Abstract
Objectives
The onset of many illnesses is confounded with age and sex. Increasing age is a risk factor for the development of many illnesses, and sexual dimorphism influences brain anatomy, function, and cognition. Here, we examine frequency-specific connectivity in resting-state networks in a large sample (n = 406) of healthy aged adults.
Method
We quantify frequency-specific connectivity in three resting-state networks known to be implicated in age-related decline: the default mode, dorsal attention, and salience networks, using multiband functional magnetic resonance imaging. Frequency-specific connectivity was quantified in four bands: low (0.015–0.027 Hz), moderately low (0.027–0.073 Hz), moderately high (0.073–0.198 Hz), and high (0.198–0.5 Hz) frequency bands, using mean intensity and spatial extent. Differences in connectivity between the sexes in each of the three networks were examined.
Results
Each network showed the largest intensity and spatial extent at low frequencies and smallest extent at high frequencies. Males showed greater connectivity than females in the salience network. Females showed greater connectivity than males in the default mode network.
Discussion
Results in this healthy aged cohort are compatible with those obtained in young samples, suggesting that frequency-specific connectivity, and differences between the sexes, are maintained into older age. Our results indicate that sex should be considered as an influencing factor in studies of resting-state connectivity.
Keywords: Functional connectivity, fMRI, Multiband fMRI, Sex, Resting-state
Evidence from human cognitive neuroimaging supports the notion of sexual dimorphism in cognition, brain anatomy, and function. Females tend to outperform males on tests of verbal abilities (Halpern, 1992) and males outperform females in tests of visuospatial ability (Maccoby & Jacklin, 1974). Sexual dimorphism is evident across the brain: even regions that show no anatomical differences between the sexes may show differences in function, neurotransmitter release, sex hormone receptor density, genetic expression, or metabolic response between the sexes (reviewed in Cahill, 2006). It is believed that many of the factors responsible for sexual dimorphism in cognition, brain structure, and brain function may be particularly susceptible to the ageing process (DeFrias, Nillson, & Herlitz, 2006). For example, men may show accelerated brain ageing (i.e., atrophy) compared to women (Gur et al., 1991).
The onset of many illnesses is confounded with age and sex. Increasing age is a risk factor for the development of illnesses such as Alzheimer’s disease and other dementias, cerebrovascular illnesses, and white matter disease, among others. Greater longevity in females than males is well established (see e.g. review Maklakov & Lummaa, 2013), however it remains unclear whether the trajectories of age-related cognitive decline differs between the sexes. Many studies report no differences in age-related cognitive decline trajectory (e.g., DeFrias et al., 2006), whereas others have reported sharper rates of decline in men (Gur & Gur, 2002; Wiederholt et al., 1993) or women (Proust-Lima et al., 2008). Within the brain, anatomical differences between males and females have long been established: males show larger overall brains (Chen, Sachdev, Wen, & Anstey, 2007), while females have proportionately more grey matter compared to white matter volume (Allen, Damasio, Grabowski, Bruss, & Zhang, 2003) and more efficiently connected white matter network (Gong et al., 2009) than males. Sex differences in brain structure have mostly been studied in younger adults; the studies that have examined sex differences in brain structure in older age suggest these differences are maintained into older age (Jäncke et al., 2015; Ruigrok et al., 2014), although the sex differences in grey to white matter volume proportion may reduce with age (Jäncke et al., 2015). The effect of sex on functional connectivity is less clear: with most (e.g., Allen, Erhardt, Damaraju, Gruner, Segall, 2011; Biswal et al., 2010; Filippi et al., 2013; Scheinost et al., 2015; Tomasi & Volkow, 2012) but not all (e.g., Weissman-Fogel, Moayedi, Taylor, Pope, & Davis, 2010) reporting sex differences in functional connectivity. Of those studies that do report sex differences in functional connectivity, sex differences are seen across most resting-state networks (Biswal et al., 2010). Most studies report greater default mode connectivity in females compared to males in young samples (Allen et al., 2011; Biswal et al., 2010; Filippi et al., 2013; Tomasi & Volkow, 2012). Others have reported differences between the sexes obtained in network lateralization (Liu et al., 2009; Tian et al., 2011) and in within versus between network connectivity (Satterthwaite et al., 2015).
Functional connectivity is often measured using resting-state functional magnetic resonance imaging (fMRI). Resting-state fMRI indexes spontaneous large-amplitude changes in blood oxygenation level dependent (BOLD) signal at low frequencies (Biswal et al., 1995; Cordes et al., 2001). Brain regions that exhibit temporal correlation between low frequency fluctuations are called resting-state networks (Beckmann, DeLuca, Devlin, & Smith, 2005; Damoiseaux et al., 2006). A number of reliable and consistent resting-state networks have been identified, reflecting intrinsic brain functional connectivity (reviewed in Laird et al., 2011). Resting-state networks show moderate-high test–retest reliability and replicability (Shehzad et al., 2009). Resting-state connectivity is typically examined in frequencies up to 0.1 Hz, for two reasons. Firstly, the sampling rate of standard resting-state fMRI protocols use a TR of around 2 s, limiting the bandwidth to 0–0.25 Hz. Recent advances in multiband fMRI sequences (Feinberg & Yacoub, 2012) have increased the temporal resolution to 0.5 s or less, effectively widening the available bandwidth up to ~5 Hz. Secondly, the neural component of the BOLD response has previously been thought to be dominated by oscillations within the 0.01–0.1 Hz range (Cordes et al., 2001). This contrasts with evidence from more recent studies that have confirmed the presence of neural oscillations at higher frequencies (e.g., Gohel & Biswal., 2015; Kalcher et al., 2014; Lee et al., 2013). Due to these reasons, very few studies have attempted to quantify resting-state connectivity at frequencies above 0.1 Hz.
Age-related reduction in connectivity in resting-state networks, particularly in the default mode, dorsal attention, and salience networks, is considered to be a hallmark of age-related cognitive decline (Geerligs, Renken, Saliasi, Maurits, & Lorist, 2015; Tomasi & Volkow, 2012). Reduction in default mode network connectivity with age is well established, and older adults are less likely to disengage the default mode network during externally guided cognition (Andrews-Hanna et al., 2007). The dorsal attention and salience networks subserve cognitive processes that are particularly susceptible to the ageing process, including selecting behaviorally relevant stimuli, directing attention towards those stimuli, and updating behavioral goals (see Grady, 2008; Uddin, 2016 for reviews). It is thought that reduced connectivity within default mode, dorsal attention and salience networks, and between these networks and other “lower order” sensory/motor networks, may contribute to the widespread changes in cognition during the ageing process (Geerligs et al., 2015). Older adults show decreased connectivity within resting-state networks, becoming less efficient and differentiated (Achard & Bullmore, 2007; Geerligs et al., 2015). While within-network connectivity may reduce with age, between-network connectivity increases with age (Betzel et al., 2014). Connectivity is reduced within networks particularly along the anterior–posterior axis (Allen et al., 2011; Andrews-Hanna et al., 2007) and the left-right axis (Zuo, Di Martino, Kelly, Shehzad, & Gee, 2010). Long-range connections (i.e. connectivity between regions located further away) show greater impairment than short-range connections within resting state networks (Tomasi & Volkow, 2012).
Sex differences in resting-state fMRI are rarely considered (Biswal et al., 2010), despite the fact that sexual dimorphism is known to affect the physiological processes that underlie the BOLD-fMRI signal, including cerebral blood flow (Aanerud, Borghammer, Rodell, Jónsdottir, & Gjedde, 2017), cerebral glucose metabolism (Gur et al., 1995), and hematocrit volume (Zeng, Yankowitz, Widness, & Strauss, 2001). These physiological factors may partly explain global differences in BOLD-fMRI intensity between the sexes, but they do not fully account for local regional differences in measures such as functional connectivity (Biswal et al., 2010). While the effect sizes tend to be small (around 5–15%, Allen et al., 2011), most studies report that young females show greater functional connectivity than males within nodes of the default mode network (Allen et al., 2011; Biswal et al., 2010; Tomasi & Volkow, 2012). A smaller number of studies report sex differences in attention, salience, sensorimotor, and visual networks (Allen et al., 2011; Filippi et al., 2013; Scheinost et al., 2015).
The aims of this study were twofold. (1) We aimed to quantify resting-state connectivity in healthy ageing using objective metrics of connectivity. We used multiband resting-state fMRI to quantify the frequency-specific connectivity in these three resting-state networks; here our imaging protocol allowed examination of connectivity up to 0.66 Hz (Supplementary Figure 2). To date, only a handful of studies have attempted to quantify the frequency-specific connectivity of resting state networks using objective metrics (e.g., Gohel & Biswal, 2015), and no study to date has quantified frequency-specific resting state connectivity in healthy ageing. (2) We aimed to explore age-related sex differences in functional connectivity in three resting-state networks relevant to cognitive ageing: the default mode network, dorsal attention network, and salience network. We hypothesized that resting-state network connectivity will differ between the sexes in this sample of healthy elders, particularly in the default mode network (Allen et al., 2011; Biswal et al., 2010; Tomasi & Volkow, 2012). Consistent with evidence from young samples, we predicted that females will show greater connectivity than males in resting-state networks.
We examined functional connectivity across five slow-wave bands in a large healthy aged sample that forms part of the ASPirin in Reducing Events in the Elderly (ASPREE) clinical trial, specifically the ASPREE-Neuro substudy (Ward et al., 2017). The ASPREE trial and ASPREE-Neuro substudy aimed to determine the effect of daily low-dose aspirin on health outcomes in healthy older adults. The ASPREE-Neuro substudy specifically aimed to determine the effect of low-dose aspirin on a range of MRI biomarkers, including cerebral microbleeds and white matter hyperintensity, over a 3-year period, in 559 adults aged 70 years and over (Ward et al., 2017).
Methods
Full details of the MR image acquisition, preprocessing, first-level, and group independent component analyses (ICA) are provided in Supplementary Material (Methods).
Participants
Data formed part of the baseline cohort (i.e. pre-medication) in the ASPREE trial, a multicentre, randomized double-blind placebo controlled trial of daily 100 mg aspirin in 19,000 healthy community dwelling older adults in Australia and the United States. Inclusion and exclusion criteria were published previously (ASPREE Investigator Group, 2013). Participants were eligible in Australia if aged 70 years and over, had no past history of occlusive vascular disease, atrial fibrillation, cognitive impairment, or disability, were not taking antithrombotic therapy, and did not have anemia or a diagnosis likely to cause death within 5 years. Baseline characteristics of the full ASPREE sample are reported in McNeil et al. (2017). Data in this sample was obtained from the ASPREE-Neuro substudy (Ward et al., 2017). At study entry, each participant had a Modified Mini Mental Status Examination (3MS, Teng & Chui, 1987) score of at least 78/100; 3MS was conducted on average 54 ± 17 days (mean, SD) prior to MRI. Additional exclusion criteria specific to ASPREE-Neuro included the presence of contraindications to MRI, including metallic or electronic implants not known to be safe at 3T, and claustrophobia.
For this analysis, participants (N = 553) were aged 70–88 years; n = 293 subjects were male, n = 260 subjects were female. Data from five patients were discarded due to a different number of time points in the resting state scan. One hundred and forty-two data sets were removed for poor MRI registration accuracy (see Supplementary Materials). In the final sample, 406 subjects (age range = 70–88 years, mean age 75 years, standard deviation 3.5 years, n = 223 male, n = 183 female) were retained. Supplementary Figure 1 provides a frequency histogram of ages in the final sample.
Image Acquisition and Preparation
ASPREE-Neuro sequence protocols are described in detail in Ward et al. (2017). Here, we used T1 and multiband rsfMRI (TR = 754 ms; TA = 5.16 min; eyes open) data. All subject data underwent standard resting-state fMRI (rsfMRI) pre-processing, implemented using tools from FSL (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FSL), and AFNI (Cox, 1996) (see Supplementary Methods).
Group ICA
Group ICA across the whole brain identified ten resting-state networks. The focus of the current report is on the default mode, dorsal attention, and salience networks. Details about the other seven networks can be found in the Supplementary Materials.
Resting-state data was parsed into five frequency bands: low frequency (slow-5, 0.015–0.027), moderately low frequency (mod-low: slow-4, 0.027–0.073), moderately high frequency (mod-high: slow-3, 0.073–0.198) high frequency (slow-2, 0.198–0.5), and very high frequency (0.5–0.66 Hz) bands. The very high frequency band resulted only in noisy components, so was removed from further analysis. A MELODIC analysis was then conducted to compute 15 independent components.
Within-Network Connectivity Analysis
To quantify within-network connectivity, we examined the mean intensity and spatial extent of subregions within the resting state networks of interest. The mean intensity of a subregion represents the strength of the connectivity. The mean intensity values reported here are Z scores, computed from the r-maps of connectivity. The spatial extent provides a measure of whether a subregion is apparent at all frequency bands (e.g., a region may be present at one but not all frequencies), and whether the size of the activated area within a region is consistent across frequencies. The spatial extent values reported here are numbers of voxels in a cluster. A seed-region based approach was used to explore the spatial extent of subregions within three major resting state networks (default mode, dorsal attention and salience networks), in five different frequency bands (whole band, low, mod-low, mod-high, and high frequency bands). The seeds used to generate the networks were: default mode (posterior cingulate cortex, PCC; 2, −56, 26), dorsal attention (inferior parietal sulcus, IPS; −24, −54, 52), and salience network (insula; −44, 12, −2), three spheres (radius 6 mm) were created. The average time-series extracted from those spheres were correlated with all brain voxels to derive a subject-level seed-based correlation map for each seed. These maps were converted to z-scores using Fisher’s r to z transformation and then further processed using MELODIC Gaussian Mixture Model correction, to account for temporal smoothness.
To quantify how the subnetwork connectivity varies with frequency, we extracted the spatial extent of activated regions and their mean intensity from the whole band and band pass filtered frequency bands using regions of interest (ROIs) from Shirer, Ryali, Rykhlevskaia, Menon, & Greicius (2012). Spatial extent (voxel counts) and mean Z-score intensity (extracted from r maps) of each ROI was calculated each subject. Then, for each subregion, one N × M matrix containing N = 406 (one per subject) measures of activated voxels across M = 4 frequency bands, was subjected to a four frequency band (low, mod-low, mod-high, high) repeated measures ANOVA, with repeated planned contrasts to determine significant differences in spatial extents of resting-state networks between frequency bands.
To control for multiple comparisons, alpha was divided by the number of regions of interest within each network. So, for the default mode network, α = 0.5/12 = 0.00416; for the dorsal attention network, α = 0.5/8 = 0.00625; and for the salience network, α = 0.5/11 = 0.00454.
Sex Analysis
The effect of sex was first tested on the spatial extent and mean intensity of the whole band data, using an independent samples t-test, equal variances not assumed. Secondly, the effect of sex on the frequency bands was examined using a 2 sex (female, male) × 4 frequency band (low, mod-low, mod-high, high) repeated measures ANOVA. As in the within-network analysis, to control for multiple comparisons, alpha was divided by the number of regions of interest within each network, yielding the same α thresholds.
Results
Quantification of Resting-State Networks
To quantify the differences in the default mode, dorsal attention, and salience networks, we compared the spatial extent (Figure 1; Supplementary Tables 1 and 2) and mean intensity (Figure 2; Supplementary Tables 3 and 4) of each network’s subregions across frequency bands.
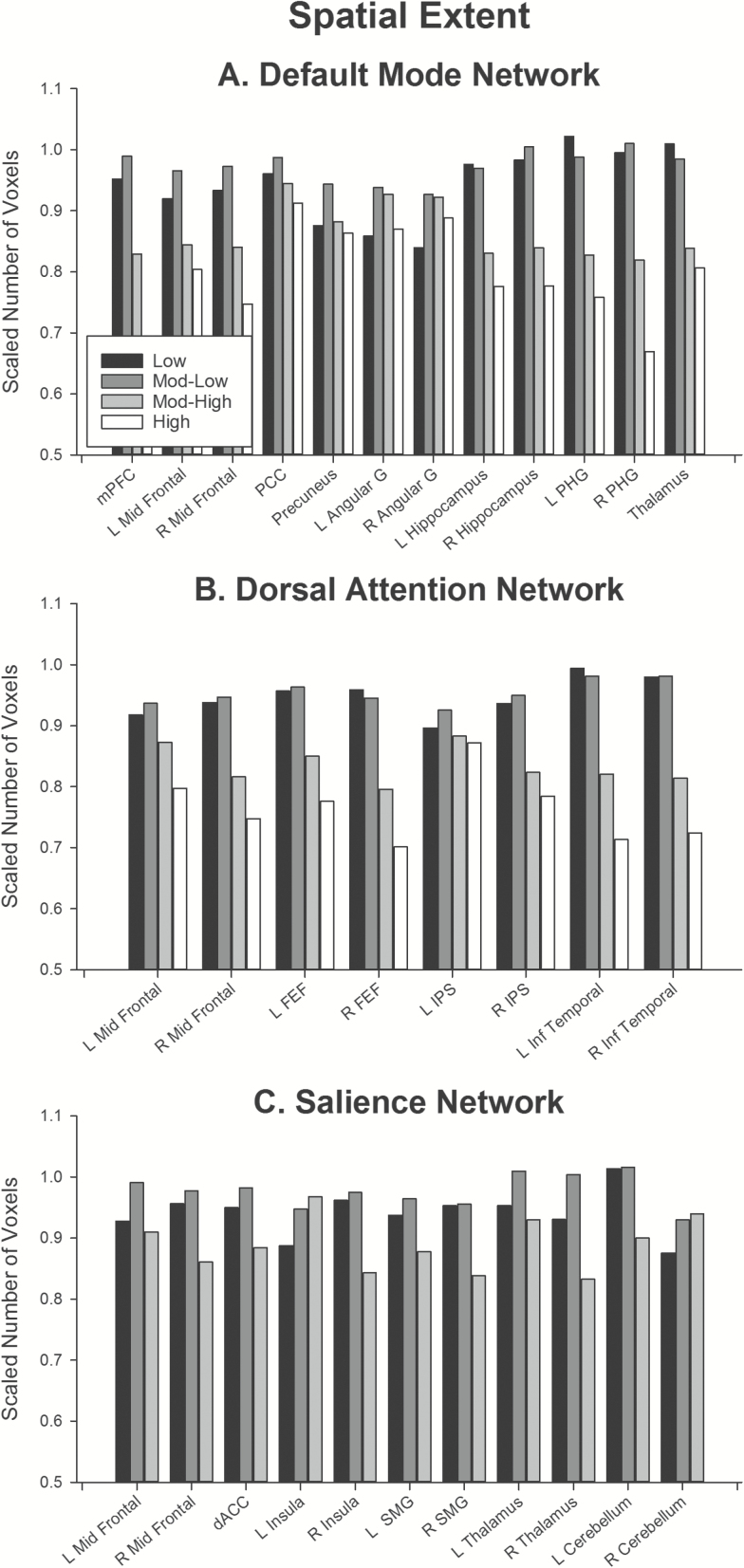
Figure 1.
Spatial extent of subregions for (A) default mode, (B) dorsal attention, and (C) salience network. To improve comparison of subregions differing substantially in size, the spatial extent of each frequency was divided by the spatial extent of that region in the whole band. Supplementary Tables 1 and 2 report raw spatial extents and statistical tests for each effect reported here. Abbreviations: angular, angular gyrus; dACC, dorsal anterior cingulate cortex; FEF, frontal eye fields; IPS, intraparietal sulcus; Inf Temporal, inferior temporal gyrus; L, left; mPFC, medial prefrontal cortex; Mid Frontal, middle frontal gyrus; PCC, posterior cingulate cortex; PHG, parahippocampal gyrus; R, right; SMG, supramarginal gyrus.
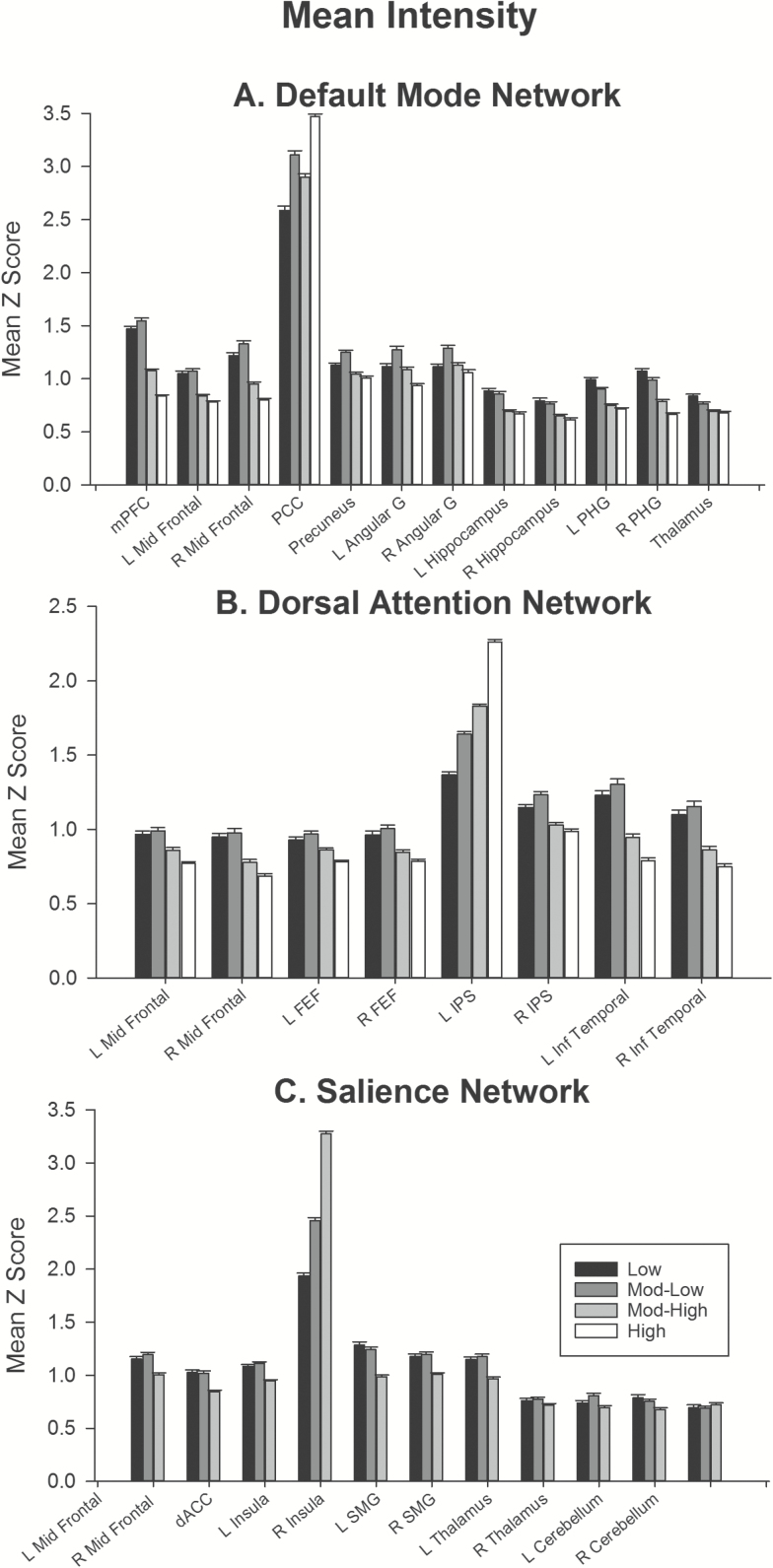
Figure 2.
Mean intensity of sub-regions for (A) default mode, (B) dorsal attention, and (C) salience network. Error bars show standard error. Supplementary Tables 3 and 4 report mean intensities and statistical tests for each effect shown here. Note that the mean intensity of the seed regions (default mode: posterior cingulate; dorsal attention: left intraparietal sulcus; salience: left insula) are larger than for all other regions. As these networks were created using a seed-based approach, the resulting maps are r-maps, which were then transformed to Z-scores. Hence, the value for each of the seed regions represents correlation of that region with itself. Abbreviations: angular, angular gyrus; dACC, dorsal anterior cingulate cortex; FEF, frontal eye fields; IPS, intraparietal sulcus; Inf Temporal, inferior temporal gyrus; mPFC, medial prefrontal cortex; mid Frontal, middle frontal gyrus; PCC, posterior cingulate cortex; PHG, parahippocampal gyrus; SMG, supramarginal gyrus; L, left; R, right.
The variation in spatial extent of regions within the dorsal attention, default mode, and salience networks across frequency bands is shown in Figure 1. To facilitate comparison of effects across regions that differ substantially in size, each frequency band for each region was normalized to the number of voxels present in the whole band. One common feature between these three networks is that for almost all subregions, the maximum spatial extent was obtained in the whole band, or in the low frequency (low and mod-low) bands. Most regions showed a linear decrease in spatial extent with increasing frequency (Supplementary Table 2, trends). Figure 2 shows the variation in mean intensity of regions within the three networks across frequency bands. Most regions showed a linear reduction in mean intensity with increased frequency band (Supplementary Table 4, trends).
In the default mode network, spatial extent was significantly lower in low than mod-low band in cortical regions: mid-prefrontal cortex, posterior cingulate, precuneus, and bilateral angular gyri. In contrast, the hippocampus, parahippocampal gyrus, and thalamus showed no difference in spatial extent between low and mod-low bands. For all regions, spatial extent reduced significantly with increase in frequency band from mod-low to high frequency bands. A similar pattern was obtained for the mean intensity of regions within the default mode network. All cortical regions showed a trend towards increased intensity in mod-low versus low frequency, although in some regions this effect was at trend level and did not survive correction for multiple comparisons. The hippocampus, parahippocampal gyrus, and thalamus showed the opposite effect, with lower intensity in mod-low versus low frequency bands. Mean intensity in all regions except posterior cingulate reduced between mod-low and high frequency bands.
A similar pattern of effects was obtained in the dorsal attention network. In all regions except the left intraparietal sulcus, low, and mod-low bands did not differ in spatial extent; and extent reduced linearly with increasing frequency band from mod-low to high frequencies. In the left intraparietal sulcus, spatial extent was larger in mod-low than low frequency and did not reduce with increasing frequency band. Mean intensity comparisons mirrored this pattern: across regions, the overall trend was for no significant difference in intensity between low and mod-low bands, with a linear reduction in intensity with increasing frequency between mod-low and high frequency bands. The left intraparietal sulcus was a notable exception to this trend, with a linear increase in intensity with increasing frequency.
In the salience network, most regions showed a trend towards smaller spatial extent in low versus mod-low bands, however this trend in many regions did not survive correction for multiple comparisons. Most regions showed trends towards linear reduction in extent with increased frequency (to mod-high band), except the right cerebellum and left insula. The right cerebellum showed no significant difference in spatial extent across frequency bands, whereas the left insula showed an increase in extent with increasing frequency. In the mean intensity comparisons, regions did not differ significantly in intensity between low mod-low frequencies, and showed a reduction in intensity between mod-low and mod-high frequencies. Again, the left insula was a notable exception to this trend, which showed the opposite effect: an increase in mean intensity with increasing frequency band.
Effect of Sex on Resting State Connectivity
Overall, the effect of sex on resting-state connectivity was modest. Figures 3 and 4 show the effect of sex on the spatial extent and mean intensity of resting state networks. Supplementary Tables 5 and 7 report effect sizes for each network and frequency band for females and males separately. Effect sizes for spatial extent for females ranged from 43 to 4366 voxels, and for males from 42 to 4098 voxels. Z scores for mean intensity ranged from 0.60 to 5.66 for females, and 0.62 to 5.38 for males. Supplementary Tables 6 and 8 report statistical results of sex and sex by frequency for each network.
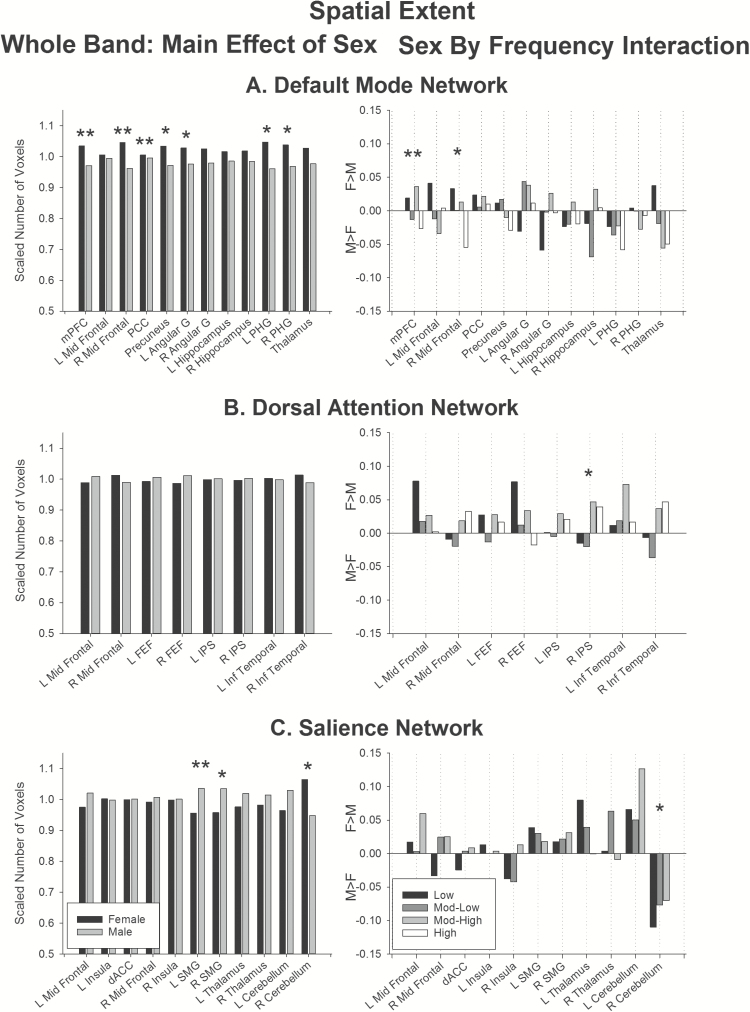
Figure 3.
Effect of sex on spatial extent. (Left) Main effect of sex in the whole band data. (Right) Sex by Frequency interaction. Bars represent female–male differences. Positive values show regions showing greater extent for females than males, negative values show regions showing greater extent for males than females. Double asterisks indicate regions significant above the threshold corrected for multiple comparisons. Single asterisks indicate regions that did not survive correction for multiple comparisons. Abbreviations: angular, angular gyrus; dACC, dorsal anterior cingulate cortex; FEF, frontal eye fields; IPS, intraparietal sulcus; Inf temporal, inferior temporal gyrus; L, left; mPFC, medial prefrontal cortex; mid frontal, middle frontal gyrus; PCC, posterior cingulate cortex; PHG, parahippocampal gyrus; SMG, supramarginal gyrus; R, right.
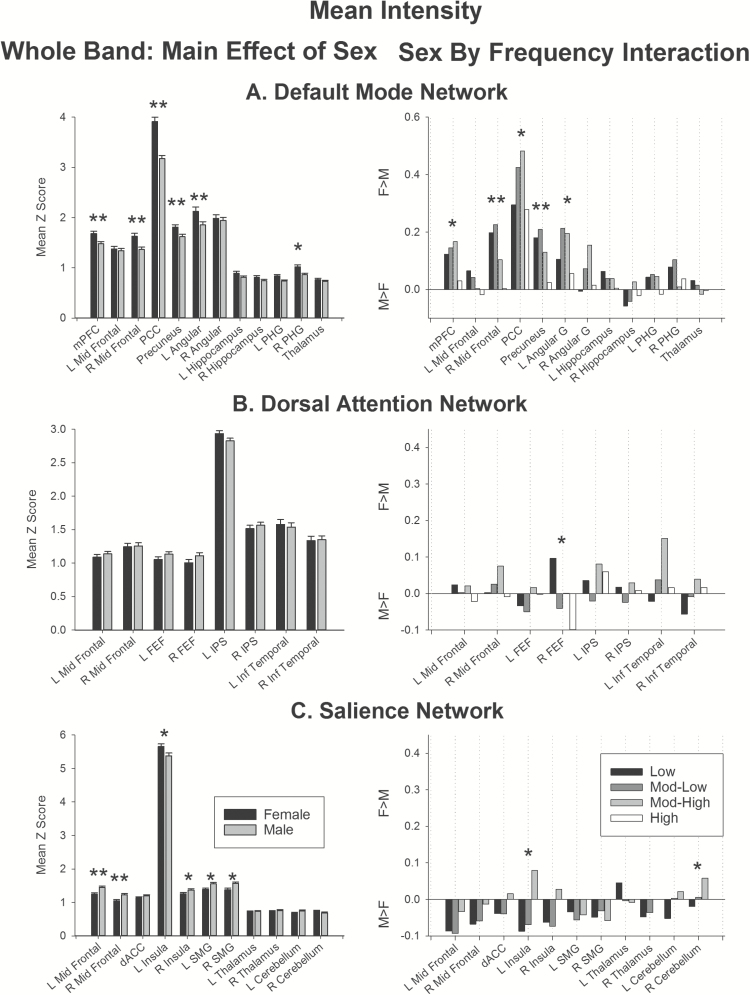
Figure 4.
Effect of sex on mean intensity. (Left) Main effect of sex in the whole band data. (Right) Sex by Frequency interaction. Bars represent female–male differences. Positive values show regions showing greater extent for females than males, negative values show regions showing greater extent for males than females. Double asterisks indicate regions significant above the threshold corrected for multiple comparisons. Single asterisks indicate regions that did not survive correction for multiple comparisons. Abbreviations: angular, angular gyrus; dACC, dorsal anterior cingulate cortex; FEF, frontal eye fields; L, left; IPS, intraparietal sulcus; inf temporal, inferior temporal gyrus; mPFC, medial prefrontal cortex; mid frontal, middle frontal gyrus; PCC, posterior cingulate cortex; PHG, parahippocampal gyrus; SMG, supramarginal gyrus; R, right.
Whole band data (Figures 3 and 4, left panels)
In the whole-band data, females showed greater mean intensity in the default mode network (Figure 4A, left), specifically in the medial prefrontal cortex, right middle frontal gyrus, precuneus, and posterior cingulate. Marginal effects were also obtained in the right parahippocampal gyrus (Figure 4A left, single asterisks). The medial prefrontal cortex, right middle frontal gyrus and posterior cingulate in this network also showed larger spatial extent (Figure 3A, left) in females than males, with marginal effects obtained in the precuneus, left angular gyrus, and bilateral parahippocampal gyrus.
In the dorsal attention network, there was no significant effect of sex on spatial extent (Figure 3B, left), or mean intensity (Figure 4B, left).
In the salience network, males showed greater mean intensity than females in the left and right middle frontal gyrus, with marginal effects in right insula and bilateral supramarginal gyrus (Figure 4C, left). In contrast, females showed greater spatial extent in the left supramarginal gyrus, and marginally in the right supramarginal gyrus, compared to males (Figure 3C, left).
In summary, the whole band data suggest that females show greater connectivity than males in the default mode network. Further, males show greater connectivity in portions of the salience network, and females show a larger spatial extent of activity in the supramarginal gyrus in the salience network.
Sex by frequency interaction (Figures 3 and 4, right panel)
The largest differential effect of sex on frequency-specific connectivity was obtained in the default mode network. This network showed largest spatial extent at low frequencies for females, and largest extent at mod-low and high frequencies in males in the medial prefrontal cortex (Figure 3A, right). Marginal effects of sex on frequency-specific connectivity were also obtained for the right mid frontal region (Figure 3A, right, single asterisks). The default mode network also showed higher mean intensity for females versus males at low, mod-low and mod-high frequencies in right middle frontal region and precuneus (Figure 4A, right); with marginal effects in the medial prefrontal cortex, posterior cingulate, and left angular gyrus.
There were no significant effects of sex on frequency-specific connectivity (Figures 3B and 4B, right panels) in the dorsal attention network. Marginal effects were obtained in the right intraparietal sulcus for spatial extent and right frontal eye fields for mean intensity.
Likewise, in the salience network, only marginal effects of sex on frequency-specific connectivity were obtained (Figures 3C and 4C, right panels), specifically in the right cerebellum for spatial extent, and left insula and right cerebellum for mean intensity.
In sum, only the default mode network showed differences in frequency-specific connectivity between the sexes after correcting for multiple comparisons. The overall trend showed increased connectivity for females compared to males at low, mod-low and mod-high frequencies in frontoparietal components of the default mode network.
Discussion
The aims of this study were twofold: (1) quantify frequency-specific connectivity in three resting-state networks relevant to cognitive ageing; and (2) examine the effects of sexual dimorphism on connectivity in those same resting-state networks.
Frequency-Specific Characteristics of Resting-State Networks
Using ICA decomposition, we identified 10 resting-state networks in healthy aged individuals. To quantify the differences in the resting state networks across frequency bands, we examined the variation in spatial extent and mean intensity for three networks relevant to cognitive ageing: the default mode, dorsal attention, and salience networks. The three networks showed relatively consistent results, with largest spatial extent and mean intensity in the lowest frequencies (low and mod-low) and smallest extent and intensity at high frequencies (mod-low and high). This reduction in connectivity with increasing frequency band was roughly linear across regions within each network.
The default mode, dorsal attention, and salience networks are particularly noteworthy in the context of cognitive ageing, as the intrinsic connectivity of these networks influences higher-order cognition. The default mode network (Raichle et al., 2001) subserves internally directed cognition and is often anticorrelated with task performance—that is, activity in this network is reduced or inhibited during the performance of many cognitive tasks (Buckner et al., 2005). The dorsal attention network (Fox, Corbetta, Snyder, Vincent, & Raichle, 2006) subserves externally driven cognition and is involved in top-down orienting of attention during cognitive tasks. The salience network (Menon, 2015; Seeley et al., 2007) is an exogenously focused network that integrates information about conflict monitoring, interoception, and reward processing centers to detect salient information in the environment. Ageing results in reduced functional connectivity of these networks (e.g., Andrews-Hanna et al., 2007; He et al., 2014), and older subjects show reduced modularity (Geerligs et al., 2015), reduced network efficiency (Achard & Bullmore, 2007), and reduced long-range connections between networks (Tomasi & Volkow, 2012). Very few studies have specifically examined age effects on the amplitude of high-frequency spontaneous fluctuations of resting-state networks using fMRI. Using standard-TR fMRI, Allen et al. (2011) found a decrease in low frequency power and mean intensity with age across 75 resting-state networks, primarily between 0 and 0.15 Hz (low to mod-high bands here). They found that default mode and attentional networks showed a slightly more significant decline in connectivity with age compared to other networks. Evidence from near infrared spectroscopy confirms that ageing reduces spontaneous and task-related fluctuations in the cerebral microvasculature: Schroeter, Schmiedel, and von Cramon (2004) demonstrated reduced hemodynamic oscillations at 0.07–0.11 Hz (approximately mod-low band here) but not at 0.01–0.05 Hz (~low band); whereas Vermeij et al. (2014) demonstrated reductions in hemodynamic oscillations across all studied frequencies (0.02–0.35 Hz).
Ageing leads to degeneration of the vascular system (Marín, 1995), which affects hemodynamic measures like BOLD fMRI (D’Esposito, Zarahn, Aguirre, & Rypma, 1999). Blood volume, total red cell mass, cerebral perfusion, cerebral metabolic rate of oxygen, and cerebral glucose utilization decrease with age (reviewed in Marín, 1995; and Schroeter et al., 2004), the interaction of which has a yet-unknown effect on the BOLD-fMRI response (D’Esposito, Deouell, & Gazzaley, 2003). The participants in this sample had no history of occlusive vascular disease, atrial fibrillation, were not currently taking antithrombotic therapy and were not anemic (Ward et al., 2017), ruling out some common issues of studying older individuals using fMRI. It remains possible that some of the modest differences identified between the current and past studies (e.g., Gohel & Biswal, 2015) may be attributable to differences in the underlying signal between young and older subjects.
Effect of Sexual Dimorphism on Resting-State Network Connectivity
Overall, the effect of sex on resting-state functional connectivity was modest, particularly in the dorsal attention network, where no effect of sex survived correction for multiple comparisons. This is consistent with some previous reports (e.g., Weissman-Fogel et al., 2010). Within the salience network, males showed greater connectivity than females. No differences were obtained in the frequency-specific analyses after correcting for multiple comparisons. This result is compatible with the results of Filippi et al. (2013) who showed that inter-network connectivity between the salience network and sensorimotor network was larger in males than females. The salience network is active when salient events change the current focus of attention (Corbetta & Shulman, 2002; Menon, 2015), and so is an exogenously focused network. Note that while males showed greater mean intensity than females throughout the salience network, females did show larger spatial extent of the supramarginal gyrus in this network. The salience network has been shown to reduce in connectivity with age and in Alzheimer’s disease, but not in mild cognitive impairment (He et al., 2014). Interestingly, Alzheimer’s disease is more prevalent in females (von Strauss, Viitanen, De Ronchi, Winblad, & Fratiglioni, 1999) suggesting that early sex differences in salience network connectivity may be a related to sexual dimorphism of Alzheimer’s disease prevalence.
The largest effects of sex were obtained in the default mode network, where females showed greater connectivity than males, particularly at low, mod-low, and high-frequencies. This is consistent with previous studies that have reported greater default mode connectivity in females than males in young samples (Allen et al., 2011; Biswal et al., 2010; Filippi et al., 2013; Scheinost et al., 2015; Tomasi & Volkow, 2012; Zuo et al., 2010; but see Weissman-Fogel et al., 2010 who did not find sex differences). Our results are compatible with previous reports of greater age-related disconnectivity in the default mode network in males compared to females. Tomasi & Volkow (2012) reported that females show greater long-range connectivity in the default mode network than males, with no significant change in older age. Scheinost et al. (2015) demonstrated that males have a greater age-related disconnectivity in the default mode network than females. Thus, evidence of sexual dimorphism in default mode connectivity in older age appears to be a continuation of differences that are evident across the lifespan.
Conclusions and Directions for Future Research
This study quantified frequency-specific resting-state network connectivity in a sample of healthy older people. We found that males show greater connectivity than females in the salience network, and females show greater connectivity than males in the default mode network. Our results in healthy older people indicate that frequency-specific connectivity and sex differences in connectivity previously reported in young adults are maintained in older age. Furthermore, our results also highlight the importance of considering sex as an influencing factor on resting-state functional connectivity, particularly in the default mode and salience networks. These sex differences may influence an individual’s risk for cognitive decline, psychiatric or neurodegenerative diseases, or response to treatment. Consistent with Cahill (2006), our results support the argument that sex influences on brain function are not negligible, and should be considered in future studies.
Our study possesses a number of strengths, including the large sample size and use of multiband fMRI protocols that allowed the examination of frequency-specific connectivity in a bandwidth significantly larger than possible using standard imaging protocols. This study does have a number of limitations, the most important of which is the lack of a young comparison sample. Thus, this study can only be considered as a characterization of frequency-specific connectivity of resting-state networks in older adulthood, rather than making inferences on the effect of age, per se. To this end, we are currently working to acquire a young comparison group. However, the strength of the ASPREE-Neuro study lies in its longitudinal nature: this paper reports the baseline results from a minimum three-year study, with follow-ups at one and three years (Ward et al., 2017). Future reports will build upon the current results to examine the effects of ageing in this large sample of healthy older adults.
Supplementary Material
Supplementary data is available at The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences online.
Funding
This work was supported by an Australian National Health and Medical Research Council Project Grant (APP1086188). ASPREE was supported by the National Institutes of Health (grant number U01AG029824); the National Health and Medical Research Council of Australia (grant numbers 334047, 1127060); Monash University (Australia) and the Victorian Cancer Agency (Australia). GE and SJ are supported by the Australian Research Council (ARC) Centre of Excellence for Integrative Brain Function (CE140100007). SJ is supported by an ARC Discovery Early Career Researcher Award (DE150100406). The Principal ASPREE study is registered with the International Standardized Randomized Controlled Trials Register, ASPirin in Reducing Events in the Elderly, Number: ISRCTN83772183 and clinicaltrials.gov number NCT01038583. ASPREE-Neuro trial is registered with Australian New Zealand Clinical Trials Registry ACTRN12613001313729.
Conflict of Interest
The authors declare no conflict of interest.
References
- Aanerud J. Borghammer P. Rodell A. Jónsdottir K. Y. & Gjedde A (2017). Sex differences of human cortical blood flow and energy metabolism. Journal of Cerebral Blood Flow and Metabolism, 37, 2433–2440. doi:10.1177/0271678X16668536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achard S. & Bullmore E (2007). Efficiency and cost of economical brain functional networks. PLoS Computational Biology, 3, e17. doi:10.1371/journal.pcbi.0030017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen J. S. Damasio H. Grabowski T. J. Bruss J. & Zhang W (2003). Sexual dimorphism and asymmetries in the gray-white composition of the human cerebrum. Neuroimage, 18, 880–894. doi:10.1016/S1053-8119(03)00034-X [DOI] [PubMed] [Google Scholar]
- Allen E. A., Erhardt E. B., Damaraju E., Gruner W., Segall J. M. et al. (2011). A baseline for the multivariate comparison of resting-state networks. Frontiers in Systems Neuroscience, 5, 2. doi:10.3389/fnsys.2011.00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna J. R. Snyder A. Z. Vincent J. L. Lustig C. Head D. Raichle M. E. & Buckner R. L (2007). Disruption of large-scale brain systems in advanced aging. Neuron, 56, 924–935. doi:10.1016/j.neuron.2007.10.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ASPREE Investigator Group (2013)Study design of ASPirin in Reducing Events in the Elderly (ASPREE): a randomized, controlled trial. Contemporary Clinical Trials, 36, 555–564. doi:10.1016/j.cct.2013.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann C. F. DeLuca M. Devlin J. T. & Smith S. M (2005). Investigations into resting-state connectivity using independent component analysis. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences, 360, 1001–1013. doi:10.1098/rstb.2005.1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betzel R. F. Byrge L. He Y. Goñi J. Zuo X. N. & Sporns O (2014). Changes in structural and functional connectivity among resting-state networks across the human lifespan. Neuroimage, 102 Pt 2, 345–357. doi:10.1016/j.neuroimage.2014.07.067 [DOI] [PubMed] [Google Scholar]
- Biswal B. Yetkin F. Z. Haughton V. M. & Hyde J. S (1995). Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magnetic Resonance in Medicine, 34, 537–541. doi:10.1002/mrm.1910340409 [DOI] [PubMed] [Google Scholar]
- Biswal B.B., Mennes M., Zuo X.N., Gohel S., Kelly C., Smith S.M., … Milham, M.P (2010). Toward discovery science of human brain function. Proceedings of the National Academy of Sciences USA, 107, 4734–4739. doi:10.1073/pnas.0911855107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner R. L., Snyder A. Z., Shannon B. J., LaRossa G., Sachs R., Fotenos A. F., …, Mintun M. A. (2005). Molecular, structural, and functional characterization of Alzheimer’s disease: evidence for a relationship between default activity, amyloid, and memory. The Journal of Neuroscience, 25, 7709–7717. doi:10.1523/JNEUROSCI.2177-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L. (2006). Why sex matters for neuroscience. Nature Reviews Neuroscience, 7, 477–484. doi:10.1038/nrn1909 [DOI] [PubMed] [Google Scholar]
- Chen X. Sachdev P. S. Wen W. & Anstey K. J (2007). Sex differences in regional gray matter in healthy individuals aged 44-48 years: a voxel-based morphometric study. Neuroimage, 36, 691–699. doi:10.1016/j.neuroimage.2007.03.063 [DOI] [PubMed] [Google Scholar]
- Corbetta M. & Shulman G. L (2002). Control of goal-directed and stimulus-driven attention in the brain. Nature reviews. Neuroscience, 3, 201–215. doi:10.1038/nrn755 [DOI] [PubMed] [Google Scholar]
- Cordes D., Haughton V. M., Arfanakis K., Carew J. D., Turski P. A., Moritz C. H., …, Meyerand M. E. (2001). Frequencies contributing to functional connectivity in the cerebral cortex in “resting-state” data. American Journal of Neuroradiology, 22, 1326–1333. [PMC free article] [PubMed] [Google Scholar]
- Cox R. W. (1996). AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, 29, 162–173. [DOI] [PubMed] [Google Scholar]
- D’Esposito M. Deouell L. Y. & Gazzaley A (2003). Alterations in the BOLD fMRI signal with ageing and disease: a challenge for neuroimaging. Nature Reviews Neuroscience, 4, 863–872. doi:10.1038/nrn1246 [DOI] [PubMed] [Google Scholar]
- D’Esposito M. Zarahn E. Aguirre G. K. & Rypma B (1999). The effect of normal aging on the coupling of neural activity to the bold hemodynamic response. Neuroimage, 10, 6–14. doi:10.1006/nimg.1999.0444 [DOI] [PubMed] [Google Scholar]
- Damoiseaux J. S., Rombouts S. A. R. B., Barkhof F., Scheltens P., Stam C. J., Smith S. M., & Beckmann C. F. 2006. Consistent resting-state networks across healthy subjects. Proceedings of the National Academy of Sciences USA, 103, 13848–13853. doi:10.1073/pnas.0601417103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFrias C. M., Nillson L-G., & Herlitz A (2006). Sex differences in cognition are stable over a 10-year period in adulthood and old age. Aging, Neuropsychology, and Cognition, 13: 574–587. doi:10.1080/13825580600678418 [DOI] [PubMed] [Google Scholar]
- Feinberg D. A. & Yacoub E (2012). The rapid development of high speed, resolution and precision in fMRI. Neuroimage, 62, 720–725. doi:10.1016/j.neuroimage.2012.01.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippi M. Valsasina P. Misci P. Falini A. Comi G. & Rocca M. A (2013). The organization of intrinsic brain activity differs between genders: a resting-state fMRI study in a large cohort of young healthy subjects. Human Brain Mapping, 34, 1330–1343. doi:10.1002/hbm.21514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M. D., Corbetta M., Snyder A. Z., Vincent J. L., & Raichle M. E (2006). Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proceedings of the National Academy of Sciences USA, 103, 10046–10051. doi:10.1073/pnas.0604187103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerligs L. Renken R. J. Saliasi E. Maurits N. M. & Lorist M. M (2015). A brain-wide study of age-related changes in functional connectivity. Cerebral cortex (New York, N.Y.: 1991), 25, 1987–1999. doi:10.1093/cercor/bhu012 [DOI] [PubMed] [Google Scholar]
- Goh J. O. S. (2011). Functional dedifferentiation and altered connectivity in older adults: neural accounts of cognitive aging. Aging and Disease, 2, 30–48. [PMC free article] [PubMed] [Google Scholar]
- Gohel S. R. & Biswal B. B (2015). Functional integration between brain regions at rest occurs in multiple-frequency bands. Brain Connectivity, 5, 23–34. doi:10.1089/brain.2013.0210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong G., Rosa-Neto P., Carbonell F., Chen Z. J., He Y., & Evans A. C (2009). Age- and gender-related differences in the cortical anatomical network. Journal of Neuroscience, 29, 15684– 15693. doi:10.1523/JNEUROSCI.2308–09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur R. C., Mozley L. H., Mozley P. D., Resnick S. M., Karp J. S., Alavi A., …, Gur R. E. (1995). Sex differences in regional cerebral glucose metabolism during a resting state. Science (New York, N.Y.), 267, 528–531. doi:10.1126/science.7824953 [DOI] [PubMed] [Google Scholar]
- Gur R. C., Mozley P. D., Resnick S. M., Gottlieb G. E., Kohn M., Zimmerman R., … Gur R. E (1991). Gender differences in age effect on brain atrophy measured by magnetic resonance imaging. Proceedings of the National Academy of Sciences USA, 88, 2845–2849. doi:10.1073/pnas.88.7.2845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur R. E. & Gur R. C (2002). Gender differences in aging: cognition, emotions, and neuroimaging studies. Dialogues in Clinical Neuroscience, 4, 197–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady C. L. (2008). Cognitive neuroscience of aging. Annals of the New York Academy of Sciences, 1124, 127–144. doi:10.1196/annals.1440.009 [DOI] [PubMed] [Google Scholar]
- Halpern D. F. (1992). Sex differences in cognitive abilities. Hillsate, NJ: L. Erlbaum Associates. [Google Scholar]
- He X., Qin W., Liu Y., Zhang X., Duan Y., Song J., …, Yu C. (2014). Abnormal salience network in normal aging and in amnestic mild cognitive impairment and Alzheimer’s disease. Human Brain Mapping, 35, 3446–3464. doi:10.1002/hbm.22414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jäncke L., Mérillat S., Liem F., & Hänggi J (2015). Brain size, sex, and the aging brain. Human Brain Mapping, 36, 150–169. doi:10.1002/hbm.22619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalcher K., Boubela R. N., Huf W., Bartova L., Kronnerwetter C., Derntl B., …, Moser E. (2014). The spectral diversity of resting-state fluctuations in the human brain. PLoS One, 9, e93375. doi:10.1371/journal.pone.0093375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird A. R., Fox P. M., Eickhoff S. B., Turner J. A., Ray K. L., McKay D. R., …, Fox P. T. (2011). Behavioral interpretations of intrinsic connectivity networks. Journal of Cognitive Neuroscience, 23, 4022–4037. doi:10.1162/jocn_a_00077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H. L. Zahneisen B. Hugger T. LeVan P. & Hennig J (2013). Tracking dynamic resting-state networks at higher frequencies using MR-encephalography. Neuroimage, 65, 216–222. doi:10.1016/j.neuroimage.2012.10.015 [DOI] [PubMed] [Google Scholar]
- Liu H., Stufflebeam S. M., Sepulcre J., Hedden T., & Buckner R. L (2009). Evidence from intrinsic activity that asymmetry of the human brain is controlled by multiple factors. Proceedings of the National Academy of Sciences USA, 106, 20499–20503. doi:10.1073/pnas.0908073106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccoby E. E., & Jacklin C. N (1974). The psychology of sex differences. Stanford, CA: Stanford University Press [Google Scholar]
- Maklakov A. A. & Lummaa V (2013). Evolution of sex differences in lifespan and aging: causes and constraints. Bioessays: news and reviews in molecular, cellular and developmental biology, 35, 717–724. doi:10.1002/bies.201300021 [DOI] [PubMed] [Google Scholar]
- Marín J. (1995). Age-related changes in vascular responses: a review. Mechanisms of Ageing and Development, 79, 71–114. [DOI] [PubMed] [Google Scholar]
- McNeil J. J., Woods R. L., Nelson M. R., Murray A. M., Reid C. M., Kirpach B., …, Grimm R. H; ASPREE Investigator Group (2017). Baseline Characteristics of Participants in the ASPREE (ASPirin in Reducing Events in the Elderly) Study. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 72, 1586–1593. doi:10.1093/gerona/glw342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V. (2015). Salience network. In Toga A.W. (Ed.) Brain Mapping, An Encyclopaedic Reference. Oxford, UK; 597–611. [Google Scholar]
- Proust-Lima C. Amieva H. Letenneur L. Orgogozo J. M. Jacqmin-Gadda H. & Dartigues J. F (2008). Gender and education impact on brain aging: a general cognitive factor approach. Psychology and Aging, 23, 608–620. doi:10.1037/a0012838 [DOI] [PubMed] [Google Scholar]
- Raichle M. E., MacLeod A. M., Snyder A. Z., Powers W. J., Gusnard D. A., & Shulman G. L, 2001. A default mode of brain function. Proceedings of the National Academy of Sciences USA. 98, 676–82. doi:10.1073/pnas.98.2.676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruigrok A. N., Salimi-Khorshidi G., Lai M. C., Baron-Cohen S., Lombardo M. V., Tait R. J., & Suckling J (2014). A meta-analysis of sex differences in human brain structure. Neuroscience & Biobehavioral Reviews, 39, 34–50. doi:10.1016/j.neubiorev.2013.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite T. D., Wolf D. H., Loughead J., Ruparel K., Elliott M. A., Hakonarson H., … Gur R. E (2012). Impact of in-scanner head motion on multiple measures of functional connectivity: relevance for studies of neurodevelopment in youth. Neuroimage, 60, 623–632. doi:10.1016/j.neuroimage.2011.12.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheinost D. Finn E. S. Tokoglu F. Shen X. Papademetris X. Hampson M. & Constable R. T (2015). Sex differences in normal age trajectories of functional brain networks. Human Brain Mapping, 36, 1524–1535. doi:10.1002/hbm.22720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeter M. L. Schmiedel O. & von Cramon D. Y (2004). Spontaneous low-frequency oscillations decline in the aging brain. Journal of Cerebral Blood Flow and Metabolism, 24, 1183–1191. doi:10.1097/01.WCB.0000135231.90164.40 [DOI] [PubMed] [Google Scholar]
- Seeley W. W., Menon V., Schatzberg A. F., Keller J., Glover G. H., Kenna H., …, Greicius M. D. (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. The Journal of neuroscience: the official journal of the Society for Neuroscience, 27, 2349–2356. doi:10.1523/JNEUROSCI.5587-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehzad Z., Kelly A. M., Reiss P. T., Gee D. G., Gotimer K., Uddin L. Q., …, Milham M. P. (2009). The resting brain: unconstrained yet reliable. Cerebral cortex (New York, N.Y.: 1991), 19, 2209–2229. doi:10.1093/cercor/bhn256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirer W. R. Ryali S. Rykhlevskaia E. Menon V. & Greicius M. D (2012). Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cerebral cortex (New York, N.Y.: 1991), 22, 158–165. doi:10.1093/cercor/bhr099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng E. L., & Chui H. C (1987). The Modified Mini-Mental State (3MS) examination. Journal of Clinical Psychiatry, 48, 314–318. [PubMed] [Google Scholar]
- Tian L., Wang J., Yan C., & He Y (2011). Hemisphere- and gender-related differences in small-world brain networks: a resting-statefunctional MRI study. Neuroimage, 54, 191–202. doi:10.1016/j.neuroimage.2010.07.066 [DOI] [PubMed] [Google Scholar]
- Tomasi D. & Volkow N. D (2012). Gender differences in brain functional connectivity density. Human Brain Mapping, 33, 849–860. doi:10.1002/hbm.21252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin L. Q. (2016). Salience Network of the Human Brain. London, UK: Academic Press. [Google Scholar]
- Vermeij A. Meel-van den Abeelen A. S. Kessels R. P. van Beek A. H. & Claassen J. A (2014). Very-low-frequency oscillations of cerebral hemodynamics and blood pressure are affected by aging and cognitive load. Neuroimage, 85 Pt 1, 608–615. doi:10.1016/j.neuroimage.2013.04.107 [DOI] [PubMed] [Google Scholar]
- von Strauss E. Viitanen M. De Ronchi D. Winblad B. & Fratiglioni L (1999). Aging and the occurrence of dementia: findings from a population-based cohort with a large sample of nonagenarians. Archives of Neurology, 56, 587–592. doi:10.1001/archneur.56.5.587 [DOI] [PubMed] [Google Scholar]
- Ward S. A., Raniga P., Ferris N. J., Woods R. L., Storey E., Bailey M. J., …, McNeil J. J; (Joint Senior authorship); on behalf of the ASPREE investigator group. (2017). ASPREE-NEURO study protocol: A randomized controlled trial to determine the effect of low-dose aspirin on cerebral microbleeds, white matter hyperintensities, cognition, and stroke in the healthy elderly. International Journal of Stroke, 12, 108–113. doi:10.1177/1747493016669848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman-Fogel I. Moayedi M. Taylor K. S. Pope G. & Davis K. D (2010). Cognitive and default-mode resting state networks: do male and female brains “rest” differently?Human Brain Mapping, 31, 1713–1726. doi:10.1002/hbm.20968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiederholt W. C. Cahn D. Butters N. M. Salmon D. P. Kritz-Silverstein D. & Barrett-Connor E (1993). Effects of age, gender and education on selected neuropsychological tests in an elderly community cohort. Journal of the American Geriatrics Society, 41, 639–647. [DOI] [PubMed] [Google Scholar]
- Zeng S. M. Yankowitz J. Widness J. A. & Strauss R. G (2001). Etiology of differences in hematocrit between males and females: sequence-based polymorphisms in erythropoietin and its receptor. The journal of gender-specific medicine: JGSM: the official journal of the Partnership for Women’s Health at Columbia, 4, 35–40. [PubMed] [Google Scholar]
- Zuo X-N., Di Martino A., Kelly C., Shehzad Z. E., Gee D. G. et al. (2010). The oscillating brain: Complex and reliable. Neuroimage, 49, 1432–1445. doi:10.1016/j.neuroimage.2009.09.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.