Abstract
Aims
Sudden cardiac death (SCD) annual incidence is 0.6–1% in post-myocardial infarction (MI) patients with left ventricular ejection fraction (LVEF)≥40%. No recommendations for implantable cardioverter-defibrillator (ICD) use exist in this population.
Methods and results
We introduced a combined non-invasive/invasive risk stratification approach in post-MI ischaemia-free patients, with LVEF ≥ 40%, in a multicentre, prospective, observational cohort study. Patients with at least one positive electrocardiographic non-invasive risk factor (NIRF): premature ventricular complexes, non-sustained ventricular tachycardia, late potentials, prolonged QTc, increased T-wave alternans, reduced heart rate variability, abnormal deceleration capacity with abnormal turbulence, were referred for programmed ventricular stimulation (PVS), with ICDs offered to those inducible. The primary endpoint was the occurrence of a major arrhythmic event (MAE), namely sustained ventricular tachycardia/fibrillation, appropriate ICD activation or SCD. We screened and included 575 consecutive patients (mean age 57 years, LVEF 50.8%). Of them, 204 (35.5%) had at least one positive NIRF. Forty-one of 152 patients undergoing PVS (27–7.1% of total sample) were inducible. Thirty-seven (90.2%) of them received an ICD. Mean follow-up was 32 months and no SCDs were observed, while 9 ICDs (1.57% of total screened population) were appropriately activated. None patient without NIRFs or with NIRFs but negative PVS met the primary endpoint. The algorithm yielded the following: sensitivity 100%, specificity 93.8%, positive predictive value 22%, and negative predictive value 100%.
Conclusion
The two-step approach of the PRESERVE EF study detects a subpopulation of post-MI patients with preserved LVEF at risk for MAEs that can be effectively addressed with an ICD.
Clinicaltrials.gov identifier
Keywords: Myocardial infarction, Preserved ejection fraction, Arrhythmic risk stratification, Two-step approach, Programmed ventricular stimulation
See page 2950 for the editorial comment on this article (doi: 10.1093/eurheartj/ehz410)
Introduction
Current guidelines suggest the prophylactic use of an implantable cardioverter-defibrillator (ICD) in post-myocardial infarction (MI) patients with left ventricular ejection fraction (LVEF) ≤35%1,2 for primary prevention of sudden cardiac death (SCD).3,4 In the presence of non-sustained ventricular tachycardia, with a LVEF between 35% and 40%, induction of sustained ventricular tachyarrhythmias during programmed ventricular stimulation (PVS) identifies an additional post-MI population at risk for SCD that may also benefit from ICD therapy.5
However, the majority of out-of-hospital SCD victims suffer from heart disease at earlier stages, exhibiting relatively well-preserved LVEFs (>35%), and would not have been candidates for ICD therapy.6,7 In a cohort of 1041 post-MI patients with LVEF of ≥40% (mean 55%), and during a follow-up period of 32 months, 18 patients presented with SCD, yielding an annual incidence of 0.6%.8 Although the risk of SCD in an individual post-MI patient with a LVEF ≥40% is relatively small, it can become significant in the overall population of post-MI patients. This is particularly applicable in the current era, where the majority of post-MI patients are revascularized and are likely to maintain a LVEF above this threshold.9,10
Early identification of potential future arrhythmic sudden death victims among patients with preserved left ventricular systolic function post-MI is a challenging task.11 In order to facilitate the design of a randomized clinical trial that will ultimately determine the role of prophylactic ICD implantation in post-MI patients with relatively preserved LVEF in clinical practice, a high-risk subgroup for major arrhythmic events (MAEs) has to be identified, to eventually be included in the randomization process.
The presence of specific non-invasive risk factors (NIRFs) in post-MI patients with relatively preserved LVEFs might identify those at high SCD risk.8,12,13 We hypothesized that, when NIRFs are present, PVS can further increase the diagnostic accuracy and value of such a risk stratification strategy in this low-risk population. Therefore, in the present study, we assessed the performance of a multifactorial, two-step, PVS-inclusive approach in identifying the high-risk post-MI patients with LVEF ≥40% who are at increased arrhythmic risk and would benefit from an ICD.
Methods
Trial oversight
The PRESERVE EF study is a multicentre, prospective, observational cohort study (clinicaltrials.gov identifier NCT02124018) with seven Cardiology departments in Greece actively participating. Study protocol was approved by all institutions’ ethics committees and was endorsed by the Hellenic Society of Cardiology with an anonymized online database created and maintained in its servers.14
Patients
Post-angiographically proven MI patients, at least 40 days after the event (90 days after surgery if they underwent coronary artery bypass grafting),15,16 with LVEF ≥40% (also assessed after 40 or 90 days, respectively from the index event), either revascularized or not—but without any evidence of active ischaemia (following negative myocardial scintigraphy/exercise treadmill test/stress echocardiography in the previous 6 months), on optimal tolerated medical therapy, were enrolled. Exclusion criteria were: (i) presence of a secondary prevention indication for ICD implantation, (ii) presence of a permanent pacemaker, due to potential effects on NIRF acquisition following pacemaker dependency and cardiac memory,17 (iii) persistent, long-standing persistent and permanent atrial fibrillation, (iv) neurological symptoms (presyncope or syncope) within the last 6 months, (v) patients with systemic illnesses (cancer, liver failure, end-stage renal disease, rheumatic diseases, and thyroid dysfunction), (vi) administration of antiarrhythmic medication other than β-blockers, and (vii) age ≥80 or ≤18 years old.
All patients provided written informed consent.
Study protocol
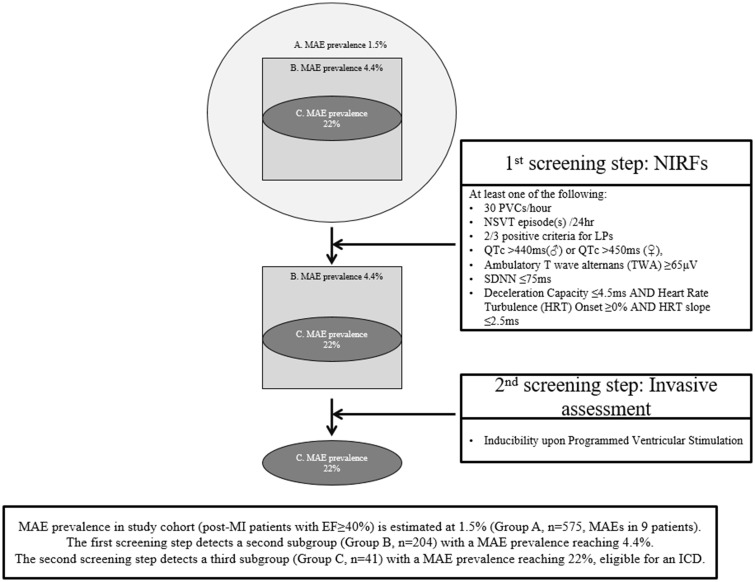
A two-step stratification algorithm was implemented.14 Upon the first step of the algorithm, an ambulatory 24 h, as well as signal-averaged electrocardiogram recordings were obtained and evaluated for the presence of the following NIRFs13,14,18–20: (i) >30 premature ventricular complexes/hour on 24-h electrocardiography, (ii) presence of non-sustained ventricular tachycardia on 24-h electrocardiography, (iii) 2/3 positive criteria for late potentials, either conventional or modified,20 (iv) QTc derived from 24-h electrocardiography >440 ms (men) or >450 ms (women)19 according to the Fridericia formula from a signal recorded in three pseudo-orthogonal leads, (v) ambulatory T-wave alternans ≥65μV in two Holter channels,18 (vi) standard deviation of normal RR intervals ≤75ms on the 24-h electrocardiography, and (7) deceleration capacity ≤4.5ms and heart rate turbulence onset ≥0% and heart rate turbulence slope ≤2.5 ms.13
In the presence of at least one NIRF, patients underwent invasive PVS and were classified as inducible if sustained monomorphic ventricular tachycardia or ventricular flutter or polymorphic ventricular tachycardia were induced. The arrhythmia induced was defined as sustained monomorphic ventricular tachycardia when a uniform morphology of QRS complexes with a rate between 120 and 220 b.p.m. was observed, while persisting for ≥30 s (or shorter, if termination was necessary due to haemodynamic instability). Faster rates of regular monomorphic ventricular tachycardia (≥220 b.p.m.) not permitting QRS complexes to be readily distinguished from T waves and without deterioration towards fibrillation, were defined as ventricular flutter, but they were included in the monomorphic category. Polymorphic ventricular tachycardia was defined if constantly changing morphologies and axis were present, eventually degenerating into fibrillation. See Supplementary material online, Appendix for PVS protocol details.
Patients with paroxysmal atrial fibrillation were evaluated for NIRF presence and submitted to PVS while on sinus, with same stimulation protocol implemented in all cases.
Risk level groups
After completion of the study protocol, patients were stratified into three groups:
Group 1—No NIRFs present—no invasive PVS performed.
Group 2—At least one NIRF present—non-inducible upon PVS.
Group 3—At least one NIRF present AND inducible upon PVS.
An ICD was offered only to Group 3 patients.
Patients declining PVS were considered not to have completed stratification and were not included in the protocol performance and survival analyses, yet were followed up as scheduled for the occurrence of any events. Patients completing the protocol but declining an offered ICD were fully included in all analyses.
Implantable cardioverter-defibrillator programming
In accordance with trials favouring prolonged detection intervals at higher rates for the avoidance of treatment of self-terminating arrhythmic events,21 ventricular tachycardia therapy cycle length was set to 330 ms and number of intervals to detect to 32. Fibrillation therapy cycle length was set to 270 ms and number of intervals to detect to 18/24. In devices with time programming, the same cycle lengths were used but intervals were set to 7 s for cycle lengths (CLs) in the 270–330 ms range and to 2.5 s for CLs < 270 ms. Ventricular tachycardia therapy consisted of several attempts of antitachycardia pacing, followed by cardioversion at progressively increasing energy. High-energy shocks were administered to terminate ventricular fibrillation. In 32 cases dual-chamber ICDs were inserted, while in the remaining 5 a single-chamber device was chosen by both the primary and implanting physicians, after excluding the presence of bradyarrhythmic aberration on the electrophysiological study.
Follow-up
Implanted patients were followed up every 3 months, and non-implanted patients every 6 months. Events included cardiac (sudden and non-sudden) and non-cardiac death. Acute coronary syndromes and/or repeat revascularization events were also recorded. All device activation were adjudicated independently by two electrophysiologists (D.T. and P.A.). In case of discrepancy, a third electrophysiologist (K.A.G.) reviewed the event.
Outcomes
The primary endpoint of the study was the occurrence of MAEs, namely either SCD/clinical ventricular tachycardia/fibrillation or/and appropriate ICD activation. Sudden cardiac death was defined as death occurring within 1 h of symptom onset if no evidence of alternative causes was present. Death was considered non-sudden cardiac if occurring in the context of heart failure deterioration. All other deaths were classified as non-cardiac. The secondary endpoint was total mortality.
Statistics
The primary goal of the study was to assess the proposed two-step PVS-inclusive risk stratification algorithm’s ability to identify a subpopulation of post-MI patients with LVEF ≥40% at risk for MAEs. To that end, the study was designed to have a statistical power of 80% for detecting free from primary endpoint occurrence survival curve divergence at the 0.05 significance level.14 All continuous variables were checked for normality of distribution, using the Shapiro–Wilk test. Regarding those with normal distribution, Student’s t-test was used for all comparisons. In case of non-normality, the Mann–Whitney U-test was used. In cases of categorical variables Fisher’s exact test was used. Binary logistic regression was used to compare the odds ratio (OR) between groups with different number of NIRFs regarding inducibility. Kaplan–Meier curves were used to visualize survival free from primary endpoint occurrence and the logrank test was applied to assess the presence of statistically significant differences. A two-sided P-value of ≤0.05 was considered statistically significant in all cases.
Power analysis14: Due to the paucity of data regarding PVS yield in similar population cohorts, it was necessary to use metrics derived from non-invasive indices included in this Study as well. After setting the type I error probability for a two-sided test at 5% and the power of our study at 80%, SDNN was found to be the variable that required the largest sample (a ratio of high SDNN patients/controls to low SDNN patients/exposed equal to 12 was anticipated). The anticipated accrual/mean follow-up intervals were 2 and 3 years, respectively. Relative risk (based on SDNN performance) of control subjects relative to exposed subjects was equal to 0.65. Based on the above, 66 exposed subjects and 792 control subjects would need to be studied to be able to reject with a probability of 80% the null hypothesis that the exposed and control survival curves are equal.
Data were analysed by K.A.G., C.-K.A., P.A., and D.T.
Results
According to the above power analysis, study design required the recruitment of at least 858 patients based on the initial power analysis. However, after 9 patients had appropriate ICD activations in a relatively short period of observation, the steering committee, considering ethical reasons suggested study termination given that a high-risk population was emerging. Of note the timing of the aforementioned post-hoc data analysis was remarkably close to the expected mean follow-up period (32 months actual follow-up vs. 36 months planned follow-up).
Thus, ultimately, from April 2014 to July 2018, 575 consecutive patients were enrolled, with the demographic characteristics shown in Table 1: mean age 57 years, 86.2% males, 66.3% ST-elevation MI (STEMI), and mean LVEF = 50.8%, with 92.7% at NYHA class I. Five-hundred and forty-two patients (94.2%) were revascularized with either percutaneous coronary intervention and/or coronary artery bypass grafting. Mean follow-up duration was 32 months.
Table 1.
Baseline clinicolaboratory data
| Parameter | All patients (n = 575) | Group 1 (no NIRFs) (n = 371) | Group 2a (NIRFs, non-inducible) (n = 111) | Group 3a (NIRFs, inducible) (n = 41) | P-value [Group 1 vs. Group (2 + 3)] | P-value (Group 2 vs. Group 3) |
|---|---|---|---|---|---|---|
| Age | 57 ± 10.4 | 55.7 ± 10.2 | 60 ± 10.9 | 61.7 ± 9.2 | <0.001 | 0.37 |
| Sex (% male) | 86.2 | 84.7 | 86.5 | 97.6 | 0.17 | 0.071 |
| Smoking (% yes) | 57.7 | 59.8 | 52.3 | 53.7 | 0.14 | 1 |
| Hypertension (% yes) | 56 | 56.5 | 56 | 63.4 | 0.77 | 0.46 |
| Dyslipidaemia (% yes) | 65.1 | 63.6 | 65.1 | 68.3 | 0.62 | 0.85 |
| Diabetes mellitus (% yes) | 17.7 | 14.9 | 15.6 | 36.6 | 0.09 | 0.007 |
| β-blockers (% yes) | 85 | 81.5 | 90.9 | 90.2 | 0.011 | 1 |
| β-blockers (mg)b | 95 ± 76 | 88 ± 73.8 | 106 ± 87.6 | 107 ± 86.7 | 0.017 | 0.95 |
| Either ACEI or ARB (% yes) | 73 | 71.6 | 70 | 80.5 | 0.83 | 0.22 |
| Statins (% yes) | 98.1 | 98.9 | 93.6 | 100 | 0.018 | 0.19 |
| Aspirin (% yes) | 97.9 | 98.4 | 97.3 | 95.1 | 0.31 | 0.61 |
| LVEF % | 50.8 (45–55) | 51.6 (45–55) | 51.1 (45–55) | 45.6 (40–50) | 0.001 | <0.001 |
| LVEDD mm | 49.8 ± 5.3 | 49.3 ± 4.9 | 50.7 ± 5.7 | 53 ± 5.7 | <0.001 | 0.031 |
| NYHA class I (%) | 92.7 | 95.8 | 87.2 | 80.5 | <0.001 | 0.4 |
| Type of infarction (% STEMI) | 66.3 | 67.6 | 54.1 | 82.9 | 0.26 | 0.001 |
| % Reperfused | 94.2 | 95.6 | 90.8 | 90.8 | 0.04 | 1 |
| Reperfusion strategy (%PCI, %CABG, %both) | 93.9, 3.8, 2.3 | 97.3, 1.35, 1.35 | 89.2, 7.2, 3.6 | 73.1, 17.1, 9.8 | <0.001 | 0.11 |
| Haemoglobin (mmol/L) | 8.75 ± 1.12 | 8.69 ± 0.87 | 8.94 ± 1.92 | 8.69 ± 0.74 | 0.17 | 0.72 |
| Blood urea nitrogen (mmol/L) | 12.21 ± 5.11 | 12.32 ± 4.79 | 12.18 ± 4.79 | 14.36 ± 6.57 | 0.33 | 0.09 |
| Creatinine (μmol/L) | 84.88 ± 20.34 | 84.88 ± 19.45 | 85.77 ± 21.22 | 87.54 ± 19.45 | 0.66 | 0.39 |
| Potassium (mmol/L) | 4.4 ± 0.41 | 4.4 ± 0.36 | 4.3 ± 0.43 | 4.4 ± 0.39 | 0.25 | 0.23 |
| Sodium (mmol/L) | 139 ± 9.1 | 139 ± 7.1 | 138 ± 11.4 | 139 ± 3 | 0.43 | 0.21 |
| Low-density lipoprotein (mmol/L) | 2.9 ± 0.99 | 2.91 ± 0.99 | 2.92 ± 1.03 | 2.53 ± 0.8 | 0.33 | 0.07 |
| High-density lipoprotein (mmol/L) | 1.06 ± 0.32 | 1.07 ± 0.26 | 1.02 ± 0.31 | 1.13 ± 0.68 | 0.59 | 0.35 |
| Follow-up duration (months) | 32 ± 13.05 | 32.2 ± 13.5 | 33.3 ± 12.4 | 28.9 ± 13.6 | 0.61 | 0.06 |
| NIRF | Prevalence (%) | |||||
| LPs | 13.8 | 0 | 31.5 | 51.2 | N/A | 0.036 |
| PVCs (>30/h) | 10.8 | 0 | 34.3 | 39 | N/A | 0.7 |
| nsVT | 8.6 | 0 | 23.1 | 46.3 | N/A | 0.009 |
| QTc prolongation | 13.6 | 0 | 40.4 | 36.6 | N/A | 0.71 |
| Abnormal heart rate turbulence/deceleration capacity | 2.8 | 0 | 9.3 | 9.8 | N/A | 1 |
| Abnormal heart rate variability (SDNN <75ms) | 2.8 | 0 | 8.3 | 9.8 | N/A | 0.75 |
| TWA ≥65μV | 6.8 | 0 | 20.4 | 24.4 | N/A | 0.66 |
All continuous variables with normal distributions shown as mean ± standard deviation. Continuous variables without normal distributions shown as mean (25th–75th quartile).
The 52 NIRF positive patients declining PVS were excluded from analysis.
B-blocker doses in metoprolol equivalents. None of the patients received neprilysin inhibitors.
ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; CABG, coronary artery bypass grafting; LP, late potentials; LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; N/A, not applicable; NIRF, non-invasive risk factor; nsVT, non-sustained ventricular tachycardia; PCI, percutaneous coronary intervention; PVC, premature ventricular complex; PVS, programmed ventricular stimulation; STEMI, ST-elevation myocardial infarction; TWA, T-wave alternans.
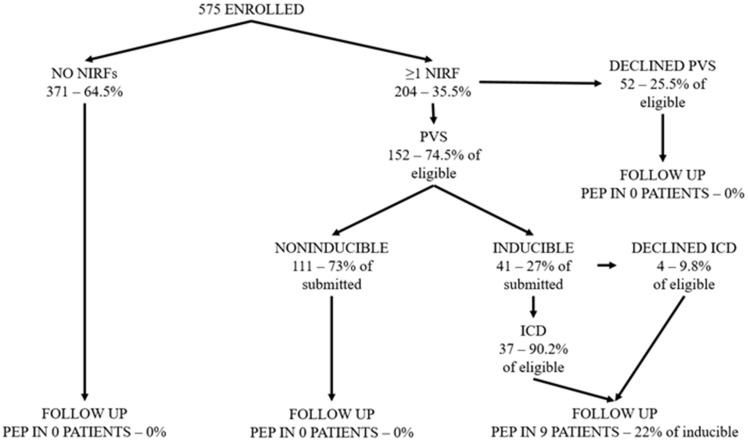
The actual flow of patients is indicated in Figure 1. Fifty-two patients declined PVS, and four patients declined ICD implantation. There were no complications following PVS, while patients could usually be discharged on the same day. There was a single case of pocket infection necessitating ICD extraction.
Figure 1.
Study flowchart and patient flow. MAE, major arrhythmic event; NIRF, non-invasive risk factors; PEP, primary endpoint (MAE occurrence); PVS, programmed ventricular stimulation—see ‘Methods’ section for more details.
Two-hundred and four patients (35.5%) had at least one NIRF. Late potentials and QTc prolongation were the most commonly encountered NIRFs (13.8% and 13.6%, respectively), followed by the ventricular burden indices of premature ventricular complexes and non-sustained ventricular tachycardia (10.8% and 8.6%, respectively), then by T-wave alternans (6.8%), and lastly by the autonomic function indices of abnormal heart rate variability and turbulence with deceleration capacity (2.8% for both).
Table 1 presents the comparison of characteristics between non-inducible and inducible groups—i.e. Groups 2 and 3, respectively. Only patients having undergone PVS are included. Patients declining PVS did not significantly differ from those consenting in any study variable, with the exception of mean number of NIRFs, being actually lower than that of patients who underwent (1.31 vs. 1.78, P = 0.015). Group 3 patients, compared with Group 2, had lower LVEF, more dilated left ventricles and more often suffered from diabetes. ST-elevation MIs were more prevalent in this group, as were late potentials and non-sustained ventricular tachycardia.
Odds ratios regarding inducibility of ventricular arrhythmias during PVS between subgroups with different number of risk factors revealed those with ≥2 to be significantly more likely to be inducible compared with those with a single factor (OR≥2factors/factor=1 = 2.5, 95% credibility interval 1.2–5.5, P = 0.02). Similarly, those with ejection fraction ≤50% were more likely to be inducible vs. those with ejection fractions >50% (OR = 10.7, 95% credibility interval 3.1–36.9). No significantly increased OR for inducibility were noted in relation with infarction type (STEMI vs. nSTEMI).
Table 2 presents PVS findings in Group 3 patients. The majority of induced arrhythmias was monomorphic ventricular tachycardias (85.4%) with a short mean cycle length (244 ms).
Table 2.
Programmed ventricular stimulation findings in inducible patients
| Induced VT | Induced polymorphic VT/VF | Site | Number of extrasystoles | Morphology | Axis | Ventricular tachycardia cycle length (ms) | Termination mode |
|---|---|---|---|---|---|---|---|
| 35 | 6 (one patient had both VT and PVT inducible) | RVA: 36 RVOT: 5 |
|
|
|
244 (216–247) |
|
Number of inducible patients = 41.
ATP, antitachycardia pacing; LAD, left axis deviation; LBBB, left bundle branch block; NWA, northwestern axis; RAD, right axis deviation; RBBB, right bundle branch block; RVA, right ventricular apex; RVOT, right ventricular outflow tract; VF, ventricular fibrillation; VT, ventricular tachycardia.
Table 3 shows the characteristics of patients with appropriate ICD activation. No inappropriate ICD activations were observed. Notably, in all patients with device activation monomorphic ventricular tachycardia had been induced upon PVS. No events occurred in patients with a LVEF > 50%. No difference whatsoever was noted between those suffering the primary endpoint and other inducible patients regarding β-blocker dose (P = 1).
Table 3.
Characteristics of patients with appropriate ICD activation
| Patient number | Age | Gender | Infarction type | Culprit vessel | NYHA class | LVEF % | LVEDD mm | β-blocker dose (metoprolol equivalents) | NIRFs present | Time after index MI (months) | Induced arrhythmia cycle length (ms) | Clinical arrhythmia cycle length (ms) | Clinical arrhythmia therapy |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 64 | Male | STEMI | LCx | 1 | 45 | 57 | 75 | LPs | 10.6 | 250 | 220 | SHOCK |
| 2 | 67 | Male | STEMI | LCx | 1 | 47 | 46 | 125 | LPs, PVCs, nsVT | 11.3 | 240 | 250 | SHOCK |
| 3 | 63 | Male | STEMI | RCA | 1 | 48 | 47 | 150 | LPs, PVCs, nsVT | 7 | 250 | 240 | SHOCK |
| 4 | 58 | Male | STEMI | LAD | 1 | 45 | 48 | 75 | LPs, QTc, nsVT | 14 | 310 | 320 | Failed ATP>SHOCK |
| 5 | 53 | Male | STEMI | RCA | 1 | 45 | 54 | 100 | LPs, PVCs,QTc,nsVT | 12 | 300 | 285 | Failed ATP>SHOCK |
| 6 | 59 | Male | STEMI | LAD/RCA | 1 | 50 | 60 | 75 | LPs, QTc, nsVT | 47.7 | 248 | 310 | ATP |
| 7 | 70 | Male | STEMI | 3 vessels | 1 | 40 | 55 | 100 | LPs,QTc,TWA | 30.7 | 280 | 270 | SHOCK |
| 8 | 72 | Male | STEMI | LCx | 1 | 50 | 52 | 200 | nsVT | 46.2 | 208 | 320 | Failed ATP>SHOCK |
| 9 | 64 | Male | STEMI | 3 vessels | 2 | 50 | 56 | 65 | QTc | 59.5 | 260 | 260 | ATP (during charging) |
| Average | 63 | — | — | — | 1.1 | 47 | 52.8 | 107 | — | 26.6 | 261 | 275 | — |
It should be highlighted that three of the above patients only had a single NIRF, with the rest having at least three NIRFs each.
ATP, antitachycardia pacing; ICD, implantable cardioverter-defibrillator; LAD, left anterior descending artery; LCx, left circumflex artery; LP, late potentials; LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NIRF, non-invasive risk factors; nsVT, non-sustained ventricular tachycardia; PVC, premature ventricular complex; RCA, right coronary artery; STEMI, ST-elevation myocardial infarction; TWA, T-wave alternans.
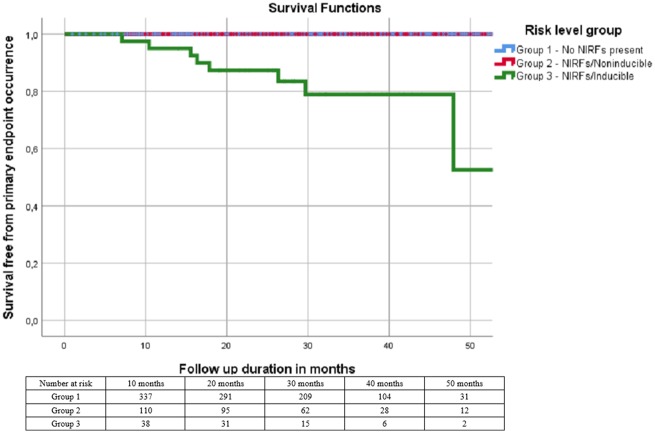
Survival analysis showed that no primary endpoint events occurred in Groups 1 and 2 while nine Group 3 patients met this endpoint in the form of appropriate device therapy, for a prevalence of 22% and an annual incidence rate of 8.2%. Mean time elapsed between device implantation and activation was 13 ± 11.9 (median 10) months. Kaplan–Meier curves for all three groups regarding primary endpoint occurrence are shown in Figure 2 (Plogrank < 0.001). Survival analysis for the endpoint of total mortality/appropriate ICD activation yielded similar results (Plogrank < 0.001). Due to the low number of events (9) a multivariable analysis was not feasible.
Figure 2.
Kaplan–Meier survival curves by risk group. No primary endpoint events occurred in Groups 1 and 2. Patients declining programmed ventricular stimulation are not depicted (unknown risk group). NIRF, non-invasive risk factor.
The secondary endpoint occurred in five patients (0.9%), with four Group 2 patients suffering non-cardiac deaths and one Group 3 ICD-bearing patient suffering non-SCD during an episode of acute renal failure, without the device interrogation offering any evidence for precipitating arrhythmic events. New acute coronary events occurred in 25 patients (4.35% of total population). Two patients exhibited worsening of NYHA functional class (moving from class I to class II), and 15 had paroxysmal atrial fibrillation episodes during follow-up (none had been inducible upon PVS), with no ischaemic strokes reported.
The performance of the proposed approach was characterized by the following metrics: Sensitivity = 100%, specificity = 93.8%, positive predictive value = 22%, and negative predictive value = 100%.
Discussion
In the present study, we examined a combined, two-step, multifactorial approach for arrhythmic risk stratification of post-MI patients with LVEF ≥40%. Indeed, a high risk post-MI subgroup within the PRESERVE EF study population exists and these patients can be identified and subsequently protected with an ICD. Our two-step approach identified 41 high-risk patients out of 575 consecutively enrolled post-MI subjects with a LVEF ≥ 40%. The primary endpoint occurred in nine out of them, all having suffered an STEMI, yielding a prevalence of 22% and an annual incidence rate of 8.2%, over a short follow-up period of 32 months. No sudden death events were observed among the study population when no NIRFs were present (Group 1), or even when risk factors were present but were not associated with tachyarrhythmia induction during PVS (Group 2). Thus, our approach accurately identified post-MI patients with LVEF ≥40% at high risk, enabling their timely protection with an ICD from any MAE.
The annual incidence of SCD in this population is about 0.6–1%, close to the observed incidence of ICD activation in the current study.8,11 Our study suggests that this relatively low annual incidence of sudden arrhythmic death is likely to be increased exponentially among those truly high-risk post-MI patients with inducible sustained ventricular tachyarrhythmias and at least one NIRF present. The prevalence of 22% ICD appropriate activation rate among them is close to the observed appropriate ICD activation rate in the SCD-HeFT stage II-III heart failure patient population with LVEFs ≤35% (21%), namely a study in which a survival benefit for the ICD group was observed.4 Although appropriate ICD activation rate might have overestimated true SCD incidence,22 it is remarkable that no SCD was observed. Furthermore, ICD programming required a relatively long detection interval in two detection zones, one at 180–220 and the second at >220 b.p.m., in accordance with relevant trials, thus minimizing the possibility of inappropriate activation and unnecessary and potentially dangerous overtreatment.21,23 Indeed, in our ICD population no inappropriate activations were observed. Moreover, the induced ventricular tachycardia cycle length, both of Group 3 (244 ms), as well as of the nine primary endpoint meeting patients (261 ms) was rather short, and, in the latter case, close to the observed cycle length of the interrupted clinical tachyarrhythmia (275 ms). Consequently, it is likely that the observed incidence of ICD activation in this study population reflects a realistic SCD risk among patients in whom sustained ventricular tachyarrhythmias were induced, despite relatively preserved LVEF.
Non-invasive risk factors, like T-wave alternans, late potentials, non-sustained ventricular tachycardia, heart rate turbulence and deceleration capacity, have been associated with SCD risk among post-MI patients with LVEF ≥35%.5,8,12,13 We additionally incorporated the burden of ventricular ectopy, along with repolarization phase prolongation in order to include all arrhythmogenic mechanisms.14,24 Previous studies did not further proceed with PVS, a technique believed not to bear an associated prognostic role among such post-MI patients, with the exception of the MUSTT.5 Contrary to this belief, we have demonstrated a significant contribution of PVS among post-MI and dilated cardiomyopathy patients with a LVEF ≥35%, when presenting with either complex ventricular ectopy and/or neurological symptoms, such as presyncope or syncope attacks.25,26 We have also supported the beneficial role of a combined non-invasive—PVS-inclusive approach in such patients with a LVEF ≥35%.27 Such observations, along with the need to better define the high-risk post-MI subpopulation that despite a relatively preserved LVEF (≥40%) will benefit from an ICD, led us to the design and performance of the present study.1,2,14
The concept of the two-step multifactorial, PVS-inclusive approach has been successfully introduced for the risk stratification and management of post-MI patients, incorporating NIRFs, such as heart rate variability, LVEF, late potentials, and complex ventricular arrhythmias.28–30 Indeed, such studies identified a high-risk group of post-MI patients, predominantly among those with reduced LVEF, that are usually offered an ICD, based on current guidelines.1,2 Our risk stratification approach provides an accurate algorithm to detect the high risk post-MI patient following a limited myocardial injury without any evidence of ongoing myocardial ischaemia or significant left ventricular dysfunction.31
The non-invasive step of our approach would necessitate PVS performance on approximately one-third of enrolled patients (35.5%). Late potentials and prolonged repolarization were the most frequently encountered NIRFs. Abnormal autonomic function indices were uncommonly met,13 reflecting the absence of heart failure symptomatology while the relatively lower prevalence of T-wave alternans8,12 may be a reflection of the strict criteria required for establishing its presence in the current study (≥65μV in two Holter channels).
The second step helped better define the associated risk by identifying patients susceptible to sustained ventricular tachyarrhythmias, without compromising patient safety. These high-risk inducible post-MI patients had a relatively lower LVEF, along with a slightly more dilated left ventricle, suffered an STEMI in the presence of diabetes, a finding reported in previous studies as well and with intricate pathophysiological mechanisms.32–34. They also had an increased prevalence of late potentials and non-sustained ventricular tachycardia.35 All these characteristics suggest that there is a critical amount of myocardial fibrosis required in order to sustain ventricular re-entry, rendering the use of imaging modalities capable of identifying and quantifying scar burden, such as cardiac magnetic resonance imaging, appealing alternatives.36 This has recently been suggested in proposed algorithm for arrhythmic risk stratification in heart failure.37 Moreover, all primary endpoint events occurred in patients in whom monomorphic ventricular tachycardias were induced. This could have been anticipated considering the absence of active ischaemia in our population. Although post-MI patients with ≥2 NIRFs were more likely to be inducible, three out of nine primary endpoint meeting patients only had a single risk factor, supporting study design.
The performance of our approach yielded absolute sensitivity and negative predictive values, with a positive predictive one of 22%. This under the limitation of the relatively high percentage of eligible patients declining PVS (n = 52, 25.5%) that may have led to inaccurate assessment of our algorithm accuracy. However, their lower NIRF number, combined with the relationship between inducibility and NIRFs renders credibility to the assumption that a lower tachyarrhythmia induction rate would have been observed, compared with the 27% of those submitted to PVS.
Notably, patients in Groups 2 and 3 had significantly higher percentages of β-blocker treatment, as well as medication dose, compared with Group 1, findings attributable to their lower ejection fraction, worse NYHA functional class, and lower reperfusion rates (Table 1). We also acknowledge the remarkably low incidence of recurrent myocardial ischaemia in our cohort of appropriately revascularized post-MI population. It can be argued that the explicit requirement for ongoing ischaemia exclusion with a negative test in the previous 6 months may partially account for this finding. Absence of SCD mortality in our patient population may be the combined result of both an adequate revascularization strategy, along with an effective antiarrhythmic protection of the truly high risk subpopulation. Given the absence of significant myocardial systolic dysfunction and left ventricular dilatation in this cohort of post-MI patients at an early heart failure stage, it is expected to observe a much better survival rate among them, compared with those ICD post-MI patients with significant ventricular dysfunction at symptomatic heart failure stages.27
The proposed evaluation of post-MI patients with preserved LVEF for the detection of NIRFs is a low-cost and widely available procedure for every practicing cardiologist in an out-of-hospital basis. Detection of NIRFs in this patient population will disclose those at high risk for SCD after the performance of PVS even on an outpatient basis. Increased awareness of the potential risk for SCD in these patients will benefit the community and every single patient as well. The above are rendered even more clinically relevant given the formal introduction of the heart failure with moderately reduced LVEF (40–49%) category in guidelines,38 given that no primary endpoint events occurred in patients with LVEF >50%. The above are illustrated in Take home figure.
Take home figure.
Outline of study design and findings. Starting with a cohort exhibiting a prevalence of major arrhythmic events at the 1.5% level (after a 32-month follow-up), the two-step, programmed ventricular stimulation-inclusive approach allowed for the identification of a high arrhythmic risk subgroup with a major arrhythmic event prevalence reaching 22% (almost 15-fold higher than baseline). LP, late potentials; MAE, major arrhythmic event; NIRF, non-invasive risk factors; nsVT, non-sustained ventricular tachycardia; PVC, premature ventricular complex.
However, our study was not a randomized clinical trial since almost all patients with a per protocol indication received a device, without the presence of a high-risk control group, thus potentially overestimating true arrhythmic risk (non-lethal events being treated by ICDs). Moreover, it is possible that some Group 2 patients (NIRFs present but non-inducible) might have experienced subclinical episodes of self-terminating ventricular arrhythmias that could also have fallen within the pre-specified therapy zone had they had a device. Thus, it is advisable to perform a randomized trial with extensive use of implantable loop recorders (offered to inducible patients after randomization to follow-up as controls as well as to those with NIRFs who are non-inducible) in order to better define the accuracy and clinical ramifications stemming from the application of the algorithm implemented in this study.
Conclusions
In summary, a truly high-risk post-MI subgroup of patients with relatively preserved LVEF, at an early heart failure stage, exists and can be identified by means of a multifactorial, PVS-inclusive two-step approach applied in the late post-infarction phase. Based on the detection of such a high arrhythmic risk subgroup, multicentre randomized controlled clinical trials could clarify the clinical significance of findings presented herein and allow for the broad application of the proposed algorithm in clinical practice.
Supplementary Material
Acknowledgements
PRESERVE EF investigators wish to acknowledge General Electric (GE) Healthcare assistance and technical support regarding 24-hour holter recorders.
Funding
PRESERVE EF is a physician-initiated study that received unrestricted funding by Medtronic Hellas S.A. Notably, all ICDs were paid for by the Hellenic State.
Conflict of interest: none declared.
References
- 1. Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, Deal BJ, Dickfeld T, Field ME, Fonarow GC, Gillis AM, Hlatky MA, Granger CB, Hammill SC, Joglar JA, Kay GN, Matlock DD, Myerburg RJ, Page RL.. AHA/ACC/HRS Guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation 2018;138:e272–e391. [DOI] [PubMed] [Google Scholar]
- 2. Priori SG, Blomstrom-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, Elliott PM, Fitzsimons D, Hatala R, Hindricks G, Kirchhof P, Kjeldsen K, Kuck KH, Hernandez-Madrid A, Nikolaou N, Norekval TM, Spaulding C, Van Veldhuisen DJ.. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J 2015;36:2793–2867. [DOI] [PubMed] [Google Scholar]
- 3. Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW, Andrews ML.. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002;346:877–883. [DOI] [PubMed] [Google Scholar]
- 4. Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, Domanski M, Troutman C, Anderson J, Johnson G, McNulty SE, Clapp-Channing N, Davidson-Ray LD, Fraulo ES, Fishbein DP, Luceri RM, Ip JH.. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med 2005;352:225–237. [DOI] [PubMed] [Google Scholar]
- 5. Buxton AE, Lee KL, DiCarlo L, Gold MR, Greer GS, Prystowsky EN, O'Toole MF, Tang A, Fisher JD, Coromilas J, Talajic M, Hafley G.. Electrophysiologic testing to identify patients with coronary artery disease who are at risk for sudden death. Multicenter Unsustained Tachycardia Trial Investigators. N Engl J Med 2000;342:1937–1945. [DOI] [PubMed] [Google Scholar]
- 6. Gorgels AP, Gijsbers C, de V-SJ, Lousberg A, Wellens HJ.. Out-of-hospital cardiac arrest–the relevance of heart failure. The Maastricht Circulatory Arrest Registry. Eur Heart J 2003;24:1204–1209. [DOI] [PubMed] [Google Scholar]
- 7. Stecker EC, Vickers C, Waltz J, Socoteanu C, John BT, Mariani R, McAnulty JH, Gunson K, Jui J, Chugh SS.. Population-based analysis of sudden cardiac death with and without left ventricular systolic dysfunction: two-year findings from the Oregon Sudden Unexpected Death Study. J Am Coll Cardiol 2006;47:1161–1166. [DOI] [PubMed] [Google Scholar]
- 8. Ikeda T, Yoshino H, Sugi K, Tanno K, Shimizu H, Watanabe J, Kasamaki Y, Yoshida A, Kato T.. Predictive value of microvolt T-wave alternans for sudden cardiac death in patients with preserved cardiac function after acute myocardial infarction: results of a collaborative cohort study. J Am Coll Cardiol 2006;48:2268–2274. [DOI] [PubMed] [Google Scholar]
- 9. Myerburg RJ, Interian A Jr, Mitrani RM, Kessler KM, Castellanos A.. Frequency of sudden cardiac death and profiles of risk. Am J Cardiol 1997;80:10f–19f. [DOI] [PubMed] [Google Scholar]
- 10. Serrao GW, Lansky AJ, Mehran R, Stone GW.. Predictors of left ventricular ejection fraction improvement after primary stenting in ST-segment elevation myocardial infarction (from the harmonizing outcomes with revascularization and stents in acute myocardial infarction trial). Am J Cardiol 2018;121:678–683. [DOI] [PubMed] [Google Scholar]
- 11. Goldberger JJ, Buxton AE, Cain M, Costantini O, Exner DV, Knight BP, Lloyd-Jones D, Kadish AH, Lee B, Moss A, Myerburg R, Olgin J, Passman R, Rosenbaum D, Stevenson W, Zareba W, Zipes DP.. Risk stratification for arrhythmic sudden cardiac death: identifying the roadblocks. Circulation 2011;123:2423–2430. [DOI] [PubMed] [Google Scholar]
- 12. Exner DV, Kavanagh KM, Slawnych MP, Mitchell LB, Ramadan D, Aggarwal SG, Noullett C, Van Schaik A, Mitchell RT, Shibata MA, Gulamhussein S, McMeekin J, Tymchak W, Schnell G, Gillis AM, Sheldon RS, Fick GH, Duff HJ.. Noninvasive risk assessment early after a myocardial infarction the REFINE study. J Am Coll Cardiol 2007;50:2275–2284. [DOI] [PubMed] [Google Scholar]
- 13. Bauer A, Barthel P, Schneider R, Ulm K, Muller A, Joeinig A, Stich R, Kiviniemi A, Hnatkova K, Huikuri H, Schomig A, Malik M, Schmidt G.. Improved stratification of autonomic regulation for risk prediction in post-infarction patients with preserved left ventricular function (ISAR-Risk). Eur Heart J 2009;30:576–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gatzoulis KA, Tsiachris D, Arsenos P, Dilaveris P, Sideris S, Simantirakis E, Efremidis M, Dagres N, Korantzopoulos P, Fragkakis N, Letsas K, Flevari P, Vasilikos V, Sideris A, Iliodromitis E, Goudevenos I, Lekakis I, Vardas P, Kallikazaros I, Stefanadis C.. Post myocardial infarction risk stratification for sudden cardiac death in patients with preserved ejection fraction: PRESERVE-EF study design. Hellenic J Cardiol 2014;55:361–368. [PubMed] [Google Scholar]
- 15. Hohnloser SH, Kuck KH, Dorian P, Roberts RS, Hampton JR, Hatala R, Fain E, Gent M, Connolly SJ.. Prophylactic use of an implantable cardioverter-defibrillator after acute myocardial infarction. N Engl J Med 2004;351:2481–2488. [DOI] [PubMed] [Google Scholar]
- 16. Steinbeck G, Andresen D, Seidl K, Brachmann J, Hoffmann E, Wojciechowski D, Kornacewicz-Jach Z, Sredniawa B, Lupkovics G, Hofgartner F, Lubinski A, Rosenqvist M, Habets A, Wegscheider K, Senges J.. Defibrillator implantation early after myocardial infarction. N Engl J Med 2009;361:1427–1436. [DOI] [PubMed] [Google Scholar]
- 17. Shvilkin A, Huang HD, Josephson ME.. Cardiac memory: diagnostic tool in the making. Circ Arrhythm Electrophysiol 2015;8:475–482. [DOI] [PubMed] [Google Scholar]
- 18. Verrier RL, Klingenheben T, Malik M, El-Sherif N, Exner DV, Hohnloser SH, Ikeda T, Martinez JP, Narayan SM, Nieminen T, Rosenbaum DS.. Microvolt T-wave alternans physiological basis, methods of measurement, and clinical utility–consensus guideline by International Society for Holter and Noninvasive Electrocardiology. J Am Coll Cardiol 2011;58:1309–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Arsenos P, Gatzoulis KA, Dilaveris P, Gialernios T, Sideris S, Lazaros G, Archontakis S, Tsiachris D, Kartsagoulis E, Stefanadis C.. The rate-corrected QT interval calculated from 24-hour Holter recordings may serve as a significant arrhythmia risk stratifier in heart failure patients. Int J Cardiol 2011;147:321–323. [DOI] [PubMed] [Google Scholar]
- 20. Gatzoulis KA, Carlson MD, Biblo LA, Rizos I, Gialafos J, Toutouzas P, Waldo AL.. Time domain analysis of the signal averaged electrocardiogram in patients with a conduction defect or a bundle branch block. Eur Heart J 1995;16:1912–1919. [DOI] [PubMed] [Google Scholar]
- 21. Gasparini M, Proclemer A, Klersy C, Kloppe A, Lunati M, Ferrer JB, Hersi A, Gulaj M, Wijfels MC, Santi E, Manotta L, Arenal A.. Effect of long-detection interval vs standard-detection interval for implantable cardioverter-defibrillators on antitachycardia pacing and shock delivery: the ADVANCE III randomized clinical trial. JAMA 2013;309:1903–1911. [DOI] [PubMed] [Google Scholar]
- 22. Ellenbogen KA, Levine JH, Berger RD, Daubert JP, Winters SL, Greenstein E, Shalaby A, Schaechter A, Subacius H, Kadish A.. Are implantable cardioverter defibrillator shocks a surrogate for sudden cardiac death in patients with nonischemic cardiomyopathy? Circulation 2006;113:776–782. [DOI] [PubMed] [Google Scholar]
- 23. Moss AJ, Schuger C, Beck CA, Brown MW, Cannom DS, Daubert JP, Estes NA 3rd, Greenberg H, Hall WJ, Huang DT, Kautzner J, Klein H, McNitt S, Olshansky B, Shoda M, Wilber D, Zareba W.. Reduction in inappropriate therapy and mortality through ICD programming. N Engl J Med 2012;367:2275–2283. [DOI] [PubMed] [Google Scholar]
- 24. Arsenos P, Gatzoulis K, Dilaveris P, Manis G, Tsiachris D, Archontakis S, Vouliotis AI, Sideris S, Stefanadis C.. Arrhythmic sudden cardiac death: substrate, mechanisms and current risk stratification strategies for the post-myocardial infarction patient. Hellenic J Cardiol 2013;54:301–315. [PubMed] [Google Scholar]
- 25. Gatzoulis KA, Vouliotis AI, Tsiachris D, Salourou M, Archontakis S, Dilaveris P, Gialernios T, Arsenos P, Karystinos G, Sideris S, Kallikazaros I, Stefanadis C.. Primary prevention of sudden cardiac death in a nonischemic dilated cardiomyopathy population: reappraisal of the role of programmed ventricular stimulation. Circ Arrhythm Electrophysiol 2013;6:504–512. [DOI] [PubMed] [Google Scholar]
- 26. Gatzoulis KA, Tsiachris D, Arsenos P, Archontakis S, Dilaveris P, Vouliotis A, Sideris S, Skiadas I, Kallikazaros I, Stefanadis C.. Prognostic value of programmed ventricular stimulation for sudden death in selected high risk patients with structural heart disease and preserved systolic function. Int J Cardiol 2014;176:1449–1451. [DOI] [PubMed] [Google Scholar]
- 27. Gatzoulis KA, Tsiachris D, Dilaveris P, Archontakis S, Arsenos P, Vouliotis A, Sideris S, Trantalis G, Kartsagoulis E, Kallikazaros I, Stefanadis C.. Implantable cardioverter defibrillator therapy activation for high risk patients with relatively well preserved left ventricular ejection fraction. Does it really work?. Int J Cardiol 2013;167:1360–1365. [DOI] [PubMed] [Google Scholar]
- 28. Pedretti R, Etro MD, Laporta A, Sarzi Braga S, Carù B.. Prediction of late arrhythmic events after acute myocardial infarction from combined use of noninvasive prognostic variables and inducibility of sustained monomorphic ventricular tachycardia. Am J Cardiol 1993;71:1131–1141. [DOI] [PubMed] [Google Scholar]
- 29. Andresen D, Steinbeck G, Bruggemann T, Muller D, Haberl R, Behrens S, Hoffmann E, Wegscheider K, Dissmann R, Ehlers HC.. Risk stratification following myocardial infarction in the thrombolytic era: a two-step strategy using noninvasive and invasive methods. J Am Coll Cardiol 1999;33:131–138. [DOI] [PubMed] [Google Scholar]
- 30. Schmitt C, Barthel P, Ndrepepa G, Schreieck J, Plewan A, Schomig A, Schmidt G.. Value of programmed ventricular stimulation for prophylactic internal cardioverter-defibrillator implantation in postinfarction patients preselected by noninvasive risk stratifiers. J Am Coll Cardiol 2001;37:1901–1907. [DOI] [PubMed] [Google Scholar]
- 31. Pascale P, Schlaepfer J, Oddo M, Schaller MD, Vogt P, Fromer M.. Ventricular arrhythmia in coronary artery disease: limits of a risk stratification strategy based on the ejection fraction alone and impact of infarct localization. Europace 2009;11:1639–1646. [DOI] [PubMed] [Google Scholar]
- 32. Kutyifa V, Beck C, Brown MW, Cannom D, Daubert J, Estes M, Greenberg H, Goldenberg I, Hammes S, Huang D, Klein H, Knops R, Kosiborod M, Poole J, Schuger C, Singh JP, Solomon S, Wilber D, Zareba W, Moss AJ.. Multicenter Automatic Defibrillator Implantation Trial-Subcutaneous Implantable Cardioverter Defibrillator (MADIT S-ICD): design and clinical protocol. Am Heart J 2017;189:158–166. [DOI] [PubMed] [Google Scholar]
- 33. Tousoulis D, Oikonomou E, Siasos G, Stefanadis C.. Diabetes mellitus and heart failure. Eur Cardiol 2014;9:37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vaduganathan M, Claggett BL, Chatterjee NA, Anand IS, Sweitzer NK, Fang JC, O’Meara E, Shah SJ, Hegde SM, Desai AS, Lewis EF, Rouleau J, Pitt B, Pfeffer MA, Solomon SD.. Sudden death in heart failure with preserved ejection fraction: a competing risks analysis from the TOPCAT trial. JACC Heart Fail 2018;6:653–661. [DOI] [PubMed] [Google Scholar]
- 35. Gatzoulis KA, Arsenos P, Trachanas K, Dilaveris P, Antoniou C, Tsiachris D, Sideris S, Kolettis TM, Tousoulis D.. Signal-averaged electrocardiography: past, present, and future. J Arrhythm 2018;34:222–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yalin K, Golcuk E, Buyukbayrak H, Yilmaz R, Arslan M, Dursun M, Bilge AK, Adalet K.. Infarct characteristics by CMR identifies substrate for monomorphic VT in post-MI patients with relatively preserved systolic function and ns-VT. Pacing Clin Electrophysiol 2014;37:447–453. [DOI] [PubMed] [Google Scholar]
- 37. Gatzoulis KA, Sideris A, Kanoupakis E, Sideris S, Nikolaou N, Antoniou CK, Kolettis TM.. Arrhythmic risk stratification in heart failure: time for the next step? Ann Noninvasive Electrocardiol 2017;22:e12430–e12438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, Gonzalez-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P.. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129–2200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.