Abstract
Background
Previous studies of hereditary retinoblastoma survivors have reported elevated mortality, particularly for sarcomas, compared with the general population. However, cause-specific mortality patterns for long-term hereditary and nonhereditary retinoblastoma survivors are poorly understood.
Methods
Among 2053 retinoblastoma patients diagnosed during 1914–2006 at two major US treatment centers and followed to 2016, we estimated cumulative mortality, standardized mortality ratios (SMRs), and absolute excess risks (AERs) compared with the US general population.
Results
Most deaths occurred in 1129 hereditary retinoblastoma patients (n = 518 deaths, cumulative mortality 70 years after retinoblastoma = 75.8%, 95% CI = 69.0% to 82.6%; SMR = 8.5, 95% CI = 7.7 to 9.2). Of these, 267 were due to subsequent cancers (SMR = 27.4, 95% CI = 24.2 to 30.9; AER = 72.3 deaths/10 000 person-years), for which SMRs were highest 15–29 years after diagnosis (n = 69, SMR = 89.9, 95% CI = 70.0 to 113.8) but remained statistically significantly elevated at 60 and more years (n = 14, SMR = 6.7, 95% CI = 3.6 to 11.2), whereas AERs increased with time (AER<15years = 38.0; AER60+years = 327.5). Increased risk of death due to cancers of pancreas, large intestines, and kidney were noted for the first time. Overall risk of subsequent cancers was greater for those treated with radiotherapy and chemotherapy compared to radiotherapy alone, although patterns varied by organ site. For 924 patients with nonhereditary retinoblastoma, we noted a modestly increased risk of death for subsequent cancers (n = 27, SMR = 1.8, 95% CI = 1.2 to 2.6) possibly due to treatment or misclassification of hereditary status. Risks of noncancer causes of death were not elevated for hereditary or nonhereditary patients.
Conclusion
Hereditary retinoblastoma survivors died mainly from an excess risk of subsequent cancers up to six decades later, highlighting the need to develop long-term clinical management guidelines for hereditary retinoblastoma survivors treated in the past.
Retinoblastoma, a rare pediatric ocular tumor (1), occurs in two forms: hereditary disease, caused by a germline mutation in RB1 typically affecting both eyes, and nonhereditary disease, caused by somatic mutations in RB1 occurring in one eye. Hereditary retinoblastoma survivors are known to have strikingly increased risks of developing subsequent cancers, likely due to a combination of genetic susceptibility and treatment (2–5).
Previous studies have shown that hereditary survivors have elevated mortality compared to age-matched individuals in the general population, with particularly elevated risk for sarcomas (6–10). In contrast, nonhereditary survivors do not appear to have elevated mortality because of subsequent cancers, and only limited evidence of excess mortality from noncancer causes has been reported (7). Limitations of previous mortality studies include lack of long-term follow-up beyond age 50 years, when risks for common epithelial cancers and numerous other diseases begin to increase; small sample sizes (<400–750 survivors), resulting in imprecise estimates of cause-specific mortality; and/or lack of individual-level treatment data on retinoblastoma survivors diagnosed across different treatment eras, because treatments for retinoblastoma have evolved substantially over time, with corresponding improvements in prognosis (11).
To address these gaps in knowledge and inform clinical surveillance guidelines, we evaluated cause-specific mortality in the National Cancer Institute Long-Term Follow-up Study of Retinoblastoma Survivors. Using data from 1129 hereditary and 924 nonhereditary retinoblastoma survivors with available treatment information, we analyzed risk of death for a broad range of malignant and nonmalignant causes by hereditary status, treatment, year of diagnosis, and time since diagnosis.
Methods
Study Population and Data Collection
As described previously (12), we identified 2136 retinoblastoma patients from medical records who were originally diagnosed and treated during 1914–2006 at major treatment centers in New York and Boston. For this analysis, we excluded survivors who were first seen at the study center more than 5 years after primary retinoblastoma diagnosis (n = 28), who lacked follow-up (n = 11), and who were non-US residents (n = 44), resulting in an eligible study population of 2053 survivors. Institutional Review Boards of the National Cancer Institute and participating treatment centers approved the study. The Institutional Review Board did not require written informed consent for the data used in this analysis (medical records and death certificates).
Standardized forms were utilized to abstract detailed data for retinoblastoma diagnosis (age, calendar year, laterality), treatment, and family history of retinoblastoma. Abstraction was completed at three separate time points for patients diagnosed during 1914–1984 and 1985–1996, as reported previously (10), and 1997–2006, as reported here for the first time. Treatment data included dates and type of surgery (eye removal, cryotherapy, or photocoagulation), radiotherapy (external beam radiation, radioactive plaque, or both), and chemotherapy (name of chemotherapeutic agents). Prior to 1960, patients with bilateral retinoblastoma were treated if possible with radiotherapy in one eye to preserve sight and surgery to remove the other eye. Addition of systemic therapy became more common since its introduction in the 1950s and gradually replaced radiotherapy by the 1980s. Since 2000, chemotherapy has been injected directly into the ophthalmic artery or intravitreally. In contrast, most unilateral survivors received surgery. Based on medical record data at the time of study inclusion, all patients with bilateral retinoblastoma and those with unilateral disease and a first- or second-degree relative with retinoblastoma (excluding children) were classified as hereditary; all other unilateral patients were classified as nonhereditary.
Death Ascertainment
We ascertained deaths from medical records, state health departments, or the National Death Index (NDI) (http://www.cdc.gov/nchs/ndi.htm), depending on the year of death. For deaths occurring before 1979 and noted in the medical records, reported by a relative, or reported by the Social Security Administration Death Master File, we retrieved copies of death certificates from state health departments. We linked cohort members not known to be deceased before 1979 with the NDI to ascertain vital status and underlying cause of death for 1979–2016.
Causes of death were coded using the International Classification of Diseases (ICD) coding version in use during the year of death (Supplementary Table 1, available online). We grouped causes of death into three broad categories: retinoblastoma, subsequent cancers, and noncancer causes of death. We further grouped subsequent cancers by topography and noncancer causes by major categories.
Statistical Analysis
Accrual of person-years began at retinoblastoma diagnosis until death, last known date of vital status before 1979, or end of study (December 31, 2016), whichever occurred earliest. If survivors were known to be alive after 1979, assumed to be living in the United States, and not identified as deceased by the NDI, they were considered alive at the end of the study.
To assess the clinical burden of mortality, we calculated cumulative mortality for all causes combined and stratified by retinoblastoma, subsequent cancers, and noncancer causes, taking competing risk of death from other causes into account (13). For hereditary survivors, we also calculated cumulative mortality by decade of retinoblastoma diagnosis and treatment.
To identify the specific causes of death contributing to increased cumulative mortality, we compared the observed number of deaths to that expected in the general population based on age-, sex-, and calendar year-specific US death rates from 1950 to 2014 for cancer and from 1969 to 2014 for noncancer causes. Standardized mortality ratios (SMRs) and corresponding Poisson exact 95% confidence intervals (CIs) compared the relative risk of death in retinoblastoma patients with the general population (SMR = observed (O)/expected (E). Statistical significance of the SMRs was determined using a two-sided P less than .05 (confidence bound of the SMR excluded 1.0).
We also estimated absolute excess risks (AERs) per 10 000 person-years (AER = 10 000 × [observed–expected)/person-years). SMRs and AERs were calculated for all causes; overall groupings of retinoblastoma, subsequent cancers, and noncancer causes; and more specific subsequent cancers and noncancer causes of death observed in at least two cohort members. Subgroup analyses were conducted by sex, time since retinoblastoma diagnosis (latency), and treatment. Because most patients were at least 2 years old at retinoblastoma diagnosis, latency is roughly equivalent to attained age.
Results
Table 1 presents descriptive characteristics of the cohort for retinoblastoma diagnosis, treatment, and vital status by hereditary status. By the end of follow-up, 518 (45.9%) deaths occurred in 1129 hereditary retinoblastoma and 172 (18.6%) deaths in 924 nonhereditary patients. In the total cohort, 25.8% of participants were followed 45–59 years after retinoblastoma diagnosis and 7.7% for 60 and more years.
Table 1.
Characteristics of 2053 survivors of retinoblastoma in the study cohort, by hereditary status*
| Characteristic | Nonhereditary* |
Hereditary* |
|---|---|---|
| (N = 924) |
(N = 1129) |
|
| No. (%) | No. (%) | |
| Laterality | ||
| Unilateral | 924 (100.0) | 29 (2.6) |
| Bilateral | 0 (0.0) | 1100 (97.4) |
| Sex | ||
| Male | 472 (51.1) | 579 (51.3) |
| Female | 452 (48.9) | 550 (48.7) |
| Calendar year of retinoblastoma diagnosis | ||
| 1914–1959 | 212 (22.9) | 304 (26.9) |
| 1960–1969 | 224 (24.2) | 297 (26.3) |
| 1970–1979 | 204 (22.1) | 248 (22.0) |
| 1980–1989 | 138 (14.9) | 162 (14.4) |
| 1990–2006 | 146 (15.8) | 118 (10.5) |
| Age at retinoblastoma, mo | ||
| <6 | 93 (10.1) | 397 (35.2) |
| 6–11 | 107 (11.6) | 264 (23.4) |
| 12–23 | 268 (29.0) | 303 (26.8) |
| 24–35 | 249 (27.0) | 120 (10.6) |
| ≥36 | 207 (22.4) | 45 (4.0) |
| Treatment for retinoblastoma | ||
| Surgery only | 636 (68.8) | 90 (8.0) |
| Radiation, no chemotherapy | 97 (10.5) | 535 (47.4) |
| Radiation and chemotherapy | 86 (9.3) | 435 (38.5) |
| Chemotherapy, no radiation | 58 (6.3) | 38 (3.4) |
| Other/unknown | 47 (5.1) | 31 (2.8) |
| Family history of retinoblastoma* | ||
| No/unknown | 924 (100.0) | 892 (79.0) |
| Yes | 0 (0.0) | 237 (21.0) |
| Latency, y† | ||
| <1 | 62 (6.7) | 30 (2.7) |
| 1–14 | 106 (11.5) | 260 (23.0) |
| 15–29 | 150 (16.2) | 213 (18.9) |
| 30–44 | 262 (28.4) | 283 (25.1) |
| 45–59 | 246 (26.6) | 283 (25.1) |
| ≥60 | 98 (10.6) | 60 (5.3) |
| Vital status at last follow-up | ||
| Alive | 716 (77.5) | 578 (51.2) |
| Deceased | 172 (18.6) | 518 (45.9) |
| Lost to follow-up | 36 (3.9) | 33 (2.9) |
| Total person-years at risk | 33 460 | 35 627 |
Hereditary retinoblastoma status was defined based on data collected from the medical record at the time of study inclusion. All patients with bilateral retinoblastoma and unilateral patients with positive family history (first- or second-degree relative with retinoblastoma, excluding children) were classified as hereditary; all other unilateral patients were classified as nonhereditary.
Years from retinoblastoma diagnosis to last follow-up.
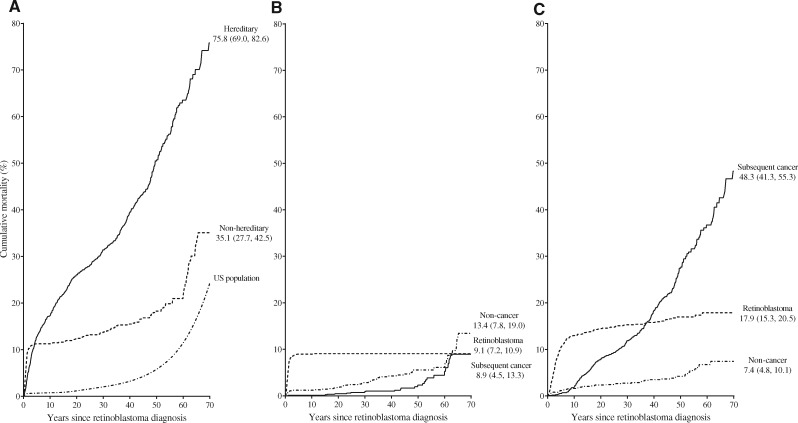
In cumulative mortality analyses (Figure 1), deaths by any cause were substantially higher for hereditary than nonhereditary patients (70 years after retinoblastoma: 75.8%, 95% CI = 69.0% to 82.6% vs 35.1%, 95% CI = 27.7% to 42.5%) (Figure 1A, Supplementary Table 2, available online). Among nonhereditary patients, cumulative mortality was slightly higher for noncancer causes (13.4%, 95% CI = 7.8% to 19.0%) than for subsequent cancers (8.9%, 95% CI = 4.5% to 13.3%) or retinoblastoma (9.1%, 95% CI = 7.2% to 10.9%) (Figure 1B, Supplementary Table 2, available online). In contrast, the highest cumulative mortality for hereditary patients was for subsequent cancers, reaching 48.3% (95% CI = 41.3% to 55.3%) at 70 years after retinoblastoma diagnosis, followed by death due to retinoblastoma (17.9%, 95% CI = 15.3% to 20.5%) and noncancer causes (7.4%, 95% CI = 4.8% to 10.1%) (Figure 1C, Supplementary Table 2, available online).
Figure 1.
Cumulative mortality by years since retinoblastoma diagnosis. Numbers represent estimated cumulative mortality (95% confidence interval) at 70 years after retinoblastoma diagnosis. A) All causes of death, by hereditary status and for the general US population. Data on cumulative mortality by age were derived from the 2000 US vital statistics for whites to serve as a reference for the general population (14). B) By cause among nonhereditary retinoblastoma patients. C) By cause among hereditary retinoblastoma patients. See Supplementary Table 2 (available online) for cumulative mortality estimates and person-years at risk.
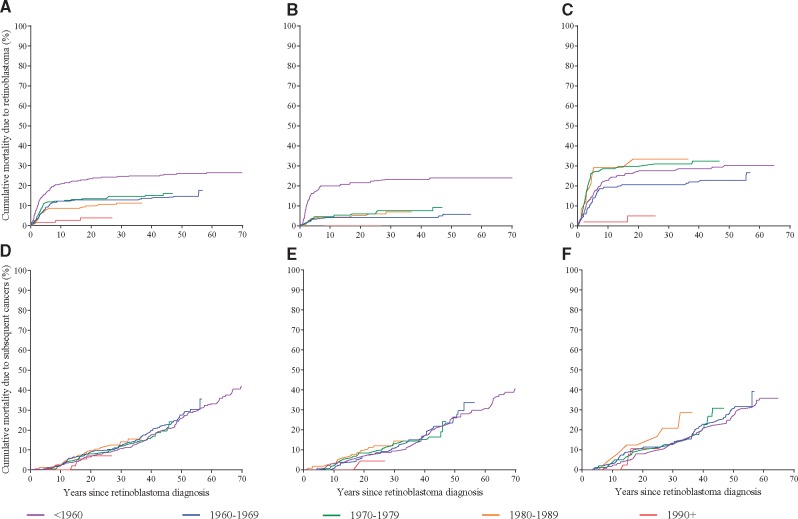
To investigate factors influencing the high cumulative mortality of subsequent cancers in hereditary patients, we conducted analyses stratified by treatment and calendar year of retinoblastoma diagnosis (Figure 2). Overall, cumulative mortality due to retinoblastoma declined for patients diagnosed more recently (Figure 2A), whereas cumulative mortality due to subsequent cancers generally was consistent regardless of decade of retinoblastoma diagnosis (Figure 2D). However, these patterns depended on treatment for retinoblastoma. For patients treated with radiotherapy alone (n = 535), those diagnosed 1960 and later had substantially lower mortality due to retinoblastoma compared with those diagnosed before 1960 (Figure 2B), whereas improvements in mortality due to subsequent cancers for patients diagnosed more recently were very modest (Figure 2E). For patients treated with both radiotherapy and chemotherapy (n = 435), those diagnosed from 1970 to 1989 had the highest mortality due to both retinoblastoma (Figure 2C) and subsequent cancers (Figure 2F). Notably, for patients treated from 1990 to 2006, when the use of brachytherapy increased, cumulative mortality due to any cause appeared substantially reduced, although data on subsequent cancers should be interpreted cautiously because of limited follow-up.
Figure 2.
Cumulative mortality by years since hereditary retinoblastoma diagnosis, stratified by decade of treatment. Cumulative mortality from retinoblastoma for (A) all treatments, (B) radiotherapy without chemotherapy, and (C) radiotherapy with chemotherapy. Cumulative mortality from subsequent cancers for (D) all treatments, (E) radiotherapy without chemotherapy, and (F) radiotherapy with chemotherapy.
To identify specific causes of deaths occurring after retinoblastoma and estimate excesses compared with the general population, we calculated SMRs and AERs (Table 2). Nonhereditary retinoblastoma survivors had a nearly twofold increased risk of death compared with the general population (O = 172, SMR = 2.4, 95% CI = 2.0 to 2.8), with 29.8 excess deaths per 10 000 person-years. This slightly increased risk was primarily due to 27 deaths from subsequent cancers (SMR = 1.8, 95% CI = 1.2 to 2.6, AER = 3.6/10 000), with statistically significantly elevated SMRs for soft tissue sarcoma and breast cancer. The risk for noncancer causes did not differ from population expectation.
Table 2.
Causes of death other than retinoblastoma in 2053 retinoblastoma survivors by hereditary status
| Causes of death* | Nonhereditary (N = 924) |
Hereditary (N = 1129) |
||||
|---|---|---|---|---|---|---|
| O | SMR* (95% CI) | AER | O | SMR* (95% CI) | AER | |
| All causes of death† | 172 | 2.4 (2.0 to 2.8) | 29.8 | 518 | 8.5 (7.7 to 9.2) | 128.2 |
| All malignant cancer excluding retinoblastoma‡ | 27 | 1.8 (1.2 to 2.6) | 3.6 | 267 | 27.4 (24.2 to 30.9) | 72.3 |
| Bone | 0 | — | — | 79 | 718.5 (568.8 to 895.4) | 22.2 |
| Soft tissue sarcoma and connective tissue | 2 | 11.1 (1.2 to 39.9) | 0.5 | 52 | 334.9 (250.1 to 439.2) | 14.6 |
| Brain and other parts of the nervous system | 2 | 3.0 (0.3 to 10.7) | 0.4 | 17 | 26.3 (15.3 to 42.1) | 4.6 |
| Nasal cavities, middle ear and sinus | 0 | — | — | 15 | >1000 (677.6 to >1000) | 4.2 |
| Melanoma | 2 | 5.7 (0.6 to 20.5) | 0.5 | 15 | 55.1 (30.8 to 90.8) | 4.1 |
| Lung and trachea | 4 | 1.1 (0.3 to 2.7) | 0.1 | 12 | 5.8 (3.0 to 10.1) | 2.8 |
| Corpus uteri | 1 | — | — | 9 | 89.5 (40.9 to 170.0) | 5.2 |
| Bladder | 0 | — | — | 7 | 59.7 (23.9 to 123.0) | 1.9 |
| Breast | 6 | 4.4 (1.6 to 9.5) | 1.4 | 5 | 5.5 (1.8 to 12.9) | 1.2 |
| Pancreas | 1 | — | — | 5 | 11.4 (3.7 to 26.7) | 1.3 |
| Large intestine | 1 | — | — | 4 | 6.9 (1.8 to 17.5) | 1.0 |
| Buccal cavity and pharynx | 0 | — | — | 4 | 24.1 (6.5 to 61.7) | 1.1 |
| Kidney | 0 | — | — | 3 | 12.3 (2.5 to 35.8) | 0.8 |
| Retroperitoneum | 1 | — | — | 2 | 112.7 (12.7 to 406.8) | 0.6 |
| Nonmelanoma | 0 | — | — | 2 | 47.8 (5.4 to 172.8) | 0.6 |
| Cervix uteri | 0 | — | — | 2 | 11.4 (1.3 to 41.0) | 1.1 |
| Ovary | 0 | — | — | 2 | 7.6 (0.9 to 27.6) | 1.0 |
| Lymphoma and hematopoietic | 1 | — | — | 2 | 1.4 (0.2 to 5.0) | 0.2 |
| Liver and gallbladder | 0 | — | — | 1 | — | — |
| Prostate | 1 | — | — | 1 | — | — |
| Thyroid | 1 | — | — | 1 | — | — |
| Benign neoplasms§ | 0 | — | — | 3 | 60.4 (12.1 to 176.6) | 0.8 |
| Brain/central nervous system | 0 | — | — | 1 | — | — |
| Other known causes of death‖ | 49 | 0.9 (0.6 to 1.2) | −2.2 | 49 | 1.0 (0.7 to 1.3) | −0.6 |
| Circulatory system | 13 | 0.9 (0.5 to 1.5) | −0.5 | 8 | 0.9 (0.4 to 1.8) | −0.2 |
| Arteriosclerotic heart disease including CHD | 6 | 0.8 (0.3 to 1.7) | −0.6 | 6 | 1.4 (0.5 to 3.0) | 0.5 |
| Vascular lesion of central nervous system | 3 | 1.4 (0.3 to 4.2) | 0.3 | 1 | — | — |
| External causes | 13 | 0.7 (0.4 to 1.3) | −1.3 | 9 | 0.5 (0.2 to 0.9) | −2.6 |
| Suicide | 4 | 1.1 (0.3 to 2.9) | 0.1 | 1 | — | — |
| Symptoms, senility, and ill-defined conditions | 10 | 6.4 (3.1 to 11.8) | 2.5 | 10 | 5.5 (2.6 to 10.1) | 2.3 |
| Digestive system | 3 | 1.0 (0.2 to 2.9) | 0.0 | 5 | 2.2 (0.7 to 5.1) | 0.8 |
| Nervous system and sense organs | 0 | — | — | 4 | 2.5 (0.7 to 6.5) | 0.7 |
| Infectious and parasitic disease | 3 | 1.2 (0.2 to 3.4) | 0.1 | 4 | 1.6 (0.4 to 4.1) | 0.4 |
| Respiratory diseases | 1 | — | — | 4 | 1.2 (0.3 to 3.2) | 0.2 |
| Allergic, endocrine, metabolic, and nutritional disease | 2 | 0.9 (0.1 to 3.1) | −0.1 | 2 | 1.2 (0.1 to 4.3) | 0.1 |
| Mental, psychoneurotic, and personality disorders | 3 | 2.5 (0.5 to 7.3) | 0.5 | 0 | — | — |
SMRs, CIs, and AERs not presented for deaths <2. AER = absolute excess risk per 10 000 person-years; CHD = coronary heart disease; CI = confidence interval; O = observed number of cancers; SMR = standardized mortality ratio.
Total all causes includes deaths due to in situ, uncertain, or secondary cancers (nonhereditary: n = 1, ICD-9 D43.2; hereditary: n = 6, ICD-9 238.1; ICD-10 D48.3, D48.9), unknown causes of death (nonhereditary: n = 12; hereditary: n = 11), and retinoblastoma (nonhereditary: n = 83; hereditary: n = 182).
Malignant cancers not listed were other or poorly specified (hereditary: n = 27, ICD-9 194.4, 195.0, 199.0, 199.1; ICD-10 C38.0, C76.2, C80, C97; nonhereditary: n = 4, ICD-9 199.1).
Total benign includes lipoma (hereditary: n = 1, ICD-9 214.9) and other benign neoplasm (hereditary: n = 1, ICD = 9 226.2).
Total includes diseases of the blood and blood-forming organs (hereditary: n = 1, ICD-10 D68.9), other diseases of the circulatory system (nonhereditary: n = 4, ICD-9 398.9, 450; ICD-10 I42.0, I42.9; hereditary: n = 1, ICD-9 427.5), and other noncancer causes of death (nonhereditary: n = 1, ICD-9 U01.1; hereditary: n = 1 ICD-10 H66.9).
Most deaths and highest mortality risks occurred in the hereditary retinoblastoma survivors (O = 518, SMR = 8.5, 95% CI = 7.7 to 9.2), with 128 excess deaths per 10 000 person-years (Table 2). Slightly over half the deaths were attributable to subsequent cancers, a statistically significantly increased risk of death compared with the general population (O = 267, SMR = 27.4, 95% CI = 24.4 to 30.9, AER = 72.3/10 000). In contrast, we did not observe excess risk of noncancer deaths in hereditary survivors compared with the general population.
Among specific types of subsequent cancers, we observed the highest SMRs (>100) for bone cancer, soft tissue sarcomas, and nasal cavity cancers. We also found statistically significantly elevated risks (SMR > 10) for melanoma and brain and central nervous system (CNS) cancers. Based on smaller numbers of deaths (O ≤ 15), we noted excess risks of death due to cancers of the lung, uterine corpus, bladder, breast, pancreas, large intestine, buccal cavity and pharynx, and kidney. Although most of these deaths were due to epithelial cancers, three of nine uterine corpus cancers, two of four large intestine cancers, and one of three kidney cancers were sarcomas of those organ sites, mostly leiomyosarcomas, based on death certificate data for a subset of patients.
To understand how the SMRs and AERs varied over time, we analyzed risks by latency (Table 3, Supplementary Figure 1, available online). SMRs for subsequent cancers overall and for sarcomas specifically were statistically significantly elevated at all latency periods. SMRs were highest 15–29 years after retinoblastoma (n = 69, SMR = 89.9, 95% CI =70.0 to 113.8) and remained elevated 60 and more years after retinoblastoma (n = 14, SMR = 6.7, 95% CI = 3.6 to 11.2). AERs for subsequent cancers increased with time (AER<15years = 38.0; AER60+years = 327.5). SMRs for melanoma were highest 15–39 years after retinoblastoma and SMRs for brain/CNS cancers were highest less than 15 years and 30–44 years after retinoblastoma. For lung, uterine corpus, and bladder cancers, SMRs generally declined with increasing time since diagnosis, whereas AERs increased with time and cases tended to occur more frequently at longer latency periods. In analyses by treatment, SMRs and AERs for most specific cancers were slightly higher after radiotherapy and chemotherapy compared with radiotherapy alone with the exception of brain/CNS and uterine corpus cancers (Table 4). In analyses by sex, females had a higher risk of death than males for melanoma (O = 10, SMR = 104.3, 95% CI = 50.0 to 191.9 vs O = 5, SMR = 28.3, 95% CI = 9.1 to 66.1) and brain/CNS cancers (O = 12, SMR = 47.0, 95% CI = 24.3 to 82.2 vs O = 5, SMR = 12.8, 95% CI = 4.1 to 29.8). In contrast, SMRs for noncancer causes of death after hereditary retinoblastoma did not differ by retinoblastoma treatment or sex.
Table 3.
SMR and AER for selected cancers by years since treatment for hereditary retinoblastoma
| Cancer type | Age, y |
||||
|---|---|---|---|---|---|
| <15 | 15–29 | 30–44 | 45–59 | ≥60 | |
| All subsequent cancers | |||||
| O | 55 | 69 | 74 | 55 | 14 |
| SMR* (95% CI) | 71.1 (53.5 to 92.5) | 89.9 (70.0 to 113.8) | 33.6 (26.4 to 42.2) | 14.1 (10.7 to 18.4) | 6.7 (3.6 to 11.2) |
| AER | 38.0 | 62.1 | 97.6 | 196.4 | 327.5 |
| Bone | |||||
| O | 31 | 33 | 9 | 5 | 1 |
| SMR* (95% CI) | 886.5 (602.2 to >1000) | 695.9 (479.0 to 977.4) | 591.4 (269.9 to >1000) | 539.3 (173.8 to >1000) | — |
| AER | 21.7 | 30.0 | 12.2 | 19.2 | — |
| Soft tissue sarcoma and connective tissue | |||||
| O | 4 | 12 | 18 | 14 | 4 |
| SMR* (95% CI) | 166.1 (44.7 to 425.2) | 316.4 (163.3 to 552.8) | 410.1 (242.9 to 648.1) | 380.5 (207.8 to 638.5) | 318.6 (85.7 to 815.6) |
| AER | 2.8 | 10.9 | 24.4 | 53.6 | 109.8 |
| Nasal cavities, middle ear and sinus | |||||
| O | 4 | 5 | 2 | 4 | 0 |
| SMR* (95% CI) | >1000 (>1000 to >1000) | >1000 (969.8 to >1000) | 521.6 (58.6 to >1000) | 916.6 (246.6 to >1000) | — |
| AER | 2.8 | 4.5 | 2.7 | 15.4 | — |
| Melanoma | |||||
| O | 1 | 8 | 4 | 2 | 0 |
| SMR* (95% CI) | — | 210.4 (90.6 to 414.5) | 37.9 (10.2 to 97.1) | 21.3 (2.4 to 77.0) | — |
| AER | — | 7.2 | 5.3 | 7.3 | — |
| Brain, and other parts of the nervous system | |||||
| O | 8 | 0 | 7 | 2 | 0 |
| SMR* (95% CI) | 44.6 (19.2 to 87.9) | — | 42.9 (17.2 to 88.4) | 12.6 (1.4 to 45.4) | — |
| AER | 5.5 | — | 9.3 | 7.1 | — |
| Lung and trachea | |||||
| O | 1 | 1 | 5 | 5 | 0 |
| SMR* (95% CI) | — | — | 15.5 (5.0 to 36.1) | 4.8 (1.5 to 11.2) | — |
| AER | — | — | 6.4 | 15.2 | — |
| Corpus uteri | |||||
| O | 0 | 0 | 4 | 4 | 1 |
| SMR* (95% CI) | — | — | 202.5 (54.5 to 518.4) | 81.2 (21.8 to 207.9) | — |
| AER | — | — | 11.7 | 31.1 | — |
| Bladder | |||||
| O | 0 | 0 | 2 | 4 | 1 |
| SMR* (95% CI) | — | — | 130.0 (14.6 to 469.4) | 79.0 (21.3 to 202.3) | — |
| AER | — | — | 2.7 | 15.2 | — |
SMRs, CIs, and AERs not presented for deaths <2. AER = absolute excess risk per 10 000 person-years; CI = confidence interval; O = observed number of cancer deaths; SMR = standardized mortality ratio.
Table 4.
SMR and AER for subsequent cancers by treatment for hereditary retinoblastoma
| Causes of death | Radiation without chemotherapy* (N = 535) |
Radiation with chemotherapy* (N = 435) |
|||||
|---|---|---|---|---|---|---|---|
| O | SMR† | (95% CI) | AER | O | SMR† (95% CI) | AER | |
| All causes of death‡ | 220 | 7.2 (6.2 to 8.2) | 106.2 | 251 | 11.7 (10.3 to 13.3) | 181.2 | |
| All malignant cancer excluding retinoblastoma§ | 123 | 25.1 (20.9 to 29.9) | 66.5 | 121 | 38.0 (31.5 to 45.4) | 93.0 | |
| Bone | 34 | 612.8 (424.3 to 856.4) | 19.1 | 41 | >1000 (765.9 to >1000) | 32.3 | |
| Soft tissue sarcoma and connective tissue | 23 | 302.1 (191.5 to 453.3) | 12.9 | 24 | 435.4 (278.9 to 647.9) | 18.9 | |
| Nasal cavities, middle ear and sinus | 8 | >1000 (559.5 to >1000) | 4.5 | 7 | >1000 (668.4 to >1000) | 5.5 | |
| Melanoma | 7 | 54.0 (21.6 to 111.3) | 3.9 | 6 | 62.1 (22.7 to 135.3) | 4.7 | |
| Brain and other parts of the nervous system | 11 | 35.2 (17.5 to 63.0) | 6.0 | 4 | 17.1 (4.6 to 43.7) | 3.0 | |
| Lung and trachea | 5 | 4.6 (1.5 to 10.7) | 2.2 | 4 | 6.7 (1.8 to 17.1) | 2.7 | |
| Corpus uteri | 5 | 102.7 (33.1 to 239.7) | 5.9 | 3 | 84.1 (16.9 to 245.7) | 4.8 | |
| Bladder | 3 | 46.0 (9.2 to 134.3) | 1.7 | 3 | 96.8 (19.5 to 282.9) | 2.3 | |
| Breast | 2 | 4.6 (0.5 to 16.8) | 0.9 | 1 | — | — | |
| Pancreas | 2 | 9.2 (1.0 to 33.3) | 1.0 | 2 | 14.3 (1.6 to 51.7) | 1.5 | |
| Large intestine | 0 | — | — | 3 | 16.7 (3.4 to 48.8) | 2.2 | |
| Buccal cavity and pharynx | 2 | 24.6 (2.8 to 88.9) | 1.1 | 2 | 36.3 (4.1 to 131.1) | 1.5 | |
| Kidney | 2 | 16.7 (1.9 to 60.2) | 1.1 | 1 | — | — | |
| Retroperitoneum | 0 | — | — | 2 | 335.4 (37.7 to >1000) | 1.6 | |
| Nonmelanoma | 2 | 96.9 (10.9 to 349.8) | 1.1 | 0 | — | — | |
| Cervix uteri | 0 | — | — | 2 | 29.1 (3.3 to 105.1) | 3.1 | |
| Lymphoma and hematopoietic | 0 | — | — | 1 | — | — | |
| Ovary | 1 | — | — | 1 | — | — | |
| Liver and gallbladder | 0 | — | — | 1 | — | — | |
| Prostate | 0 | — | — | 1 | — | — | |
| Thyroid | 0 | — | — | 1 | — | — | |
| Benign neoplasms‖ | 2 | 80.2 (9.0 to 289.5) | 1.1 | 1 | — | — | |
| Brain/central nervous system | 1 | — | — | 0 | — | — | |
| Other known causes of death¶ | 26 | 1.0 (0.7 to 1.5) | 0.2 | 15 | 0.8 (0.5 to 1.4) | −2.5 | |
| Circulatory system | 4 | 0.9 (0.2 to 2.2) | −0.4 | 1 | — | — | |
| Arteriosclerotic heart disease including CHD | 3 | 1.3 (0.3 to 3.7) | 0.3 | 1 | — | — | |
| External causes | 4 | 0.4 (0.1 to 1.1) | −2.8 | 4 | 0.6 (0.2 to 1.6) | −1.9 | |
| Suicide | 0 | — | — | 1 | — | — | |
| Symptoms, senility, and ill-defined conditions | 8 | 8.8 (3.8 to 17.4) | 4.0 | 1 | — | — | |
| Digestive system | 1 | — | — | 2 | 2.4 (0.3 to 8.6) | 0.9 | |
| Nervous system and sense organs | 2 | 2.5 (0.3 to 8.9) | 0.7 | 2 | 3.8 (0.4 to 13.7) | 1.2 | |
| Infectious and parasitic disease | 0 | — | — | 3 | 3.0 (0.6 to 8.8) | 1.6 | |
| Respiratory diseases | 2 | 1.2 (0.1 to 4.4) | 0.3 | 2 | 1.9 (0.2 to 6.7) | 0.7 | |
| Allergic, endocrine, metabolic, and nutritional disease | 2 | 2.4 (0.3 to 8.7) | 0.7 | 0 | — | — | |
Median follow-up, 33.4 and 29.4 years for hereditary survivors treated with radiation alone and radiation plus chemotherapy, respectively. AER = absolute excess risk per 10 000 person-years; CHD = coronary heart disease; CI = confidence interval; O = observed number of cancers; SMR = standardized mortality ratio.
SMRs, CIs, and AERs not presented for deaths <2.
Total all causes includes deaths due to in situ, uncertain, or secondary cancers (radiation without chemotherapy: n = 2, ICD-9 238.1, ICD-10 D48.9; radiation and chemotherapy: n = 4, ICD-9 238.1, ICD-10 D48.3) and unknown causes of death (radiation without chemotherapy: n = 10; radiation and chemotherapy: n = 1). Deaths due to retinoblastoma includes 57 treated with radiation without chemotherapy and 109 treated with radiation and chemotherapy.
Sixteen malignant cancers not listed for hereditary retinoblastoma survivors treated with radiation without chemotherapy include other malignant cancer (n = 16, ICD-9 194.4, 195.0, 199.1; ICD-10, C69.6, C80, C97). Eleven malignant cancers not listed for hereditary retinoblastoma survivors treated with radiation and chemotherapy include other malignant cancer (n = 11, ICD-9 199.0, 199.1; ICD-10 C38.0, C76.2, C80, C97).
Total benign includes lipoma (radiation and chemotherapy: n = 1, ICD-9 214.9) and other benign neoplasm (radiation without chemotherapy: n = 1, ICD-9 226.2).
Total includes diseases of the blood and blood-forming organs (radiation without chemotherapy: n = 1, ICD-10 D68.9), disease of the vascular lesion of central nervous system (radiation without chemotherapy: n = 1, ICD-9 430.9), and other noncancer causes of death (radiation without chemotherapy: n = 1, ICD-10 H66.9).
Discussion
In this large-scale study of retinoblastoma survivors, we demonstrate that mortality patterns substantially differ by hereditary status, cause of death, calendar year of retinoblastoma diagnosis, treatment, and time since diagnosis. Among survivors of nonhereditary retinoblastoma, long-term mortality was largely comparable to age-matched individuals in the general population overall and for both cancer- and noncancer-related deaths specifically. Hereditary retinoblastoma survivors also did not appear to have excess risk of death due to noncancer causes. However, with 13 additional years of follow-up and more than twice as many survivors older than 50 years since our previous report (10), we demonstrated substantially increased risk of death due to subsequent cancers persisting up to 70 years after hereditary retinoblastoma diagnosis, particularly for sarcomas. Our current analyses identified for the first time excess mortality risks for cancers of the pancreas, large intestines, and kidney, as well as more precisely estimating excess mortality risks for melanoma and cancers of the brain/CNS, lung, uterine corpus, breast, and bladder (reported previously based on <5 deaths). Additionally, by adding a cohort of 143 retinoblastoma survivors diagnosed during 1997–2006, we show continued improvements in mortality for more recently treated survivors, albeit based on small numbers. Because of advances in clinical practice, mortality for more recently treated survivors may also be influenced by surveillance for subsequent cancers and/or treatment options, but we lack data to evaluate this.
Our results provide reassuring evidence that survivors of nonhereditary retinoblastoma do not have elevated risk of death due to subsequent cancers overall or noncancer causes, even in long-term survivors (6–10). The elevated risk for sarcomas based on two deaths likely represents misclassified hereditary status rather than a true increased risk because we relied on medical records to determine hereditary status due to lack of RB1 mutation data for the entire cohort, although treatment exposures could play a role. We also observed weakly elevated risk of death due to breast cancer after both nonhereditary and hereditary retinoblastoma, based on six and five deaths, respectively. Although ionizing radiation exposure is known to increase breast cancer risk, the inconsistent results in previous studies (3, 15) and low dose to the breast from scatter radiation (16) suggest that further research is needed to clarify the risk of breast cancer after retinoblastoma.
Among hereditary retinoblastoma survivors, patterns of cause-specific mortality differed substantially by calendar year of retinoblastoma diagnosis and treatment. Most hereditary survivors in this cohort received radiotherapy, with about half also treated with systemic chemotherapy. Although risk for death due to subsequent cancers was strikingly elevated in both groups, the SMRs and AERs were slightly higher for those survivors who received radiotherapy with systemic chemotherapy versus radiotherapy alone, consistent with previous incidence-based analyses (2). Reduced mortality due to retinoblastoma among more recently treated patients supports known improvements in retinoblastoma treatment approaches, specifically the increased use of chemotherapy in place of radiotherapy since the 1970s (17). In our data, the only exception to this pattern was a higher mortality rate from retinoblastoma for patients treated with radiotherapy and chemotherapy combined during 1970–1989, likely due to improved short-term survival among patients with more advanced retinoblastoma. In contrast, deaths due to subsequent cancers did not change appreciably for hereditary retinoblastoma survivors diagnosed prior to 1990, but were reduced for the most recently treated patients. These results are consistent with data from the United Kingdom (7), although longer-term follow-up is needed in both studies to confirm the findings. Additionally, because our study only includes survivors diagnosed with retinoblastoma through 2006, we cannot evaluate the impact of ophthalmic artery chemosurgery on mortality (18).
We also observed important differences in mortality patterns with very long-term follow-up. Although hereditary retinoblastoma patients are known to have strikingly increased SMRs for bone and soft tissue sarcomas as well as nasal cavity cancers (6–8), the large sample size and long-term follow-up in this analysis enabled us to demonstrate that these risks persist 60 years and more following retinoblastoma. Most of the nasal cavity cancers were likely sarcomas, based on an earlier incidence analysis that included histology (19). We also found that several deaths from common adult cancer sites such as uterine corpus, kidney, and large intestines were sarcomas. Increased risks for incident leiomyosarcoma of the uterus had been reported previously (20), however, sarcomas of the latter two organ sites have not been reported. The strikingly elevated sarcoma risk after hereditary retinoblastoma has been attributed to a combination of genetic susceptibility and treatment (2, 21).
We identified a novel increased risk for pancreatic cancer in this cohort and confirmed previous reports of increased melanoma risk after retinoblastoma (3, 4, 22). RB1 is in the same cell cycle control pathway as other major melanoma and pancreatic cancer susceptibility genes, including CDKN2A and CDK4 (23, 24). Melanoma-prone families with CDKN2A germline mutations also have increased pancreatic cancer risk (24). Whereas pancreatic cancer deaths occurred later in our cohort, the highest mortality risks due to melanoma occurred at ages 15–29 years, earlier than in the general population but similar to familial melanoma. The higher SMR for melanoma in women could reflect their lower general population melanoma mortality rates (1).
Although the evidence is less clear, germline RB1 mutations also may contribute to the excess risk of lung, bladder, and brain/CNS cancers that we and others (3) observed, because RB1 has been shown to be somatically altered in a small proportion of these tumors (25). Whereas the lung and bladder cancer deaths tended to occur later in life, the increased risk of death due to brain/CNS tumors in young retinoblastoma survivors could be due to a combination of genetic susceptibility and ionizing radiation exposure (26). Notably, although cigarette smoking is associated with increased risks for lung, bladder, and pancreatic cancers, past analyses of smoking behavior in this cohort indicated that smoking rates did not differ by hereditary status and were similar to the general population (27, 28).
In this and other mortality studies that rely on NDI linkage, limitations include a lack of detailed information on causes of death (eg, histology for cancers). We may have underestimated mortality because of potential incomplete ascertainment of deaths, particularly before 1979, and because we lacked information on emigration. In addition, concurrent ICD coding for eye/orbit made it difficult to distinguish between deaths due to retinoblastoma and subsequent orbital sarcomas before 1979, which likely led to the underestimation of sarcoma risks and may have contributed to death reports attributed to retinoblastoma many years after initial diagnosis. To evaluate the amount of potential misclassification due to coding, one of us (MAT) reviewed all 165 death certificates coded 1901 before 1979 and found that 11 (6.7%) were likely orbital sarcomas rather than retinoblastoma. Even with our large patient population, we had limited numbers of patients whose deaths were due to most specific causes other than sarcomas, limiting our ability to statistically compare SMRs and AERs among subgroups of survivors. Finally, our cohort covered a limited geographic region, because most survivors resided in the northeastern United States.
The results of this study are strengthened by availability of treatment information, length of follow-up, large number of survivors, and comparison of mortality for hereditary and nonhereditary retinoblastoma survivors. Despite the large sample size, some risk estimates remain imprecise and would benefit from combining data with other similar cohorts, particularly for patients treated more recently. The lack of excess mortality due to subsequent cancers among survivors with nonhereditary retinoblastoma and to noncancer causes among both hereditary and nonhereditary survivors is very encouraging. Hereditary retinoblastoma survivors died mainly from an excess risk of subsequent cancers up to six decades later, highlighting the need to develop long-term clinical management guidelines for hereditary retinoblastoma survivors treated in the past.
Funding
This work was supported in part by the Intramural Research Program, National Cancer Institute, National Institutes of Health (RAK, LMM, MAT, BSS).
Notes
Affiliations of authors: Division of Cancer Epidemiology and Genetics, Department of Health and Human Services, National Cancer Institute, National Institutes of Health, Bethesda, MD (RAK, MAT, BSS, LMM); Ocular Oncology Service, Department of Surgery, Memorial Sloan Kettering Cancer Center, New York, NY (DHA); Department of Ophthalmology and Visual Sciences, University of Massachusetts Medical School, Worcester, MA (JMS)
The authors have no conflicts of interest to report. The funders had no role in the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication. The lead and corresponding authors had full access to the data in the study and final responsibility for the decision to submit for publication.
Supplementary Material
References
- 1. Noone AM,, Howlader N, Krapcho M, eds, et al. SEER Cancer Statistics Review, 1975–2015. Bethesda, MD: National Cancer Institute. https://seer.cancer.gov/csr/1975_2015/. Accessed April 2018.
- 2. Wong JR, Morton LM, Tucker MA, et al. Risk of subsequent malignant neoplasms in long-term hereditary retinoblastoma survivors after chemotherapy and radiotherapy. J Clin Oncol. 2014;3229:3284–3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Marees T, Moll AC, Imhof SM, et al. Risk of second malignancies in survivors of retinoblastoma: more than 40 years of follow-up. J Natl Cancer Inst. 2008;10024:1771–1779. [DOI] [PubMed] [Google Scholar]
- 4. MacCarthy A, Bayne AM, Brownbill PA, et al. Second and subsequent tumours among 1927 retinoblastoma patients diagnosed in Britain 1951-2004. Br J Cancer. 2013;10812:2455–2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Temming P, Arendt M, Viehmann A, et al. Incidence of second cancers after radiotherapy and systemic chemotherapy in heritable retinoblastoma survivors: a report from the German reference center. Pediatr Blood Cancer. 2017;641:71–80. [DOI] [PubMed] [Google Scholar]
- 6. Marees T, van Leeuwen FE, de Boer MR, et al. Cancer mortality in long-term survivors of retinoblastoma. Eur J Cancer. 2009;4518:3245–3253. [DOI] [PubMed] [Google Scholar]
- 7. Fidler MM, Reulen RC, Winter DL, et al. Long term cause specific mortality among 34 489 five year survivors of childhood cancer in Great Britain: population based cohort study. BMJ. 2016;354:i4351.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Acquaviva A, Ciccolallo L, Rondelli R, et al. Mortality from second tumour among long-term survivors of retinoblastoma: a retrospective analysis of the Italian retinoblastoma registry. Oncogene. 2006;2538:5350–5357. [DOI] [PubMed] [Google Scholar]
- 9. Temming P, Arendt M, Viehmann A, et al. How eye-preserving therapy affects long-term overall survival in heritable retinoblastoma survivors. J Clin Oncol. 2016;3426:3183–3188. [DOI] [PubMed] [Google Scholar]
- 10. Yu CL, Tucker MA, Abramson DH, et al. Cause-specific mortality in long-term survivors of retinoblastoma. J Natl Cancer Inst. 2009;1018:581–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Abramson DH, Shields CL, Munier FL, et al. Treatment of retinoblastoma in 2015: agreement and disagreement. JAMA Ophthalmol. 2015;13311:1341–1347. [DOI] [PubMed] [Google Scholar]
- 12. Kleinerman RA, Tucker MA, Tarone RE, et al. Risk of new cancers after radiotherapy in long-term survivors of retinoblastoma: an extended follow-up. J Clin Oncol. 2005;2310:2272–2279. [DOI] [PubMed] [Google Scholar]
- 13. Gooley TA, Leisenring W, Crowley J, et al. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;186:695–706. [DOI] [PubMed] [Google Scholar]
- 14. Arias E. United States life tables 2000. Natl Vital Stat Rep. 2002;513:1–38. [PubMed] [Google Scholar]
- 15. Reulen RC, Frobisher C, Winter DL, et al. Long-term risks of subsequent primary neoplasms among survivors of childhood cancer. JAMA. 2011;30522:2311–2319. [DOI] [PubMed] [Google Scholar]
- 16. Little MP, Schaeffer ML, Reulen RC, et al. Breast cancer risk after radiotherapy for heritable and non-heritable retinoblastoma: a US-UK study. Br J Cancer. 2014;11010:2623–2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shinohara ET, DeWees T, Perkins SM.. Subsequent malignancies and their effect on survival in patients with retinoblastoma. Pediatr Blood Cancer. 2014;611:116–119. [DOI] [PubMed] [Google Scholar]
- 18. Habib LA, Francis JH, Fabius AW, et al. Second primary malignancies in retinoblastoma patients treated with intra-arterial chemotherapy: the first 10 years. Br J Ophthalmol. 2018;1022:272–275. [DOI] [PubMed] [Google Scholar]
- 19. Kleinerman RA, Tucker MA, Abramson DH, et al. Risk of soft tissue sarcomas by individual subtype in survivors of hereditary retinoblastoma. J Natl Cancer Inst. 2007;991:24–31. [DOI] [PubMed] [Google Scholar]
- 20. Francis JH, Kleinerman RA, Seddon JM, et al. Increased risk of secondary uterine leiomyosarcoma in hereditary retinoblastoma. Gynecol Oncol. 2012;1242:254–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wong FL, Boice JD Jr, Abramson DH, et al. Cancer incidence after retinoblastoma. Radiation dose and sarcoma risk . JAMA. 1997;27815:1262–1267. [DOI] [PubMed] [Google Scholar]
- 22. Kleinerman RA, Yu CL, Little MP, et al. Variation of second cancer risk by family history of retinoblastoma among long-term survivors. J Clin Oncol. 2012;309:950–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Witkiewicz AK, McMillan EA, Balaji U, et al. Whole-exome sequencing of pancreatic cancer defines genetic diversity and therapeutic targets. Nat Commun. 2015;6:6744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tucker MA, Elder DE, Curry M, et al. Risks of melanoma and other cancers in melanoma-prone families over 4 decades. J Invest Dermatol. 2018; 138(7):1620–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lawrence MS, Stojanov P, Mermel CH, et al. Discovery and saturation analysis of cancer genes across 21 tumour types. Nature. 2014;5057484:495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Neglia JP, Robison LL, Stovall M, et al. New primary neoplasms of the central nervous system in survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2006;9821:1528–1537. [DOI] [PubMed] [Google Scholar]
- 27. Foster MC, Kleinerman RA, Abramson DH, et al. Tobacco use in adult long-term survivors of retinoblastoma. Cancer Epidemiol Biomarkers Prev. 2006;158:1464–1468. [DOI] [PubMed] [Google Scholar]
- 28. Kleinerman RA, Tarone RE, Abramson DH, et al. Hereditary retinoblastoma and risk of lung cancer. J Natl Cancer Inst. 2000;9224:2037–2039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.