Abstract
Background
Physical function declines during hospitalization in geriatric patients, increasing the risk of loss of independence. There is a need for evidence-based, pragmatic interventions to improve functional recovery of older adults following acute hospitalization. Here, we report the results of a Phase I randomized clinical trial designed to determine safety and effect size of protein supplementation, exercise, and testosterone interventions on 30-day post-discharge functional recovery and readmissions in geriatric patients.
Methods
A total of 100 patients admitted to the University of Texas Medical Branch hospital for an acute medical illness were randomized to one of five intervention groups: isocaloric placebo, whey protein supplement, in-home rehabilitation + placebo, in-home rehabilitation + whey protein, or testosterone. Primary outcome measure was the change from baseline in short physical performance battery score at 1 and 4 weeks post-discharge. Secondary outcomes were changes in body composition, activities of daily living, and 30-day readmissions. Comparisons were made across study groups and between placebo and all active intervention groups.
Results
Four weeks post-discharge, the short physical performance battery total score and balance score increased more in active intervention groups than placebo group (p < .05). There were no significant differences in change in body composition or activities of daily living across groups or between active intervention groups and placebo group. Readmission rates were highest in placebo (28%), followed by rehabilitation + placebo (15%), whey protein (12%), rehabilitation + whey protein (11%), and testosterone (5%). There was a trend for lower readmission rates in all active intervention groups (11%) versus placebo group (28%).
Conclusions
Findings from this Phase I clinical trial suggest that pragmatic, evidence-based interventions may accelerate recovery from acute hospitalization in geriatric patients. These data provide essential information to design larger randomized controlled trials to test the effectiveness of these interventions.
Keywords: Protein, Rehabilitation, Testosterone, Hospitalization
Older adults admitted to the hospital often experience a significant reduction in physical function (1,2). Functional decline leads to loss of independence and nursing home placement (3). Hospital-associated functional loss has been attributed to several factors occurring during the hospital stay, including physical inactivity, malnutrition, and polypharmacy (4–10). These factors contribute to muscle dysfunction, increased fall risk, and loss of independence (11–13). The inability to recover function after hospitalization also increases the risk of hospital readmission and mortality (3).
Evidence-based strategies to improve recovery in acutely ill geriatric patients are lacking. Protein supplementation, exercise, and testosterone have been identified as potential means to improve physical functioning in healthy older adults as they stimulate muscle anabolism and can increase function (14–16). The effect of these interventions may be larger in malnourished, inactive, and ill older patients than in healthy, independent, and protein replete older adults. However, studies of nutritional or exercise interventions in hospitalized older patients have so far focused on select diseases or conditions, and their results are not generalizable to the broader complex inpatient geriatric population.
We have designed the current Phase I randomized clinical trial to test if protein supplementation, intensive physical therapy, or testosterone interventions could be feasible after hospital discharge and determine their safety and effect size in improving the patients’ 30-day post-hospital functional recovery. We have previously reported that these interventions are feasible and acceptable in geriatric patients after a hospital admission for an acute medical condition (17). Here, we report the safety and effect size of post-hospital protein supplementation, intensive physical therapy, and testosterone supplementation on physical function, body composition, and 30-day readmission rates. The goal of this study was to inform the design of larger, multisite, pragmatic clinical trials aimed at determining the effectiveness of these interventions on post-hospital outcomes in geriatric patients.
Methods
This study was approved by the University of Texas Medical Branch (UTMB) Institutional Review Board and is registered at ClinicalTrials.gov (NCT02203656). Written informed consent was obtained from each participant before any study procedure. All data were collected from a single university hospital. Design, methods, and adherence to the intervention were previously published (17,18).
Participants
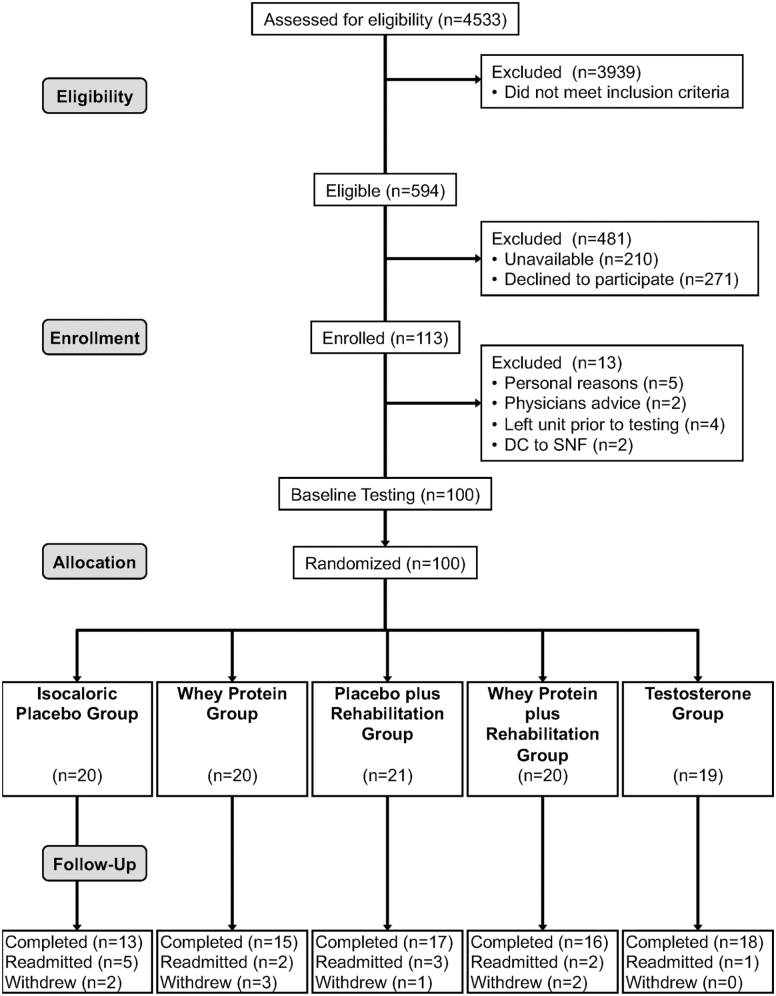
Over 3 years, we chart-screened 4,553 and enrolled 100 patients aged at least 65 years admitted to the UTMB hospital for an acute medical illness (Figure 1). Inclusion and exclusion criteria are reported in Figure 1. Enrolled patients were consented, stratified by gender, and randomized into one of five post-hospital interventions: (i) whey protein supplementation, (ii) in-home rehabilitation with placebo supplementation, (iii) in-home rehabilitation with protein supplementation, (iv) single testosterone injection, or (v) isocaloric placebo supplementation. Participants followed the protocol for their intervention for 4 weeks (27–33 days) after discharge unless readmitted to the hospital or withdrawing from the study.
Figure 1.
CONSORT flow diagram. We assessed for eligibility (chart screening) 4,533 patients admitted to the University of Texas Medical Branch hospital with an acute onset medical disease or condition. Inclusion criteria were age at least 65 years, residing at home before and after hospitalization, ability to provide informed consent, self-reported ability to walk across a small room (with or without an assistive device) 2 weeks before hospitalization, ability to stand independently at baseline testing, no medical contraindication to wearing a step activity monitor on one ankle. Exclusion criteria for all patients were residing at a nursing home, hospice, uncontrolled blood pressure, history of stroke with motor disability, renal insufficiency, aspartate transaminase/alanine aminotransferase two times above the normal limit or hyperbilirubinemia, treatment with anabolic steroids within 3 months, planned/elective hospitalization within 30 days of discharge, cognitive dysfunction including delirium, living more than 30 miles from the hospital. Additional exclusion criteria for the testosterone arm were history of breast or prostate cancer, palpable prostate nodule or induration or prostate specific antigen at least 4 ng/ml (≥ 3 ng/ml in men at high risk of prostate cancer), hematocrit at least 50%, or decompensated heart failure.
Intervention Groups
Whey protein supplements consisted of 20 g whey protein (22 g BiPro; Eden Prairie, MN). Placebo supplements, 20 g maltodextrin, were isocaloric to the whey protein supplement. Supplements were provided in single-dose containers. All individual supplement doses contained one single-serve packet of a sugar-free drink mix to mask flavor and color. Participants were instructed to take one supplement dose twice daily, morning and evening, after mixing it with 8 oz of water.
Participants randomized to the rehabilitation groups took whey or placebo supplements as described above and underwent a progressive in-home rehabilitation training program 3 days per week for 4 weeks. The experimental rehabilitation program was added to and scheduled whenever possible on different days than any physical and/or occupational therapy ordered by the treating provider. The program was prescribed and overseen by a physical therapist and supervised one to two times per week by research staff. The exercises included chair rises, toe stands, and three exercises using TheraBands including seated knee extensions, seated rows, and seated arm extensions.
Participants randomized to the testosterone group received a single dose of testosterone enanthate (men: 200 mg; women: 100 mg) as an intramuscular injection within 24 hours of discharge. Previous studies by UTMB and other investigators have demonstrated the safety of weekly testosterone injections in older men and women (17,19). We did not test the combination of testosterone plus exercise and/or nutrition to contain the scope of this pilot investigation due to the complete lack of data on the feasibility and effect size of a testosterone single injection in this patient population.
Intervention adherence was previously published in detail (17). Briefly, adherence was adequate across all arms. The overall mean supplement adherence score was 75%, the rehabilitation adherence score was 77%, and adherence to testosterone injection was 100% (17).
Outcome Measures
The aim of this study was to determine the interventions’ safety, effect size, and variability on physical function in community-dwelling older adults discharged home after hospitalization for a medical illness. The primary outcome measure was the short physical performance battery (SPPB). Secondary outcome measures were body composition, activities of daily living, and 30-day readmissions. Functional testing was conducted at baseline (during hospitalization), 1 week, and 4 weeks post-discharge.
The SPPB consists of three tests of lower body function: a short, timed walk at usual gait speed, five repeated chair stands, and a standing balance exercise (20,21). To measure usual gait speed, participants were asked to walk (using an assistive device if needed) at their normal pace. This test was repeated and the quickest time used for the analysis. To determine the safety of the repeated chair stands, participants were first asked to fold their arms across their chest and stand up once from a straight-backed, regular-height chair. If successful, they were asked to stand up from the chair and sit back down five times as quickly as possible. The five chair rises were timed from the initial sitting position to the final standing position at the end of the fifth stand. To measure balance, participants were asked to hold three standing positions (side-by-side, semi-tandem, and tandem) for 10 seconds each. SPPB total score was calculated by summing each component score (0–4 points for gait, chair rise, and balance) for a range of 0–12 total point.
Body composition was measured at baseline and at 4 week post-discharge testing time points. Appendicular and whole-body composition were determined by dual-energy X-ray absorptiometry (DEXA, GE Lunar iDXA). The coefficient of variation for repeated measures of lean tissue is less than 1% (22).
Activities of daily living (ADLs) and instrumental activities of daily living (IADLs) were determined at all testing time points. ADL consisted of bathing, using the toilet, transferring from bed to chair, walking across a small room, personal grooming, dressing, and eating (13). Scoring was 0–7 points with a higher score for participants requiring more help with ADLs. IADL consisted of using the telephone, driving a car, shopping, cooking, cleaning, taking medication, handling money, climbing stairs, and walking ½ mile. Scoring was 0–10 points with a lower score, indicating participants needing more help with IADLs.
Readmission within 30 days of discharge was determined by staff communication with the patients/caregivers and adjudicated through review of electronic medical records. Doctors’ appointments and emergency room visits without admission to the hospital were not considered readmissions. Withdrawal from the study was considered a hard endpoint and those participants were not included in readmission analysis.
Statistical Analyses
All statistical analyses were performed using SAS (version 9.2; SAS Inst. Inc., Cary, NC). A p-value of less than .05 was considered statistically significant. Comparisons were made across study groups (one-way analysis of variance), between placebo and all active intervention groups (AIG; independent sample t-test), and with intention-to-treat analysis (carrying the most recent outcome values forward). Sample sizes necessary to detect an observed effect with an alpha equal to .05 and beta equal to .8 were calculated for our primary outcome, the SPPB. All comparisons were analyzed using both parametric and nonparametric statistical tests. Because the results of the two sets of analyses were very similar, we presented only the parametric results. Two-sided p-value of less than .05 was considered statistically significant. Data are reported as the mean (standard deviation).
Results
Baseline Characteristics
Patients admitted to UTMB (n = 4,533) were chart-screened from January 2014 to July 2016. Of these patients, 13.1% were eligible for study participation (Figure 1). Of the eligible patients, 35.3% were unavailable at the time of approach due to their diagnostic or treatment plan. Approximately 29% approached patients agreed to participate and were enrolled (n = 113). However, 13 participants were not randomized for the following reasons: personal reasons (n = 5), treating physician’s advice based on the nature their diagnosis (n = 2), discharged or transferred from the unit before the completion of testing (n = 4), and discharged to skilled nursing facilities (n = 2).
Participants (n = 100) randomized into this study (Table 1) were on average 78.1 years old (SD = 8.0), 70% female, and 74% white (11% Hispanic, 13% black, and 2% other). They were highly educated with 93% having a high school diploma and 53% having a college degree. Average SPPB total score was 6.9 (SD = 3.3), indicating functional limitation. Seventy-four percent of the cohort had a SPPB score less than 10. Ten percent required assistance with at least one ADL and 58% required assistance with at least one IADL. Discharge diagnoses were not significantly different across intervention groups. Most common diagnoses were 28% cardiovascular complications (atrial fibrillation, hypotension, peripheral arterial disease, congestive heart failure exacerbation, etc.); 23% pulmonary (chronic obstructive pulmonary disease exacerbation, pulmonary embolism, pneumonia, etc.); 22% gastrointestinal (gastrointestinal bleed, diverticulitis, gastroenteritis, pancreatitis, etc.). Average Charlson Comorbidity Index score was 5.3 (SD = 1.5) and average length of stay was 3.1 days (SD = 2.0). There were no differences across groups in demographic factors or clinical characteristics.
Table 1.
Baseline Characteristics of Participants
| Variable | All sample (n = 100) | Placebo (n = 20) | Whey protein (n = 20) | Rehabilitation + placebo (n = 21) | Rehabilitation + protein (n = 20) | Testosterone (n = 19) | All active intervention groups (n = 80) |
|---|---|---|---|---|---|---|---|
| Age, y | 78.08 ± 7.95 | 75.70 ± 7.12 | 80.00 ± 8.65 | 77.57 ± 7.53 | 80.00 ± 8.77 | 77.11 ± 7.42 | 78.68 ± 7.95 |
| Female | 70 (70) | 14 (70) | 14 (70) | 14 (67) | 14 (70) | 14 (74) | 56 (70) |
| Education, y | 13.44 ± 2.71 | 12.60 ± 2.39 | 13.70 ± 2.00 | 13.67 ± 1.93 | 13.45 ± 2.87 | 13.79 ± 4.04 | 13.65 ± 2.71 |
| Middle school degree | 7 (7) | 2 (10) | 0 (0) | 0 (0) | 2 (10) | 3 (16) | 5 (6) |
| High school degree | 40 (40) | 10 (50) | 9 (45) | 9 (42) | 7 (35) | 5 (26) | 30 (38) |
| College degree | 42 (42) | 7 (35) | 11 (55) | 10 (48) | 9 (45) | 5 (26) | 35 (44) |
| Advanced education | 11 (11) | 1 (5) | 0 (0) | 2 (10) | 2 (10) | 6 (32) | 10 (13) |
| Non-Hispanic white | 74 (74) | 12 (60) | 17 (85) | 16 (76) | 18 (90) | 11 (58) | 62 (78) |
| Black | 13 (13) | 5 (25) | 3 (15) | 2 (10) | 1 (5) | 2 (11) | 8 (11) |
| Hispanic | 11 (11) | 3 (15) | 0 (0) | 2 (10) | 1 (5) | 5 (26) | 8 (10) |
| Other | 2 (2) | 0 (0) | 0 (0) | 1 (5) | 0 (0) | 1 (5) | 2 (3) |
| Weight, kg | 74.79 ± 17.49 | 78.61 ± 8.00 | 78.34 ± 16.30 | 73.39 ± 19.03 | 71.42 ± 20.27 | 72.10 ± 13.06 | 73.83 ± 17.49 |
| Height, m | 1.64 ± 8.62 | 1.64 ± 8.99 | 1.65 ± 10.29 | 1.64 ± 7.68 | 1.65 ± 8.58 | 1.64 ± 8.11 | 164.31 ± 8.62 |
| BMI, kg/m2 | 27.68 ± 6.02 | 28.99 ± 5.33 | 28.90 ± 6.43 | 27.38 ± 6.39 | 26.05 ± 6.56 | 27.05 ± 5.28 | 27.35 ± 6.02 |
| DEXA weight, kg | 73.99 ± 17.23 | 77.93 ± 8.74 | 75.24 ± 15.84 | 72.87 ± 18.40 | 72.82 ± 20.70 | 71.44 ± 13.15 | 73.10 ± 17.23 |
| DEXA fat, kg | 29.82 ± 11.05 | 30.99± 11.11 | 30.79 ± 10.19 | 27.93 ± 10.84 | 30.33 ± 13.14 | 29.23 ± 10.89 | 29.56 ± 11.05 |
| DEXA lean mass, kg | 41.87 ± 8.91 | 44.57 ± 9.81 | 42.12 ± 8.89 | 42.67 ± 9.75 | 40.34 ± 9.66 | 39.88 ± 6.36 | 41.27 ± 8.91 |
| DEXA appendicular lean mass, kg | 17.79 ± 4.45 | 19.02 ± 4.97 | 18.30 ± 4.19 | 18.21 ± 4.93 | 16.79 ± 4.74 | 16.72 ± 3.37 | 17.52 ± 4.45 |
| Grip Strength, kg | 18.92 ± 8.63 | 21.39 ± 8.70 | 19.30 ± 7.03 | 18.65 ± 10.17 | 17.20 ± 9.60 | 18.02 ± 7.38 | 18.30 ± 8.63 |
| SPPB total score | 6.93 ± 3.29 | 7.75 ± 3.68 | 6.20 ± 3.09 | 7.14 ± 2.92 | 6.20 ± 3.53 | 7.37 ± 3.22 | 6.73 ± 3.29 |
| SPPB gait speed score | 2.37 ± 1.25 | 2.55 ± 1.15 | 2.05 ± 1.32 | 2.67 ± 1.32 | 2.00 ± 1.17 | 2.58 ± 1.26 | 2.33 ± 1.25 |
| SPPB chair rise score | 1.83 ± 1.60 | 2.30 ± 1.49 | 1.60 ± 1.67 | 1.81 ± 1.50 | 1.50 ± 1.61 | 1.95 ± 1.78 | 1.71 ± 1.60 |
| SPPB balance score | 2.73 ± 1.29 | 2.90 ± 1.41 | 2.55 ± 1.39 | 2.67 ± 1.28 | 2.70 ± 1.22 | 2.84 ± 1.26 | 2.69 ± 1.29 |
| Gait speed, m/s | 0.66 ± 0.28 | 0.64 ± 0.27 | 0.59 ± 0.27 | 0.75 ± 0.30 | 0.56 ± 0.22 | 0.75 ± 0.32 | 0.67 ± 0.28 |
| Mobility aid use | 25 (25) | 6 (30) | 7 (35) | 5 (24) | 3 (15) | 4 (21) | 19.00 (24) |
| None | 75 (75) | 14 (70) | 13 (65) | 16 (76) | 17 (85) | 15 (79) | 61 (76) |
| Cane | 8 (8) | 2 (10) | 2 (10) | 3 (14) | 1 (5) | 0 (0) | 6 (8) |
| Walker | 17 (17) | 4 (20) | 5 (25) | 2 (10) | 2 (10) | 4 (21) | 13 (16) |
| Geriatric Depression Scale score | 3.03 ± 2.60 | 3.50 ± 2.61 | 2.60 ± 1.79 | 2.62 ± 2.52 | 3.80 ± 3.38 | 2.63 ± 2.48 | 2.91 ± 2.60 |
| Normal (<5) | 74 (74) | 14 (70) | 17 (85) | 18 (86) | 12 (60) | 13 (68) | 60 (75) |
| Mild depression (5–8) | 20 (20) | 5 (25) | 3 (15) | 2 (10) | 5 (25) | 5 (26) | 15 (19) |
| Moderate depression(9–11) | 6 (6) | 1 (5) | 0 (0) | 1 (5) | 3 (15) | 1 (5) | 5 (6) |
| Severe depression (12–15) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Any falls in past year | 49 (49) | 11 (55) | 10 (48) | 10 (48) | 10 (50) | 8 (42) | 38 (48) |
| None | 51 (51) | 9 (45) | 10 (50) | 11 (52) | 10 (50) | 11 (58) | 42 (53) |
| One | 22 (22) | 5 (25) | 5 (25) | 5 (24) | 6 (30) | 1 (5) | 17 (21) |
| Two | 14 (14) | 3 (15) | 4 (20) | 3 (14) | 1 (5) | 3 (16) | 11 (14) |
| Three or more | 13 (13) | 3 (15) | 1 (5) | 2 (10) | 3 (15) | 4 (21) | 10 (13) |
| ADL | 0.12 ± 0.38 | 0.10 ± 0.45 | 0.20 ± 0.52 | 0.10 ± 0.30 | 0.10 ± 0.31 | 0.11 ± 0.32 | 0.13 ± 0.38 |
| Independent (0) | 90 (90) | 19 (95) | 17 (85) | 19 (90) | 18 (90) | 17 (89) | 71 (89) |
| Partially dependent (1–2) | 10 (10) | 1 (5) | 3 (15) | 2 (10) | 2 (10) | 2 (11) | 9 (11) |
| Significantly dependent (3–6) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| IADL | 8.46 ± 1.99 | 8.55 ± 2.06 | 8.75 ± 1.45 | 8.05 ± 2.62 | 8.50 ± 2.21 | 8.47 ± 1.39 | 8.44 ± 1.99 |
| Independent (9–10) | 61 (61) | 13 (65) | 14 (70) | 12 (57) | 12 (60) | 10 (53) | 48 (60) |
| Partially dependent (6–8) | 33 (33) | 5 (25) | 6 (30) | 6 (29) | 7 (35) | 9 (47) | 28 (35) |
| Dependent (1–5) | 6 (6) | 2 (10) | 0 (0) | 3 (14) | 1 (5) | 0 (0) | 4 (5) |
| Comorbidity score | 5.28 ± 1.49 | 5.15 ± 1.42 | 5.70 ± 1.45 | 5.10 ± 1.61 | 5.75 ± 1.55 | 4.68 ± 1.25 | 5.31 ± 1.49 |
| Length of stay, d | 3.10 ± 2.05 | 3.55 ± 2.11 | 2.85 ± 2.06 | 2.90 ± 2.26 | 3.40 ± 2.06 | 2.79 ± 1.81 | 2.99 ± 2.05 |
Note: All data are mean ± standard deviation or number of participants (percentage). ADL, activities of daily living; DEXA, dual-energy X-ray absorptiometry; IADL, instrumental activities of daily living; SPBB, short physical performance battery.
Short Physical Performance Battery
Changes in SPPB score 1 and 4 weeks after hospital discharge are reported in Table 2. There were no significant differences in SPPB across groups or between placebo and AIG at baseline. Four weeks post-discharge, SPPB total score (p = .03) and balance score (p = .004) increased more in AIG compared with placebo group. The percentage of participants who had a clinically meaningful improvement in SPPB (≥1 point change) was higher in AIG than placebo group (p = .01). With intention-to-treat analysis, the effect sizes of AIG versus placebo group were larger as more data points were missing in the placebo group due to readmissions. The sample sizes necessary to detect a statistically significant effect in SPPB outcomes are reported in Table 2. A sample size of 64 participants/group would be necessary to detect a clinically meaningful SPPB change with alpha equal to .05 and beta equal to .80.
Table 2.
Improvement in Short Physical Performance Battery (SPPB) Score at Week-1 and Week-4 Testing
| All sample | Placebo (P) | Whey protein (W) | Rehabilitation + placebo (R + P) | Rehabilitation + protein (R + W) | Testosterone (T) | Active intervention groups (AIG) | p-Value (across groups) | p-Value (P vs. AIG) | |
|---|---|---|---|---|---|---|---|---|---|
| Results | |||||||||
| Baseline testing “n” | 100 | 20 | 20 | 21 | 20 | 19 | 80 | ||
| Total score, mean (SD) | 6.93 (3.29) | 7.75 (3.68) | 6.20 (3.09) | 7.14 (2.92) | 6.20 (3.53) | 7.37 (3.22) | 6.73 (3.29) | .46 | .22 |
| Gait speed score, mean (SD) | 2.37 (1.25) | 2.55 (1.15) | 2.05 (1.32) | 2.67 (1.32) | 2.00 (1.17) | 2.58 (1.26) | 2.33 (1.25) | .27 | .48 |
| Balance score, mean (SD) | 1.83 (1.60) | 2.30 (1.49) | 1.60 (1.67) | 1.81 (1.50) | 1.50 (1.61) | 1.95 (1.78) | 1.71 (1.60) | .92 | .51 |
| Chair rise score, mean (SD) | 2.73 (1.29) | 2.90 (1.41) | 2.55 (1.39) | 2.67 (1.28) | 2.70 (1.22) | 2.84 (1.26) | 2.69 (1.29) | .55 | 0.14 |
| Week-1 testing “n” | 93 | 19 | 18 | 19 | 19 | 18 | 74 | ||
| Total score change, mean (SD) | 1.03 (2.30) | 0.84 (1.80) | 1.33 (2.20) | 0.00 (2.26) | 1.05 (2.72) | 2.00 (2.20) | 1.08 (2.30) | .11 | .21 |
| Gait speed score change, mean (SD) | 0.40 (1.07) | 0.37 (0.83) | 0.56 (0.92) | –0.21 (1.36) | 0.58 (1.12) | 0.72 (0.89) | 0.41 (1.07) | .07 | .37 |
| Balance score change, mean (SD) | 0.28 (1.15) | 0.26 (0.56) | 0.39 (1.50) | 0.21 (0.98) | 0.05 (1.31) | 0.50 (1.25) | 0.28 (1.15) | .81 | .47 |
| Chair rise score change, mean (SD) | 0.35 (1.27) | 0.21 (1.32) | 0.39 (1.46) | 0.00 (1.00) | 0.42 (1.17) | 0.78 (1.35) | 0.39 (1.27) | .44 | .24 |
| Clinically meaningful Improvement, n (%) | 54 (58) | 8 (42) | 12 (67) | 8 (42) | 12 (63) | 14 (78) | 46 (62) | .11 | .06 |
| Week-4 testing “n” | 79 | 13 | 15 | 17 | 16 | 18 | 66 | ||
| Total score change, mean (SD) | 2.37 (2.24) | 1.31 (1.93) | 2.73 (2.28) | 2.00 (1.90) | 2.75 (2.79) | 2.83 (2.07) | 2.58 (2.24) | .29 | .03 |
| Gait speed score change, mean (SD) | 0.67 (0.89) | 0.54 (0.78) | 0.87 (0.83) | 0.47 (0.87) | 0.75 (0.86) | 0.72 (1.07) | 0.70 (0.89) | .73 | .36 |
| Balance score change, mean (SD) | 0.66 (0.14) | –0.08 (0.14) | 0.87 (0.45) | 0.71 (0.31) | 0.75 (0.31) | 0.89 (0.21) | 0.80 (0.15) | .20 | .004* |
| Chair rise score change, mean (SD) | 1.04 (0.14) | 0.85 (0.34) | 1.00 (0.29) | 0.82 (0.31) | 1.25 (0.35) | 1.22 (0.32) | 1.08 (0.16) | .81* | .32* |
| Clinically meaningful improvement, n (%) | 62 (78) | 7 (54) | 12 (80) | 14 (82) | 12 (75) | 17 (94) | 55 (83) | .10* | .01* |
| Sample size needed per group to achieve α = .05, β = .8 vs. placebo | |||||||||
| SPPB total score difference (week 4–baseline) | — | 35 | 117 | 38 | 29 | ||||
| Clinically meaningful SPPB change (≥1) | — | 64 | 64 | 64 | 64 |
Note: Change scores are improvement from baseline score *p < .05 with intention to treat analysis. All data are mean ± standard deviation or number of participants (percentage).
Body Composition
Changes in body composition between baseline and week 4 post-discharge are reported in Table 3. There were no significant differences for changes in weight, fat mass, muscle mass, or appendicular lean mass across groups, or between AIG and placebo.
Table 3.
Change in Body Composition (Weight, Fat, Muscle Mass) at Week-4 Testing
| Variable | All sample (n = 63) | Placebo (n = 10) | Whey protein (n = 11) | Rehabilitation + placebo (n = 13) | Rehabilitation + protein (n = 12) | Testosterone (n = 17) | All active intervention groups (n = 53) | p-Value (across groups) | p-Value (placebo vs. interventions) |
|---|---|---|---|---|---|---|---|---|---|
| Weight, kg | 0.290 ± 1.74 | 0.380 ± 1.27 | 0.273 ± 1.93 | 0.777 ± 2.26 | 0.992 ± 1.21 | –0.618 ± 1.49 | 0.274 ± 1.74 | .38 | .67 |
| Loss | 24 (38) | 3 (30) | 6 (55) | 4 (31) | 1 (8) | 10 (59) | 21 (40) | ||
| No change | 2 (3) | 1 (10) | 0 (0) | 0 (0) | 0 (0) | 1 (6) | 1 (2) | ||
| Gain | 37 (59) | 6 (60) | 5 (45) | 9 (69) | 11 (92) | 6 (35) | 31 (58) | ||
| Fat, kg | –0.056 ± 1.23 | –0.110 ± 0.77 | 0.108 ± 1.50 | –0.140 ± 1.42 | 0.328 ± 1.27 | –0.338 ± 1.13 | –0.046 ± 1.23 | .69 | .98 |
| Loss | 33 (52) | 5 (50) | 5 (45) | 8 (62) | 6 (50) | 9 (53) | 28 (53) | ||
| No change | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| Gain | 30 (48) | 5 (50) | 6 (55) | 5 (38) | 6 (50) | 8 (47) | 25 (47) | ||
| Muscle mass, kg | 0.331 ± 1.53 | 0.493 ± 0.92 | 0.087 ± 1.60 | 0.905 ± 1.84 | 0.641 ± 1.59 | –0.265 ± 1.40 | 0.300 ± 1.53 | .27 | .72 |
| Loss | 25 (40) | 2 (20) | 5 (45) | 4 (31) | 4 (33) | 10 (59) | 23 (43) | ||
| No change | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| Gain | 38 (60) | 8 (80) | 6 (55) | 9 (69) | 8 (67) | 7 (41) | 30 (57) | ||
| Appendicular lean mass, kg | –0.106 ± 0.99 | –0.212 ± 0.77 | –0.065 ± 1.04 | 0.317 ± 1.10 | 0.001 ± 1.10 | –0.469 ± 0.98 | –0.086 ± 0.99 | .29 | .59 |
| Loss | 37 (59) | 7 (70) | 6 (55) | 6 (46) | 5 (42) | 13 (76) | 30 (57) | ||
| No change | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| Gain | 26 (41) | 3 (30) | 5 (45) | 7 (54) | 7 (58) | 4 (24) | 23 (43) |
Note: All data are mean ± standard deviation or number of participants (percentage). A positive number is an increase in mass.
Activities of Daily Living
Changes in ADL and IADL between baseline, week 1, and week 4 post-discharge are reported in Table 4. There were no significant differences in ADL or IADL recovery across groups or between AIG and placebo.
Table 4.
Improvement in Activities of Daily Living (ADL) and Instrumental Activities of Daily Living (IADL) Score at Week-1 and Week-4 Testing
| Variable | All sample | Placebo | Whey protein | Rehabilitation + placebo | Rehabilitation + protein | Testosterone | All active intervention groups | p-Value (across groups) | p-Value (placebo vs. interventions) |
|---|---|---|---|---|---|---|---|---|---|
| Week-1 testing, number of patients | 93 | 19 | 18 | 19 | 19 | 18 | 74 | ||
| ADL point change | –0.05 ± 0.43 | –0.05 ± 0.40 | –0.11 ± 0.32 | –0.21 ± 0.71 | 0.11 ± 0.32 | 0.00 ± 0.00 | –0.05 ± 0.43 | .20 | .28 |
| Loss | 6 (6) | 2 (11) | 2 (11) | 2 (11) | 0 (0) | 0 (0) | 4 (5) | ||
| No change | 84 (90) | 16 (84) | 16 (89) | 17 (81) | 17 (89) | 18 (100) | 68 (92) | ||
| Gain | 3 (3) | 1 (5) | 0 (0) | 0 (0) | 2 (11) | 0 (0) | 2 (3) | ||
| IADL point change | –0.51 ± 1.77 | 0.05 ± 1.47 | –0.28 ± 1.45 | –0.89 ± 2.38 | –1.32 ± 2.06 | –0.06 ± 0.80 | –0.65 ± 1.77 | .15 | .25 |
| Loss | 30 (32) | 4 (21) | 3 (17) | 7 (37) | 12 (63) | 4 (22) | 26 (35) | ||
| No change | 45 (48) | 10 (53) | 11 (61) | 7 (37) | 7 (37) | 10 (56) | 35 (47) | ||
| Gain | 18 (19) | 5 (26) | 4 (22) | 5 (26) | 0 (0) | 4 (22) | 13 (18) | ||
| Week-4 testing, number of patients | 79 | 13 | 15 | 17 | 16 | 18 | 66 | ||
| ADL point change | –0.04 ± 0.49 | –0.08 ± 0.28 | 0.07 ± 0.46 | –0.24 ± 0.75 | 0.13 ± 0.34 | –0.06 ± 0.42 | –0.03 ± 0.49 | .26 | .52 |
| Loss | 6 (8) | 1 (8) | 1 (7) | 2 (12) | 0 (0) | 2 (11) | 5 (8) | ||
| No change | 68 (86) | 12 (92) | 12 (80) | 15 (88) | 14 (88) | 15 (83) | 56 (85) | ||
| Gain | 5 (6) | 0 (0) | 2 (13) | 0 (0) | 2 (13) | 1 (6) | 5 (8) | ||
| IADL point change | 0.15 ± 1.11 | 0.08 ± 1.12 | 0.27 ± 1.03 | –0.06 ± 1.25 | 0.13 ± 0.81 | 0.33 ± 1.33 | 0.17 ± 1.11 | .86 | .79 |
| Loss | 13 (16) | 1 (8) | 2 (13) | 5 (29) | 3 (19) | 2 (11) | 12 (18) | ||
| No change | 39 (49) | 9 (69) | 8 (53) | 6 (35) | 9 (56) | 7 (39) | 30 (45) | ||
| Gain | 27 (34) | 3 (23) | 5 (33) | 6 (35) | 4 (25) | 9 (50) | 24 (36) |
Note: All data are mean ± standard deviation or number of participants (percentage). A positive value indicates an improvement in score.
30-Day Readmissions
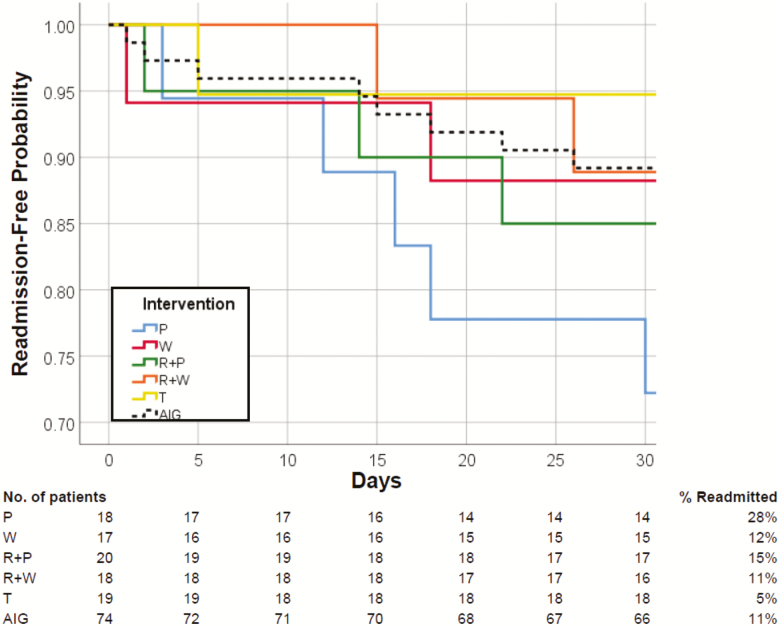
Of the 100 participants randomized in this study, 76 completed the 30-day intervention, 13 were rehospitalized, and 8 withdrew from the study and were not included in the analysis (Figure 2). The readmission rate was highest in the placebo group (28%), followed by rehabilitation + placebo (15%), whey (12%), rehabilitation + whey (11%), and testosterone (5%). There were no significant differences in readmission rates across groups. However, there was a trend for lower readmission rate in AIG (11%) compared with the placebo group (28%), p = .065. Readmission rates tended to be improved across groups (p = .09) and in AIG compared with placebo group (p = .06).
Figure 2.
Readmission-free Kaplan–Meier curves. Cumulative readmission-free Kaplan–Meier curves of geriatric patients discharged home following a hospital admission for an acute medical illness. None of the patients died during the 30-day intervention period. Dropouts (n = 8) are excluded. Patients were randomized to 20 g twice daily whey protein supplementation (W) for 30 days, twice daily isocaloric placebo (P) for 30 days, intensive rehabilitation (R) 3 times weekly for 30 days, combined intensive rehabilitation plus whey protein (R + W) for 30 days, combined intensive rehabilitation plus placebo (R + P) for 30 days, or single testosterone injection at discharge (T). AIG: all intervention groups.
Safety
Adverse event rates were not significantly different between groups.
Discussion
The main finding of this Phase 1 clinical trial was that interventions that increase muscle anabolism and strength in healthy older adults can improve functional recovery and may reduce 30-day readmission rates in geriatric patients following an acute hospitalization for a medical illness. Compared with the placebo group, the pooled interventions (AIG) accelerated the recovery of physical function after acute hospitalization as measured by the SPPB. Specifically, the total SPPB score, balance score, and the percent of participants with a clinically meaningful improvement in SPPB score increased more in the pooled intervention groups compared with placebo group. There was also a trend for interventions to decrease the readmission rates. However, we could not detect significant improvements in body composition or ADL/IADL scores. Using the effect size and variability data measured in this study, we estimated the sample size necessary to detect a significant difference in the primary outcome, the SPPB score, between each individual intervention and placebo, with alpha value of .05 and a power of 0.8. We found that by increasing the sample size to 64 per group, we could detect a significant difference in the proportion of patients who had a clinically meaningful increase in SPPB score between any of the interventions and placebo. A smaller sample size per group would be needed to detect significant differences in SPPB scores between the whey protein interventions with or without rehabilitation and placebo (n = 35–38), and between testosterone and placebo (n = 29). Conversely, to detect a significant difference in SPPB scores between the rehabilitation group with placebo and placebo alone, we would need to scale up the trial to include at least 113 patients per group. These sample size estimates should be tempered by the wide confidence intervals about the purported effects. Sample sizes are based on the effect sizes we observed in this small pilot study; however, it is important to acknowledge that they have a substantial degree of variability as indicated by large confidence intervals. From these preliminary data, it appears that protein supplementation or testosterone injection could be more effective means to accelerate functional recovery from hospitalization in geriatric patients. Larger, multisite clinical trials will be necessary to demonstrate efficacy and effectiveness of these interventions.
A few studies have examined whether nutritional supplementation can improve physical function in older adults. Nutritional supplementation was found to lower the incidence of falls (23), reduce inflammation (during concomitant rehabilitation) (24), increase handgrip strength (25,26), and reduce 90-day readmission and mortality (27). However, these studies did not report benefits of protein supplementation on measures of physical function or muscle size. A study carried out in frail, older adults found that adequate doses of high-quality protein throughout the day (15 g protein supplement at breakfast and lunch) can increase measures of physical function (28). However, a longer-term randomized trial in frail elderly did not find improvements in lean body mass with high-protein energy supplementation (29). Taken together, these studies are consistent with our findings of improvements in physical function, but not body composition, with protein supplementation.
Exercise or rehabilitation therapy has been used to improve physical function in older adults. We recently reported that in healthy, low active, and independent older adults, a 6-month aerobic exercise training program with essential amino acid supplementation could increase muscle strength and quality (30). In sarcopenic frail older women, a 3-month strength, balance, and gait resistance exercise training program combined with supplementation increased not only muscle strength but also muscle mass (31). However, in this study, we found that the intensive rehabilitation alone tended to produce smaller improvements in physical function than the other interventions and did not appear to enhance the beneficial effects of protein supplementation. This may be due to the inability of our patients to complete the prescribed exercise routine, particularly at the beginning of the intervention, due to lingering symptoms of their acute illness, fear of falling, or pain (17). Nonetheless, all participants completing the program did show improvements in intensity, number of repetitions completed, and strength of the Theraband used.
Testosterone increases skeletal muscle protein synthesis and muscle mass. In younger men, androgen therapy increases skeletal muscle strength and size (32–34). In older men, results of long-term testosterone treatment studies are less clear, particularly regarding the effects of testosterone on physical performance measures. However, testosterone treatment can increase lean mass, decrease fat mass, and increase strength (35–38). In a long-term randomized clinical trial, testosterone supplementation significantly increased a composite timed test of physical function (39). Moreover, testosterone may have positive effects on quality of life, energy, mood, and appetite (33,40). At present, the Testosterone Trials three main studies reported that testosterone therapy provided small gains in physical performance, mood, and depression, with no differences in vitality between groups (41). Our initial finding that a single testosterone dose at discharge may accelerate functional recovery and reduce readmissions after hospitalization could be related to its effects on mood and strength and deserves further investigation in a larger trial.
A major strength of this Phase I study was the design including block randomization and intervention masking. We used comprehensive and multicomponent interventions to ensure a high rate of acceptance and adherence to the interventions. The main limitation is exclusion of cognitively impaired patients. Approximately one-fourth of the patients admitted to the hospital had to be excluded due to various degrees of cognitive dysfunction, including delirium. To increase inclusiveness, future studies should consider consent by proxy particularly for those patients admitted with no history of dementia and an acute onset cognitive problem (confusion or delirium). Another limitation was the heterogeneity of diagnoses and comorbidities in this study population. Much larger sample sizes than those calculated in this pilot study will be necessary for future studies to determine if the tested interventions are more or less beneficial to any specific disease or condition. Further, we did not track patient participation in home health because we felt it unlikely to differentially affect study outcomes. This speaks to the unclear efficacy of home health rehab in a very impaired post-discharge sample. The lack of improvement in SPPB during the first-week post-discharge may also suggest the need for a different outcome assessment in the early phase of recovery in this functionally impaired population. Finally, our cohort included a larger proportion of women than men. However, considering that the average age was 78, the gender distribution was not very different from that found in the local population aged 75 and older.
Conclusion
Post-hospital protein supplementation, in-home exercise, and testosterone interventions are safe, can accelerate recovery, and may reduce readmission rates in geriatric patients. This Phase I study provides essential effect size and sample size information to inform the design and power larger multisite clinical trials aimed to improve post-hospital outcomes in geriatric patients. It is encouraging to note that the sample size needed to detect a significant difference in SPPB between each of the individual interventions and placebo is not excessively large, ranging between 29 and 113 per group. This increases the likelihood of a rapid development of future larger trials to assess efficacy, safety, and cost-effectiveness of these interventions.
Funding
This work was supported by the National Dairy Council (1229); University of Texas Medical Branch (UTMB) Claude D. Pepper OAIC (P30 AG024832) from the National Institute on Aging; and the UTMB Clinical and Translational Science Award (UL1 TR001439 and TL1 TR001440) from the National Center for Advancing Translational Sciences. Whey protein supplements were provided in kind by BiPro, Eden Prairie, MN. The funding sources had no role in the design and conduct of the study; collection, management, analysis or interpretation of the data; or preparation, review or approval of the manuscript.
Acknowledgments
We thank Shawn Goodlett, lead study coordinator, for her invaluable assistance with recruitment and functional testing, and the University of Texas Medical Branch Institute for Translational Sciences Clinical Research Center for providing nursing support.
Conflict of interest
The authors declare that they have no conflicts of interest.
References
- 1. Lamont CT, Sampson S, Matthias R, Kane R. The outcome of hospitalization for acute illness in the elderly. J Am Geriatr Soc. 1983;31:282–288. [DOI] [PubMed] [Google Scholar]
- 2. Gillick MR, Serrell NA, Gillick LS. Adverse consequences of hospitalization in the elderly. Soc Sci Med. 1982;16:1033–1038. [DOI] [PubMed] [Google Scholar]
- 3. Krumholz HM. Post-hospital syndrome–an acquired, transient condition of generalized risk. N Engl J Med. 2013;368:100–102. doi: 10.1056/NEJMp1212324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fisher SR, Goodwin JS, Protas EJ, et al. . Ambulatory activity of older adults hospitalized with acute medical illness. J Am Geriatr Soc. 2011;59:91–95. doi: 10.1111/j.1532-5415.2010.03202.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fisher SR, Galloway RV, Kuo YF, et al. . Pilot study examining the association between ambulatory activity and falls among hospitalized older adults. Arch Phys Med Rehabil. 2011;92:2090–2092. doi: 10.1016/j.apmr.2011.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brown CJ, Redden DT, Flood KL, Allman RM. The underrecognized epidemic of low mobility during hospitalization of older adults. J Am Geriatr Soc. 2009;57:1660–1665. doi: 10.1111/j.1532-5415.2009.02393.x [DOI] [PubMed] [Google Scholar]
- 7. Gariballa SE, Parker SG, Taub N, Castleden M. Nutritional status of hospitalized acute stroke patients. Br J Nutr. 1998;79:481–487. [DOI] [PubMed] [Google Scholar]
- 8. Sullivan DH. The role of nutrition in increased morbidity and mortality. Clin Geriatr Med. 1995;11:661–674. [PubMed] [Google Scholar]
- 9. Sullivan DH, Walls RC, Bopp MM. Protein-energy undernutrition and the risk of mortality within one year of hospital discharge: a follow-up study. J Am Geriatr Soc. 1995;43:507–512. [DOI] [PubMed] [Google Scholar]
- 10. Sullivan DH, Sun S, Walls RC. Protein-energy undernutrition among elderly hospitalized patients: a prospective study. JAMA. 1999;281:2013–2019. [DOI] [PubMed] [Google Scholar]
- 11. Vellas B, Conceicao J, Lafont C, et al. . Malnutrition and falls. Lancet. 1990;336:1447. [DOI] [PubMed] [Google Scholar]
- 12. Johnson CS. The association between nutritional risk and falls among frail elderly. J Nutr Health Aging. 2003;7:247–250. [PubMed] [Google Scholar]
- 13. Brown CJ, Friedkin RJ, Inouye SK. Prevalence and outcomes of low mobility in hospitalized older patients. J Am Geriatr Soc. 2004;52:1263–1270. doi: 10.1111/j.1532-5415.2004.52354.x [DOI] [PubMed] [Google Scholar]
- 14. Deer RR, Volpi E. Protein intake and muscle function in older adults. Curr Opin Clin Nutr Metab Care. 2015;18:248–253. doi: 10.1097/MCO.0000000000000162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Paddon-Jones D, Sheffield-Moore M, Zhang XJ, et al. . Amino acid ingestion improves muscle protein synthesis in the young and elderly. Am J Physiol Endocrinol Metab. 2004;286:E321–E328. doi: 10.1152/ajpendo.00368.2003 [DOI] [PubMed] [Google Scholar]
- 16. Ferrando AA, Sheffield-Moore M, Yeckel CW, et al. . Testosterone administration to older men improves muscle function: molecular and physiological mechanisms. Am J Physiol Endocrinol Metab. 2002;282:E601–E607. doi: 10.1152/ajpendo.00362.2001 [DOI] [PubMed] [Google Scholar]
- 17. Deer RR, Goodlett SM, Fisher SR, et al. . A randomized controlled pilot trial of interventions to improve functional recovery after hospitalization in older adults: feasibility and adherence. J Gerontol A Biol Sci Med Sci. 2018;73:187–193. doi: 10.1093/gerona/glx111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Deer RR, Dickinson JM, Fisher SR, Ju H, Volpi E. Identifying effective and feasible interventions to accelerate functional recovery from hospitalization in older adults: a randomized controlled pilot trial. Contemp Clin Trials. 2016;49:6–14. doi: 10.1016/j.cct.2016.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dillon EL, Basra G, Horstman AM, et al. . Cancer cachexia and anabolic interventions: a case report. J Cachexia Sarcopenia Muscle. 2012;3:253–263. doi: 10.1007/s13539-012-0066-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fisher S, Ottenbacher KJ, Goodwin JS, Graham JE, Ostir GV. Short physical performance battery in hospitalized older adults. Aging Clin Exp Res. 2009;21:445–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Guralnik JM, Simonsick EM, Ferrucci L, et al. . A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. [DOI] [PubMed] [Google Scholar]
- 22. Schroeder ET, Jaque SV, Hawkins SA, Olsson C, Wiswell RA, Sattler FR. Regional DXA and MRI in assessment of muscle adaptation to anabolic stimuli. J Clin Exercise Physiol. 2001;3:199–206. [Google Scholar]
- 23. Neelemaat F, Lips P, Bosmans JE, Thijs A, Seidell JC, van Bokhorst-de van der Schueren MA. Short-term oral nutritional intervention with protein and vitamin D decreases falls in malnourished older adults. J Am Geriatr Soc. 2012;60:691–699. doi: 10.1111/j.1532-5415.2011.03888.x [DOI] [PubMed] [Google Scholar]
- 24. Paillaud E, Bories PN, Le Parco JC, Campillo B. Nutritional status and energy expenditure in elderly patients with recent hip fracture during a 2-month follow-up. Br J Nutr. 2000;83:97–103. [PubMed] [Google Scholar]
- 25. McMurdo ME, Price RJ, Shields M, Potter J, Stott DJ. Should oral nutritional supplementation be given to undernourished older people upon hospital discharge? A controlled trial. J Am Geriatr Soc. 2009;57:2239–2245. doi: 10.1111/j.1532-5415.2009.02568.x [DOI] [PubMed] [Google Scholar]
- 26. Price R, Daly F, Pennington CR, McMurdo ME. Nutritional supplementation of very old people at hospital discharge increases muscle strength: a randomised controlled trial. Gerontology. 2005;51:179–185. doi: 10.1159/000083991 [DOI] [PubMed] [Google Scholar]
- 27. Deutz NE, Matheson EM, Matarese LE, Luo M, Baggs GE, Nelson JL, et al. . Readmission and mortality in malnourished, older, hospitalized adults treated with a specialized oral nutritional supplement: a randomized clinical trial. Clin Nutr. 2016;35:18–26. doi: 10.1016/j.clnu.2015.12.010 [DOI] [PubMed] [Google Scholar]
- 28. Tieland M, van de Rest O, Dirks ML, et al. . Protein supplementation improves physical performance in frail elderly people: a randomized, double-blind, placebo-controlled trial. J Am Med Dir Assoc. 2012;13:720–726. doi: 10.1016/j.jamda.2012.07.005 [DOI] [PubMed] [Google Scholar]
- 29. Bonnefoy M, Cornu C, Normand S, et al. . The effects of exercise and protein-energy supplements on body composition and muscle function in frail elderly individuals: a long-term controlled randomised study. Br J Nutr. 2003;89:731–739. doi: 10.1079/BJN2003836 [DOI] [PubMed] [Google Scholar]
- 30. Markofski MM, Jennings K, Timmerman KL, Dickinson JM, Fry CS, Borack MS, et al. . Effect of aerobic exercise training and essential amino acid supplementation for 24 weeks on physical function, body composition and muscle metabolism in healthy, independent older adults: a randomized clinical trial. J Gerontol A Biol Sci Med Sci. 2018. May 10. doi: 10.1093/gerona/gly109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kim H, Kim M, Kojima N, et al. . Exercise and nutritional supplementation on community-dwelling elderly Japanese women with sarcopenic obesity: a randomized controlled trial. J Am Med Dir Assoc. 2016;17:1011–1019. doi: 10.1016/j.jamda.2016.06.016 [DOI] [PubMed] [Google Scholar]
- 32. Bhasin S, Storer TW, Berman N, Yarasheski KE, Clevenger B, Phillips J, et al. . Testosterone replacement increases fat-free mass and muscle size in hypogonadal men. J Clin Endocrinol Metab. 1997;82:407–413. doi: 10.1210/jcem.82.2.3733 [DOI] [PubMed] [Google Scholar]
- 33. Wang C, Swerdloff RS, Iranmanesh A, et al. ; Testosterone Gel Study Group Transdermal testosterone gel improves sexual function, mood, muscle strength, and body composition parameters in hypogonadal men. J Clin Endocrinol Metab. 2000;85:2839–2853. doi: 10.1210/jcem.85.8.6747 [DOI] [PubMed] [Google Scholar]
- 34. Brodsky IG, Balagopal P, Nair KS. Effects of testosterone replacement on muscle mass and muscle protein synthesis in hypogonadal men–a clinical research center study. J Clin Endocrinol Metab. 1996;81:3469–3475. doi: 10.1210/jcem.81.10.8855787 [DOI] [PubMed] [Google Scholar]
- 35. Kenny AM, Prestwood KM, Gruman CA, Marcello KM, Raisz LG. Effects of transdermal testosterone on bone and muscle in older men with low bioavailable testosterone levels. J Gerontol A Biol Sci Med Sci. 2001;56:M266–M272. [DOI] [PubMed] [Google Scholar]
- 36. Snyder PJ, Peachey H, Hannoush P, et al. . Effect of testosterone treatment on body composition and muscle strength in men over 65 years of age. J Clin Endocrinol Metab. 1999;84:2647–2653. doi: 10.1210/jcem.84.8.5885 [DOI] [PubMed] [Google Scholar]
- 37. Sih R, Morley JE, Kaiser FE, Perry HM 3rd, Patrick P, Ross C. Testosterone replacement in older hypogonadal men: a 12-month randomized controlled trial. J Clin Endocrinol Metab. 1997;82:1661–1667. doi: 10.1210/jcem.82.6.3988 [DOI] [PubMed] [Google Scholar]
- 38. Borst SE. Interventions for sarcopenia and muscle weakness in older people. Age Ageing. 2004;33:548–555. doi: 10.1093/ageing/afh201 [DOI] [PubMed] [Google Scholar]
- 39. Page ST, Amory JK, Bowman FD, et al. . Exogenous testosterone (T) alone or with finasteride increases physical performance, grip strength, and lean body mass in older men with low serum T. J Clin Endocrinol Metab. 2005;90:1502–1510. doi: 10.1210/jc.2004-1933 [DOI] [PubMed] [Google Scholar]
- 40. Kenny AM, Fabregas G, Song C, Biskup B, Bellantonio S. Effects of testosterone on behavior, depression, and cognitive function in older men with mild cognitive loss. J Gerontol A Biol Sci Med Sci. 2004;59:75–78. [DOI] [PubMed] [Google Scholar]
- 41. Snyder PJ, Bhasin S, Cunningham GR, et al. ; Testosterone Trials Investigators Effects of testosterone treatment in older men. N Engl J Med. 2016;374:611–624. doi: 10.1056/NEJMoa1506119 [DOI] [PMC free article] [PubMed] [Google Scholar]