Abstract
Background
The vast majority of women diagnosed with ductal carcinoma in situ (DCIS) undergo treatment. Therefore, the risks of invasive progression and competing death in the absence of locoregional therapy are uncertain.
Methods
We performed survival analyses of patient-level data from DCIS patients who did not receive definitive surgery or radiation therapy as recorded in the US National Cancer Institute’s Surveillance, Epidemiology, and End Results program (1992–2014). Kaplan-Meier curves were used to estimate the net risk of subsequent ipsilateral invasive cancer. The cumulative incidences of ipsilateral invasive cancer, contralateral breast cancer, and death were estimated using competing risk methods.
Results
A total of 1286 DCIS patients who did not undergo locoregional therapy were identified. Median age at diagnosis was 60 years (inter-quartile range = 51–74 years), with median follow-up of 5.5 years (inter-quartile range = 2.3–10.6 years). Among patients with tumor grade I/II (n = 547), the 10-year net risk of ipsilateral invasive breast cancer was 12.2% (95% confidence interval [CI] = 8.6% to 17.1%) compared with 17.6% (95% CI = 12.1% to 25.2%) among patients with tumor grade III (n = 244) and 10.1% (95% CI = 7.4% to 13.8%) among patients with unknown grade (n = 495). Among all patients, the 10-year cumulative incidences of ipsilateral invasive cancer, contralateral breast cancer, and all-cause mortality were 10.5% (95% CI = 8.5% to 12.4%), 3.9% (95% CI = 2.6% to 5.2%), and 24.1% (95% CI = 21.2% to 26.9%), respectively.
Conclusion
Despite limited data, our findings suggest that DCIS patients without locoregional treatment have a limited risk of invasive progression. Although the cohort is not representative of the general population of patients diagnosed with DCIS, the findings suggest that there may be overtreatment, especially among older patients and patients with elevated comorbidities.
The detection and clinical management of ductal carcinoma in situ (DCIS) poses a critical public health challenge. Each year, more than 50 000 women in the United States are diagnosed with DCIS (1), and 98% of them undergo surgery in the form of a lumpectomy (with or without radiation therapy), mastectomy, or bilateral mastectomy (2). There is concern that a clinically significant fraction of these women may be overtreated for indolent or slowly growing disease that would, in the absence of treatment, not develop into symptomatic or clinically significant breast cancer during their remaining lifetime (3,4). Importantly, overtreated patients are at risk of experiencing treatment-associated harms—including pain and sensory disturbances, psychological distress, and radiation-induced malignancies—without any cancer-related benefits (5,6).
To prevent potentially harmful overtreatment of DCIS, it is essential to identify well-defined subgroups of patients who are at minimal risk for progression to invasive breast cancer. The risk of breast cancer-specific mortality in the absence of locoregional treatment has been previously reported (7). However, there are few available data on the risk of invasive progression of DCIS in the absence of definitive surgery and radiation with which to inform risk stratification (8). To address this clinical question, three randomized clinical trials are currently enrolling low-risk patients to surgery or active surveillance, some of whom will undergo treatment with endocrine therapy (9–11). The trials have slightly different definitions of “low-risk” DCIS (12), yet all include criteria based on age at diagnosis and tumor pathology.
Because the progression dynamics of low-risk DCIS are expected to be slow, clinically actionable follow-up data from these trials will be available in 10 years at the earliest. In the meantime, it is important to summarize available evidence that could provide insights into the risk of progression to invasive disease. Despite the fact that observational data are subject to selection biases, they can inform plausible ranges of absolute risks, and they provide opportunity to estimate relative risks with respect to patient and tumor features.
In this study we extracted data from the US National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program (13) to characterize outcomes of DCIS patients in the absence of definitive surgery and radiation therapy. The aims of this study were to quantify the risks of ipsilateral invasive breast cancer, contralateral cancer, and competing death and to identify patient and tumor characteristics associated with these risks.
Methods
Data Extraction
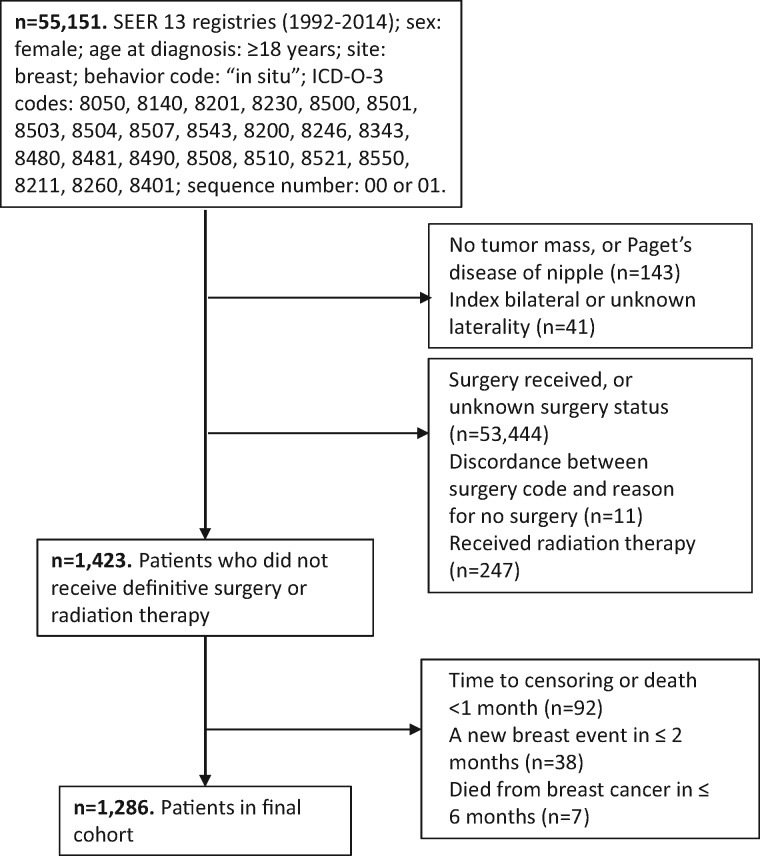
The coding rules for SEER require that all contralateral breast cancers (CBC) and all ipsilateral invasive breast cancers (iIBC) occurring at least 60 days after diagnosis with DCIS be recorded as multiple primaries (14). This enables ascertainment of the time from index DCIS to subsequent breast cancers as well as the laterality of these new breast events. The SEER 13 Registries database (1992–2014) was queried for female breast cancer patients age 18 years or older diagnosed with behavior code “in situ” and ICD-O-3 histology codes associated with DCIS (Figure 1). Only patients with unilateral DCIS and no reported record of definitive surgery or radiation therapy prior to the diagnosis of invasive cancer were included. Excluded were patients with a first cancer diagnosis other than DCIS, those with unknown laterality of the index lesion, and those with 0 months to death or censoring. Due to difficulties in distinguishing between cancers present at baseline and early new breast events, patients with a second breast event (invasive or in situ) diagnosed within 2 months were excluded. Furthermore, because it is unlikely for DCIS patients to die from breast cancer within 6 months we excluded patients with such a sequence of events. Patient and tumor features (age, year of diagnosis, tumor grade, estrogen receptor [ER] and progesterone receptor [PR] status, tumor size) and individual patient histories were extracted with the SEER*Stat software (15). Time from diagnosis to the first of the following events was extracted: iIBC, CBC (includes invasive and in situ cancers), death, or right-censoring (ie, loss to follow-up and alive without any of the above events at last date of disease assessment). Institutional review board approval for this study was obtained at Duke University.
Figure 1.
US National Cancer Institute’s Surveillance, Epidemiology, and End Results program (1992–2014): selection of ductal carcinoma in situ patients without locoregional treatment.
Sensitivity Analysis
Previous studies have found that a fraction of DCIS patients in SEER die from breast cancer without first experiencing an invasive breast cancer diagnosis (16). Due to the nonlethal nature of DCIS, this suggests the possibility of undiagnosed and/or unrecorded invasive cancers in these patients. Because this may lead to underestimation of the true rate of iIBC, we performed a sensitivity analysis where we assumed that all DCIS patients whose death was attributable to the DCIS diagnosis must have experienced an ipsilateral breast cancer at some time between the DCIS diagnosis and death. The corresponding risk estimates were computed using interval-censored event times in the Kaplan-Meier analyses. That is, rather than entering a precise time to ipsilateral breast cancer for these patients, we provided only an interval ranging from the time of diagnosis to the time of breast cancer death.
Guideline-Concordant Care Patients
To evaluate generalizability of our findings with respect to the overall DCIS patient population, we characterized guideline-concordant care patients in SEER (1992–2014). Patient selection was performed as outlined in “Data Extraction,” except that only patients who had received surgery (and possibly radiation therapy) were included. Patient and tumor characteristics were recorded, and competing risk analyses were performed. Finally, expected overall survival of the treatment and no-treatment cohorts was estimated in a survival session in SEER*Stat using the Kaplan-Meier estimator and the respective cohort’s distribution of age at diagnosis (binned into 5-year intervals).
Previously Published Cohort Studies
To compare our findings against those from previously published studies, we performed a literature search and a pooled patient-level analysis of included studies; see Supplementary Methods (available online) for details. In brief, the literature search yielded 5 cohorts of interest; two were excluded due to very short follow-up (17,18), and a third was excluded because its case-control design was not suitable for the purposes of absolute risk estimation (19). Finally, two retrospective cohort studies were included: cohort 1 from Italy (20) and cohort 2 from the United States (21–24). Both cohorts were based on retrospective pathology reviews, which identified patients whose biopsies had been classified as benign on clinical diagnosis and were subsequently upgraded to DCIS during a systematic review of pathology archives. The majority of patients were diagnosed before the introduction of widespread mammography screening (ie, 1964–1978 for the Italian and 1950–1989 for the US cohort), suggesting symptomatic presentation at time of diagnosis. None of the patients in these cohorts had received definitive surgery or radiation therapy after clinical diagnosis. Biopsy procedures were not specified; however, based on the dates of diagnosis, patients had likely undergone excisional biopsies.
Statistical Analyses
Net iIBC risks were estimated using the Kaplan-Meier estimator, with CBC diagnoses and deaths right-censored. Differences in iIBC risk by tumor grade (nonhigh: grade I/II, high: grade III), ER status (positive, negative), tumor size (≤1 cm, >1 cm), and age group (younger: <55 years, older: ≥55 years) were evaluated using log-rank tests. Hazard ratios (HRs) were calculated based on Cox proportional hazard models; the models were adjusted for age at diagnosis (continuous time), ER status (positive, negative, unknown), tumor grade (nonhigh, high, unknown), and tumor size (≤1 cm, >1 cm, unknown). The models’ proportionality assumptions were assessed graphically with a log cumulative hazard plot and using standard tests (25). The cumulative incidence of competing events (iIBC, CBC, and death) were estimated in competing risk analyses, and Gray’s test was used to compare incidence within patient and tumor feature subgroups (26). An additional subgroup analysis was performed for “low-risk” patients who met three of the eligibility criteria of the prospective COMET active surveillance trial (11): age 40 years or older at diagnosis, nonhigh-grade lesions, and ER or PR positive status.
Risk estimates were reported so long as at least 10% of the original patient cohort remained at risk. All P values were calculated as two-sided, with statistical significance declared for P less than .05. All statistical analyses were performed in R (version 3.4.2, R Foundation for Statistical Computing, Vienna, Austria) using the packages “survival” (version 2.41–3) and “cmprsk” (version 2.2–7).
Results
Cohort Characteristics
A total of 1286 patients diagnosed with DCIS without definitive surgery or radiation therapy were included in the analyses (Table 1). Median follow-up was 5.5 years (interquartile range [IQR] = 2.3–10.6 years). Most patients were white (n = 901, 70.1%) and median age at diagnosis was 60 years (IQR = 51–74 years). Among the 791 (61.5%) patients with known tumor grade 69.2% had a nonhigh-grade lesion, and among the 471 (36.6%) patients with known ER status 86.4% were ER positive. In the entire cohort, 111 (8.6%) patients were diagnosed with an iIBC (64 localized, 26 regional, 15 distant, 6 unstaged), 42 (3.3%) patients had a contralateral breast diagnosis (33 invasive, 8 situ, 1 unstaged), and 290 (22.6%) patients died before experiencing a new breast cancer diagnosis.
Table 1.
Patient characteristics*
| Characteristics | SEER (1992–2014) | Pooled cohort studies |
|---|---|---|
| No. (%) | No. (%) | |
| Patients, n | 1286 | 87 |
| Median follow-up (IQR), y | 5.5 (2.3–10.6) | 17.0 (7.5–24.0) |
| Age, y | ||
| 18–39 | 42 (3.3) | 12 (13.8) |
| 40–54 | 421 (32.7) | 35 (40.2) |
| 55–69 | 415 (32.3) | 24 (27.6) |
| ≥70 | 408 (31.7) | 16 (18.4) |
| Race | ||
| White | 901 (70.1) | — |
| Black | 192 (14.9) | — |
| Other/unknown | 193 (15.0) | — |
| Tumor grade | ||
| Nonhigh (I/II) | 547 (42.3) | 73 (83.9) |
| High (III/IV) | 244 (19.0) | 14 (16.1) |
| Unknown | 495 (38.5) | 0 (0) |
| Tumor size | ||
| ≤1 cm | 266 (20.7) | — |
| >1 cm | 187 (14.5) | — |
| Unknown | 833 (64.8) | — |
| ER status | ||
| Positive | 407 (31.6) | — |
| Negative | 64 (5.0) | — |
| Unknown | 815 (63.4) | — |
| PR status | ||
| Positive | 321 (25.0) | — |
| Negative | 104 (8.1) | — |
| Unknown | 861 (66.9) | — |
| Laterality | ||
| Left | 663 (51.6) | — |
| Right | 623 (48.4) | — |
ER = estrogen receptor; IQR = interquartile range; PR = progesterone receptor; SEER = US National Cancer Institute’s Surveillance, Epidemiology, and End Results program.
Risk of iIBC
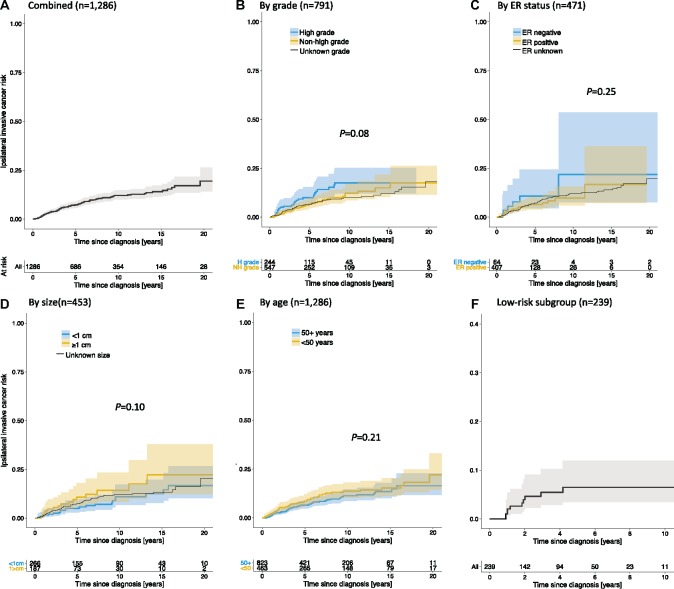
After 10 years, the overall net risk of iIBC was 12.1% (95% CI = 10.0% to 14.7%) (Figure 2A;Table 2). Among patients diagnosed with nonhigh-grade DCIS (n = 547), the 10-year risk of iIBC was 12.2% (95% CI = 8.6% to 17.1%) compared with 17.6% (95% CI = 12.1% to 25.2%) among patients diagnosed with high-grade DCIS (n = 244) (Figure 2B;Table 2); the difference was not statistically significant (log-rank test, P = .08). Among patients of unknown tumor grade (n = 495) the 10-year risk of iIBC was 10.1% (95% CI = 7.4% to 13.8%) (Supplementary Table 1, available online). Similarly, there were no statistically significant differences in subsequent ipsilateral invasive cancer risk by ER status, tumor size, and age at diagnosis (Figure 2, C–E; Table 2). Based on Cox proportional hazard models, we found that the risk of iIBC trended higher in patients with the following characteristics: high-grade vs nonhigh-grade lesions (HR = 1.44, 95% CI = 0.87 to 2.37), ER negative vs ER positive lesions (HR = 1.40, 95% CI = 0.56 to 3.50), and more than 1 cm vs 1 cm or less lesions (HR = 1.62, 95% CI = 0.84 to 3.10) (Table 2).
Figure 2.
Net risk of ipsilateral invasive breast cancer (iIBC) in patients without locoregional treatment, based on US National Cancer Institute’s Surveillance, Epidemiology, and End Results program (SEER) (1992–2014). The cumulative net risk of iIBC in the SEER no-treatment cohort is shown for all cases combined (A) and stratified by tumor grade (B), estrogen receptor (ER) status (C), tumor size (D), and age at diagnosis (E). The net risk (up to 10 years only) of iIBC in “low-risk” patients (40 years or older at diagnosis, nonhigh-grade, and ER and/or progesterone receptor-positive ductal carcinoma in situ) is shown in (F). The number of patients at risk is shown beneath the figures. Subgroup comparisons (excluding unknowns) were performed using a log-rank test.
Table 2.
Net risk of iIBC in DCIS patients without locoregional treatment, based on SEER (1992–2014)*
| Risk measure | All (n = 1286) | Tumor grade |
ER status |
Tumor size |
Age at diagnosis |
||||
|---|---|---|---|---|---|---|---|---|---|
| Nonhigh (n = 547) | High | Positive | Negative | ≤1 cm | >1 cm | <55 y | ≥55 y | ||
| (n = 244) | (n = 407) | (n = 64) | (n = 266) | (n = 187) | (n = 463) | (n = 823) | |||
| Net risk of iIBC, % (95% CI) | |||||||||
| 5 y | 7.3 | 6.7 | 10.0 | 6.5 | 10.7 | 5.1 | 10.7 | 8.7 | 6.5 |
| (5.8 to 9.1) | (4.6 to 9.8) | (6.4 to 15.3) | (4.1 to 10.2) | (4.5 to 24.3) | (2.8 to 9.1) | (6.3 to 18.0) | (6.2 to 12.0) | (4.8 to 8.7) | |
| 10 y | 12.1 | 12.2 | 17.6 | NA | NA | 10.9 | 14.2 | 13.6 | 11.3 |
| (10.0 to 14.7) | (8.6 to 17.1) | (12.1 to 25.2) | (6.8 to 17.3) | (8.4 to 23.4) | (10.2 to 18.0) | (8.6 to 14.7) | |||
| 15 y | 14.0 | NA | NA | NA | NA | 12.5 | NA | 15.2 | NA |
| (11.4 to 17.2) | (7.8 to 19.8) | (11.4 to 20.3) | |||||||
| Cox Proportional Hazard Models | |||||||||
| Univariate HR (95% CI) | — | 1.00 (Ref) | 1.56 | 1.00 (Ref) | 1.65 | 1.00 (Ref) | 1.67 | 0.99† | |
| (0.96 to 2.55) | (0.67 to 4.06) | (0.88 to 3.17) | (0.98 to 1.01) | ||||||
| Multivariat HR (95% CI) | — | 1.00 (Ref) | 1.44 | 1.00 (Ref) | 1.40 | 1.00 (Ref) | 1.62 | 0.99† | |
| (0.87 to 2.37) | (0.56 to 3.50) | (0.84 to 3.10) | (0.98 to 1.01) | ||||||
For risk estimates and HRs in patients with unknown tumor features, see Supplementary Table 1 (available online). CI = confidence interval; DCIS = ductal carcinoma in situ; HR = hazard ratio; iIBC = ipsilateral invasive breast cancer; NA = estimates not reported when less than 10% of initial patients remain at risk; Ref = reference factor; SEER = US National Cancer Institute’s Surveillance, Epidemiology, and End Results program.
Age as continuous variable (years) in Cox models.
Competing Risks
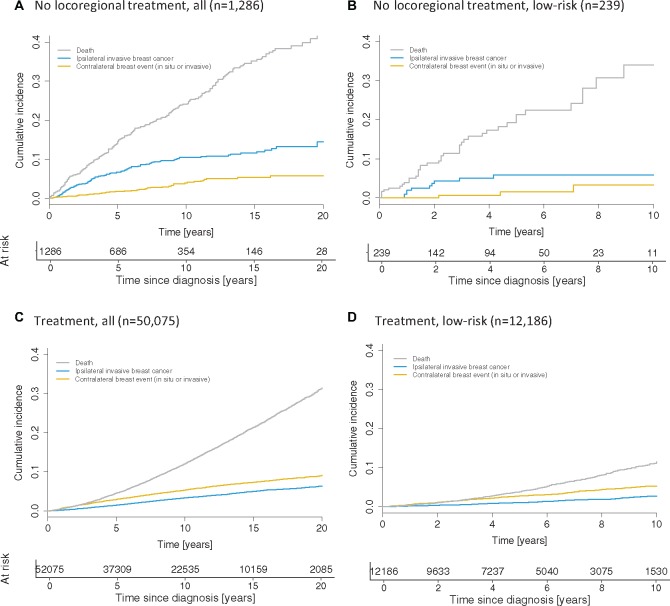
Overall, the 10-year cumulative incidence rates of ipsilateral invasive and CBC were 10.5% (95% CI = 8.5% to 12.4%) and 3.9% (95% CI = 2.6% to 5.2%), respectively (Figure 3A;Supplementary Table 2, available online). The 10-year cumulative incidence of death as a competing risk was 24.1% (95% CI = 21.2% to 26.9%), which was higher than the combined incidence of in situ and invasive cancers (14.4%, 95% CI = 12.0% to 16.8%), with similar patterns observed in a subgroup analysis by tumor grade (Supplementary Table 2, available online). With the exception of the CBC rate, the magnitude of the competing risks depended on age at diagnosis: compared with patients aged 55 years or older, those younger than 55 years had a lower competing risk of death (Gray’s test: P < .001; 8.0% [95% CI = 5.0% to 11.1%) vs 32.8% [95% CI = 28.9% to 36.7%] at 10 years) but a higher cumulative incidence of iIBC (P = .03; 13.0% [95% CI = 9.3% to 16.6%] vs 9.1% [95% CI = 6.7% to 11.4%] at 10 years).
Figure 3.
Competing risks in ductal carcinoma in situ (DCIS) patients, based on US National Cancer Institute’s Surveillance, Epidemiology, and End Results program (SEER) (1992–2014). The cumulative incidence of competing events (ipsilateral invasive breast cancer, any contralateral breast cancer, and competing death) in the study cohort is shown for all cases combined (A) and for “low-risk” patients (40 years or older at diagnosis, nonhigh-grade, and estrogen receptor and/or progesterone receptor-positive DCIS) (B). Similarly, the cumulative incidence of competing events among SEER who underwent guideline-concordant care is shown for all cases combined (C) and for “low-risk” patients (D). Please note the difference in follow-up shown: 20 years in A and C, and 10 years in B and D.
Low-Risk Subgroup
Low-risk DCIS was defined as those lesions that were nonhigh-grade and ER or PR positive, diagnosed in women 40 years or older. A total of 239 patients satisfied the criteria of the low-risk subgroup. Limited follow-up precluded risk estimation beyond 7.5 years. The cumulative net risk of iIBC at 7.5 years after diagnosis was 6.5% (95% CI = 3.5% to 12.0%) (Figure 2F). In a competing risk analysis (Figure 3B), the 7.5-year cumulative incidences of iIBC, CBC, and death were 5.9% (95% CI = 2.3% to 9.5%), 3.3% (95% CI = 0.0% to 7.3%), and 28.2% (95% CI = 19.1% to 37.1%), respectively.
Sensitivity Analysis
Assuming that patients whose death was attributable to breast cancer must have developed ipsilateral invasive cancer at some time between diagnosis with DCIS and death, we calculated interval-censored Kaplan-Meier estimates of the iIBC rate (Table 3; Supplementary Figure 1; Supplementary Table 3, available online). As expected, the resulting estimates were higher compared with the main analyses (Table 2), with an absolute risk increase of the order of 5% for most subgroups. However, among patients with high-grade lesions, the increase was even larger, from a 10-year iIBC rate of 17.6% (95% CI = 12.1% to 25.2%) to a corresponding rate of 27.9% (95% CI = 20.3% to 34.7%). In the low-risk subgroup, the 7.5-year iIBC rate increased to 8.5% (95% CI = 4.0% to 12.8%).
Table 3.
iIBC rate in DCIS patients without locoregional treatment, based on SEER (1992–2014): sensitivity analysis using cause of death information*
| Time since diagnosis, years | Net risk of iIBC, % (95% CI) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| All (n = 1286) | Tumor grade |
ER status |
Tumor size |
Age at diagnosis |
|||||
| Nonhigh (n = 547) | High (n = 244) | Positive (n = 407) | Negative (n = 64) | ≤1cm (n = 266) | >1cm (n = 187) | <55 y (n = 463) | ≥55 y (n = 823) | ||
| 5 | 11.6 | 9.1 | 19.0 | 9.6 | 18.3 | 8.0 | 16.3 | 13.0 | 10.7 |
| (9.6 to 13.5) | (6.1 to 11.9) | (13.3 to 24.4) | (6.1 to 13.0) | (6.5 to 28.6) | (4.3 to 11.5) | (9.6 to 22.6) | (9.5 to 16.3) | (8.3 to 13.1) | |
| 1 | 17.2 | 14.9 | 27.9 | NA | NA | 14.5 | 20.1 | 18.8 | 16.2 |
| (14.5 to 19.7) | (10.4 to 19.2) | (20.3 to 34.7) | (8.8 to 19.9) | (11.9 to 27.6) | (14.4 to 23.0) | (12.8 to 19.5) | |||
| 15 | 19.1 | NA | NA | NA | NA | 16.0 | NA | 20.5 | NA |
| (16.0 to 22.1) | (9.6 to 22.0) | (15.5 to 25.1) | |||||||
For risk estimates in patients with unknown tumor features, see Supplementary Table 3 (available online). CI = confidence interval; DCIS = ductal carcinoma in situ; ER = estrogen receptor; iIBC = ipsilateral invasive breast cancer; NA = estimates not reported when less than 10% of initial patients remain at risk; SEER = US National Cancer Institute’s Surveillance, Epidemiology, and End Results program.
Comparison with Guideline-Concordant Care Patients
A comparison between characteristics of DCIS patients who did and did not receive guideline-concordant care revealed differences with respect to age, race, and tumor grade, but not tumor size and ER and PR status (Supplementary Table 4, available online). Competing risk analyses (Figure 3C) revealed a substantially lower 10-year cumulative all-cause mortality among guideline concordant patients (12.1%, 95% CI = 11.8% to 12.4%) compared with patients who did not receive locoregional treatment (24.1%, 95% CI = 21.2% to 26.9%; Figure 3A,P < .001). Among low-risk patients, the difference in all-cause mortality was more pronounced (Figure 3, B and D) but subject to increased residual uncertainty in the no-treatment cohort. Finally, a comparison of expected and observed overall survival between the no-treatment and treatment cohorts (Supplementary Figure 2, available online) indicated that the difference in observed survival between the two cohorts exceeded the difference in expected survival.
Pooled Cohort Studies
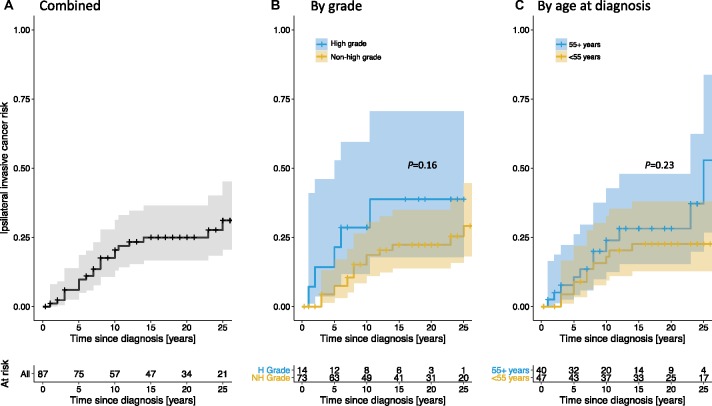
Of 125 cases extracted from the two included cohorts, 87 (70.0%) cases were included in the final analysis (Table 1). Median age at diagnosis was 53 years (IQR = 45–63), and median follow-up was 17.0 years (IQR = 7.5–24.0). The 10-year net risk of iIBC in the pooled retrospective cohorts was 18.7% (95% CI = 11.0% to 30.6%) in nonhigh-grade (n = 73) and 28.6% (95% CI = 11.8% to 59.4%) in high-grade (n = 14) patients (Figure 4; Supplementary Table 5, available online). Both estimates were higher than the corresponding estimates in our study (Table 2); however, wide confidence intervals preclude definitive conclusions. Importantly, due to the extended follow-up in the retrospective cohorts, 20-year iIBC rates could be estimated; they were 22.3% (95% CI = 13.8% to 34.8%) and 38.8% (95% CI = 17.9% to 70.6%) in nonhigh-grade and high-grade patients, respectively (Supplementary Table 5, available online).
Figure 4.
Net risk of ipsilateral invasive breast cancer (iIBC) based on retrospective cohort studies of ductal carcinoma in situ patients without locoregional treatment. The cumulative net risk of iIBC in the pooled retrospective cohort studies is shown for all cases combined (A) and stratified by tumor grade (B) and age at diagnosis (C). The number of patients at risk is shown beneath the figures. Subgroup comparisons were performed using a log-rank test.
Discussion
The natural history of DCIS in patients who do not receive locoregional therapy after biopsy remains poorly understood. We sought to address this knowledge gap by analyzing the few available data sources that might shed light on this critical question. Given the potential for overtreatment among women diagnosed with nonhigh-grade DCIS, the risk of subsequent ipsilateral breast cancer in this subgroup is of particular interest.
How the absolute risk estimates of iIBC for patients without definitive surgery and radiation therapy for DCIS compare against the iIBC risk for patients treated by breast-conserving surgery directly affects the question of whether active surveillance is a viable management strategy for newly detected DCIS (27). The ECOG-ACRIN E5194 study reported a 10-year net risk of iIBC of 6.4% (95% CI = 4.2% to 8.6%) for patients with nonhigh-grade DCIS treated by lumpectomy without radiation (28). However, this estimate does not account for the 6%–10% (no CI given) of patients who are upstaged to an invasive cancer diagnosis after surgical excision of the lesion (12). Combining these risks, the upstaging-adjusted 10-year risk of ipsilateral cancer is expected to be on the order of 12%–16%. This is comparable to our estimate of the risk of iIBC in the absence of definitive surgery and radiation therapy, which was 12.2% (95% CI = 8.6% to 17.1%) in the main analysis and 14.9% (95% CI = 10.4% to 19.2%) in a sensitivity analysis. Although our estimates are close to those in the ECOG-ACRIN cohort after adjustment for upstaging, the two study populations are not directly comparable. Elevated mortality in the SEER no-treatment cohort likely reflects higher prevalence of comorbidities, which may have influenced clinical recommendations and patient treatment preferences; these selection factors may also have influenced the underlying risk and ascertainment of subsequent invasive disease.
The absolute level of invasive breast cancer risk that is acceptable in clinical practice is not a fixed rate and depends on each patient’s personal risk tolerance and their competing risks (29,30). In the SEER no-treatment cohort, the 10-year risk of dying was greater than the combined 10-year risk of experiencing an ipsilateral or contralateral event. This finding emphasizes the importance of contrasting the risk of invasive disease against competing risks, especially among older patients and for patients with substantial comorbidities.
It is unclear how SEER patients who did not receive definitive locoregional treatment compare to their guideline-concordant counterparts. In addition to differences in age, race, and tumor grade distributions, an elevated all-cause mortality rate among patients without locoregional treatment compared with those who received it suggests that no-treatment patients may have had additional comorbidities leading to excess cumulative mortality. Indeed, comparing expected and observed overall survival between the two cohorts, we found that survival was higher than expected in the treatment cohort and lower than expected in the no-treatment cohort. At 10 years after diagnosis, about half of the absolute difference in observed survival between the two cohorts was attributable to the differential age distributions (as witnessed by the difference in expected survival), with the remainder likely due to factors such as comorbidity levels and differential screening and surveillance patterns. Importantly, due to the above measured and implied cohort differences and a lack of knowledge about the specific comorbidities present in the no-treatment cohort, it is not possible to make causal inferences about counterfactual no-treatment outcomes in the general DCIS patient population.
The estimated rates of iIBC in the SEER no-treatment cohort were lower than those from the pooled analysis of previously published studies. This discrepancy may be due to a number of factors. First, the retrospective cohorts predated the introduction of widespread mammography screening, whereas the majority of SEER cases are expected to have been screen-detected. Because screen detection brings the diagnosis forward in time, the expected time from diagnosis to ipsilateral invasive cancer is longer, which could explain the lower risk of ipsilateral cancer in our study compared with the retrospective cohorts. Second, although patients in the retrospective cohorts are unlikely to have received endocrine therapy, an (albeit unknown) fraction of SEER patients may have received endocrine therapy. Due to the expected benefit of endocrine therapy (31), these differences may also have contributed to the lower breast cancer risks in the SEER no-treatment cohort.
Our study is subject to a number of limitations. First, SEER contains limited information about the biopsy type used. Due to differential upstaging rates by biopsy technique (12,32), our estimates may not accurately reflect the risk of contemporary cohorts. Second, because endocrine therapy uptake has not been measured, our findings may overestimate the risk of iIBC in patients who are eligible for and choose to undergo endocrine therapy. Third, SEER does not capture new cancer events that occur outside the registry where the index lesion was recorded. Therefore, invasive ipsilateral disease in patients diagnosed and treated outside the initial registry may have been missed due to incomplete ascertainment, potentially leading to underestimation of the risk of new breast events. This issue was partially addressed through sensitivity analyses that incorporated cause of death information to estimate adjusted iIBC risks. Furthermore, a comparison between the estimated 10-year Kaplan-Meier risk of contralateral cancer in the SEER no-treatment cohort (5.1%, 95% CI = 3.6% to 7.2%) against the corresponding risks in the NSABP-24 trial (33) of patients undergoing either lumpectomy and radiation alone (6.9%, 95% CI = 5.1% to 8.7%) or in conjunction with endocrine therapy (4.7%, 95% CI = 3.0 to 6.7%) suggests that this bias may be limited in magnitude.
In summary, although the risk estimates differed substantially between sources and were subject to wide confidence intervals, our findings summarize the highest quality data currently available about the course of DCIS in the absence of definitive surgery and radiation therapy. In particular, our results indicate a limited propensity for invasive progression in contemporary patients aged 40 years and older who are diagnosed with nonhigh-grade, ER positive DCIS and thus a potential for overtreatment in these patients. Although an elevated rate of all-cause mortality in this cohort precludes generalizations to the general DCIS patient population, it suggests that a more conservative approach such as active surveillance may be of value for older patients and patients with elevated comorbidities.
Funding
This work was supported by the National Institutes of Health (K99 CA207872 to MDR, R01 CA185138-01 to ESH, R01CA165301 to FZ, 5P30CA014236 to TH, U01CA199218 and R01CA165301 to SJL, R50 CA221836 to RG), the National Science Foundation (DMS-1614838 to MDR), the Patient-Centered Outcomes Research Institute (1505–30497 to ESH and TH), the Department of Defense (BC132057 to ESH), and the Breast Cancer Research Fund (to ESH). This work was also supported in part by National Institutes of Health grants U01 CA152958 and U01 CA157224 to the Cancer Intervention and Surveillance Modeling Network Breast and Prostate Cancer Working Groups, respectively, for important background discussions relevant to the scientific areas covered in this paper.
Notes
Affiliations of authors: Department of Population Health Sciences, Duke University Medical Center, Durham, NC (MDR), Department of Surgery, Division of Advanced Oncologic and GI Surgery, Duke University Medical Center, Durham, NC (MDR, ESH); Department of Mathematics, Duke University, Durham, NC (MDR); Department of Pathology and Laboratory Medicine, University of Vermont and UVM Cancer Center, Burlington, VT (DLW); Department of Biostatistics and Computational Biology, Dana-Farber Cancer Institute, Harvard Medical School, Boston, MA (FZ, SJL); Department of Biostatistics, Harvard T.H. Chan School of Public Health, Boston, MA (FZ, SJL); Department of Visceral Surgery and Medicine, Inselspital, Bern University Hospital, University of Bern, Switzerland (MW); Department of Radiology, Duke University Medical Center, Durham, NC (LG); Division of Public Health Sciences, Fred Hutchinson Cancer Research Center, Seattle, WA (RG, RE); Department of Biostatistics & Bioinformatics, Duke University Medical Center, Durham, NC (TH).
The funders had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication.
The authors have no conflicts of interest to disclose.
Supplementary Material
References
- 1. Ward EM, DeSantis CE, Lin CC, et al. Cancer statistics: breast cancer in situ. CA Cancer J Clin. 2015;656:481–495. [DOI] [PubMed] [Google Scholar]
- 2. Worni M, Akushevich I, Greenup R, et al. Trends in treatment patterns and outcomes for ductal carcinoma in situ. J Natl Cancer Inst. 2015;10712:djv263.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Erbas B, Provenzano E, Armes J, et al. The natural history of ductal carcinoma in situ of the breast: a review. Breast Cancer Res Treat. 2006;972:135–144. [DOI] [PubMed] [Google Scholar]
- 4. Groen EJ, Elshof LE, Visser LL, et al. Finding the balance between over- and under-treatment of ductal carcinoma in situ (DCIS). Breast. 2017;31:274–283. [DOI] [PubMed] [Google Scholar]
- 5. Mertz BG, Duriaud HM, Kroman N, et al. Pain, sensory disturbances and psychological distress are common sequelae after treatment of ductal carcinoma in situ: a cross-sectional study. Acta Oncol. 2017;565:724–729. [DOI] [PubMed] [Google Scholar]
- 6. Mertz BG, Duriaud HM, Kroman N, et al. Pain, sensory disturbances, and psychological distress among Danish women treated for ductal carcinoma in situ: an exploratory study. Pain Manag Nurs. 2017;185:309–317. [DOI] [PubMed] [Google Scholar]
- 7. Sagara Y, Mallory MA, Wong S, et al. Survival benefit of breast surgery for low-grade ductal carcinoma in situ: a population-based cohort study. JAMA Surg. 2015;1508:739–745. [DOI] [PubMed] [Google Scholar]
- 8. Maxwell AJ, Clements K, Hilton B, et al. Risk factors for the development of invasive cancer in unresected ductal carcinoma in situ. Eur J Surg Oncol. 2018;444:429–435. [DOI] [PubMed] [Google Scholar]
- 9. Elshof LE, Tryfonidis K, Slaets L, et al. Feasibility of a prospective, randomised, open-label, international multicentre, phase III, non-inferiority trial to assess the safety of active surveillance for low risk ductal carcinoma in situ—the LORD study. Eur J Cancer. 2015;5112:1497–1510. [DOI] [PubMed] [Google Scholar]
- 10. Francis A, Thomas J, Fallowfield L, et al. Addressing overtreatment of screen detected DCIS; the LORIS trial. Eur J Cancer. 2015;5116:2296–2303. [DOI] [PubMed] [Google Scholar]
- 11. Youngwirth LM, Boughey JC, Hwang ES.. Surgery versus monitoring and endocrine therapy for low-risk DCIS: the COMET trial. Bull Am Coll Surg. 2017;1021:62–63. [PubMed] [Google Scholar]
- 12. Grimm LJ, Ryser MD, Partridge AH, et al. Surgical upstaging rates for vacuum assisted biopsy proven DCIS: implications for active surveillance trials. Ann Surg Oncol. 2017;2412:3534–3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Surveillance Epidemiology and End Results (SEER) Program (www.seer.cancer.gov). SEER*Stat Database: MP-SIR Multiple Event Analysis—SEER 13 Regs Research Data, Nov 2016 Sub (1992-2014) <Katrina/Rita Population Adjustment>—Linked To County Attributes - Total U.S., 1969-2015 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2017, based on the November 2016 submission. Accessed February 22, 2018.
- 14. Johnson C, Peace S, Adamo P, Fritz A, Percy-Laurry A, Edwards BK. The 2007 Multiple Primary and Histology Coding Rules. Bethesda, MD: National Cancer Institute, Surveillance, Epidemiology and End Results Program; 2007. [Google Scholar]
- 15.Surveillance Research Program. National Cancer Institute SEER*Stat software (seer.cancer.gov/seerstat) version 8.3.4. Accessed February 22, 2018.
- 16. Narod SA, Iqbal J, Giannakeas V, et al. Breast cancer mortality after a diagnosis of ductal carcinoma in situ. JAMA Oncol. 2015;17:888–896. [DOI] [PubMed] [Google Scholar]
- 17. Grimm LJ, Ghate SV, Hwang ES, et al. Imaging features of patients undergoing active surveillance for ductal carcinoma in situ. Acad Radiol. 2017;2411:1364–1371. [DOI] [PubMed] [Google Scholar]
- 18. Meyerson AF, Lessing JN, Itakura K, et al. Outcome of long term active surveillance for estrogen receptor-positive ductal carcinoma in situ. Breast. 2011;206:529–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Collins LC, Tamimi RM, Baer HJ, et al. Outcome of patients with ductal carcinoma in situ untreated after diagnostic biopsy: results from the nurses’ health study. Cancer. 2005;1039:1778–1784. [DOI] [PubMed] [Google Scholar]
- 20. Eusebi V, Feudale E, Foschini MP, et al. Long-term follow-up of in situ carcinoma of the breast. Semin Diagn Pathol. 1994;113:223–235. [PubMed] [Google Scholar]
- 21. Page DL, Dupont WD, Rogers LW, et al. Continued local recurrence of carcinoma 15-25 years after a diagnosis of low grade ductal carcinoma in situ of the breast treated only by biopsy. Cancer. 1995;767:1197–1200. [DOI] [PubMed] [Google Scholar]
- 22. Page DL, Dupont WD, Rogers LW, et al. Intraductal carcinoma of the breast: follow-up after biopsy only. Cancer. 1982;494:751–758. [DOI] [PubMed] [Google Scholar]
- 23. Sanders ME, Schuyler PA, Dupont WD, et al. The natural history of low-grade ductal carcinoma in situ of the breast in women treated by biopsy only revealed over 30 years of long-term follow-up. Cancer. 2005;10312:2481–2484. [DOI] [PubMed] [Google Scholar]
- 24. Sanders ME, Schuyler PA, Simpson JF, et al. Continued observation of the natural history of low-grade ductal carcinoma in situ reaffirms proclivity for local recurrence even after more than 30 years of follow-up. Mod Pathol. 2015;285:662–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Grambsch PM, Therneau TM.. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;813:515–526. [Google Scholar]
- 26. Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;163:1141–1154. [Google Scholar]
- 27. Ryser MD, Worni M, Turner EL, et al. Outcomes of active surveillance for ductal carcinoma in situ: a computational risk analysis. J Natl Cancer Inst. 2016;1085:djv372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Solin LJ, Gray R, Hughes LL, et al. Surgical excision without radiation for ductal carcinoma in situ of the breast: 12-year results from the ECOG-ACRIN E5194 study. J Clin Oncol. 2015;3333:3938–3944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Muhsen S, Barrio AV, Miller M, et al. Outcomes for women with minimal-volume ductal carcinoma in situ completely excised at core biopsy. Ann Surg Oncol. 2017;2413:3888–3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ryser MD, Horton JK, Hwang ES.. How low can we go-and should we? Risk reduction for minimal-volume DCIS. Ann Surg Oncol. 2018;252:354–355. [DOI] [PubMed] [Google Scholar]
- 31. Cuzick J, Sestak I, Pinder SE, et al. Effect of tamoxifen and radiotherapy in women with locally excised ductal carcinoma in situ: long-term results from the UK/ANZ DCIS trial. Lancet Oncol. 2011;121:21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Brennan ME, Turner RM, Ciatto S, et al. Ductal carcinoma in situ at core-needle biopsy: meta-analysis of underestimation and predictors of invasive breast cancer. Radiology. 2011;2601:119–128. [DOI] [PubMed] [Google Scholar]
- 33. Wapnir IL, Dignam JJ, Fisher B, et al. Long-term outcomes of invasive ipsilateral breast tumor recurrences after lumpectomy in NSABP B-17 and B-24 randomized clinical trials for DCIS. J Natl Cancer Inst. 2011;1036:478–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.