Abstract
Lengthening the annual low-dose computed tomography (CT) screening interval for individuals at lowest risk of lung cancer could reduce harms and improve efficiency. We analyzed 23 328 participants in the National Lung Screening Trial who had a negative CT screen (no ≥4-mm nodules) to develop an individualized model for lung cancer risk after a negative CT. The Lung Cancer Risk Assessment Tool + CT (LCRAT+CT) updates “prescreening risk” (calculated using traditional risk factors) with selected CT features. At the next annual screen following a negative CT, risk of cancer detection was reduced among the 70% of participants with neither CT-detected emphysema nor consolidation (median risk = 0.2%, interquartile range [IQR] = 0.1%–0.3%). However, risk increased for the 30% with CT emphysema (median risk = 0.5%, IQR = 0.3%–0.8%) and the 0.6% with consolidation (median = 1.6%, IQR = 1.0%–2.5%). As one example, a threshold of next-screen risk lower than 0.3% would lengthen the interval for 57.8% of screen-negatives, thus averting 49.8% of next-screen false-positives among screen-negatives but delaying diagnosis for 23.9% of cancers. Our results support that many, but not all, screen-negatives might reasonably lengthen their CT screening interval.
Although efficacious, low-dose computed tomography (CT) lung cancer screening carries harms including false-positives (1–4) and radiation-induced cancers (2,5). Screening uptake has been low (6) and there is need to improve efficiency (7). Fortunately, multiple studies show that screen-negative individuals have reduced lung-cancer risk over subsequent screens (8–10). This suggests the possibility of lengthening screening intervals after a negative CT (11–14).
However, not all screen-negatives have sufficiently low risk to lengthen intervals (15). It is unclear how to identify appropriate candidates, because existing risk models for screening either combine individuals with negative and abnormal screens (16) or only predict current risk (17). We previously developed the Lung Cancer Risk Assessment Tool (LCRAT) to predict prescreening lung cancer risk. Here, we build on this work to develop a simple model, LCRAT+CT, that predicts short-term lung cancer risk following a negative CT screen. LCRAT+CT accounts for both prescreening risk factors and negative-CT features. We suggest how LCRAT+CT could identify candidates for longer screening intervals.
We analyzed 23 328 CT-arm participants in the U.S. National Lung Screening Trial (NLST) (1) who had at least one negative CT (defined as the absence of any nodules ≥4 mm in longest diameter). Among these participants, most had a negative result at all three screens, and 43 interval cancers and 138 next-screen cancers occurred (Supplementary Table 1, available online). First, we calculated individual one-year baseline “prescreening risk” based on risk factors using the LCRAT (18, 19). Next, we selected features of a negative CT that modify the relationship between prescreening and future lung cancer risk (Supplementary Table 2, available online). Specifically, we fit first-order Markov transition models using log-binomial regression (20, 21). LCRAT+CT outputs future risk by raising prescreening risk to an exponent determined by negative-CT features. We fit separate models for risk between screens (interval-cancer risk) and at the next annual screen (next-screen risk). The Supplementary Methods (available online) describes methodological details for LCRAT, feature selection, and LCRAT+CT model definition. The NLST was approved by the institutional review board at each study site, and all participants provided informed consent.
LCRAT+CT accounts for four properties of NLST screening that we observed during model development (Supplementary Methods, available online). First, prescreening risk strongly affected risk during screening. Second, prescreening risk encapsulated the effects of individual risk factors. Third, risk calculations were similar across NLST screens. Fourth, risk calculations were similar among individuals with a recent negative CT, regardless of their prior CT result.
A total of 43 interval cancers arose after 56 921 negative screens, yielding 0.08% mean risk (Supplementary Figure 1, available online). For next-screen cancer, 138 cases were detected following 35 530 negative screens, yielding 0.4% mean risk. The next-screen risk model included terms for CT-detected emphysema and consolidation. It had good cross-validated internal calibration (138 and 138.5 cases observed and predicted, P = .93) and reasonable discrimination (optimism-corrected area under the receiver operating characteristic curve = 0.73).
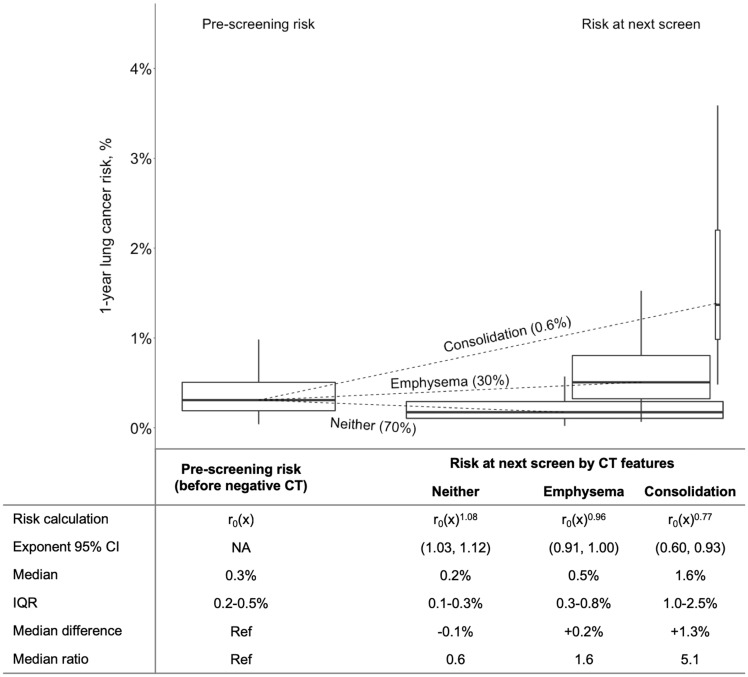
Due to variation in prescreening risk and CT features, next-screen lung cancer risk was heterogeneous (Figure 1). Among the 70% of screen-negatives with neither emphysema nor consolidation on their negative CT, median next-screen risk was reduced from 0.3% prescreening risk to 0.2% (interquartile range [IQR] = 0.1%–0.3%, risk ratio = 0.6). In contrast, for the 30% with CT-detected emphysema, risk increased 1.6-fold (median risk = 0.5%, IQR = 0.3%–0.8%). For the 0.6% with consolidation, risk increased 5-fold (median risk = 1.6%, IQR = 1.0%–2.5%).
Figure 1.
Effect of features noted on a negative computed tomography (CT) screen on risk of next-screen lung cancer detection among participants in the National Lung Screening Trial. For illustration, this figure was constructed using data from individuals who had a negative result at the first U.S. National Lung Screening Trial screen (T0) and were subsequently at risk for lung cancer at the second screen (T1) (n = 18 245). Among these individuals, 30% (n = 5484) had emphysema noted on their negative CT, 0.6% (n = 106) had consolidation, and 70% (12 691) had neither (n = 36 when both emphysema and consolidation were included in both risk distributions). Prescreening risk [r0(x)] was calculated using the Lung Cancer Risk Assessment Tool (18). Outliers are not included in the figure but are included in the calculations in the table. Within each group of boxplots, boxplot widths are scaled by the percentage of the population represented, boxplot heights represent the interquartile range (IQR), and the vertical lines (whiskers) represent the range of data excluding outliers. CI, confidence interval; Ref, reference.
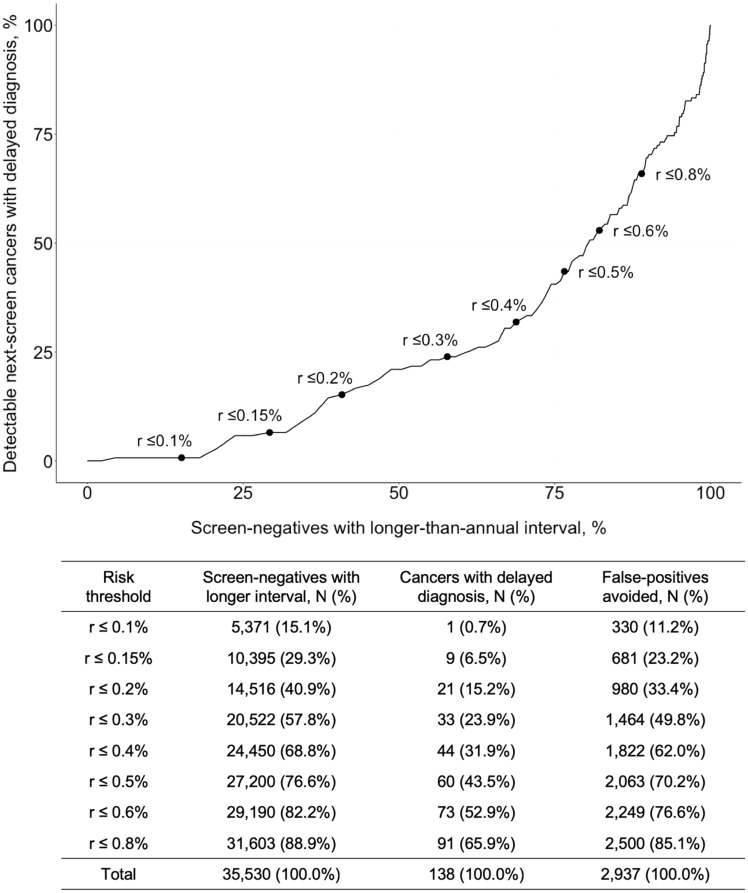
We examined potential risk thresholds to identify candidates for longer screening intervals (Figure 2). We considered next-screen risk only, because CT features did not meaningfully stratify interval-cancer risk (Supplementary Methods, available online). First, we considered a threshold of 0.3% risk, below which screening is highly preference sensitive (22). Risk was below this threshold for 20 522, or 57.8%, of screen-negatives, in whom 33 of the 138 next-screen cancers were detected (23.9%) and 1464 of the 2937 next-screen false-positives occurred (49.8%). Therefore, if the screening interval were lengthened for 58% of screen-negatives, then 50% of the false-positives among screen-negatives could have been avoided, but diagnosis would have been delayed for 24% of the cancers. Lower risk thresholds would reduce delayed diagnosis but avert fewer false-positives. For example, at a 0.15% threshold, only 6.5% of cancer diagnoses would be delayed, but only 29.3% would lengthen their interval and only 23.2% of false-positives would be avoided (Figure 2).
Figure 2.
Potential effect of Lung Cancer Risk Assessment Tool with computed tomography (LCRAT+CT) risk thresholds for longer screening intervals among screen-negative participants in the National Lung Screening Trial. Points and labels indicate potential next-screen risk thresholds for lengthening CT screening intervals beyond one year. For example, if the interval were lengthened for those with a predicted next-screen risk 0.3% or less, then the interval would be lengthened for 57.8% of screen-negatives. Among them, 33 cancers were detected at the next screen and would therefore have their diagnosis delayed (ie, 33 of 138 or 23.9% of all next-screen cancers in screen-negatives). Screen-negatives at both T0 and T1 (and corresponding cancers at T1 and T2) were included in this analysis, such that individuals with a negative result at both screens may be included twice. The denominator for percentages is the total number of screen-negatives, the number of next-screen cancers among screen-negatives, and the number of false-positives at the next screen among screen-negatives, respectively.
Our findings indicate that reassurance from a negative CT is insufficient to recommend a longer interval for all screen-negatives. Instead, the decision requires comprehensive risk calculations incorporating prescreening risk and individual CT findings. In practice, to update a screen-negative’s lung cancer risk with LCRAT+CT, one would simply apply the appropriate exponent (corresponding to CT-emphysema and/or consolidation; Figure 1) to the LCRAT prescreening risk. If a risk threshold were established, then a longer interval could be offered to individuals below it, with use of a decision tool.
A risk-based approach to lengthen screening intervals for low-risk participants could substantially improve the efficiency of CT screening. Using a threshold of 0.3% next-screen risk, we found that 57.8% of NLST screen-negatives could lengthen their interval, thereby avoiding 49.8% of next-screen false-positives among screen-negatives. Other benefits that we could not quantify would include reductions in overdiagnosed lung cancers, radiation-induced cancers, and invasive procedures. However, this threshold would have delayed diagnosis for 23.9% of detectable next-screen cancers among screen-negatives. Of these cancers, 55% were stage I, and these in particular might have become incurable if the screening interval had been extended, for example, to two years (diagnosis delayed by one year).
Risk thresholds between 0.10% and 0.40% are well within the range of annual risks for 53-year-old, 30-or-more-pack-year smokers (19), who are currently recommended to begin screening in two years (23). Because such people have a de facto two-year “lengthened interval,” their range of one-year risks implicitly identifies potential thresholds for longer intervals that underlie existing guidelines. We note that the proportions in Figure 2 are specific to the NLST and may vary with the population risk distribution and over time (24), though the individual risk-benefit trade-off that they represent might be maintained.
In relation to prescreening risk, next-screen risk after a negative CT is driven by opposing forces: reduced risk from a negative screen countered by increased detection, some of which is screening-induced overdiagnosis. Estimates of overdiagnosis in CT screening vary, but modeling of long follow-up estimates 9% (25). Because we cannot know which cancers are overdiagnosed, LCRAT+CT estimates total next-screen risk.
Our study has limitations. External validation of LCRAT+CT is needed to determine its portability outside the NLST. We did not investigate whether other prescreening risk models can be substituted for LCRAT. We could not determine the specific length that longer intervals should be, because the NLST used only annual screening. Data from the NELSON and MILD trials support extending to two years but no longer (26–28). Our calculations do not consider that some individuals with deleterious CT features may have reduced life expectancy and thus lower benefit from annual screening. LCRAT+CT only applies to individuals who fit the NLST definition of screen-negative (ie, no ≥4-mm nodules). Finally, we did not estimate the reduction in screening effectiveness from lengthening intervals.
When considering for whom to lengthen screening intervals, guidelines committees might consider the benefit-harm tradeoff we presented within the broader context of feasibility, acceptability to patients, potential reduction in screening effectiveness, and costs. Like the decision to screen, the decision to lengthen intervals may be highly preference-sensitive for many patients (22). Ultimately, the individualized decision-making offered by our approach may provide an important avenue to improve efficiency and reduce harms in CT screening.
Funding
This study was supported in part by the Intramural Research Program of the U.S. National Institutes of Health/National Cancer Institute. H. Robbins was supported by the Cancer Epidemiology, Prevention, and Control Training Grant (NCI T32 CA009314), an individual National Research Service Award (NCI F31 CA210660), and the INTEGRAL program (NCI U19 CA203654).
Notes
Affiliations of authors: Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD (HAR); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (CDB, LCC, AKC, HAK).
Present affiliation: International Agency for Research on Cancer, Lyon, France (HAR).
HAR, LCC, AKC, and HAK report no conflict of interest. CDB receives consulting fees from Medial Early Sign, LLC and GRAIL, Inc.
The NIH approved the final version of the manuscript but had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation of the manuscript. The authors alone are responsible for the views expressed in this article and they do not necessarily represent the views, decisions, or policies of the institutions with which they are affiliated.
We thank Dr Scott Zeger and Dr Rebecca Landy for their helpful comments on this manuscript. We are also grateful to the anonymous reviewers whose thoughtful suggestions improved this manuscript. Hilary A. Robbins had full access to all of the data in the study and takes full responsibility for the integrity of the data and the accuracy of the data analysis.
Supplementary Material
References
- 1. Aberle DR, Adams AM, Berg CD, et al. National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;3655:395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bach PB, Mirkin JN, Oliver TK, et al. Benefits and harms of CT screening for lung cancer: a systematic review. JAMA. 2012;30722:2418–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bach PB. Raising the bar for the U.S. Preventive Services Task Force. Ann Intern Med. 2014;1605:365–366. [DOI] [PubMed] [Google Scholar]
- 4. Kinsinger LS, Anderson C, Kim J, et al. Implementation of lung cancer screening in the Veterans Health Administration. JAMA Intern Med. 2017;1773:399–406. [DOI] [PubMed] [Google Scholar]
- 5. de Koning HJ, Meza R, Plevritis SK, et al. Benefits and harms of computed tomography lung cancer screening strategies: a comparative modeling study for the U.S. Preventive Services Task Force. Ann Intern Med. 2014;1605:311–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jemal A, Fedewa SA.. Lung cancer screening with low-dose computed tomography in the United States– 2010 to 2015. JAMA Oncol. 2017;39:1278–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Green AK, Bach P.. Model-based eligibility for lung cancer screening: where theory meets practice. Ann Intern Med. 2018;1683:223–224. [DOI] [PubMed] [Google Scholar]
- 8. Patz EF, Greco E, Gatsonis C, Pinsky P, Kramer BS, Aberle DR.. Lung cancer incidence and mortality in National Lung Screening Trial participants who underwent low-dose CT prevalence screening: a retrospective cohort analysis of a randomised, multicentre, diagnostic screening trial. Lancet Oncol. 2016;175:590–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tammemagi MC, Schmidt H, Martel S, et al. Participant selection for lung cancer screening by risk modelling (the Pan-Canadian Early Detection of Lung Cancer [PanCan] study): a single-arm, prospective study. Lancet Oncol. 2017;1811:1523–1531. [DOI] [PubMed] [Google Scholar]
- 10. Horeweg N, van Rosmalen J, Heuvelmans MA, et al. Lung cancer probability in patients with CT-detected pulmonary nodules: a prespecified analysis of data from the NELSON trial of low-dose CT screening. Lancet Oncol. 2014;1512:1332–1341. [DOI] [PubMed] [Google Scholar]
- 11. van der Aalst CM, Ten Haaf K, de Koning HJ.. Lung cancer screening: latest developments and unanswered questions. Lancet Respir Med. 2016;49:749–761. [DOI] [PubMed] [Google Scholar]
- 12. Field JK, Duffy SW.. Lung cancer CT screening: is annual screening necessary? Lancet Oncol. 2016;175:543–544. [DOI] [PubMed] [Google Scholar]
- 13. Karachaliou N, Sosa AE, Rosell R.. Annual or biennial lung cancer CT screening? J Thorac Dis. 2016;89:2424–2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ridge CA, Boiselle PM.. Optimizing the lung cancer screening interval: the world is waiting. J Thorac Dis. 2016;810:E1369–E1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kavanagh J, Liu G, Menezes R, et al. Importance of long-term low-dose CT follow-up after negative findings at previous lung cancer screening. Radiology. 2018;2891:218–224. [DOI] [PubMed] [Google Scholar]
- 16. Schreuder A, Schaefer-Prokop CM, Scholten ET, Jacobs C, Prokop M, van Ginneken B.. Lung cancer risk to personalise annual and biennial follow-up computed tomography screening. Thorax. 2018;73:626–633. [DOI] [PubMed] [Google Scholar]
- 17. McWilliams A, Tammemagi MC, Mayo JR, et al. Probability of cancer in pulmonary nodules detected on first screening CT. N Engl J Med. 2013;36910:910–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Katki HA, Kovalchik SA, Berg CD, Cheung LC, Chaturvedi AK.. Development and validation of risk models to select ever-smokers for CT lung cancer screening. JAMA. 2016;31521:2300–2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. National Cancer Institute. Lung cancer risk assessment tool. https://analysistools.nci.nih.gov/lungCancerRiskAssessment/. Accessed April 17, 2018.
- 20. Diggle P, Heagerty P, Liang KY, Zeger S. Chapter 10: Transition models. In: Atkinson AC, Carroll RJ, Hand DJ, Wang J-L, eds. Analysis of Longitudinal Data 2nd ed. 2013. Oxford, UK: Oxford University Press.
- 21. Wacholder S. Binomial regression in GLIM: estimating risk ratios and risk differences. Am J Epidemiol. 1986;1231:174–184. [DOI] [PubMed] [Google Scholar]
- 22. Caverly TJ, Cao P, Hayward RA, Meza R.. Identifying patients for whom lung cancer screening is preference-sensitive: a microsimulation study. Ann Intern Med. 2018;1691:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Moyer VA. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;1605:330–338. [DOI] [PubMed] [Google Scholar]
- 24. Cheung LC, Katki HA, Chaturvedi AK, Jemal A, Berg CD.. Preventing lung cancer mortality by computed tomography screening: the effect of risk-based versus U.S. Preventive Services Task Force eligibility criteria, 2005-2015. Ann Intern Med. 2018;1683:229–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ten Haaf K, de Koning HJ.. Overdiagnosis in lung cancer screening: why modelling is essential. J Epidemiol Community Health. 2015;6911:1035–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Horeweg N, van der Aalst CM, Thunnissen E, et al. Characteristics of lung cancers detected by computed tomography screening in the randomized NELSON trial. Am J Respir Crit Care Med. 2013;1878:848–854. [DOI] [PubMed] [Google Scholar]
- 27. Yousaf-Khan U, van der Aalst C, de Jong PA, et al. Final screening round of the NELSON lung cancer screening trial: the effect of a 2.5-year screening interval. Thorax. 2017;721:48–56. [DOI] [PubMed] [Google Scholar]
- 28. Sverzellati N, Silva M, Calareso G, et al. Low-dose computed tomography for lung cancer screening: comparison of performance between annual and biennial screen. Eur Radiol. 2016;2611:3821–3829. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.