Abstract
Objective
While schemas aid memory for schematically related information, the gist induced by the schema can also lead to high rates of false memories, especially in older adults. The neural mechanisms that support and differentiate true and false memories in aging are not well understood. The current study sought to clarify this, using a novel scene paradigm to investigate the role of schemas on true and false memories in older adults.
Methods
Healthy older adults encoded schematic scenes (e.g., bathroom). At retrieval, participants were tested on their memory for both schematic and nonschematic targets and lures while functional magnetic resonance imaging data was collected.
Results
Results indicate that true memories were supported by the typical retrieval network, and activity in this network was greater for true than false memories. Schema specific retrieval was supported by medial prefrontal cortex, extending this common finding to aging. While no region differentiated false memories compared to correct rejections, results showed that individual differences in false memory rates were associated with variability in neural activity.
Discussion
The findings underscore the importance of elucidating the neural basis of cognition within older adults, as well as the specific contribution of individual differences to the neural basis of memory errors in aging.
Keywords: False memory, functional magnetic resonance imaging, Schema memory
Schemas provide useful frameworks for organizing large amounts of information, which in turn facilitates memory encoding and retrieval (Alba & Hasher, 1983). The extraction of schematic gist helps guide attention during scene processing and facilitates memory for schema-consistent (i.e., schematic) information (Brewer & Treyens, 1981; Hess & Slaughter, 1990; Miller & Gazzaniga, 1998; Oliva & Torralba, 2006; Webb, Turney, & Dennis, 2016). Despite this, reliance on schemas can induce false memories for new, but schematically related, information (Lampinen, Copeland, & Neuschatz, 2001; Miller & Gazzaniga, 1998; Neuschatz, Lampinen, Preston, Hawkins, & Toglia, 2002; Pezdek, Whetstone, Reynolds, Askari, & Dougherty, 1989). This can be particularly problematic for older adults who show an overreliance on gist (Koutstaal & Schacter, 1997; Tun, Wingfield, Rosen, & Blanchard, 1998) and schema based processing in memory tasks (e.g., Castel, 2005; Hess & Slaughter, 1990; Waddell & Rogoff, 1981). Given this, the current study sought to examine the role of schematic gist in both true and false memories in older adults, as well as identify the neural correlates that support and differentiate each type of memory in aging.
While schemas can facilitate true memory for schematic information (Brewer & Treyens, 1981; Hess & Slaughter, 1990; Miller & Gazzaniga, 1998), the neural mechanisms of this processing advantage have only recently begun to be investigated (e.g., Aminoff, Schacter, & Bar, 2008; van Kesteren, Rijpkema, Ruiter, & Fernandez, 2010). For example, work by van Kesteren and colleagues (2010) in younger adults has suggested a role of ventral medial prefrontal cortex (mPFC) in contributing to successful encoding and retrieval of schematic information, due to the fact that it is consistent with prior knowledge. In addition, recent evidence from our lab showed higher rates of accurate retrieval of schematic compared to nonschematic information, and this memory advantage was supported by activity in occipital cortices and hippocampus in younger adults (Webb et al., 2016). Further, both true and false retrieval of schematic information was associated with neural processing in the lateral temporal cortices, a region associated with processing and retrieval of semantic gist (Simons et al., 2005; Wise & Price, 2006); however, this activation was greater for false memories. This suggests that false recognition of schematically related information arose primarily as a result of the gist shared between the schema and related lures. Because older adults show an increased reliance on schematic processing in memory tasks compared with younger adults (Hess & Slaughter, 1990; Koutstaal & Schacter, 1997; Tun et al., 1998), older adults’ false memories to schematic lures may also be reliant on gist-processing regions, such as the lateral temporal cortices.
While a handful of studies have examined false memories in aging, the majority of these studies have reported results in reference to activation differences between older and younger adults, or have examined neural activity that is common across true and false memories (e.g., Dennis, Bowman, & Peterson, 2014; Dennis, Kim, & Cabeza, 2008; Duarte, Graham, & Henson, 2010). As a result, they only provide a partial understanding of how true and false memories are represented within older adults themselves. These studies generally find that while true recognition in older adults is supported by activity in middle and medial prefrontal, medial temporal, and parieto-occipital regions (Dennis et al., 2008; Duarte et al., 2010), false recognition of new items is supported by activity in dorsal prefrontal, lateral temporal, and inferior parietal regions (Dennis et al., 2008; Duarte et al., 2010). Recent evidence from our lab also suggests that activity in middle and superior temporal gyri (MTG; STG) predicts false memory rates in older adults (Dennis, Bowman, & Vandekar, 2012; Dennis, Kim, & Cabeza, 2007; Dennis & Turney, 2018), with higher rates of false memories associated with increased activity in this region. This is thought to reflect the influence of gist-based processing leading to false recognition of related lures that share semantic or conceptual features with studied information (Dennis et al., 2007; Dennis et al., 2008; Dennis et al., 2014; Duarte et al., 2010; Webb et al., 2016). This result aligns with behavioral studies suggesting that an overreliance on gist processing contributes to increased rates of false memories in older adults.
Given that much of everyday memory is embedded within schemas (e.g., memory for a birthday party or a trip to the beach), it is important to understand if what has been observed in past research investigating semantic and perceptual false memories extends to more complex stimuli. In line with previous research, we expect that older adults will utilize the encoded schemas to support true memories, but also that the strong gist will lead to high rates of false memories for schematically related lures. We expect neuroimaging results to show that true memories are supported by neural activation in the medial temporal lobes (MTL) and frontal and parietal cortices, typically found to underlie accurate memories. These regions should exhibit greater activity for true compared to false memories, reflecting recapitulation and retrieval of item-specific details from encoding. Further, we expect that successful retrieval of the schema (compared to nonschematic items), as well as false recognition of schematically related lures should elicit activation in lateral temporal cortices as retrieval of the strong schematic gist should influence both memory processes. Critically, in line with previous false memory research in aging, we also expect that variability in the rate of false memory will play a critical role in modulating neural activity, specifically in the lateral temporal cortices. Results from the current study will help clarify the role of schemas in true and false memory in aging, as well as how neural activity is influenced by these processes within older adults.
Methods
Participants
Thirty right-handed native English speaking older adults from the State College community completed this experiment. Participants were screened for history of neurological disorders and psychiatric illness, alcoholism, drug abuse, and/or learning disabilities. Three older adults were excluded from the analysis due to head motion in excess of 4 mm and two were excluded for excessive atrophy. Five additional older adults were also excluded for poor behavioral performance (two for greater than 50% miss rate for schematic targets; three for a no response rate greater than 30%), leaving data from 20 participants (16 females; mean age = 74.55 years [SD = 7.21]). See Table 1 for complete participant demographics. As part of a secondary analysis regarding effects of age, the current results in older adults were examined in comparison with a previously published sample of younger adults who completed the same task. This sample included 22 younger adults (13 females; mean age = 22.91 years [SD = 3.01]). For complete results in younger adults, refer to Webb et al. (2016). All participants provided written informed consent and received financial compensation for their participation. All experimental procedures were approved by The Pennsylvania State University’s Institutional Review Board for the ethical treatment of human participants.
Table 1.
Participant Demographics and Behavioral Rates
| Older adults (N = 20) | Younger adults (N = 22) | |
|---|---|---|
| Participant Demographics | ||
| Age | 74.55 (7.21) | 22.91 (3.01) |
| Education (years) | 17.50 (1.82) | - |
| Cognitive assessment tasks | ||
| MMSE | 29.60 (0.60) | - |
| WAIS-III | ||
| Symbol Search | 13.70 (2.49) | - |
| Digit Symbol Coding | 12.85 (2.58) | - |
| Symbol Copy | 102.55 (23.22) | - |
| Digit Span | 11.95 (2.26) | - |
| Arithmetic | 11.25 (1.68) | - |
| Letter Number Sequencing | 11.53 (3.37) | - |
| Vocabulary | 14.10 (2.94) | - |
| GDS | 0.70 (1.08) | - |
| Memory Task—Retrieval Rates | ||
| All “Old” Responses | ||
| Related Hits | 0.70 (0.13) | 0.69 (0.11) |
| Unrelated Hits | 0.56 (0.15) | 0.54 (0.14) |
| Related False Alarms | 0.56 (0.17) | 0.54 (0.13) |
| Unrelated False Alarms | 0.26 (0.19) | 0.24 (0.17) |
Note: All numbers indicate means, with standard deviations in parentheses; all scores represent scaled scores with the exception of Symbol Copy; MMSE = Mini-Mental State Exam; GDS = Geriatric Depression Scale; Neither detailed demographic characteristics nor cognitive assessment measures were collected for the younger adult data reported in Webb et al. 2016.
Stimuli
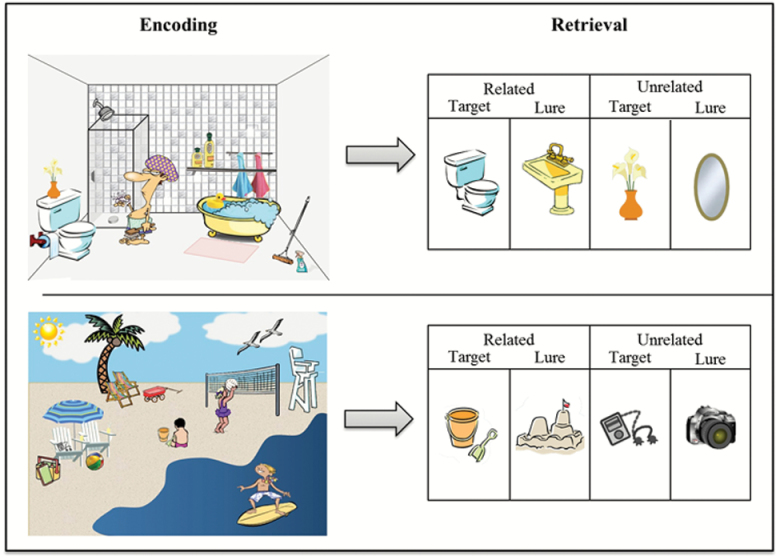
Stimuli consisted of 26 schematic scenes (e.g., Bathroom, Farm), comprised of objects commonly related to each schema (schematic targets: e.g., Farm: pig, barn; Bathroom: toilet, tub) as well as items unrelated to the schema (e.g., Farm: bush; flower pot; Bathroom: vase; spray bottle) (see Figure 1). Lures also consisted of both items commonly related to each schema (schematic lures: e.g., Farm: rooster, tractor; Bathroom: sink, plunger), as well as unrelated lures (e.g., purse, hammer). All backgrounds and images were obtained from an Internet image search. All items included in testing were normed for their association with each scene in a multi-step process (for details regarding norming and a list of all included items, see Webb et al., 2016). At encoding, 26 schematic scenes were presented focally in the same order for all participants. Three hundred individual items were presented one at a time at retrieval, including (a) 104 related targets (4 from each scene); (b) 62 unrelated targets (2–3 from each scene); (c) 104 related lures (4 associated with each scene); and (d) 30 unrelated lures.
Figure 1.
Schematic scene memory paradigm. During encoding, participants viewed schematic scenes (e.g., Bathroom, Beach). During retrieval, in the MRI scanner, participants made “Remember,” “Know,” or “New” responses to both schematically related targets (e.g., toilet, sand pail/shovel) and lures (e.g., sink, sandcastle), as well as unrelated targets (e.g., flower vase, music player) and lures (e.g., mirror, camera).
Task and Procedure
An intentional encoding task took place outside of the scanner during which participants were asked to look at each scene and try to remember as much as they could for a later memory task. The 26 scenes were presented for 10 s each across two runs (13 scenes per run). Retrieval occurred in the scanner during which individual items from the scenes were presented in the center of the screen. The order of stimulus presentation at both encoding and retrieval was the same for all participants. Behavioral responses were recorded on a four-button response box. Images were displayed by COGENT in MATLAB (Mathworks Inc., Natick, MA) and were displayed at a screen resolution of 1,024 (H) × 768 (V) at 75 Hz.
During retrieval participants completed six runs, each approximately 7 min in length. Each image was displayed for 3 s during which participants made memory responses using the “Remember/Know/New” paradigm. A jittered interstimulus interval (2–8 s) separated the presentation of each image. The images were pseudorandomly sorted, ensuring that no more than three images from any one trial type appeared in a row and no two images associated with a given scene appeared in a row. In accord with typical Remember/Know/New task instructions, participants were told to respond “Remember” if they could recollect specific details about the object such as its shape, color, placement in the scene or their thoughts or feelings during its initial presentation. Participants were told to respond “Know” if the picture looked familiar, but they could not recollect any specific details of its prior presentation. They were told to respond “New” if they believed the picture was not presented during the encoding session.
Image Acquisition
Structural and functional images were acquired using a Siemens 3T scanner equipped with a 12-channel head coil, parallel to the AC–PC plane. Structural images were acquired with a 1,650 ms TR, 2.03 ms TE, 256 mm field of view (FOV), 2562 matrix, 160 axial slices, and 1.0 mm slice thickness for each participant. Echo-planar functional images were acquired using a descending acquisition, 2,500 ms TR, 25 ms TE, 240 mm FOV, a 802 matrix, 90° flip angle, 42 axial slices with 3.0 mm slice thickness resulting in 3.0 mm isotropic voxels.
Image Processing
Preprocessing of all imaging data was carried out in SPM8 (Wellcome Institute of Cognitive Neurology, London, UK. www.fil.ion.ucl.ac.uk), using MATLAB (Mathworks Inc). The functional time series were first corrected for differences in slice timing acquisition. Functional images were then realigned to the first image using a six-parameter rigid body affine transformation and then spatially normalized to the standard Montreal Neurological Institute (MNI) template implemented in SPM8. To do this, the raw structural image was coregistered to the mean realigned functional image, and then this coregistered image was segmented and registered to the MNI template. Lastly, the parameters from this registration process were applied to the slice time corrected and realigned functional images (3 mm isotropic voxels) to normalize them to the MNI template, with coordinates later converted into Talairach space (Talairach & Tournoux, 1988) for reporting. As a final preprocessing step, all of the normalized functional images were smoothed using a 6 mm full-width-half-maximum Gaussian smoothing kernel.
Functional Magnetic Resonance Imaging Analyses
Trial-related activity was modeled at the first-level using the general linear model with a stick function corresponding to trial onset, convolved with a canonical hemodynamic response function. A second-level random effects model was created and one sample t-tests were conducted to investigate contrasts of interest. The current analyses focused on five trial types of interest: (a) Schematic Hits, which were defined as both “Remember” and “Know” responses to related targets; (b) Schematic Misses, which were defined as “New” responses to related targets; (c) Schematic False Alarms (FA), which were defined as both “Remember” and “Know” responses to related lures; (d) Schematic Correct Rejections (CR), which were defined as “New” responses to related lures; and (e) Nonschematic Hits, which were defined as both “Remember” and “Know” responses to unrelated targets. All other trial types, along with no response trials, were coded with their own regressors (as were movement parameters) and treated as regressors of no interest.
To examine effects of true and false memory in aging, we first examined neural regions that supported veridical and false schematic retrieval by contrasting schematic hits with schematic misses and schematic FA with schematic CR, respectively. Then, in order to identify neural resources that were differentially recruited for each type of retrieval response, we directly compared activity supporting schematic hits and FA, within regions that were significantly activated for each type of memory. In order to further characterize this activity, we compared the foregoing activation differences in older adults with that of younger adults using a 2 (age: old, young) × 2 (veracity: schematic hit, schematic FA) analysis of variance (ANOVA) on peak activity in the regions that were shown to differentiate veracity in older adults. Additionally, schematic memory effects in older adults were examined by contrasting schematic and nonschematic hit activity to identify regions that specifically contributed to accurate memory for the schema above and beyond that of general memory processes. Similar to above, age differences were assessed with a 2 (age: old, young) × 2 (schema type: schematic hit, nonschematic hit) repeated measures ANOVA on peak activity in the regions that were shown in older adults to support schematic compared to nonschematic memory.
Finally, individual difference analyses were conducted to identify neural activity associated with variability in older adults’ behavioral performance supporting both true and false schematic retrieval. Specifically, we regressed the contrast of schematic hits > misses on hit rates to schematic targets to identify regions in which behavioral performance predicted the magnitude of activation for true recognition. A similar analysis was conducted for false memories in which the schematic FA > CR contrast was regressed with false alarm rates to schematic lures in order to identify regions in which behavioral performance predicted the magnitude of activation for false recognition.
To identify significant results in our contrasts of interest, we employed Monte Carlo simulations as implemented by 3dClustSim in AFNI version 16.0 (Cox & Hyde, 1997), to determine activation that was corrected for multiple comparisons at p < .05. The input to this simulation was the search space [grey matter mask using the Wake Forest University aal pickatlas (Lancaster et al., 2000)], across-subject average intrinsic smoothness obtained from the residual time-series (12.0 mm), and the uncorrected p threshold (p < .005). An additional simulation was run to determine a correction specific to our a priori regions of interest (ROIs), including the MTG/STG and the MTL (hippocampus and parahippocampal gyrus), which were defined by using anatomical masks of each region (bilaterally) within the aal pickatlas toolbox in SPM8.
Results
Behavioral Results
A 2 (memory: hit, FA) × 2 (schema type: schematic, nonschematic) ANOVA calculated on “old” response rates (collapsed across “Remember” and “Know”) revealed significant main effects of both memory (F[1,19] = 104.72, p < .001) and schema type (F[1,19] = 78.89, p < .001), as well as a significant memory by schema type interaction (F[1,19] = 17.18, p < .001). Follow-up t-tests indicated that older adults made more hits (t[19] = 6.22, p < .001) as well as more FAs to schematic than nonschematic items (t[19] = 7.86, p < .001). It should be noted that while unrelated lures included both plausible and nonplausible items, nonschematic target were always plausible items that fit within the scene with which they were associated. In addition, older adults made more hits than FA to both schematic (t[19] = 6.90, p < .001) and nonschematic items (t[19] = 8.64, p < .001), indicating discriminability between both targets and lures for both schema types. Separate analyses with age showed no significant differences between age groups across any trial type (all ps > .05; see Table 1 for all means).
Neuroimaging Results
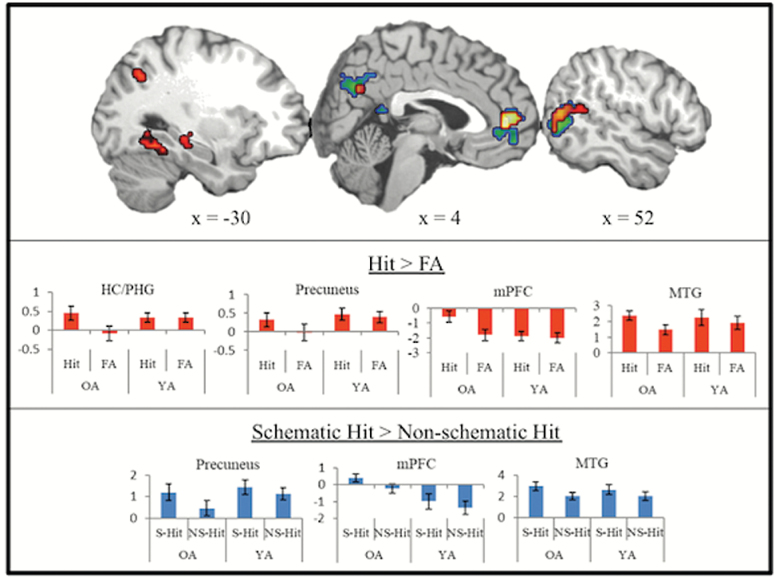
True schematic memories (schematic hits > misses) exhibited neural activity in the typical retrieval network including mPFC, bilateral hippocampus/parahippocampal gyrus, bilateral inferior parietal lobe, precuneus, fusiform gyrus, and right MTG. Furthermore, activity in frontal-partial regions, left hippocampus, right MTG, and left fusiform distinguished schematic hits from schematic FA (Figure 2; Table 2). Within all regions that distinguished true from false memory in older adults, significant interactions were observed between age and veracity (all Fs > 5, all ps < .05). Specifically, while older adults varied activity based on veracity, younger adults did not across all regions.
Figure 2.
Effect of schemas on true and false recognition. Activation map in red depicts regions showing greater activation for true (Hit) compared to false memory (FA) in regions masked by true retrieval (red) and activation map in blue depicts regions showing greater activation for schematic hits (S-Hit) compared to nonschematic hits (NS-Hit) in older adults. Bar graphs are included to indicate age differences within regions activated by older adults for each contrast (OA = older adults; YA = younger adults).
Table 2.
True Retrieval
| All hit vs miss | All hit vs all FA (masked with all hit vs miss) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Region | H | BA | Coordinates | t | mm3 | Coordinates | t | mm3 | ||||
| x | y | z | x | y | z | |||||||
| Medial Prefrontal Cortex | M | 32/10 | −6 | 57 | 1 | 7.37 | 2889 | −6 | 55 | 4 | 7.14 | 2,889 |
| Hippocampus | R | -- | 33 | −22 | −8 | 4.32 | 459 | |||||
| Hippocampus/Parahippocampal Gyrus | L | -- | −24 | −33 | −5 | 5.29 | 7776 | −30 | −30 | −5 | 3.98 | 810 |
| Parahippocampal Gyrus | R | 30 | 27 | −39 | −5 | 4.95 | 2430 | |||||
| Inferior Parietal Lobe | R | 40 | 42 | −40 | 39 | 6.30 | 2997 | |||||
| Middle Temporal Gyrus | R | 21 | 62 | −48 | 1 | 4.53 | 2916 | 53 | −64 | 12 | 4.86 | 1,296 |
| Precuneus/Cuneus | R | 7/31 | 12 | −61 | 32 | 5.50 | 5454 | 18 | −49 | 28 | 5.03 | 4,239 |
| Inferior Temporal Gyrus/Fusiform | L | 37 | −39 | −62 | −4 | 5.03 | 3267 | −36 | −48 | −14 | 6.79 | 2,511 |
| Inferior Parietal Lobe | L | 7 | −33 | −69 | 46 | 4.42 | 5643 | −39 | −57 | 40 | 4.35 | 891 |
Note: Italics represent a priori ROIs. FA = False Alarm; H = hemisphere (L = left; R = right; M = medial); BA = Brodmann’s area; x,y,z represent peak Talairach coordinates; t: statistical t value; mm3 represents voxel extent.
Neural activity supporting accurate retrieval of schematic compared to nonschematic items in older adults was identified in mPFC, precuneus, right MTG, and occipitotemporal cortex (Figure 2; Table 3). Age by schema type ANOVAs within each region revealed a main effect of schema, such that older and younger adults similarly showed greater activity in these regions for schematic compared to nonschematic hits (all Fs > 8, all ps < .05). Only activity in mPFC exhibited a main effect of age, such that older adults showed greater activation across both schematic and nonschematic hits compared to younger adults (F[1, 19] = 6.76, p <. 05). No interactions between age and schema type were identified.
Table 3.
Schema Specific Retrieval
| Schematic hit > Nonschematic hit | |||||||
|---|---|---|---|---|---|---|---|
| Region | H | BA | Coordinates | t | mm3 | ||
| x | y | z | |||||
| Medial Prefrontal Cortex | M | 10/11 | 3 | 54 | −12 | 4.04 | 2,727 |
| Precuneus | R | 31 | 12 | −61 | 29 | 4.23 | 4,536 |
| Middle Temporal Gyrus | R | 39 | 53 | −65 | 10 | 4.86 | 2,673 |
| Occipitotemporal Cortex | L | 19 | −48 | −73 | 10 | 6.74 | 2,052 |
Note: Italics represent a priori ROIs. FA = False Alarm; H = hemisphere (L = left; R = right; M = medial); BA = Brodmann’s area; x,y,z represent peak Talairach coordinates; t: statistical t value; mm3 represents voxel extent; No region showed greater activity for nonschematic compared to schematic hits.
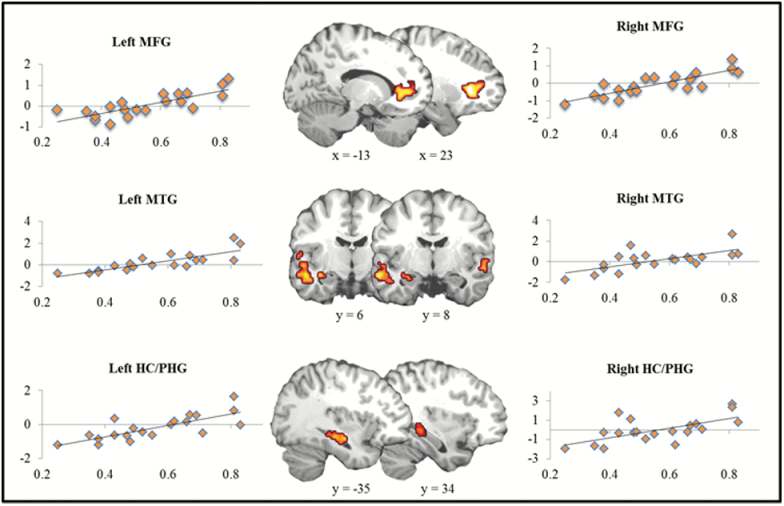
Using correct rejections as a baseline, false memory retrieval exhibited no distinguishing neural activity. However, when regressing false alarm rates with the foregoing analysis, results showed that neural activity in bilateral middle frontal gyrus, bilateral MTG, and bilateral hippocampus/parahippocampal gyrus increased as a function of false memory rate (Figure 3). For complete results, see Table 4. No significant results were identified for the true memory regression.
Figure 3.
Individual differences in false memory. Activation maps represent regions, including bilateral middle frontal gyrus (MFG), bilateral middle temporal gyrus (MTG), and bilateral hippocampus/parahippocampal gyrus (HC/PHG), where neural activity was correlated with false memory rate. X-axis represents beta values from neural signal. Y-axis represents percentage of related false alarms.
Table 4.
Individual Differences in False Retrieval
| Coordinates | |||||||
|---|---|---|---|---|---|---|---|
| Region | H | BA | x | y | z | t | mm3 |
| Middle Frontal Gyrus | L | 10/46/45 | −21 | 37 | 7 | 5.40 | 4,104 |
| R | 10/46/45 | 24 | 31 | 2 | 6.33 | 6,561 | |
| Middle Temporal Gyrus | L | 21 | −59 | −10 | −14 | 5.75 | 3,726 |
| R | 21 | 59 | −10 | −6 | 3.85 | 810 | |
| Hippocampus/Parahippocampal Gyrus | L | -- | −39 | −25 | −11 | 5.06 | 1,485 |
| R | -- | 33 | −33 | 0 | 3.52 | 837 | |
| Insula | R | 31 | 30 | −29 | 16 | 4.97 | 2,133 |
| Occipitotemporal/Fusiform | R | 37/19 | 24 | −54 | −4 | 3.99 | 2,835 |
| Middle Occipital Gyrus | L | 18 | −21 | −85 | 19 | 4.09 | 4,050 |
Note: Italics represent a priori ROIs. H = hemisphere (L = left; R = right); BA = Brodmann’s area; x,y,z represent Talairach coordinates; t: statistical t value; mm3 represents voxel extent.
Discussion
Consistent with past research investigating schematic memory, the schema supported veridical memory for schematically related information, leading to a higher hit rate for schematic items compared to nonschematic items. At the same time, the schematic gist induced by the scenes led to an increased rate of false memories for schematically related lures compared to unrelated lures. The imaging analyses showed that true memories were mediated by the typical retrieval network, including frontal-parietal and medial temporal regions, as well as the MTG, a region implicated in gist processing. Moreover, a comparison of schematic and nonschematic retrieval indicated that older adults also elicit ventral mPFC when retrieving schema specific information, extending a key finding in schema memory research in younger adults to aging. While false memories did not exhibit any unique recruitment compared to correct rejections, individual differences in rates of false memories were found to be a critical indicator of neural recruitment supporting these memory errors in aging, specifically within bilateral MTL and MTG. Each finding is discussed in turn below.
True Schematic Retrieval
True memory for schematic targets (schematic hits vs misses) exhibited activation throughout the typical retrieval network, including mPFC, bilateral hippocampus/PHG, bilateral inferior parietal lobe, right MTG and left fusiform gyrus (see Spaniol et al., 2009 for meta-analyis of retrieval activity in younger adults). The presence of this network in a group of older adults highlights its stability in supporting successful retrieval throughout aging. Activity in frontal-partial regions, left hippocampus, right MTG, and left fusiform distinguished hits from false alarms, indicating a stronger role of these retrieval-related regions in true compared to false memories in older adults (Figure 2). Frontal-parietal, and specifically dorsal PFC, activity has been shown to support memory monitoring and decision making, leading to successful recognition (e.g., Yonelinas, Otten, Shaw, & Rugg, 2005). The fact that these regions contributed to a greater degree to true versus false memories reflects the idea that demands may be greater for accurate compared to illusory memories in older adults. Interestingly, false memory studies in younger adults have often found the opposite pattern (i.e., greater frontal activation for false compared to true memories; Dennis et al., 2012; Kim & Cabeza, 2007; Okado & Stark, 2003), with prior studies attributing this pattern of activity to increased monitoring demands required when presented with highly related lures (Dennis et al., 2012; Kahn, Davachi, & Wagner, 2004). If one views the difference between true and false retrieval as reflecting differences in retrieval monitoring demands, then the fact that we observed greater frontal activity for true memories is consistent with the scaffolding theory of cognitive aging, wherein older adults engage frontal resources at an earlier level of difficulty compared to younger adults (Park & Reuter-Lorenz, 2009). A comparison with younger adults who completed the current task further supports this notion in that younger adults did not exhibit any effect of veracity on mPFC or parietal activation.
The comparison between schematic and nonschematic hits also indicated a role of the mPFC in supporting memory for schema specific information. While the loci of activation in the two analyses overlapped in extent, the activation elicited for schema specific memory extended out ventrally from the memory success cluster (see Figure 2). This is consistent with previous research in younger adults, which commonly finds that ventral mPFC is critical to integrating and retrieving schematic information (e.g., Aminoff et al., 2008; van Kesteren et al., 2010); whereas more dorsal mPFC, as mentioned above, is supportive of memory monitoring and decision making (Buckner & Wheeler, 2001; Yonelinas et al., 2005). Importantly, to our knowledge, this is the first study to indicate that ventral mPFC plays a specific role in supporting memory for schematic information in older adults. Moreover, our results showed that older adults recruited this region for schematic retrieval to an even greater degree than younger adults in order to support an equivalent level of memory for schematic targets. This may be indicative of the fact that older adults rely on schematic information to a greater degree in order to support accurate memory. Future research should aim to further delineate specific differences between general monitoring and schema specific prefrontal-based processes in both older and younger adults with the goal of clarifying the role of mPFC in schema memory across age.
In addition to regions supporting memory monitoring, older adults also elicited greater activity in memory-related medial temporal regions, specifically left posterior hippocampus/PHG, for true compared to false retrieval. While greater MTL for true compared to false memories is often observed in younger adults (for review see Dennis, Bowman, & Turney, 2015), it is not often reported in studies of older adults, likely due in part to the fact that past research has reported only age differences. The ability of the MTL to differentiate veracity of memories has been posited to reflect the retrieval of episodic details supporting veridical memories (Cabeza, Rao, Wagner, Mayer, & Schacter, 2001; Kahn et al., 2004; Okado & Stark, 2003). The current results are consistent with this interpretation, and suggest that the MTL maintains the ability to differentiate veridical from illusory retrieval throughout the life span. Furthermore, this appears to be the case regardless of the level of schema relatedness, as no differences in hippocampal activation were identified between schematic and nonschematic hits. Interestingly, the exact locus of this process in the MTL does not appear to be consistent across age. While younger adults exhibited differentiation of veracity in anterior hippocampus (Webb & Dennis, 2016), older adults exhibited this difference in a more posterior region, with younger showing no differentiation at this locus. This highlights the critical importance of examining subdivisions of the MTL when characterizing true and false memory retrieval in older and younger adults. Future research using more fine-grained high resolution and neuroimaging pattern analyses will be able to further characterize these delineations.
Reinstatement of encoding-related sensory details has also been shown to play a key role in differentiating between retrieval of veridical and false information (Dennis et al., 2012; Slotnick & Schacter, 2004). Aside from activity in left fusiform gyrus, older adults did not exhibit differential activation in occipital cortex supporting true memories. This differs from prior false memory studies in younger adults (Dennis et al., 2012; Slotnick & Schacter, 2004), including younger adults in this same paradigm (Webb et al., 2016), where early occipital cortex supported the distinction between true and false memories. The current results are, however, consistent with cognitive aging research showing reduced differentiation in occipital cortices (Goh, 2011) and support the conclusion that older adults do not experience recapitulation of sensory details during retrieval to support veridical memory in the same manner as is seen in younger adults. Given the fact that older and younger adults showed equivalent memory for schematic targets, this may indicate that older adults show a greater reliance on other processing resources, such as mPFC to support veridical memory.
Finally, right MTG was found to support veridical retrieval in older adults. This region, and specifically right-lateralized activity, has been noted in meta-analyses of subjective recollection (Spaniol et al., 2009), yet its role in relation to true retrieval has not been previously explored. As noted, past work in language and semantic memory has shown a role of lateral temporal gyri in semantic gist processing (Simons et al., 2005; Wise & Price, 2006). For example, STG and MTG have been shown to support integration of semantic information, and patients with damage to right MTG fail to comprehend the gist of stories or conversations (Jung-Beeman, 2005). Given that, in the current study, schematic gist is inherently part of the associations between items within the scenes, results indicate that activity in lateral temporal gyri may facilitate retrieval of the schema, and in turn, memory for the related target item (Webb et al., 2016). In fact, a largely overlapping region of the MTG (see Figure 2) was identified as supporting retrieval of schema specific (compared to unrelated) information, further highlighting the contribution of schematic gist to supporting successful retrieval in older adults. Moreover, younger adults did not differentially recruit this region based on veracity, suggesting that retrieval of schematic gist information may be more critical to supporting equivalent levels of accurate memory for the schema in aging.
False Schematic Retrieval
In addition to providing an opportunity to assess true memories, the current scene paradigm also allowed for the investigation of neural processes underlying schematic false memories. When using correct rejections as a baseline, no region exhibited greater activity for false memories (and thus no region differentiated false from true memories). The absence of such activity for older adults is not without precedent (Dennis et al., 2014; Dennis & Turney, 2018; Duarte et al., 2010; Gutchess, Ieuji, & Federmeier, 2007). We have previously suggested that the failure to observe neural activity that is greater for false memories is reflective of the idea that false retrieval does not engage the retrieval network to the same extent as true recognition (e.g., Dennis et al., 2012). Given the current results, we propose that this may be especially pronounced in older adults who traditionally show both high rates of false memories (Devitt & Schacter, 2016) and dedifferentiation in cognitive and neural processing (Goh, 2011). Previous work in our lab has shown that, unlike younger adults, older adults fail to differentially recruit retrieval-related regions to capitalize on the salient differences between target and lure stimuli (Bowman & Dennis, 2015), indicating that older adults process lures in a highly similar manner to targets. This is likely the case here where targets and lures share a strong conceptual gist, resulting in reduced differentiation based on subjective memory for the item. Given this, it is interesting to consider the fact that both older and younger adults made equal amounts of true and false memories; yet younger adults showed differential activity for false compared to true memories (see Webb, et al., 2016) and older adults did not. This again highlights the fact that memory networks contributing to equivalent levels of behavior may fundamentally differ between older and younger adults. This also provides further motivation for aging studies to focus on both within and between group comparisons to obtain a full picture of cognitive processing in older adults.
Unfortunately, we were not able to compare the neural correlates of schematic and nonschematic false alarms due to too few nonschematic false alarm trials. While this comparison would provide important insight into schema specific false alarm activity, false memories for unrelated items do not naturally occur in rates high enough to conduct neuroimaging analyses. Future work that can modulate degree of relatedness of lures to encoded information, such as in relation to a schema, would help address this limitation.
Nevertheless, our investigation of false memories in older adults did reveal significant modulation of neural activity when accounting for individual differences in the rate of false memories. This result is in accord with recent work from our lab (Dennis et al., 2007; Dennis et al., 2014; Dennis & Turney, 2018), indicating a significant relationship between individual differences in false memory performance and neural activation in older adults. In the current study, neural activity in bilateral middle frontal gyrus, bilateral MTG, and bilateral hippocampus/parahippocampal gyrus increased with respect to increases in false memory rate across individuals (Figure 3). As noted, the MTG and MTL have been posited to reflect divergent processes with respect to memory (i.e., retrieval of gist and episodic details, respectively). The fact that activation in both regions was associated with increased rates of false memories in older adults indicates that both processes play an important role in contributing to false memories in older adults.
Several previous studies have indicated that the MTL can play a role in supporting false memories, as individuals recollect the studied episode and/or studied details that, in turn, support the (incorrect) endorsement of related lures (Cabeza et al., 2001; Dennis et al., 2012). Such misattribution of studied details to lures has also been posited to underscore false memories in older adults (Koutstaal & Schacter, 1997). The current neural findings lend support to this position and further suggest that such processing may be a strong contributor to memory errors in older adults. Additionally, increased recruitment of the MTG in association with higher false memory rates has been observed in several previous studies (Dennis et al., 2007; Dennis et al., 2014; Dennis & Turney, 2018). Given that these previous studies have included both semantic and perceptually related stimuli, we have posited that such activity reflects the role of the lateral temporal cortex in gist processing beyond that of pure semantic gist (Dennis et al., 2014; Turney & Dennis, 2017). Given that the current paradigm utilized lures that were conceptually related to presented schemas (i.e., toilet plunger; bathroom), MTG activity supporting false memories likely reflects the shared schematic gist that encompasses both targets and lures. Increased engagement of this region appears to be detrimental to memory performance, contributing to consistently high rates of false memories in older adults. In line with this, suppression of gist influences, reflected by disengagement of MTG, may help older adults to avoid making high rates of false memories. A more comprehensive investigation of individual differences in false memories and associated neural correlates is needed to further elucidate these mechanisms.
Conclusions
The current results indicate that the schematic gist evoked by the scenes both supported and hindered older adults’ memory performance. In addition, the neuroimaging findings help to elucidate the mechanisms underlying both accurate and false memory for schematic information in aging. While true memories were supported by a general retrieval network, results indicated that, within the context of schematic false memories, neural mechanisms separating false retrieval from a correct rejection are minimal. Importantly, results continue to highlight the significance of interindividual differences in performance in accounting for false memory activation, specifically with respect to recruitment of lateral temporal gyri. As such, the results emphasize the strong role of gist processing in false memories across the life span.
Funding
This work was supported by a National Science Foundation grant (BCS1025709) awarded to NAD. C. E. Webb was partially supported by National Institute on Aging Grant T32 (AG049676) awarded to The Pennsylvania State University.
Conflict of Interest
None reported.
Acknowledgments
The authors wish to thank Courtney Allen for help with stimulus development and Kristina Peterson for assistance in data collection. The authors also wish to thank the Penn State Social, Life, & Engineering Sciences Imaging Center (SLEIC), 3T MRI Facility.
References
- Alba J. W., & Hasher L (1983). Is memory schematic?Psychological Bulletin, 93, 203–231. doi:10.1037/0033-2909.93.2.203 [Google Scholar]
- Aminoff E. Schacter D. L. & Bar M (2008). The cortical underpinnings of context-based memory distortion. Journal of Cognitive Neuroscience, 20, 2226–2237. doi:10.1162/jocn.2008.20156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman C. R. & Dennis N. A (2015). Age differences in the neural correlates of novelty processing: The effects of item-relatedness. Brain Research, 1612, 2–15. doi:10.1016/j.brainres.2014.08.006 [DOI] [PubMed] [Google Scholar]
- Brewer W. F., & Treyens J. C (1981). Role of schemata in memory for places. Cognitive Psychology, 13, 207–230. [Google Scholar]
- Buckner R. L. & Wheeler M. E (2001). The cognitive neuroscience of remembering. Nature reviews Neuroscience, 2, 624–634. doi:10.1038/35090048 [DOI] [PubMed] [Google Scholar]
- Cabeza R., Rao S. M., Wagner A. D., Mayer A. R., & Schacter D. L (2001). Can medial temporal lobe regions distinguish true from false? An event-related functional MRI study of veridical and illusory recognition memory. Proceedings of the National Academy of Sciences of the United States of America, 98, 4805–4810. doi:10.1073/pnas.081082698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castel A. D. (2005). Memory for grocery prices in younger and older adults: The role of schematic support. Psychology and Aging, 20, 718–721. doi:10.1037/0882-7974.20.4.718 [DOI] [PubMed] [Google Scholar]
- Cox R. W. & Hyde J. S (1997). Software tools for analysis and visualization of fMRI data. NMR in Biomedicine, 10, 171–178. doi:10.1002/(Sici)1099–1492(199706/08)10:4/5<171::Aid-Nbm453>3.0.Co;2-L [DOI] [PubMed] [Google Scholar]
- Dennis N. A. Bowman C. R. & Peterson K. M (2014). Age-related differences in the neural correlates mediating false recollection. Neurobiology of Aging, 35, 395–407. doi:10.1016/j.neurobiolaging.2013.08.019 [DOI] [PubMed] [Google Scholar]
- Dennis N. A., Bowman C. R., & Turney I. C (2015). Functional neuroimaging of false memories. In Addis D. R., Barense M., & Duarte A. (Eds.), The wiley handbook on the cognitive neuroscience of memory. Chichester, UK: John Wiley & Sons, Ltd. [Google Scholar]
- Dennis N. A. Bowman C. R. & Vandekar S. N (2012). True and phantom recollection: An fMRI investigation of similar and distinct neural correlates and connectivity. Neuroimage, 59, 2982–2993. doi:10.1016/j.neuroimage.2011.09.079 [DOI] [PubMed] [Google Scholar]
- Dennis N. A. Kim H. & Cabeza R (2008). Age-related differences in brain activity during true and false memory retrieval. Journal of Cognitive Neuroscience, 20, 1390–1402. doi:10.1162/jocn.2008.20096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis N. A., Kim H. K., & Cabeza R (2007). Effects of aging on the neural correlates of true and false memory formation. Neuropsychologia, 45, 3157–3166. doi:10.1016/j.neuropsychologia.2007.07.003 [DOI] [PubMed] [Google Scholar]
- Dennis N. A. & Turney I. C (2018). The influence of perceptual similarity and individual differences on false memories in aging. Neurobiology of Aging, 62, 221–230. doi:10.1016/j.neurobiolaging.2017.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devitt A. L. & Schacter D. L (2016). False memories with age: Neural and cognitive underpinnings. Neuropsychologia, 91, 346–359. doi:10.1016/j.neuropsychologia.2016.08.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte A. Graham K. S. & Henson R. N (2010). Age-related changes in neural activity associated with familiarity, recollection and false recognition. Neurobiology of Aging, 31, 1814–1830. doi:10.1016/j.neurobiolaging.2008.09.014 [DOI] [PubMed] [Google Scholar]
- Goh J. O. (2011). Functional dedifferentiation and altered connectivity in older adults: Neural accounts of cognitive aging. Aging and Disease, 2, 30–48. [PMC free article] [PubMed] [Google Scholar]
- Gutchess A. H. Ieuji Y. & Federmeier K. D (2007). Event-related potentials reveal age differences in the encoding and recognition of scenes. Journal of Cognitive Neuroscience, 19, 1089–1103. doi:10.1162/jocn.2007.19.7.1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess T. M., & Slaughter S. J (1990). Semantic knowledge influences on memory for scene information in young and older adults. Developmental Psychology, 26, 855–865. doi:10.1037/0012-1649.26.5.855 [Google Scholar]
- Jung-Beeman M. (2005). Bilateral brain processes for comprehending natural language. Trends in Cognitive Sciences, 9, 512–518. doi:10.1016/j.tics.2005.09.009 [DOI] [PubMed] [Google Scholar]
- Kahn I. Davachi L. & Wagner A. D (2004). Functional-neuroanatomic correlates of recollection: Implications for models of recognition memory. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 24, 4172–4180. doi:10.1523/JNEUROSCI.0624-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. & Cabeza R (2007). Trusting our memories: Dissociating the neural correlates of confidence in veridical versus illusory memories. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 27, 12190–12197. doi:10.1523/JNEUROSCI.3408-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutstaal W., & Schacter D. L (1997). Gist-based false recognition of pictures in older and younger adults. Journal of Memory & Language, 37, 555–583. doi:10.1006/jmla.1997.2529 [Google Scholar]
- Lampinen J. M. Copeland S. M. & Neuschatz J. S (2001). Recollections of things schematic: Room schemas revisited. Journal of Experimental Psychology. Learning, Memory, and Cognition, 27, 1211–1222. doi:10.1037//0278-7393.27.5.1211 [DOI] [PubMed] [Google Scholar]
- Lancaster J. L. Woldorff M. G. Parsons L. M. Liotti M. Freitas C. S. Rainey L.…Fox P. T (2000). Automated talairach atlas labels for functional brain mapping. Human Brain Mapping, 10, 120–131. doi:10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M. B. & Gazzaniga M. S (1998). Creating false memories for visual scenes. Neuropsychologia, 36, 513–520. doi:10.1016/S0028-3932(97)00148-6 [DOI] [PubMed] [Google Scholar]
- Neuschatz J. S., Lampinen J. M., Preston E. L., Hawkins E. R., & Toglia M. P (2002). The effect of memory schemata on memory and the phenomenological experience of naturalistic situations. Applied Cognitive Psychology, 16, 687–708. doi:10.1002/Acp.824 [Google Scholar]
- Okado Y. & Stark C (2003). Neural processing associated with true and false memory retrieval. Cognitive, Affective & Behavioral Neuroscience, 3, 323–334. doi:10.3758/CABN.3.4.323 [DOI] [PubMed] [Google Scholar]
- Oliva A., & Torralba A (2006). Building the gist of a scene: The role of global image features in recognition. Visual Perception, Pt 2: Fundamentals of Awareness: Multi-Sensory Integration and High-Order Perception, 155, 23–36. doi:10.1016/S0079-6123(06)55002-2 [DOI] [PubMed] [Google Scholar]
- Park D. C. & Reuter-Lorenz P (2009). The adaptive brain: Aging and neurocognitive scaffolding. Annual Review of Psychology, 60, 173–196. doi:10.1146/annurev.psych.59.103006.093656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezdek K., Whetstone T., Reynolds K., Askari N., & Dougherty T (1989). Memory for real-world scenes: The role of consistency with schema expectation. Journal of Experimental Psychology: Learning, Memory, and Cognition, 15, 587–595. doi:10.1037//0278-7393.15.4.587 [Google Scholar]
- Simons J. S. Verfaellie M. Hodges J. R. Lee A. C. Graham K. S. Koutstaal W.…Budson A. E (2005). Failing to get the gist: Reduced false recognition of semantic associates in semantic dementia. Neuropsychology, 19, 353–361. doi:10.1037/0894-4105.19.3.353 [DOI] [PubMed] [Google Scholar]
- Slotnick S. D. & Schacter D. L (2004). A sensory signature that distinguishes true from false memories. Nature Neuroscience, 7, 664–672. doi:10.1038/nn1252 [DOI] [PubMed] [Google Scholar]
- Spaniol J. Davidson P. S. Kim A. S. Han H. Moscovitch M. & Grady C. L (2009). Event-related fMRI studies of episodic encoding and retrieval: Meta-analyses using activation likelihood estimation. Neuropsychologia, 47, 1765–1779. doi:10.1016/j.neuropsychologia.2009.02.028 [DOI] [PubMed] [Google Scholar]
- Talairach J., & Tournoux P (1988). Co-planar stereotaxic atlas of the human brain. Stuttgart, Germany: Thieme. [Google Scholar]
- Tun P. A. Wingfield A. Rosen M. J. & Blanchard L (1998). Response latencies for false memories: Gist-based processes in normal aging. Psychology and Aging, 13, 230–241. doi:10.1037//0882-7974.13.2.230 [DOI] [PubMed] [Google Scholar]
- Turney I. C. & Dennis N. A (2017). Elucidating the neural correlates of related false memories using a systematic measure of perceptual relatedness. Neuroimage, 146, 940–950. doi:10.1016/j.neuroimage.2016.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kesteren M. T. Rijpkema M. Ruiter D. J. & Fernández G (2010). Retrieval of associative information congruent with prior knowledge is related to increased medial prefrontal activity and connectivity. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 30, 15888–15894. doi:10.1523/JNEUROSCI.2674-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddell K. J., & Rogoff B (1981). Effect of contextual organization on spatial memory of middle-aged and older women. Developmental Psychology, 17, 878–885. doi:10.1037/ 0012-1649.17.6.878 [Google Scholar]
- Webb C. E. Turney I. C. & Dennis N. A (2016). What’s the gist? The influence of schemas on the neural correlates underlying true and false memories. Neuropsychologia, 93, 61–75. doi:10.1016/j.neuropsychologia.2016.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise R. J. S., & Price C. J (2006). Functional imaging of language. In Cabeza R. & Kingstone A. (Eds.), Handbook of functional neuroimaging of cognition (2nd ed, pp. 191–228). Cambridge, MA: MIT Press. [Google Scholar]
- Yonelinas A. P. Otten L. J. Shaw K. N. & Rugg M. D (2005). Separating the brain regions involved in recollection and familiarity in recognition memory. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 25, 3002–3008. doi:10.1523/JNEUROSCI.5295-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]