ABSTRACT
According to the World Health Organization, Salmonella is one of the most important zoonotic foodborne pathogens. Poultry products are thought to be the main source of Salmonella, which means that it is necessary to control Salmonella at the pre-harvest stage. Bacteriophages, acting as host-specific parasites of bacterial cells, represent one of the alternatives to antibiotics that can contribute to food safety and security. The present study evaluated the effectiveness of the bacteriophage cocktail SalmoFREE® to control Salmonella on a commercial broiler farm. We assessed the relationship between the use of SalmoFREE® and productivity parameters (feed conversion, weight gain, homogeneity). Two field trials (trial 1 n = 34,986; trial 2 n = 34,680) were carried out under commercial rearing conditions on a Colombian broiler farm with a record of Salmonella presence. Each trial comprised 2 control chicken houses and 2 experimental ones. SalmoFREE® and a control suspension were delivered in the drinking water at 3 time points in the production cycle, and the presence of Salmonella was assessed in cloacal swabs the day before and after the treatments. Results revealed that SalmoFREE® controls the incidence of Salmonella and does not affect the animals nor the production parameters, demonstrating its efficacy and innocuity at the production scale. We detected phage-specific genes in samples of total DNA extracted from ceca after the treatment with SalmoFREE®, and tested for the appearance of cocktail-resistant Salmonella, which showed to be an uncommon event. These results contribute relevant information to the adoption of phage therapy as an alternative to growth-promoter antibiotics on poultry farms.
Keywords: phage therapy, Salmonella reduction, broilers, broiler farm
INTRODUCTION
Salmonella (non-typhoid) is a gram-negative zoonotic bacterium that is considered one of the 4 principal causes of diarrheal disease worldwide (WHO, 2016). Each year, 93.8 million cases of acute gastroenteritis in humans are attributed to this foodborne pathogen, in addition to 155,000 deaths (CDC, 2015). Based on these data, this pathogen has become an important public health concern with a significant economic impact on society (WHO, 2016). The consumption of poultry products is considered to be the main source of Salmonella infections. This fact necessitates the control of Salmonella at the pre-harvest stage, preventing the introduction of this pathogen into the food chain and, consequently, reducing food poisoning among consumers (Wegener et al., 2003).
Control of Salmonella at the farm level is carried out using a multi-factorial approach. When Salmonella infection occurs in broilers, several simultaneous or sequential procedures are applied. Good agricultural practices based on hazard analysis and critical control, vaccination, probiotics, prebiotics, and antimicrobial treatments are the most common actions carried out by farmers (Chambers and Gong, 2011; Tellez et al., 2012). Moreover, as a prevention measure, the supplementation of animal feed with antimicrobials at sub-therapeutic levels is still carried out (Castanon, 2007).
The use of antimicrobials in animal feed leads to selective pressure and the dominance of antimicrobial-resistant bacteria. This problem is one of the most important current threats to public health, markedly reducing the number of antimicrobials available for the effective treatment of infectious diseases in humans and animals (WHO, 2015a). The frequent appearance of antimicrobial-resistant Salmonella provides strong motivation to find new and effective prophylactic and therapeutic means to control Salmonella from poultry farms(Wegener et al., 2003).
Bacteriophages (phages), defined as host-specific parasites of bacterial cells, stand out as an alternative to antibiotics in animal therapy, prophylaxis, and reduction of pathogen loads in food products of animal origin (Carrillo and Abedon, 2011; Irshad et al., 2012). Phages may provide a natural, non-toxic, feasible, and inexpensive technology for the pre-harvest control of Salmonella in poultry. Phage therapy has been shown to have advantages over other bacteria control alternatives that have been described in detail in the literature (Carrillo and Abedon, 2011; Nilsson, 2014). Phages are highly specific; thus, their use in treatment offers the specific targeting of a group of bacteria without affecting the normal microbiota. Another advantage is their higher efficiency in comparison to antibiotics due to the fact that they multiply when their host is present. Hence, phages have the ability to increase their density in situ. Likewise, following the infection, and once the concentration of the host has been reduced, the population of phages diminishes as well. Furthermore, immunomodulatory activity has been attributed to phages; this activity helps to resolve the infection via the phage's non-specific effects on different functions of immune cell populations, involved in both innate and adaptive immune responses (Górski et al., 2012). In addition, phages can be effective against sensitive bacteria as well as those strains that are resistant to antibiotics (Carrillo and Abedon, 2011; Nilsson, 2014).
Trials with phages in poultry have been successful in killing foodborne pathogens such as Salmonella (Filho et al., 2007; Borie et al., 2008; Sillankorva et al., 2010; Wong et al., 2014). Most of these studies were conducted using germ-free chickens, reared in battery cages under tightly controlled conditions. In all cases, phages were administered orally, either as a feed supplement, in water, or using a gavage once the birds had been infected with a given concentration of the pathogen. Nevertheless, research into the effectiveness of phages under the commercial conditions of factory farming is still required (Clavijo and Florez, 2017). To the best of our knowledge, the only study that used bacteriophages in commercial broiler flocks studied Campylobacter phages (Kittler et al., 2013). The authors reported a reduction of up to 3.2 CFU/g of Campylobacter load in the cecal content in comparison to the control.
A patent has recently been granted for SalmoFREE®, a mixture of 6 Salmonella lytic bacteriophages, to be used as a potential therapy for Salmonella control in poultry products (Holguin et al., 2017). Data about the cocktail's phages genome sequence, host range, in vitro efficiency, and stability in chlorinated water have already been documented. Additionally, a safety trial has been carried out using chickens reared in battery cages where the SalmoFREE® cocktail was delivered via the drinking water. These in vivo experiments showed that the phage cocktail is safe for the chicks because no differences in mortality, weight gain, and feed intake were observed in comparison to the untreated group. Furthermore, there was greater weight homogeneity in chicks that were treated with the phage cocktail. Altogether, SalmoFREE® has been demonstrated to be innocuous for its use in broilers, under controlled conditions (Holguin et al., 2017).
The present study aimed at testing the effectiveness of SalmoFREE® in reducing Salmonella on a larger scale, in the setting of a productive farm. Additionally, it assessed the relationship between the use of phages and the productivity parameters such as feed conversion, weight gain, and weight homogeneity at this scale. Altogether, this research expands on current knowledge about the performance of phages under the commercial conditions of factory farming.
MATERIALS AND METHODS
Broiler Flocks and Farm Management
This study was approved by the Institutional Committee on Care and Use of Experimental Animals (CICUAL) from the Universidad de los Andes, Ref. CICUAL 15–008, within the framework of Colombian Law 84/89 and Resolution 8430/93.
Two field trials were carried out under commercial rearing conditions in a commercial broiler farm in Colombia. This farm belongs to an integrated poultry company that handles the entire production and processing cycle of a chicken (hatching, feed, production, processing, and marketing). Table 1 presents the general information on the farm, including the biosecurity and composition of the litter. The farm was divided into 11 houses, and all chickens were of the same age and housed on the same day. The houses had a fabric separation for different sexes when it contained both male and female chickens. During both trials, the poultry company's permanent staff carried out maintenance of the houses and animal care. The animals were provided with water and feed ad libitum.
Table 1.
Farm general rearing conditions during the field assays performed in this work.
| Characteristic | Description |
|---|---|
| Numberof houses | 11 |
| Litter composition | Ground |
| Average number of chickens/m2 | 13.86 |
| Average house area (m2) | 645.61 |
| Biosecurity | Farmers live in a house within the farm |
| Buddle for washing boots at the entrance of each house | |
| Use of showers for changing clothes for external personnel | |
| Litter treatment | Litters are changed twice per year |
| Litter is disinfected between cycles using high temperature (flaming), fumigation, and disinfection with acetic acid |
Experimental Design
Four production houses (labeled houses 4, 8, 9, and 10) were selected based on the existing record of Salmonella detection during the 2 previous production cycles (data not shown). Chickens in houses 4 and 8 were treated with a control suspension, whereas thoseinhouses 9 and 10 were treated with the bacteriophage cocktail SalmoFREE®. Treated flocks were separated from the control ones by a distance of approximately 300 m.Table 2 gives the information about houses used in the present study: size, breed line, sex, and antimicrobial therapy per house and trial.
Table 2.
Rearing conditions in houses employed for the phage trials.
| Trial | House1 | Sex | Chickens/m2 | Number of chickens | Breed line | Antibiotic therapy | Slaughter day |
|---|---|---|---|---|---|---|---|
| 1 | 4 | Mixed | 13.76 | 7,956 | Cobb | None | 36 |
| 8 | Female | 13.91 | 6,120 | Ross | None | ||
| 9 | Mixed | 14.24 | 7,446 | Cobb | Cipr2 | ||
| 10 | Female | 13.61 | 13,464 | Cobb | Cipr2 | ||
| 2 | 4 | Mixed | 13.61 | 7,956 | Cobb | None | 35 |
| 8 | Female | 13.45 | 5,916 | Ross | None | ||
| 9 | Mixed | 14.04 | 7,344 | Cobb | None | ||
| 10 | Female | 13.61 | 13,464 | Ross | None |
1Houses 4 and 8 are the control houses, and houses 9 and 10 correspond to SalmoFREE®-treated houses.
2Antibiotic treatment between days 15 and 21 of the cycle.
Cipr: ciprofloxacin.
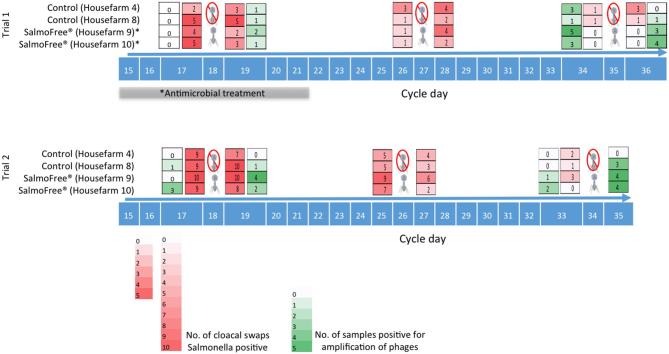
The broiler production cycle in Colombia is carried out in 2stages. The first stage comprises the period from day 1, when 1-day-old chicks are delivered to the farm, until days 13 to 17. Chickens in this stage are fed with a starter diet. Then, the second stage corresponds to days 14 to 18 until days 35 to 42, where chickens receive a grower diet. At the end of the second stage, the chickens are sent to the slaughterhouse. During the second stage, SalmoFREE® and the control suspensions were delivered to the animals via the drinking water: once at the beginning of the cycle (day 18 for both trials), the middle (days 27 and 26 for the first and second trials, respectively), and 1 D before slaughter (days 35 and 34 for the first and second trials, respectively) (Figure 1).
Figure 1.
Heat map for Salmonella reduction and phage detection throughout the production cycle for trials 1 and 2. The number in the blue boxes corresponds to the cycle day. The last day presented is the slaughter date. Phage figures indicate the days the treatments were provided to the animals in experimental houses 9 and 10. Phages were not delivered to the animals in control houses 4 and 8. Intensity of color red shows the incidence of Salmonella (number of cloacal swaps positive for Salmonella). Intensity of green color shows the detection of phages by PCR (number of cecal samples positive for phages). An accidental spillage of the samples occurred on day 35 in trial 2.
Preparation of the Phage and Control Treatments
SalmoFREE® was prepared following a standard liquid lysate procedure (Kutter and Sulakvelidze, 2004). Typically, the preparation was carried out individually for each phage in a nutritive broth (Sharlau, Spain), using an multiplicity of infection of 0.1. Each lysate was centrifuged at 4°C at 13.000 g for 20 min,and the supernatant was filtered through a 0.22-μm filter. Approximately 5.5 L of each phage was produced per trial and was stored at 4°C. The cocktail was mixed according to the adequate concentration and volume immediately prior to each treatment.
Quality standards for the phage preparation of sterility (zero bacteria) and a concentration of at least 1010 PFU/mL were established. All treatments were evaluated under these criteria. Sterility was evaluated by incubating a 10 mL aliquot of the cocktail at 37°C for 24 h.Additionally, this culture was streaked onto a nutrient agar plate. After 24 h of incubation, the absence of any type of growth was verified. Cocktail concentration was determined by carrying out serial dilutions from the cocktail suspension and plating the dilutions using the double agar overlay plaque assay (Kutter and Sulakvelidze, 2004).
The control suspension was prepared using a grown bacterial culture that was lysed by adding 0.1% chloroform. The lysate was centrifuged and filtered as described above in the phage lysate procedure. The final suspension was confirmed to be free of bacteria and phages. This control guarantees that the cell residuals found in the normal lysate do not affect the results.
Treatment Delivery to the Animals
The drinking water supply was suspended 30 min before dosing the treatments. This is a common practice in poultry production, which is carried out in order to facilitate the uptake of the treatment due to the temporary shortage of hydration. The water-supply tanks from each farmhouse can store up to 1,000 L of water. SalmoFREE® and control suspensions were added to these tanks in a 100:1 water:treatment ratio. Thus, the final concentration of the phage suspension was 108 PFU/mL. Treatments were delivered to the animals for 2.5 h. The total quantity of water taken up by the animals was calculated based on the number of animals per house prior to the treatment. The average water consumption per chicken is shown in Table 3.
Table 3.
Average of water drunk (in milliliters) per animal during the treatment period (2.5 h) discriminated by house and doses. Doses were delivered in the drinking water at a final concentration of 1 × 108 FPU/mL.
| Trial | House | Dose 11 | Dose 22 | Dose 33 | |
|---|---|---|---|---|---|
| 1 | Control houses | 4 | 38.7 | 51.6 | 94.9 |
| 8 | 26.2 | 38.9 | 50.7 | ||
| Treated houses | 9 | 35.2 | 44.5 | 110.3 | |
| 10 | 42.9 | 53.7 | 64.1 | ||
| 2 | Control houses | 4 | 30.1 | 45.7 | 60.3 |
| 8 | 32 | 50.1 | 49.7 | ||
| Treated houses | 9 | 44.6 | 57.1 | 52.6 | |
| 10 | 60.1 | 60.9 | 58.9 |
1Day 18 for both trials.
2Days 27 and 26 for the first and second trials, respectively.
3Days 35 and 34 for the first and second trials, respectively.
Sampling Methods
A total of 5 and 10 individual cloacal swabs were collected per house in the first and second trials, respectively, using swabs with Stuart Transport Medium (Copan, Italia) for all sampling days except for day 35 of the second trial, when swaps were hydrated with 15 mL of 3M buffered peptone water (BPW). Then, swaps were transported at 4°C and analyzed immediately upon arrival at the laboratory. Sampling was carried out first in control houses in order to prevent carryover of the phages and cross-contamination of the samples. Additionally, 5 female chickens from each of the 4 houses were randomly selected. These chickens were sacrificed and their ceca were removed. Each cecum was collected in a sterile plastic bag (Nasco, Saugerties, NY USA) and transported in liquid nitrogen to the laboratory, where it was stored at −80°C and promptly processed for DNA extraction.
Salmonella Detection
Samples were processed for the detection of Salmonella using the 3MMolecular Detection System (MDS) at the Microbiology Lab in the Colombian Corporation of Agricultural Research – Agrosavia (formerly Corpoica). This method uses isothermal amplification of specific nucleic acid sequences using a high-fidelity polymerase; amplification is detected by bioluminescence. Processing of samples started immediately upon arrival at the laboratory. Each swap was pre-enriched in 15 mL of 3M buffered peptone water and was incubated at 37°C for 18 to 24 h. After incubation, a pre-enrichment aliquot was removed and tested in the 3M MDS by taking an aliquot of 20 μL and transferring it into an individual lysis tube. The aliquot was heated on a dry block heater for 15 min at 100°C, followed by prompt chilling for 10 min on a pre-chilled 3M Molecular Detection Chill Block. Following chilling, the tubes were mixed by inverting them multiple times and were then left to stand for 5 min at room temperature. Subsequently, sample lysates of 20 μL were transferred to reagent tubes containing lyophilized pellets and were pipetted to mix them gently. The closed reagent tubes were transferred to a 3MMolecular Detection Speed Loader Tray. The samples were labeled in the 3M Molecular Detection software following the arrangement of reagent tubes on the speed loader tray and were placed into the 3M MDS. A blank (uninoculated, sterile 3M BPW-ISO), a Salmonella-positive process control (Salmonella Typhimurium ATCC 13,311), a Salmonella-negative process control (Escherichia coli ATCC 25,922), and a negative-reagent control were run along with the samples. The 3M MDS utilizes LAMP and bioluminescence to amplify and detect Salmonella concurrently, in which positive Salmonella results were reported in real-time, whereas negative Salmonella results were shown after a 75-min run. Fifty percent of Salmonella-positive samples were confirmed using the traditional methodology (USDA, 2008). These isolates were preserved in 10% glycerol and maintained at −80°C.
Phage Susceptibility Test for Salmonella Isolates
Thirty-one Salmonella isolates from both surveys were tested in vitro for their susceptibility to the SalmoFREE® cocktail. Susceptibility tests were performed using the spot test with the recovered isolates (Kutter and Sulakvelidze, 2004). A volume of 100 μL of an overnight culture was added to 3 mL lysogeny broth (LB) soft agar and then poured onto LB plates. The plates were dried for 30 min at room temperature to form the overlay. Serial dilutions of the phage suspension in SM buffer (10 mM Tris Base pH 7.4, 10 mM MgSO4, 100 mM NaCl) were prepared, and afterwards 5 μL of each dilution was spotted onto the bacteria overlay. They were then allowed to dry for 15 to 20 min and incubated for 24 h at 37°C. All quantifications were performed in duplicate. The PFU/mL was calculated by considering the dilution factor and the sample volume.
DNA Extraction
180 to 200 mg of the cecal content was aseptically collected under cold conditions, to avoid thawing the samples. The samples were immediately processed with the QIAamp DNA Stool Mini Kit (Qiagen, Valencia, CA) according to the manufacturer's directions. Samples were measured in a Nanodrop ND-1000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA) to assess the DNA quality and were quantified using a Qubitfluorometer (Life Technologies, Paisley, UK).
Phage Tail Fiber Protein Gene Amplification
In order to verify the presence of phages in the chickens’ ceca, the PCR amplification of a gene fragment, encoding a phage tail fiber protein, was standardized. This gene is shared by 3 of the SalmoFREE® phages and has a length of 2,520 nucleotides (Holguin et al., 2017). Fourprimer search tool programs were used for its design: Primer BLAST (Ye et al., 2012), Primer 3 (Untergasser et al., 2012), PrimerQuest (Integrated DNA Technologies, Inc. Coralville, Iowa, USA), and OligoPerfect (Thermo Fisher Scientific). One primer pair was selected according to the values of optimal melting temperature, GC clamp (1 to 4 bp), base runs (less than or equal to 4), and product length (50 to 250 bp). Self-dimerization was also evaluated. Selected primers correspond to S2TF (TGGTGGTCATTATGGTGGTG)/S2TR (GTTTCCGTTAGAGGCAGCAG). The expected length of the product is 130 bp and the melting temperature 60°C. PCR was standardized using different primer concentrations (0.1 μM, 0.3 μM, 0.6 μM, and 0.9 μM); different cecal DNA volumes (1.25 μL and 5 μL correspond to 84 ng and 335 ng, respectively); and the number of denaturing–annealing–extension cycles (28 and 34). Finally, PCR primers and reagent conditions were set to 12.5 μLDreamTaq PCR Master Mix 2X (Thermo Fisher Scientific),0.1 μM forward and reverse primers, 5 μL (on average 255 ng) template DNA, and water to a 25 μL final volume. Cycling conditions were set as follows: initial denaturation at 95°C for 3 min, 34 cycles of denaturation at 95°C for 30 s, annealing at 55°C for 30 s,and extension at 72°C for 1 min, followed by a final extension step at 72°C for 7 min.
For phage detection, PCR amplification of the tailfiberprotein gene was carried out using the total DNA extracted from cecum samples, taken before and after the first and third doses, both from control and treated animals (days 17, 19, 34, and 36 for trial 1; days 17, 19, 33, and 35 for trial 2). Phage ΦSan23 was used as a positive control. PCR products were visualized with GelRed in a 1.8% (w/v) agarose gel.
Data Management and Statistical Analysis
Weight, feed intake, and mortality were recorded, discriminating by sex, for the 4 houses. Data on Salmonella incidence (number of cloacal swabs testing positive for Salmonella/number of total cloacal swabs) were obtained, and a heat map was built in Microsoft Excel using a color scale for Salmonella incidence values. Additionally, feed conversion (feed consumption/weight per animal) and a weight homogeneity test (weight standard deviation) were calculated for all production houses. Statistical variance analysis was performed to compare the parameters of the houses treated with SalmoFREE® with those of the control houses, using the statistical software R 3.4.1.
RESULTS
Production Parameters
In order to determine whether the addition of phages to the drinking water affects broiler production, production parameters of the control and the treated houses were compared. Firstly, in order to make the parameters comparable, data were analyzed, discriminating by sex. Results for weight, feed conversion, weight homogeneity, and mortality for females only for both trials are shown in Figure 2. The mean weights of chickens treated with the phage cocktail and those of the control group were not significantly different across both cycles (P ≤ 0.05) (Figure 2A). Concerning feed-conversion and weight-homogeneity results, Figure 2B shows that non-treated chickens and those treated with SalmoFREE® do not have significantly different conversion indexes and weight homogeneity (P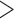 0.05) (Figures 2B and 2D). Similar results were found for mortality rate, where the slope of the line was calculated and compared, indicating that mortality rate is the same for both groups (P
0.05) (Figures 2B and 2D). Similar results were found for mortality rate, where the slope of the line was calculated and compared, indicating that mortality rate is the same for both groups (P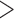 0.05) (Figure 2C). Overall, these results confirmed the safety of the cocktail because its incorporation into broiler production did not affect the normal growth and behavior of the animals.
0.05) (Figure 2C). Overall, these results confirmed the safety of the cocktail because its incorporation into broiler production did not affect the normal growth and behavior of the animals.
Figure 2.
Production parameters analyzed for female chickens of trials 1 and 2. (A) Weight (g). (B) Mortality rate (%) = number of chicks that died in a group/total number of chicks in the group x100. (C) Feed conversion = feed consumption/weight per animal. (D) Weight homogeneity (weight standard deviation).
Salmonella Reduction
Field Trial 1
Results concerning Salmonella reduction for this trial are presented in Figure 1. In brief, Salmonella incidence in treated houses drops to zero on day 34 in comparison with the control houses where the bacterium was still detected. However, an antibiotic intervention (not planned for the experimental design) was carried out by the veterinarian in charge of the farm in the houses treated with SalmoFREE®, houses 9 and 10 (between days 15 and 21) (Table 2, Figure 1), due to the high rate of chicken mortality observed in house 10. Antibiotics were applied to houses 9 and 10 because their chickens came from the same egg batch, despite the fact that only house 10 showed high mortality (Figure 2B; trial 1). Due to this intervention, it is not possible to assure that the reduction of Salmonella is the result of the phage activity alone. Instead, it can be attributed to the combined action of both the antibiotic and the phage cocktail.
Nevertheless, looking at the heat map on day 17 (Figure 1) (1D before starting the phage treatment), houses 9 and 10 exhibited a higher prevalence of Salmonella compared to the control houses, despite having already received 3Dof antibiotic treatment. This result indicates that, although the mortality was reduced, this antibiotic is not effective against the circulating Salmonella. Also, we are aware that, in Colombia, Salmonella has a high resistance to the administered antimicrobial (Donado-Godoy et al., 2015). Nevertheless, due to the antimicrobial intervention, it was necessary to repeat the trial in order to confirm the controlling effect of SalmoFREE®.
Field Trial 2
The number of cloacal swabs per house was increased from 5 to 10 for the second trial, given the valuable information obtained from the swab samples in the first trial. Results of the second trial exhibited a similar behavior to that obtained in the first. At the beginning of the cycle, all 4 houses had a high Salmonella incidence. Then, a reduction in Salmonella detection was observed from the second dose of the phage treatment; finally, at the end of the cycle, Salmonella was reduced to 0% on day 33. Data from day 35 were lost due to the spillage of the peptone water during transportation (Figure 1). In spite of the fact that this reduction of the pathogen is a good result, it is hard to attribute the reduction of Salmonella to the phage cocktail treatment without additional information because the control houses also exhibited a significant reduction. We postulate that there were possible phage residuals in the houses deriving from the first trial. To confirm this hypothesis, we looked for the detection of the phages through the amplification of phage-specific regions using the total DNA extracted from ceca.
Phage Tail Fiber Protein Gene Amplification
Field Trial 1
There was no amplification of the phagetail gene in any of the ceca samples on day 17 (before treatments), indicating no detection of the phages present in SalmoFREE®. The amplification product appeared on day 19, after the first phage dose in all farmhouses, in 1 or 2 samples of the 5 tested in each house. Ondays 34 and 36, phages were also detected in both houses, treated and non-treated. However, more phage-positive samples were found in the treated houses (Figure 1).
Field Trial 2
Amplification products were detected on day 17—before treatments—in both treated and control houses. Detection of the gene encoding the phage tail fiber protein continued until the end of the cycle in both treated and control groups. Similarly to the first trial, the number of positive samples was larger in the treated houses after the first dose was delivered (Figure 1).
Phage Susceptibility Testfor Salmonella Isolates
A total of 20 isolates from trial 1 and 11 from trial 2 were tested for susceptibility to SalmoFREE®; 24 (77.4%) of the Salmonella isolates tested were found to be susceptible to the cocktail phage. From these isolates, 14 came from trial 1 and 11 from trial 2.
DISCUSSION
To the best of our knowledge, this study presents the first results from the use of Salmonella phages at a large scale, in a poultry production system. Most of the previous studies were carried out under controlled conditions (Bardina et al., 2012; Gonçalves et al., 2013; Wong et al., 2014). Results obtained here are evidence of the fact that(a) there are no negative effects on the production parameters for the groups treated with the phage cocktail SalmoFREE®; and(b) a reduction in Salmonella incidence in the houses, which could be partially attributed to the phage treatment.
Regarding production parameters, the incorporation of SalmoFREE® in the drinking water did not have any negative effect on the weight, feed conversion, weight homogeneity, and mortality rate of the chickens (Figure 2). These findings are consistent with other reports under battery conditions (Oliveira et al., 2009; Kim et al., 2013; Tsonos et al., 2014). However, there are some studies that suggest a positive effect on the growth performance of chickens (Atterbury et al., 2007; Miller et al., 2010; Li et al., 2012; Kaikabo et al., 2017). Similarly, the safety assessment carried out in battery cages with SalmoFREE® suggested that chickens treated with bacteriophages have a more homogeneous weight than the control ones (Holguin et al., 2017). Nevertheless, it was observed in this study that on a large scale, there is no difference in this parameter between treatments (Figure 2). These differences might be attributed to all the variables that are present at the productive scale such as feed composition, litter replacement, feeding and antibiotic intervention, vaccination scheme, and animal density, among others. Accordingly, treatment with phages follows the same trend as reported for prebiotics, probiotics, and phytobiotics (Chambers and Gong, 2011; Diaz-Sanchez et al., 2015), where the beneficial properties of these biological products are masked by the variables of the productive scale (Timmerman et al., 2006; Yang et al., 2015).
Our field experiments in a commercial poultry farm evidenced a reduction in Salmonella incidence for both trials of up to 100% in the phage-treated group, whereas the presence of the pathogen was still detected in the control. However, our data also suggested a decrease in the Salmonella-positive samples in the control houses at the end of the cycle (Figure 1). This result could be explained by phage cross-contamination between houses and a residual effect of the phages from the first trial. This hypothesis was confirmed by the amplification of the tail phage protein in control house farms. We proposed that the entry of phages to the control houses might have occurred via rubber boots (which are not changed during the work day) or other conditions we could not control. Likewise, the entry of phages to the control flocks was reported in the assay with Campylobacter phages on a commercial farm (Kittler et al., 2013).
The antimicrobial intervention in the first trial was an unfortunate event because it made it impossible to attribute the reduction of Salmonella to the phage activity alone. However, this drawback allowed us to evidence that the combination of antibiotics and phages is compatible and altogether suggested an effective action in controlling Salmonella. This result is in agreement with an increasing number of studies suggesting that both of these antimicrobial agents in combination are more effective in controlling multidrug-resistant bacteria than either one by itself (Huff et al., 2004). Moreover, combined treatment in vivo and in vitro with a lytic phage and an antibiotic resulted not only in a better control or eradication of bacteria, but also in the complete prevention of the emergence of resistant variants, compared to a single antimicrobial treatment (Torres-Barceló and Hochberg, 2016). A combined therapy reduces the development of drug resistance and, as a consequence, would help to diminish the use of antimicrobials in animal production. Hence, a mixed therapy has important implications for the serious problem of infections by antibiotic-resistant pathogens and is in line with the global action plan that promotes the reduction of antibiotics used at the pre-harvest level (WHO, 2015b). Moreover, it is probable that the animal feed provided by the company contained antibiotics as growth promoters because in Colombia it is a legal and common practice.
Concerning phage detection, results confirmed that phages were not previously present in the ceca of the farm chickens, for both treated and control animals. This result suggested that SalmoFREE® phages are not frequently found in the farm environment and validated that phages detected correspond to those provided in the treatments. Phages were detected from the beginning of the second trial in treated and control groups, showing that phages from the cocktail persisted in the environment and survived after the cleaning and disinfection process between cycles. This result leads us to propose that Salmonella could also be controlled by treating the litter as a preventive treatment. The phage cross-contamination observed between treated and non-treated flocks in the second trial is problematic for a clear-cut conclusion on the effect of the cocktail. However, this field experiment was carried out under commercial rearing conditions where it is difficult to control all the variables. Nonetheless, our results revealed that the use of the bacteriophage cocktail SalmoFREE® reduces Salmonella loads at the pre-harvest level. In order to better understand the interactions that occurred between the host system and the phage system in the chickens’ gut, as well as how they affect the parameters measured in this study, a metagenomic approach should be performed in the near future.
An additional, important aspect of this in vivo assessment was the trust gained from the poultry producers, who allowed us to test the phage technology at a production scale. Nevertheless, working at the production scale is difficult, not only due to the variation of several factors that are out of our control, but also due to the interventions that producers carry out in favor of their investment. We think that the reduction in the incidence of Salmonella on the farm would decrease the detection of the pathogen at slaughterhouses, taking into account that a 50% reduction in Salmonella prevalence at the pre-harvest level prevents the introduction of this pathogen into the food chain at the same scale (Alali and Hofacre, 2016). Consequently, this would reduce food poisoning among consumers.
Overall, this study obtained compelling evidence that SalmoFREE® does not affect the animals nor the production parameters, demonstrating its safety for broilers. It also contributes to the reduction of the presence of Salmonella, when it is used in multiple doses. These results also suggest that the use of the bacteriophage cocktail SalmoFREE® may constitute an efficient prevention measure to avoid food-poisoning outbreaks associated with Salmonella.
ACKNOWLEDGMENTS
The authors thank Colciencias for funding the project “Uso de bacteriófagos nativos como alternativa para el control de la salmonelosis en Colombia” code 1204-569-34190, contract 0845-2013; and for the doctoral student scholarship awarded to VC. Also, they would like to thank the School of Sciences, Universidad de los Andes, for funding the research project, code INV-2017-25-1117. The authors also gratefully acknowledge the Universidad de los Andes and Instituto Tecnológico de Estudios Superiores de Monterrey for their financial support (project code P16.700099.001/02-04). The authors thank Natalia Portilla and Carolina Páez from the Universidad de los Andes for their help with some laboratory assays, also to Alejandro Reyes from Universidad de los Andes for his contribution with the experimental design.
CONFLICT OF INTEREST
The authors V. Clavijo and M. Vives-Florez declare a conflict of interest as they are members of the spin-off company SciPhage S.A.S., which works for the development of phage therapy in Colombia.
REFERENCES
- Alali W. Q., Hofacre C. L.. 2016. Preharvest food safety in broiler chicken production. Microbiol. Spectr. 4. [DOI] [PubMed] [Google Scholar]
- Atterbury R., Van Bergen M., Ortiz F., Lovell M., Harris J., De Boer A., Wagenaar J., Allen V., Barrow P.. 2007. Bacteriophage therapy to reduce Salmonella colonization of broiler chickens. Appl. Environ. Microbiol. 73:4543–4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardina C., Spricigo D. A., Cortés P., Llagostera M.. 2012. Significance of the bacteriophage treatment schedule in reducing Salmonella colonization of poultry. Appl. Environ. Microbiol. 78:6600–6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borie C., Albala I., Sánchez P., Sánchez M. L., Ramírez S., Navarro C., Robeson J.. 2008. Bacteriophage treatment reduces Salmonella colonization of infected chickens. Avian Dis. 52:64–67. [DOI] [PubMed] [Google Scholar]
- Carrillo C., Abedon S. T.. 2011. Pros and cons of phage therapy. Bacteriophage. 1:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castanon J. I. R. 2007. History of the use of antibiotic as growth promoters in European poultry feeds. Poult. Sci. 86:2466–2471. [DOI] [PubMed] [Google Scholar]
- CDC. 2015. Annual listing of foodborne disease outbreaks. Available at http://www.cdc.gov/salmonella/outbreaks.html. [Google Scholar]
- Chambers J. R., Gong J.. 2011. The intestinal microbiota and its modulation for Salmonella control in chickens. Food Res. Int. 44: 3149–3159. [Google Scholar]
- Clavijo V., VivesFlorez M. J.. 2018. The gastrointestinal microbiome and its association with the control of pathogens in broiler chicken production: a review. Poult. Sci. 97:1006–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Sanchez S., D'Souza D., Biswas D., Hanning I.. 2015. Botanical alternatives to antibiotics for use in organic poultry production. Poult. Sci. 94:1419–1430. [DOI] [PubMed] [Google Scholar]
- Donado-Godoy P., Byrne B. A., León M., Castellanos R., Vanegas C., Coral A., Tafur M.. 2015. Prevalence, resistance patterns, and risk factors for antimicrobial resistance in bacteria from retail chicken meat in Colombia. J. Food Prot. 78:751–759. [DOI] [PubMed] [Google Scholar]
- Filho R. L., Higgins J. P., Higgins S. E., Gaona G., Wolfenden A. D., Tellez G., Hargis B. M.. 2007. Ability of bacteriophages isolated from different sources to reduce Salmonella enterica serovar enteritidis in vitro and in vivo. Poult. Sci. 86:1904–1909. [DOI] [PubMed] [Google Scholar]
- Gonçalves G. A. M., Donato T. C., Baptista A. A. S., Corrêa I. M. D. O., Garcia K. C. O. D., Filho R. L. A.. 2014. Bacteriophage-induced reduction in Salmonella Enteritidis counts in the crop of broiler chickens undergoing preslaughter feed withdrawal. Poult. Sci. 93:216–220. [DOI] [PubMed] [Google Scholar]
- Górski A., Międzybrodzki R., Borysowski J., Dąbrowska K., Wierzbicki P., Ohams M., Korczak-Kowalska G., Olszowska-Zaremba N., Łusiak-Szelachowska M., Kłak M.. 2012. Phage as a modulator of immune responses: practical implications for phage therapy.Pages 41–71 in Advances in Virus Research. Łobocka M., Szybalski W., eds. Academic Press, Cambridge, USA. [DOI] [PubMed] [Google Scholar]
- Holguín-Moreno A. V., Vives-Flores M. J., Jimenez-Sanchez A. P.. 2017. Composition comprising bacteriophages for reducing, eliminating and/or preventing Salmonella Enteritidis, Salmonella Typhimurium and Salmonella Paratyphi b (original in Spanish as Composición que comprende bacteriófagos para reducir, eliminar y/o prevenir Salmonella Enteritidis, Salmonella Typhimurium y Salmonella Paratyphi b). Universidad de los Andes, assignee. Colombian Pat. No. 15281747. [Google Scholar]
- Huff W., Huff G., Rath N., Balog J., Donoghue A.. 2004. Therapeutic efficacy of bacteriophage and Baytril (enrofloxacin) individually and in combination to treat colibacillosis in broilers. Poult. Sci. 83:1944–1947. [DOI] [PubMed] [Google Scholar]
- Irshad U. H., Waqas N. C., Mah A. N. A., Saadia A., Ishtiaq Q.. 2012. Bacteriophages and their implications on future biotechnology: a review. Virol. J. 9:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaikabo A., AbdulKarim S., Abas F.. 2017. Evaluation of the efficacy of chitosan nanoparticles loaded ΦKAZ14 bacteriophage in the biological control of colibacillosis in chickens. Poult. Sci. 96:295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K., Lee G., Jang J., Kim J., Kim Y.. 2013. Evaluation of anti-SE bacteriophage as feed additives to prevent Salmonella enteritidis (SE) in broiler. Asian Australas. J. Anim. Sci. 26:386–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittler S., Fischer S., Abdulmawjood A., Glünder G., Klein G.. 2013. Effect of bacteriophage application on Campylobacter jejuni loads in commercial broiler flocks. Appl. Environ. Microbiol. 79:7525–7533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutter E., Sulakvelidze A.. 2004. Bacteriophages: Biology andApplications. CRC Press, Boca Raton, USA. [Google Scholar]
- Li H., Ma M.-L., Xie H.-J., Kong J.. 2012. Biosafety evaluation of bacteriophages for treatment of diarrhea due to intestinal pathogen Escherichia coli 3-2 infection of chickens. World J. Microbiol. Biotechnol. 28:1–6. [DOI] [PubMed] [Google Scholar]
- Miller R. W., Skinner J., Sulakvelidze A., Mathis G. F., Hofacre C. L.. 2010. Bacteriophage therapy for control of necrotic enteritis of broiler chickens experimentally infected with Clostridium perfringens. Avian Dis. 54:33–40. [DOI] [PubMed] [Google Scholar]
- Nilsson A. S. 2014. Phage therapy–constraints and possibilities. Ups J. Med. Sci. 119:192–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira A., Sereno R., Nicolau A., Azeredo J.. 2009. In vivo toxicity study of phage lysate in chickens. Br. Poult. Sci. 50:558–563. [DOI] [PubMed] [Google Scholar]
- Sillankorva S., Pleteneva E., Shaburova O., Santos S., Carvalho C., Azeredo J., Krylov V.. 2010. Salmonella Enteritidis bacteriophage candidates for phage therapy of poultry. J. Appl. Microbiol. 108:1175–1186. [DOI] [PubMed] [Google Scholar]
- Tellez G., Pixley C., Wolfenden R., Layton S., Hargis B.. 2012. Probiotics/direct fed microbials for Salmonella control in poultry. Food Res. Int. 45:628–633. [Google Scholar]
- Timmerman H., Veldman A., Van den Elsen E., Rombouts F., Beynen A.. 2006. Mortality and growth performance of broilers given drinking water supplemented with chicken-specific probiotics. Poult. Sci. 85:1383–1388. [DOI] [PubMed] [Google Scholar]
- Torres-Barceló C., Hochberg M. E.. 2016. Evolutionary rationale for phages as complements of antibiotics. Trends Microbiol. 24:249–256. [DOI] [PubMed] [Google Scholar]
- Tsonos J., Oosterik L. H., Tuntufye H. N., Klumpp J., Butaye P., De Greve H., Hernalsteens J.-P., Lavigne R., Goddeeris B. M.. 2014. A cocktail of in vitro efficient phages is not a guarantee for in vivo therapeutic results against avian colibacillosis. Vet. Microbiol. 171:470–479. [DOI] [PubMed] [Google Scholar]
- Untergasser A., Cutcutache I., Koressaar T., Ye J., Faircloth B. C., Remm M., Rozen S. G.. 2012. Primer3-new capabilities and interfaces. Nucleic Acids Res. 40:e115–e115. 10.1093/nar/gks596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- USDA. 2008. Laboratory Guidebook: Isolation and Identification of Salmonella from Meat, Poultry, and Egg Products. USDA, Athens, GA. [Google Scholar]
- Wegener H. C., Hald T., Lo Fo Wong D., Madsen M., Korsgaard H., Bager F., Gerner-Smidt P., Mølbak K.. 2003. Salmonella control programs in Denmark. Emerg. Infect. Dis. 9:774–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. 2015a. Antibiotic resistance: multi-country public awareness survey. Accessed April 2019. http://apps.who.int/medicinedocs/documents/s22245en/s22245en.pdf. [Google Scholar]
- WHO. 2015b. Global action plan on antimicrobial resistance.Accessed April 2019. http://apps.who.int/iris/bitstream/10665/193736/1/9789241509763_eng.pdf?ua = 1. [Google Scholar]
- WHO. 2016. Interventions for the control of non-typhoidal Salmonella spp. Pages 1–10 inBeef and Pork: Meeting Report and Systematic Review. Thorgeir L., ed. Microbiological Risk Assessment Series No. 30, Rome, Italy. [Google Scholar]
- Wong C. L., Sieo C. C., Tan W. S., Abdullah N., Hair-Bejo M., Abu J., Ho Y. W.. 2014. Evaluation of a lytic bacteriophage, Φ st1, for biocontrol of Salmonella enterica serovar Typhimurium in chickens. Int. J. Food Microbiol. 172:92–101. [DOI] [PubMed] [Google Scholar]
- Yang C., Chowdhury M., Huo Y., Gong J.. 2015. Phytogenic compounds as alternatives to in-feed antibiotics: potentialsand challenges in application. Pathogens 4:137–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J., Coulouris G., Zaretskaya I., Cutcutache I., Rozen S., Madden T. L.. 2012. Primer-BLAST: a tool to design target-specific primers for polymerase chain reaction. BMC Bioinform. 1:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]