Abstract
Objectives
Physical activity (PA) is a modifiable health behavior that can protect against age-related gray matter atrophy and cognitive dysfunction. Current studies of PA and gray matter failed to utilize device measures of PA and do not focus on adults >80 years. Thus, the purpose of this secondary analysis was to examine cross-sectional associations between accelerometer lifestyle PA and (a) gray matter volumes and (b) cognitive function, controlling for demographics, and health status.
Method
Participants were 262 older adults without dementia or mild cognitive impairment from Rush Memory and Aging Project, an epidemiological cohort study. Participants wore an accelerometer to assess total daily lifestyle PA, and completed anatomical magnetic resonance imaging to assess gray matter volumes and a neurocognitive test battery to assess cognitive function.
Results
Multivariate linear regression indicated that higher levels of total daily lifestyle PA was significantly related to larger gray matter volumes, F(2, 215) = 3.61, p = .027, including subcortical gray matter (β = 0.17, p = .007) and total gray matter (β = 0.11, p = .049), with no significant associations between lifestyle PA and cognitive function.
Discussion
These findings may inform future lifestyle PA interventions in order to attenuate age-related gray matter atrophy.
Keywords: Brain, Cognition, Exercise, Neuroimaging, Prevention
Over 15% of adults 65 years or older develop cognitive dysfunction, or decline in one or more dimensions of cognition (e.g., memory, visuospatial ability, perceptual speed), progressing to nearly 40% of adults 85 years or older (Hugo & Ganguli, 2014). Of older adults with cognitive dysfunction, nearly 33% progress to dementia (Mitchell & Shiri-Feshki, 2009), a distressing chronic condition that impacts quality of life, independence, and health care costs (Hugo & Ganguli, 2014). Age-related cognitive dysfunction can be attributed in part to structural changes in the brain, such as declines in gray matter volumes (A.-L. Lin, Laird, Fox, & Gao, 2012). Declines in gray matter volumes often precedes declines detected in tests of cognition and memory (Erickson, Leckie, & Weinstein, 2014) and atrophy of gray matter is associated with the development of mild cognitive impairment and Alzheimer’s disease and related dementias (Pini et al., 2016). The rate and extent of gray matter volume decline varies among older adults, and depends on a variety of factors, including genetics, biological factors, and lifestyle behaviors.
Physical activity (PA) is a modifiable health behavior associated with a host of health benefits (Bauman, Merom, Bull, Buchner, & Fiatarone Singh, 2016), including improved cognitive function in older adults without dementia (Watson, 2016). PA is any bodily movement that increases energy expenditure. There are two subcategories of PA: structured exercise and lifestyle PA. Structured exercise is planned, repetitive, purposive, and aimed to improve or maintain physical fitness, while lifestyle PA refers to activities that a person carries out in the course of daily life, including household, transportation, and occupational activities (Centers for Disease Control and Prevention, 2015).
Nine studies published between 2010 and 2017 examined the association between self-report of structured exercise and gray matter volumes in adults without mild cognitive impairment or dementia (Benedict et al., 2013; Brown et al., 2014; Bugg & Head, 2011; K. Erickson et al., 2010; Head, Singh, & Bugg, 2012; Kooistra et al., 2014; Rovio et al., 2010; Ruscheweyh et al., 2011; Tamura et al., 2015). Findings of these studies indicated that higher levels of structured exercise were associated with greater gray matter volumes, including subcortical gray matter, cortical gray matter, and total cortex volumes, independent of demographics, and health status. In addition, four of the five studies that examined cognitive function found positive associations between structured exercise and cognitive function, consistent with a body of literature that has established this association (Bherer, Erickson, & Liu-Ambrose, 2013; Colcombe & Kramer, 2003). However, these nine studies were limited by including participants primarily aged 55–80 years (M = 69 years), with few studies focusing on adults 80 years or older. This is problematic due to the unique health concerns of this age group including their increased risk for chronic conditions and lower rates PA (Bauman et al., 2016). In addition, these nine studies focused on structured exercise only, and did not include lifestyle PA.
Lifestyle PA is often preferred over structured exercise programs in older adult populations, with higher adherence and satisfaction (Darden, Richardson, & Jackson, 2013). Higher levels of lifestyle PA as measured by accelerometer are associated with: (a) greater hippocampal volumes in healthy older adults (Varma, Chuang, Harris, Tan, & Carlson, 2015; Varma, Tang, & Carlson, 2016), (b) greater hippocampal volumes in older adults with mild cognitive impairment (Makizako et al., 2015), and (c) greater subcortical brain volume and higher levels of cognitive function in patients with heart failure (Alosco et al., 2015). Another body of literature focuses on the relation between lifestyle PA (also measured by accelerometer) and cognitive function. These studies demonstrate the positive impact of lifestyle PA on cognitive function, including lowered risks of cognitive dysfunction, dementia, and Alzheimer’s disease in older adults (Buchman et al., 2012; Buchman, Wilson, & Bennett, 2008; Kerr et al., 2013; Stubbs, Chen, Chang, Sun, & Ku, 2017; Zhu et al., 2017).
One characteristic of these lifestyle PA studies is that they uniformly utilized accelerometer data to measure lifestyle PA. Devices such as accelerometers increase the likelihood of obtaining reliable and valid assessment of total PA, including both structured exercise and lifestyle PA (Edwards & Loprinzi, 2016).
Lifestyle PA interventions, particularly those focused on moderate or vigorous-intensity PA, can increase aerobic fitness (X. Lin et al., 2015). In sedentary adults, even small increases in low-intensity lifestyle PA can improve aerobic fitness that may result in health benefits (Murtagh et al., 2015). Although earlier meta-analyses reported inconsistent findings regarding the effects of aerobic fitness on cognitive function (Colcombe & Kramer, 2003; Etnier, Nowell, Landers, & Sibley, 2006), a more recent analysis showed that interventions of moderate-intensity PA that aim to improve aerobic fitness have positive effects on cognitive function (Northey, Cherbuin, Pumpa, Smee, & Rattray, 2017). Results from a review of correlational and intervention studies also indicated independent positive associations between aerobic fitness and gray matter volumes (Erickson et al., 2014). These associations are potentially due to the fitness effects on the brain, such as increased cerebral blood flow, vascularization, neurogenesis, and neuroplasticity (Watson, 2016). Thus, the benefits of lifestyle PA on cognitive function and gray matter volumes in older adults are likely to be mediated through increased aerobic fitness.
The most common covariates included in existing studies of lifestyle PA, brain structure, and cognitive function were demographics, including age, sex, education, and income. However, most studies failed to include other health status covariates, such as depressive symptoms, body mass index (BMI), and physical disability. This failure persists in spite of evidence that older adults with depression had a significantly lower gray matter volumes compared to those without depression (Du et al., 2014). This finding may be explained by impaired neuroplasticity (Manji, Drevets, & Charney, 2001). Second, higher BMI was related to lower gray matter volumes in older adults (Kharabian Masouleh et al., 2016), which may be mediated by impaired neuronal growth (Papenberg et al., 2016). By increasing lifestyle PA, we can potentially attenuate these biological effects of depressive symptoms and BMI. Finally, while there is limited evidence suggesting an association between physical disability and gray matter volumes in the healthy older adults, physical disability may impact both lifestyle PA (Blodgett, Theou, Kirkland, Andreou, & Rockwood, 2015) and cognitive function (Kim, 2016). Therefore, the effects of physical disability must be considered.
The purpose of this secondary analysis was to explore the association between an accelerometer measure of lifestyle PA and (a) MRI measures of gray matter volumes (subcortical gray matter, total cortex, total gray matter) and (b) cognitive function (visuospatial ability, perceptual speed, episodic memory, semantic memory, working memory, and global cognition), while controlling for demographics, depressive symptoms, BMI, and physical disability. We aimed to address the gaps of the existing studies by (a) examining lifestyle PA, cognitive function, and gray matter volumes in a cohort of older adults that include those 80 years and older, an age group not always included in previous studies; (b) including depressive symptoms, BMI, and a measure of physical disability (basic activities of daily living) as covariates in addition to age, sex, education, and income, which are known to be related to gray matter volumes and cognitive function; and (c) utilizing a device measure of lifestyle PA. We hypothesize that those who have higher levels of lifestyle PA have greater gray matter volumes and higher levels of cognitive function.
Methods
Design
This is a secondary analysis of data from the Rush Memory and Aging Project, an epidemiological study that examines common chronic conditions of old age with questionnaires, performance tests, and clinical evaluations (Bennett et al., 2005). The Rush Memory and Aging Project began in 1997 and a MRI sub-study with biennial neuroimaging was added in 2009. For the present analyses, we use cross-sectional data from participants’ first MRI assessment obtained between 2009 and 2011, which were all completed on the same scanner.
Participants
As reported earlier, participants from this dataset were recruited from retirement communities throughout northeastern Illinois and the Chicago metropolitan area (Bennett et al., 2005, 2012). Older adults without known dementia provided written informed consent for data collection, including agreeing to: (a) an assessment of risk factors; (b) blood donation; (c) detailed clinical evaluation each year; and (d) donation of brain, the entire spinal cord, and selected nerve and muscles at the time of death. Participants in these analyses also agreed to biennial brain MRI scans. Participants underwent standardized clinical evaluations, including medical history and neurological examination, at the participant’s home (Bennett et al., 2005, 2012). The study was approved by the Rush University Medication Center Institutional Review Board, and waiver of consent was obtained for the secondary analysis.
Inclusion criteria were the following: (a) living in the retirement communities or housing facilities that were recruitment sites, and (b) have cognitive ability to complete the informed consent process at the time of study enrollment. For the purposes of these analyses, participants must have been scanned on the same MR scanner in use between 2009 and 2011 and have accelerometer data within that same year. Exclusion criteria for these analyses included: (a) dementia or mild cognitive impairment, (b) inability to sign the Anatomical Gift Act, (c) history of brain surgery, (d) large infarcts or structural brain abnormalities (e.g., tumors) atypical of aging and visible with MRI (Arfanakis et al., 2016), and (d) invalid accelerometer data (device failure or less than 7 days of data; Buchman et al., 2012; Lim et al., 2016). Since participants were not excluded due to health conditions, co-morbidities common in population-based epidemiologic studies are well represented. Thus, the “healthy volunteer effect” seen in many cohort studies was minimized.
Based on a clinical evaluation, a diagnosis of dementia or Alzheimer’s disease was determined utilizing the criteria from the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (McKhann et al., 1984). Dementia was diagnosed in a three-step process, including (a) a battery of 19 cognitive tests scored by a computer; (b) review of cognitive test results by a neuropsychologist; and (c) evaluation by a physician who used all cognitive and clinical data to diagnose dementia (Bennett et al., 2005). Participants were considered to have mild cognitive impairment when there was evidence of cognitive impairment, but did not meet the criteria for dementia (Boyle, Wilson, Aggarwal, Tang, & Bennett, 2006). Participants were not excluded from the original study for dementia or mild cognitive impairment diagnoses, but were excluded for the purpose of these analyses.
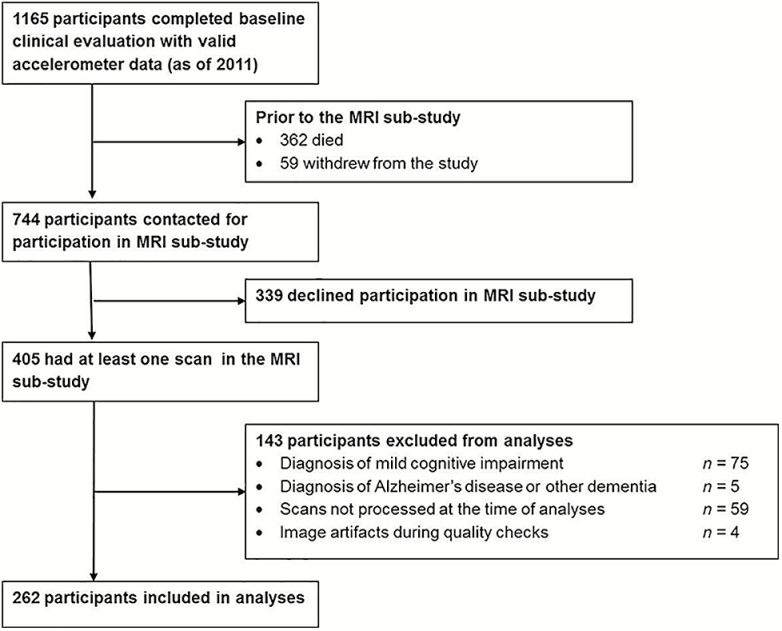
A total 1,165 participants completed the baseline clinical evaluation and had valid Actical accelerometer data prior to 2011 (Figure 1). Of these, 362 died and 59 withdrew from the study before the MRI sub-study began in February of 2009. Of the remaining 744 participants, 339 did not agree to participate in the MRI sub-study. A total of 405 had at least one scan during the study period on the same scanner (used between 2009 and 2011). Of these 405, 143 participants were excluded from analyses due to a diagnosis of mild cognitive impairment (n = 75), a diagnosis of Alzheimer’s disease or other dementia (n = 5), scans not processed at the time of analyses (October 2016; n = 59), and image artifacts discovered during quality checks (n = 4). Analyses were completed on the remaining 262 participants.
Figure 1.
Flowchart of subject participation.
Measures
Lifestyle PA
Lifestyle PA was measured by an Actical(C) (Mini Mitter, Bend, OR), a device with a piezo‐electric accelerometer. The Actical is a battery-operated activity monitor worn on the wrist similar to a wrist watch, and is waterproof and can be worn while bathing or swimming. The Actical provides valid estimates of free-living PA (Crouter et al., 2011). Participants were instructed to wear the Actical continuously for 10 days on the nondominant wrist. Upon retrieval of the Actical, raw data were downloaded and viewed using software provided by Respironics, Inc (Bend, OR). Non-wear time was identified in a two-stage process. First, trained technicians visually examined the accelerometer data, and periods of suspected removal were noted. Second, automatic algorithms noted periods of 4 h or greater (over the 24-h period) with no movement, and additional visual examinations were completed if necessary. To be included in the dataset participants must have had a minimum of 7 days of data without any noted non-wear periods. When more than 7 days of data were available, only the first 7 days were analyzed to ensure only 5 weekdays and two weekend days were included (Lim et al., 2016).
Data then were partitioned into 24-h periods from the time of placement to the time of retrieval, and only data from complete 24-h periods were used to determine average total daily lifestyle PA. Activity counts represent the area calculated by integrating the activity curve for each 1‐s sample, where non‐zero values reflected activity. Activity counts are summed for each epoch (15 s; Buchman et al., 2012). Total daily lifestyle PA is the sum of all activity counts during a 24-h period, averaged over the number of days that the individual wore the device. To correct for positively skewed data, total daily lifestyle PA was log-transformed. To facilitate the presentation and interpretation of result, total daily PA was converted to a z-score.
Image acquisition
High-resolution T1-weighted anatomical data were obtained on a 1.5 Tesla General Electric MRI scanner (Waukesha, WI) using a 3D IR-FSPGR sequence with: echo-time (TE)= 2.8 ms, repetition time (TR) = 6.3 ms, preparation time=1000 ms, flip-angle=8°, field-of-view (FOV)=24 cm×24 cm, 160 sagittal slices, slice thickness=1 mm, 224 × 192 acquisition matrix, and two repetitions. A conventional transmit/receive birdcage quadrature single channel coil was used.
Image processing
All MR images were reviewed by a neuroradiologist. For each participant, the two copies of the anatomical data were averaged to increase signal to noise ratio. The averaged T1-weighted data were automatically segmented with Freesurfer version 5 (http://surfer.nmr.mgh.harvard.edu). All results were reviewed and manual corrections were performed when necessary. White matter hyperintensities were classified as white matter (Arfanakis et al., 2016). The intracranial volume, subcortical and cortical gray matter volumes, as well as the total gray matter volume were obtained. All gray matter volumes were normalized by the intracranial volume. Variables for this analysis included subcortical gray matter, total cortex, and total gray matter normalized volumes.
Cognitive function
As reported earlier (Bennett et al., 2005, 2012; Wilson et al., 2005), cognitive function was assessed using a battery of 19 neurocognitive tests. All neurocognitive tests were conducted annually. The 19 cognitive tests assessed visuospatial ability (two tests; Line Orientation, Progressive Matrices), perceptual speed (four tests; Numbers Comparison, Symbol Digit Test, Stroop Word Reading, Stroop Color Naming), episodic memory (seven tests; Logical Memory Immediate Recall, Logical Memory Delayed Recall, Word List Memory, Word List Recall, East Boston Memory Test, East Boston Delayed Recall, Word List Recognition), semantic memory (three tests; Verbal Fluency, 15-item Boston Naming Test, 15-item Reading Test), and working memory (three tests; Digit Span Forward, Digit Span Backward, Digit Ordering; Bennett et al., 2005, 2012; Wilson et al., 2005). The raw scores of all tests were converted to z-scores standardized relative to the sample mean and SD. Composite scores for the five cognitive domains (visuospatial ability, perceptual speed, episodic memory, semantic memory, working memory) were calculated by averaging the z-scores of the corresponding individual tests. A global cognition score was the average of the z-scores of all 19 neurocognitive tests (Bennett et al., 2005, 2012; Wilson et al., 2005).
Covariates
Covariates included demographics and health status. Demographics included age, sex, education, and income. Age in years utilizing self-reported birth year was documented at time of MRI scan. Sex, education (number of years completed), and income (self-report of personal annual income divided in categories 1–10, with 1 = $0–$4,999 and 10 = $75,000 and over) were documented at study entry.
Health status included depressive symptoms, BMI, and physical disability. Depressive symptoms were assessed with a modified, 10-item version of the Center of Epidemiologic Studies Depression scale (Bennett et al., 2012). Participants were asked whether or not (yes or no) they experienced each of 10 symptoms much of the time in the past week. The score is the total number of symptoms experienced (possible range 0–10) with higher scores indicating more depressive symptoms. Due to the low levels of depressive symptoms in this cohort, depressive symptoms was dichotomized at the mean (M = 0.79) for analyses. BMI was based on measured weight and height, calculated as weight in kilograms divided by height in meters squared.
Physical disability was a composite measure of basic activities of daily living, measured with the Katz Activities of Daily Living Scale. The scale measures six basic physical abilities: walking across a small room, bathing, dressing, eating, getting from bed to chair, and toileting. Participants are asked to report need for help/assistance in performing the activities of daily living. Responses are dichotomized into 0 = no help and 1 = requires help or unable to do. The composite measure ranges from 0 to 6 and is the sum of the number of items for which participants report the need for help/assistance, with higher scores indicating greater disability.
Analysis
All analyses were calculated using SPSS version 23 (IBM Corp., 2015) with an alpha value of p ≤ .05 denoting statistical significance. Descriptive statistics (means and standard deviations) were calculated for the continuous variables in the models. First, two separate multivariate general linear models were conducted to examine the following associations: (a) total daily lifestyle PA with the three outcomes of gray matter volumes (subcortical gray matter, total cortex, and total gray matter volumes), and (b) total daily lifestyle PA with the six outcomes of cognitive function (visuospatial ability, perceptual speed, episodic memory, semantic memory, working memory, and global cognition). Demographics (age, sex, education, and income) and health status (depressive symptoms, BMI, and physical disability) were included as covariates. All covariates were tested for collinearity. In the presence of significant multivariate effects, separate follow-up regression analyses were conducted to examine the association between total daily lifestyle PA and the three gray matter volume outcomes and six cognitive function outcomes individually.
Results
There were 262 participants with a mean age of 81 years (SD = 7), 76% were female, and on average had 15 years of education (SD = 1.0; Table 1). Participants reported low levels of depressive symptoms, M = 0.8 (SD = 1.3). A total of 14 participants (5.3%) were above the threshold that indicates high risk for major depression (Kohout, Berkman, Evans, & Cornoni-Huntley, 1993). The mean BMI of 27 (SD = 5.0) was in the overweight range. Participants wore the Actical for an average of 9.3 days (SD = 1.1). Examination of participant characteristics by sex revealed males had significantly higher levels of education (16.5 vs. 15.0 years completed; t(260) = −3.7, p < .001), higher reported income levels ($35,000–$49,999 vs. $30,000–$34,999; t(249) = −3.5, p < .001), and lower levels of depressive symptoms (0.5 vs. 0.9; t(260) = 2.2, p = .031) than females.
Table 1.
Characteristics of Older Adults without Dementia or Mild Cognitive Impairment (N = 262)
| Femalesa | Malesb | Totalc | ||||
|---|---|---|---|---|---|---|
| Characteristic | M/N | SD/% | M/N | SD/% | M/N | SD/% |
| Age (years at time of MRI scan) | 80.7 | 7.3 | 81.7 | 6.7 | 80.9 | 7.2 |
| Race (percent white) | 189 | 94.5% | 60 | 96.8% | 249 | 95.0% |
| Education (number of years completed)*** | 15.0 | 2.7 | 16.5 | .5 | 15.4 | 1.1 |
| Income (self-report level range 1–10)** | 7.2 | 2.3 | 8.3 | 1.9 | 7.5 | 2.3 |
| Depressive symptoms (percent with more depressive symptoms)* | 82 | 41.0% | 16 | 25.8% | 98 | 37.4% |
| BMI (kg/m2) | 26.9 | 5.3 | 27.1 | 4.0 | 27.0 | 5.0 |
| Physical disability (activities of daily living) | 0.7 | 1.4 | 0.7 | 1.4 | .7 | 1.4 |
| Total daily lifestyle physical activity | 0.03 | 1.0 | −0.08 | 1.1 | <.01 | 1.0 |
Note. BMI = body mass index; MRI = magnetic resonance imaging.
a n = 200. bn = 62. cn = 262.
*p < .05, **p < .01, ***p < .001 (for t-tests comparing females vs. males).
Total lifestyle PA was moderately correlated with all three gray matter volumes (r = 0.30–0.34) and generally with the cognitive function variables (r = 0.12–0.25; see online Supplementary Material). The three variables of gray matter volumes were generally highly correlated (r = 0.64–0.98). The five cognitive function subscales were moderately correlated (r = 0.29–0.60), and were correlated with the global cognition score (r = 0.62–0.77). Correlations between gray matter volumes and cognitive function subscales ranged from −0.01 to 0.32.
The multivariate general linear models indicated that higher levels of lifestyle PA were significantly related to higher gray matter volumes, F(2, 215) = 3.61, p = .027. There were no significant relations between lifestyle PA and cognitive function, F(6, 207) = 1.99, p = .068. In order to better interpret the multivariate results for gray matter volumes, three separate follow-up univariate regression analyses were conducted (Table 2). These analyses indicated significant independent positive associations between lifestyle PA and subcortical gray matter (β = 0.17 p = .007) and total gray matter volume (β = 0.11, p = .049), controlling for demographics (age, sex, education, and income) and health status (depressive symptoms, BMI, and physical disability). Age and sex were significantly associated with all three measures of gray matter volume, such that younger age and male sex were associated with greater gray matter volumes, and higher self-reported income was significantly associated with greater total cortex volume only. In addition, fewer depressive symptoms and lower BMI were significantly associated with greater subcortical gray matter only, and less physical disability was significantly associated with greater total cortex and total gray matter volumes.
Table 2.
Multiple Linear Regression of Gray Matter Volumes on Lifestyle Physical Activity in Older Adults without Dementia or Mild Cognitive Impairment (N = 262)
| Subcortical Gray Matter Volume | Total Cortex Volume | Total Gray Matter Volume | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | βa | p b | d | β | p | d | β | p | d |
| Age (years at time of MRI scan) | −0.35 | <.001 | −0.47 | <.001 | −0.47 | <.001 | |||
| Sex (0 = female, 1 = male) | −0.17 | .006 | −0.34 | −0.30 | <.001 | −0.63 | −0.28 | <.001 | −0.58 |
| Education (number of years completed) | 0.01 | .871 | −0.02 | .683 | −0.02 | .775 | |||
| Income (self-report level range 1–10) | −0.03 | .602 | 0.13 | .031 | 0.09 | .108 | |||
| Depressive symptoms | −0.16 | .008 | −0.32 | −0.06 | .263 | −0.12 | −0.09 | .093 | −0.18 |
| BMI (kg/m2) | 0.18 | .003 | <0.01 | .999 | 0.05 | .397 | |||
| Physical disability (activities of daily living) | −0.06 | .280 | −0.14 | .010 | −0.13 | .016 | |||
| Total daily lifestyle physical activity | 0.17 | .007 | 0.09 | .143 | 0.11 | .049 | |||
| R 2 | 0.30 | 0.39 | 0.40 | ||||||
Note. BMI = body mass index.
aThe standardized regression coefficient (β) represents the change (in standard deviation [SD] units) in the dependent variable (gray matter volumes) uniquely attributable to a 1 SD change in the predictor. For dichotomous outcomes, β was converted to a Cohen’s d.
b p-values are unadjusted.
*p < .05, **p < .01, ***p < .001.
Discussion
We examined the associations between (a) lifestyle PA and gray matter volumes (subcortical gray matter volume, total cortex volume, total gray matter volume), and (b) lifestyle PA and cognitive function (visuospatial ability, perceptual speed, episodic memory, semantic memory, working memory, and global cognition), controlling for demographics (age, sex, education, and income), and health status (depressive symptoms, BMI, and physical disability). Overall, we found significant independent relations between lifestyle PA as measured using an accelerometer and two gray matter volumes (subcortical gray matter and total gray matter volumes) as measured by MRI in this population.
Consistent with earlier studies of structured exercise and gray matter volumes (Benedict et al., 2013; Brown et al., 2014; Bugg & Head, 2011; K. Erickson et al., 2010; Head et al., 2012; Kooistra et al., 2014; Rovio et al., 2010; Ruscheweyh et al., 2011; Tamura et al., 2015), we found significant associations between lifestyle PA and subcortical gray matter and total gray matter volumes when controlling for demographics and health status. Unexpectedly, the relation between lifestyle PA and total cortex volume in our analyses was not significant. However, we controlled for depressive symptoms, BMI, and physical disability, which earlier studies neglected. In our analysis, less physical disability was associated with greater total cortex volume. This emphasizes the importance of controlling for health status in studies of lifestyle PA and gray matter volumes, including physical disability, which may impact both PA and brain health in older adults (Blodgett et al., 2015; Kim, 2016).
We did not find a significant relation between lifestyle PA and cognitive function, a surprising result given the abundant evidence supporting increasing lifestyle PA to prevent cognitive dysfunction and dementia in older adults (Kerr et al., 2013; Stubbs et al., 2017; Zhu et al., 2017). Two previous analyses also using data from the Rush Memory and Aging Project showed the following: (a) higher levels of lifestyle PA (as measured by accelerometer) were significantly related to cognitive function in cross-sectional analyses (Buchman et al., 2008), and (b) higher levels of lifestyle PA were significantly related to decreased risk of cognitive decline and Alzheimer’s disease in longitudinal analyses (Buchman et al., 2012). However, the samples were larger (521 and 716 vs. 262 participants, respectively), and appear to be more diverse in terms of age and total lifestyle PA, which may also contribute to the difference in results. In addition, we included BMI as a covariate due to the negative association between BMI and cognitive function in adults. Yet, evidence suggests that this association may reverse with age due to frailty (Sabia, Kivimaki, Shipley, Marmot, & Singh-Manoux, 2009), which may also impact our findings.
Our results may be explained by a number of mechanisms. A review of both animal and human studies (Voelcker-Rehage & Niemann, 2013) indicates multifaceted effects of PA on brain health, including cognition and brain structure. First, the coordination components of lifestyle PA (balance, gross and fine motor) improve brain health by augmenting neuroplasticity (Cotman, Berchtold, & Christie, 2007; Raz & Lindenberger, 2013). The metabolic components of PA increase cerebral blood flow and vascularization of brain structures (Holzschneider, Wolbers, Röder, & Hötting, 2012; Pereira et al., 2007). Because total lifestyle PA was assessed in our analysis, we cannot differentiate whether the coordination or the metabolic components of PA were involved; it is likely that both components contributed to our effects.
In our recent systematic review, four of six studies showed significant effects of aerobic PA interventions on brain structure, with limited effects to cognitive function (Halloway, Wilbur, Schoeny, & Arfanakis, 2016). This may indicate that changes in MRI measures of brain structure may precede improved performance on neurocognitive tests. Another possible explanation is that a larger dose of PA is required in order to obtain significant effects on cognitive function (Loprinzi, Edwards, Crush, Ikuta, & Del Arco, 2017).
This sample was similar to the majority of the earlier cross-sectional studies (Benedict et al., 2013; Brown et al., 2014; Bugg & Head, 2011; Ho et al., 2011; Kooistra et al., 2014). Participants were mostly white (95.0 vs. 98.6%), but significantly older (M, SD = 80.9, 7.2 vs. M, SD = 70.4, 12.5) with a greater proportion of females (76.3 vs. 58.3%). As with earlier cross-sectional studies, this sample had higher levels of education than the United States average (50 vs. 25% of older adults had at least 16 years of education; Ryan & Bauman, 2016). Consistent with the general population, males completed significantly more years of education than females (Ryan & Bauman, 2016), males reported higher income levels than females (Cameron, Song, Manheim, & Dunlop, 2010), and females were more likely to report more depressive symptoms than males (Barry, Allore, Guo, Bruce, & Gill, 2008).
These analyses were unique in the older age of the study participants (mean 81 years vs. 70 years of earlier studies). The characteristics and health needs of the older adult population greatly differ depending on the specific age subgroup: the young–old (65–74 years), middle–old (75–84 years), or the old–old (85 years and greater; Administration on Aging, 2014). Unfortunately, there is a paucity of research focused on the middle–old and old–old subgroups, with most research focused on the young–old subgroup (Bauman et al., 2016). Yet, the middle–old and old–old age groups are the fastest rising segments of the population (Administration on Aging, 2014), with the highest risk of developing of cognitive dysfunction and dementia. Given that over 83% of the total sample was considered middle–old or old–old, our analysis also shows that the benefits of lifestyle PA for gray matter volumes continue with age, including the growing middle–old and old–old age subgroups. However, knowledge is still developing that focuses on the specific behavioral, biological, or social factors that increase risk of cognitive dysfunction and influence survival in advanced age (I. N. Miller et al., 2015; Spira, Rebok, Stone, Kramer, & Yaffe, 2012).
Limitations of this study should be noted. First, this analysis was cross-sectional, which precludes the ability to determine directionality of relations. Second, the measure of lifestyle PA available in this secondary dataset did not include PA intensity categories (e.g., light and moderate-vigorous). PA intensity categories can provide important information for implementation and clinical application (N. E. Miller, Strath, Swartz, & Cashin, 2010). Third, we did not include a measure of aerobic fitness, which can provide insight on the fitness effects on brain structure and cognition. Finally, the sample consisted of a cohort of volunteers who were disproportionately female, white, and with higher than U.S. average education level, limiting the generalizability particularly to other racial ethnic groups and those with lower education levels. There may also have been a selection bias since these analyses represent a sub-sample who volunteered to participate in the MRI sub-study.
To our knowledge, this is the first study to examine the effects of a device measure of lifestyle PA (accelerometers) on gray matter volumes in older adults without dementia or mild cognitive impairment. The device measure provides a more accurate representation of total lifestyle PA (Koster et al., 2016) compared to self-report questionnaires. Our findings with a device measure of lifestyle PA not only confirmed results from earlier self-report structured exercise and gray matter studies (Benedict et al., 2013; Brown et al., 2014; Bugg & Head, 2011; Ho et al., 2011; Kooistra et al., 2014), but also provide insight on these relations in the growing middle–old and old–old populations.
Our findings should be further explored in a longitudinal study or randomized controlled intervention trial. There are three existing trials that tests the effects of structured exercise on gray matter volumes: one tested aerobic training (Colcombe et al., 2006), one tested walking and low-intensity gymnastics (Ruscheweyh et al., 2011), and one tested calisthenics (Tamura et al., 2015). All three showed significant modest increases in gray matter volumes. In addition, a recent systematic review identified 11 studies that examined the effects of structured exercise interventions on regional brain volumes or brain volumes of specific structures. Findings of these studies showed significant effects in brain structures, including the hippocampus, cerebellum, and prefrontal cortex (Batouli & Saba, 2017).
To date, the effects of lifestyle PA interventions on gray matter volumes have been tested almost exclusively in the young–old age subgroup. Future research is needed to develop and test lifestyle PA interventions in order to prevent loss of gray matter volumes and prevent cognitive dysfunction in the middle–old and old–old populations without dementia or mild cognitive impairment. Further, future intervention research should target lifestyle PA, which is generally preferred and more engaging for older adults (Darden et al., 2013).
Supplementary Material
Supplementary data is available at The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences online.
Funding
This secondary analysis was supported by the Rush Alzheimer’s Disease Center grant NIH/NIA P30 AG010161.
Supplementary Material
References
- Administration on Aging (2014). A profile of older Americans: 2014. Washington, DC: U.S. Department and Human Services; Retrieved from https://aoa.acl.gov/aging_statistics/profile/2014/docs/2014-profile.pdf [Google Scholar]
- Alosco M. L., Brickman A. M., Spitznagel M. B., Sweet L. H., Josephson R., Griffith E. Y., … Gunstad J (2015). Daily physical activity is associated with subcortical brain volume and cognition in heart failure. Journal of the International Neuropsychological Society, 21, 851–860. doi:10.1017/S1355617715000697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arfanakis K., Wilson R. S., Barth C. M., Capuano A. W., Vasireddi A., Zhang S., …, Bennett D. A. (2016). Cognitive activity, cognitive function, and brain diffusion characteristics in old age. Brain Imaging and Behavior, 10, 455–463. doi:10.1007/s11682-015-9405-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry L. C. Allore H. G. Guo Z. Bruce M. L. & Gill T. M (2008). Higher burden of depression among older women: The effect of onset, persistence, and mortality over time. Archives of General Psychiatry, 65, 172–178. doi:10.1001/archgenpsychiatry.2007.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batouli S. A. H., & Saba V (2017). At least eighty percent of brain grey matter is modifiable by physical activity: A review study. Behavioural Brain Research, 332(Supplement C), 204–217. doi:10.1016/j.bbr.2017.06.002 [DOI] [PubMed] [Google Scholar]
- Bauman A., Merom D., Bull F. C., Buchner D. M., & Fiatarone Singh M. A (2016). Updating the evidence for physical activity: Summative reviews of the epidemiological evidence, prevalence, and interventions to promote “active aging.” The Gerontologist, 56 (Suppl 2), S268–280. doi:10.1093/geront/gnw031 [DOI] [PubMed] [Google Scholar]
- Benedict C., Brooks S. J., Kullberg J., Nordenskjöld R., Burgos J., Le Grevès M., …, Schiöth H. B. (2013). Association between physical activity and brain health in older adults. Neurobiology of Aging, 34, 83–90. doi:10.1016/j.neurobiolaging.2012.04.013 [DOI] [PubMed] [Google Scholar]
- Bennett D. A. Schneider J. A. Buchman A. S. Barnes L. L. Boyle P. A. & Wilson R. S (2012). Overview and findings from the rush Memory and Aging Project. Current Alzheimer Research, 9, 646–663. doi:10.2174/156720512801322663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett D. A. Schneider J. A. Buchman A. S. Mendes de Leon C. Bienias J. L. & Wilson R. S (2005). The rush memory and aging project: Study design and baseline characteristics of the study cohort. Neuroepidemiology, 25, 163–175. doi:10.1159/000087446 [DOI] [PubMed] [Google Scholar]
- Bherer L. Erickson K. I. & Liu-Ambrose T (2013). A review of the effects of physical activity and exercise on cognitive and brain functions in older adults. Journal of Aging Research, 2013. doi:10.1155/2013/657508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blodgett J. Theou O. Kirkland S. Andreou P. & Rockwood K (2015). The association between sedentary behaviour, moderate-vigorous physical activity and frailty in NHANES cohorts. Maturitas, 80, 187–191. doi:10.1016/j.maturitas.2014.11.010 [DOI] [PubMed] [Google Scholar]
- Boyle P. A. Wilson R. S. Aggarwal N. T. Tang Y. & Bennett D. A (2006). Mild cognitive impairment: Risk of Alzheimer disease and rate of cognitive decline. Neurology, 67, 441–445. doi:10.1212/01.wnl.0000228244.10416.20 [DOI] [PubMed] [Google Scholar]
- Brown B. M., Bourgeat P., Peiffer J. J., Burnham S., Laws S. M., Rainey-Smith S. R., … AIBL Research Group. (2014). Influence of BDNF Val66Met on the relationship between physical activity and brain volume. Neurology, 83, 1345–1352. doi:10.1212/WNL.0000000000000867 [DOI] [PubMed] [Google Scholar]
- Buchman A. S. Boyle P. A. Yu L. Shah R. C. Wilson R. S. & Bennett D. A (2012). Total daily physical activity and the risk of AD and cognitive decline in older adults. Neurology, 78, 1323–1329. doi:10.1212/WNL.0b013e3182535d35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchman A. S. Wilson R. S. & Bennett D. A (2008). Total daily activity is associated with cognition in older persons. The American Journal of Geriatric Psychiatry, 16, 697–701. doi:10.1097/JGP.0b013e31817945f6 [DOI] [PubMed] [Google Scholar]
- Bugg J. M. & Head D (2011). Exercise moderates age-related atrophy of the medial temporal lobe. Neurobiology of Aging, 32, 506–514. doi:10.1016/j.neurobiolaging.2009.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron K. A. Song J. Manheim L. M. & Dunlop D. D (2010). Gender disparities in health and healthcare use among older adults. Journal of Women’s Health (2002), 19, 1643–1650. doi:10.1089/jwh.2009.1701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (2015). Glossary of Terms: Physical Activity Retrieved from https://www.cdc.gov/physicalactivity/basics/glossary/index.htm
- Colcombe S. J., Erickson K. I., Scalf P. E., Kim J. S., Prakash R., McAuley E., …, Kramer A. F. (2006). Aerobic exercise training increases brain volume in aging humans. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 61, 1166–1170. doi:10.1093/gerona/61.11.1166 [DOI] [PubMed] [Google Scholar]
- Colcombe S. & Kramer A. F (2003). Fitness effects on the cognitive function of older adults: A meta-analytic study. Psychological Science, 14, 125–130. doi:10.1111/1467-9280.t01-1-01430 [DOI] [PubMed] [Google Scholar]
- Cotman C. W. Berchtold N. C. & Christie L. A (2007). Exercise builds brain health: Key roles of growth factor cascades and inflammation. Trends in Neurosciences, 30, 464–472. doi:10.1016/j.tins.2007.06.011 [DOI] [PubMed] [Google Scholar]
- Crouter S. E. Dellavalle D. M. Horton M. Haas J. D. Frongillo E. A. & Bassett D. R. Jr (2011). Validity of the Actical for estimating free-living physical activity. European Journal of Applied Physiology, 111, 1381–1389. doi:10.1007/s00421-010-1758-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darden D., Richardson C., & Jackson E. A (2013). Physical activity and exercise for secondary prevention among patients with cardiovascular disease. Current Cardiovascular Risk Reports, 7. doi:10.1007/s12170-013-0354-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du M., Liu J., Chen Z., Huang X., Li J., Kuang W., … Gong Q (2014). Brain grey matter volume alterations in late-life depression. Journal of Psychiatry & Neuroscience, 39, 397–406. doi:10.1503/jpn.130275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards M. K. & Loprinzi P. D (2016). Associations between accelerometer-assessed sedentary behavior, physical activity and objectively-measured cardiorespiratory fitness with red blood cell distribution width. International Journal of Cardiology, 221, 755–758. doi:10.1016/j.ijcard.2016.07.137 [DOI] [PubMed] [Google Scholar]
- Erickson K. I., Leckie R. L., & Weinstein A. M (2014). Physical activity, fitness, and gray matter volume. Neurobiology of Aging, 35 (Suppl 2), S20–28. doi:10.1016/j.neurobiolaging. 2014.03.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson K., Raji C., Lopez O., Becker J., Rosano C., Newman A., … Kuller L (2010). Physical activity predicts gray matter volume in late adulthood: The cardiovascular health study. Neurology, 75, 1415–1422. doi:10.1212/WNL.0b013e3181f88359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etnier J. L. Nowell P. M. Landers D. M. & Sibley B. A (2006). A meta-regression to examine the relationship between aerobic fitness and cognitive performance. Brain Research Reviews, 52, 119–130. doi:10.1016/j.brainresrev.2006.01.002 [DOI] [PubMed] [Google Scholar]
- Halloway S., Wilbur J., Schoeny M. E., & Arfanakis K (2016). Effects of endurance-focused physical activity interventions on brain health: A systematic review. Biological Research For Nursing, doi:10.1177/1099800416660758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head D. Singh T. & Bugg J. M (2012). The moderating role of exercise on stress-related effects on the hippocampus and memory in later adulthood. Neuropsychology, 26, 133–143. doi:10.1037/a0027108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho A. J., Raji C. A., Becker J. T., Lopez O. L., Kuller L. H., Hua X., …, Thompson P. M. (2011). The effects of physical activity, education, and body mass index on the aging brain. Human Brain Mapping, 32, 1371–1382. doi:10.1002/hbm.21113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzschneider K. Wolbers T. Röder B. & Hötting K (2012). Cardiovascular fitness modulates brain activation associated with spatial learning. NeuroImage, 59, 3003–3014. doi:10.1016/j.neuroimage.2011.10.021 [DOI] [PubMed] [Google Scholar]
- Hugo J. & Ganguli M (2014). Dementia and cognitive impairment: Epidemiology, diagnosis, and treatment. Clinics in Geriatric Medicine, 30, 421–442. doi:10.1016/j.cger.2014.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- IBM Corp (2015). IBM SPSS Statistics for Windows (Version 23.0). Armonk, NY: IBM, Corp. [Google Scholar]
- Kerr J., Marshall S. J., Patterson R. E., Marinac C. R., Natarajan L., Rosenberg D., …, Crist K. (2013). Objectively measured physical activity is related to cognitive function in older adults. Journal of the American Geriatrics Society, 61, 1927–1931. doi:10.1111/jgs.12524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharabian Masouleh S., Arélin K., Horstmann A., Lampe L., Kipping J. A., Luck T., … Witte A. V (2016). Higher body mass index in older adults is associated with lower gray matter volume: Implications for memory performance. Neurobiology of Aging, 40, 1–10. doi:10.1016/j.neurobiolaging.2015.12.020 [DOI] [PubMed] [Google Scholar]
- Kim D. (2016). Correlation between physical function, cognitive function, and health-related quality of life in elderly persons. Journal of Physical Therapy Science, 28, 1844–1848. doi:10.1589/jpts.28.1844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohout F. J. Berkman L. F. Evans D. A. & Cornoni-Huntley J (1993). Two shorter forms of the CES-D (Center for Epidemiological Studies Depression) depression symptoms index. Journal of Aging and Health, 5, 179–193. doi:10.1177/089826439300500202 [DOI] [PubMed] [Google Scholar]
- Kooistra M. Boss H. M. van der Graaf Y. Kappelle L. J. Biessels G. J. & Geerlings M. I; SMART-MR Study Group (2014). Physical activity, structural brain changes and cognitive decline. The SMART-MR study. Atherosclerosis, 234, 47–53. doi:10.1016/j.atherosclerosis.2014.02.003 [DOI] [PubMed] [Google Scholar]
- Koster A. Shiroma E. J. Caserotti P. Matthews C. E. Chen K. Y. Glynn N. W. & Harris T. B (2016). Comparison of sedentary estimates between activPAL and Hip- and Wrist-Worn ActiGraph. Medicine and Science in Sports and Exercise, 48, 1514–1522. doi:10.1249/MSS.0000000000000924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim A. S. Fleischman D. A. Dawe R. J. Yu L. Arfanakis K. Buchman A. S. & Bennett D. A (2016). Regional neocortical gray matter structure and sleep fragmentation in older adults. Sleep, 39, 227–235. doi:10.5665/sleep.5354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin A. L. Laird A. R. Fox P. T. & Gao J. H (2012). Multimodal MRI neuroimaging biomarkers for cognitive normal adults, amnestic mild cognitive impairment, and Alzheimer’s disease. Neurology Research International, 2012, doi:10.1155/ 2012/907409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X., Zhang X., Guo J., Roberts C. K., McKenzie S., Wu W.-C., … Song Y (2015). Effects of exercise training on cardiorespiratory fitness and biomarkers of cardiometabolic health: A systematic review and meta-analysis of randomized controlled trials. Journal of the American Heart Association: Cardiovascular and Cerebrovascular Disease, 4. doi:10.1161/JAHA.115.002014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loprinzi P. D., Edwards M. K., Crush E., Ikuta T., & Del Arco A (2017). Dose–response association between physical activity and cognitive function in a national sample of older adults. American Journal of Health Promotion. [Epub ahead of print]. doi:10.1177/0890117116689732 [DOI] [PubMed] [Google Scholar]
- Makizako H., Liu-Ambrose T., Shimada H., Doi T., Park H., Tsutsumimoto K., Suzuki T (2015). Moderate-intensity physical activity, hippocampal volume, and memory in older adults with mild cognitive impairment. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 70, 480–486. doi:10.1093/gerona/glu136 [DOI] [PubMed] [Google Scholar]
- Manji H. K. Drevets W. C. & Charney D. S (2001). The cellular neurobiology of depression. Nature Medicine, 7, 541–547. doi:10.1038/87865 [DOI] [PubMed] [Google Scholar]
- McKhann G. Drachman D. Folstein M. Katzman R. Price D. & Stadlan E. M (1984). Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA work group under the auspices of department of health and human services task force on Alzheimer’s Disease. Neurology, 34, 939–944. [DOI] [PubMed] [Google Scholar]
- Miller I. N. Himali J. J. Beiser A. S. Murabito J. M. Seshadri S. Wolf P. A. & Au R (2015). Normative data for the cognitively intact oldest-old: The framingham heart study. Experimental Aging Research, 41, 386–409. doi:10.1080/0361073X.2015.1053755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller N. E. Strath S. J. Swartz A. M. & Cashin S. E (2010). Estimating absolute and relative physical activity intensity across age via accelerometry in adults. Journal of Aging and Physical Activity, 18, 158–170. doi:10.1123/japa.18.2.158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell A. J. & Shiri-Feshki M (2009). Rate of progression of mild cognitive impairment to dementia–meta-analysis of 41 robust inception cohort studies. Acta Psychiatrica Scandinavica, 119, 252–265. doi:10.1111/j.1600-0447.2008.01326.x [DOI] [PubMed] [Google Scholar]
- Murtagh E. M., Nichols L., Mohammed M. A., Holder R., Nevill A. M., & Murphy M. H (2015). The effect of walking on risk factors for cardiovascular disease: An updated systematic review and meta-analysis of randomised control trials. Preventive Medicine, 72(Supplement C), 34–43. doi:10.1016/j.ypmed.2014.12.041 [DOI] [PubMed] [Google Scholar]
- Northey J. M., Cherbuin N., Pumpa K. L., Smee D. J., & Rattray B (2017). Exercise interventions for cognitive function in adults older than 50: A systematic review with meta-analysis. British Journal of Sports Medicine, 52, 154–160. doi:10.1136/bjsports-2016–096587 [DOI] [PubMed] [Google Scholar]
- Papenberg G., Ferencz B., Mangialasche F., Mecocci P., Cecchetti R., Kalpouzos G., … Bäckman L (2016). Physical activity and inflammation: Effects on gray-matter volume and cognitive decline in aging. Human Brain Mapping, 37, 3462–3473. doi:10.1002/hbm.23252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira A. C., Huddleston D. E., Brickman A. M., Sosunov A. A., Hen R., McKhann G. M., … Small S. A (2007). An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proceedings of the National Academy of Sciences of the United States of America, 104, 5638–5643. doi:10.1073/pnas.0611721104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pini L., Pievani M., Bocchetta M., Altomare D., Bosco P., Cavedo E., …, Frisoni G. B. (2016). Brain atrophy in Alzheimer’s Disease and aging. Ageing Research Reviews, 30, 25–48. doi:10.1016/j.arr.2016.01.002 [DOI] [PubMed] [Google Scholar]
- Raz N., & Lindenberger U (2013). Life-span plasticity of the brain and cognition: From questions to evidence and back. Neuroscience & Biobehavioral Reviews, 37(9, Part B), 2195–2200. doi:10.1016/j.neubiorev.2013.10.003 [DOI] [PubMed] [Google Scholar]
- Rovio S., Spulber G., Nieminen L. J., Niskanen E., Winblad B., Tuomilehto J., …, Kivipelto M. (2010). The effect of midlife physical activity on structural brain changes in the elderly. Neurobiology of Aging, 31, 1927–1936. doi:10.1016/j.neurobiolaging.2008.10.007 [DOI] [PubMed] [Google Scholar]
- Ruscheweyh R., Willemer C., Krüger K., Duning T., Warnecke T., Sommer J., …, Flöel A. (2011). Physical activity and memory functions: An interventional study. Neurobiology of Aging, 32, 1304–1319. doi:10.1016/j.neurobiolaging.2009.08.001 [DOI] [PubMed] [Google Scholar]
- Ryan C. L., & Bauman K. B (2016). Educational attainment in the United States: 2015. Washington, DC: United States Census Bureau; Retrieved from https://www.census.gov/content/dam/Census/library/publications/2016/demo/p20-578.pdf [Google Scholar]
- Sabia S. Kivimaki M. Shipley M. J. Marmot M. G. & Singh-Manoux A (2009). Body mass index over the adult life course and cognition in late midlife: The Whitehall II Cohort Study. The American Journal of Clinical Nutrition, 89, 601–607. doi:10.3945/ajcn.2008.26482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spira A. P. Rebok G. W. Stone K. L. Kramer J. H. & Yaffe K (2012). Depressive symptoms in oldest-old women: Risk of mild cognitive impairment and dementia. The American Journal of Geriatric Psychiatry, 20, 1006–1015. doi:10.1097/JGP.0b013e318235b611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbs B. Chen L. J. Chang C. Y. Sun W. J. & Ku P. W (2017). Accelerometer-assessed light physical activity is protective of future cognitive ability: A longitudinal study among community dwelling older adults. Experimental Gerontology, 91, 104–109. doi:10.1016/j.exger.2017.03.003 [DOI] [PubMed] [Google Scholar]
- Tamura M., Nemoto K., Kawaguchi A., Kato M., Arai T., Kakuma T., … Asada T (2015). Long-term mild-intensity exercise regimen preserves prefrontal cortical volume against aging. International Journal of Geriatric Psychiatry, 30, 686–694. doi:10.1002/gps.4205 [DOI] [PubMed] [Google Scholar]
- Varma V. R., Chuang Y.-F., Harris G. C., Tan E. J., & Carlson M. C (2015). Low-intensity daily walking activity is associated with hippocampal volume in older adults. Hippocampus, 25, 605–615. doi:10.1002/hipo.22397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma V. R. Tang X. & Carlson M. C (2016). Hippocampal sub-regional shape and physical activity in older adults. Hippocampus, 26, 1051–1060. doi:10.1002/hipo.22586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voelcker-Rehage C. & Niemann C (2013). Structural and functional brain changes related to different types of physical activity across the life span. Neuroscience and Biobehavioral Reviews, 37, 2268–2295. doi:10.1016/j.neubiorev.2013.01.028 [DOI] [PubMed] [Google Scholar]
- Watson R. R. (2016). Physical activity and the aging brain: Effects of exercise on neurological function. Cambridge, Massachusetts: Academic Press. [Google Scholar]
- Wilson R. S. Barnes L. L. Krueger K. R. Hoganson G. Bienias J. L. & Bennett D. A (2005). Early and late life cognitive activity and cognitive systems in old age. Journal of the International Neuropsychological Society, 11, 400–407. [PubMed] [Google Scholar]
- Zhu W. Wadley V. G. Howard V. J. Hutto B. Blair S. N. & Hooker S. P (2017). Objectively measured physical activity and cognitive function in older adults. Medicine and Science in Sports and Exercise, 49, 47–53. doi:10.1249/MSS.0000000000001079 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.