Abstract
Background
Gender equity in global health is a target of the Sustainable Development Goals and a requirement of just societies. Substantial progress has been made towards control and elimination of neglected tropical diseases (NTDs) via mass drug administration (MDA). However, little is known about whether MDA coverage is equitable. This study assesses the availability of gender-disaggregated data and whether systematic gender differences in MDA coverage exist.
Methods
Coverage data were analyzed for 4784 district-years in 16 countries from 2012 through 2016. The percentage of districts reporting gender-disaggregated data was calculated and male–female coverage compared.
Results
Reporting of gender-disaggregated coverage data improved from 32% of districts in 2012 to 90% in 2016. In 2016, median female coverage was 85.5% compared with 79.3% for males. Female coverage was higher than male coverage for all diseases. However, within-country differences exist, with 64 (3.3%) districts reporting male coverage >10 percentage points higher than female coverage.
Conclusions
Reporting of gender-disaggregated data is feasible. And NTD programs consistently achieve at least equal levels of coverage for women. Understanding gendered barriers to MDA for men and women remains a priority.
Keywords: equity, gender, mass drug administration, neglected tropical diseases, public health, Sustainable Development Goals
Introduction
Neglected tropical diseases (NTDs) and gender equity are both identified as priorities in the United Nations’ Sustainable Development Goals (SDGs). SDG 3 includes objectives to ‘end the epidemics of AIDS, tuberculosis, malaria and neglected tropical diseases’ (Target 3.3) and to ‘achieve universal health coverage, including…access to safe, effective, quality and affordable essential medicines…for all’ (Target 3.8).1 SDG 5 aims to ‘achieve gender equality and empower all women and girls.’1 The intersection of these two goals is the focus of this paper.
A focus on gender equity reflects society’s understanding that many causes of unequal outcomes result from remediable, unjust circumstances or a lack of attention to differences in biological susceptibility. Women, particularly those with lower incomes, face barriers to utilizing health care services including: the low status and priority of women’s health; lack of access to transport and financial resources; opportunity costs; being made to feel unwelcome in health facilities; and, in many settings, the lack of power women have to make decisions about their own health; also, gender may intersect with disability, ethnicity, or other sources of societal disempowerment.2–8 At the same time, a growing body of literature has identified gendered barriers to services for men, including social constructions of ‘idealized’ masculinity that constrain treatment-seeking and expectations of temporary migration for employment.9
Delivery of preventive chemotherapy (PC) to contribute to the control or elimination of five of the most common NTDs—lymphatic filariasis (LF), onchocerciasis (OV), schistosomiasis (SCH), trachoma (TR), and a group of three soil-transmitted helminths (STH)10—is one of the world’s largest public health programs. In 2016 alone, more than 1.4 billion treatments were delivered to more than 1 billion people, using drugs donated by pharmaceutical companies.11 Led by ministries of health (MOH), annual or biannual mass drug administration (MDA) of treatments to all eligible people at risk of disease is the backbone intervention strategy in endemic countries.10
Between 2002 and 2012, global health stakeholders increasingly emphasized the need for gender-disaggregated data and included the collection and use of gender-disaggregated data in best-practice recommendations and in their funding criteria.12–14 Policy, guidelines and programs began to require data that were disaggregated at a minimum by gender, and often by age and other pertinent social stratifications.14 The WHO’s Joint Reporting Form for NTDs, which is used by national MOHs to report their coverage for MDA for LF, OV, SCH and/or STH, and in support of their requests for annual donations of PC NTD drugs, has requested—but does not require—gender-disaggregation of aggregate treatment results since it was introduced in 2009.15
Nonetheless, little data are available about gender-specific coverage in MDA programs. A recent review found ‘some evidence that...in general, MDA program coverage is gender equal at the national-level...but may not hold when examined at sub-national levels.’ It specifically highlighted the lack of ‘[h]igh quality, comprehensive sex- and age-disaggregated data,’ which suggests that planners and implementers often may not be able to monitor gender equity.16 A lack of routinely disaggregated data is important because gendered barriers to PC may manifest differently in specific contexts and thus require locally designed interventions to address inequities. For example, one recent Indonesian study found that within-family gender dynamics influenced whether women accepted PC.17 Similarly, a Ugandan study found that different gendered barriers existed for men and for women. It found that men tend to miss PC because of occupational travel and that, in some instances, pregnant women are denied treatment because distributors hold erroneous safety concerns.18 In other settings, male drug distributors may not be able to administer treatment to women in the household.2
This paper presents analysis of data from MDA that administered 1.14 billion cumulative PC treatments from fiscal year (FY) 2012 through FY 2016 in 16 countries. The goal of this analysis was to determine whether PC for NTDs, conducted through MDA campaigns, reaches females and males equally. While ascertaining equity requires examining whether differences in experiences are unjust, we assume that consistent gender inequality in treatment would provide prima facie evidence of inequity resulting from societally influenced access or expectations.
We seek to answer two questions. First, to what extent do countries report gender-disaggregated data at the subnational level, which are necessary to identify inequality, probe for inequity, and design and implement responsive interventions? Second, to what extent do NTD programs achieve subnational gender-equality in MDA coverage?
Materials and methods
Source of data
We approached the MOHs of all 19 countries where MDA is supported by the United States Agency for International Development (USAID) through its projects ENVISION (managed by RTI International) and END Neglected Tropical Diseases in Africa (END in Africa) (managed by FHI 360) to use their data in this analysis. Sixteen countries granted permission: Benin, Burkina Faso, Ethiopia, Ghana, Guinea, Haiti, Indonesia, Mozambique, Nepal, Niger, Nigeria, Senegal, Sierra Leone, Tanzania, Togo and Uganda. Three countries did not grant permission and were excluded. We then examined district-level coverage data from the included countries for five USAID FYs: FY 2012 through FY 2016, which run from October to September. The diseases targeted via USAID-supported MDA varied for epidemiologic reasons and USAID priorities by country, district and year, but in all cases included one or more of the five major PC NTDs: LF, OV, SCH, TR and a group of three STH (ascaris, trichuris and hookworm).
Drug distributors, which include community volunteers, community health workers and/or teachers, collected and reported data using MOH-standard reporting. Depending on the country, the drug distributors may use tally sheets or registers, both of which are completed by hand. These are then aggregated, in paper or electronic format, through each administrative unit to the central-level MOH. The MOH shares these data with the local representatives of the USAID-supported project (either ENVISION or END in Africa) for entry into Microsoft Excel workbooks (Microsoft Corp., Redmond, WA, USA), which are then sent to the ENVISION project RTI International headquarters in Washington, DC, to be cleaned, incorporated into a database and reported to USAID. We received permission from each country’s MOH to disseminate their data and use it in this analysis. The data represent a complete accounting of MDA coverage in USAID-supported programs in the included countries.
Measures and analyses
We first assessed the availability of gender-disaggregated data. For the USAID-supported districts from each of the 16 countries, we conducted a district-level analysis to determine if treatment data had been reported separately for males and females. We calculated the overall percentage of districts reporting gender-specific data, as well as by disease, district and year. We checked reported gender-disaggregated data to make sure that they were not just a retrospective application of the fraction of the at-risk population expected to be each gender, but rather gender-specific treatment tallies. We did this by calculating the fraction of recipients that was female in each country-year; if the fraction did not vary (i.e. had a standard deviation of <1 percentage points within the country-year), MDAs were classified as non-disaggregated. A single year’s data for each of two countries were accordingly reclassified.
In analyses that combined MDA for multiple diseases, districts were counted as reporting gender-disaggregated data only if they reported it for all diseases treated with USAID funding that year. A district that conducted two rounds of MDA for one disease in the same FY was counted as reporting with gender-disaggregation for that disease, provided that at least one of those rounds was reported with gender-disaggregation.
We then measured whether there were differences in coverage by gender in each district that separately reported the numbers of males and females treated. To do so, we used data reported for MDA conducted in FY 2016 to calculate coverage for males and females because it was the most recent year for which data were available. If a district administered MDA twice within the year, both MDA campaigns were included in the analysis, as separate MDA episodes.
Our main outcome of interest was the gender-specific proportion of the at-risk population that received treatment via MDA. We estimated the gender-specific size of the at-risk population as follows. District population sizes were based on local estimates, as shared in Microsoft Excel workbooks. For SCH and STH, the at-risk population was school-aged children; for the other diseases, it was the total population. When using the total population, district-level gender ratios from the most recent national census projection, or from pre-MDA program censuses, were applied, based on the standard practice used by each country’s MOH. For school-aged children, the national gender ratio of youth aged 5–14 years from the most recent Demographic and Health Survey or Malaria Indicator Survey was used. Female population at-risk was then adjusted for LF and OV to exclude pregnant women.10 The number of women expected to be pregnant was estimated by applying the percentage of women pregnant in the country’s most recent Demographic and Health Survey or Malaria Indicator Survey.
Statistical analyses
We provide standard descriptive statistics. To describe the extent of gender-disaggregated data, we calculated the proportion of districts with disaggregated data, overall and stratified by year and country. We describe gender equality by calculating and displaying the median and interquartile range of districts’ coverage separately for men and women, overall and disaggregated. We then present the distribution of within-district differences in coverage by gender as the median difference and its interquartile range, analyzed separately by disease and country. Because analyses are conducted on the total universe of districts in included countries, we do not present measures of statistical uncertainty. We used SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) for analyses and R version 3.3.1 (R Foundation for Statistical Computing, Vienna, Austria) for figures.
This study analyzed only aggregate data from which individuals cannot be identified, so institutional review board approval was not required. We report this study following the STROBE guidelines.
Results
Availability of gender-disaggregated data
A total of 4784 district-years of treatment data from 16 countries were analyzed. In FY 2012, 32% of districts reported gender-disaggregated data (Table 1). By FY 2016, that had increased to 90%. By FY 2016, 11 of 16 countries (69%) reported gender-disaggregated data from all districts, a marked increase from 3 of 11 (27%) in FY 2012. All countries reported at least some disaggregated data. Two countries contradicted the general trend: Uganda, which held approximately constant at 70% in FY 2012 and 66% in FY 2016, and Niger, which dropped from 100% to 8% over that period.
Table 1.
Percentage of USAID-supported districts in each country that reported gender-disaggregated data for all diseases treated, by year
| FY 2012 | FY 2013 | FY 2014 | FY 2015 | FY 2016 | |
|---|---|---|---|---|---|
| % (n/N) | % (n/N) | % (n/N) | % (n/N) | % (n/N) | |
| Benin | – | 100 | 100 | 100 | 100 |
| (68/68) | (68/68) | (70/70) | (71/71) | ||
| Burkina Faso | 17 | 90 | 33 | 100 | 100 |
| (11/64) | (47/52) | (20/61) | (44/44) | (64/64) | |
| Ethiopia | – | 100 | – | 66 | 100 |
| (70/70) | (57/86) | (234/234) | |||
| Ghana | 0 | 38 | 100 | 87 | 74 |
| (0/188) | (52/137) | (188/188) | (136/157) | (151/205) | |
| Guinea | 100 | 100 | 100 | 100 | 100 |
| (7/7) | (2/2) | (11/11) | (12/12) | (16/16) | |
| Haiti | 0 | 100 | 100 | 100 | 74 |
| (0/106) | (106/106) | (97/97) | (56/56) | (17/23) | |
| Indonesia | 52 | 100 | 100 | 100 | 100 |
| (14/27) | (36/36) | (38/38) | (50/50) | (51/51) | |
| Mozambique | – | 100 | 100 | 100 | 100 |
| (10/10) | (21/21) | (22/22) | (24/24) | ||
| Nepal | 0 | 23 | 100 | 100 | 100 |
| (0/46) | (13/56) | (41/41) | (18/18) | (18/18) | |
| Niger | 100 | 100 | 100 | 0 | 8 |
| (65/65) | (2/2) | (55/55) | (0/57) | (4/53) | |
| Nigeria | – | 76 | 79 | 100 | 100 |
| (94/124) | (110/139) | (135/135) | (185/185) | ||
| Senegal | – | 95 | 100 | 100 | 100 |
| (60/63) | (76/76) | (76/76) | (54/54) | ||
| Sierra Leone | 100 | 14 | 100 | 100 | 100 |
| (14/14) | (2/14) | (12/12) | (12/12) | (14/14) | |
| Tanzania | 76 | 81 | 100 | 100 | 99 |
| (65/86) | (78/96) | (54/54) | (80/80) | (126/127) | |
| Togo | 0 | 100 | 100 | 100 | 100 |
| (0/30) | (35/35) | (4/4) | (35/35) | (35/35) | |
| Uganda | 70 | 81 | 85 | 69 | 66 |
| (47/67) | (55/68) | (66/78) | (47/68) | (33/50) | |
| Total | 32 | 78 | 91 | 87 | 90 |
| (223/700) | (730/939) | (861/943) | (850/978) | (1097/1224) |
Gender-specific coverage levels
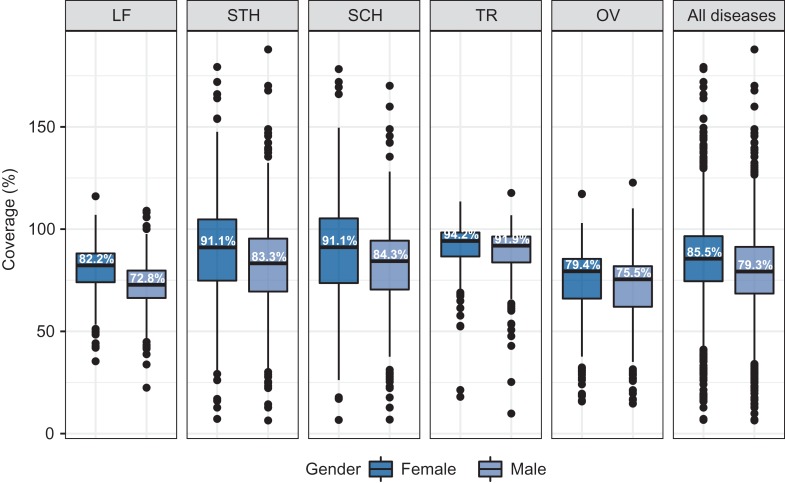
For FY 2016, median male-specific coverage for all districts reporting disaggregated data was 79.3% combining MDA for all diseases, while it was 85.5% for females (Figure 1). The median district’s coverage was slightly higher for females than males for all diseases, with the difference ranging from 9.4 percentage points for LF to 2.3 percentage points for TR. Coverage exceeded 100% of the estimated target population in 13% of instances, suggesting occasional imprecision in the target population estimates or coverage that includes non-target populations.
Figure 1.
Distribution of district level coverage by disease and gender in FY 2016. Boxes represent the median and interquartile range, whiskers represent observations within 1.5 times the interquartile range from the first or third quartile, and dots represent observations outside that range. Percentages denote the median value. FY, fiscal year; LF, lymphatic filariasis; STH, soil-transmitted helminths; SCH, schistosomiasis; TR, trachoma; OV, onchocerciasis.
Within-district differences in coverage demonstrate a similar pattern (Figure 2). The median difference in coverage in FY 2016 ranged from 2.6 percentage points higher among females for OV to 6.7 percentage points for STH. Among the 1915 MDA episodes conducted in districts for which disaggregated data were available, 64 (3.3%) produced male coverage >10 percentage points higher than female coverage. Female coverage was >10 percentage points higher for 492 (25.7%) MDAs. A ≥10 percentage points difference favoring males was most common for SCH (7%); for females, it was STH (34%).
Figure 2.
Distribution of within-district gender differences in coverage by disease, FY 2016. FY, fiscal year; LF, lymphatic filariasis; STH, soil-transmitted helminths; SCH, schistosomiasis; TR, trachoma; OV, onchocerciasis.
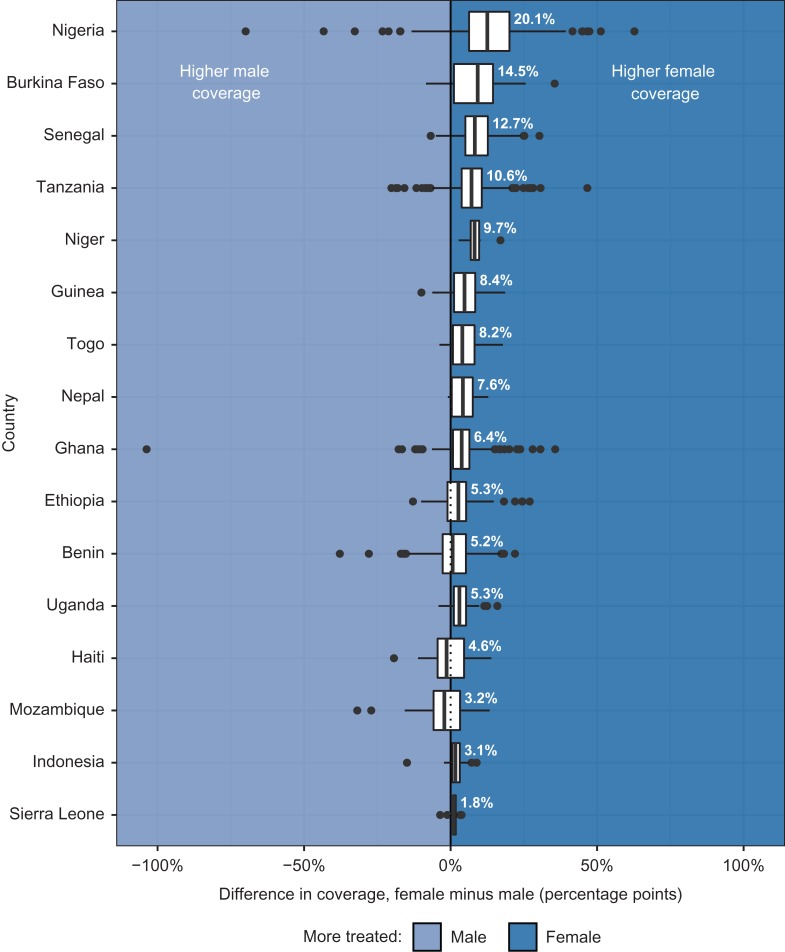
By country
Differences in district-level MDA coverage tended towards females in 14 of 16 countries (Figure 3). Female coverage was higher than male coverage by >10 percentage points in Nigeria (12.5) and >5 percentage points in Burkina Faso (9.2), Senegal (8.3), Niger (8.2) and Tanzania (7.1). Median differences tended towards men in Mozambique (2.1 percentage points) and Haiti (1.4 percentage points).
Figure 3.
Distribution of within-district gender differences in coverage by country, FY 2016. FY, fiscal year.
When districts had differences in coverage of at least 10 percentage points between males and females, female coverage was usually higher. Only Benin had multiple diseases for which coverage favored men by 10 percentage points in more districts than women: SCH (26% vs 9% of districts) and STH (25% vs 13% of districts). Differences in coverage for LF of at least 10 percentage points favored men slightly in two countries, Haiti (12% of districts were at least 10 percentage points higher for men, compared with 6% of districts for women) and Indonesia (2% vs 0% of districts). In Mozambique, more districts’ TR coverage favored men by at least 10 percentage points than women (17% vs 13% of districts). Discrepancies of >10 percentage points for all other countries and diseases either balanced across genders or were more common among females.
Discussion
Using an extensive programmatic dataset, we identified two principal findings. First, the majority of countries included in our analysis present gender-disaggregated MDA coverage, and such reporting increased over time. Second, we find no evidence that females are systematically disadvantaged in MDA coverage in places where disaggregated data are available; in fact, men are missed more often than women. Understanding gendered barriers to MDA coverage will be important for completing the remaining steps to NTD elimination.
Availability of gender-disaggregated data
Our results demonstrate that collecting and reporting gender-disaggregated MDA data is feasible with the proper support and incentives. The trend towards greater disaggregated reporting was likely driven by several factors, including WHO recommendations and, especially, USAID policies requiring funded projects to ‘collect and use sex-disaggregated data.’19,20 A principal catalyst was USAID’s 2012 request for disaggregated reporting, which supplanted its earlier approach to NTD program support. Other donors also have strategies that require sex-disaggregated reporting in line with the SDGs to ensure equity of access. Requesting disaggregated reporting should be the standard for all national programs and funders.
Transitioning to reporting gender-disaggregated data required both national leadership and the support of partners. Data collection forms (e.g. tally sheets and registers) had to be revised and reprinted, and data collection and compilation procedures had to be changed at each level (distribution, district, regional and central) of the health system. This required initial training, refresher training in many cases, and more intensive field supervision during the first year of disaggregated collection. It also required concerted collaboration among countries and their technical and financing partners. The authors observed that during the transition to disaggregated reporting, MOHs reported several challenges, including the time burden placed on distributors, greater complexity to forms, requests for additional compensation, and complications arising from other simultaneous changes to data systems (such as, in Haiti, the concurrent adoption of age disaggregation in STH data which caused confusion among some distributors). On the other hand, some programs reported better data quality and easier quality assurance because data were disaggregated.
As illustrated by a few countries in this dataset, gender-disaggregated data are, however, still not universally reported, and practice within countries can vary by year. Reasons reported by program managers—who are the authors of this article—include the additional burden of gender-disaggregated reporting for drug distributors and for health workers at all levels; time constraints in meeting the WHO’s deadlines for drug applications and partner reporting deadlines; changes in partner support (e.g. if a previous partner did not prioritize gender-disaggregated reporting); whether MOHs mandate disaggregated reporting; and, in long-running NTD programs, a developed habit of reporting data without disaggregation (an especially acute challenge in programs that are nearing MDA completion and thus will only use new systems a few times) is harder to change.
Gender equality in utilization of PC services
In the 16 countries represented here, female coverage is generally higher than male, a finding that is consistent with limited existing evidence.18 Considering the systematic disempowerment women encounter relative to men in many settings, this is an important accomplishment for which national programs and their partners deserve credit. We suspect that it is partially due to the community-based nature of MDA being able to overcome differential access to transportation, resources and power, all of which may cause women to have poorer access to treatment in other settings.21
At the same time, we note that a significant number of MDAs—more than a quarter—resulted in male coverage which was lower by at least 10 percentage points. For virtually all diseases and countries, male coverage lagged behind female coverage. This suggests that gendered barriers to MDA participation likely impede full coverage, and these barriers may be context-specific and require the incorporation of location-specific knowledge. Prior research, and feedback from programs involved in this study, have suggested that men may be less likely to participate in MDA, in part due to lack of access when occupational travel draws them away from MDA sites.18
Pregnant women are sometimes formally or informally excluded from certain types of MDA for which they are eligible under WHO guidelines. The WHO encourages inclusion of pregnant and lactating women in SCH MDA, yet in practice many national programs still do not target them for treatment, either because of perceived risks or because much SCH MDA is targeted at school-age children and/or uses school-based platforms to administer the drugs.10,22 One country, Mozambique, had slightly lower female coverage because pregnant women, although eligible as per WHO guidelines, are ineligible for TR MDA under national guidelines because of concerns that pregnancy complications after MDA could cause public fears and undermine treatment campaigns. Nonetheless, we included pregnant women in our denominator for MDA for TR, because pregnancy is not a medical contraindication for treatment.
Although women do not encounter systematically lower coverage, reasons for lower coverage in specific districts should be investigated. Additionally, even when coverage levels are equal, women may still face relative disadvantage. First, in some settings, social dynamics drive higher prevalence for certain NTDs in females, such as TR and related blindness (because of their greater contact with children, who harbor the highest level of infection)23 or SCH (because of more common contact with water in some communities).24 Second, the biological consequences of infection are sometimes more severe for women, such as helminth-caused anemia during pregnancy25 or genital SCH infection and with it greater susceptibility to HIV infection.26 Third, the social consequences of infection may fall differently on men and women, such as devaluation because of physical disfigurement.27,28 When the prevalence or consequences of infection fall more heavily on women, equal MDA coverage may not fully remediate differential harm.
Our findings underscore the feasibility of collecting gender-disaggregated MDA data and that MDA programs generally achieve at least equal coverage for women. At the same time, achieving disease elimination will require a better understanding of gendered reasons for men’s lower coverage. This, as well as a fuller understanding of why some specific districts have large gender imbalances in coverage, will require both researchers and program implementers to understand specific gendered contextual factors that influence MDA success. Further, qualitative analysis would enable a better understanding of whether, how, and to what benefit countries have used their gender-disaggregated MDA data to strengthen their programs.
Limitations
This study has several limitations. First, we do not have complete data from all countries with PC NTD programs, and in the countries included in the study we use data only for districts supported by USAID. The sample of countries and districts was selected for convenience, based on access to treatment data. For these reasons, we cannot assess the extent to which our results generalize to programs that are not supported by USAID.
Second, all data are programmatic, which entails more messiness than data collected for research purposes. Denominators are based on the estimated size of populations at risk in each district, and there is error in these estimates (resulting, for example, in estimated coverage >100% in some districts). Some countries reported data quality challenges in areas of instability or frequent population migration. However, we have no expectation that error is systematically related to gender or otherwise creates differential bias in our estimates. Similarly, numerators are calculated from routinely collected programmatic data. While past studies have found such data to be reasonably accurate,29 errors are inevitable. However, we have no reason to expect bias from errors being correlated with gender.
Third, our method for identifying gender-disaggregated data was reported using a formula that has yet to be validated and needs further testing.
Fourth, we are not able to investigate specific subpopulations. For example, without age-by-gender-disaggregated data, we cannot examine whether girls and adolescent women are disadvantaged by lower school attendance rates in some jurisdictions that employ school-based MDA. Similarly, without pregnancy data, we cannot investigate whether pregnant women are systematically underreached.18 Finally, we cannot investigate intersectional marginalization—for example, interactions between gender and ethnic minority status—without further disaggregated data. Qualitative inquiry on intersectional marginalization would be valuable.
Fifth, while in most settings distributors directly observe treatment, so coverage and treatment are approximately identical, in some countries treatment is provided to households but not directly observed. In such settings, prior research has found that within-household and societal gender relations can increase the risk that women who receive PC do not ingest the drugs.17,30 We are not able to determine whether this is the case from programmatic coverage, but future research using coverage surveys will be able to investigate this.
Acknowledgements
We wish to acknowledge the Ministries of Health of Benin, Burkina Faso, Ethiopia, Ghana, Guinea, Haiti, Indonesia, Mozambique, Nepal, Niger, Nigeria, Senegal, Sierra Leone, Tanzania, Togo and Uganda for their efforts to control and eliminate NTDs, and for permission to share these data and results. Ethiopia’s Federal MOH would also like to acknowledge the support of the Children’s Investment Fund Foundation (CIFF), The End Neglected Tropical Diseases Fund (END Fund) and Schistosomiasis Control Initiative for other aspects of its NTD program. We thank Lisa Ghaffari for support organizing and preparing citations. We thank the in-country and home office teams of RTI International, FHI 360, Health & Development International, Helen Keller International, IMA WorldHealth, Light for the World, The Carter Center and The Fred Hollows Foundation for their work liaising with MOHs and reviewing the data.
Disclaimer
The authors’ views expressed in this publication do not necessarily reflect the views of the US Agency for International Development or the United States Government.
Authors’ contributions
DAC, MPK, KB and MCB designed the study. WEB, FD, NN, BM, AG, JFL, SG, MM, PR, IG, INA, MN, YMB, UJM, MSA and EMT lead the national NTD programs and were responsible for data collection in this study. LAR, BP and JBK supported data collection, analysis and interpretation. MPK led the data analysis, supported by DAC, KB, KLZ, JDK and MCB, who also contributed to data interpretation. MPK and RDS prepared the figures and MPK prepared the table. DAC was responsible for writing the manuscript with support from JDK and PCM. All authors reviewed drafts of the manuscript, read the final version of the manuscript, provided final approval of the manuscript and agree to be accountable for the work.
Funding
This work was supported by the United States Agency for International Development through its projects ENVISION, led by RTI International under [cooperative agreement number AID-OAA-A-11-00048], and End Neglected Tropical Diseases in Africa (END in Africa), led by FHI 360 under [cooperative agreement number AID-OAA-A-10-00050]. JDK was a fellow at RTI International. The funder had no role in project design, in project implementation, in analysis or interpretation of this manuscript, or in the decisions where, how or when to publish in the peer-review press. For more information, go to www.NTDenvision.org.
Competing interests
None declared.
Ethical approval
Not required, as the study utilized data collected for routine programmatic purposes.
References
- 1. United Nations General Assembly RES/70/1. Transforming our world: the 2030 agenda for sustainable development, 2015.
- 2. Theobald S, MacPherson EE, Dean L, et al. 20 years of gender mainstreaming in health: lessons and reflections for the neglected tropical diseases community. BMJ Glob Health. 2017;2:e000512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vlassoff C. Gender differences in determinants and consequences of health and illness. J Health Popul Nutr. 2007;25:47–61. [PMC free article] [PubMed] [Google Scholar]
- 4. Sen G, Östlin P, George A Unequal, Unfair, Ineffective and Inefficient Gender Inequity in Health: Why it exists and how we can change it, 2007. [DOI] [PubMed]
- 5. Merten S, Martin Hilber A, Biaggi C, et al. Gender Determinants of Vaccination Status in Children: Evidence from a Meta-Ethnographic Systematic Review. PLoS One. 2015;10:e0135222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Steketee RW, Eisele TP. Is the Scale Up of Malaria Intervention Coverage Also Achieving Equity? PLoS One. 2009;4:e8409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hudelson P. Gender differentials in tuberculosis: the role of socio-economic and cultural factors. Tuber Lung Dis. 1996;77:391–400. [DOI] [PubMed] [Google Scholar]
- 8. Ghanotakis E, Peacock D, Wilcher R. The importance of addressing gender inequality in efforts to end vertical transmission of HIV. J Int AIDS Soc. 2012;15(Suppl 2):17385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Baker P, Dworkin SL, Tong S, et al. The men’s health gap: men must be included in the global health equity agenda. Bull World Health Organ. 2014;92:618–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. World Health Organization Preventive chemotherapy in human helminthiasis. World Health Organization, Geneva, Switzerland; 2006. [Google Scholar]
- 11. World Health Organization. Global programme to eliminate lymphatic filariasis: progress report, 2016. Wkly Epidemiol Rec. 2017;92:589–608.28984120 [Google Scholar]
- 12. World Health Organization Strategy for integrating gender analysis and actions into the work of WHO. World Health Organization, Geneva, Switzerland; 2009. [Google Scholar]
- 13. WHO Regional Office for Africa Women’s Health: a Strategy for the African Region: Report of the Regional Director, 2003.
- 14. World Health Organization WHO gender policy: Integrating gender perspectives in the work of WHO. World Health Organization, Geneva, Switzerland; 2002. [Google Scholar]
- 15. World Health Organization Neglected tropical diseases, Planning, requesting medicines and reporting. http://www.who.int/neglected_diseases/preventive_chemotherapy/reporting/en/ [accessed 7 November 2018].
- 16. Arakaki L, Kidane L, Kwan-Gett TS Neglected Tropical Diseases: Women and Girls in Focus, 2016.
- 17. Krentel A, Wellings K. The role of gender relations in uptake of mass drug administration for lymphatic filariasis in Alor District, Indonesia. Parasit Vectors. 2018;11:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rilkoff H, Tukahebwa EM, Fleming FM, et al. Exploring Gender Dimensions of Treatment Programmes for Neglected Tropical Diseases in Uganda. PLoS Negl Trop Dis. 2013;7:e2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. USAID Gender equality and female empowerment, 2012.
- 20. USAID ADS Chapter 205: Integrating Gender Equality and Female Empowerment in USAID’s Program Cycle, 2013.
- 21. Gabrysch S, Campbell OM. Still too far to walk: literature review of the determinants of delivery service use. BMC Pregnancy Childbirth. 2009;9:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Friedman JF, Olveda RM, Mirochnick MH, et al. Praziquantel and human pregnancy Praziquantel for the treatment of schistosomiasis during human pregnancy. Bull World Health Organ. 2017;1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. World Health Organization Trachoma: Key Facts. World Health Organization, Geneva, Switzerland; 2018. [Google Scholar]
- 24. World Health Organization Women and Health : Today’s Evidence Tomorrow’s Agenda. World Health Organization, Geneva, Switzerland; 2009. [Google Scholar]
- 25. Aderoba AK, Iribhogbe OI, Olagbuji BN, et al. Prevalence of helminth infestation during pregnancy and its association with maternal anemia and low birth weight. Int J Gynecol Obstet. 2015;129:199–202. [DOI] [PubMed] [Google Scholar]
- 26. Poggensee G, Kiwelu I, Weger V, et al. Female Genital Schistosomiasis of the Lower Genital Tract: Prevalence and Disease‐Associated Morbidity in Northern Tanzania. J Infect Dis. 2000;181:1210–3. [DOI] [PubMed] [Google Scholar]
- 27. Vlassoff C, Weiss M, Ovuga EB, et al. Gender and the stigma of onchocercal skin disease in Africa. Soc Sci Med. 2000;50:1353–68. [DOI] [PubMed] [Google Scholar]
- 28. Bandyopadhyay L. Lymphatic filariasis and the women of India. Soc Sci Med. 1996;42:1401–10. [DOI] [PubMed] [Google Scholar]
- 29. Kabatereine NB, Tukahebwa E, Kazibwe F, et al. Progress towards countrywide control of schistosomiasis and soil-transmitted helminthiasis in Uganda. Trans R Soc Trop Med Hyg. 2006;100:208–15. [DOI] [PubMed] [Google Scholar]
- 30. Talbot JT, Viall A, Direny A, et al. Predictors of compliance in mass drug administration for the treatment and prevention of lymphatic filariasis in Leogane, Haiti. Am J Trop Med Hyg. 2008;78:283–8. [PubMed] [Google Scholar]