ABSTRACT
Feed additives that promote gastrointestinal health may complement coccidiosis vaccination programs in antibiotic-free broiler production systems. This study examined the effects of a commercial feed additive blend (FA) on intestinal histomorphology and inflammatory biomarkers in vaccinated Ross 708 cockerels (N = 2,160). The study was a randomized complete block design (12 blocks) with 3 dietary treatments: CON (negative control), AGP (positive control: 55 ppm of bacitracin methylene disalicylate), and FA (1.5 kg/MT in starter; 1.0 kg/MT in grower; and 0.5 kg/MT in finisher). Birds were reared on re-used litter and fed a 3-phase feeding program (starter, 0 to 14 D; grower, 15 to 28 D; finisher, 29 to 36 D). One master batch of basal feed for each feeding phase was prepared and final experimental diets were manufactured by mixing the basal feed with the respective test ingredient prior to pelleting. Growth measurements, including pen body weight and feed intakes, and fresh fecal samples were taken throughout the study. On day 20, samples of intestinal tissue were collected from a subset of birds (n = 72, 1 block) for histomorphology and mRNA expression of tight junction and inflammatory genes. In the duodenum, the ratio of villi length to crypt depth was significantly lower in FA (and AGP) fed birds than those consuming the CON diet. Relative mRNA expressions of iNOS, IFNƔ, and claudin-1 were upregulated in the jejunum of FA and AGP treatment groups compared to those in the CON group; the response in the FA was of lesser magnitude than AGP. Together, these results demonstrated that the FA treatment altered the microstructure of the duodenum and affected the expression of inflammatory genes in the jejunum. The timing of these changes coincided with peak oocyte shedding in feces and an observed reduction in feed efficiency in all dietary treatment groups.
Keywords: coccidiosis, antibiotic free, feed additives, gastrointestinal tract
INTRODUCTION
Coccidiosis is a parasitic disease in livestock and poultry caused by single-celled protozoan parasites of the genus Eimeria spp. The parasites proliferate in the gastrointestinal tract (GIT) causing bloody diarrhea, dehydration, and intestinal lesions (Trees, 2008). In broiler chickens, the lesions induced by coccidiosis are a predisposing factor for necrotic enteritis caused by Clostridium perfringens. The global economic losses associated with necrotic enteritis, such as reduced growth performance and increased condemnations, are estimated at 6 billion USD in broiler chickens (Wade and Keyburn, 2015). In conventional production, coccidiosis is prevented and managed through in-feed (or water) anti-coccidial and antibiotic administration. However, with increased pressure to reduce (remove) antibiotics and ionophore use in poultry production, alternative strategies are needed.
One approach to coccidiosis prevention in antibiotic-free production is vaccination. Vaccines composed of live sporulated oocytes induce adaptive immunity, and by consequence cause damage to the intestinal epithelium and induce localized inflammation (Williams, 2002; Dalloul and Lillehoj, 2005; Stringfellow et al., 2011). This stress has been associated with reduced growth performance (Li et al., 2005). Feed additives that support intestinal integrity and function, mitigate inflammation, or possess microbial modification properties may complement vaccination, offering an alternative approach to disease management in antibiotic-free poultry production. Indeed, several natural alternatives to antibiotic growth promoters (AGP) exist and have been investigated in broiler chickens, including prebiotics, probiotics, organic acids, enzymes, and phytochemicals (reviewed by Sethiya, 2016). Blended additives that incorporate several ingredients are available commercially and may provide an opportunity to influence GIT health through multiple mechanisms.
A proprietary commercial blend, “FA”, of fatty acids, organic acids, and phytochemicals has been developed and tested in broiler chickens raised in antibiotic-free production systems. Studies conducted in our laboratory have observed increased body weight and decreased feed to gain (F:G) when the FA was fed throughout the production cycle (unpublished). It was hypothesized that enhanced growth performance was attributed, in part, to improved intestine integrity and function, yet this notion remains to be confirmed. The objective of this study was to examine the effects of the FA on markers of GIT integrity and inflammatory response in broiler chickens vaccinated in hatchery for coccidiosis. Gastrointestinal tract integrity was evaluated by histomorphology and tight junction genes. The relative expression of genes involved in the inflammatory response was also examined. It was hypothesized that feeding the commercial product (FA) would support GIT health and function.
MATERIALS AND METHODS
Animals and Housing
The animal experiment took place between August 22, 2017 and September 28, 2017 at the Trouw Nutrition Broiler Research Unit (Burford, ON, Canada). Animal procedures were approved by the Institutional Animal Care Committee of Trouw Nutrition in accordance with Canadian Council of Animal Care guidelines (1993). A total of 2,160 Ross 708 cockerels were purchased from Maple Leaf Foods’ hatchery (Hanover, ON, Canada) where they were vaccinated for Marek's disease, infectious bronchitis, and coccidiosis (Immucox3, CEVA, Cambridge, ON, Canada). Day-old chicks were received at the Trouw Nutrition Broiler Research Unit and randomly allocated to 36 floor pens (60 birds/pen). Each pen provided 0.07 m2/bird with wood shavings litter retained from the previous commercial flock, and used to induce an environmental stress. The litter was treated with PLT Poultry Litter Treatment (Jones-Hamilton, Walbridge OH) at the recommended application of 49 to 73 kg/100 m2 prior to placement to control ammonia levels. Ammonia concentration did not exceed 25 ppm at any point in the study, and litter humidity was maintained (with water) at 25 to 35%.
Experimental Design
The study was a randomized complete block design with 12 blocks and 3 experimental treatments. The treatments consisted of a commercial product (FA) (Selko B.V., Tilburg, the Netherlands), a negative control (CON) that contained no additives or medications, and a medicated positive control (AGP). Birds were subject to a 3-phase commercial feeding program: starter (0 to 14 D), grower (15 to 28 D), and finisher (29 to 36 D). One master batch of basal feed was made for each feeding phase (Yantzi Feed and Seed, Tavistock, ON, Canada) that met or exceeded the energy and nutrient requirements of the birds outlined by NRC (1994) (Table 1). Experimental diets were manufactured by mixing subbatches of basal feeds with their respective test ingredient. For the AGP treatment, bacitracin methylene disaclicyclate (Zoetis Canada Inc., Kirkland, QC, Canada) was included in each diet (0.5 kg/MT; 55 ppm bacitracin). The FA was included in the starter diet at 1.5 kg/MT, grower diet at 1.0 kg/MT, and finisher diet at 0.5 kg/MT as per the manufacturer's recommendations. No coccidiostats, additional medications, or additives were included in the diets. Ten equally spaced samples of diet were taken from each production lot, mixed, and subsampled to provide a representative composite for later nutrient analysis by near-infrared spectroscopy (NIRS DS2500, Foss, Hilleroed, Denmark) (Shur-Gain Laboratory, St. Hyacinthe, QC, Canada).
Table 1.
Proximate analysis and ingredient composition of the basal diets.
| Starter | Grower | Finisher | |
|---|---|---|---|
| Composition | |||
| Dry matter (%) | 87.5 | 87.1 | 87.4 |
| Crude protein (%) | 18.4 | 18.4 | 16.1 |
| Crude fat (%) | 3.8 | 3.5 | 4.7 |
| Crude fiber (%) | 3.1 | 3.2 | 2.9 |
| Sodium (%) | 0.14 | 0.13 | 0.16 |
| Calcium (%) | 0.68 | 0.72 | 0.61 |
| Phosphorus (%) | 0.55 | 0.51 | 0.45 |
| Copper (PPM) | 18.2 | 16.3 | 11.3 |
| Zinc (PPM) | 111 | 96 | 98 |
| Ingredient, % DM | |||
| Ground corn | 48.85 | 53.91 | 55.45 |
| Soybean meal | 23.30 | 19.44 | 16.63 |
| Wheat | 7.50 | 7.50 | 7.50 |
| Wheat shorts | 7.50 | 7.50 | 7.50 |
| Bakery product | 6.30 | 5.07 | 6.76 |
| Feather meal | 1.50 | 2.50 | 2.50 |
| Calcium carbonate | 1.33 | 1.27 | 1.09 |
| Animal/vegetable oil blend | 1.00 | 1.00 | 1.00 |
| Corn distillers dried grains | 0.71 | 0.00 | 0.00 |
| Dicalcium phosphate | 0.67 | 0.50 | 0.31 |
| Sodium bicarbonate | 0.36 | 0.39 | 0.47 |
| Lysine | 0.34 | 0.35 | 0.34 |
| Vitamin and mineral premix | 0.25 | 0.20 | 0.13 |
| Methionine | 0.24 | 0.20 | 0.17 |
| Threonine | 0.09 | 0.09 | 0.07 |
| Choline chloride | 0.04 | 0.07 | 0.06 |
| Econase (NSPase) | 0.01 | 0.01 | 0.01 |
| Quantum Blue phytase | 0.02 | 0.02 | 0.02 |
Feed was administered to individual pens, and birds had free access to feed and water. Pen feed intakes and body weights (BW) were recorded on day 7, 14, 20, 28, and 36 for calculation of average BW, average daily feed intake, and feed conversion ratio (F:G). General health of the animals was monitored daily and mortalities were recorded, with cause of death determined by a licensed veterinarian. As an index of vaccine uptake, the number of oocytes in the feces was determined on day 7, 20, and 28 using the McMaster technique by the University of Guelph (MAFF, 1986). On the final day of study, 5 birds in each pen were randomly selected and foot pad integrity was scored using a 4-point scale (Ekstrand et al., 1998), where 0 indicates completely intact foot pad and 3 represents severe lesion.
Intestine Sampling and Analysis
On day 20, a subsample of 24 birds from an entire block (n = 72) were euthanized by cervical dislocation. Tissue samples (1.5 to 2.0 cm) were immediately harvested from the duodenum at the duodenal loop, the ileum at the Meckel's diverticulum, and ceca (mid-section), rinsed in PBS, and stored in 10% neutral-buffered formalin. After 72 h, tissues were transferred into PBS and stored at 21°C for later histological analysis. A sample of tissue (300 to 350 mg) was taken from the jejunum at the Meckel's diverticulum placed in RNA later solution and stored at 4°C for 24 h and then transferred to –20°C until subsequent analysis for mRNA expression.
For histological analyses (Department of Pathobiology, University of Guelph), intestinal tissue segments were sectioned (4 μm thick) on charged slides and stained with hematoxylin and eosin. Villi measurements were taken from the surface of the epithelium at the tip of the villus to the base of the villus as delimited by the beginning of the epithelium in adjacent crypts. Crypts that were clearly present at the base of one villus or between 2 consecutive villi were chosen; an effort was made to select crypts that showed a straight profile. In the cecum, the length of the mucous gland was measured. Measurements were taken using the cellSens software (Olympus Canada Inc., Richman Hill, ON, Canada). Villi and crypts measurements were taken at ×40 magnification in duodenum (Supplement Figure S1) and ×100 magnification in ileum (Supplement Figure S2) and cecum. As many villi from the same field as possible were taken. Additional images were consecutively taken until measurements for up to 6 villi and 6 crypts were completed per sample.
Gene expression analysis was completed as has been described in detail previously (Peppler et al., 2016). Prior to RNA extraction, samples were blotted dry of RNA later and placed in homogenization tubes. Samples were homogenized in 1 mL of QIAzol, and RNA was extracted using an RNeasy mini kit, including DNase-free treatment. Complementary DNA was synthesized from 2 μg of RNA using a High Capacity cDNA Reverse Transcription Kit with addition of RNaseOUT. PCR plates were run on a BioRad CFX Real-Time PCR instrument and prepared using iTaq Universal SYBR Green Supermix and RNase-free water. Primers for interleukin-2 (IL-2), interleukin-6 (IL-6), interleukin-10 (IL-10), inducible nitric oxide synthase (iNOS), interferon gamma (IFNγ), occludin, claudin-1 (CLDN-1), claudin-2 (CLDN-2), and scaffold protein ZO2 were created by University of Guelph Laboratory Services (Supplement Table S1) from published literature (Wang et al., 2012; Osselaere et al., 2013; Yu et al., 2015; Song et al., 2017; Zhang et al., 2017). The 2-ΔΔCT method was used to quantify gene expression (Livak and Schmittgen, 2001), with Gapdh used as a housekeeping gene.
Calculations and Statistical Analyses
Feed conversion ratio was adjusted to a common body weight (raw F: G—[(BW–2.41 kg) x 0.24]). Data were analyzed in SAS Studio (v 9.4). Pen was considered the experimental unit for growth analyses and statistical significance was declared at P < 0.05. Continuous data were modeled in the GLIMMIX procedure of SAS with treatment and time and their interaction considered fixed effects and block a random source of variation. Repeated measures on pens over time were modeled, and the covariance structure that best fit the model (lowest AIC value) was used. Means were separated using the Slice function and P values were adjusted for multiplicity using the Simulate method. For all models, conditional studentized residuals were checked for normality and linearity. The simulation method was used for multiple pairwise comparisons. Foot pad scores were analyzed using a multinomial distribution. Histological data and mRNA expression data were modeled in non-parametric Kruskal–Wallis test with a Dunnet's test for multiple comparisons, and bird was the experimental unit.
RESULTS
Histology
Villi lengths and crypt depths for sections of the duodenum and ileum and length of the mucous gland of the cecum are provided in Table 2. Birds fed FA had significantly shorter villi in the duodenum than those fed CON. Villi lengths of the duodenum were similar between FA and AGP. Birds fed FA had significantly deeper crypts than CON birds, yet significantly shorter crypts than birds in AGP group. The ratio of villi length to crypt depth (VCR) for FA fed birds was significantly lower than birds fed CON, yet not different from birds fed AGP. There were no differences between dietary treatments for villi lengths and crypt depths (and ratio) of the ileum and cecum mucous gland length.
Table 2.
Intestinal histomorphology of 20 day-old broiler chickens vaccinated in hatchery for coccidiosis fed a commercial additive (FA), no additive (CON), or a medicated feed (AGP) (bacitracin methylene disalicylate).
| CON | FA | AGP | P | |
|---|---|---|---|---|
| Duodenum | ||||
| Villi, μm | 1953 ± 45a | 1793 ± 52b | 1836 ± 37a,b | 0.03 |
| Crypt, μm | 214 ± 11a | 265 ± 9b | 314 ± 8c | <0.001 |
| VCR2 | 9.6 ± 0.4a | 6.9 ± 0.3b | 5.9 ± 0.2b | <0.001 |
| Ileum | ||||
| Villi, μm | 608 ± 19 | 631 ± 22 | 676 ± 22 | 0.10 |
| Crypt, μm | 206 ± 5 | 206 ± 6 | 223 ± 6 | 0.11 |
| VCR | 3.0 ± 0.1 | 3.1 ± 0.1 | 3.0 ± 0.1 | 0.83 |
| Ceca, μm | 340 ± 9 | 320 ± 11 | 327 ± 8 | 0.29 |
1Means and standard errors, means within a row with no common superscripts differ (P < 0.05).
2VCR, villus to crypt length ratio.
Gene Expression Analysis
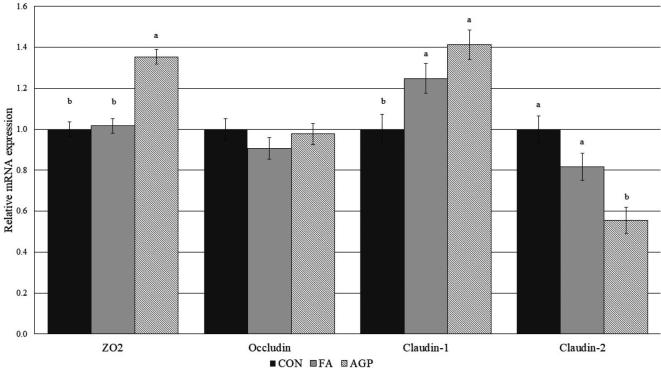
Relative mRNA expressions of tight junction proteins, including CLDN-1, CLDN-2, occludin, and ZO2, are shown in Figure 1. Claudin-1 mRNA expression was significantly upregulated in birds fed FA relative to CON, with no statistical difference between FA and AGP. The relative expressions of CLDN-2, occludin, and ZO2 were not different between FA and CON. Claudin-2 was significantly downregulated and ZO2 upregulated in the AGP group compared to FA and CON. These findings demonstrate that AGP treatment affected the expression of several tight junction genes, whereas the FA only induced the expression of CLDN-1.
Figure 1.
Relative mRNA expressions of claudin-1, claudin-2, occludin, and scaffolding protein ZO2 in jejunum of 20-day-old broiler chickens vaccinated in hatchery for coccidiosis fed a commercial additive (FA), no additive (CON), or a medicated feed (AGP) (bacitracin methylene disalicylate). 1Means without a common superscript differ across treatments (P < 0.05).
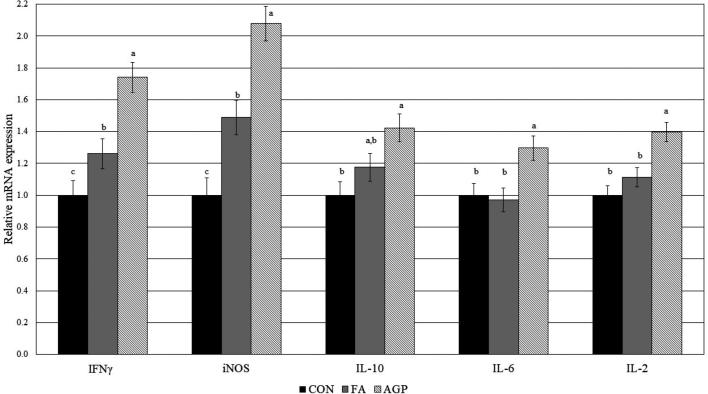
Cytokine mRNA expressions of IL-2, IL-6, and IL-10 are presented in Figure 2. For IL-1 and IL-2, no significant difference in mRNA expression between FA and CON group was observed; however, expression was significantly upregulated in the AGP group compared to FA and CON. mRNA expression of IL-10 was not different between birds fed FA and CON and birds fed FA and AGP (AGP was significantly greater than CON). iNOS and IFNƔ relative mRNA expression was significantly upregulated in FA fed birds compared to CON, yet remained significantly lower than birds fed AGP diet. Together, these findings suggest that the degree of intestinal inflammation induced by FA is less than that of the AGP.
Figure 2.
Relative mRNA expressions of interleukin-2 (IL-2), interleukin-6 (IL-6), interleukin-10 (IL-10), inducible nitric oxide synthase (iNOS), and interferon gamma (IFNƔ) in jejunum of 20-day-old broiler chickens vaccinated in hatchery for coccidiosis fed a commercial additive (FA), no additive (CON), or a medicated feed (AGP) (bacitracin methylene disalicylate). Means without a common superscript differ across treatments (P < 0.05).
Animal Health and Growth
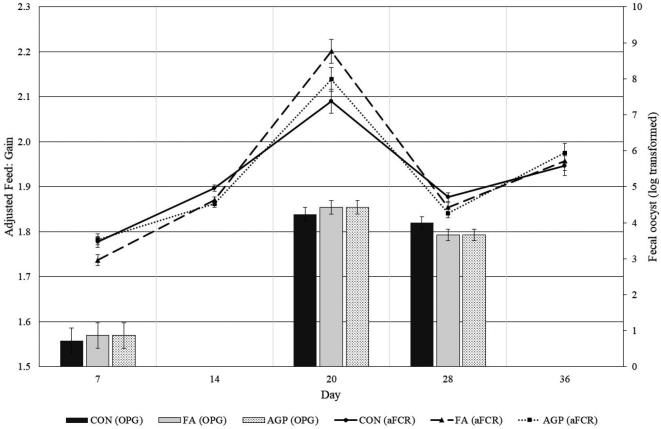
No clinical disease outbreaks were observed during the study. Cumulative mortality was not significantly different between dietary treatments (CON 3.6%, FA 3.2%, and AGP 2.3%). Similarly, foot pad scores (day 36) were not significantly different between treatments (Table 3). The majority of animals (56%) had a score of 0, indicative of a completely intact foot pad. Treatment had no significant impact on fecal oocyst counts (Figure 3), and there was no interaction between treatment and time. Overall, dietary treatment did not significantly impact animal health parameters.
Table 3.
Frequency of foot pad dermatitis scores1 in 36 day-old broiler chickens vaccinated in hatchery for coccidiosis fed a commercial additive (FA), no additive (CON), or a medicated feed (AGP) (bacitracin methylene disalicylate).
| Score | CON3 | FA | AGP3 | Sum |
|---|---|---|---|---|
| 0 | 31 | 33 | 28 | 92 (56%) |
| 1 | 7 | 9 | 12 | 28 (17%) |
| 2 | 17 | 13 | 15 | 45 (27%) |
| 3 | 0 | 0 | 0 | 0 (0%) |
1Scoring scale of Ekstrand et al. (1998), where 0 indicates completely intact foot pad and 3 represents severe lesion.
Figure 3.
Feed conversion ratio1 (primary axis) and oocyte per gram of feces2 (secondary axis) in broiler chickens vaccinated in hatchery for coccidiosis fed a commercial additive (FA), no additive (CON), or a medicated feed (AGP) (bacitracin methylene disalicylate). 1Data are presented as least square mean, feed to gain was adjusted to common body weight. 2Data were log transformed and measurements were taken on days 7, 20, and 28.
Repeated measures analysis of the time-course growth performance data (Table 4) found a significant interaction between treatment and time for BW (P = 0.005). Specifically, birds fed the FA treatment had significantly higher BW in the starter period (day 14) than CON, yet not significantly different from AGP (Table 4). The interaction between treatment and time was also significant for adjusted F: G (Table 4, Figure 3). Birds fed the FA had significantly lower F: G in the starter period (day 7 and 14) than CON. In contrast, on day 20 birds fed the FA diet had significantly higher F: G than those fed the CON diet. There were no differences in F: G between FA and CON or AGP treatments on day 28 or 36. Irrespective of dietary treatment, F: G was highest on day 20, coincident with peak oocyst shedding (Figure 3). Together, these findings suggest that birds fed the FA had improved growth (BW and F: G) in the starter period (day 0 to 14) compared to CON. The beneficial effects of the FA on growth performance were diminished in the grower and finisher feeding phases.
Table 4.
Growth measures over time in broiler chickens vaccinated in hatchery for coccidiosis fed a commercial additive (FA), no additive (CON), or a medicated feed (AGP) (bacitracin methylene disalicylate).
| CON | FA | AGP | SEM | P trt | P trt × d | |
|---|---|---|---|---|---|---|
| Body weight, kg | ||||||
| Day 7 | 153.2 | 155.6 | 154.3 | 0.9 | 0.85 | 0.02 |
| Day 14 | 408.1a | 417.5b | 415.7b | 1.9 | ||
| Day 20 | 700.8 | 689.5 | 694.9 | 4.7 | ||
| Day 28 | 1353.8 | 1347.4 | 1352.0 | 6.4 | ||
| Day 36 | 2030.0 | 2028.2 | 2010.9 | 15.3 | ||
| Average daily feed intake, g/d | ||||||
| Day 7 | 19.05 | 18.98 | 19.53 | 0.16 | 0.19 | 0.09 |
| Day 14 | 51.49 | 51.86 | 51.17 | 0.33 | ||
| Day 20 | 80.86 | 80.56 | 80.12 | 0.37 | ||
| Day 28 | 131.81 | 131.27 | 130.69 | 1.01 | ||
| Day 36 | 156.38 | 158.16 | 153.10 | 1.59 | ||
| Feed: gain1 | ||||||
| Day 7 | 1.78a | 1.74b | 1.78a | 0.01 | 0.86 | <0.01 |
| Day 14 | 1.90a | 1.87b | 1.86b | 0.01 | ||
| Day 20 | 2.09 a | 2.20 b | 2.14 a,b | 0.03 | ||
| Day 28 | 1.88 a | 1.85 a,b | 1.84 b | 0.01 | ||
| Day 36 | 1.95 | 1.96 | 1.98 | 0.02 | ||
1Feed to gain adjusted to a common body weight (raw F: G—[(BW–2.41 kg) × 0.24).
DISCUSSION
Coccidiosis is a major intestinal disease affecting broiler chickens. Live vaccines introduce low doses of Eimeria initiating cellular responses against the parasite (Dalloul and Lillehoj, 2006). In addition to the energetic cost of immune activation, recycling of the parasite in the intestine creates damage and inflammation predisposing the animal for secondary infection (Moore, 2016). The present study investigated the potential efficacy of FA, a blend of fatty acids, organic acids, and phytochemicals, to help mitigate the negative impacts of vaccination in broiler chickens raised without antibiotics or ionophores. Results of the study demonstrated that the FA treatment altered the microstructure of the duodenum and in the jejunum induced an inflammatory response that was blunted in comparison to birds fed an AGP.
Samples of intestine were collected on day 20 to evaluate the impact of the dietary treatment on intestinal morphology. In the duodenum, birds fed the FA (and AGP) had shorter villus lengths and deeper crypt depths resulting in reduced VCR compared to birds fed CON. Deeper crypts and lower VCR are indicative of increased cellular proliferation to renew villi (Gao et al., 2008). In agreement, oocyte shedding was highest on day 20 and may have contributed to increased epithelia turnover. Indeed, typically, 3 cycles of re-ingestion of excreted vaccine strain oocysts in the feces are necessary to achieve acquired immunity (Peek and Landman, 2011). This hypothesis cannot be confirmed in the present study as microbiota were not quantified and furthermore, lesion scoring was not completed. However, damage to the duodenum epithelium and localized inflammation has been documented in poultry infected with coccidiosis (Lillehoj and Trout, 1996). Eimeria acervulina preferentially colonizes the duodenum and was a component of the vaccine used in this study. The vaccine was also composed of sporulated oocytes of Eimeria tenella and Eimeria maxima. Eimeria tenella typically infects ceca, whereas E. maxima colonize the ileum (Trees, 2008). However, no differences in ileal and cecal gland morphology were noted between dietary treatments in the present study. Histomorphology measures in the jejunum were not done in this study and should be considered in future experiments, as E. maxima and E. acervulina can infect the jejunum (Trees, 2008).
The jejunum is the major site of nutrient digestion and absorption (Svihus, 2014). Given this important role, samples of jejunum were collected in this study to examine markers of intestinal barrier function and inflammation. The relative mRNA expression of the tight junction protein, CLDN-1, was upregulated in the birds fed FA and AGP compared to CON. In the AGP-treated birds, CLDN-2 was downregulated and ZO-2 upregulated compared to CON and FA. CLDN-1 has a predominantly barrier function, whereas CLDN-2 has a pore-forming role (Gunzel and Yu, 2013). How an upregulation of CLDN-1, as observed with FA and AGP, is associated with barrier integrity and permeability cannot be answered in this study. Future work that includes measures of intestinal permeability in combination with gene expression analyses is warranted. In AGP-treated birds, remodeling of the intestinal barrier may be further accelerated by a downregulation of CLDN-2 and upregulation of scaffolding protein ZO-2. Together, these findings suggest that the magnitude of the response in FA fed birds was less than that of the AGP-treated birds. However, the interpretation of these findings is limited by the small number of tight junction genes examined.
Consistent with changes in morphology and tight junction gene expression, inflammatory gene expression was also altered by dietary treatment. The mRNA expressions of proinflammatory cytokines, IFNƔ and iNOS, were upregulated in birds fed FA compared to CON. Birds fed AGP demonstrated an even greater upregulation of both cytokines. In addition, birds in the AGP treatment had a significant induction of IL-6 relative to CON. Induction of IL-6 may relate, in part, to the observed upregulation of CLDN-2 expression discussed previously (Al-Sadi et al., 2014). Previous studies in broiler chickens have demonstrated an upregulation of proinflammatory cytokines expression in response to an Eimeria challenge (Laurent et al., 2001). In acute Eimeria challenge studies, IFNƔ increased up to 200-fold post-infection and remained elevated for 7 D. Further evidence demonstrated that IFNƔ has a direct inhibitory effect on Eimeria through disruption of their replication (Lillihoj and Choi, 1998). For these reasons, upregulation of IFNƔ has been considered to be a critical component of a robust immune response to parasitic infection (Lillihoj and Choi, 1998). Of equal importance to cytokine induction is the magnitude and duration of the response. An exacerbated inflammatory response comes at a high energetic cost. In rapidly growing animals, like broiler chickens, the energy required to mount the immune response decreases the availability of energy and nutrients for growth and maintenance (Lochmiller and Deerenburg, 2000; Humphrey and Klasing, 2004).
Growth performance in FA fed birds was better than CON and AGP groups in the starter period (0 to 14 D). Interestingly, the improved efficiency observed in FA group was lost at day 20 during peak parasite replication and then later restored at day 28. These findings suggest that birds fed the FA are capable of recovering from coccidiosis challenge rapidly. It is unclear if the body weight and feed efficiency advantages observed in the starter phase were related to FA dose. The dose of FA was decreased in the grower phase and lowered again in the finisher phase as per the manufacturer's recommendations. It is possible that improvements in growth performance are dose specific or there are critical time periods of product efficacy. These hypotheses warrant further investigation. AGP treatment did not improve growth performance in this study. Lack of response may be related to lower sensitivity due to overuse (Slavić et al., 2011). Bacitracin is a narrow-spectrum antibiotic used for the prevention of necrotic enteritis via reducing intestinal Clostridia. The efficacy of bacitracin in coccidiosis-vaccinated broilers, particularly in the absence of secondary bacterial infection, requires further evaluation. Alternatively, immune activation may have impaired growth performance in the AGP birds. AGP-treated birds exhibited a 2-fold increase in proinflammatory cytokine expression, whereas FA fed birds had a more blunted response, compared to CON birds. Furthermore, markers of intestinal remodeling were increased in AGP fed birds compared to CON and FA.
CONCLUSIONS
The present study investigated the effects of FA, a blend of fatty acids, organic acids, and phytochemicals, as an alternative to antimicrobials in coccidiosis-vaccinated broiler chickens. Results indicated that birds fed the FA containing diet had reduced VCR in the duodenum and an induction of proinflammatory cytokines in the jejunum compared to birds fed the CON diet. The magnitude of these responses in FA fed birds was dampened in comparison to birds consuming a diet containing AGP. Coincident with changes in GIT structure and inflammatory response, the FA treatment experienced an impairment in feed efficiency (day 20) that was later restored in the grower feeding phase. Together, these results suggest energy is diverted from lean growth to the immune system to achieve innate immunity to Eimeria. However, a more comprehensive assessment of intestinal integrity and associated immune activation is warranted.
SUPPLEMENTARY DATA
Supplement Table S1. Primers used for the quantification of genes of interest and housekeeping gene (GAPDH).
Supplement Figure S1. Duodenum histomorphology of 20-day-old broiler chickens.
Supplement Figure S2. Ileum histomorphology of 20-day-old broiler chickens.
Supplementary Material
Acknowledgements
This study was funded by Trouw Nutrition, and L. McKnight, G. Page, and Y. Han are employees of Trouw Nutrition. D. Wright received funding from the National Science and Engineering Council of Canada ENGAGE program (EGP 517522–17).
We thank the staff at Trouw Nutrition Agresearch Burford in particular Shayne Stankov, Jennifer Norton, Heidi Laderoute, Cara Cargo-Froom, James Ovington, and Haley Belliveau for caring for the animals and assisting with animal procedures. Thank you to Dr. Zahid Nasir and Dr. Douglas Castagnino of Trouw Nutrition for their assistance with bird sampling and fecal collections. Thank you to Ryan Synder of Dr. Barta's laboratory, University of Guelph, for completing the oocyte counts.
REFERENCES
- Al-Sadi R., Ye D., Boivin M., Guo S., Hashimi M., Ereifej L., Ma T.Y.. 2014. Interleukin-6 modulation of intestinal epithelial tight junction permeability is mediated by JNK pathway activation of claudin-2 gene. Biol. Trace Elem. Res. 9:3096–3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canadian Council on Animal Care 1993. Guide to the Care and Use of Experimental Animals. Vol. 1 Offert E. D., Cross B. M., McWilliam A. A., ed. CCAC, Ontario, Ottawa, Canada. [Google Scholar]
- Dalloul R. A., Lillehoj H. S.. 2005. Recent advances in immunomodulation and vaccination strategies against coccidiosis. Avian Dis. 49:1–8. [DOI] [PubMed] [Google Scholar]
- Dalloul R. A., Lillehoj H. S.. 2006. Poultry coccidiosis: recent advancements in control measures and vaccine development. Expert Rev. Vaccines. 5:143–163. [DOI] [PubMed] [Google Scholar]
- Ekstrand C., Carpenter T. E., Anderson I., Algers B.. 1998. Prevalence and control of foot-pad dermatitis in broilers in Sweden. Br. Poult. Sci. 39:318–324. [DOI] [PubMed] [Google Scholar]
- Gao J., Zhang H. J., Yu S. H., Wu S. G., Yoon I., Quigley J., Gao Y. P., Qi G. H.. 2008. Effects of yeast culture in broiler diets on performance and immunomodulatory functions. Poult. Sci. 87:1377–1384. [DOI] [PubMed] [Google Scholar]
- Gunzel D., Yu A. S. L.. 2013. Claudin and the modulation of tight junction permeability. Physiol. Rev. 93:525–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey B. D., Klasing K. C.. 2004. Modulation of nutrient metabolism and homeostasis by the immune system. World's Poult. Sci. J. 60:90–100. [Google Scholar]
- Laurent F., Mancassola R., Lacroix S., Menezes R., Naciri M.. 2001. Analysis of chicken mucosal immune response to eimeria tenella and eimeria maxima infection by quantitative reverse transcription-PCR. Infect. Immun. 69:2527–2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G. Q., Kanu S., Xiao S. M., Xiang F. Y.. 2005. Responses of chickens vaccinated with a live attenuated multi-valent ionophore-tolerant Eimeria vaccine. Vet. Parasitol. 129:179–186. [DOI] [PubMed] [Google Scholar]
- Lillehoj H. S., Choi K. D.. 1998. Recombinant chicken interferon-gamma-mediated inhibition of Eimeria tenella development in vitro and reduction of oocyst production and body weight loss following Eimeria acervulina challenge infection. Avian Dis. 42:307–314. [PubMed] [Google Scholar]
- Lillehoj H. S., Trout J. M.. 1996. Avian gut-associated lymphoid tissues and intestinal immune responses to Eimeria parasites. Clin. Microbiol. Rev. 9:349–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K. J., Schmittgen T. D.. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. [DOI] [PubMed] [Google Scholar]
- Lochmiller R. L., Deerenburg C.. 2000. Trade-offs in evolutionary immunology: Just what is the cost of immunity? Oikos. 88:87–98. [Google Scholar]
- Ministry of Agriculture, Fisheries and Food (MAFF) 1986. Manual Veterinary Parasitological Laboratory Techniques Third Edition. H. M. S. O., London, Great Britain. [Google Scholar]
- Moore R. J. 2016. Necrotic enteritis predisposing factors in broiler chickens. Avian Pathol. 45:275–281. [DOI] [PubMed] [Google Scholar]
- Osselaere A., Santos R., Hautekiet V., De Backer P., Chiers K., Ducatelle R., Croubels S.. 2013. Deoxynivalenol impairs hepatic and intestinal gene expression of selected oxidative stress, tight junction and inflammation proteins in broiler chickens, but addition of an adsorbing agent shifts the effects to the distal parts of the small intestine. PLoS One. 8:e69014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peek H.W., Landman W. J. M.. 2011. Coccidiosis in poultry: anticoccidial products, vaccines and other prevention strategies. Vet. Q. 31:143–161. [DOI] [PubMed] [Google Scholar]
- Peppler W.T., Anderson Z.G., Sutton C.D., Rector R.S., Wright D.C.. 2016. Voluntary wheel running attenuates lipopolysaccharide-induced liver inflammation in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 310:R934–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethiya N. K. 2016. Review on natural growth promoters available for improving gut health of poultry: an alternative to antibiotic growth promoters. Asian J. Poult. Sci. 10:1–29. [Google Scholar]
- Slavić D., Boerlin P., Fabri M., Klotins K. C., Zoethout J. K., Weir P. E., Bateman D.. 2011. Antimicrobial susceptibility of Clostridium perfringens isolates of bovine, chicken, porcine, and turkey origin from Ontario. Can. J. Vet. Res. 75:89–97. [PMC free article] [PubMed] [Google Scholar]
- Song Z., Cheng K., Zhang L., Wang T.. 2017. Dietary supplementation of enzymatically treated Artemisia annua could alleviate the intestinal inflammatory response in heat-stressed broilers. J. Therm. Biol. 69:184–190. [DOI] [PubMed] [Google Scholar]
- Stringfellow K., Caldwell D., Lee J., Mohnl M., Beltran R., Schatzmayr G., Fitz-Coy S., Broussard C., Farnell M.. 2011. Evaluation of probiotic administration on the immune response of coccidiosis-vaccinated broilers. Poult. Sci. 90:1652–1658. [DOI] [PubMed] [Google Scholar]
- Svihus B. 2014. Function of the digestive system. J. Appl. Poult. Res. 23:306–314. [Google Scholar]
- Trees A. J. 2008. Parasitic diseases. Pages 444–469 in Poultry Diseases, 6th ed. Pattison M., McMullin P. F., Bradbury J. M., Alexander D. J., eds. Saunders Elsevier Limited, Philadelphia, PA. [Google Scholar]
- Voeten A. C., Braunius W. W., Orthel F. W., van Rijen M. A.. 1988. Influence of coccidiosis on growth rate and feed conversion in broilers after experimental infections with Eimeria acervulina and Eimeria maxima. Vet. Q. 10:256–264. [DOI] [PubMed] [Google Scholar]
- Wade B., Keyburn A. L.. 2015. The true cost of necrotic enteritis. World Poult. 31:16–17. [Google Scholar]
- Wang Y. C., Deng J. L., Xu S. W., Peng X., Zuo Z. C., Cui H. M., Ren Z. H.. 2012. Effects of zearalenone on IL-2, IL-6, and IFN-gamma mRNA levels in the splenic lymphocytes of chickens. ScientificWorldJournal. 2012:567327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. B. 2002. Anticoccidial vaccines for broiler chickens: pathways to success. Avian Pathol. 31:317–353. [DOI] [PubMed] [Google Scholar]
- Yu J., Yao H., Gao X., Zhang Z., Wang J. F., Xu S. W.. 2015. The role of nitric oxide and oxidative stress in intestinal damage induced by selenium deficiency in chickens. Biol. Trace Elem. Res. 163:144–153. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Eicher S. D., Ajuwon K. M., Applegate T. J.. 2017. Development of a chicken ileal explant culture model for measurement of gut inflammation induced by lipopolysaccharide. Poult. Sci. 96:3096–3103. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.