Abstract
Background
Low-grade chronic inflammation, characterized by elevations in plasma Interleukin-6 (IL-6), is an independent risk factor of impaired mobility in older persons. Angiotensin receptor blockers and omega-3 polyunsaturated fatty acids (ω-3) may reduce IL-6 and may potentially improve physical function. To assess the main effects of the angiotensin receptor blocker losartan and ω-3 as fish oil on IL-6 and 400 m walking speed, we conducted the ENRGISE Pilot multicenter randomized clinical trial.
Methods
The ENRGISE Pilot enrolled participants between April 2016 and June 2017, who participated for 12 months. Participants were aged ≥70 years with mobility impairment, had IL-6 between 2.5 and 30 pg/mL, and were able to walk 400 m at baseline. Participants were randomized in three strata 2 × 2 factorial to: (i) losartan 50–100 mg/d or placebo (n = 43), (ii) fish oil 1,400–2,800 mg/d or placebo (n = 180), and (iii) with both (n = 66).
Results
Two hundred eighty-nine participants were randomized (mean age 78.3 years, 47.4% women, 17.0% black). There was no effect of losartan (difference of means = −0.065 ± 0.116 [SE], 95% confidence interval [CI]: −0.293–0.163, p = .58) or fish oil (−0.020 ± 0.077, 95% CI: −0.171–0.132, p = .80) on the log of IL-6. Similarly, there was no effect of losartan (−0.025 ± 0.026, 95% CI: −0.076–0.026, p = .34) or fish oil (0.010 ± 0.017, 95% CI: −0.025–0.044, p = .58) on walking speed (m/s).
Conclusions
These results do not support the use of these interventions to prevent mobility loss in older adults at risk of disability with low-grade chronic inflammation.
Registration
Clinicaltrials.gov NCT02676466.
Keywords: Inflammation, Aging, Walking speed, Factorial design, Multicenter trial
Preserving mobility is central to maintaining a high quality of life and participation in activities to be fully independent in the community (1). Mobility limitation is associated with subsequent hospitalization, nursing home placement, increased healthcare costs, and death (2,3). Low-grade chronic inflammation, characterized by elevations in plasma C-reactive protein, tumor necrosis factor-alpha (TNF-alpha), and particularly interleukin-6 (IL-6), is an independent risk factor of disability, impaired mobility, and slow walking speed (4). Low-grade chronic inflammation is a modifiable risk factor. However, it is unknown whether interventions that reduce the levels of inflammatory markers in younger healthier individuals are effective in the older people with mobility limitations; if so, will reduction in inflammation per se improve mobility, or avert decline in mobility in older persons.
To address this gap in evidence, we have conducted the randomized clinical trial ENRGISE (ENabling Reduction of low-Grade Inflammation in SEniors) Pilot study to test the ability of anti-inflammatory interventions for improving or preserving walking ability. We chose interventions that we expected to be safe, tolerable, acceptable, and affordable for vulnerable older persons. We have tested the efficacy of the angiotensin receptor blocker (ARB) losartan (LO) and omega-3 polyunsaturated fatty acids (ω-3) as fish oil.
Methods
Study Design
The study design has been described in detail elsewhere (5). Briefly, ENRGISE was a multicenter double-blind placebo-controlled three strata clinical trial testing losartan and fish oil in isolation and in a 2 × 2 factorial. This pilot study was implemented to obtain preliminary data allowing refinement of a set of key trial design considerations, including the primary outcome of major mobility disability, sample size calculations, methods for recruitment, participant retention, adherence to and safety of the interventions, and organizational infrastructure, and to provide internal validity concerning the efficacy of the losartan and fish oil interventions by assessing their effects on IL-6 and 400-meter walking speed.
The study was conducted at five clinical centers (Northwestern University, Tufts University, University of Florida, University of Pittsburgh, and Wake Forest School of Medicine [WFSM]) between April 2016 and June 2018.
Data management and statistical analyses were performed at WFSM, and the Administrative Coordinating Center was at the University of Florida. Both study entry and outcome IL-6 level were measured at the Central Laboratory at the University of Vermont. The study was approved by the local institutional review boards. A data and safety monitoring board monitored safety and the conduct of the trial; participants gave written informed consent. The protocol is consistent with the principles of the Declaration of Helsinki and is registered at Clinicaltrials.gov with identifier NCT02676466.
Participants and Assessments
We enrolled men and women aged 70 years and older who self-reported difficulty walking one-quarter of a mile or climbing a flight of stairs, had a 4 m walking speed at usual pace of less than 1 m/s but were able to complete the 400 m walk, and had a plasma IL-6 of 2.5–30 pg/mL based on the average of two measures taken 1–3 weeks apart, with the first measure being between 2.3 and 30 pg/mL. Participants were excluded if they reported smoking, acute infection within 1 month, autoimmune disease, severe arthritis, a neurological condition causing low walking speed, or other conditions that may interfere with the participation in the trial (see details in Manini et al. (5)). Participants taking an angiotensin-converting enzyme inhibitor (ACEI), ARB, or potassium-sparing diuretic, or those with bilateral renal artery stenosis, liver cirrhosis, serum potassium ≥5.0 mEq/l, intolerance or allergy to ARBs, hypotension, estimated glomerular filtration rate <15 mL/min per 1.73 m2, congestive heart failure with ejection fraction <40%, or with type 2 diabetes and taking aliskerin were excluded from the losartan strata. Those who had eaten more than two servings per week of fatty fish in the past year, were taking fish oil, had intolerance or allergy to fish oil or fish/shellfish, or with history of paroxysmal or persistent atrial fibrillation were excluded from the fish oil strata. Temporary exclusion criteria also included acute myocardial infarction, deep venous thrombosis, pulmonary embolism, major arrhythmias, or stroke within 6 months, recent major surgery, uncontrolled hypertension, and uncontrolled diabetes. The recruitment target was 300 participants approximately 69% female, 20% racial minorities, and 5% Hispanic.
Participants were recruited primarily by means of mass mailing, community outreach, and media advertising (6). Potential participants were screened by telephone interviews to assess the main inclusion/exclusion criteria. Those who qualified were invited for the first screening visit during which a brief informed consent was obtained and the 4 m walk at usual pace was administered. Plasma IL-6 was tested in those with a walking speed <1 m/s and >0.14 m/s.
IL-6 was initially measured with a sandwich immunoassay (Human IL-6 Quantikine ELISA Kit, catalog #HS600B, R & D Systems, Minneapolis, MN). Levels greater than 10 pg/mL were measured by using the Human IL-6 QuantiGlo ELISA Kit, catalog #Q6000B, R & D Systems. Inconsistency in lot-to-lot reagents and the frequency of reruns required a shift in format. Therefore, later in the study, IL-6 was measured using the Meso Scale Discovery (MDS) platform (Mesoscale Discovery, Rockville, MD), using a singleplex format (Proinflammatory Panel 1 NHP IL-6, catalog #K156QXG). This assay has a sensitivity of 0.095 pg/mL and was run in ENRGISE with an overall coefficient of variation of 8%. With a greater dynamic range, no dilutions or reruns with a different assay were needed. The correlation coefficient of this assay correlated to the ELISA was 0.9373 using test serum sets, and 0.8557 for direct comparison of ENRGISE samples (influenced by the use of the two different ELISA assay formats). There was a shift in standardization, corrected by adjusting all MSD data to the original scale with the regression equation ELISA-IL-6 = MSD-IL-6/0.3868, based on direct comparison of ENRGISE samples (n = 80).
Participants with qualifying IL-6 levels were invited for the second screening visit to complete blood testing (CBC, lipids, chemical panel, from Quest Diagnostics and second IL-6 measure), blood pressure, and pulse. Validated diet (7–9) and physical activity (10) questionnaires were distributed for completion at home and reviewed at the baseline visit. If there were no safety concerns, participants attended the baseline visit during which we administered the full informed consent, the 400 m walk, the Mini-Mental State Examination (MMSE) (11), a complete medical and health history (including the drug inventory for medication use and the items to assess frailty) (12), the short physical performance battery, and we measured height, weight, and grip strength. If there were no exclusion criteria identified, the participants were randomized, and the study drugs were dispensed. The baseline measures (except height, MMSE, diet, and physical activity) were repeated every 3 months for 12 months of follow-up in all participants. Blood pressure, and pulse, blood tests, and adverse events were measured 1–2 weeks after randomization and after each visit when the losartan dose was modified.
Interventions
Participants were assigned a stratum based on eligibility and then randomized to losartan or placebo (target n = 75), fish oil or placebo (target n = 75), or one of the four combinations in the 2 × 2 factorial of losartan and fish oil with their placebos (target n = 150).
Losartan and matching placebo were obtained from Almac Group, Souderton, PA, in 25 and 50 mg capsules; they had identical shape, color, taste, and weight. We initiated participants at a dose of 25 mg/d. If tolerated, we increased the participant dose to 50 mg/d after 1–2 weeks. If there were no safety concerns, we continued the participant on 50 mg/d until the 6-month visit. If the average of IL-6 measured at 3- and 6-month visits did not decrease by >40% from baseline, we further increased the dose to 100 mg/d. Losartan was reduced in dose or discontinued if there were adverse events, such as hypotension, hyperkalemia, reduction in glomerular filtration rate, or other events that could be related to losartan.
Fish oil and placebo (corn oil) were obtained from Epax, Aalesund, Norway, in 0.7 g gel caps; they had identical shape, color, and weight. Each 0.7 g of fish oil contained 400 mg eicosapentaenoic acid and 200 mg docosahexaenoic acid. The purity and composition of fish oil was assessed by means of nuclear magnetic resonance spectroscopy. We initiated participants at a dose of 1.4 g/d of fish oil and continued until the 6-month visit. The fish oil or placebo intervention was discontinued if there was incident atrial fibrillation, intolerance to fish oil, or otherwise unexplained increases in fasting glucose or low density lipoproteins, or anemia. If the average of IL-6 levels measured at 3- and 6-month visits did not decrease by >40% from baseline, we increased the dose to 2.8 g/d. If there was an acute illness within 1 month prior to the visit, the 3-month or the 6-month IL-6 measure was postponed or excluded from titration algorithms.
Sample Size, Power, Statistical Analyses, and Study Outcomes
Details of the study design have been previously published (5). The primary outcomes were plasma IL-6 and walking speed over 400 m at usual pace at 12 months. The primary comparisons of losartan and fish oil were made using contrasts at 12 months from a mixed model using all three strata with adjustment for the baseline level of the outcome, visit, strata, and clinical site. Inflammation markers were log-transformed prior to analysis. All participants with baseline and at least one follow-up visit were included in the analysis according to their randomized group, consistent with the intention to treat principle. We report here point estimates and 95% confidence intervals of main effects as well as p-values of two-sided tests. The target sample size of 300 was based on marginal comparisons (135 and 165/group) of each active intervention and placebo using one-sided tests at the 10% level. We did not expect definitive evidence; our goal was to exclude small effects with little clinical value. For IL-6, we had 91% power to detect an effect if the difference (on the natural log scale) was at least 0.1625; this is equivalent to the difference between 4.20 and 3.57 pg/mL (or a 15% effect). We had 66% power for a 10% effect and 99% power for a 20% effect. For 400 m walk speed, we had >99% power to detect a difference of 0.095 m/s (a substantial meaningful change), and 86% power for a difference of 0.038 m/s (a small meaningful change).
Results
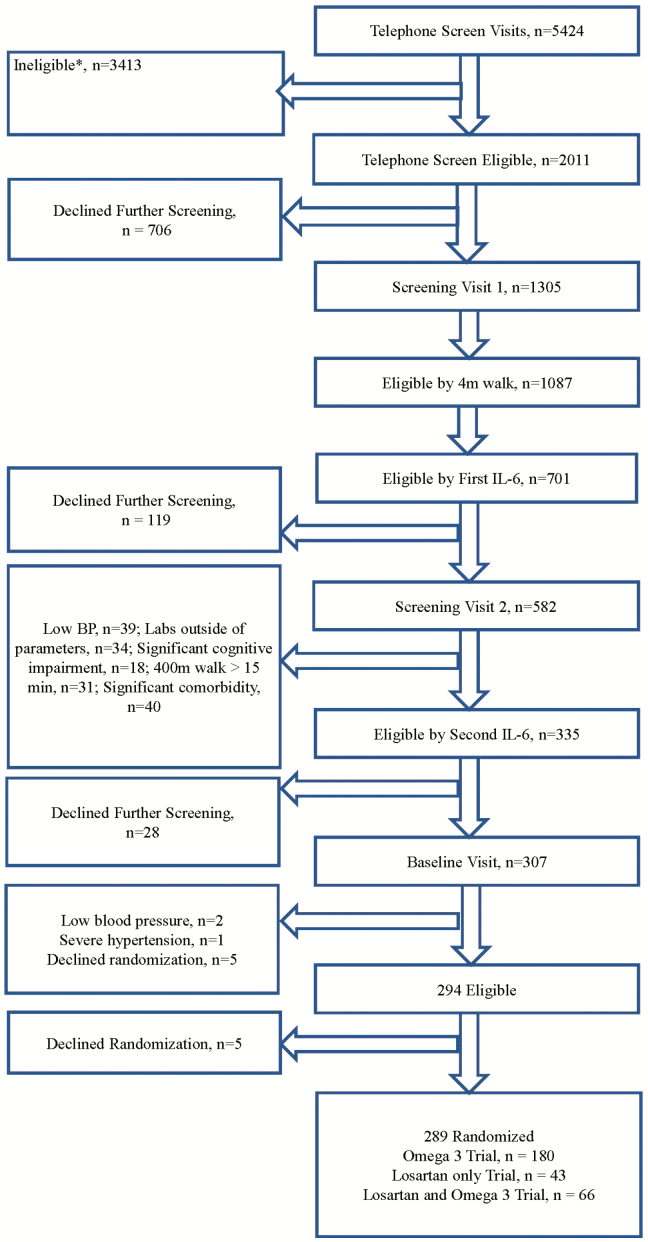
Of the 5,424 persons who were initially screened by phone, a total of 290 (5.3%) were ultimately randomized. The main reasons for exclusion are outlined in Figure 1. Among 1,087 participants who had a slow 4 m walking speed (<1 m/s), 781 (71.8%) qualified by first IL-6 level. The mean age of the 290 participants was 77.6 years (SD 5.4 years); 47.4% were women, 22.1% were racial/ethnic minorities, and had a 400 m walking speed of 0.8 m/s and a BMI of 31.4 kg/m2. The randomized groups had similar baseline characteristics (Table 1).
Figure 1.
Consort flow chart. Potential participants could be ineligible for more than one reason. *No self-reported difficulty walking ¼ mile or climbing steps, n = 1,183. *Current use of ACEI or ARB for those ineligible for both Losartan and Fish Oil trials, n = 623. *Currently taking omega-3/fish oil for those ineligible for both Losartan and Fish Oil trials, n = 344. *Usually use a walker to get around, n = 246. * Known/active inflammatory disease, n = 230. *Prior or current atrial fibrillation, for those ineligible for both Losartan and *Unable to walk one block, n = 204. *Current smoker, n = 185. *Currently receiving physical therapy for gait, balance, n = 123. *Use of potassium sparing diuretics for those ineligible for both Losartan and Fish Oil trials, n = 96. *Lives outside area/relocations, n = 86. *Neurologic conditions, impaired mobility, n = 75. *Allergy to fish, shell fish, and/or fish oil for those ineligible for both Losartan and Fish Oil trials, n = 70. *Acute Infection, n = 66. *>14 alcohol drinks/week, n = 53. *Severe pulmonary disease, n = 47. *Participation in another intervention study, n = 47. *Consumed more than two servings per week of fish in the past year for those ineligible for both Losartan and Fish Oil trials, n = 44. *Age <70, n = 41.
Table 1.
Baseline Characteristics by Randomized Group
| Treatment | |||||
|---|---|---|---|---|---|
| Overall (N = 289) | Placebo (N = 102) | Losartan Only (N = 39) | Fish oil (N = 122) | Combination (N = 26) | |
| Age in years | 77.6 ± 5.4 | 77.4 ± 5.3 | 77.3 ± 4.9 | 78.0 ± 5.6 | 77.2 ± 5.6 |
| Age | |||||
| 70–79 y | 186 (87.3%) | 67 (90.5%) | 25 (89.3%) | 76 (81.7%) | 18 (100%) |
| 80–89 y | 20 (9.4%) | 6 (8.1%) | 3 (10.7%) | 11 (11.8%) | 0 (0.0%) |
| 90+ y | 7 (3.3%) | 1 (1.4%) | 0 (0.0%) | 6 (6.5%) | 0 (0.0%) |
| Female % | 137 (47.4%) | 49 (48.0%) | 18 (46.2%) | 58 (47.5%) | 12 (46.2%) |
| White % | 225 (77.9%) | 81 (79.4%) | 27 (69.2%) | 95 (77.9%) | 22 (84.6%) |
| African American % | 49 (17.0%) | 17 (16.7%) | 8 (20.5%) | 21 (17.2%) | 3 (11.5%) |
| Other/Mixed Race % | 15 (5.2%) | 4 (3.9%) | 4 (10.3%) | 6 (4.9%) | 1 (3.8%) |
| Hispanic % | 7 (2.4%) | 2 (2.0%) | 2 (5.1%) | 3 (2.5%) | 0 (0.0%) |
| Education | |||||
| Elementary School (K-08) | 3 (1.0%) | 1 (1.0%) | 1 (2.6%) | 1 (0.8%) | 0 (0.0%) |
| High School/Equivalent (09−12) | 87 (30.1%) | 26 (25.5%) | 9 (23.1%) | 46 (37.7%) | 6 (23.1%) |
| College (13−16) | 120 (41.5%) | 48 (47.1%) | 18 (46.2%) | 42 (34.4%) | 12 (46.2%) |
| Post Graduate | 69 (23.9%) | 24 (23.5%) | 9 (23.1%) | 28 (23.0%) | 8 (30.8%) |
| Other | 10 (3.5%) | 3 (2.9%) | 2 (5.1%) | 5 (4.1%) | 0 (0.0%) |
| BMI | 31.4 ± 5.7 | 31.1 ± 5.2 | 32.1 ± 6.9 | 31.5 ± 5.6 | 31.3 ± 6.0 |
| Weight in kg | 87.0 ± 17.8 | 86.5 ± 16.7 | 90.3 ± 19.5 | 86.4 ± 17.2 | 86.8 ± 21.8 |
| MMSE | 28.0 ± 1.7 | 27.9 ± 1.7 | 28.2 ± 1.6 | 28.1 ± 1.6 | 28.0 ± 1.9 |
| 400 m Walk Speed | 0.8 ± 0.2 | 0.8 ± 0.2 | 0.9 ± 0.2 | 0.8 ± 0.2 | 0.8 ± 0.2 |
| SPPB Total Score | 8.7 ± 2.1 | 8.4 ± 2.2 | 8.6 ± 2.1 | 8.9 ± 1.9 | 8.7 ± 2.0 |
| IL6: median and IQ range | 3.7 (2.8, 4.9) | 4.0 (2.8, 5.0) | 3.8 (3.1, 4.5) | 3.7 (2.8, 5.1) | 3.2 (2.4, 4.1) |
| Systolic Blood Pressure mmHg | 135.5 ± 18.0 | 133.2 ± 17.7 | 139.8 ± 13.8 | 135.1 ± 19.8 | 139.7 ± 14.0 |
| Diastolic Blood Pressure mmHg | 72.2 ± 10.1 | 71.4 ± 10.8 | 77.5 ± 8.5 | 70.3 ± 9.3 | 75.8 ± 9.2 |
| Grip Strength Right Hand kg | 25.4 ± 8.8 | 25.4 ± 8.1 | 26.6 ± 10.1 | 25.2 ± 8.6 | 24.1 ± 10.0 |
| Grip Strength Left Hand kg | 24.6 ± 8.6 | 24.5 ± 8.3 | 26.0 ± 9.6 | 24.2 ± 8.3 | 25.0 ± 10.0 |
| Hypertension | 200 (69.2%) | 71 (69.6%) | 22 (56.4%) | 96 (78.7%) | 11 (42.3%) |
| Cancer | 109 (37.7%) | 38 (37.3%) | 9 (23.1%) | 51 (41.8%) | 11 (42.3%) |
| Diabetes | 68 (23.5%) | 27 (26.5%) | 3 (7.7%) | 37 (30.3%) | 1 (3.8%) |
| Myocardial Infarction | 26 (9.0%) | 10 (9.8%) | 2 (5.1%) | 13 (10.7%) | 1 (3.8%) |
| Congestive Heart Failure | 8 (2.8%) | 2 (2.0%) | 0 (0.0%) | 6 (4.9%) | 0 (0.0%) |
| Stroke | 13 (4.5%) | 6 (5.9%) | 2 (5.1%) | 4 (3.3%) | 1 (3.8%) |
| Palpitations | 44 (15.2%) | 20 (19.6%) | 8 (20.5%) | 14 (11.5%) | 2 (7.7%) |
Note: BMI = Body mass index; MMSE = Mini-Mental State Examination; SPPB = short physical performance battery.
Self-reported adherence at 12 months is presented in Table 2. It was excellent for fish oil and modest for losartan. Retention and the treatment effects are presented in Table 3.
Table 2.
Self-reported Adherence to the Interventions at 12 mo of Follow-up, Regardless of Stratum
| Losartan N = 57 |
Placebo losartan N = 42 |
Fish oil N = 138 |
Placebo fish oil N = 88 |
|
|---|---|---|---|---|
| Excellent/very good/good N (%) |
34 (60%) | 19 (50%) | 115 (85%) | 68 (77%) |
| Fair/poor/very poor N (%) |
2 (4%) | 1 (3%) | 5 (4%) | 3 (3%) |
| Discontinued N (%) |
21 (37%) | 18 (47%) | 16 (12%) | 17 (19%) |
Table 3.
Retention of the Study Participants for IL-6 and 400 m Walk Assessments, and Effect of Losartan, Fish Oil, and Combination of Losartan + Fish Oil on IL-6 and 400 m Walk Speed
| Losartan | Fish Oil | ||||||
|---|---|---|---|---|---|---|---|
| Month | Active N = 65 N (%) | Placebo N = 44 N (%) | Effect ± SE (95% CI); p-value | Active N = 148 N (%) | Placebo N = 98 N (%) | Effect (SE), 95% CI: p-value | |
| Natural log of il6 | 3 | 58 (89.2%) | 40 (90.9%) | −0.152 ± 0.096 (−0.342, 0.038); p = .12 | 137 (92.6%) | 92 (93.9%) | 0.098 ± 0.063 (−0.026, 0.222); p = .12 |
| 6 | 57 (87.7%) | 37 (84.1%) | −0.201 ± 0.116 (−0.429, 0.026); p = .08 | 134 (90.5%) | 87 (88.8%) | 0.019 ± 0.075 (−0.130, 0.167); p = .80 | |
| 9 | 52 (80.0%) | 34 (77.3%) | 0.047 ± 0.114 (−0.178, 0.271); p = .68 | 130 (87.8%) | 78 (79.6%) | 0.044 ± 0.074 (−0.102, 0.190); p = .55 | |
| 12 | 54 (83.1%) | 37 (84.1%) | −0.065 ± 0.116 (−0.293, 0.163); p = .58 | 129 (87.2%) | 80 (81.6%) | −0.020 ± 0.077 (−0.171, 0.132); p = .80 | |
| Average | −0.093 ± 0.081 (−0.253, 0.067); p = .26 | 0.035 ± 0.053 (−0.069, 0.140); p = .51 | |||||
| 400 m walking speed | 3 | 54 (83.1%) | 37 (84.1%) | 0.016 ± 0.020 (−0.023, 0.055); p = .43 | 131 (88.5%) | 86 (87.8%) | 0.005 ± 0.013 (−0.021, 0.030); p = .72 |
| 6 | 51 (78.5%) | 33 (75.0%) | 0.009 ± 0.021 (−0.033, 0.051); p = .68 | 122 (82.4%) | 79 (80.6%) | 0.019 ± 0.014 (−0.008, 0.047); p = .17 | |
| 9 | 48 (73.8%) | 30 (68.2%) | −0.030 ± 0.026 (−0.082, 0.022); p = .26 | 115 (77.7%) | 72 (73.5%) | 0.013 ± 0.017 (−0.021, 0.047); p = .45 | |
| 12 | 44 (67.7%) | 34 (77.3%) | −0.025 ± 0.026 (−0.076, 0.026); p = .34 | 113 (76.4%) | 67 (68.4%) | 0.010 ± 0.017 (−0.025, 0.044); p = .58 | |
| Average | −0.007 ± 0.020 (−0.048, 0.033); p = .71 | 0.012 ± 0.013 (−0.015, 0.038); p = .39 | |||||
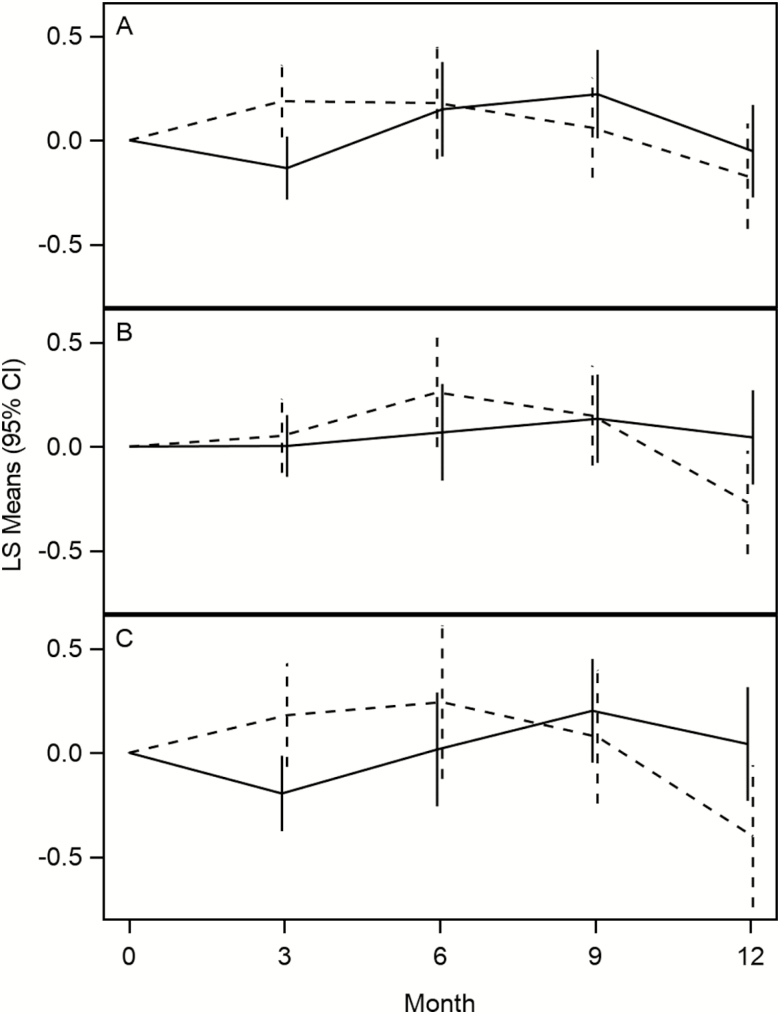
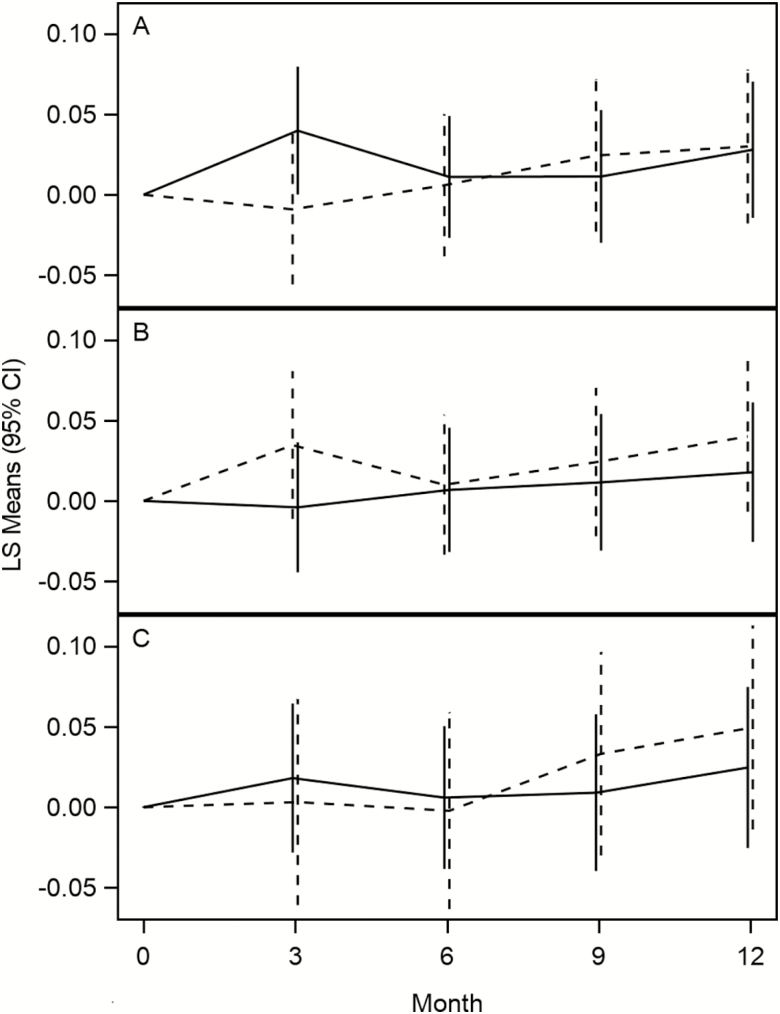
When analyzing the main effects after 12 months of intervention, there was no effect of losartan (−0.065 ± 0.116 [SE], 95% CI: −0.293–0.163, p = .58) or fish oil (−0.020 ± 0.077, 95% CI: −0.171–0.132, p = .80) on the log of IL-6. Similarly, there was no effect of losartan (−0.025 ± 0.026, 95% CI: −0.076–0.026, p = .34) or fish oil (0.010 ± 0.017, 95% CI: −0.025–0.044, p = .58) on walking speed (m/s). The main effects at other times are presented in Table 3. There was also no evidence of an interaction of losartan and fish oil in the group that was eligible to be randomized to both losartan + fish oil for either the log of IL-6 (p = .75) or walking speed (p = .75). Plots of adjusted means over time are presented for IL-6 and walking speed in Figures 2 and 3. As an exploratory analysis, we have also compared the combination of fish oil and losartan to placebo from the third stratum (Figures 2C and 3C). Serious adverse events and adverse events may be seen in Table 4; the rates were generally low.
Figure 2.
Change in IL-6 pg/mL over time. (A) Losartan (solid line) vs no losartan (dotted line). (B) Fish oil (solid line) vs no fish oil (dotted line). (C) Losartan + fish oil (solid line) vs placebo (dotted line).
Figure 3.
Change in 400 m walk speed m/s over time. (A) Losartan (solid line) vs no losartan (dotted line). (B) Fish oil (solid line) vs no fish oil (dotted line). (C) Losartan + fish oil (solid line) vs placebo (dotted line).
Table 4.
SAE and AE After 12 mo of Follow-up
| MedDRA Category (Or Predefined) | Overall (N = 289) | Placebo (N = 102) | Losartan (N = 39) | Fish Oil (N = 122) | Combination (N = 26) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Events | People (%) | Events | People (%) | Events | People (%) | Events | People (%) | Events | People (%) | ||
| SAE | Cardiac disorders | 15 | 14 (4.8%) | 9 | 8 (7.8%) | 1 | 1 (2.6%) | 5 | 5 (4.1%) | 0 | 0 (0.0%) |
| Gastrointestinal disorders | 5 | 4 (1.4%) | 0 | 0 (0.0%) | 0 | 0 (0.0%) | 5 | 4 (3.3%) | 0 | 0 (0.0%) | |
| General disorders and administration site conditions | 4 | 4 (1.4%) | 3 | 3 (2.9%) | 0 | 0 (0.0%) | 1 | 1 (0.8%) | 0 | 0 (0.0%) | |
| Hepatobiliary disorders | 2 | 1 (0.3%) | 2 | 1 (1.0%) | 0 | 0 (0.0%) | 0 | 0 (0.0%) | 0 | 0 (0.0%) | |
| Infections and infestations | 8 | 8 (2.8%) | 2 | 2 (2.0%) | 0 | 0 (0.0%) | 4 | 4 (3.3%) | 2 | 2 (7.7%) | |
| Injury, poisoning and procedural complications | 9 | 9 (3.1%) | 2 | 2 (2.0%) | 1 | 1 (2.6%) | 4 | 4 (3.3%) | 2 | 2 (7.7%) | |
| Metabolism and nutrition disorders | 2 | 2 (0.7%) | 1 | 1 (1.0%) | 0 | 0 (0.0%) | 1 | 1 (0.8%) | 0 | 0 (0.0%) | |
| Musculoskeletal and connective tissue disorders | 11 | 9 (3.1%) | 1 | 1 (1.0%) | 1 | 1 (2.6%) | 9 | 7 (5.7%) | 0 | 0 (0.0%) | |
| Neoplasms benign, malignant and unspecified (including cysts and polyps) | 3 | 3 (1.0%) | 1 | 1 (1.0%) | 0 | 0 (0.0%) | 1 | 1 (0.8%) | 1 | 1 (3.8%) | |
| Nervous system disorders | 12 | 11 (3.8%) | 5 | 5 (4.9%) | 3 | 2 (5.1%) | 3 | 3 (2.5%) | 1 | 1 (3.8%) | |
| Renal and urinary disorders | 8 | 5 (1.7%) | 4 | 3 (2.9%) | 0 | 0 (0.0%) | 3 | 1 (0.8%) | 1 | 1 (3.8%) | |
| Respiratory, thoracic and mediastinal disorders | 2 | 2 (0.7%) | 2 | 2 (2.0%) | 0 | 0 (0.0%) | 0 | 0 (0.0%) | 0 | 0 (0.0%) | |
| Skin and subcutaneous tissue disorders | 2 | 2 (0.7%) | 0 | 0 (0.0%) | 0 | 0 (0.0%) | 2 | 2 (1.6%) | 0 | 0 (0.0%) | |
| Spinal stenosis-lumbar | 1 | 1 (0.3%) | 0 | 0 (0.0%) | 0 | 0 (0.0%) | 1 | 1 (0.8%) | 0 | 0 (0.0%) | |
| Surgical and medical procedures | 7 | 7 (2.4%) | 4 | 4 (3.9%) | 0 | 0 (0.0%) | 1 | 1 (0.8%) | 2 | 2 (7.7%) | |
| Vascular disorders | 4 | 4 (1.4%) | 0 | 0 (0.0%) | 0 | 0 (0.0%) | 3 | 3 (2.5%) | 1 | 1 (3.8%) | |
| AE | Cardiac disorders | 11 | 9 (3.1%) | 4 | 3 (2.9%) | 3 | 2 (5.1%) | 4 | 4 (3.3%) | 0 | 0 (0.0%) |
| Dizziness/presyncope | 20 | 18 (6.2%) | 2 | 2 (2.0%) | 11 | 9 (23.1%) | 3 | 3 (2.5%) | 4 | 4 (15.4%) | |
| Eye disorders | 13 | 12 (4.2%) | 4 | 4 (3.9%) | 1 | 1 (2.6%) | 6 | 5 (4.1%) | 2 | 2 (7.7%) | |
| Fall (mechanical) | 22 | 19 (6.6%) | 9 | 9 (8.8%) | 1 | 1 (2.6%) | 9 | 8 (6.6%) | 3 | 1 (3.8%) | |
| Gastrointestinal disorders | 10 | 9 (3.1%) | 3 | 3 (2.9%) | 2 | 2 (5.1%) | 5 | 4 (3.3%) | 0 | 0 (0.0%) | |
| General disorders and administration site conditions | 21 | 19 (6.6%) | 8 | 8 (7.8%) | 7 | 5 (12.8%) | 3 | 3 (2.5%) | 3 | 3 (11.5%) | |
| GI upset | 17 | 17 (5.9%) | 7 | 7 (6.9%) | 0 | 0 (0.0%) | 7 | 7 (5.7%) | 3 | 3 (11.5%) | |
| Infections and infestations | 69 | 54 (18.7%) | 26 | 19 (18.6%) | 11 | 9 (23.1%) | 29 | 24 (19.7%) | 3 | 2 (7.7%) | |
| Injury, poisoning, and procedural complications | 38 | 33 (11.4%) | 12 | 10 (9.8%) | 6 | 5 (12.8%) | 17 | 15 (12.3%) | 3 | 3 (11.5%) | |
| Investigations | 25 | 17 (5.9%) | 9 | 7 (6.9%) | 2 | 1 (2.6%) | 12 | 7 (5.7%) | 2 | 2 (7.7%) | |
| Metabolism and nutrition disorders | 10 | 10 (3.5%) | 4 | 4 (3.9%) | 2 | 2 (5.1%) | 3 | 3 (2.5%) | 1 | 1 (3.8%) | |
| Musculoskeletal and connective tissue disorders | 85 | 65 (22.5%) | 21 | 16 (15.7%) | 15 | 14 (35.9%) | 41 | 30 (24.6%) | 8 | 5 (19.2%) | |
| Nervous system disorders | 32 | 29 (10.0%) | 13 | 10 (9.8%) | 7 | 7 (17.9%) | 11 | 11 (9.0%) | 1 | 1 (3.8%) | |
| Renal and urinary disorders | 10 | 8 (2.8%) | 7 | 5 (4.9%) | 2 | 2 (5.1%) | 1 | 1 (0.8%) | 0 | 0 (0.0%) | |
| Respiratory, thoracic, and mediastinal disorders | 18 | 16 (5.5%) | 3 | 3 (2.9%) | 4 | 3 (7.7%) | 9 | 8 (6.6%) | 2 | 2 (7.7%) | |
| Surgical and medical procedures | 44 | 33 (11.4%) | 11 | 7 (6.9%) | 9 | 7 (17.9%) | 18 | 14 (11.5%) | 6 | 5 (19.2%) | |
| Vascular disorders | 13 | 12 (4.2%) | 4 | 4 (3.9%) | 4 | 3 (7.7%) | 4 | 4 (3.3%) | 1 | 1 (3.8%) | |
| Other | 52 | 51 (17.6%) | 19 | 19 (18.6%) | 10 | 10 (25.6%) | 19 | 18 (14.8%) | 4 | 4 (15.4%) | |
Note: AE = Adverse events; GI = gastrointestinal; SAE = Serious adverse events.
Discussion
There was a high rate of inflammation in persons screened for this study who had slow gait speed (Figure 1). Also, we demonstrated excellent retention and good adherence to the fish oil intervention. In this study, Losartan or fish oil did not demonstrate any significant effects on our primary outcomes of IL-6 or walking speed over 400 m.
These findings were unexpected as preliminary review of the literature and our recent systematic review and meta-analysis (13) suggested that both losartan and fish oil may reduce chronic low-grade inflammation and potentially improve or avert decline in walking speed.
ARBs are primarily indicated for the treatment of hypertension heart failure with reduced ejection fraction. Losartan is also a partial PPAR-γ agonist (14), yielding anti-inflammatory effects (15). Losartan has been shown to reduce IL-6 in another human study at similar doses as those used in ENRGISE pilot (16), and in an animal study (17), possibly through PPAR-γ/AMPK pathways, blocking proinflammatory angiotensin II AT1 signaling, or both (15,18). ARBs have also shown to mitigate lipopolysaccharide-mediated inflammation, and inhibit TNF-α-mediated endothelial Receptor for Advanced Glycosylation Endproduct (RAGE) expression (19). Losartan has been shown to improve skeletal muscle related activity measures in older mice (17,20).
Fish oil and ω-3 fatty acids are indicated to reduce triglyceride levels in adults with severe hypertriglyceridemia. Fish oil and ω-3 fatty acids are also potentially anti-inflammatory (21) via a specific ω-3 fatty acid receptor, GP120 (22) which blocks NF-KB and JNK signaling, counter to saturated fatty acid-mediated proinflammatory TLR2 signaling (23). Saturated fats are generally proinflammatory (24), and can cause a decline in muscle protein synthesis via ER stress (25). ω-3 supplementation has shown to downregulate inflammation (26) in part through decreased TNF-α expression and ω-3 fatty acids are likely to improve mTOR signaling, thereby stimulating muscle protein synthesis in humans (27). A meta-analysis of several randomized clinical trials has shown that ω-3 reduces both IL-6 and C-reactive protein in chronic nonautoimmune disease (28).
In summary, inflammation plays a major role in the loss of physical function through a wide variety of mechanisms, many of which offer interventional targets. Losartan (14,18,29) and ω-3 (30,31), in addition to potentially averting inflammation, may also affect vasculature, coagulation, metabolism, and skeletal muscle, all of which may benefit mobility.
The lack of effect of losartan and fish oil on IL-6 and walking speed we found in ENRGISE may be due to several factors possibly related to the characteristics of population recruited into this study including older persons with multiple morbidities and frailty. Insufficient dosage, the high rate of study drug withdrawals in the losartan group, and the high prevalence of overweight or obesity may also have played a role. However, regarding the latter point, in stratified analyses according to low/normal and high BMI the results were unchanged (data not shown). The very strict safety monitoring criteria we have applied to the losartan interventions may have prevented the increase in losartan dosage and caused a large number of withdrawals from the losartan group. To support this hypothesis, in the losartan group, both active losartan and placebo arms had similar rates of withdrawals, suggesting that the withdrawals from the losartan group were due to the very strict safety criteria and to factors related to the participants, not to losartan safety (Table 2). In addition, in the fish oil only group, participants may have been on an ACE inhibitors or ARB already for health reasons, and despite the chronic ACE/ARB use, still had low-grade inflammation. That may have indicated that they were likely to be resistant to the relatively weaker anti-inflammatory effects of the fish oil. Publication bias, may have also played a role in the favorable findings of the meta-analyses (13,28).
The ENRGISE pilot study has several strengths including the double blinded randomized clinical trial design, the intention to treat analyses, recruitment of high risk subjects who are often excluded from clinical trials, use of walking speed to efficiently screen subjects in the field, and excellent retention. The study has also a number of limitations, including failure to meet losartan enrollment goals due to high prevalence of use of angiotensin receptor blockers in the community and low adherence to losartan and placebo, and potentially limited 1 year duration of the trial.
In conclusion, neither losartan nor fish oil modified IL-6 or walking speed in older adults with low-grade chronic inflammation and mobility limitations. These results do not support use of these interventions to prevent mobility loss or reduce levels of inflammation in older adults at risk of disability with low-grade chronic inflammation.
Funding
The ENRGISE Pilot Study is funded by a National Institutes of Health/National Institute on Aging Cooperative Agreement U01AG050499. The research is partially supported by the Claude D. Pepper Older Americans Independence Centers at the University of Florida (P30AG028740), Wake Forest University (P30AG021332), Tufts University (P30AG031679), and University of Pittsburgh (P30AG024827). Tufts University is also supported by the Boston Rehabilitation Outcomes Center (R24HD065688-01A1). R.A.F. (Tufts University) is partially supported by the U.S. Department of Agriculture, under agreement No. 58-1950-0-014. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the U.S. Department of Agriculture. Abbott Laboratories provided funding for the purchase of study drug and matching placebo. The ENRGISE Pilot Study Acknowledgement List is available online as a supplementary file.
Supplementary Material
The ENRGISE investigators are listed in Supplementary Appendix 1.
References
- 1. Shumway-Cook A, Patla A, Stewart A, Ferrucci L, Ciol MA, Guralnik JM. Environmental components of mobility disability in community-living older persons. J Am Geriatr Soc. 2003;51:393–398. [DOI] [PubMed] [Google Scholar]
- 2. Newman AB, Simonsick EM, Naydeck BL, et al. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA. 2006;295:2018–2026. doi: 10.1001/jama.295.17.2018 [DOI] [PubMed] [Google Scholar]
- 3. Hardy SE, Kang Y, Studenski SA, Degenholtz HB. Ability to walk ¼ mile predicts subsequent disability, mortality, and health care costs. J Gen Intern Med. 2011;26:130–135. doi: 10.1007/s11606-010-1543-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Penninx BW, Kritchevsky SB, Newman AB, et al. Inflammatory markers and incident mobility limitation in the elderly. J Am Geriatr Soc. 2004;52:1105–1113. doi: 10.1111/j.1532-5415.2004.52308.x [DOI] [PubMed] [Google Scholar]
- 5. Manini TM, Anton SD, Beavers DP, et al. ; ENRGISE Pilot study investigators. Enabling reduction of low-grade inflammation in seniors pilot study: concept, rationale, and design. J Am Geriatr Soc. 2017;65:1961–1968. doi: 10.1111/jgs.14965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cauley JA, Manini TM, Lovato L, et al. The enabling reduction of low-grade inflammation in seniors (ENRGISE) pilot study: screening methods and recruitment results. J Gerontol A Biol Sci Med Sci. 2018; in press. doi: 10.1093/gerona/gly204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Diet History Questionnaire. Version 1.0. Bethesda, MD: National Institutes of Health, Applied Research Program, National Cancer Institute; 2007. [Google Scholar]
- 8. Tasevska N, Park Y, Jiao L, Hollenbeck A, Subar AF, Potischman N. Sugars and risk of mortality in the NIH-AARP diet and health study. Am J Clin Nutr. 2014;99:1077–1088. doi: 10.3945/ajcn.113.069369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Subar AF, Thompson FE, Kipnis V, et al. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires: the eating at America’s table study. Am J Epidemiol. 2001;154:1089–1099. [DOI] [PubMed] [Google Scholar]
- 10. Stewart AL, Mills KM, King AC, Haskell WL, Gillis D, Ritter PL. CHAMPS physical activity questionnaire for older adults: outcomes for interventions. Med Sci Sports Exerc. 2001;33:1126–1141. [DOI] [PubMed] [Google Scholar]
- 11. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 12. Fried LP, Tangen CM, Walston J, et al. ; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. [DOI] [PubMed] [Google Scholar]
- 13. Custodero C, Mankowski RT, Lee SA, et al. Evidence-based nutritional and pharmacological interventions targeting chronic low-grade inflammation in middle-age and older adults: a systematic review and meta-analysis. Ageing Res Rev. 2018;46:42–59. doi: 10.1016/j.arr.2018.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Koh EJ, Yoon SJ, Lee SM. Losartan protects liver against ischaemia/reperfusion injury through PPAR-γ activation and receptor for advanced glycation end-products down-regulation. Br J Pharmacol. 2013;169:1404–1416. doi: 10.1111/bph.12229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. An J, Nakajima T, Kuba K, Kimura A. Losartan inhibits LPS-induced inflammatory signaling through a PPARgamma-dependent mechanism in human THP-1 macrophages. Hypertens Res. 2010;33:831–835. doi: 10.1038/hr.2010.79 [DOI] [PubMed] [Google Scholar]
- 16. Trevelyan J, Brull DJ, Needham EW, Montgomery HE, Morris A, Mattu RK. Effect of enalapril and losartan on cytokines in patients with stable angina pectoris awaiting coronary artery bypass grafting and their interaction with polymorphisms in the interleukin-6 gene. Am J Cardiol. 2004;94:564–569. doi: 10.1016/j.amjcard.2004.05.017 [DOI] [PubMed] [Google Scholar]
- 17. Lin CH, Yang H, Xue QL, et al. Losartan improves measures of activity, inflammation, and oxidative stress in older mice. Exp Gerontol. 2014. doi: 10.1016/j.exger.2014.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kim JE, Choi HC. Losartan inhibits vascular smooth muscle cell proliferation through activation of AMP-activated protein kinase. Korean J Physiol Pharmacol. 2010;14:299–304. doi: 10.4196/kjpp.2010.14.5.299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Grossin N, Boulanger E, Wautier MP, Wautier JL. The different isoforms of the receptor for advanced glycation end products are modulated by pharmacological agents. Clin Hemorheol Microcirc. 2010;45:143–153. doi: 10.3233/CH-2010-1292 [DOI] [PubMed] [Google Scholar]
- 20. Burks TN, Andres-Mateos E, Marx R, et al. Losartan restores skeletal muscle remodeling and protects against disuse atrophy in sarcopenia. Sci Transl Med. 2011;3:82ra37. doi: 10.1126/scitranslmed.3002227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lucas L, Russell A, Keast R. Molecular mechanisms of inflammation. Anti-inflammatory benefits of virgin olive oil and the phenolic compound oleocanthal. Curr Pharm Des. 2011;17:754–768. [DOI] [PubMed] [Google Scholar]
- 22. Oh DY, Walenta E, Akiyama TE, et al. A Gpr120-selective agonist improves insulin resistance and chronic inflammation in obese mice. Nat Med. 2014;20:942–947. doi: 10.1038/nm.3614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Snodgrass RG, Huang S, Choi IW, Rutledge JC, Hwang DH. Inflammasome-mediated secretion of IL-1β in human monocytes through TLR2 activation; modulation by dietary fatty acids. J Immunol. 2013;191:4337–4347. doi: 10.4049/jimmunol.1300298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Forbes SC, Little JP, Candow DG. Exercise and nutritional interventions for improving aging muscle health. Endocrine. 2012;42:29–38. doi: 10.1007/s12020-012-9676-1 [DOI] [PubMed] [Google Scholar]
- 25. Deldicque L, Cani PD, Philp A, et al. The unfolded protein response is activated in skeletal muscle by high-fat feeding: potential role in the downregulation of protein synthesis. Am J Physiol Endocrinol Metab. 2010;299:E695–E705. doi: 10.1152/ajpendo.00038.2010 [DOI] [PubMed] [Google Scholar]
- 26. Raphael W, Sordillo LM. Dietary polyunsaturated fatty acids and inflammation: the role of phospholipid biosynthesis. Int J Mol Sci. 2013;14:21167–21188. doi: 10.3390/ijms141021167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Smith GI, Atherton P, Reeds DN, et al. Dietary omega-3 fatty acid supplementation increases the rate of muscle protein synthesis in older adults: a randomized controlled trial. Am J Clin Nutr. 2011;93:402–412. doi: 10.3945/ajcn.110.005611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li K, Huang T, Zheng J, Wu K, Li D. Effect of marine-derived n-3 polyunsaturated fatty acids on C-reactive protein, interleukin 6 and tumor necrosis factor alpha: a meta-analysis. PLoS One. 2014;9:e88103. doi: 10.1371/journal.pone.0088103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. de Gasparo M, Catt KJ, Inagami T, Wright JW, Unger T. International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol Rev. 2000;52:415–472. [PubMed] [Google Scholar]
- 30. Siddiqui RA, Shaikh SR, Sech LA, Yount HR, Stillwell W, Zaloga GP. Omega 3-fatty acids: health benefits and cellular mechanisms of action. Mini-Rev Med Chem. 2004;4:859–871. [DOI] [PubMed] [Google Scholar]
- 31. Calder PC. Mechanisms of action of (n-3) fatty acids. J Nutr. 2012;142:592S–599S. doi: 10.3945/jn.111.155259 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.