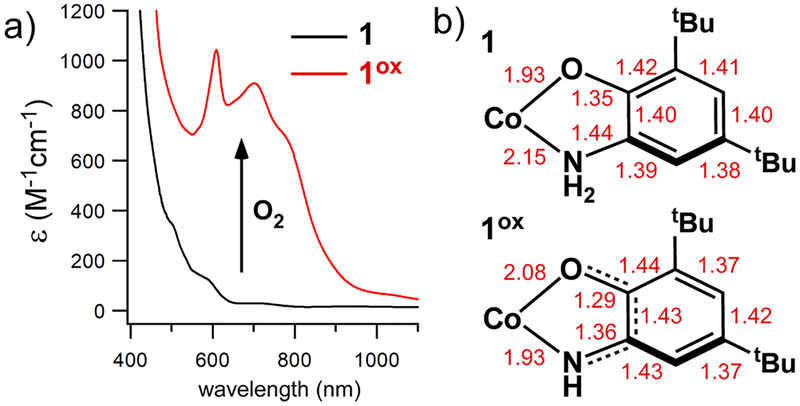
Complex 1 reacts slowly with O2 to yield a stable, dark green species (1ox; Figure 2a). X-ray crystallography determined that 1ox, like its precursor, is a neutral five-coordinate complex. The Co−NTp bond distances change only slightly from 1 to 1ox (Table S1), suggesting that the Co center remains divalent and high-spin. Despite these similarities, comparison of the two structures reveals that the tBu2APH ligand of 1 has been oxidized to an ISQ radical in 1ox. The change is apparent in the shorter O1−C1 and N2−C2 bond distances of 1ox, as well as the quinoidal distortion of its C−C bonds (Figure 2b). Using the “metrical oxidation state” method developed by Brown,10 the tBu2ISQ ligand of 1ox carries a charge of −0.95, near the ideal value of −1.0 for an ISQ ligand. The presence of an tBu2ISQ radical is also evident from characteristic π → π* features in the 600−800 nm region of the absorption spectrum that overlap with Co(II) d-d bands (Figure 2a).11 Complex 1ox is EPR-silent and the observed magnetic moment of 2.9 μB (S = 1) is indicative of antiferromagnetic coupling between the Co(II) and tBu2ISQ spins.
Figure 2.
(a) UV−vis absorption spectra of 1 and 1ox in CH2Cl2 at 20 °C. (b) Selected bond distances (Å) for the tBu2APH and tBu2ISQ ligands in X-ray structures of 1 and 1ox, respectively.
Likewise, exposure of 2 to O2 at room temperature (RT) yields a green species (2ox) with spectral and magnetic properties similar to 1ox (Figure S4). A notable difference, however, is that 2ox decays within minutes at 20 °C, which hindered the growth of suitable crystals. Significantly, 2ox can also be generated under anaerobic conditions by treating 2 with one equivalent of 2,4,6-tri-tert-butylphenoxy radical (TTBP•), a well-known H atom abstractor (Figure S4). This result demonstrates that the conversion of 2 → 2ox involves loss of a proton and electron to generate [CoII(TpMe2)-(tBu2ISQ)]. Nuclear magnetic resonance (NMR) analysis of the reaction mixture after decay of 2ox in air found that 3,5-di-tert-butyl-o-benzoquinone (DTBQ) is the only product derived from the tBu2APH ligand (Figure S5). Thus, the overall O2 reaction does not result in oxygenated or ring-cleaved products; instead, the tBu2APH ligand undergoes two-electron oxidation to the corresponding o-iminobenzoquinone, followed by hydrolysis to DTBQ upon aqueous workup.
While the O2 reactivity of 1 and 2 at RT is dominated by HAT chemistry, we found that it is possible to observe novel Co/O2 adducts at reduced temperatures. Reaction of 2 with O2 at −78 °C in CH2Cl2 or THF generates a metastable pink species (2-O2) with absorption features at λmax = 505 and 800 nm (Figure 3a). Purging the solution with Ar does not regenerate 2, and warming causes 2-O2 to convert to 2ox. The X-band EPR spectrum of 2-O2 presents a S = 1/2 signal with g-values of 2.084, 2.007, 1.957 and 59Co hyperfine splitting of 28 G (Figure 3b). Quantification of the EPR signal indicates that 2-O2 accounts for ~80% of the Co in the sample, with the remainder being starting complex. Both the UV−vis and EPR spectra of 2-O2 are strikingly similar to those previously reported for Co/O2 adducts.12 In particular, the clustering of the g-values near 2.0 and the small ACo-value of 2-O2 (relative to its Co(II) precursor) are distinctive characteristics of cobalt(III)-superoxo species, reflecting localization of the unpaired electron on the superoxo ligand. The presence of a superoxo-to-Co(III) charge transfer (CT) transition near 500 nm, as observed for 2-O2, is also a common feature of known cobalt(III)-superoxo complexes in noncorrinoid environments.12 Interestingly, complex 1 is unreactive with O2 at low temperatures, suggesting that sterics modulate the energetics of O2 binding.
Figure 3.
(a) UV−vis absorption spectrum of 2-O2 obtained by reaction of 2 with O2 in THF at −70 °C. [2]initial = 1.25 mM. (b) X-band EPR spectrum (red) of 2-O2 in frozen THF at 77 K. Parameters for the simulated spectrum (gray) are provided in the SI.
The geometric and electronic structures of 2-O2 were further analyzed using density functional theory (DFT) calculations. The geometry-optimized structure (Figure S6) features an end-on superoxo ligand in a bent conformation. The superoxo nature of the O2 ligand is reflected in the computed O−O distance of 1.278 Å. The six-coordinate Co(III) center is low-spin and nearly all of the unpaired spin density resides on the superoxo ligand, consistent with the EPR data. The computed 59Co A-tensor is anisotropic with a dominant hyperfine splitting of 26 G, in excellent agreement with the experimental value. The g-values predicted by CASSCF/NEVPT2 calculations (g1,2,3 = 2.060, 1.991 and 1.979) reproduce the weak anisotropy of the 2-O2 signal. Thus, the computational data further corroborate the assignment of 2-O2 as a cobalt(III)-superoxo species.
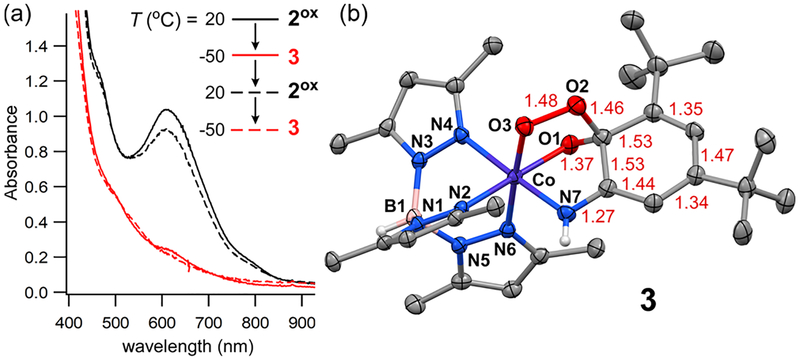
Interestingly, we found that a second Co/O2 adduct with spectroscopic features distinct from 2-O2 is generated when 2ox is treated with O2 at reduced temperatures. Aerobic solutions of 2ox in CH3CN and CH2Cl2 change from dark green to light brown upon cooling. Monitoring the process by UV−vis spectroscopy revealed that the species generated at low temperature (3) lacks well-defined absorption features in the visible region (Figure 4a). The absorption features of 2ox return when the solution is warmed to RT, but full intensity is not recovered due to its instability. No color change is observed during cooling if 2ox is generated anaerobically via reaction with TTBP•, indicating that formation of 3 requires O2. The characteristics of the 2ox → 3 conversion are reminiscent of those previously reported for the O2 reaction of cobalt(II)-semiquinonate complexes at reduced temperatures.8b,9b Because of this, we prepared and structurally characterized [CoII(TpMe2)(tBu2SQ)] (4; Figure S7), the SQ analogue of 2ox. Complex 4 reacts with O2 at T < −40 °C to give a brown chromophore (5) with absorption features similar to those of 3 (Figure S8). Like 3, species 5 is EPR-silent and variable-temperature NMR experiments indicate that both Co/O2 adducts are diamagnetic (Figures S9−S11).
Figure 4.
(a) UV−vis spectral changes for the thermal interconversion of 2ox (black) and 3 (red) in CH2Cl2 in the presence of O2. [Co] = 0.7 mM (b) Ellipsoid plot derived from the X-ray structure of 3. Selected bond lengths (Å) are provided in red.
Due to its stability at temperatures below −25 °C, we succeeded in growing light brown crystals of 3 for X-ray analysis. The resulting crystal structure revealed a neutral cobalt-alkylperoxo complex in which the O2-derived atoms form a bridge between Co and C1 of the ligand (Figure 4b), thereby generating a five-membered metallocycle. The O2−O3 distance of 1.482(3) Å is typical of alkylperoxo ligands, and the sp3 hybridization of the C1-atom is evident from its average bond angle of 110° ± 7°. The Co−N/O bond distances in 3 are shorter than those of 2 by an average of 0.15 Å, indicating a change from high-spin Co(II) to low-spin Co(III). Comparison of 3 to 1ox reveals that the quinoidal distortion of the ligand is far more pronounced in the former complex, and the metric parameters observed for 3 are characteristic of iminobenzoquinone ligands.10 Thus, formation of 3 is a two- electron process involving oxidation of both the Co center and tBu2ISQ ligand (Scheme 2).
Scheme 2.
Species Generated by Reaction of Complexes 1 and 2 with O2
The “spiroendoperoxide” structure of 3 is the first of its kind among first-row transition metal complexes; indeed, it represents the only X-ray structure to date of a synthetic dioxygen adduct with direct relevance to ring-cleaving dioxygenases. The closest analogues are Rh(III)- and Ir(III)-alkylperoxo complexes generated by O2 addition to a 9,10-phenanthrene-catecholate(2-) ligand.13 Similarly, Gade recently reported a square-planar nickel(II) complex that features an alkylperoxometallocycle derived from O2.14 In main-group chemistry, Abakumov showed that a series of Sb(V)-amidophenolate complexes reversibly bind O2 to yield an alkylperoxo donor.15 As for biological precedents, the structure 3 closely resembles the iron-alkylperoxo intermediate observed by Lipscomb in a crystal structure of HPCD.16
As summarized in Scheme 2, we have explored the O2 reaction landscape of two cobalt(II)-aminophenolate complexes. These studies led to the isolation and characterization of cobalt(III)-superoxo (2-O2) and -alkylperoxo (3) species that mimic proposed intermediates of ring-cleaving dioxygenases. It is instructive that subtle differences between these synthetic Co/O2 adducts and their enzymatic counterparts account for the lack of ring-cleavage activity exhibited by our synthetic models. Specifically, the low-spin Co(III) center of 3 stabilizes the alkylperoxo ligand and prevents subsequent O−O bond cleavage, whereas the high-spin Co(II) ion in the putative enzymatic intermediate facilitates insertion of the distal O atom into the ring via Criegee rearrangement. In our models, the inability of 2-O2 to convert to the requisite cobalt(II)-alkylperoxo intermediate is likely due to the lack of unpaired spin density within the [Co3+-tBu2APH] unit, which hinders O−C bond formation. The enzyme avoids this scenario by coupling O2 binding to a proton transfer from the substrate to a conserved second-sphere His residue. According to computational studies, this process yields a superoxo-Co(II)-substrate radical species (B in Scheme 1) that is primed for alkylperoxo formation.17 Future efforts in our laboratory will be directed toward the design of functional active-site mimics that replicate the ability of the enzyme to control both H+ transfer and O2 binding.
Supplementary Material
ACKNOWLEDGMENTS
This research received financial support from the National Institutes of Health (GM126522). Improvements to the X-band EPR instrument at Marquette University were funded through a grant from the National Science Foundation (CHE-1532168). We thank Dr. Sheng Cai for assistance with the variable-temperature NMR experiments.
Footnotes
The authors declare no competing financial interest.
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/jacs.9b05320.
Experimental procedures, computational methods and models, spectroscopic data (EPR, 1H NMR, UV-vis) (PDF)
Crystallographic information (CIF)
REFERENCES
- (1).Wang Y; Li J; Liu A Oxygen activation by mononuclear nonheme iron dioxygenases involved in the degradation of aromatics. JBIC, J. Biol. Inorg. Chem 2017, 22, 395–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Vaillancourt FH; Bolin JT; Eltis LD The ins and outs of ring-cleaving dioxygenases. Crit. Rev. Biochem. Mol. Biol 2006, 41, 241–267. [DOI] [PubMed] [Google Scholar]
- (3).(a) Koehntop KD; Emerson JP; Que L The 2-His-1-carboxylate facial triad: a versatile platform for dioxygen activation by mononuclear non-heme iron(II) enzymes. JBIC, J. Biol. Inorg. Chem 2005, 10, 87–93. [DOI] [PubMed] [Google Scholar]; (b) Bruijnincx PCA; van Koten G; Klein Gebbink RJM Mononuclear non-heme iron enzymes with the 2-His-1-carboxylate facial triad: recent developments in enzymology and modeling studies. Chem. Soc. Rev 2008, 37, 2716–2744. [DOI] [PubMed] [Google Scholar]
- (4).(a) Emerson JP; Kovaleva EG; Farquhar ER; Lipscomb JD; Que L Swapping metals in Fe- and Mn-dependent dioxygenases: Evidence for oxygen activation without a change in metal redox state. Proc. Natl. Acad. Sci. U. S. A 2008, 105, 7347–7352. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Fielding AJ; Kovaleva EG; Farquhar ER; Lipscomb JD; Que L Jr. A hyperactive cobalt-substituted extradiol-cleaving catechol dioxygenase. JBIC, J. Biol. Inorg. Chem 2011, 16, 341–355. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Fielding AJ; Lipscomb JD; Que L Characterization of an O2 adduct of an active cobalt-substituted extradiol-cleaving catechol dioxygenase. J. Am. Chem. Soc 2012, 134, 796–799. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Fiedler AT; Fischer AA Oxygen activation by mononuclear Mn, Co, and Ni centers in biology and synthetic complexes. JBIC, J. Biol. Inorg. Chem 2017, 22, 407–424. [DOI] [PubMed] [Google Scholar]
- (5).Fielding AJ; Lipscomb JD; Que L Jr. A two-electron-shell game: intermediates of the extradiol-cleaving catechol dioxygenases. JBIC, J. Biol. Inorg. Chem 2014, 19, 491–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).(a) Lipscomb JD Mechanism of extradiol aromatic ring-cleaving dioxygenases. Curr. Opin. Struct. Biol 2008, 18, 644–649. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Kovaleva EG; Lipscomb JD Versatility of biological non-heme Fe(II) centers in oxygen activation reactions. Nat. Chem. Biol 2008, 4, 186–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).(a) Dong G; Lu J; Lai W Insights into the Mechanism of Aromatic Ring Cleavage of Noncatecholic Compound 2-Amino-phenol by Aminophenol Dioxygenase: A Quantum Mechanics/Molecular Mechanics Study. ACS Catal. 2016, 6, 3796–3803. [Google Scholar]; (b) Machonkin TE; Doerner AE Substrate Specificity of Sphingobium chlorophenolicum 2,6-Dichlorohydroquinone 1,2-Dioxygenase. Biochemistry 2011, 50, 8899–8913. [DOI] [PubMed] [Google Scholar]
- (8).(a) Wang P; Yap GPA; Riordan CG Five-coordinate MII-semiquinonate (M = Fe, Mn, Co) complexes: reactivity models of the catechol dioxygenases. Chem. Commun 2014, 50, 5871–5873. [DOI] [PubMed] [Google Scholar]; (b) Wang P; Yap GPA; Riordan CG Synthesis, characterization and O2 reactivity of a bioinspired cobalt(II)-catecholate complex. Inorg. Chim. Acta 2019, 488, 49–55. [Google Scholar]
- (9).(a) Agake S.-i.; Komatsuzaki H; Satoh M; Agou T; Tanaka Y; Akita M; Nakazawa J; Hikichi S A monomeric manganese(II) catecholato complex: Synthesis, crystal structure, and reactivity toward molecular oxygen. Inorg. Chim. Acta 2019, 484, 424–429. [Google Scholar]; (b) Ikeda A; Hoshino K; Komatsuzaki H; Satoh M; Nakazawa J; Hikichi S O2 activation and external substrate oxidation capability of a Co(II)-semiquinonato complex. New J. Chem 2013, 37, 2377–2383. [Google Scholar]
- (10).Brown SN Metrical Oxidation States of 2-Amidophenoxide and Catecholate Ligands: Structural Signatures of Metal-Ligand pi Bonding in Potentially Noninnocent Ligands. Inorg. Chem 2012, 51, 1251–1260. [DOI] [PubMed] [Google Scholar]
- (11).Bittner MM; Kraus D; Lindeman SV; Popescu CV; Fiedler AT Synthetic, Spectroscopic, and DFT Studies of Iron Complexes with Iminobenzo(semi)quinone Ligands: Implications for o-Aminophenol Dioxygenases. Chem.-Eur. J 2013, 19, 9686–9698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).(a) Wang C-C; Chang H-C; Lai Y-C; Fang H; Li C-C; Hsu H-K; Li Z-Y; Lin T-S; Kuo T-S; Neese F; Ye S; Chiang Y-W; Tsai M-L; Liaw W-F; Lee W-Z A Structurally Characterized Nonheme Cobalt-Hydroperoxo Complex Derived from Its Superoxo Intermediate via Hydrogen Atom Abstraction. J. Am. Chem. Soc 2016, 138, 14186–14189. [DOI] [PubMed] [Google Scholar]; (b) Oddon F; Chiba Y; Nakazawa J; Ohta T; Ogura T; Hikichi S Characterization of Mononuclear Non-heme Iron(III)-Superoxo Complex with a Five-Azole Ligand Set. Angew. Chem., Int. Ed 2015, 54, 7336–7339. [DOI] [PubMed] [Google Scholar]; (c) Corona T; Padamati SK; Acuna-Pares F; Duboc C; Browne WR; Company A Trapping of superoxido cobalt and peroxido dicobalt species formed reversibly from CoII and O2. Chem. Commun 2017, 53, 11782–11785. [DOI] [PubMed] [Google Scholar]; (d) Gordon JB; Vilbert AC; Siegler MA; Lancaster KM; Moenne-Loccoz P; Goldberg DP A Nonheme Thiolate-Ligated Cobalt Superoxo Complex: Synthesis and Spectroscopic Characterization, Computational Studies, and Hydrogen Atom Abstraction Reactivity. J. Am. Chem. Soc 2019, 141, 3641–3653. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Fischer AA; Lindeman SV; Fiedler AT Spectroscopic and computational studies of reversible O2 binding by a cobalt complex of relevance to cysteine dioxygenase. Dalton Trans 2017, 46, 13229–13241. [DOI] [PubMed] [Google Scholar]; (f) Jones RD; Summerville DA; Basolo F Synthetic oxygen carriers related to biological systems. Chem. Rev 1979, 79, 139–79. [Google Scholar]
- (13).(a) Barbaro P; Bianchini C; Mealli C; Meli A Synthetic models for catechol 1,2-dioxygenases. Interception of a metal catecholate-dioxygen adduct. J. Am. Chem. Soc 1991, 113, 3181–3. [Google Scholar]; (b) Dutta S; Peng S-M; Bhattacharya S Ligand Control on Molecular Oxygen Activation by Rhodium Quinone Complexes. Inorg. Chem 2000, 39, 2231–2234. [DOI] [PubMed] [Google Scholar]
- (14).Rettenmeier CA; Wadepohl H; Gade LH Structural Characterization of a Hydroperoxo Nickel Complex and Its Autoxidation: Mechanism of Interconversion between Peroxo, Superoxo, and Hydroperoxo Species. Angew. Chem., Int. Ed 2015, 54, 4880–4884. [DOI] [PubMed] [Google Scholar]
- (15).Abakumov GA; Poddel’sky AI; Grunova EV; Cherkasov VK; Fukin GK; Kurskii YA; Abakumova LG Reversible binding of dioxygen by a non-transition-metal complex. Angew. Chem., Int. Ed 2005, 44, 2767–2771. [DOI] [PubMed] [Google Scholar]
- (16).Kovaleva EG; Lipscomb JD Crystal structures of Fe2+ dioxygenase superoxo, alkylperoxo, and bound product intermediates. Science 2007, 316, 453–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Cao L; Dong G; Lai W Reaction Mechanism of Cobalt-Substituted Homoprotocatechuate 2,3-Dioxygenase: A QM/MM Study. J. Phys. Chem. B 2015, 119, 4608–4616. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.