Abstract
Background
Brief periods of physical inactivity can compromise muscle health. Increasing dietary protein intake is potentially beneficial but complicated by difficulties reconciling anabolic potential with a realistic food volume and energy intake. We sought to determine whether increasing dietary protein quality could reduce the negative effects of physical inactivity.
Methods
Twenty healthy, older men and women completed 7 days of bed rest followed by 5 days of rehabilitation. Volunteers consumed a mixed macronutrient diet (MIXED: N = 10; 68 ± 2 years; 1,722 ± 29 kcal/day; 0.97 ± 0.01 g protein/kg/day) or an isoenergetic, whey-augmented, higher protein quality diet (WHEY: N = 10; 69 ± 1 years; 1,706 ± 23 kcal/day; 0.90 ± 0.01 g protein/kg/day). Outcomes included body composition, blood glucose, insulin, and a battery of physical function tests.
Results
During bed rest, both groups experienced a 20% reduction in knee extension peak torque (p < .05). The WHEY diet partially protected leg lean mass (−1,035 vs. −680 ± 138 g, MIXED vs. WHEY; p = .08) and contributed to a greater loss of body fat (−90 vs. −233 ± 152 g, MIXED vs. WHEY; p < .05). Following rehabilitation, knee extension peak torque in the WHEY group fully recovered (−10.0 vs. 2.2 ± 4.1 Nm, MIXED vs. WHEY; p = .05). Blood glucose, insulin, aerobic capacity, and Short Physical Performance Battery (SPPB) changes were similar in both dietary conditions (p > .05).
Conclusions
Improving protein quality without increasing total energy intake has the potential to partially counter some of the negative effects of bed rest in older adults.
Keywords: Bed rest, Atrophy, Nutrition, Whey protein
The negative, catabolic effects of physical inactivity and bed rest have been well documented (1–4). When muscular disuse is accompanied by illness, injury, inadequate nutrition, or simply increased age, substantial muscle atrophy and functional impairment can occur in a matter of days (5,6). Without focused rehabilitation, this often results in prolonged or chronic disability (7,8).
We have previously argued that optimizing nutritional support during periods of catabolic crisis should be the cornerstone of efforts to preserve and optimize the restoration of muscle health (2,9). In clinical environments, physical activity and pharmaceutical interventions can clearly be beneficial, but may also be contraindicated, inefficient, or place a burden on patients and the health care delivery system (10,11). Although nutritional support can be provided almost universally, factors such as energy requirements and protein quality/quantity vary considerably and have the potential to positively and negatively influence health outcomes. For example, supplementation with large quantities of protein or amino acids provides a robust anabolic stimulus and can partially protect muscle mass and function during inactivity (9,12). However, a blunt increase in dietary protein and/or energy can also contribute to metabolic dysregulation and pose practical challenges (eg, satiety, cost) for clinical populations (13).
We recently demonstrated that consuming a whole-food, mixed animal- and plant-protein diet, which slightly exceeds the recommended dietary allowance for protein (ie, 0.9 g protein/kg body weight) during 7 days of bed rest, was unable to prevent the loss of knee extensor peak torque (−24 ± 4 Nm) or lean leg mass (−1.2 ± 0.1 kg) in middle age adults (9). However, supplementing the same mixed-meals with a small amount of leucine (~4 g per meal) partially protected lean mass and strength outcomes (9). While encouraging, we recognize that a more practical, food-focused strategy to improve dietary protein quality has the potential to benefit a broader selection of older adults.
The effect of leucine on skeletal muscle protein synthesis is well documented (14,15). Whey protein isolate is a high-quality protein with the highest proportion of leucine (12%) of all foods readily available to consumers (16). Whey can acutely stimulate muscle protein synthesis (17) and chronically improve nutritional status and health outcomes in clinical populations (18). Whey supplementation can also modestly enhance muscle hypertrophy and strength gains in many, but not all, exercise training interventions (19). Whey protein is low in lactose (<1%) (20) and has a relatively neutral taste profile that permits incorporation into many common meals and menus (21).
Our objective was to use bed rest to model the physical inactivity associated with hospitalization in older adults and to determine whether a whey protein-augmented, higher protein quality diet can (i) preserve lean mass and function and (ii) facilitate the recovery of functional and metabolic capacity during 5 days of rehabilitation.
Methods
Participants
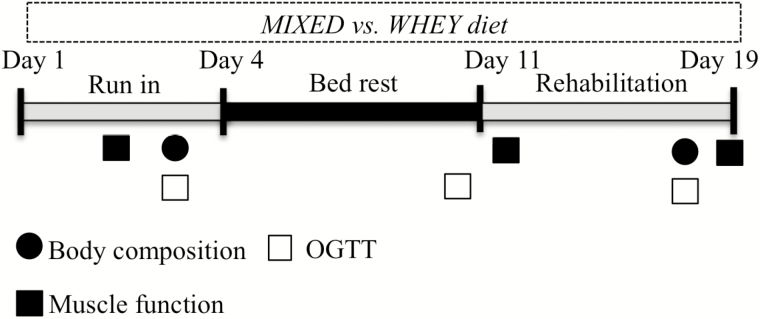
Twenty healthy, older adults of ages 60–80 were recruited, provided written informed consent, medically screened, and compensated for their time. All participants were generally healthy, modestly active with activities of daily living, but not engaged in formal exercise or athletic pursuits. The study protocol was conducted in accordance with the Declaration of Helsinki and approved by the University of Texas Medical Branch (UTMB) Institutional Review Board. The CONSORT diagram is presented in Supplementary Figure 1. Study sponsors were not involved in protocol development, data collection, analysis, or manuscript preparation. This study was registered at clinicaltrials.gov (NCT01846130). Volunteers were assigned to the MIXED or WHEY experimental conditions. All completed 7 days of horizontal bed rest and 5 days of rehabilitation in the Institute for Translational Sciences–Clinical Research Center (ITS-CRC). The general experimental design is depicted in Figure 1. The groups did not differ in age, weight, or height, although WHEY participants tended to have a higher body mass index (p = .056; Table 1).
Figure 1.
Study timeline. During the 7-d bed rest phase and 5-d rehabilitation phase, participants received either diet with 70% of protein from high-quality whey protein isolate (WHEY) or a control diet with a mixture of plant and animal protein (MIXED).
Table 1.
Baseline Participant Characteristics.
| Age (y) | Sex (M/F) | Height (cm) | Body Mass (kg) | BMI (kg/m2) | |
|---|---|---|---|---|---|
| MIXED | 68 ± 2 | 7/3 | 167 ± 1.8 | 72.1 ± 3.0 | 25.2 ± 0.7 |
| WHEY | 69 ± 1 | 5/5 | 166 ± 0.0 | 75.7 ± 2.9 | 27.4 ± 0.8 |
Notes: BMI = body mass index. Values are means ± SEM.
Bed Rest
Continuous participant monitoring, safety, and comfort provisions were consistent with our previous horizontal bed rest studies (9). All bathing and toiletry activities were performed without bearing weight.
Rehabilitation
Following bed rest, participants completed daily 45-minute bouts of supervised, progressive rehabilitation consisting of stretching and balance/strength-focused exercises. The intensity and duration of all rehabilitation exercises were standardized across all volunteers and are summarized in Supplementary Table 1.
Diet
The MIXED and WHEY groups consumed isoenergetic diets (55% carbohydrate, 29% fat, and 16% protein) with protein and energy intake evenly distributed across three daily meals (0800, 1300, and 1800; Table 2; Supplementary Table 4). Daily energy requirements were estimated using the Harris–Benedict equation with activity factors of 1.6 and 1.3 used for the ambulatory and bed rest period, respectively (9). Water was provided ad libitum.
Table 2.
Mean Daily Energy and Macronutrient Intake in Healthy Adults During 7 d of Bed Rest and 5 d of Rehabilitation
| Phase | Group | Energy (kcal) | Protein (g) | Protein (g/kg) | Carbohydrate (g) | Fat |
|---|---|---|---|---|---|---|
| Bed rest | MIXED | 1,722 ± 29 | 71 ± 1.2 | 0.97 ± 0.01 | 241 ± 4.1 | 56 ± 1.0 |
| WHEY | 1,706 ± 23 | 68 ± 1.0* | 0.90 ± 0.01* | 242 ± 3.7 | 56 ± 0.7 | |
| Rehabilitation | MIXED | 2,112 ± 37 | 86 ± 1.6 | 1.17 ± 0.01 | 296 ± 5.2 | 70 ± 1.4 |
| WHEY | 2,135 ± 28 | 80 ± 1.6* | 1.06 ± 0.02* | 1.06 ± 0.02* | 72 ± 1.3 |
Note: *Significantly different from MIXED group (p < .05).
The MIXED group consumed a diet containing protein from a variety of whole-food, plant, and animal sources (68 ± 1.2% animal; 32 ± 1.6% plant protein). In the WHEY cohort, whey protein isolate (BiPro, Agropur, Eden Prairie, MN) replaced some of the whole-food sources of protein (74 ± 1.0% animal; 26 ± 1.0% plant; see Supplementary Table 2).
On the mornings of the oral glucose tolerance tests (OGTT), the energy and macronutrient content of the breakfast was adjusted to account for the additional glucose load. Macronutrient intake and plate waste were analyzed by using Nutrition Data System for Research software (version 2011, Nutrition Coordinating Center, Minneapolis, MN).
Body Composition
Whole-body lean mass, leg lean mass, and whole-body fat mass were assessed using dual-energy x-ray absorptiometry (Lunar iDXA; GE Medical Systems, Madison, WI). In vivo precision errors for appendicular lean soft tissue mass range from approximately 1% to 3.0% (22). As part of our quality control process, a Body Composition Phantom was completed daily. Participants remained in a supine position for 10 minutes before each scan.
Muscle Function
Unilateral knee extensor peak torque was assessed using isokinetic dynamometry (Biodex System 4; Biodex Medical Systems, Shirley, NY). Familiarization sessions were conducted prior to admission. Peak isokinetic torque was assessed via three maximal repetitions at 60°/s.
Peak Aerobic Capacity
A graded exercise test on a cycle ergometer (Monark Ergomedic 828E; Monark Exercise, Vansbro, Sweden) and metabolic cart (VMax Encore 29; CareFusion, Yorba Linda, CA) was used to assess peak oxygen uptake. Data were expressed in absolute (L/min) and relative terms (mL/kg body mass/min and mL/kg lean mass/min) to account for potential changes in body composition during bed rest.
Short Physical Performance Battery
Participants completed a Short Physical Performance Battery (SPPB) to provide a clinically focused assessment of functional capacity. Participants were scored 0–4 based on their ability to complete each test (0 meaning the participant was unable to complete the task, and 4 indicates the highest level of function) using standardized cutoffs (23), and the score of each test was summed to calculate a composite SPPB score.
Oral Glucose Tolerance Test
Participants completed standard 2-hour OGTT, which included a 75-g glucose load (Glucola, Azer Scientific, Morgantown, PA). Whole-blood samples (0, 30, 60, 90, and 120 minutes) were analyzed on an YSI Bioanalyzer (YSI, Yellow Springs, OH). Serum insulin was measured using a commercially available enzyme-linked immunosorbent assay (MilliporeSigma, Burlington, MA). Glucose and insulin area under the curve were calculated using the trapezoidal method (24). Metabolic clearance rate was calculated based on work of Stumvoll and colleagues (25).
Statistical Analysis
Statistical analyses were performed using SPSS v24 software (IBM, Chicago, IL). Two-factor mixed ANOVA was used to analyze dependent variables with fixed effects of time (baseline/post-bed rest/post-rehabilitation time points) as the within-participant factor and diet (MIXED/WHEY) as the between-participant factor. For blood glucose OGTT statistical analysis, a three-way ANOVA of diet, time, and time point (0, 30, 60, 90, and 120 min) was performed. If the interaction of diet by time was significant (p < .05), individual post hoc tests with a Bonferroni adjustment were performed. Residual normality was tested using the Shapiro-Wilk test (p < .05), and Levene’s test of equality of error variance was used to check for equal variance. Data points greater than 2 SD from the average were excluded from the analysis to better meet model assumptions. All data are expressed as mean ± SEM; significance was set at p value less than .05.
Results
MIXED and WHEY participants consumed a similar quantity of carbohydrates and fat during bed rest and rehabilitation (Table 2). The WHEY group consumed slightly less protein than the MIXED group. In absolute terms, this represented a relatively trivial 3- to 6-g difference in total daily protein intake during bed rest and rehabilitation. Total branch chain amino acid intake was similar in both diets. However, the WHEY group consumed significantly more leucine at each meal (Table 3; Supplementary Table 5).
Table 3.
Mean Daily Branch Chain Amino Acid Intake in Healthy Adults During 7 d of Bed Rest and 4 d of Rehabilitation
| Phase | Group | Isoleucine (g) | Leucine (g) | Valine (g) |
|---|---|---|---|---|
| Bed rest | MIXED | 3.08 ± 0.07 | 5.15 ± 0.11 | 3.41 ± 0.07 |
| WHEY | 3.14 ± 0.04 | 5.93 ± 0.08* | 3.33 ± 0.05* | |
| Rehabilitation | MIXED | 3.76 ± 0.07 | 6.30 ± 0.11 | 4.15 ± 0.08 |
| WHEY | 3.71 ± 0.07 | 6.98 ± 0.14* | 3.94 ± 0.07* |
Note: *Significantly different from MIXED group (p < .05).
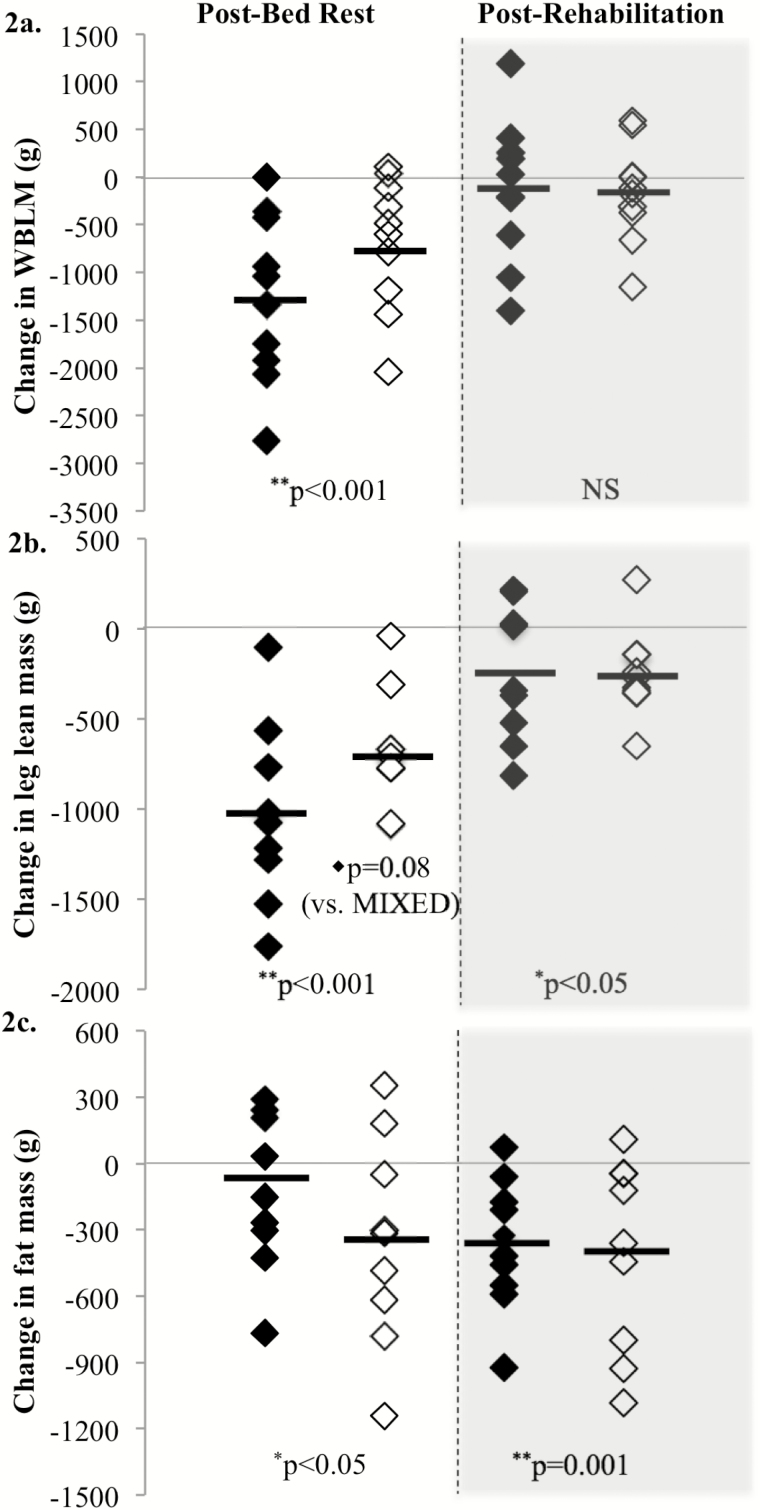
Bed rest resulted in a significant decrease in whole-body and leg lean mass (Figure 2). The WHEY diet demonstrated potential to partially protect leg lean mass (p = .08). Following rehabilitation, both the WHEY and MIXED groups had recovered some leg lean mass, but remained significantly below baseline (Figure 2).
Figure 2.
Body composition measurements were assessed with dual-energy x-ray absorptiometry on days 3 (baseline), 10 (post-BR), and 17 (post-Rehab) and are presented as change from baseline of each individual response. MIXED participants are showing in black diamonds, and the WHEY participants are in open diamonds. Whole-body lean mass was significantly decreased in both the MIXED and WHEY groups following bed rest (p < .001) but returned to baseline following rehabilitation (a). Leg lean mass was significantly decreased following disuse (p < .001) and was not yet fully restored following rehabilitation (p < .05; b). In addition, WHEY participants tended (p = .08) to lose less leg lean mass than participants in the MIXED group (b). Fat mass was significantly different from baseline following disuse (p < .05) and rehabilitation (p < .001; c).
Despite similar energy intakes, the WHEY group lost more whole-body fat mass than the MIXED group during bed rest (Figure 2c). At the end of the 5-day rehabilitation period, total fat loss was similar in both groups.
Bed rest resulted in a ~20% decrease in muscle strength (peak knee extension torque) in both groups. Following rehabilitation, strength in the WHEY group was fully recovered, whereas the decrement in the MIXED group persisted (Table 4).
Table 4.
Muscle Strength and Aerobic Capacity (Absolute and Relative to Body Mass) for MIXED and WHEY Measured at Baseline and the Change Following Bed Rest (ΔPost-BR) and Rehabilitation (ΔPost-Rehab)
| Knee Extensor Strength at 60°/s (Nm) | VO2 peak (L/min) | Relative VO2 peak (mL/kg/min) | |
|---|---|---|---|
| Baseline | |||
| MIXED | 133 ± 10.8 | 1.79 ± 0.16 | 24.5 ± 1.85 |
| WHEY | 106 ± 8.7 | 1.39 ± 0.08 | 18.7 ± 1.33† |
| Post-BR (Δ) | |||
| MIXED | −16.3 ± 5.5* | −0.12 ± 0.08 | −1.15 ± 0.95 |
| WHEY | −9.1 ± 5.5* | −0.09 ± 0.09 | −0.94 ± 1.06 |
| Post-Rehab (Δ) | |||
| MIXED | −10.0 ± 4.1 | −0.12 ± 0.07 | −1.19 ± 0.89 |
| WHEY | 2.2 ± 4.1† | −0.02 ± 0.07 | −0.20 ± 0.99 |
Notes: *Significant change from baseline (p < .05).
†Significantly different from MIXED group (p < .05).
The WHEY group had a lower aerobic capacity (relative VO2peak) than the MIXED group at baseline (Table 4). There was no effect of bed rest or rehabilitation on aerobic capacity in either group.
General functional status (SPPB) decreased in both groups following bed rest (Table 5). The change in scores was primarily the result of a decrease in chair rise time and gait speed scores, as balance remained unchanged. SPPB scores returned to baseline following rehabilitation.
Table 5.
Composite and Categorical Scores From Short Performance Physical Battery (SPPB) Testing Performed on Days 2 (Baseline), 12 (Post-BR), and 19 (Post-Rehab)
| SPPB | Balance | Chair Rise | Gait | |
|---|---|---|---|---|
| Baseline | ||||
| MIXED | 11.5 ± 0.2 | 3.8 ± 0.1 | 3.7 ± 0.5 | 4.0 ± 0.0 |
| WHEY | 10.4 ± 0.5 | 3.8 ± 0.1 | 2.9 ± 0.4 | 3.7 ± 0.3 |
| Post-BR | ||||
| MIXED | 10.5 ± 0.4* | 3.8 ± 0.1 | 3.1 ± 0.4* | 3.5 ± 0.3* |
| WHEY | 9.9 ± 0.6* | 3.7 ± 0.1 | 2.7 ± 0.4* | 3.5 ± 0.3* |
| Post-Rehab | ||||
| MIXED | 11.4 ± 0.3 | 3.9 ± 0.1 | 3.5 ± 0.3 | 4.0 ± 0.0 |
| WHEY | 10.8 ± 0.4 | 4.0 ± 0.0 | 3.2 ± 0.3 | 3.8 ± 0.2 |
Note: *Significant change from baseline (p < .05).
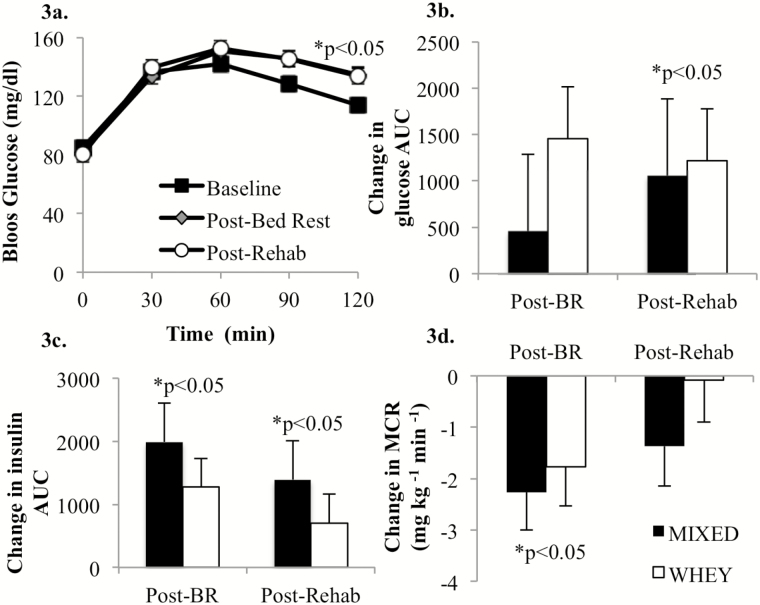
There was no impact of diet (MIXED vs. WHEY) on the blood glucose or insulin response to inactivity or rehabilitation. At both the post-bed rest and post-rehabilitation OGTT, the 120-minute blood glucose concentrations were significantly increased relative to the baseline (pre-bed rest) 120-minute value (Figure 3a). The OGTT area under the curve following bed rest was not different from baseline (Figure 3b). However, following rehabilitation, blood glucose area under the curve was significantly elevated (Figure 3b). The insulin area under the curve response to the OGTT was elevated following inactivity and rehabilitation (Figure 3c). Together, these data suggest a sustained insulin resistance initiated by inactivity, which is supported by significant decrease in metabolic clearance rate following 7 days of bed rest (Figure 3d).
Figure 3.
There was no effect of diet on blood glucose concentration response to the oral glucose tolerance test following disuse and rehabilitation (Time × Trt, p = .39), so for clarity of presentation, the groups were collapsed at each time point (a). Blood glucose concentration was characteristically elevated in response to the 75-g oral glucose challenge and was significantly elevated (p < .001) at 30, 60, 90, and 120 min compared with 0 min at baseline, post-bed rest, and post-rehabilitation (a). The 120-min glucose concentration following bed rest and rehabilitation was significantly higher than baseline (p < .05; a). Blood glucose area under the curve (AUC) did not change during bed rest (p = .36), but significantly increased following rehabilitation (p < .05; b). Insulin AUC was significantly increased (p < .05) following bed rest and remained elevated (p < .05) following rehabilitation (c). The metabolic clearance rate (MCR) significantly decreased during bed rest (p < .05), but was no longer significantly different from baseline following 7 d of rehabilitation (d).
Discussion
We have demonstrated that improving dietary protein quality has a modest protective effect on leg lean mass and promotes fat loss during short-term physical inactivity in older adults. During rehabilitation, a higher protein quality diet also contributed to an improved recovery of muscle strength.
Although individual patient populations are exposed to a variety of metabolic stressors, most experience a degree of physical inactivity and disuse atrophy (5,6). In this study, we recruited healthy older adults and subjected them to a controlled 7-day bed rest protocol to mimic this physical inactivity. This approach avoids the confounding catabolic influence of injury or illness while allowing for tight control of dietary intake.
Strategies to protect muscle health during inactivity can be broadly grouped into three categories: (i) nutrition, (ii) exercise/physical activity, and (iii) pharmacology. Previous clinical trials exploring these interventions, alone or in concert, demonstrate at least some potential to preserve lean mass, strength, physical function, and/or blood glucose regulation (9,26,27). Unfortunately, the clinical translation and implementation is easily compromised by feasibility, practicality, and even safety issues. For example, we recently reported that a 2,000 step/day intervention has only a modest protective effect on markers of muscle health in older adults during bed rest (26). Similarly, we have previously demonstrated that supplemental leucine has a moderate protective effect on markers of muscle health during bed rest over a relatively short (7 days) time period (9). However, palatability, accessibility, and cost could be barriers to widespread clinical use. In the present study, we did not perform a cost analysis or track objective markers of dietary/food preferences. However, dietary compliance was extremely high in both cohorts, with no palatability concerns.
The primary, novel outcome of this study was the greater loss of whole-body fat during bed rest in the WHEY group. This occurred despite a closely matched macronutrient profile and energy intake in the two experimental diets. Specifically, the MIXED group lost over 1 kg of whole-body lean mass, with minimal change in fat mass. This is consistent with body composition changes we have observed in previous bed rest studies with the same base dietary conditions (9,26). In contrast, the WHEY group lost approximately 50% less lean mass while experiencing increased fat loss. Although fat loss in some patient populations and circumstances may be undesirable (28), as an index of the overall change in body composition (ie, muscle-to-fat ratio), the WHEY cohort fared considerably better.
Protein supplementation is used extensively in exercise training studies (19). Although bed rest and disuse protocols are less common, several dietary/protein interventions have also been conducted. In each instance, protein was added to a standardized research diet, providing more protein and energy (9). The consequences of overfeeding in a critical care environment are well documented and bear little translational similarity to our bed rest study model (29). However, strategies that improve protein anabolic efficiency while avoiding the need for additional food/energy intake continue to have broad clinical applicability (30). Potential benefits include reduced meal costs, lower food/meal volume, improved satiety/hunger management, and improved metabolic-and-muscle health outcomes.
In this study, total protein intake (quantity) was similar in both diet conditions, although it trended lower in the WHEY group (0.90 vs. 0.97 ± 0.01 g protein/kg/day, MIXED vs. WHEY). This amount of protein consumed each day exceeded the recommended dietary allowance for protein, but fell short of the 1.2 g protein/kg/day recommendation for older adults and (noncritical) patient populations (31,32).
Protein quality is a function of the (i) amino acid composition, (ii) digestibility, (iii) amino acid bioavailability, and (iv) quantity of the limiting essential amino acid in a particular protein source (33). Several methods have been developed to assess protein quality and have been reviewed extensively (34,35). In broadest terms, animal-derived proteins have a higher protein quality score compared with most plant-based sources of protein (34). Whey protein isolate consistently ranks as one of the highest quality proteins. Examples of the macronutrient and amino acid profiles of the MIXED and WHEY diets are presented in Tables 2 and 3. Specifically, Table 3 demonstrates how whey protein was used to improve protein quality without overtly changing the total energy content or macronutrient profile.
A second novel and potentially clinically relevant finding was the improved recovery of muscle strength in the WHEY cohort following rehabilitation. During bed rest, both groups experienced a characteristic 20% reduction in peak knee extension torque (2,26,36). The ability to influence the recovery of muscle strength by manipulating dietary protein quality probably reflects the partial preservation of lean mass, but did not extend to other functional outcomes such as the SPPB and aerobic capacity.
Despite rigorous inclusion/exclusion criteria and standardized physical environment, our volunteers exhibited considerable heterogeneity in their responses to bed rest and study diets. This variability reduced study power and limited our ability to perform some comparisons (eg, male vs. female). Future examination of mechanisms and biomarkers differentiating “responders vs. non-responders” to disuse atrophy could have considerable clinical relevance. Other inherent limitations of our study design relate to the translation and implementation of the results. For example, our research model clearly cannot account for the various catabolic stimuli present in clinical populations. Similarly, the whey protein-augmented, research diet was not designed to be used in any real-world or clinical setting. Rather, the entire experimental model was developed to test a protein quality/inactivity hypothesis.
In conclusion, improving dietary protein quality without increasing total energy or protein intake has the potential to partially counter some of the negative effects of bed rest on body composition and may contribute to the recovery of muscle strength following rehabilitation.
Funding
The work was supported by the National Dairy Council, the Dairy Council of California, the National Institute of Nursing Research (NINR) at the National Institutes of Health (NIH) (R01 NR012973 to D.P.J.) and in part the Claude D. Pepper Older Americans Independence Center (P30 AG024832); and the National Center for Research Resources (1UL1RR029876). The study was conducted with the support of UTMB’s Institute for Translational Sciences, supported by Clinical and Translational Science Awards (UL1TR000071 and UL1TR001439) from the National Center for Advancing Translational Sciences.
Author Contributions
D.P.J. and E.A.L. designed the study. E.A.L., E.G., A.W., J.E., and D.P.J. participated in subject management, data collection, and sample analysis. E.A.L. and D.P.J. participated in data analysis and manuscript preparation. All authors read and approved the final manuscript.
Conflict of Interest
D.P.J. has participated on scientific advisory panels, provided educational seminars, and received travel reimbursements and honoraria from the American Egg Board, Leprino Foods, National Cattlemens Beef Association, National Dairy Council, Sabra Wellness and Nutrition, and the U.S. Dairy Export Council. E.A.L., E.G., J.E., and A.W. report no conflicts of interest.
Supplementary Material
Acknowledgments
We sincerely thank Glenda Blaskey, Rachel Deer, Syed Husaini, Adetutu Odejimi, Sneha Nagamma Jessica Spahn, Elena Volpi, and staff of the ITS-CRC for their assistance. We also thank Agropur for generously donating the whey protein isolate used in this study (BiPro, Agropur, Eden Prairie, MN).
References
- 1. Arentson-Lantz EJ, English KL, Paddon-Jones D, Fry CS. Fourteen days of bed rest induces a decline in satellite cell content and robust atrophy of skeletal muscle fibers in middle-aged adults. J Appl Physiol. 2016;120:965–975. doi: 10.1152/japplphysiol.00799.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. English KL, Paddon-Jones D. Protecting muscle mass and function in older adults during bed rest. Curr Opin Clin Nutr Metab Care. 2010;13:34–39. doi: 10.1097/MCO.0b013e328333aa66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. MacInnis MJ, McGlory C, Gibala MJ, Phillips SM. Investigating human skeletal muscle physiology with unilateral exercise models: when one limb is more powerful than two. Appl Physiol Nutr Metab. 2017;42:563–570. doi: 10.1139/apnm-2016-0645 [DOI] [PubMed] [Google Scholar]
- 4. Wall BT, Dirks ML, Snijders T, Senden JM, Dolmans J, van Loon LJ. Substantial skeletal muscle loss occurs during only 5 days of disuse. Acta Physiol (Oxf). 2014;210:600–611. doi: 10.1111/apha.12190 [DOI] [PubMed] [Google Scholar]
- 5. Baldwin C, van Kessel G, Phillips A, Johnston K. Accelerometry shows inpatients with acute medical or surgical conditions spend little time upright and are highly sedentary: systematic review. Phys Ther. 2017;97:1044–1065. doi: 10.1093/ptj/pzx076 [DOI] [PubMed] [Google Scholar]
- 6. McCullagh R, Dillon C, Dahly D, Horgan NF, Timmons S. Walking in hospital is associated with a shorter length of stay in older medical inpatients. Physiol Meas. 2016;37:1872–1884. doi: 10.1088/0967-3334/37/10/1872 [DOI] [PubMed] [Google Scholar]
- 7. Fisher SR, Goodwin JS, Protas EJ, et al. Ambulatory activity of older adults hospitalized with acute medical illness. J Am Geriatr Soc. 2011;59:91–95. doi: 10.1111/j.1532-5415.2010.03202.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Whyte J, Hart T. It’s more than a black box; it’s a Russian doll: defining rehabilitation treatments. Am J Phys Med Rehabil. 2003;82:639–652. doi: 10.1097/01.PHM.0000078200.61840.2D [DOI] [PubMed] [Google Scholar]
- 9. English KL, Mettler JA, Ellison JB, et al. Leucine partially protects muscle mass and function during bed rest in middle-aged adults. Am J Clin Nutr. 2016;103:465–473. doi: 10.3945/ajcn.115.112359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Morley JE. Undernutrition: a major problem in nursing homes. J Am Med Dir Assoc. 2011;12:243–246. doi: 10.1016/j.jamda.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 11. Rolland Y, Onder G, Morley JE, Gillette-Guyonet S, Abellan van Kan G, Vellas B. Current and future pharmacologic treatment of sarcopenia. Clin Geriatr Med. 2011;27:423–447. doi: 10.1016/j.cger.2011.03.008 [DOI] [PubMed] [Google Scholar]
- 12. Paddon-Jones D, Sheffield-Moore M, Urban RJ, et al. Essential amino acid and carbohydrate supplementation ameliorates muscle protein loss in humans during 28 days bedrest. J Clin Endocrinol Metab. 2004;89:4351–4358. doi: 10.1210/jc.2003-032159 [DOI] [PubMed] [Google Scholar]
- 13. Nieuwenhuizen WF, Weenen H, Rigby P, Hetherington MM. Older adults and patients in need of nutritional support: review of current treatment options and factors influencing nutritional intake. Clin Nutr. 2010;29:160–169. doi: 10.1016/j.clnu.2009.09.003 [DOI] [PubMed] [Google Scholar]
- 14. Casperson SL, Sheffield-Moore M, Hewlings SJ, Paddon-Jones D. Leucine supplementation chronically improves muscle protein synthesis in older adults consuming the RDA for protein. Clin Nutr. 2012;31:512–519. doi: 10.1016/j.clnu.2012.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. van Loon LJ. Leucine as a pharmaconutrient in health and disease. Curr Opin Clin Nutr Metab Care. 2011;1:71–7. doi: 10.1097/MCO.0b013e32834d617a [DOI] [PubMed] [Google Scholar]
- 16. Rutherfurd SM, Fanning AC, Miller BJ, Moughan PJ. Protein digestibility-corrected amino acid scores and digestible indispensable amino acid scores differentially describe protein quality in growing male rats. J Nutr. 2015;145:372–379. doi: 10.3945/jn.114.195438 [DOI] [PubMed] [Google Scholar]
- 17. Paddon-Jones D, Sheffield-Moore M, Katsanos CS, Zhang XJ, Wolfe RR. Differential stimulation of muscle protein synthesis in elderly humans following isocaloric ingestion of amino acids or whey protein. Exp Gerontol. 2006;41:215–219. doi: 10.1016/j.exger.2005.10.006 [DOI] [PubMed] [Google Scholar]
- 18. Rondanelli M, Klersy C, Terracol G, et al. Whey protein, amino acids, and vitamin D supplementation with physical activity increases fat-free mass and strength, functionality, and quality of life and decreases inflammation in sarcopenic elderly. Am J Clin Nutr. 2016;103:830–840. doi: 10.3945/ajcn.115.113357 [DOI] [PubMed] [Google Scholar]
- 19. Morton RW, Murphy KT, McKellar SR, et al. A systematic review, meta-analysis and meta-regression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults. Br J Sports Med. 2018;52:376–384. doi: 10.1136/bjsports-2017-097608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Morr CV, Ha EY. Whey protein concentrates and isolates: processing and functional properties. Crit Rev Food Sci Nutr. 1993;33:431–476. doi: 10.1080/10408399309527643 [DOI] [PubMed] [Google Scholar]
- 21. Carter BG, Drake MA. Invited review: the effects of processing parameters on the flavor of whey protein ingredients. J Dairy Sci. 2018;101:6691–6702. doi: 10.3168/jds.2018-14571 [DOI] [PubMed] [Google Scholar]
- 22. Hangartner TN, Warner S, Braillon P, Jankowski L, Shepherd J. The official positions of the international society for clinical densitometry: acquisition of dual-energy X-ray absorptiometry body composition and considerations regarding analysis and repeatability of measures. J Clin Densitom. 2013;16:520–536. doi: 10.1016/j.jocd.2013.08.007 [DOI] [PubMed] [Google Scholar]
- 23. Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. [DOI] [PubMed] [Google Scholar]
- 24. Tai MM. A mathematical model for the determination of total area under glucose tolerance and other metabolic curves. Diabetes Care. 1994;17:152–154. [DOI] [PubMed] [Google Scholar]
- 25. Stumvoll M, Mitrakou A, Pimenta W, et al. Use of the oral glucose tolerance test to assess insulin release and insulin sensitivity. Diabetes Care. 2000;23:295–301. [DOI] [PubMed] [Google Scholar]
- 26. Arentson-Lantz E, Galvan E, Wacher A, Fry CS, Paddon-Jones D. 2000Steps/day does not fully protect skeletal muscle health in older adults during bed rest. J Aging Phys Act. 2018;10:1–25. doi: 10.1123/japa.2018-0093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Deutz NE, Pereira SL, Hays NP, et al. Effect of β-hydroxy-β-methylbutyrate (HMB) on lean body mass during 10 days of bed rest in older adults. Clin Nutr. 2013;32:704–712. doi: 10.1016/j.clnu.2013.02.011 [DOI] [PubMed] [Google Scholar]
- 28. Bouillanne O, Dupont-Belmont C, Hay P, Hamon-Vilcot B, Cynober L, Aussel C. Fat mass protects hospitalized elderly persons against morbidity and mortality. Am J Clin Nutr. 2009;90:505–510. doi: 10.3945/ajcn.2009.27819 [DOI] [PubMed] [Google Scholar]
- 29. Hoffer LJ. High-protein hypocaloric nutrition for non-obese critically ill patients. Nutr Clin Pract. 2018;33:325–332. doi: 10.1002/ncp.10091 [DOI] [PubMed] [Google Scholar]
- 30. Paddon-Jones D, Coss-Bu JA, Morris CR, Phillips SM, Wernerman J. Variation in protein origin and utilization: research and clinical application. Nutr Clin Pract. 2017;32(suppl 1):48S–57S. doi: 10.1177/0884533617691244 [DOI] [PubMed] [Google Scholar]
- 31. Bauer J, Biolo G, Cederholm T, et al. Evidence-based recommendations for optimal dietary protein intake in older people: a position paper from the PROT-AGE Study Group. J Am Med Dir Assoc. 2013;14:542–559. doi: 10.1016/j.jamda.2013.05.021 [DOI] [PubMed] [Google Scholar]
- 32. Paddon-Jones D, Rasmussen BB. Dietary protein recommendations and the prevention of sarcopenia. Curr Opin Clin Nutr Metab Care. 2009;12:86–90. doi: 10.1097/MCO.0b013e32831cef8b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lee WT, Weisell R, Albert J, Tome D, Kurpad AV, Uauy R. Research approaches and methods for evaluating the protein quality of human foods proposed by an FAO expert working group in 2014. J Nutr. 2016;146:929–932. doi: 10.3945/jn.115.222109 [DOI] [PubMed] [Google Scholar]
- 34. Mathai JK, Liu Y, Stein HH. Values for digestible indispensable amino acid scores (DIAAS) for some dairy and plant proteins may better describe protein quality than values calculated using the concept for protein digestibility-corrected amino acid scores (PDCAAS). Br J Nutr. 2017;117:490–499. doi: 10.1017/S0007114517000125 [DOI] [PubMed] [Google Scholar]
- 35. Schaafsma G. Advantages and limitations of the protein digestibility-corrected amino acid score (PDCAAS) as a method for evaluating protein quality in human diets. Br J Nutr. 2012;108(suppl 2):S333–S336. doi: 10.1017/s0007114512002541 [DOI] [PubMed] [Google Scholar]
- 36. Dirks ML, Backx EM, Wall BT, Verdijk LB, van Loon LJ. May bed rest cause greater muscle loss than limb immobilization? Acta Physiol (Oxf). 2016;218:10–12. doi: 10.1111/apha.12699 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.