ABSTRACT
Objective: Insulin resistance (IR) plays an important role in the development of many diseases, such as diabetes mellitus. Therefore, the aim of the present study was to evaluate the effects of the extracts from fruits native to Brazil on metabolic parameters and hepatic oxidative markers in an animal model of insulin resistance induced by dexamethasone (DEX).
Methods: Wistar rats received water or extracts of Eugenia uniflora or Psidium cattleianum, once a day for 21 days. For the last 5 days, the rats received an intraperitoneal injection of saline or DEX.
Results: DEX caused a reduction in body weight gain and relative pancreatic weight, as well as glucose intolerance, and an increase in serum glucose and triacylglycerol levels. The extracts were found to prevent hyperglycemia and hypertriglyceridemia. DEX caused an increase in the levels of thiobarbituric acid-reactive substances and reactive oxygen species production in the liver of rats, and both extracts prevented these changes. In addition, hepatic glutathione peroxidase activity was reduced by DEX. However, total thiol content and activities of catalase, superoxide dismutase, and delta-aminolevulinate dehydratase were not altered in any of the tested groups.
Conclusion: Fruit extracts of E. uniflora and P. cattleianum exhibited considerable antihyperglycemic, antidyslipidemic, and antioxidant effects, and may be useful in the therapeutic management of alterations due to IR.
KEYWORDS: Insulin resistance, redox status, Eugenia uniflora, Psidium cattleianum, fruits, rats
1. Introduction
Insulin resistance (IR) is a reduction of the responses of insulin target cells and tissues to a physiological concentration of this hormone, and is characterized by insulin insensitivity or alterations of cellular and tissue response, including those of muscle, adipose, and liver tissue [1]. IR is widely associated with abdominal obesity, type 2 diabetes (T2D), and metabolic syndrome (MetS). Additionally, it can be a risk factor for the development of dyslipidemia, cardiovascular diseases, and steatosis [2,3].
Some authors have suggested that IR contributes to increased oxidative stress, probably due to an alteration of mitochondrial function, and this increase may also further aggravate IR [4,5]. It is well known that the oxidative stress caused by an imbalance in redox state, owing to either excessive production of reactive species or disturbance of the antioxidant system, can lead to the damage of cell membranes and other functional components such as proteins, lipids, and DNA. In aerobic metabolism, reactive oxygen species (ROS) and reactive nitrogen species (RNS) are produced. To limit oxidative damage, the body produces antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx). Additionally, substances acquired through diet, such as flavonoids, vitamins, and minerals, form an important defense barrier [6,7].
Eugenia uniflora (popularly known as pitanga) and Psidium cattleianum (popularly known as araçá) are fruits native to Brazil and belong to the Myrtaceae family. These genera have been studied for their potential health benefits, such as antioxidant, anti-inflammatory, diuretic, and hypoglycemic properties. Some authors relate these effects to the secondary metabolites present in both species, mainly phenolic compounds [7–9].
To the best of our knowledge, there are very few studies on E. uniflora and P. cattleianum fruits. Therefore, the aim of this study was to evaluate the effects of these native fruit extracts on metabolic parameters and hepatic oxidative stress markers in an animal model of IR induced by dexamethasone (DEX).
2. Materials and methods
2.1. Preparation of fruit extracts
E. uniflora and P. cattleianum fruits were harvested from an orchard belonging to Embrapa Clima Temperado (Brazilian Agricultural Research Corporation) Pelotas/RS, Brazil (31°40′47"S and 52°26′24"W). After picking, the fruits were immediately frozen at −20°C and protected from light. The extracts were prepared according to the method described by Bordignon et al. [10] with minor modifications. Briefly, unprocessed frozen fruits were sonicated for 30 minutes at 25°C in 90 ml 70:30 v/v ethanol–water (pH 1.0). The crude extracts were filtered; the ethanol was removed under reduced pressure and then lyophilized. These procedures were performed in triplicate and sheltered from light.
2.2. Phytochemical characterization
2.2.1. Total phenolic, flavonoid, and anthocyanin content
The total phenolic content was determined according to the method described by Singleton et al. [11] with minor modifications and expressed as milligrams of gallic acid per 1 g of dried extract. The total flavonoid content was determined according to the method described by Miliauskas et al. [12]. Data were presented as expressed as milligrams of rutin per 1 g of dried extract. Anthocyanins were quantified by the pH differential method [13] and expressed as milligrams of cyanidin-3-glucoside (C3G) per 1 g of dried extract. All data are presented as mean ± SD values and analyses were performed in triplicate.
2.3. Animals and drug treatments
Forty-eight adult male Wistar rats were obtained from the Central Animal House of the Universidade Federal de Pelotas (UFPel), Pelotas, Rio Grande do Sul, Brazil, where they were kept under appropriate conditions (e.g. controlled temperature of 22 ± 1°C, on a 12-hour light/dark cycle), and received commercial standard chow and water ad libitum. All animal procedures used were approved by UFPel’s Ethics Committee on Animal Experimentation (protocol no. CEEA 5747/2015).
The rats were divided into six experimental groups (n = 8 in each group): I (control/vehicle), II (control/E. uniflora), III (control/P. cattleianum), IV (DEX/vehicle), V (DEX/E. uniflora), and VI (DEX/P. cattleianum). Groups I and IV received distilled water; Groups II and V received 200 mg/kg/day E. uniflora extract; and Groups III and VI received 200 mg/kg/day P. cattleianum. The distilled water and extracts were administered orally once daily for 21 days. For the last 5 days, Groups IV, V, and VI received DEX injection (1 mg/kg), intraperitoneally (i.p.). The doses of fruit extracts and DEX were chosen according to Oliveira et al. [14] and Rafacho et al. [15], respectively.
2.4. Body weight gain
Changes in the rats’ body weight were measured throughout the experimental period. The weight of each rat was recorded on day 0 and at weekly intervals throughout the course of the study.
2.5. Sample collection and biochemical assay
At the end of the 21-day experimental period, all rats were killed. Blood was collected and centrifuged at 800g for 15 minutes and the resulting serum was frozen in liquid nitrogen and stored at −80°C for further biochemical analysis. The liver was dissected and stored for further determination of oxidative stress parameters. The pancreas was dissected and weighed.
2.5.1. Glucose tolerance test
The glucose tolerance test was performed on day 21. The rats received 50% glucose solution (2 mg/g b.w. i.p.); 0, 30, 60, and 120 minutes after injection, blood from the tail was collected via a small tail puncture and the glucose levels were measured using a glucometer (AccuChek® Active, Roche Diagnostics, Indianapolis, IA, U.S.A.).
2.5.2. Biochemical parameters
Measurements of serum glucose, total cholesterol, and triacylglycerol level, as well as aspartate aminotransferase (AST) and alanine aminotransferase (ALT) activity, were performed using the commercially available diagnostic kits supplied by Labtest® (Labtest, MG, Brazil).
2.6. Oxidative stress parameters
2.6.1. Reactive oxygen species (ROS)
ROS formation was determined according to the method employed by Ali et al. [16], with some modifications. In this assay, the oxidation of dichlorodihydrofluorescein diacetate (DCFH-DA) to fluorescent dichlorofluorescein (DCF) was measured for the detection of intracellular ROS. ROS levels are expressed as µmol DCF/mg protein.
2.6.2. Thiobarbituric acid-reactive substances (TBARS)
For the determination of lipid peroxidation, TBARS levels were measured according to the method described by Ohkawa et al. [17] and reported as nmol TBARS/mg protein.
2.6.3. Total thiol content assay
This assay was performed as described by Aksenov and Markesbery [18]. The method is based on the reduction of DTNB by thiols, which, in turn, become oxidized (disulfide), generating a yellow derivative (TNB) the absorption of which is measured spectrophotometrically at 412 nm. Results are reported as nmol TNB/mg of protein.
2.6.4. Superoxide dismutase (SOD) activity
SOD activity was measured by the method described by Misra and Fridovich [19] and the specific activity is reported as units/mg protein.
2.6.5. Catalase (CAT) activity
CAT activity was assayed according to the method described by Aebi [20] based on the decomposition of H2O2 monitored at 240 nm at ambient temperature. The specific activity is reported as units/mg protein.
2.6.6. Glutathione peroxidase (GPx) activity
GPx activity was measured using a commercial kit (RANSEL®; Randox Lab, Antrim, Northern Ireland). The specific activity is reported as units/mg protein.
2.6.7. Aminolevulinic acid dehydratase (ALA-D) activity
ALA-D activity was assayed according to the method of Sassa [21] by measuring the rate of porphobilinogen (PBG) formation and data are expressed as nmol PBG/h/mg protein.
2.6.8. Protein determination
Protein was determined by the method of Lowry et al. [22] using bovine serum albumin as standard.
2.7. Statistical analysis
Statistical analysis was performed using the software GraphPad Prism 5.0 (GraphPad Software, San Diego, CA, U.S.A.). Glucose tolerance was analyzed by repeated-measures ANOVA and Bonferroni’s post hoc test. Parametric variables were tested by one-way ANOVA and Bonferroni post hoc test, with P < 0.05 considered to represent a significant difference in the analysis.
3. Results
The total phytochemical determination of E. uniflora and P. cattleianum extracts is summarized in Table 1.
Table 1. Phytochemical characterization of E. uniflora and P. cattleianum fruit extracts.
| Total phenolic | Flavonoid | Anthocyanin | |
|---|---|---|---|
| E. uniflora | 7.92 ± 0.23 | 5.50 ± 0.68 | 1.72 ± 0.05 |
| P. cattleianum | 16.72 ± 0.26 | 15.24 ± 2.09 | 2.48 ± 0.09 |
Data are expressed as mean ± S.D. Total phenolic content is reported as mg of gallic acid/g of dried extract. Flavonoid content is reported as mg of rutin/g of dried extract. Anthocyanins are expressed as mg of cyanidin-3-glucoside/g of dried extract.
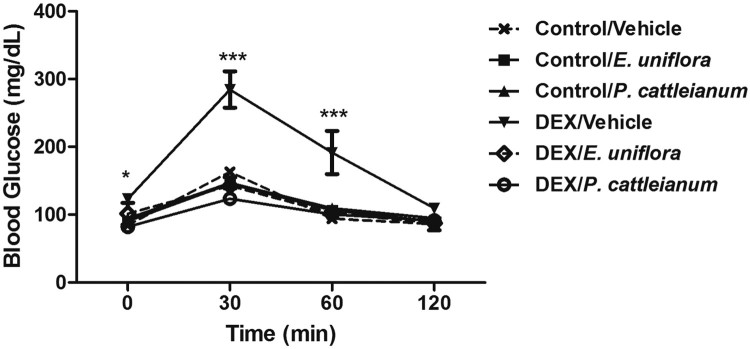
As shown in Figure 1, DEX impaired glucose tolerance (P < 0.01) and treatment with E. uniflora and P. cattleianum extracts were found to prevent this change.
Figure 1.
Glucose tolerance test performed at baseline (0) and at 30, 60, and 120 minutes after glucose injection (2 mg/g body weight). Data are expressed as mean ± SEM. Repeated-measures ANOVA followed by Bonferroni post hoc test; *P < 0.05 and ***P < 0.001 vs. control/vehicle. DEX: dexamethasone.
The metabolic parameters were measured after treatment with the extracts. Table 2 shows that there was a reduction of weight gain [F(5,39) = 12.84, P < 0.001], as well as relative pancreatic weight in the DEX/vehicle group [F(5,26) = 11.80, P < 0.001]. However, the extracts did not prevent these alterations. With respect to serum biochemical parameters, the DEX group showed an increase in glucose [F(5,36) = 10.08, P < 0.001] and triacylglycerol level [F(5,19) = 104.7, P < 0.001] compared with the control group; treatment with E. uniflora and P. cattleianum prevented these changes. No difference was observed in total cholesterol level [F(5,38) = 1.59, P > 0.05] and AST [F(5,27) = 1.48, P > 0.05], and ALT activities [F(5,38) = 1.58, P > 0.05].
Table 2. Effect of E. uniflora and P. cattleianum fruit extract treatment on metabolic parameters of control and dexamethasone-treated rats.
| Control/vehicle | Control/ E. uniflora | Control/ P. cattleianum | DEX/Vehicle | DEX/ E. uniflora | DEX/P. cattleianum | |
|---|---|---|---|---|---|---|
| Weight gain (g) | 103.7 ± 9.6 | 103.0 ± 3.9 | 88.0 ± 5.5 | 50.2 ± 4.7*** | 59.5 ± 10.3** | 50.4 ± 5.45*** |
| Relative pancreatic weight (g/100 g b.w.) | 0.75 ± 0.05 | 0.64 ± 0.04 | 0.61 ± 0.02 | 0.50 ± 0.03*** | 0.46 ± 0.02*** | 0.49 ± 0.03*** |
| Glucose (mg/dL) | 102.27 ± 8.93 | 96.30 ± 4.53 | 107.79 ± 2.74 | 156.55 ± 8.20*** | 102.36 ± 6.05### | 104.23 ± 7.64### |
| Total cholesterol (mg/dL) | 155.42 ± 7.18 | 152.0 ± 8.57 | 157.11 ± 4.76 | 142.57 ± 1.61 | 141.2 ± 3.04 | 145.38 ± 2.20 |
| Triacylglycerol (mg/dL) | 63.19 ± 2.88 | 68.83 ± 3.49 | 74.07 ± 2.67 | 177.40 ± 3.57*** | 91.67 ± 4.81***,### | 93.98 ± 2.84***,### |
| AST (U/L) | 138.5 ± 3.5 | 166.4 ± 11.4 | 148.4 ± 14.6 | 127.6 ± 20.5 | 105.8 ± 6.16 | 160.0 ± 27.6 |
| ALT (U/L) | 36.2 ± 2.6 | 38.4 ± 3.9 | 42.0 ± 3.2 | 31.7 ± 4.2 | 26.7 ± 4.5 | 33.5 ± 4.1 |
Data are expressed as mean ± S.E.M. (n = 4–8). ***P < 0.001 as compared to the control/vehicle. **P < 0.01 as compared to control/vehicle. ###P < 0.001 as compared to DEX/vehicle. One-way ANOVA followed by Bonferroni post hoc test. DEX: dexamethasone. AST: aspartate aminotransferase; ALT: alanine aminotransferase.
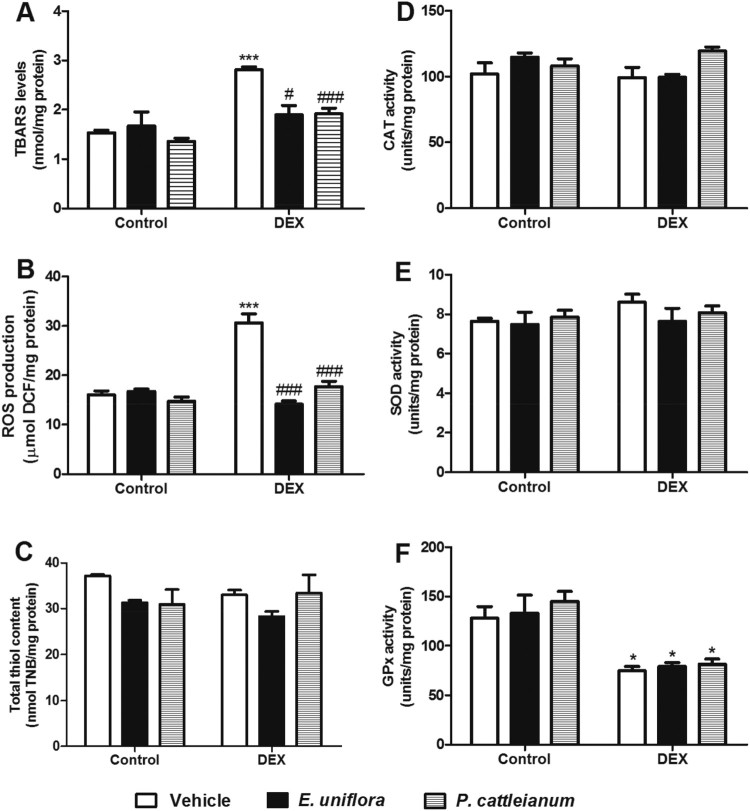
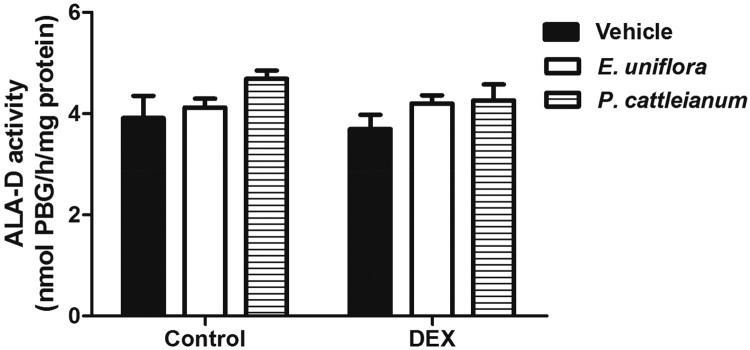
The oxidative stress parameters evaluated in the liver of rats treated with E. uniflora and P. cattleianum are shown in Figure 2. The TBARS values [F(5,19) = 10.02, P < 0.001] and ROS production [F(5,23) = 26.78, P < 0.001] were enhanced by DEX administration and treatment with E. uniflora and P. cattleianum extracts prevented these effects. GPx activity was reduced in the DEX group compared with control values [F(5,18) = 9.78, P < 0.001]. However, sulfhydryl (SH) content [F(5,19) = 2.51, P > 0.05] and the enzymatic activities of SOD [F(5,18) = 0.81, P > 0.05] and CAT [F(5,18) = 2.27, P > 0.05] were not significantly different in any group. Furthermore, ALA-D activity was altered neither by fruit extracts, nor by DEX treatment [F(5,18) = 1.51, P > 0.05] (Figure 3).
Figure 2.
Effect of E. uniflora and P. cattleianum fruit extract treatment on oxidative stress parameters in the liver of control and dexamethasone-treated rats. Data are expressed as mean ± SEM (n = 4–5). One-way ANOVA followed by Bonferroni post hoc test; ***P < 0.001 and *P < 0.05 vs. control/vehicle; ###P < 0.001 and #P < 0.05 vs. DEX/vehicle. TBARS: thiobarbituric acid-reactive substances; ROS: reactive oxygen species; CAT: catalase; SOD: superoxide dismutase; GPx: glutathione peroxidase; DEX: dexamethasone.
Figure 3.
Effect of E. uniflora and P. cattleianum fruit extract treatment on aminolevulinic acid dehydratase (ALA-D) activity in the liver of control and dexamethasone-treated rats. Data are expressed as mean ± SEM (n = 4). No statistically significant difference was found between groups (one-way ANOVA followed by Bonferroni post hoc test). DEX: dexamethasone.
4. Discussion
Health benefits of dietary flavonoids, including the management of MetS, obesity, and diabetes mellitus, are widely known [23]. In our study, we demonstrated that E. uniflora and P. cattleianum contain potentially important phenolic compounds, mainly anthocyanins. The total phenolic and flavonoid contents of small fruits, such as blackberries, blueberries, and strawberries, are 100–820 and 14–290 mg/100 g, respectively [24]. These compounds may have various antidiabetic effects such as modification of glucagon-like peptide-1 (GLP-1), alteration of peroxisome proliferator-activated receptor (PPAR) activities, protection against glucolipotoxicity, and modification of endogenous antioxidants [25]. Khoo and collaborators [26] demonstrated that an extract of Canarium odontophyllum, a fruit rich in anthocyanins, inhibited oxidative stress, further inhibited binding of low-density lipoprotein to endothelial cells, and showed protective effect against lipid peroxidation.
In addition, anthocyanins have been reported to enhance the endogenous antioxidant defense system [25]. The purified anthocyanin C3G increased glutathione (i.e. antioxidant) synthesis in the liver of diabetic db/db mice through upregulation of glutamate–cysteine ligase catalytic subunit expression and enhanced serum levels of SOD and CAT after injections of pelargonidin (i.e. anthocyanidin) in streptozotocin-induced diabetic rats [27,28]. Another study demonstrated that C3G reduced cell death in pancreatic β-cells induced by oxidative stress, acting as a protective factor against diabetes [29]. Schumacher et al. [7] demonstrated that E. uniflora aqueous extract reduced the incidence of T2D, inflammatory cell infiltration, and oxidative stress, as well as increased hepatic glutathione levels and serum insulin, in non-obese diabetic mice. These results may be associated with phenolic compounds (gallic acid, rutin, and ellagic acid) identified using chromatographic analyses of the extract used.
The consumption of fatty food and a sedentary lifestyle, as well as the use of glucocorticoids (including DEX), have been associated with IR. Glucocorticoids are widely used in the treatment of allergies, asthma, and rheumatoid arthritis, among other pathologies, and their chronic use is associated with various negative effects in the metabolism of carbohydrates, causing IR, glucose intolerance, T2D, and dyslipidemia [15,30].
In this study, we evaluated the effects of two extracts from fruits of the Myrtaceae family on metabolic and oxidative stress parameters in an animal model of IR induced by DEX. This experimental model is considered suitable for the investigation of drugs influencing the mechanisms involved in the pathogenesis of T2D and MetS. The relation between DEX and IR can be explained by an increase in gene expression, mainly of FKBP5, which decreases glucose uptake [31]. In addition, Dionísio et al. [32] showed that DEX decreases IRS-1, AKT levels, and GLUT-4 translocation to the membrane. Another possible explanation for this relationship is based on reduction in β-cell mass pancreatic in rats [33] and in humans [34], which may be a cofactor for the development of T2D, directly related to the insulin secretion. This decrease may occur due to the continuous apoptosis caused by several factors, including excessive production of ROS, resulting in loss of β-cells and decreased function of these cells [35,36]. Similarly, in our study, we verified a reduction of relative pancreatic weight in rats that received DEX.
It is well known that IR can induce dyslipidemia; triacylglycerol (TAG), total cholesterol (TC), and high-density lipoprotein (HDL) can, therefore, be used as markers of IR. Nevertheless, hypertriglyceridemia is the most common feature of dyslipidemia in cases of IR and T2D. In our study, we demonstrated that serum levels of TAG increased in animals treated with DEX and both the extracts prevented this increase. In this context, several studies have reported a correlation between this lipid and IR based on the compensatory hyperinsulinemia caused by IR, which induces the flow of free fatty acid to the liver and adipose tissue, providing a substrate for TAG production [37,38]. This could also be attributable to the ability of DEX to increase gluconeogenesis, downregulate GLUT2 expression on the cell membrane, and increase TAG and VLDL through activation of the expression of several genes encoding enzymes in TAG synthesis; this causes lipid redistribution along with lipolysis in adipocytes as well as TAG accumulation in the liver [39].
Mitochondrion plays a crucial role in DEX-induced oxidative stress. DEX may lead to an increase in ROS production that directly causes mitochondrial dysfunction, reduces cellular energy yield, promotes the release of cytochrome C, decreases mitochondrial permeability, and leads to oxidative stress and apoptosis [40]. In our study, administration of DEX did not alter SH content, nor did it affect the activity of CAT, SOD, or ALA-D. However, it reduced GPx activity, caused lipid peroxidation, and increased production of ROS, indicating a dysregulation of redox homeostasis in the liver of these rats. These findings are important since the oxidative damage plays a critical role in the pathogenesis of obesity, atherosclerosis, T2D, and IR. The major end-product of lipid peroxidation 4-hydroxynonenal may induce the IR by inactivating critical components of the insulin signaling pathway [41]. Additionally, we demonstrated that E. uniflora and P. cattleianum fruit extracts prevented lipid peroxidation and ROS formation in the liver, suggesting an important antioxidant action of these extracts in the experimental model of IR.
In conclusion, our results suggest that E. uniflora and P. cattleianum extracts prevent the hyperglycemia and hypertriglyceridemia caused by DEX-induced IR and exert an antioxidant action by preventing the formation of ROS and TBARS in the liver.
Funding Statement
The Brazilian research funding agencies FAPERGS, CAPES, and CNPq supported this study.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Juliane de Souza Cardoso http://orcid.org/0000-0002-2726-4159
Vanessa Plasse Ramos http://orcid.org/0000-0003-0844-2762
References
- 1.Yu J, Zheng J, Liu XF, et al. . Exercise improved lipid metabolism and insulin sensitivity in rats fed a high-fat diet by regulating glucose transporter 4 (GLUT4) and musclin expression. Braz J Med Biol Res. 2016;49:1414–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown AE, Walker M.. Genetics of insulin resistance and the metabolic syndrome. Curr Cardiol Rep. 2016;18:1–8. doi: 10.1007/s11886-016-0755-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Capeau J.Insulin resistance and steatosis in humans. Diabetes Metab. 2008;34:649–657. doi: 10.1016/S1262-3636(08)74600-7 [DOI] [PubMed] [Google Scholar]
- 4.Hagve M, Gjessing PF, Fuskevag OM, et al. . Skeletal muscle mitochondria exhibit decreased pyruvate oxidation capacity and increased ROS emission during surgery-induced acute insulin resistance. Am J Physiol Endoc M. 2015;308:E613–E620. [DOI] [PubMed] [Google Scholar]
- 5.Matsuzawa-Nagata N, Takamura T, Ando H, et al. . Increased oxidative stress precedes the onset of high-fat diet-induced insulin resistance and obesity. Metab Clin Exp. 2008;57:1071–1077. doi: 10.1016/j.metabol.2008.03.010 [DOI] [PubMed] [Google Scholar]
- 6.Rahal A, Kumar A, Singh V, et al. . Oxidative stress, prooxidants, and antioxidants: The interplay. Biomed Res Int. 2014;2014:1–19. doi: 10.1155/2014/761264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schumacher NSG, Colomeu TC, Figueiredo D, et al. . Identification and antioxidant activity of the extracts of Eugenia uniflora leaves. characterization of the anti-inflammatory properties of aqueous extract on diabetes expression in an experimental model of spontaneous type 1 diabetes (NOD mice). Antioxidants. 2015;4:662–680. doi: 10.3390/antiox4040662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Figuerôa EO, Silva LCN, Melo CML, et al. . Evaluation of antioxidant, immunomodulatory, and cytotoxic action of fractions from Eugenia uniflora L. and Eugenia malaccensis L.: correlation with polyphenol and flavonoid content. Scientific World J. 2013;2013:1–7. doi: 10.1155/2013/125027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scur MC, Pinto FGS, Pandini JA, et al. . Antimicrobial and antioxidant activity of essential oil and different plant extracts of psidium cattleianum sabine. Braz J Biol. 2016;76:101–108. doi: 10.1590/1519-6984.13714 [DOI] [PubMed] [Google Scholar]
- 10.Bordignon JRCL, Francescatto V, Nienow AA, et al. . Influência do pH da solução extrativa no teor de antocianinas em frutos de morango. Ciência e Tecnologia de Alimentos. 2009;29:183–188. doi: 10.1590/S0101-20612009000100028 [DOI] [Google Scholar]
- 11.Singleton VL, Orthefer R, Lamuela-Ranventós R.. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteau reagent. Methods Enzymol. 1999;299:152–178. doi: 10.1016/S0076-6879(99)99017-1 [DOI] [Google Scholar]
- 12.Miliauskas G, Venskutonis PR, Van TA.. Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chem. 2004;85:231–237. doi: 10.1016/j.foodchem.2003.05.007 [DOI] [Google Scholar]
- 13.Lee J, Durst RW, Wrolstad RE.. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants and wines by the pH differential method: collaborative study. J AOAC Int. 2005;8:1269–1278. [PubMed] [Google Scholar]
- 14.Oliveira PS, Gazal M, Flores NP, et al. . Vaccinium virgatum fruit extract as an important adjuvant in biochemical and behavioral alterations observed in animal model of metabolic syndrome. Biomed Pharmacother. 2017;88:939–947. doi: 10.1016/j.biopha.2017.01.121 [DOI] [PubMed] [Google Scholar]
- 15.Rafacho A, Gonçalves-Neto LM, Santos-Silva JC, et al. . Pancreatic alpha-cell dysfunction contributes to the disruption of glucose homeostasis and compensatory insulin hypersecretion in glucocorticoid-treated rats. Plos One. 2014;9:e93531. doi: 10.1371/journal.pone.0093531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ali SF, Lebel CP, Bond SC.. Reactive oxygen species formation as a biomarker of methylmercury and trimethyltin neurotoxicity. Neurotoxicology. 1992;13:637–648. [PubMed] [Google Scholar]
- 17.Ohkawa H, Ohishi N, Yagi K.. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Annual Rev Med. 1979;95:351–358. [DOI] [PubMed] [Google Scholar]
- 18.Aksenov MY, Markesbery WR.. Changes in thiol content and expression of glutathione redox system genes on the hippocampus and cerebellum in Alzheimeŕs disease. Neurosci Lett. 2001;302:141–145. doi: 10.1016/S0304-3940(01)01636-6 [DOI] [PubMed] [Google Scholar]
- 19.Misra HP, Fridovich I.. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170–3175. [PubMed] [Google Scholar]
- 20.Aebi H.Catalase in vitro. Meth Enzymol. 1984;105:121–126. doi: 10.1016/S0076-6879(84)05016-3 [DOI] [PubMed] [Google Scholar]
- 21.Sassa S.Delta-aminolevulinic acid dehydratase assay. Enzyme. 1982;28:133–145. doi: 10.1159/000459097 [DOI] [PubMed] [Google Scholar]
- 22.Lowry OH, Rosebrough NJ, Farr AL, et al. . Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 23.Vinayagam R, Xu B.. Antidiabetic properties of dietary flavonoids: a cellular mechanism review. Nutr Metab. 2015;12:1–20. doi: 10.1186/s12986-015-0057-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Biegelmeyer R, Andrade JMM, Aboy AL, et al. . Comparative analysis of the chemical composition and antioxidant activity of red (Psidium cattleianum) and yellow (psidium cattleianum var. lucidum) strawberry guava fruit. J. Food Sci. 2011;76:C991-C996. doi: 10.1111/j.1750-3841.2011.02319.x [DOI] [PubMed] [Google Scholar]
- 25.Stull AJ.Blueberries’ impact on insulin resistance and glucose intolerance. Antioxidants. 2016;5:1–11. doi: 10.3390/antiox5040044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khoo HE, Azlan A, Ismail A, et al. . Inhibition of oxidative stress and lipid peroxidation by anthocyanins from defatted Canarium odontophyllum pericarp and peel using in vitro bioassays. Plos One. 2014;e81447. doi: 10.1371/journal.pone.0081447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu W, Jia Q, Wang Y, et al. . The anthocyanin cyanidin-3-O-glucoside, a flavonoid, increases hepatic glutathione synthesis and protects hepatocytes against reactive oxygen species during hyperglycemia: involvement of a cAMP-PKA-dependent signaling pathway. Free Radical Biol Med. 2012;52:314–327. doi: 10.1016/j.freeradbiomed.2011.10.483 [DOI] [PubMed] [Google Scholar]
- 28.Roy M, Sen S, Chakraborti AS.. Action of pelargonidin on hyperglycemia and oxidative damage in diabetic rats: implication for glycation-induced hemoglobin modification. Life Sci. 2008;82:1102–1110. doi: 10.1016/j.lfs.2008.03.011 [DOI] [PubMed] [Google Scholar]
- 29.Lee JS, Kim YR, Song IG, et al. . Cyanidin-3-glucoside isolated from mulberry fruit protects pancreatic β-cells against oxidative stress-induced apoptosis. Int J Mol Med. 2015;35:405–412. doi: 10.3892/ijmm.2014.2013 [DOI] [PubMed] [Google Scholar]
- 30.Bighetti BB, Assis GF, Vieira DC, et al. . Long-term dexamethasone treatment alters the histomorphology of acinar cells in rat parotid and submandibular glands. Int J Exp Pathol. 2014;95:351–363. doi: 10.1111/iep.12092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pereira MJ, Palming J, Svensson MK, et al. . FKBP5 expression in human adipose tissue increases following dexamethasone exposure and is associated with insulin resistance. Metab Clin Exp. 2014;63:1198–1208. doi: 10.1016/j.metabol.2014.05.015 [DOI] [PubMed] [Google Scholar]
- 32.Dionísio TJ, Louzada JCA, Viscelli BA, et al. . Aerobic training prevents dexamethasone-induced peripheral insulin resistance. Endocr Res. 2014;46:484–489. [DOI] [PubMed] [Google Scholar]
- 33.Shen C-N, Seckl JR, Slack JMW, et al. . Glucocorticoids suppress β-cell development and induce hepatic metaplasia in embryonic pancreas. Biochem Soc. 2003;375:41–50. doi: 10.1042/bj20030140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Henquin JC, Rahier J.. Pancreatic alpha cell mass in European subjects with type 2 diabetes. Diabetologia. 2011;54:1720–1725. doi: 10.1007/s00125-011-2118-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gesina E, Tronche F, Herrera P, et al. . Dissecting the role of glucocorticoids on pancreas development. Diabetes. 2004;53:2322–2329. doi: 10.2337/diabetes.53.9.2322 [DOI] [PubMed] [Google Scholar]
- 36.Meier JJ, Bonadonna RC.. Role of reduced b-cell mass versus impaired b- cell function in the pathogenesis of type 2 diabetes. Diabetes Pathophysiol. 2013;36:113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li N, Fu J, Koonen DP, et al. . Are hypertriglyceridemia and low HDL causal factors in the development of insulin resistance? Atherosclerosis. 2014;233:130–138. doi: 10.1016/j.atherosclerosis.2013.12.013 [DOI] [PubMed] [Google Scholar]
- 38.Park SY, Cho YJ, Lee SR, et al. . Triglyceride is a useful surrogate marker for insulin resistance in Korean women with polycystic ovary syndrome. Yonsei Med J. 2015;56:785–792. doi: 10.3349/ymj.2015.56.3.785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tovchiga OV.The influence of goutweed (Aegopodium podagraria L.) tincture and metformin on the carbohydrate and lipid metabolism in dexamethasone-treated rats. BMC Complement Altern Med. 2016;16:1–11. doi: 10.1186/s12906-016-1221-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yi J, Zhu R, Wu J, et al. . In vivo protective effect of betulinic acid on dexamethasone induced thymocyte apoptosis by reducing oxidative stress. Pharmacol Rep. 2016;68:95–100. doi: 10.1016/j.pharep.2015.07.003 [DOI] [PubMed] [Google Scholar]
- 41.Murdolo G, Piroddi M, Luchetti F, et al. . Oxidative stress and lipid peroxidation by-products at the crossroad between adipose organ dysregulation and obesity-linked insulin resistance. Biochimie. 2013;95:585–594. doi: 10.1016/j.biochi.2012.12.014 [DOI] [PubMed] [Google Scholar]