Abstract
Background
Essential amino acids (EAA) and aerobic exercise (AE) acutely and independently stimulate skeletal muscle protein anabolism in older adults.
Objective
In this Phase 1, double-blind, placebo-controlled, randomized clinical trial, we determined if chronic EAA supplementation, AE training, or a combination of the two interventions could improve muscle mass and function by stimulating muscle protein synthesis.
Methods
We phone-screened 971, enrolled 109, and randomized 50 independent, low-active, nonfrail, and nondiabetic older adults (age 72 ± 1 years). We used a 2 × 2 factorial design. The interventions were: daily nutritional supplementation (15 g EAA or placebo) and physical activity (supervised AE training 3 days/week or monitored habitual activity) for 24 weeks. Muscle strength, physical function, body composition, and muscle protein synthesis were measured before and after the 24-week intervention.
Results
Forty-five subjects completed the 24-week intervention. VO2peak and walking speed increased (p < .05) in both AE groups, irrespective of supplementation type, but muscle strength increased only in the EAA + AE group (p < .05). EAA supplementation acutely increased (p < .05) muscle protein synthesis from basal both before and after the intervention, with a larger increase in the EAA + AE group after the intervention. Total and regional lean body mass did not change significantly with any intervention.
Conclusions
In nonfrail, independent, healthy older adults AE training increased walking speed and aerobic fitness, and, when combined with EAA supplementation, it also increased muscle strength and EAA-stimulated muscle protein synthesis. These increases occurred without improvements in muscle mass.
Keywords: Body composition, Exercise, Nutrition, Physical performance
Nutritional factors and inactivity are important contributors to sarcopenia, the involuntary loss of skeletal muscle mass and function with aging (1,2). With aging, skeletal muscle protein synthesis becomes resistant to the anabolic stimulation by feeding and resistance exercise (anabolic resistance) (3,4). The intake of relatively large amounts of essential amino acids (EAA) (3) or a single bout of aerobic exercise (AE) (5) can acutely overcome the muscle anabolic resistance of aging in healthy older individuals. If the positive acute effects of EAA and AE on skeletal muscle protein anabolism accumulate over time, it is reasonable to expect that prolonged EAA supplementation, AE training, or a combination of the two may lead to improvements in lean body mass and function in older adults. Indeed, a study in frail, sarcopenic older women reported that EAA supplementation when combined with strength and balance exercise improved muscle strength more than exercise alone (6). However, we do not know if EAA supplementation, AE training, or a combination of the two can enhance skeletal muscle mass and function and prevent sarcopenia in healthier, nonfrail older men, and women with adequate habitual protein intake.
The primary aim of this study was to determine in a placebo-controlled, randomized, 2 × 2 factorial design, phase 1 clinical trial if EAA supplementation and/or AE training can increase skeletal muscle mass, strength, and function in healthy, low-active, independent older adults. We also sought to test if acute changes in muscle protein synthesis can predict the treatments’ long-term effect, and whether the chronic intervention induced positive or negative adaptations to the acute response to treatment. Our hypothesis was that interventions that acutely activate muscle protein synthesis and anabolism will increase muscle mass, strength, and function when administered chronically. Here, we report the primary outcome results.
Materials and Methods
The protocol was approved by the Institutional Review Board (IRB) of the University of Texas Medical Branch (UTMB) and registered in ClinicalTrials.gov (NCT00872911).
Subjects
We included healthy, nonfrail, independent, normal to mildly obese (body mass index, BMI: 19–33 kg/m2), low-active (less than 7500 steps/day weekly average) older adults (age range: 65–82 years) not engaged in an exercise program. Exclusion criteria were: history of falls; dietary protein intake below the estimated average requirement (0.66 g/kg/day) or above 1.5 g/kg/day (reported intake 0.95 ± 0.04 g/kg/day); active cancer; diabetes; significant or unexplained weight loss in the previous year; uncontrolled blood pressure; current tobacco or illicit drug use; significant heart, lung, kidney, liver, or hematologic disease; infections; and any other condition that would preclude exercise. Screening tests included: medical history; physical exam; food recall questionnaire; 3-day food diary; comprehensive metabolic panel; complete blood count; urinalysis including drug screening; 75 g, 2 h oral glucose tolerance test to screen for undiagnosed diabetes; step activity monitoring; electrocardiogram; and exercise stress test.
Of 971 interested subjects screened over the phone, 109 were enrolled, provided informed written consent, and were screened at the Institute for Translational Sciences Clinical Research Center (ITS-CRC). Fifty participants met the inclusion criteria and were randomized into one of the four intervention groups.
Randomization
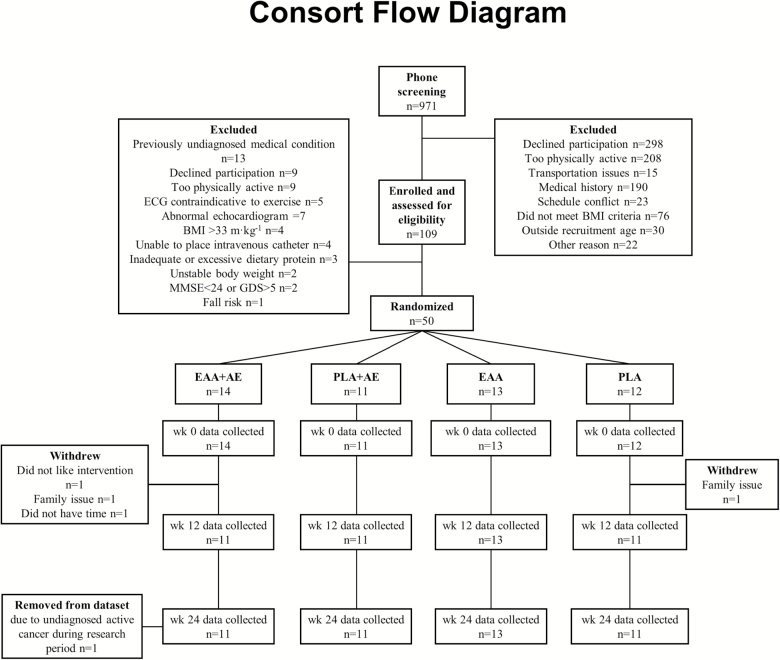
We used a block randomization scheme with stratification by sex. To improve treatment adherence and fidelity, couples participating together were assigned to a third stratum allowing for randomization to the same intervention. Subjects were randomized into one of four intervention groups: AE with EAA supplementation (EAA + AE; n = 14), AE with placebo supplementation (PLA + AE; n = 11), EAA supplementation only (EAA; n = 13), or placebo only (PLA; n = 12). Assignment to EAA or PLA was double-blinded. The AE intervention was unblinded. Of the 50 randomized participants, 45 subjects (30 women, 15 men) completed the study and were included in the dataset (Figure 1, CONSORT Flow Diagram).
Figure 1.
CONSORT flow diagram.
Study Design
Randomized subjects underwent strength, fitness, and functional testing, and measurement of body composition and muscle protein synthesis before and after 24 weeks of intervention with the assigned treatment (Supplementary Figure 1).
Strength, Fitness, and Functional Tests
Tests were completed 10–14 days prior to the baseline muscle protein experiment and 7–14 days prior to the final muscle protein experiment. Leg strength was assessed with a dynamometer (Biodex, Shirley, New York). After warm-up, subjects performed an isokinetic (120°/s) peak torque strength test on the right leg. Muscle quality was calculated as isokinetic peak torque divided by whole leg lean mass. Subjects also performed 20 m walk, 20 m walk with carry (~10% body weight load), and 400 m walk tests at normal and fast pace. Approximately 1–2 weeks prior to the first and the last muscle metabolism experiment, we measured VO2peak using the modified Bruce incremental treadmill test (7). Physical activity was monitored for 1 week prior to the first acute experiment, and 1 week every 4 weeks throughout the intervention period using a step activity monitor (StepWatch™, OrthoCare, Edmonds, WA). All days of the week were averaged and reported as daily average steps.
Body Composition
Body composition was measured with dual-energy X-ray absorptiometry (Lunar iDXA, GE Healthcare, Chicago, IL). The instrument was calibrated before each scan. All scans were analyzed by the same blinded technician.
Muscle Protein Synthesis
To determine if the interventions’ baseline acute effects on muscle protein synthesis could predict their chronic effect on muscle mass and function, and if chronic EAA supplementation or exercise induced adaptations to the acute stimuli, we measured the acute effects of the treatments on muscle protein synthesis using stable isotope tracers before and at the end of the 24-week intervention (Supplementary Figure 2). Subjects were admitted to the ITS-CRC the day prior to the acute experiment. They were fed a standardized dinner (10 kcal/kg; 60% carbohydrate, 20% fat, and 20% protein) and an evening snack at 22:00 h. Water consumption was ad libitum. After an overnight fast, a retrograde catheter was placed in a hand or wrist vein. The hand was heated for arterialized blood sampling. A second catheter was placed in an antecubital vein of the opposite arm for stable isotope tracer infusion (Supplementary Figure 1). After collection of background blood samples, a primed continuous infusion of L-[ring-13C6]phenylalanine (priming dose: 2μmol/kg, infusion rate: 0.05μmol/kg/min) (time 0) was started. Approximately 120 min after starting the infusion, a basal muscle biopsy was collected from the vastus lateralis of one leg. A second muscle biopsy was collected at time 240 min from the same leg.
Immediately after the second muscle biopsy, EAA + AE and PLA + AE subjects walked on a treadmill for 45 min at ~70% of heart rate reserve (HRreserve), whereas EAA and PLA subjects rested in bed. The exercise dose was based on our preliminary data indicating it improves anabolic sensitivity to nutrients (5). After the AE bout or rest, subjects consumed the supplement to which they were randomized (EAA or PLA). Those randomized to the EAA arms consumed 0.2g/kg body weight of an EAA mix (40% L-leucine, 16.7% L-lysine, 11% L-valine, 10.7% L-isoleucine, 9.3% L-threonine, 6.7% L-phenylalanine, 3.3% L-methionine, 1.7% histidine, and 0.7% L-tryptophan; Amino L40, Ajinomoto, Kawasaki, Japan). Subjects randomized to the PLA arms consumed 2 g of potassium bitartrate and 8 g of soluble wheat dextrin. Supplements and placebo were dissolved in 350 mL of water with sugar-free flavoring. To prevent changes in blood tracer dilution due to the ingestion of unlabeled amino acids, we enriched the EAA supplement with L-[ring-13C6]phenylalanine to achieve the expected blood value (7%). A third and fourth muscle biopsy were collected 60 and 180 min after supplement consumption.
Muscle Samples
Muscle biopsies of the vastus lateralis were obtained using aseptic procedure, local anesthesia, and a 5-mm Bergström needle. They were immediately rinsed with ice-cold saline, and blotted. After removal of any visible connective or adipose tissue, samples were frozen in liquid nitrogen and stored at −80°C until analysis. Two subjects in the placebo group elected to not participate in the muscle biopsy component of the study.
Measurement of Muscle Protein Synthesis
Mixed muscle proteins and tissue free amino acids were separated and analyzed by gas chromatography mass spectrometry (Agilent Technologies, Palo Alto, CA) (8). Muscle protein fractional synthetic rate (FSR) was calculated using the precursor–product method (8). Basal muscle protein FSR was calculated using enrichments measured in the first and second biopsy; post-treatment FSR was calculated using enrichments measured in the third and fourth biopsy.
Exercise Training
Subjects randomized to the EAA + AE or PLA + AE group participated in three nonconsecutive days per week of progressive AE training for 24 weeks. Subjects walked on a treadmill for 45 min at 70% HRreserve, plus 5-min cool down. All sessions were supervised by a study team member. Speed and incline of the treadmill was adjusted to keep the subject within 5% of the target heart rate during the entire exercise session (Polar FS1, Polar USA).
Chronic Supplementation
Subjects consumed their assigned supplement daily, between meals, and at the same time of day. Subjects in the exercise arms consumed the exercise day supplements within 1 h after the training session, and kept the timing consistent on nonexercise days. The EAA + AE and EAA subjects consumed 15 g of the same EAA mix used in the muscle experiment, and PLA + AE and PLA subjects consumed the same placebo supplement of the muscle experiment. The EAA dose (15 g) was selected based on previous data suggesting it can stimulate muscle protein anabolism (9). Supplements were dissolved in a calorie-free beverage. Every 14 days subjects met with a study team member to receive fourteen individual supplement containers and return the previous 14 containers, regardless of whether the containers were empty or full. Container count was used to measure adherence.
Statistical Analysis
For all comparisons, data were transformed using the Box-Cox family of transformations to improve model fit. To analyze the outcomes at the pre-time point, a two-way ANOVA model was used with contrasts to test the differences among treatment groups. For the comparison at the post time point, all effect sizes and means were estimated after being standardized using an ANCOVA model to the mean pre value. Contrasts were used to test group effects. Variables with known sex differences (leg strength, body composition) were adjusted for sex. All statistical analyses were conducted with statistical software (R, ver.3.2.2). Level of significance was set at p < .05. All reported values are mean ± standard error (SE).
Results
Safety
No adverse events related to the interventions were reported. One subject was diagnosed with stage 3 lung cancer immediately after study completion, which was deemed study unrelated, and excluded from final analysis.
Adherence
Supplement adherence was 92% with no differences between groups (data not shown). Exercise training adherence was 94% with no differences between the two groups randomized to the exercise arms (data not shown). Subjects randomized to the nonexercise arms did not modify their average physical activity level during the study (EAA: pre-treatment 3,733 ± 302, during treatment 3,512 ± 373; PLA: pre-treatment 4,335 ± 308, during treatment 4,051 ± 317; steps/day). Those randomized to the exercise arms significantly (p = .01941) increased their average physical activity level (EAA + AE: pre-treatment 3,819 ± 480, during treatment 5,181 ± 248; PLA + AE: pre-treatment 4,159 ± 480, during treatment 4,982 ± 428; steps/day based on weekly average including exercise and nonexercise days).
Body Composition
There were no baseline body composition differences between groups (Table 1). With the intervention, body weight (p = .026), BMI (p = .0153), and total fat mass (p = .00199) decreased in the PLA + AE group, and fat mass increased by 1 kg in the EAA group, but the change did not reach statistical significance (p = .060). Total and leg lean mass did not change in any group.
Table 1.
Body Composition, Aerobic Fitness, Strength, and Walking Speed Pre- and Post-Intervention
| EAA + AE (n = 10) |
EAA (n = 13) |
PLA + AE (n = 11) |
PLA (n = 11) |
|||||
|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | |
| Body weight (kg) | 74.5 ± 3.8 | 74.3 ± 3.7 | 71.7 ± 3.5 | 72.7 ± 3.6 | 69.8 ± 2.4 | 68.2 ± 2.2* | 75.9 ± 3.0 | 76.7 ± 3.2 |
| BMI (kg/m2) | 26.6 ± 0.8 | 26.5 ± 0.7 | 26.5 ± 1.0 | 26.9 ± 1.0 | 26.0 ± 1.0 | 25.4 ± 0.9* | 28.0 ± 0.8 | 28.3 ± 0.9 |
| Total lean mass (kg) | 44.2 ± 2.6 | 44.3 ± 2.4 | 41.7 ± 2.4 | 42.1 ± 2.6 | 40.7 ± 2.0 | 40.8 ± 2.0 | 42.0 ± 2.6|| | 42.3 ± 2.7|| |
| Total fat mass (kg) | 26.9 ± 1.7 | 26.6 ± 1.9 | 26.7 ± 2.0 | 27.7 ± 2.2‡ | 25.8 ± 2.0 | 24.1 ± 1.8* | 30.4 ± 2.3|| | 30.7 ± 2.4|| |
| Leg lean mass (kg) | 15.2 ± 1.1 | 15.3 ± 1.0 | 13.9 ± 0.9 | 13.9 ± 0.9 | 13.4 ± 0.6 | 13.3 ± 0.7 | 14.0 ± 0.9|| | 14.0 ± 0.9|| |
| VO2peak (mL/kg/min) | 24.5 ± 1.8 | 28.3 ± 2.7* | 22.0 ± 1.0 | 22.9 ± 1.4 | 22.5 ± 1.0 | 26.0 ± 1.6* | 21.8 ± 1.1 | 22.9 ± 1.7 |
| Isokinetic leg strength (Nm) | 72.8 ± 9.8 | 84.6 ± 10.9* | 65.2 ± 7.4 | 66.4 ± 6.9 | 52.7 ± 3.9 | 58.3 ± 5.2 | 64.5 ± 8.6 | 71.6 ± 7.3 |
| Muscle quality (Nm/kg) | 9.4 ± 0.9 | 10.7 ± 0.8* | 9.4 ± 0.8 | 9.7 ± 0.7 | 8.0 ± 0.6 | 8.8 ± 0.6 | 9.8 ± 0.9¶ | 10.4 ± 0.7‡,¶ |
| 400 m walk, normal (m/s) | 1.22 ± 0.07§ | 1.24 ± 0.08§ | 1.15 ± 0.05 | 1.14 ± 0.05 | 1.18 ± 0.06 | 1.20 ± 0.06 | 1.15 ± 0.04 | 1.15 ± 0.05 |
| 400 m walk, fast (m/s) |
1.38 ± 0.08§ | 1.46 ± 0.09*,§ | 1.38 ± 0.04# | 1.39 ± 0.04# | 1.35 ± 0.07 | 1.41 ± 0.06* | 1.37 ± 0.06 | 1.35 ± 0.06 |
| 20 m walk, no carry (m/s) | 1.83 ± 0.14§ | 1.89 ± 0.14§ | 1.72 ± 0.09 | 1.79 ± 0.09 | 1.68 ± 0.12 | 1.76 ± 0.10 | 1.69 ± 0.10 | 1.77 ± 0.11 |
| 20 m walk, with carry (m/s) |
1.83 ± 0.15§ | 2.08 ± 0.21*,§ | 1.68 ± 0.07 | 1.82 ± 0.11* | 1.67 ± 0.11 | 1.77 ± 0.10‡ | 1.72 ± 0.10 | 1.76 ± 0.12 |
Note: All reported values are mean ± SE. AE = aerobic exercise; BMI = body mass index; EAA = essential amino acids; PLA = placebo supplementation.
*Post value is different (p < .05) than pre value.
‡Trend for post value to be different (p < .10) than pre value.
§ n = 9 due to missing data for one subject.
|| n = 10 due to missing data for one subject.
¶ n = 9 due to missing data for one subject and one excluded outlier.
# n = 12 due to missing data for one subject.
Functional Measures
VO2peak was not different between groups before intervention (Table 1). V02peak increased (p < .05) in the AE groups following training. There were no changes in 400 m walking speed at normal pace (p > .05). The 400-m walking speed at fast pace increased (p < .05) in the AE groups. The 20-m walking speed with no carry did not change in any group (p > .05). The 20-m walking speed with carry increased (p < .05) in both EAA groups, and tended to increase in PLA + AE (p = .08). Leg isokinetic extension (120°/s) increased only in the EAA + AE (p = .0336) group. Muscle quality (leg strength/leg lean mass) also increased in the EAA + AE group (p = .00238).
Muscle Protein Synthesis
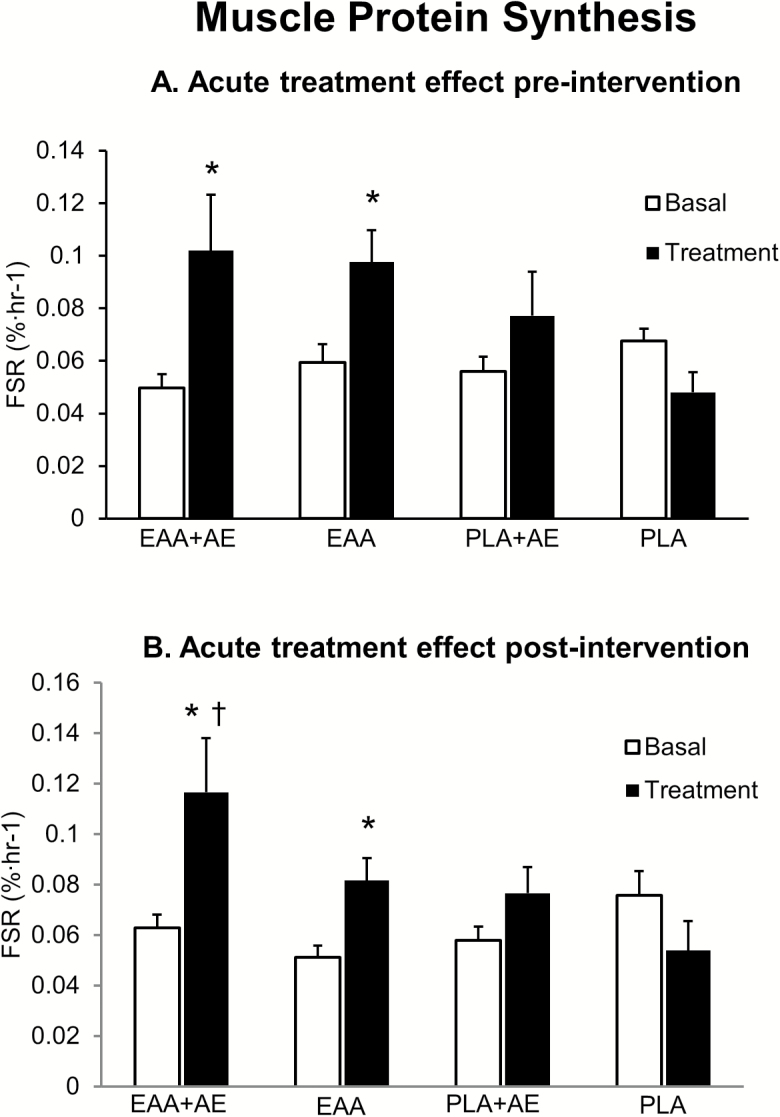
Basal muscle protein synthesis was not different between groups before or after chronic intervention (Figure 2). Acute treatment increased muscle protein synthesis only in the two groups randomized to EAA before (EAA + AE: from 0.0497 ± 0.01 to 0.1020 ± 0.02%/h, p = .0222; EAA from 0.0593 ± 0.01 to 0.0977 ± 0.02%/h, p = .00527) and after chronic intervention (EAA + AE: from 0.0629 ± 0.01 to 0.1166 ± 0.01%/h, p = .00175; EAA: from 0.0511 ± 0.01 to 0.0816 ± 0.01%/h, p = .0223). The absolute acute increase in muscle protein synthesis after intervention was larger (p < .05) than that measured before chronic intervention in the EAA + AE group only. There were no relationships between treatment-induced acute changes in muscle protein synthesis and chronic changes in leg lean mass (R2 = −0.0008) or strength (R2 = −0.0323).
Figure 2.
Muscle protein fractional synthetic rate (FSR) in the basal state and in response to acute treatment with the nutritional supplement [essential amino acids EAA, or placebo placebo supplementation (PLA)] with or without aerobic exercise (AE), before (A) and after (B) 24 weeks of chronic treatment with the same intervention. Treatment effect, *p < .05 compared to basal; time effect, †p < .05 compared to pre-intervention.
Discussion
The main finding of our study was that muscle strength and quality increased with AE training when combined with daily EAA supplementation in generally healthy, low active, independent older adults consuming an adequate protein intake. These results confirm in part our hypothesis that interventions that acutely improve muscle protein anabolism can chronically enhance muscle function. They also suggest that moderate intensity aerobic training when combined with increased EAA intake may be a viable alternative to resistance exercise training for improvement of strength and physical function in independent older individuals.
However, contrary to our original hypothesis, the functional improvement attained with aerobic training plus EAA supplementation was not associated with an increase in lean mass. While disappointing, this result does not substantially detract from our main finding. Several observational studies have shown that strength, gait speed, and endurance, but not muscle mass, are associated with mobility limitation and mortality in older adults (10,11). A meta-analysis of resistance exercise training and protein/amino acid supplementation studies also shows that increases in muscle strength and physical function may not be directly associated with increases in muscle mass (12). There are several potential reasons for the lack of changes in lean body mass in our study: inclusion of healthy, independent older individuals with adequate protein intake; a possible increase in muscle protein breakdown with AE offsetting the increases in protein synthesis (13); weight loss with aerobic training; need for a longer intervention (1–2 years); sensitivity of the DXA instrument; or insufficient mechanical stain to promote growth with the exercise routine. A previous study of sarcopenic frail older women found that a 3-month strength, balance, and gait training combined with EAA supplementation increased not only muscle strength, consistent with our findings, but also muscle mass (6). However, the exercise in that study included resistance training, which increases muscle mass (14,15). Unfortunately, there are greater barriers for older adults to participate in resistance training programs as compared to aerobic training (16): need for specialized equipment and professional supervision, psychological barriers (17), and cost (18). Additionally, compliance with home-based resistance training is low (19). Conversely, aerobic training using the walk/run method is preferred by older adults (20), and does not require specialized equipment or costly gym memberships. This type of training can be safely performed independently or in groups using common spaces (eg, sidewalks, malls, trails), which may improve adherence. Thus, aerobic training with EAA supplementation can be an effective intervention when strength gains are desirable and increases in muscle mass not necessary. If increases in muscle mass are needed, cycling exercise may be another alternative to resistance training, as it can induce muscle hypertrophy in older adults (21).
The chronic changes in physical function and lean mass were unrelated to the baseline acute effects of the treatments on muscle protein synthesis. Both groups receiving EAA exhibited an acute increase in muscle protein synthesis, but only the EAA + AE group had a chronic increase in strength. This is consistent with recent data in young (22) and older (23) adults suggesting the anabolic stimulus during the initial days of exercise training may not predict the long-term changes in muscle and lean mass (24). Nonetheless, the muscle protein synthetic response to AE plus amino acid supplementation increased with chronic treatment, suggesting a positive adaptation to the anabolic stimulus that might have contributed to the overall positive response to treatment. Anabolic resistance to nutrient ingestion (25,26) and resistance (4,27), but not aerobic (28), exercise has been reported in older adults. We used an amino acid dose large enough to stimulate muscle protein synthesis, but the larger protein synthetic response of the EAA + AE group after the 24-week intervention indicates an improvement in anabolic sensitivity. The opposite occurs with resistance and multicomponent exercise training, which shortens the duration but not magnitude of the response (29).
In our study, muscle protein synthesis or mass did not improve with EAA supplementation alone. Previous studies have yielded mixed results, reporting increases in lean mass and basal but not amino acid-stimulated muscle protein synthesis (9), increases in basal and amino acid-stimulated protein synthesis with no change in lean mass (30), or increases in lean mass and function (31). Variable duration, composition, and doses of the amino acid intervention may have contributed to the conflicting results. Overall, chronic amino acid supplementation in healthy older adults may not increase lean mass, even when the muscle protein synthetic response is enhanced (32,33), possibly due to proteostasis (34,35). Benefits of EAA supplementation may be greater in ill, malnourished, or frail elders (32), but future research is warranted.
Interestingly, fat mass and weight tended to increase in the EAA group, while they did not in the EAA + AE group, and decreased in the PLA + AE group. Loss of fat mass and total weight with aerobic training is not surprising (36) and should not be a concern in this geriatric population considering the significant gain in physical function. We speculate that EAA supplementation in older adults with habitual adequate dietary protein intake may stimulate lipogenesis and lead to fat gain unless the supplement is combined with AE (37). In our study, EAA supplementation prevented the exercise induced weight and fat loss.
Aerobic training improved aerobic capacity and the 400-m walking speed at fast pace, regardless of supplement type, as expected. Gait speed is a key component in the diagnosis of sarcopenia (38) and a critical factor influencing independence in older adults (39). While the baseline walking speed of all our subjects was higher than the cut-off for sarcopenia (>0.8–1 m/s), the increases in walking speed and aerobic capacity indicate that our interventions increased mobility and functional reserve. Interestingly, EAA supplementation with or without exercise improved also the 20-m walk with carry performance, possibly due to increased muscle remodeling: EAA stimulated muscle protein synthesis in the absence of changes in lean mass, suggesting concomitant acceleration of proteolysis and turnover.
Average weekly physical activity (encompassing both exercise and nonexercise days) increased in the two exercise groups, indicating that training did not substantially reduce the spontaneous nonexercise activity. This is an important finding, which underscores how structured progressive aerobic training does not significantly impact the nonexercise usual behavior in healthy older adults as it occurs with resistance training (15). Subjects were monitored to exercise at ~70% HRreserve during each session. To achieve this heart rate at a walking pace, they had to walk at an incline, which might have contributed to the increases in leg strength. However, since only the EAA + AE group, not the AE only group, had an increase in strength, we believe that the treadmill incline was not responsible for the strength gains in the EAA + AE group.
In addition to the novelty of testing the effects of EAA and AE for 6 months in healthy, independent older adults on physical function, body composition, and muscle metabolism, strengths of our study were: careful selection of participants to exclude protein malnourished individuals; supervising all exercise sessions; double-blinded, placebo-controlled nutritional intervention; high (>90%) adherence rate with all treatments. Limitations were the stringent inclusion criteria selected to narrow the study cohort to truly healthy, nonfrail, and independent older adults with adequate protein intake and no metabolic impairments or contraindications to exercise training. This approach restricted the potential subject pool, as demonstrated by the 971 phone calls we fielded to enroll 109 subjects and randomize 50. Future studies including frail and/or undernourished older adults with mobility limitations are warranted (40).
In conclusion, EAA supplementation when combined with AE training increased muscle strength and physical function in healthy, independent older adults. AE per se, regardless of supplementation, improved endurance, and aerobic fitness, while EAA supplementation improved walking speed with carry and prevented weight and fat loss in exercisers. However, lean body mass was unaffected by the interventions and the acute changes in muscle protein synthesis did not predict the long-term treatment effects.
Supplementary Material
Supplementary data is available at The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences online.
Funding
This work was supported by National Institutes of Health (grant numbers R01AG030070, R01AG030070S1, P30AG024832) and National Center for Advancing Translational Sciences (UL1TR001439).
Conflict of Interest
None declared.
Supplementary Material
Acknowledgments
The authors acknowledge the contributions to this project made by study coordinators Jennifer Timmerman, Paula Skinkis, and Roxana Hirst; laboratory research staff Ming-Qian Zheng, Shelley Medina, and Susan Wilson; and the staff at UTMB ITS-Clinical Research Center.
ClinicalTrials.gov Identifier: NCT00872911
References
- 1. Houston DK, Nicklas BJ, Ding J, et al. ; Health ABC Study Dietary protein intake is associated with lean mass change in older, community-dwelling adults: the Health, Aging, and Body Composition (Health ABC) Study. Am J Clin Nutr. 2008;87:150–155. doi: 10.1093/ajcn/87.1.150 [DOI] [PubMed] [Google Scholar]
- 2. Drummond MJ, Dickinson JM, Fry CS, et al. Bed rest impairs skeletal muscle amino acid transporter expression, mTORC1 signaling, and protein synthesis in response to essential amino acids in older adults. Am J Physiol Endocrinol Metab. 2012;302:E1113–E1122. doi: 10.1152/ajpendo.00603.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am J Physiol Endocrinol Metab. 2006;291:E381–E387. doi: 10.1152/ajpendo.00488.2005 [DOI] [PubMed] [Google Scholar]
- 4. Fry CS, Drummond MJ, Glynn EL, et al. Aging impairs contraction-induced human skeletal muscle mTORC1 signaling and protein synthesis. Skelet Muscle. 2011;1:11. doi: 10.1186/2044-5040-1-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Timmerman KL, Dhanani S, Glynn EL, et al. A moderate acute increase in physical activity enhances nutritive flow and the muscle protein anabolic response to mixed nutrient intake in older adults. Am J Clin Nutr. 2012;95:1403–1412. doi: 10.3945/ajcn.111.020800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kim HK, Suzuki T, Saito K, et al. Effects of exercise and amino acid supplementation on body composition and physical function in community-dwelling elderly Japanese sarcopenic women: a randomized controlled trial. J Am Geriatr Soc. 2012;60:16–23. doi: 10.1111/j.1532-5415.2011.03776.x [DOI] [PubMed] [Google Scholar]
- 7. American College of Sports Medicine., Franklin BA, Whaley MH, Howley ET, Balady GJ.. ACSM’s Guidelines for Exercise Testing and Prescription. 6th ed Philadelphia, PA: Lippincott Williams & Wilkins; 2000. [Google Scholar]
- 8. Wolfe RR, Chinkes DL.. Isotope Tracers in Metabolic Research: Principles and Practice of Kinetic Analysis. 2nd ed Hoboken, NJ: Wiley-Liss; 2005. [Google Scholar]
- 9. Dillon EL, Sheffield-Moore M, Paddon-Jones D, et al. Amino acid supplementation increases lean body mass, basal muscle protein synthesis, and insulin-like growth factor-I expression in older women. J Clin Endocrinol Metab. 2009;94:1630–1637. doi: 10.1210/jc.2008-1564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Newman AB, Kupelian V, Visser M, et al. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Gerontol A Biol Sci Med Sci. 2006;61:72–77. [DOI] [PubMed] [Google Scholar]
- 11. Newman AB, Simonsick EM, Naydeck BL, et al. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA. 2006;295:2018–2026. doi: 10.1001/jama.295.17.2018 [DOI] [PubMed] [Google Scholar]
- 12. Finger D, Goltz FR, Umpierre D, Meyer E, Rosa LH, Schneider CD. Effects of protein supplementation in older adults undergoing resistance training: a systematic review and meta-analysis. Sports Med. 2015;45:245–255. doi: 10.1007/s40279-014-0269-4 [DOI] [PubMed] [Google Scholar]
- 13. Louis E, Raue U, Jemiolo B, Trappe S. Time course of proteolytic, cytokine, and myostatin gene expression after acute exercise in human skeletal muscle. J Appl Physiol. 2007;103:1744–1751.doi: 10.1152/japplphysiol.00679.2007 [DOI] [PubMed] [Google Scholar]
- 14. Peterson MD, Sen A, Gordon PM. Influence of resistance exercise on lean body mass in aging adults: a meta-analysis. Med Sci Sports Exerc. 2011;43:249–258. doi: 10.1249/MSS.0b013e3181eb6265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hunter GR, McCarthy JP, Bamman MM. Effects of resistance training on older adults. Sports Med. 2004;34:329–348. [DOI] [PubMed] [Google Scholar]
- 16. Picorelli AM, Pereira DS, Felício DC, et al. Adherence of older women with strength training and aerobic exercise. Clin Interv Aging. 2014;9:323–331. doi: 10.2147/CIA.S54644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yardley L, Donovan-Hall M, Francis K, Todd C. Attitudes and beliefs that predict older people’s intention to undertake strength and balance training. J Gerontol B Psychol Sci Soc Sci. 2007;62:P119–P125. [DOI] [PubMed] [Google Scholar]
- 18. Reichert FF, Barros AJ, Domingues MR, Hallal PC. The role of perceived personal barriers to engagement in leisure-time physical activity. Am J Public Health. 2007;97:515–519. doi: 10.2105/AJPH.2005.070144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hong SY, Hughes S, Prohaska T. Factors affecting exercise attendance and completion in sedentary older adults: a meta-analytic approach. J Phys Act Health. 2008;5:385–397. [DOI] [PubMed] [Google Scholar]
- 20. Dallosso HM, Morgan K, Bassey EJ, Ebrahim SB, Fentem PH, Arie TH. Levels of customary physical activity among the old and the very old living at home. J Epidemiol Commun Health. 1988;42:121–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Konopka AR, Harber MP. Skeletal muscle hypertrophy after aerobic exercise training. Exerc Sport Sci Rev. 2014;42:53–61. doi: 10.1249/JES.0000000000000007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mitchell CJ, Churchward-Venne TA, Parise G, et al. Acute post-exercise myofibrillar protein synthesis is not correlated with resistance training-induced muscle hypertrophy in young men. PLoS One. 2014;9:e89431. doi: 10.1371/journal.pone.0089431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Phillips BE, Williams JP, Greenhaff PL, Smith K, Atherton PJ. Physiological adaptations to resistance exercise as a function of age. JCI Insight. 2017;2:e95581.doi: 10.1172/jci.insight.95581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Damas F, Phillips SM, Libardi CA, et al. Resistance training-induced changes in integrated myofibrillar protein synthesis are related to hypertrophy only after attenuation of muscle damage. J Physiol. 2016;594:5209–5222. doi: 10.1113/JP272472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Volpi E, Mittendorfer B, Rasmussen BB, Wolfe RR. The response of muscle protein anabolism to combined hyperaminoacidemia and glucose-induced hyperinsulinemia is impaired in the elderly. J Clin Endocrinol Metab. 2000;85:4481–4490. doi: 10.1210/jcem.85.12.7021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Moore DR, Churchward-Venne TA, Witard O, et al. Protein ingestion to stimulate myofibrillar protein synthesis requires greater relative protein intakes in healthy older versus younger men. J Gerontol A Biol Sci Med Sci. 2015;70:57–62. doi: 10.1093/gerona/glu103 [DOI] [PubMed] [Google Scholar]
- 27. Kumar V, Atherton PJ, Selby A, et al. Muscle protein synthetic responses to exercise: effects of age, volume, and intensity. J Gerontol A Biol Sci Med Sci. 2012;67:1170–1177. doi: 10.1093/gerona/gls141 [DOI] [PubMed] [Google Scholar]
- 28. Sheffield-Moore M, Yeckel CW, Volpi E, et al. Postexercise protein metabolism in older and younger men following moderate-intensity aerobic exercise. Am J Physiol Endocrinol Metab. 2004;287:E513–E522. doi: 10.1152/ajpendo.00334.2003 [DOI] [PubMed] [Google Scholar]
- 29. Damas F, Phillips S, Vechin FC, Ugrinowitsch C. A review of resistance training-induced changes in skeletal muscle protein synthesis and their contribution to hypertrophy. Sports Med. 2015;45:801–807. doi: 10.1007/s40279-015-0320-0 [DOI] [PubMed] [Google Scholar]
- 30. Casperson SL, Sheffield-Moore M, Hewlings SJ, Paddon-Jones D. Leucine supplementation chronically improves muscle protein synthesis in older adults consuming the RDA for protein. Clin Nutr. 2012;31:512–519. doi: 10.1016/j.clnu.2012.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ispoglou T, White H, Preston T, McElhone S, McKenna J, Hind K. Double-blind, placebo-controlled pilot trial of L-leucine-enriched amino-acid mixtures on body composition and physical performance in men and women aged 65-75 years. Eur J Clin Nutr. 2016;70:182–188. doi: 10.1038/ejcn.2015.91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tieland M, Franssen R, Dullemeijer C, et al. The impact of dietary protein or amino acid supplementation on muscle mass and strength in elderly people: individual participant data and meta-analysis of RCT’s. J Nutr Health Aging. 2017;21:994–1001. doi: 10.1007/s12603-017-0896-1 [DOI] [PubMed] [Google Scholar]
- 33. Komar B, Schwingshackl L, Hoffmann G. Effects of leucine-rich protein supplements on anthropometric parameter and muscle strength in the elderly: a systematic review and meta-analysis. J Nutr Health Aging. 2015;19:437–446. doi: 10.1007/s12603-014-0559-4 [DOI] [PubMed] [Google Scholar]
- 34. Robinson MM, Dasari S, Konopka AR, et al. Enhanced protein translation underlies improved metabolic and physical adaptations to different exercise training modes in young and old humans. Cell Metab. 2017;25:581–592. doi: 10.1016/j.cmet.2017.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Johnson ML, Irving BA, Lanza IR, et al. Differential effect of endurance training on mitochondrial protein damage, degradation, and acetylation in the context of aging. J Gerontol A Biol Sci Med Sci. 2015;70:1386–1393. doi: 10.1093/gerona/glu221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ross R, Janssen I, Dawson J, et al. Exercise-induced reduction in obesity and insulin resistance in women: a randomized controlled trial. Obes Res. 2004;12:789–798. doi: 10.1038/oby.2004.95 [DOI] [PubMed] [Google Scholar]
- 37. Salinas-Rubio D, Tovar AR, Noriega LG. Emerging perspectives on branched-chain amino acid metabolism during adipocyte differentiation. Curr Opin Clin Nutr Metab Care. 2018;21:49–57. doi: 10.1097/MCO.0000000000000429 [DOI] [PubMed] [Google Scholar]
- 38. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. ; European Working Group on Sarcopenia in Older People Sarcopenia: European consensus on definition and diagnosis: report of the European working group on Sarcopenia in older people. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Graham JE, Fisher SR, Bergés IM, Kuo YF, Ostir GV. Walking speed threshold for classifying walking independence in hospitalized older adults. Phys Ther. 2010;90:1591–1597. doi: 10.2522/ptj.20100018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Deer RR, Goodlett SM, Fisher SR, et al. A randomized controlled pilot trial of interventions to improve functional recovery after hospitalization in older adults: feasibility and adherence. J Gerontol A Biol Sci Med Sci. 2018;73:187–193. doi: 10.1093/gerona/glx111 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.