Abstract
Background
The age-related decline in mass, strength, and performance of skeletal muscle is associated with loss of independence, falls risk, disability, institutionalization, and death.
Methods
To determine whether a cocoa supplement enriched in flavonoids can improve plasma markers of oxidative stress and inflammation, physical performance and frailty in middle-aged and older subjects, we conducted a two-phase, randomized, double-blind, clinical trial. The initial study included 60 subjects (55- to 70-year-old) allocated into placebo (P), highly alkalinized (no-flavonoid; NF), or flavonoid-rich natural cocoa (F) beverage groups. The follow-up study included 74 older subjects (65- to 90-year-old) randomly distributed into NF or F groups. Subjects were instructed to consume the beverages once/day for up to 12-weeks. A comprehensive (aging relevant) set of end points were assessed, which included mean change in blood plasma metabolic and oxidative stress indicators, in physical performance tests and quality of life (QoL).
Results
In the initial study, the F group showed improved glycemia, triglyceridemia, High-density lipoprotein cholesterol, Low-density lipoprotein cholesterol, triglyceridemia/HDL index, and oxidative markers. Performance on the Up and Go test, skeletal muscle index, and quality of life also improved. In the follow-up study, F treatment was associated with significant improvements in metabolic, oxidative stress, and inflammatory endpoints and positive effects on physical performance, frailty indicators, and quality of life (F vs. NF group).
Conclusions
Regular flavonoids consumption positively affects blood oxidative stress and inflammation end points, cardiometabolic risk markers, physical performance, and quality of life. The sum of such effects may help to mitigate the extent of frailty development in the elderly people.
Trial Registration
Keywords: Functional performance, Metabolism, Sarcopenia, Preventative health care
Aging is frequently associated with the development of multiple morbid age-related conditions, decreased physical and mental abilities, increased dependence, and reduced quality of life (QoL). Age-related conditions such as physical and mental deficiencies constitute a heavy burden for the elderly people, their families, and the public health care systems (1). The expanded incidence and prevalence of frailty due to demographic aging of the population is a global concern (2). Frailty typically comprises unintentional weight loss, decreased grip strength (ie, muscle weakness), exhaustion or fatigue, slowed walking, and reduced physical activity, contributing to an increased risk for falls, disability, hospitalizations, and mortality (3). The prevalence of frailty in Latin America and the Caribbean ranges from 7.7% to 42.6% (4); in Mexico, a prevalence of 14.1%–37.2% has been reported (5).
Critical to the development of frailty in the elderly population is the loss of muscle mass and strength, which characterize a condition known as sarcopenia. Sarcopenia is usually accompanied by decreased physical activity, low mobility, slow gait, and poor exercise capacity, all of which are features of frailty (6). In the setting of aging, increased levels of oxidative stress (OS) occur and can activate multiple pro-inflammatory pathways that worsen the condition (7). Up to 15% of people aged more than 65 years and 50% of those aged more than 80 years have sarcopenia (8). Ruiz et al. (9) recently reported that muscle strength is inversely and independently associated with all-cause mortality in men. Thus, strategies that limit muscle mass loss and increase strength may counteract frailty. Currently, only exercise is recognized to effectively counteract sarcopenia (10). However, physical activity and exercise in the older population are often restricted by the various conditions mentioned earlier and by diseases such as osteoarthritis and obesity.
Evidence indicates that the consumption of cocoa flavonoids improves dyslipidemia, insulin resistance, and systemic inflammation (11). We previously reported that oral dietary supplementation with a flavonoid-rich dark chocolate improves the exercise capacity of sedentary middle-aged subjects while stimulating upstream regulators of mitochondrial structure and function and reducing OS (12). In murine studies, these effects can be triggered by the administration of pure epicatechin (13,14).
However, nothing is known about the effects of high flavonoid dietary supplementation on end points associated with the development of frailty in the elderly population. Therefore, this study was designed to determine whether the consumption of high flavonoid cocoa (of which the most abundant flavonoid is epicatechin) can improve serum markers of OS, inflammation, and cardiometabolic health and indicators of frailty and QoL in two population samples, one comprised middle-aged subjects (55–70 years old) and the second comprised older subjects (65–90 years old).
Methods
Study Design
The initial study used a double-blind, placebo-controlled design enrolling male and female subjects aged 55–70 years. Supplementary Table 1 delineates the end points measured (collected at the beginning and end of the study). All volunteers were evaluated for dietary habits and instructed to maintain their usual lifestyle, limiting high caloric foods, and the intake of flavonoid-containing foods and beverages (ie, chocolate and tea). Subjects were also instructed to walk for 30 min/day as vigorously as possible. Participants were randomly assigned to consume once a day a powder-based beverage for 12 weeks containing either (i) a cocoa-free skim milk-based powder (with coloring and flavoring) beverage, placebo (P); (ii) an alkalinized natural cocoa powder without flavonoids (0 mg), no flavonoids (NF) or; (iii) a natural cocoa powder rich in flavonoids (179 mg), flavonoids (F). All beverage powders were prepared and donated by Hershey and had as best possible, similar physical characteristics and flavor. Beverages were provided in individual sachets containing 22 g of a dry powder that was reconstituted with water just before consumption. Table 1 summarizes detailed compositions of the beverages.
Table 1.
Placebo and Cocoa Beverage Powder Composition
| Per serving | Placebo | No-flavonoid | Flavonoids |
|---|---|---|---|
| Calories | 60 | 56 | 56 |
| Total fat (g) | 0 | 1 | 1 |
| Carbohydrates (g) | 9 | 9 | 10 |
| Dietary fiber (g) | 0 | 2 | 2 |
| Sugar (g) | 8 | 6 | 6 |
| Protein (g) | 5 | 5 | 5 |
| Natural cocoa (g) | 0 | 0 | 5 |
| Alkalinized cocoa (g) | 0 | 5 | 0 |
| Caffeine (mg) | 0 | 6 | 12 |
| Theobromine (mg) | 0 | 103 | 103 |
| Proanthocyanidins (Degree of Polymerization 1–10; mg) | 0 | 0 | 154 |
| Epicatechin (mg) | 0 | 0 | 25 |
On the basis of the results obtained from the initial study, a follow-up study was used in older population (male and female subjects, 65–90 years old), comparing the effects of NF versus F beverages to be consumed as described earlier for only 8 weeks. The purpose of the study was to examine the effects of a shorter exposure to a flavonoid beverage in an elderly population, which is more susceptible to the development of pre-frail or frail conditions. Subjects were instructed as described earlier to maintain their usual lifestyle, to limit intake of high caloric foods and flavonoid-containing foods and beverages, and to walk for 30 min/day. End points measured including indicators of frailty are listed in Supplementary Table 1.
All subjects were instructed to consume the beverages during morning fasting and to return the empty sachets to verify compliance. Both studies were conducted in Mexico City, between January and December 2016. The study was approved by the institutional ethics and research committees of the Escuela Superior de Medicina, Instituto Politecnico Nacional, Mexico.
Study Participants
Participants were recruited through convenience sampling, among assistants to community centers in Coyoacan, an urban district of Mexico City. The inclusion criteria for the initial and follow up studies are listed in Supplementary Table 2.
Non-inclusion criteria were habitual consumption of antioxidant supplements, cocoa products, benzodiazepines, or protein supplements. Participants signed an informed consent indicating their willingness to be part of the study and allowing the use of the collected data for research purposes.
For the initial study, a total of 115 subjects were screened by evaluation of medical history, anthropometry, blood samples, and performance tests (hand strength and Up and Go test). Twenty-eight subjects did not meet eligibility criteria. Thus, 87 individuals were initially enrolled in the study (Supplementary Figure 1). For the follow-up, a total of 150 subjects were screened as described earlier. Seventy-six subjects did not meet eligibility criteria and performance tests (hand strength and Up and Go test, skeletal muscle index [SMI]) and frailty indicators). Thus, 74 individuals were initially enrolled in the study. Subjects participating in the initial study did not participate in the follow-up study (Supplementary Figure 2).
Randomization and Blinding
Beverage interventions were masked with an alphabetic key, and this list was concealed by the principal investigator until completion of the study. See Supplementary file for details.
Outcome Measures
Anthropometric measures
See Supplementary file for details.
Clinical chemistry
After 12 hours of fasting, a venous blood sample was obtained to determine glycemia and a lipid profile. The triglyceridemia (TG)/HDL index reflecting coronary risk (15) was calculated. See Supplementary file for details.
Serum OS and inflammatory biomarkers
Lipid oxidation was assessed by the malondialdehyde assay (16). Protein carbonylation was measured using 2,4-dinitrophenylhydrazine as a substrate (17). Serum interleukin-6 and tumor necrosis factor-α levels were measured using commercially available ELISA kits (Cayman Chemical Company).
Skeletal muscle index
On the basis of the definition of the European Working Group on Sarcopenia in Older People, we calculated the SMI by dividing the estimated muscle mass (kg) by the square of the height (m2). SMI values less than 8.87 kg/m2 (men) and less than 6.42 kg/m2 (women) were considered as indicative of sarcopenia.
Handgrip strength
See Supplementary file for details.
Quality of life
We assessed QoL using the two-part health-related QoL (EQ-5D) questionnaire, as described by Golicki et al. (18). See Supplementary file for details.
Mobility Assessment
Six-minute walk test
Subjects were instructed to walk at their normal gait and pace during 6 minutes, in a straight 30 m long indoor corridor, on a flat and hard surface covered by tiles 33 cm2. See Supplementary file for details.
Two-minute step in place test
Subjects in a standing up straight position marched in place during 2 minutes.
Sit-up test
Subjects seated in a standard chair were instructed to fully rise from a sitting position using no assistance and sit down as quickly as possible during a 30 seconds period
Up and Go test
Subjects seated back in an armchair, on the word “go” they got up and walked through a 3 m line on the floor, turned around, and walked back to the chair and sat down.
Frailty Determination
We used the five-criteria approach proposed by Fried frailty phenotype (3). See Supplementary file for details.
Statistical Analysis
Data are reported as mean ± standard deviation (SD) or percentages when appropriate. For the initial study, analysis of variance (intergroup analysis) and Student’s t test (paired, before and after) were used to compare means of the groups for continuous variables. Chi-square test was used for categorical variables. For the follow-up study, Student’s t test (paired, before and after) or Student’s t test (unpaired) for intergroup analysis was used. Nonparametric tests were used when necessary. Statistical significance was considered when p value is of less than 0.05. Analyses were performed with Graph Pad Prism, v7.0 and SPSS, v24.
Results
Initial Study
Supplementary Table 3 summarizes the general characteristics of the participants. P, NF, and F groups were comparable at baseline. Mean age was 62.4 ± 3.2 and 80% of participants were women (48/60). Average scholarship was less than or equal to 9 years (75%). Most subjects had a history of smoking (61.6% formerly and 26.6% currently), took more 2.97 ± 0.06 medications, and had 2.3 ± 0.1 comorbidities. Sixty percent subjects in all groups had low muscle strength and 30%–40 % of them had more than 10 seconds in the Up and Go test, no differences were found between groups (Supplementary Table 3).
At the conclusion of the initial study, there was a significant decrease in body weight in all groups, which was on average greater in the F group (2.6 ± 1.7 kg). Abdominal circumference decreased in all groups (Table 2), as did body fat (data not shown). SMI increased modestly but significantly by 0.8 ± 0.3 kg/m2 in the F group (Table 3).
Table 2.
Initial Study Results
| Placebo | NF | Flavonoids | ANOVA | |
|---|---|---|---|---|
| Anthropometric | ||||
| Body weight (kg) | ||||
| Baseline | 84.5 ± 14.3 | 78.9 ± 10.8 | 83.5 ± 12.5 | 0.34 |
| 12 wk | 82.7 ± 14.1 | 77.7 ± 11 | 80.9 ± 12.3 | 0.43 |
| p | .003 | <.0001 | <.0001 | |
| Change | –1.8 ± 2.3 | –1.3 ± 1.1 | –2.6 ± 1.7 | |
| Waist (cm) | ||||
| Baseline | 104.6 ± 13.1 | 103.4 ± 11.1 | 107.3 ± 12.3 | 0.58 |
| 12 wk | 102.1 ± 13 | 101 ± 11.2 | 103.2 ± 11.7 | 0.85 |
| p | <.0001 | .0004 | <.0001 | |
| Change | –2.5 ± 2 | –2.3 ± 2.5 | –4.1 ± 3.5 | |
| Metabolic (serum) | ||||
| Glycemia (mg/dL) | ||||
| Baseline | 98.7 ± 10.1 | 98.8 ± 16.7 | 96.1 ± 13.5 | 0.78 |
| 12 wk | 95.4 ± 9.8 | 97.1 ± 15.2 | 90.2 ± 9.1 | 0.16 |
| p | NS | NS | .04 | |
| Change | –3.3 ± 13.6 | –1.7 ± 7.7 | –5.9 ± 12.4 | |
| Cholesterol (mg/dL) | ||||
| Baseline | 200.6 ± 32.5 | 186.4 ± 21.5 | 188.6 ± 39.3 | 0.32 |
| 12 wk | 189.2 ± 27.7 | 179.3 ± 17 | 177.6 ± 21.8 | 0.22 |
| p | .01 | NS | NS | |
| Change | –11.4 ± 19.6 | –7.1 ± 18.5 | –11 ± 26.4 | |
| LDL-c (mg/dL) | ||||
| Baseline | 127.9 ± 35.3 | 108.3 ± 31.3 | 109.4 ± 47.7 | 0.21 |
| 12 wk | 124.1 ± 34.9 | 104.8 ± 27.9 | 97.9 ± 33.7 | 0.03 |
| p | .04 | .04 | .01 | |
| Change | –3.7 ± 7.7 | –3.5 ± 7.3 | –11.5 ± 18.6 | |
| HDL-c (mg/dL) | ||||
| Baseline | 42 ± 4.1 | 41.7 ± 4.3 | 41.6 ± 3.6 | 0.93 |
| 12 wk | 43.3 ± 4.1 | 42.6 ± 5 | 44.8 ± 5.4 | 0.36 |
| p | NS | NS | .003 | |
| Change | 1.2 ± 2.6 | 0.9 ± 3.9 | 3.2 ± 4.3 | |
| Triglycerides (mg/dL) | ||||
| Baseline | 189.8 ± 38.3 | 176.6 ± 41.9 | 189.5 ± 40.6 | 0.50 |
| 12 wk | 183 ± 31.2 | 168.4 ± 35.2 | 165.8 ± 25.4 | 0.17 |
| p | NS | NS | .01 | |
| Change | –6.8 ± 15.7 | –8.2 ± 23.7 | –23.6 ± 38 |
p Values denote comparisons of intragroup data before versus after treatment by paired t test. Analysis of variance (ANOVA) for intergroup analysis. Results are shown as mean ± SD. n = 20/group.
Table 3.
Physical Performance
| Placebo | NF | Flavonoids | ANOVA | |
|---|---|---|---|---|
| Physical performance | ||||
| Up and Go test (s) | ||||
| Baseline | 8.3 ± 1.8 | 8.5 ± 1.9 | 9.2 ± 1.1 | 0.20 |
| 12 wk | 8.1 ± 1.5 | 8.2 ± 1.7 | 8.5 ± 1 | 0.66 |
| p | NS | NS | .04 | |
| Change | –0.2 | –0.3 | –0.7 | |
| SMI (kg/m2) | ||||
| Baseline | 9.1 ± 2.4 | 8.8 ± 2.2 | 9 ± 2.6 | 0.92 |
| 12 wk | 9.2 ± 2.5 | 9 ± 2.5 | 9.8 ± 2.2 | 0.55 |
| p | NS | NS | .03 | |
| Baseline | 0.1 | 0.2 | 0.8 | |
| Handgrip strength (kg) | ||||
| Baseline | 23.8 ± 5.7 | 23.5 ± 5.3 | 22.7 ± 5 | 0.80 |
| 12 wk | 24±5.1 | 23.6±5.2 | 23±4.8 | 0.55 |
| p | NS | NS | NS | |
| Change | 0.2 | 0.1 | 0.3 |
p Values denote comparisons of intragroup data before vs. after treatment by paired t test. Analysis of variance (ANOVA) for intergroup analysis. Results are shown as mean ± SD. n = 20/group. SMI = skeletal muscle index.
The F group displayed a significant decrease in glycemia values (5.9 ± 12.4 mg/dL), whereas total serum cholesterol levels showed a significant decrease only in the P group (11.4 ± 19.6 md/dL). LDL cholesterol levels decreased in all groups, reaching a greater decrease (11.5 ± 18.6 mg/dL) in the F group. HDL cholesterol levels increased significantly (3.2 ± 4.3 mg/dL) only in the F group. Triglyceride levels reached a significant decrease of 23.6 ± 38 mg/dL in the F group (Table 2), and the TG/HDL ratio decreased (results not shown) significantly only in the F group. A significant decrease in the Up and Go test and an increase in SMI were observed in the F groups versus the P and NF groups (Table 3). Handgrip strength was constant across the groups.
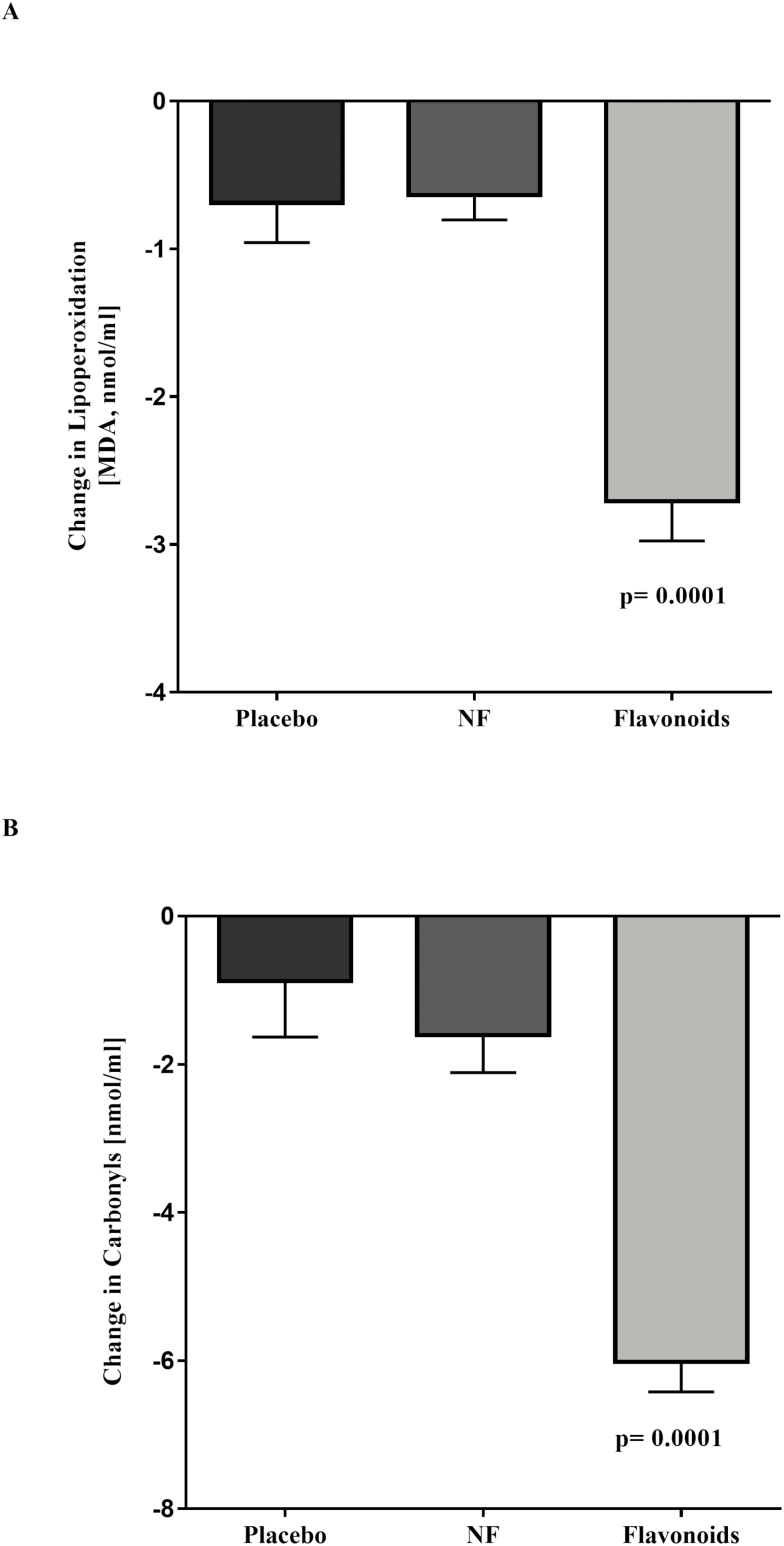
Figure 1 shows changes in serum OS biomarkers: malondialdehyde (lipid peroxidation) and protein carbonyls. Significant changes (decreased) occurred only in the F group.
Figure 1.
(A) Changes in malondialdehyde contents between treatments in initial study. (B) Changes in carbonyls contents between treatments in initial study. Data are presented as means ± SE.
Quality of Life
QoL indices improved significantly in the visual analog scale only in the F group (Supplementary Table 4); no differences were noted using the EQ-5D scale.
Follow-up Study
Supplementary Table 5 summarizes the characteristics of the patient cohort for the follow-up study. The two groups were comparable at baseline (no significant differences). Mean age was 75.9 ± 5.7 years and 78.7% of participants were women (48/61). The average scholarship was less than or equal to 9 years (68.8%). Most of the subjects had a history of smoking (68.8% formerly and 18% currently), took four medications (4 ± 0.8 drugs), and had at least two comorbidities (2.8 ± 0.8 illness).
There were significant improvements in all anthropometric and metabolic endpoints in the F group (Supplementary Table 6). Significant changes in waist and LDLc and TG/HDL were also detected in the NF group (Supplementary Table 6).
With respect to markers of OS, flavonoid treatment decreased malondialdehyde (lipid peroxidation marker) and protein carbonylation (Supplementary Figure 3). Assessments of markers of inflammation showed a significant effect of flavonoid treatment to decrease in interleukin-6 levels but not levels of necrosis factor-α (p = .06; Supplementary Figure 4).
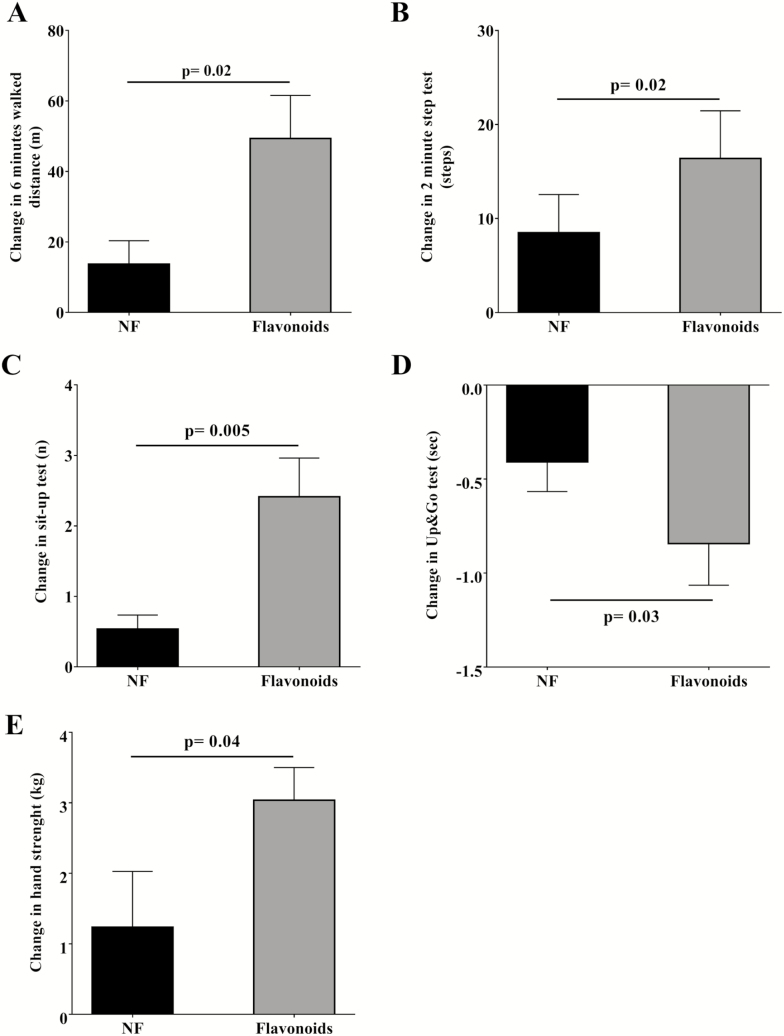
The flavonoid treatment was also associated with significant changes in end points of physical performance (Figure 2). In the 6 minutes walk test, the step test, the sit-up test, the Up and& Go test, and the handgrip strength assessment, the improvements in the F group compared to the NF group were all significant. No significant changes in individual frailty indicators (slowness, weakness, exhaustion, low physical activity, weight loss) were detected (Supplementary Table 7). However, in the F group, the number of pre-frail subjects decreased significantly by 29.4% (Supplementary Table 7). No changes were found in NF group.
Figure 2.
Physical performance tests in follow-up study. (A) Change in 6 minute walked distance. (B) Change in 2 minutes step test. (C) Change in sit-up test. (D) Change in Up and Go test. (E) Change in hand strength test. Data are presented as means ± SE.
As noted in Supplementary Table 8, when using the EQ-visual analog scale, the F group significantly improved their perception of QoL (before vs. after). With the EQ-5D scale, subjects in the F group demonstrated a significant reduction of 66% in the perception of problems in mobility and pain/discomfort, with no change noted in self-care, usual activity or anxiety/depression (data not shown). No differences were noted in the NF group.
Discussion
Because of medical advances and changes in lifestyle, life expectancy over the last decades increased much faster than the years spent in good health (ie, healthy life years). Many countries, such as the United Kingdom and Mexico, have seen major increases in the incidence of obesity in ever younger populations, which over time significantly facilitate the development of cardiometabolic diseases and musculoskeletal disorders such as osteoarthritis.
A large percentage of those aged more than 70 years have a low level of physiological function due to factors such as obesity, low muscle mass, and impaired cardiometabolic function. The declines in the functioning of physiological systems inevitably lead to slower walking speed, difficulty balancing and rising from seated positions. To better quantify such limitations, standardized tests such as the 6 minutes walk test and the 30 seconds chair-rise have been developed and validated (19). In this study, we have used integrated scoring of these tests in addition, to those such as the time for Up and Go to identify physical impairments associated with conditions such as sarcopenia, pre-frailty, and frailty.
The structure/function of the cardiovascular, respiratory, metabolic, and musculoskeletal systems is amenable to maintenance or improvement through exercise training (20). Several studies have demonstrated that a trajectory toward frailty can be modified through physical activity (21). Unfortunately, exercise participation declines progressively through adult life as well as the desire to participate. Minimum recommended activity levels for those aged more than 65 years are only met by approximately 25% of the population. Achieving more than 150 min/week of moderate-to-intense aerobic exercise such as walking is associated with at least a 30% lower risk of morbidity, mortality, and dependence versus those that are inactive (22,23). Thus, the identification of low cost and effective approaches to improve a healthy physiological status of the elderly population is urgent.
Our research group has previously evaluated the capacity of cocoa flavonoids (specifically, epicatechin; Epi) to favorably modify the structure and function of the cardiovascular and musculoskeletal system of young healthy, older, and diseased animal models with convincing results. We have measured the positive impact that the daily provision of oral Epi at low doses (1 mg/kg/day) has on the functional status of such systems leading to greater levels of tolerance to fatigue and tissue damage from challenges such as ischemia (24). Also, recent evidence showed that Epi supplementation slowed down the aging of skeletal muscle in old mice (25). The current report extends such studies to the elderly population in whom loss of muscle mass and other effects of aging decrease exercise capacity.
Moderate and high-intensity strength training increases muscle size, strength, and power in elderly and frail subjects (26). However, many studies have failed to demonstrate improvements in mobility end points such as walking or chair rising when only select groups of muscles are targeted (6). Thus, it has been proposed that older frail subjects undergo muscle (eg, leg) strengthening as well as functional training such as walking, chair rising, and balancing (27) 2–3 times week in sessions lasting approximately 45 minutes. Indeed, the combination of strength and endurance training improves muscle, cardiovascular, and metabolic health leading to improved QoL in middle-aged subjects (28). In sedentary subjects aged 70–89 years, a combined 1 year training program significantly improved mobility (29). In addition, the combination of aerobic and strength plus balance and flexibility training is recognized as even more effective in improving global measures of physical function. However, many of these training programs require periods of 6 months or more. Furthermore, the effects of training quickly disappear upon its discontinuation; thus, successful conditioning programs require sustained regimens.
In this study, we demonstrate that the consumption of flavonoid-rich cocoa for only 8 weeks in older subjects is sufficient to significantly improve mobility and physical performance measures that are accompanied by improved blood cardiometabolic end points (30). Specifically, an improvement of about 35 m was noted in the 6 minutes walk test, about 13 steps (step test), about 1.8 repetitions (sit up test), about 0.6 seconds (Up and Go test), and +1.7 kg in grip strength while reducing blood-related end points of, for example, glucose by about 9 mg/dL and LDL-c by about 10 mg/dL. These effects led to improvements in measures of frailty. As such, for the first time, a natural food rich in flavonoids appears to trigger effects similar to those reported by long-term physical conditioning in the elderly population.
Excess ROS (ie, OS) is known to contribute to the aging process via the accumulation of mitochondrial DNA mutations, which compromise optimal organelle function (31). Excess Reactive Oxygen Species can also stimulate the production of pro-inflammatory cytokines such as necrosis factor-α, which can further facilitate the decline of cell health. Aging is also associated with a lower renewal of mitochondria (ie, impaired mitochondrial biogenesis), which further limits the organelle’s ability to produce sufficient amounts of Adenosine triphosphate so as to maintain optimal cell health (32). Thus, the stimulation of mitochondrial biogenesis and an adequate function of the organelle are critical to counteract aging. Currently, only exercise is known to effectively stimulate mitochondria biogenesis and organelle function leading to improved cellular bioenergetics and metabolism. In preclinical and clinical studies, we have provided data supporting the concept that the beneficial effects of Epi may be mediated by improvements in mitochondrial function and biogenesis (33). In recent study performed in sedentary middle-aged subjects, we found that the consumption of dark chocolate for 12 weeks activated upstream signaling pathways (LKB1, AMPK, PGC1α) associated with the stimulation of mitochondrial biogenesis and/or function in skeletal muscle. Muscle citrate synthase activity was increased while OS indicators improved. Similar results were reported by us in patients with heart failure and type 2 diabetes (34). Thus, on the basis of the results observed in this study, the consumption of flavonoid-rich cocoa for 8 weeks appears sufficient to trigger similar effects in older subjects and possibly account for improvements in indicators of physical performance, mobility, and QoL.
Conclusions
Results of this intervention indicate that consumption of flavonoid-rich cocoa improves cardiometabolic status and physical performance/mobility, with accompanying improvements in QoL. Reductions noted in OS and inflammation biomarkers may partly account for the observed effects. To the extent that the modest consumption of flavonoid-rich cocoa eliminates or reduces barriers and/or conditions muscles toward an improved functional profile, it may also facilitate the active engagement of individuals in physical activities leading to the development a self-reinforcing positive feedback loop. The evidence provided in these small studies is provocative and opens up new possibilities in the management of aging-induced sarcopenia, decline in physical performance, and frailty, which should be further validated in large clinical trials.
Limitations
Because of the characteristics of the operation of community centers in Mexico City and that the inclusion of subjects was done by invitation, subjects were included on a first-to-come first-to-be-included; this resulted in a gender bias toward female subjects. In addition, sampling was not based on a power calculation. There is the possibility that differences in the chemical composition of NF and F powders may partly account for the effects noted. In this regard, a limitation is the lack of inclusion of a pure flavonoid group in the study design, which would allow us to conclude that cocoa flavanols (in particular, Epi) are responsible for the observed effects similar to our previous reports in senile animals (33). Furthermore, blood levels of Epi and metabolites were not measured.
Funding
This work was supported by the National Institute of Health, (NIH DK98717, AG47326, AG52593) to Dr. Villarreal and Consejo Nacional de Ciencia y Tecnologia (253769) to Dr. Ceballos.
Conflict of Interest
Dr. Villarreal is a co-founder and stockholder (Dr. Ceballos) of Cardero Therapeutics, Inc. The co-authors Munguia, Rubio-Gayosso, Ramirez-Sanchez, Ortiz, Hidalgo, Gonzalez, Meaney and Najera have no disclosures.
Supplementary Material
Acknowledgments
We appreciate the stimulating interest of Dr. Laurence L. Brunton (Department of Pharmacology, UC San Diego School of Medicine) in these experiments and are grateful for his critiques of drafts of this manuscript.
References
- 1. Goodpaster BH, Park SW, Harris TB, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61:1059–1064. doi: 10.1093/gerona/61.10.1059 [DOI] [PubMed] [Google Scholar]
- 2. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381:752–762. doi: 10.1016/S0140-6736(12)62167-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fried LP, Tangen CM, Walston J, et al. ; Cardiovascular Health Study Collaborative Research Group Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.M146 [DOI] [PubMed] [Google Scholar]
- 4. Da Mata FAF, Pereira PPdS, de Andrade KRC, Figueiredo ACMG, Silva MT, Pereira MG. Prevalence of frailty in Latin America and the Caribbean: a systematic review and meta-analysis. PLoS One. 2016;11:e0160019;doi: 10.1371/journal.pone.0160019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. García-Peña C, Ávila-Funes JA, Dent E, Gutiérrez-Robledo L, Pérez-Zepeda M. Frailty prevalence and associated factors in the Mexican health and aging study: a comparison of the frailty index and the phenotype. Exp Gerontol. 2016;79:55–60;doi: 10.1016/j.exger.2016.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McPhee JS, French DP, Jackson D, Nazroo J, Pendleton N, Degens H. Physical activity in older age: perspectives for healthy ageing and frailty. Biogerontology. 2016;17:567–580. doi: 10.1007/s10522-016-9641-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Davalli P, Mitic T, Caporali A, Lauriola A, D’Arca D. ROS, cell senescence, and novel molecular mechanisms in aging and age-related diseases. Oxid Med Cell Longev. 2016;2016:3565127. doi: 10.1155/2016/3565127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. von Haehling S, Morley JE, Anker SD. An overview of sarcopenia: facts and numbers on prevalence and clinical impact. J Cachexia, Sarcopenia Muscle. 2010;1:129–133. doi: 10.1007/s13539-010-0014-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ruiz JR, Sui X, Lobelo F, et al. Association between muscular strength and mortality in men: prospective cohort study. BMJ. 2008;337:a439. doi: 10.1136/bmj.a439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Iolascon G, Di Pietro G, Gimigliano F, et al. Physical exercise and sarcopenia in older people: position paper of the Italian Society of Orthopaedics and Medicine (OrtoMed). Clin Cases Miner Bone Metab. 2014;11:215–221. [PMC free article] [PubMed] [Google Scholar]
- 11. Lin X, Zhang I, Li A, et al. Cocoa flavanol intake and biomarkers for cardiometabolic health: a systematic review and meta-analysis of randomized controlled trials. J Nutr. 2016;146:2325–2333. doi: 10.3945/jn.116.237644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Taub PR, Ramirez-Sanchez I, Patel M, et al. Beneficial effects of dark chocolate on exercise capacity in sedentary subjects: underlying mechanisms. A double blind, randomized, placebo controlled trial. Food Funct. 2016;7:3686–3693. doi: 10.1039/c6fo00611f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mafi F, Biglari S, Afousi AG, Gaeini AA. Epicatechin supplementation and resistance training-induced improvement of muscle strength and circulatory levels of plasma follistatin and myostatin in sarcopenic older adults. J Aging Phys Act. 2018:1–27. doi: 10.1123/japa.2017-0389 [DOI] [PubMed] [Google Scholar]
- 14. Hüttemann M, Lee I, Perkins GA, Britton SL, Koch LG, Malek MH. (-)-Epicatechin is associated with increased angiogenic and mitochondrial signalling in the hindlimb of rats selectively bred for innate low running capacity. Clin Sci (Lond). 2013;124:663–674. doi: 10.1042/CS20120469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Onat A, Can G, Kaya H, Hergenç G. “Atherogenic index of plasma” (log10 triglyceride/high-density lipoprotein-cholesterol) predicts high blood pressure, diabetes, and vascular events. J Clin Lipidol. 2010;4:89–98. doi: 10.1016/j.jacl.2010.02.005 [DOI] [PubMed] [Google Scholar]
- 16. Gérard-Monnier D, Erdelmeier I, Régnard K, Moze-Henry N, Yadan JC, Chaudière J. Reactions of 1-methyl-2-phenylindole with malondialdehyde and 4-hydroxyalkenals. Analytical applications to a colorimetric assay of lipid peroxidation. Chem Res Toxicol. 1998;11:1176–1183. doi: 10.1021/tx9701790 [DOI] [PubMed] [Google Scholar]
- 17. Levine RL, Garland D, Oliver CN, et al. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990;186:464–478. doi: 10.1016/0076-6879(90)86141-H [DOI] [PubMed] [Google Scholar]
- 18. Golicki D, Niewada M. General population reference values for 3-level EQ-5D (EQ-5D-3L) questionnaire in Poland. Pol Arch Med Wewn. 2015;125:18–26. doi: 10.20452/pamw.2638 [DOI] [PubMed] [Google Scholar]
- 19. Rikli RE, Jones CJ. The reliability and validity of a 6-minute walk test as a measure of physical endurance in older adults. J Aging Phys Activity. 1998;6:363–375. doi: 10.1123/japa.6.4.363 [DOI] [Google Scholar]
- 20. Lexell J, Taylor CC, Sjöström M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurol Sci. 1988;84:275–294. doi: 10.1016/0022-510X(88)90132–3 [DOI] [PubMed] [Google Scholar]
- 21. Tak E, Kuiper R, Chorus A, Hopman-Rock M. Prevention of onset and progression of basic ADL disability by physical activity in community dwelling older adults: a meta-analysis. Ageing Res Rev. 2013;12:329–338. doi: 10.1016/j.arr.2012.10.001 [DOI] [PubMed] [Google Scholar]
- 22. Chou WT, Tomata Y, Watanabe T, Sugawara Y, Kakizaki M, Tsuji I. Relationships between changes in time spent walking since middle age and incident functional disability. Prev Med. 2014;59:68–72. doi: 10.1016/j.ypmed.2013.11.019 [DOI] [PubMed] [Google Scholar]
- 23. Paterson DH, Warburton DE. Physical activity and functional limitations in older adults: a systematic review related to Canada’s Physical Activity Guidelines. Int J Behav Nutr Phys Act. 2010;7:38. doi: 10.1186/1479-5868-7-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nogueira L, Ramirez-Sanchez I, Perkins GA, et al. (-)-Epicatechin enhances fatigue resistance and oxidative capacity in mouse muscle. J Physiol. 2011;589(Pt 18):4615–4631. doi: 10.1113/jphysiol.2011.209924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Si H, Wang X, Zhang L, Parnell LD, Admed B, LeRoith T, et al. Dietary epicatechin improves survival and delays skeletal muscle degeneration in aged mice. FASEB J. 33(1):965–977. doi: 10.1096/fj.201800554RR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cannon J, Kay D, Tarpenning KM, Marino FE. Comparative effects of resistance training on peak isometric torque, muscle hypertrophy, voluntary activation and surface EMG between young and elderly women. Clin Physiol Funct Imaging. 2007;27:91–100. doi: 10.1111/j.1475-097X.2007.00719.x [DOI] [PubMed] [Google Scholar]
- 27. Forster A, Lambley R, Young JB. Is physical rehabilitation for older people in long-term care effective? Findings from a systematic review. Age Ageing. 2010;39:169–175. doi: 10.1093/ageing/afp247 [DOI] [PubMed] [Google Scholar]
- 28. Holviala J, Kraemer WJ, Sillanpää E, et al. Effects of strength, endurance and combined training on muscle strength, walking speed and dynamic balance in aging men. Eur J Appl Physiol. 2012;112:1335–1347. doi: 10.1007/s00421-011-2089-7 [DOI] [PubMed] [Google Scholar]
- 29. Pahor M, Blair SN, Espeland M, et al. ; LIFE Study Investigators Effects of a physical activity intervention on measures of physical performance: results of the lifestyle interventions and independence for Elders Pilot (LIFE-P) study. J Gerontol A Biol Sci Med Sci. 2006;61:1157–1165. [DOI] [PubMed] [Google Scholar]
- 30. Yanagita M, Shiotsu Y. Role of resistance training for preventing frailty and metabolic syndromes in aged adults. J Phys Fitness Sports Med. 2014;3:35–42. doi: 10.7600/jpfsm.3.35 [DOI] [Google Scholar]
- 31. Chinnery PF, Samuels DC, Elson J, Turnbull DM. Accumulation of mitochondrial DNA mutations in ageing, cancer, and mitochondrial disease: is there a common mechanism? Lancet. 2002;360:1323–1325. doi: 10.1016/S0140-6736(02)11310-9 [DOI] [PubMed] [Google Scholar]
- 32. Gomez-Cabrera MC, Sanchis-Gomar F, Garcia-Valles R, et al. Mitochondria as sources and targets of damage in cellular aging. Clin Chem Lab Med. 2012;50:1287–1295. doi: 10.1515/cclm-2011-0795 [DOI] [PubMed] [Google Scholar]
- 33. Moreno-Ulloa A, Nogueira L, Rodriguez A, et al. Recovery of indicators of mitochondrial biogenesis, oxidative stress, and aging with (-)-epicatechin in senile mice. J Gerontol A Biol Sci Med Sci. 2015;70:1370–1378. doi: 10.1093/gerona/glu131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ramirez-Sanchez I, Taub PR, Ciaraldi TP, et al. (-)-Epicatechin rich cocoa mediated modulation of oxidative stress regulators in skeletal muscle of heart failure and type 2 diabetes patients. Int J Cardiol. 2013;168:3982–3990. doi: 10.1016/j.ijcard.2013.06.089 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.