Abstract
Background
Oropharyngeal squamous carcinoma (OPSC) continues to increase in incidence secondary to human papillomavirus (HPV) infection. Despite the good overall prognosis for these patients, treatment with chemoradiation is associated with morbidity and treatment failure. Better predictors for disease outcome are needed to guide de-intensification regimens. We hypothesized that estrogen receptor α (ERα), a prognostic biomarker in oncology with therapeutic implications, might have similar utility in OPSC.
Methods
To investigate associations among ERα and demographics, HPV status, and survival, we analyzed ERα mRNA expression of head and neck squamous carcinomas (HNSC) from The Cancer Genome Atlas (TCGA) and immunohistochemistry (IHC) of pretreatment biopsy specimens from an independent group of 215 OPSC patients subsequently treated with primary chemoradiation (OPSC-CR). Associations among variables were evaluated with Fisher exact tests and logistic regression; associations with survival were evaluated with log-rank tests and Cox proportional hazards regression.
Results
Among 515 patients in TCGA, ERα mRNA expression was highest in HPV-positive OPSC. High ERα mRNA expression was associated with improved survival among those receiving chemoradiation (hazard ratio adjusted for HPV status = 0.44, 95% confidence interval = 0.21 to 0.92). In OPSC-CR, ERα was positive by IHC in 51.6% of tumors and was associated with improved overall, disease-specific, progression-free, and relapse-free survival (log-rank tests: P < .001, P < .001, P = .002, P = .003, respectively); statistically significant associations of ERα positivity with improved survival were maintained after adjusting for clinical risk factors including HPV status.
Conclusion
In two independent cohorts, ERα is a potential biomarker for improved survival that also may represent a therapeutic target in OPSC.
Unlike most types of head and neck squamous carcinoma (HNSC), oropharyngeal squamous carcinoma (OPSC) has been increasing in incidence worldwide (1–3) because of human papillomavirus (HPV) infection. Although patients with HPV-positive OPSC have an excellent prognosis with overall survival rates greater than 80% (4,5), many patients still suffer disease recurrence and treatment-related side effects (6). Therefore, there has been a growing interest to try to identify low-risk patients who may be candidates for de-intensification of therapy without compromising survival.
We investigated estrogen receptor α (ERα), one of the most commonly used biomarkers in oncology, as a biomarker in HNSC and OPSC. In breast cancer, ERα is used as both a prognostic biomarker and a therapeutic target. As ERα has been described in secondary lymphoid tissue (7) and has been implicated in carcinogenesis of HPV-positive cervical cancer (8–12), we hypothesized that ERα might have utility as a biomarker in OPSC.
Methods
Study Population, The Cancer Genome Atlas (TCGA) Cohort
Clinical data from TCGA HNSC project were downloaded in biotab format from TCGA legacy archive (https://portal.gdc.cancer.gov/legacy-archive/). Survival data in the patient file were updated from the follow-up files; therapy annotations were processed as described in Mroz et al. (13). HPV status was determined by mapping RNA-seq reads against the HPV genome as described by The Cancer Genome Atlas Network (14). Normalized RNA-seq by Expectation-Maximization (RSEM) data for TCGA HNSC were downloaded from http://firebrowse.org. The TCGA cohort included 515 patients with both clinical and nucleic acid sequencing data. ERα expression in this cohort was evaluated as ESR1 mRNA expression.
Study Population, OPSC Chemoradiation (OPSC-CR) Cohort
With permission from the Massachusetts Eye and Ear Human Studies Committee for retrospective chart review with waiver of informed consent (protocol 11-024H), we identified patients treated at Massachusetts Eye and Ear or Massachusetts General Hospital with a diagnosis of OPSC or cancer of unknown primary from 1997 to 2011. Patients were included if they had a biopsy-proven squamous cell carcinoma, an available pretreatment biopsy specimen for analysis, no prior head and neck irradiation or prior treatment, and definitive chemoradiation as the primary treatment modality. Demographic data including sex, age, race, tobacco use, alcohol use, site of primary tumor, and TNM classification following the 7th edition of the American Joint Committee on Cancer (AJCC) were collected. Race was self-reported. Alcohol abuse was defined as a history of more than five drinks per day or described clinically as alcoholic and/or alcohol abuse.
Immunohistochemistry (IHC) for ERα and p16
Formalin-fixed paraffin-embedded pretreatment biopsy specimens were sectioned at 5-µm thickness, deparaffinized, and reconstituted for pathologic evaluation. For ERα IHC, antigen retrieval was performed in a Borg retrieval waterbath with Borg Decloaker (Biocare Medical, Pacheco, CA) at 95°C–97°C for 45 minutes. Slides were blocked with BLOXALL HRP/AP solution for 10 minutes (Vector Laboratories, Burlingame, CA), followed by additional blocking with Horse Serum Rabbit HRP Immpress (Vector Laboratories) for 20 minutes. The solution was decanted and slides incubated with 1 to 100 anti-ERα (Abcam AB16660, rabbit monoclonal, clone SP1, Cambridge, MA) in 1% bovine serum albumin (BSA) in phosphate buffer saline (PBS) at 4°C overnight. After rinsing, secondary staining was performed using Immpress polymer anti-rabbit HRP for 30 minutes at room temperature (Vector Laboratories). Slides were then developed in ImmPact (Vector Laboratories) and terminated with double-distilled H2O. The slides were then counterstained in hematoxylin, dehydrated, and prepared for microscopy.
We used p16, the accepted and clinically recommended surrogate biomarker, to determine HPV status (15). Antigen retrieval was performed with EDTA for 24 minutes followed by automated staining with a mouse antibody against p16 (Ventana Discovery Ultra, Ventana, Oro Valley, AZ). Slides were developed with the Ventana OmniMap anti-mouse HRP system (Ventana).
A dedicated head and neck pathologist (WCF) and head and neck surgeon (JWR), blinded to outcomes, reviewed slides to evaluate ERα and p16. Slides were considered p16-positive if at least 70% of the tumor cells had both nuclear and cytoplasmic positivity (15). ERα presence was scored using a modified Allred score (16–18), considered positive if greater than 1% of cancer cells had nuclear staining following established guidelines for breast cancer (19). A known ERα positive breast cancer specimen and an endometrial specimen were used as positive controls. An independent blinded review of 20% of slides selected to include a range of positive and negative ERα scores by a second pathologist (KG) demonstrated excellent agreement for ERα (κ = 0.85, P < .001) and perfect agreement for p16.
Statistical Analyses
Single-sample gene set expression analysis (ssGSEA), following the approach of Barbie et al. (20), was performed with the Bioconductor GSVA package (https://bioconductor.org/packages/release/bioc/html/GSVA.html) on TCGA HNSC RNA-seq data (21) against the Protein Interaction Database’s curated gene set for ERα nuclear receptor signaling (with ESR1 removed from the gene set to avoid bias; Supplementary Table 1, available online) (22).
The relationship between demographic data and ERα status was evaluated by the Fisher exact test, the χ2 test, and logistic regression. Survival analysis was performed using log-rank and Cox proportional hazards regression. Study endpoints were overall survival (OS), disease-specific survival (DSS), progression-free survival (PFS), and relapse-free survival (RFS), expressed relative to time of first diagnostic biopsy. DSS was defined as time to death from OPSC, with patients dying from other or unknown causes censored at time of death. PFS was defined as the time to first documented relapse or progression. RFS was defined as the time until biopsy-proven recurrence. For RFS and PFS, we excluded patients with baseline metastatic disease. Analysis was performed with R v3.4 (https://cran.r-project.org) or SPSS v24 (IBM Corp., Armonk, NY) software. All statistical tests were two-sided and a P value of less than 0.05 was considered statistically significant. The cox.zph function in the R survival package was used to test the proportional hazards assumption. Survival models were validated and calibrated with the rms package in R.
Results
ERα Expression in the TCGA HNSC Cohort and Its Relationship to ERα Signaling and to Outcome
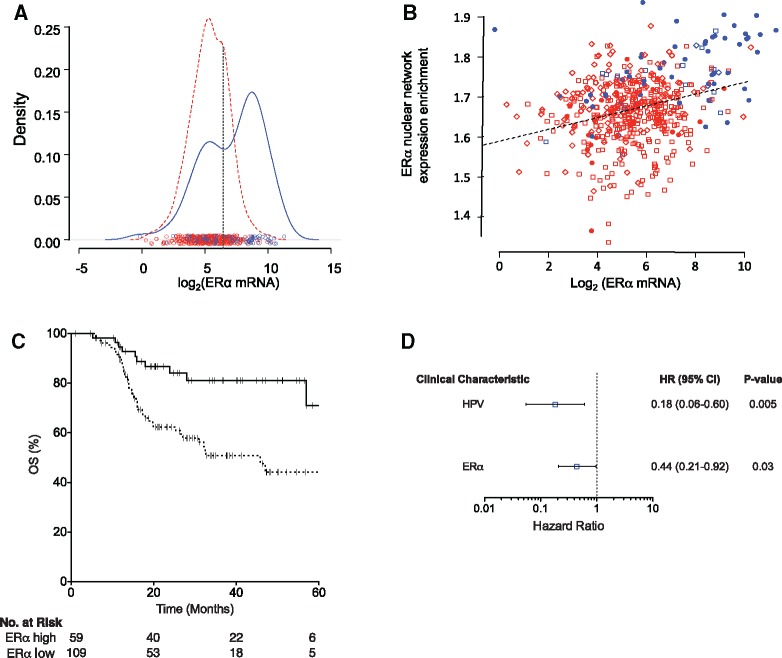
ERα expression was highest in HPV-positive tumors (Figure 1A), with a strong indication of two subpopulations, particularly in HPV-positive tumors. Multiple linear regression on several clinical variables indicated that HPV status was the major factor associated with ERα expression (Table 1).
Figure 1.
Estrogen receptor alpha (ERα) expression in The Cancer Genome Atlas (TCGA) head and neck squamous carcinoma (HNSC) cohort and its associations with human papillomavirus (HPV) status, ERα signaling, and outcome. A) Density plot of ERα mRNA expression stratified by HPV status; circles indicate individual values. Blue = HPV-positive tumors; red = HPV-negative tumors. The dashed vertical line at the dip in the distribution of expression values among HPV-positive HNSC indicates the value of 6.5 on the log2 scale used to distinguish low from high ERα expression. B) ERα network expression enrichment versus ERα expression. Blue symbols represent HPV-positive tumors; anatomic sites distinguished by shape (triangles = larynx; squares = oral cavity; circles = oropharynx). Dashed line represents linear regression relationship. C) Kaplan-Meier plot of relationship between ERα expression and overall survival of TCGA HNSC patients who received chemoradiation. Solid line = ERα-high; dashed line = ERα-low; hazard ratio = 0.29 (95% confidence interval = 0.14 to 0.59, P = .001. D) Hazard ratios determined by Cox regression incorporating HPV status and high ERα expression as predictors for TCGA HNSC patients who received chemoradiation. P values based on two-sided Wald tests. HR = hazard ratio.
Table 1.
Multiple regression analysis of relationships between ERα expression and clinical characteristics*
| Clinical characteristic | All TCGA HNSC |
OPSC-CR |
||
|---|---|---|---|---|
| Ratio of ERα mRNA (95% CI) | P * | OR for ERα-positive/negative (95% CI) | P * | |
| HPV status | ||||
| Negative | 1.00 (Referent) | 1.00 (Referent) | ||
| Positive | 3.71 (2.57 to 5.36) | <.001 | 4.04 (1.26 to 13.01) | .02 |
| N classification | ||||
| <N2b | 1.00 (Referent) | 1.00 (Referent) | ||
| ≥N2b | 0.75 (0.57 to 0.98) | .04 | 1.85 (0.97 to 3.54) | .06 |
| Sex | ||||
| Male | 1.00 (Referent) | 1.00 (Referent) | ||
| Female | 1.17 (0.91 to 1.50) | .22 | 2.00 (0.87 to 4.60) | .1 |
| Primary site tonsil | ||||
| No | 1.00 (Referent) | 1.00 (Referent) | ||
| Yes | 0.90 (0.57 to 1.43) | .66 | 1.44 (0.75 to 2.75) | .27 |
| Smoker | ||||
| Never | 1.00 (Referent) | 1.00 (Referent) | ||
| Ever | 0.76 (0.59 to 0.98) | .03 | 0.73 (0.37 to 1.44) | .36 |
| Age, y | ||||
| ≤60 | 1.00 (Referent) | 1.00 (Referent) | ||
| >60 | 1.04 (0.84 to 1.29) | .70 | 1.21 (0.60 to 2.44) | .59 |
| Metastasis at presentation | ||||
| No | 1.00 (Referent) | 1.00 (Referent) | ||
| Yes | 0.80 (0.31 to 2.07) | .65 | 0.88 (0.26 to 2.91) | .83 |
| T classification | ||||
| T≤2 | 1.00 (Referent) | 1.00 (Referent) | ||
| T >2 | 1.17 (0.90 to 1.52) | .25 | 0.92 (0.45 to 1.87) | .82 |
| Alcohol abuse | ||||
| No | — | — | 1.00 (Referent) | |
| Yes | 0.83 (0.34 to 2.04) | .69 | ||
| Keratinizing tumor | ||||
| No | — | — | 1.00 (Referent) | |
| Yes | 0.45 (0.20 to 0.99) | .048 | ||
P values were calculated using two-sided Wald tests. For The Cancer Genome Atlas (TCGA) head and neck squamous carcinoma (HNSC) cohort, multiple linear regression of log-transformed mRNA levels against the indicated variables; relationships with clinical variables are expressed as mRNA expression ratios on the nontransformed scale. For oropharyngeal squamous carcinoma chemoradiation (OPSC-CR) cohort, multiple logistic regression of positive/negative immunohistochemical staining against the indicated variables. CI = confidence interval; ERα = estrogen receptor α; OR = odds ratio.
To evaluate whether higher ERα expression was associated with activated ERα pathway signaling, we examined RNA-seq data on 63 genes in the curated Protein Interaction Database nuclear ERα-signaling gene set (22). ssGSEA showed that higher ERα expression was associated with higher expression of that gene set (P < .001), supporting functional relevance of ERα expression in these tumors. HPV-positive OPSC were enriched among the HNSC patients having both high ERα expression and greater than median activation of the nuclear ERα-signaling gene set (Fisher test, odds ratio = 10.43, 95% confidence interval [CI] = 5.40 to 20.54, P < .001; Figure 1B).
As a continuous predictor, log-transformed ERα mRNA expression was statistically significantly related to longer overall survival among TCGA HNSC patients who received chemoradiation as primary therapy or adjuvant to surgery; the hazard ratio (HR) per doubling of ERα mRNA was 0.75 (95% CI = 0.64 to 0.87, Wald test, P < .001). Spline fitting indicated no nonlinearity in the relationship between log-transformed ERα mRNA expression and survival (not shown). Clinical variables and single-variable associations with ERα expression for TCGA HNSC patients receiving chemoradiation are shown in Table 2. The relationship of ERα mRNA expression with survival is illustrated in Figure 1C, with expression stratified by the cutoff displayed in Figure 1A.
Table 2.
Clinical characteristics and their individual relationships with ERα status*
| Clinical characteristic | TCGA-chemoradiation subset No. (%) |
OPSC-CR No. (%) |
||||||
|---|---|---|---|---|---|---|---|---|
| Total (n = 168) | ERα-low† (n = 110) | ERα-high† (n = 56) | P ‡ | Total (n = 215) | ERα- (n = 104) | ERα+ (n = 111) | P ‡ | |
| Sex | ||||||||
| Male | 137 (81.5) | 88 (80.0) | 49 (87.5) | .28 | 174 (80.9) | 87 (83.7) | 87 (78.4) | .38 |
| Female | 31 (18.5) | 22 (20.0) | 7 (12.5) | 41 (19.1) | 17 (16.3) | 24 (21.6) | ||
| Age, y | ||||||||
| ≤60 | 111 (66.1) | 68 (61.8) | 42 (75.0) | .12 | 141 (65.6) | 67 (64.4) | 74 (66.7) | .78 |
| >60 | 57 (33.9) | 42 (38.2) | 14 (25.0) | 74 (34.4) | 37 (35.6) | 37 (33.3) | ||
| Race | ||||||||
| Caucasian | 141 (83.9) | 88 (82.2) | 51 (91.1) | .17 | 195 (90.7) | 91 (94.8) | 104 (97.2) | .48 |
| Non-Caucasian | 24 (14.3) | 19 (17.8) | 5 (8.9) | 8 (3.7) | 5 (5.2) | 3 (2.8) | ||
| Unknown | 3 (1.8) | — | — | 12 (5.6) | — | — | ||
| Primary site | ||||||||
| Tonsil | 27 (16.1) | 11 (10.0) | 15 (26.8) | .03 | 95 (44.2) | 40 (38.8) | 55 (49.6) | .003 |
| Base of tongue | 16 (9.5) | 10 (9.1) | 6 (10.7) | 103 (47.9) | 49 (47.6) | 54 (48.6) | ||
| Other OP | 6 (3.6) | 4 (3.6) | 2 (3.6) | 16 (7.4) | 14 (13.6) | 2 (1.8) | ||
| Non-OP | 119 (79.8) | 85 (77.2) | 33 (58.9) | — | — | — | ||
| Unknown | 0 | — | — | 1 (0.5) | — | — | ||
| Smoke Ever | ||||||||
| Never (<1 PY) | 43 (25.6) | 25 (22.7) | 17 (30.4) | .35 | 72 (33.5) | 29 (28.7) | 43 (39.4) | .11 |
| Ever (≥1 PY) | 125 (74.4) | 85 (77.3) | 39 (69.6) | 138 (64.2) | 72 (71.3) | 66 (60.6) | ||
| Unknown | 0 | — | — | 5 (2.3) | — | — | ||
| Alcohol abuse | ||||||||
| No | 64 (38.1) | 38 (69.1) | 25 (92.6) | .02 | 160 (74.4) | 75 (76.5) | 85 (85.0) | .15 |
| Yes | 19 (11.3) | 17 (30.9) | 2 (7.4) | 38 (17.7) | 23 (23.5) | 15 (15.0) | ||
| Unknown | 85 (50.6) | — | — | 17 (7.9) | — | — | ||
| Tumor classification | ||||||||
| T0–2 | 53 (31.5) | 28 (25.5) | 24 (42.9) | .03 | 146 (67.9) | 64 (65.3) | 82 (75.9) | .09 |
| T3–T4 | 115 (68.5) | 82 (74.5) | 32 (57.1) | 60 (27.9) | 34 (34.7) | 26 (24.1) | ||
| Unknown | 0 | — | — | 9 (4.2) | — | — | ||
| Nodal Classification | ||||||||
| N1–N2a | 63 (37.5) | 42 (38.2) | 21 (37.5) | 1 | 89 (41.4) | 48 (48.5) | 41 (38.0) | .16 |
| N2b–N3 | 105 (62.5) | 68 (61.8) | 35 (62.5) | 118 (54.9) | 51 (51.5) | 67 (62.0) | ||
| Unknown | 0 | — | — | 8 (3.7) | — | — | ||
| Metastasis at presentation | ||||||||
| No | 165 (98.2) | 107 (98.2) | 56 (100) | .55 | 197 (91.6) | 94 (94.9) | 103 (98.1) | .27 |
| Yes | 2 (1.2) | 2 (1.8) | 0 (0) | 7 (3.3) | 5 (5.1) | 2 (1.9) | ||
| Unknown | 1 (0.6) | — | 11 (5.1) | — | — | |||
| Keratinizing | ||||||||
| No | — | — | — | — | 165 (76.8) | 70 (68.6) | 95 (85.6) | .005 |
| Yes | 48 (22.3) | 32 (31.4) | 16 (14.4) | |||||
| Unknown | 2 (0.9) | — | — | |||||
| HPV status | ||||||||
| Negative | 123 (73.2) | 95 (86.4) | 28 (50.0) | <.001 | 38 (17.7) | 30 (28.8) | 8 (7.2) | <.001 |
| Positive | 43 (25.6) | 15 (13.6) | 28 (50.0) | 177 (82.3) | 74 (71.2) | 103 (92.8) | ||
| Unknown | 2 (1.2) | — | — | — | — | — | ||
“Unknown” omitted from analyses. ERα = estrogen receptor α; HPV = human papillomavirus; OP = oropharyngeal; OPSC-CR = oropharyngeal squamous carcinoma chemoradiation cohort; PY = pack-years; TCGA = The Cancer Genome Atlas
High versus low ERα based on mRNA expression cutoff shown in Figure 1A.
P values were calculated using a two-sided Fisher test.
A statistically significant association to survival was maintained when HPV status was taken into account in a Cox two-variable regression (HR per doubling of ERα mRNA, 0.82, 95% CI = 0.69 to 0.97, P = .02). Results of Cox two-variable regression based on the high vs low distinction of ERα mRNA expression are illustrated in Figure 1D; high ERα mRNA expression was associated with improved survival among those receiving chemoradiation (HR adjusted for HPV status = 0.44, 95% CI = 0.21 to 0.92). Proportional hazards assumptions were met (two-sided χ2 test for association of residuals with time, P > .6 for all coefficients).
ERα Expression and Outcomes in an OPSC-CR Cohort
We assessed the relationships among ERα expression, HPV status, and outcomes in an independent cohort of OPSC patients who had been treated with primary chemoradiation. We used antibodies validated for clinical use to assess HPV status (marked by expression of p16 (15) and ERα (23)).
Cohort Characteristics
Of 234 individuals in the OPSC-CR cohort who met inclusion criteria, 215 had adequate tumor specimen for p16 and ERα IHC. Clinical data for those patients are shown in Table 2. Specific chemotherapeutic regimen data were available for 183 patients (85.1%); the most common treatment regimens were carboplatin with paclitaxel, cetuximab, or cisplatin alone (Supplementary Table 2, available online). Radiation therapy dose information was available for 160 patients (74.4%), with a median 70 grays (Gy) total radiation (interquartile range [IQR] = 68–72). Therapy was in compliance with the National Comprehensive Cancer Network (NCCN) guidelines in place at the time of treatment.
The median follow-up of surviving patients was 7.0 years (IQR = 5.0–9.5 years). There were 51 deaths (23.7%) during the study period with a median time to death of 2.1 years (IQR = 1.3–3.7 years). Forty-one patients died from OPSC and two patients had progressive, unresectable disease before they proceeded with outside follow-up, totaling forty-three patients whose deaths were attributed to OPSC. There were 208 patients without metastatic disease at baseline of which 14 had progression of their disease with treatment (6.7%) and 31 had disease relapse (14.9%). Distributions of event and censoring times for OS, DSS, PFS, and RFS are shown in Supplementary Figure 1 (available online); censoring times were typically much later than most event times.
ERα Staining and Its Association With Outcome
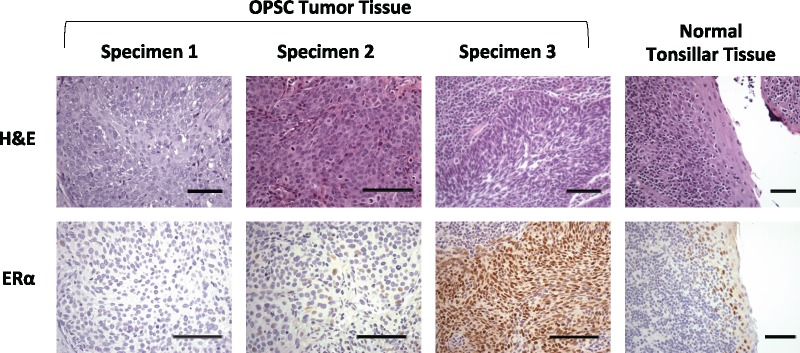
Over half of the OPSC-CR tumors (111/215, 51.6%) were ERα-positive based on accepted criteria for ERα IHC in breast cancer (19). Examples of ERα immunostaining are shown in Figure 2; controls and examples of p16 staining are shown in Supplementary Figure 2 (available online). A small subset (n = 14) had strong diffuse nuclear staining within the entire tumor (Figure 2G). More commonly, samples had either patchy regions with strong staining or diffuse regions with less intense staining (Figure 2F). In addition, the squamous epithelial component of the non-neoplastic lymphoepithelial crypt lining also tended to show a low level of nuclear staining (Figure 2H). We observed no ERα staining in the surrounding stromal tissue.
Figure 2.
Estrogen receptor alpha (ERα) staining in oropharyngeal tumor specimens and in normal tonsillar epithelium. Three oropharyngeal squamous carcinoma (OPSC) tumor specimens and one normal tonsillar tissue specimen were stained with hematoxylin and eosin (H&E) or underwent ERα immunohistochemistry. Specimen 1 is an example of an OPSC tumor that was scored negative for ERα; specimens 2 and 3, which are OPSC, scored positive for ERα. On the far right, normal tonsillar lymphoepithelial crypt specimen with ERα-positive cells. Bars represent 200 µm.
ERα expression was independent of sex, patient age, tumor size, nodal status, or baseline metastatic disease as single predictors (Table 2). ERα expression did not differ between patients receiving cetuximab compared to those receiving other therapies (P = .33). Complete smoking history with pack-years was available for 83.7% of the cohort. When stratified by pack-year history (0–10, 11–20, and ≥21), there was no statistically significant relationship between ERα expression and smoking (χ2, P = .14). ERα expression was infrequent in patients with a primary tumor site other than tongue base or tonsil (P = .003). ERα was expressed more commonly in HPV-positive tumors (P < .001) and in nonkeratinizing tumors (P = .005). A logistic regression model accounting for 10 clinical factors showed only HPV positivity and absence of tumor keratinization to be statistically significantly related to ERα positivity by IHC (Table 1).
The relationship of ERα status with HPV status was statistically indistinguishable between the TCGA and OPSC-CR cohorts, despite the difference in ERα measures. Among HPV-positive tumors, 62.5% of TCGA (45/72) and 58.2% of OPSC-CR (103/177) were ERα-positive (Fisher test, P = .57), whereas among HPV-negative tumors, corresponding values for ERα-positivity were 22.3% (99/443, TCGA) and 21.1% (8/38, OPSC-CR) (Fisher test, P = 1.0). The similar prevalence between the two cohorts of high ERα expression within each HPV status suggested that high ERα based on mRNA values (Figure 1A) and ERα-positivity by IHC (Figure 2) captured the same underlying biologic processes.
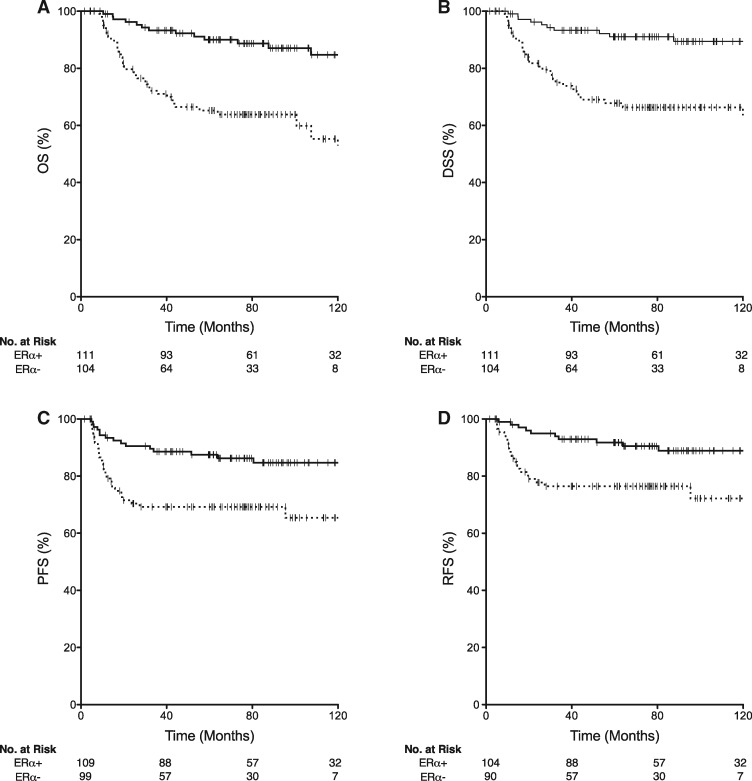
Patients with ERα-positive tumors had improved OS (log-rank, P < .001; Figure 3A), DSS (log-rank, P < .001; Figure 3B), PFS (log-rank, P = .002; Figure 3C), and RFS (log-rank, P = .003; Figure 3D) compared with those with ERα-negative tumors. Kaplan-Meier estimates of five-year survival for the ERα-positive and ERα-negative groups were OS, 90.0% (95% CI = 84.1 to 95.9) versus 64.0% (95% CI = 54.2 to 73.8); DSS, 91.0% (95% CI = 85.1 to 96.9) versus 66.5% (95% CI = 56.7 to 76.3); PFS, 86.2% (95% CI = 80.3 to 92.1) versus 69.5% (95% CI = 59.7 to 79.3); RFS, 90.4% (95% CI = 84.5 to 96.3) versus 76.7% (95% CI = 66.9 to 86.5) (Supplementary Table 3, available online). Among the 29 patients with information available on relapse site (95% of all recurrences), there was no statistically significant difference associated with ERα status for locoregional recurrence (73.6% [14/19] in ERα-negative versus 60.0% [6/10] ERα-positive, Fisher test P = .70) or distant recurrence (31.6% [6/19] in ERα-negative versus 40.0% [4/10] ERα-positive Fisher test, P = .68) associated with ERα status.
Figure 3.
Survival in the oropharyngeal squamous carcinoma chemoradiation (OPSC-CR) cohort stratified by estrogen receptor alpha (ERα) status. Kaplan-Meier plots stratified by ERα status for the OPSC-CR cohort are shown for (A) overall survival (OS, n = 215), (B) disease-specific survival (DSS, n = 215), (C) progression-free survival (PFS, n = 208), and (D) relapse-free survival (RFS, n = 194). Dashed lines represent ERα-negative patients, and solid lines represent ERα-positive patients. Hashes represent censoring times.
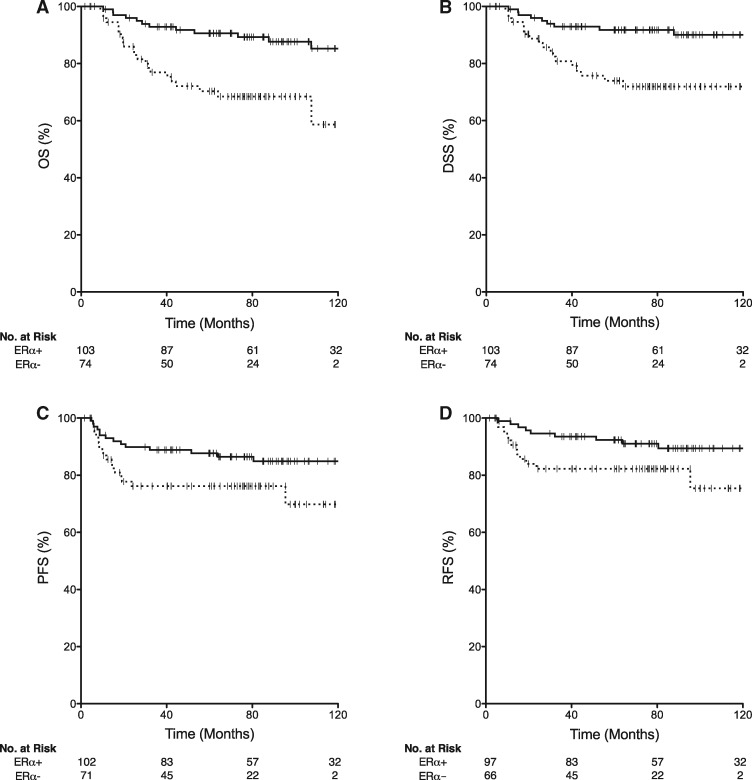
This relationship between ERα and survival in the OPSC-CR cohort went beyond its association with HPV positivity. Notably, the relationship between ERα expression and survival following chemoradiation was maintained within the subset of patients whose tumors were HPV-positive. Demographics of this subset of patients were similar to those of the entire cohort (Supplementary Table 4, available online). Among patients with HPV-positive OPSC, those whose tumors were ERα-positive had improved OS (log-rank, P = .001; Figure 4A), DSS (log-rank, P = .003; Figure 4B), PFS (log-rank, P = .03; Figure 4C), and RFS (log-rank, P = .04; Figure 4D) compared with those with HPV-positive, ERα-negative tumors.
Figure 4.
Survival in the human papillomavirus (HPV)-positive subset of the oropharyngeal squamous carcinoma chemoradiation (OPSC-CR) cohort stratified by estrogen receptor alpha (ERα) status. Kaplan-Meier plots stratified by ERα status for the HPV-positive subset of the OPSC-CR cohort are shown for (A) overall survival (OS, n = 177), (B) disease-specific survival (DSS, n = 177), (C) progression-free survival (PFS, n = 173), and (D) recurrence-free survival (RFS, n = 163). Dotted lines represent ERα-negative; solid lines represent ERα-positive. Hashes represent censoring times.
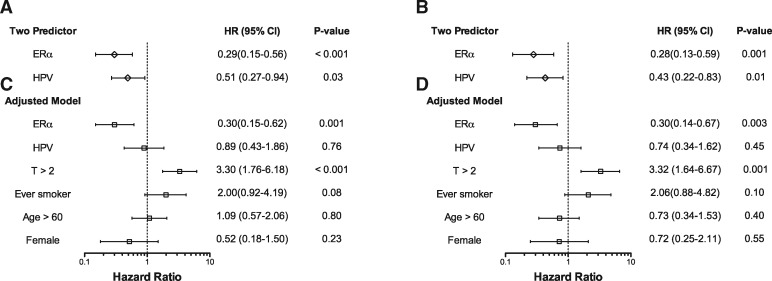
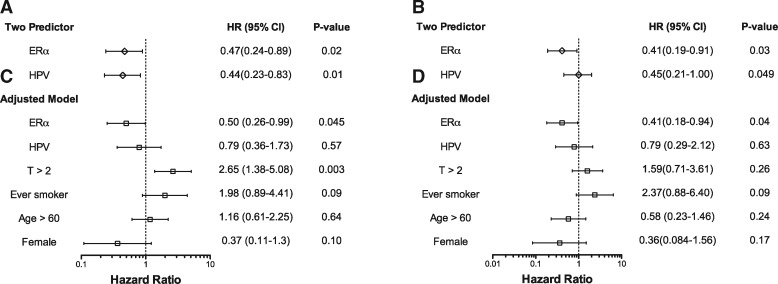
Furthermore, ERα expression was associated with improved outcome following chemoradiation for the entire OPSC-CR cohort when other clinical variables were taken into account. In two-predictor Cox proportional hazard analysis, both HPV and ERα status were statistically significantly related to outcome (OS, Figure 5A; DSS, Figure 5B; PFS, Figure 6A; RFS, Figure 6B). After accounting additionally for T classification, smoking history, age, and sex (4,24,25), ERα positivity remained associated with improved OS (HR = 0.30, 95% CI = 0.15 to 0.62, P = .001; Figure 5C), DSS (HR = 0.30, 95% CI = 0.14 to 0.67, P = .003; Figure 5D), PFS (HR = 0.50, 95% CI = 0.26 to 0.99, P = .045; Figure 6C), and RFS (HR = 0.41, 95% CI = 0.18 to 0.94, P = .04; Figure 6D). Concordance, bootstrap validation, and calibration of these models are shown in Supplementary Table 5 (available online).
Figure 5.
Cox multiple regression analysis of overall survival and disease-specific survival in the oropharyngeal squamous carcinoma chemoradiation (OPSC-CR) cohort. Hazard ratios (HR), 95% confidence intervals (CI), and Wald-test P values for overall survival (n = 215) (panels A, C) and disease-specific survival (n = 215) (panels B, D) for a two-variable model including estrogen receptor alpha (ERα) and human papillomavirus (HPV) status as predictors (panels A, B) and for a model adjusted for additional clinical variables (panels C, D). HPV = human papillomavirus; ERα = estrogen receptor alpha.
Figure 6.
Cox multiple regression analysis of progression-free survival and relapse-free survival in the oropharyngeal squamous carcinoma chemoradiation (OPSC-CR) cohort. Hazard ratios (HR), 95% confidence intervals (CI), and Wald test P values for progression-free survival (n = 208) (A, C) and relapse-free survival (n = 194), (B, D) for a two-variable model including estrogen receptor alpha (ERα) and human papillomavirus (HPV) status as predictors (A, B), and for a model adjusted for additional clinical variables (C, D).
Discussion
OPSC patients with ERα-positive tumors treated with chemoradiation therapy had improved overall, disease-free, progression-free, and relapse-free survival over those with ERα-negative tumors, with hazard ratios similar to the relationship between HPV status and survival in OPSC (26). ERα positivity was strongly associated with improved survival even after accounting for HPV status and known clinical risk factors. This novel and somewhat surprising result has potential implications both for patient stratification in trials and for therapeutic approaches in OPSC.
As patients with ERα-positive OPSC have improved outcomes following standard-of-care chemoradiation therapy, ERα staining may help determine candidacy for de-intensification of therapy. To date, there have been no well-documented biomarkers to help guide selection for de-intensification among the typically younger and healthier population with HPV-positive OPSC. ERα may provide such a biomarker.
ERα is also a potential therapeutic target. In breast cancer, ERα positivity is associated with response to endocrine therapy, lower mortality, and decreased disease recurrence (27). The anti-estrogen tamoxifen has been shown to increase apoptosis in HNSC cell lines (28,29), despite reported increased baseline invasiveness of ERα positive tumors (30). If ERα plays a critical role in tumorigenesis and tumor maintenance, endocrine-related therapy may enhance cytotoxic therapy in HNSC. The addition of endocrine therapy may allow for safe dose-reduction of cytotoxic treatment or as an alternative systemic treatment that could address metastatic disease.
In HPV-associated cervical cancer, estrogen signaling has been described as central to both development and maintenance of cancer progression (9,31,32). In K14-E6/E7 transgenic mice, an HPV model, development of carcinoma only occurred after sustained exposure to estradiol (8,9). Although the exact mechanisms by which HPV and estrogen signaling interact are incompletely understood, there may be a synergistic effect with HPV activating ERα response elements and ERα in turn inducing transcription of the HPV genome (10,33,34). If similar mechanisms are at work in HPV-positive OPSC, then anti-estrogen therapies could be considered as preventative measures for high-risk individuals.
Our findings that non-neoplastic tonsil crypt epithelium exhibits ERα staining and that ERα staining is enriched in HPV-positive tumors are consistent with an interplay of ERα and HPV in OPSC. The reticulated crypt epithelium has been previously implicated in carcinogenesis as a potential HPV reservoir that might favor tumor invasion (35–37). We found that the epithelium did not have uniform expression of ERα (Figure 2H), a mosaicism that could tend to favor ERα–positive normal epithelial cells for HPV infection and genomic integration, leading to OPSC.
To our knowledge, this is the first report of ERα expression being associated with improved outcomes in a head and neck cancer population. Despite extensive study in cervical and breast cancer (9,27,31,32,38–41), ERα has received little attention in head and neck cancer (30,42–48). Early studies failed to identify clinically significant quantities of estrogen receptor in HNSC (42), perhaps because those studies predated the increased incidence of HPV-positive disease, which we have shown is highly related to ERα expression. Although ERα has been identified previously in HNSC specimens with a wide range of reported incidence [10%–76% in laryngeal cancer (44–46); 11%–50% in oral cavity cancer (43,47,48)], it has not been described as a biomarker for HNSC outcomes (30,43).
Our study is not without limitations. Our observation of ERα in the epithelial crypt cells of adult tonsils differs from a previous study, which found no ERα expression within the epithelial cells of children’s tonsils (7). Our findings of patchy staining in normal regions of tonsil epithelium may represent a change during normal maturation or specifically in the development of carcinoma. With normal tonsil cells expressing ERα, careful pathologic analysis is needed to distinguish positivity within the tumor versus normal surrounding tissue. Second, this work, although based on two large cohorts, is retrospective and needs further validation within prospective clinical trials that have uniform chemotherapeutic regimens. Additionally, mRNA expression and ERα IHC expression need to be directly compared to determine the best assessment of ERα positivity. Finally, in other malignancies it is becoming more apparent that, beyond the role of ERα in cell cycle and proliferation, its interactions with estrogen receptor beta, progesterone receptor, and the tumor microenvironment may be important in tumorigenesis (11,49). Although outside the scope of this initial investigation, future work on responses of oropharyngeal cancer to cytotoxic treatment should re-investigate such roles of estrogen signaling.
In summary, we have shown in two independent HNSC cohorts that ERα is a biomarker for better survival following chemoradiation and may merit investigation as a therapeutic target. With the growing emphasis on de-intensification of treatment for HPV-related OPSC, with multiple ongoing clinical trials (50), identifying this clinical and potential therapeutic biomarker may improve patient selection for such trials and help develop novel de-intensification regimens.
Funding
This work was supported by National Institutes of Health grant (no. R01 DE022087), institutional funds of The Ohio State University and of Massachusetts Eye and Ear, the Massachusetts Eye and Ear Bacardi Biobank Fund, the Mary E. and John W. Alford Research Chair in Head and Neck Cancer at The Ohio State University, and the Joan Levy Bisesi Foundation for Head and Neck Oncology Research. Pathology core services utilized at the Dana-Farber/Harvard Cancer Center are supported in part by a National Cancer Institute Cancer Center Grant (no. NIH 5 P30 CA06516).
Notes
Affiliations of authors: Department of Otolaryngology – Head and Neck Surgery, The James Cancer Hospital and Solove Research Institute, The Ohio State University Wexner Medical Center, Columbus, OH (MBK, KBP, EAM, JWR); Department of Otolaryngology, Massachusetts Eye and Ear, Boston, MA (ALT, JBV, DGD, KSE, RJH, DTL); Division of Hematology-Oncology, Department of Medicine, University of Tennessee Health Sciences Center, Memphis, TN (DNH); Department of Pathology, Massachusetts Eye and Ear, Massachusetts General Hospital, Boston, MA (KG, WCF); Department of Radiation Oncology, Massachusetts General Hospital, Boston, MA (PMB, AWC); Division of Hematology-Oncology, Department of Medicine, Massachusetts General Hospital, Boston, MA (JRC, LJW); The Ohio State University Comprehensive Cancer Center-James, Columbus, OH (JWR).
The funders played no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
We thank the patients and, also, the investigators who participated in The Cancer Genome Atlas. We thank Dana-Farber/Harvard Cancer Center in Boston, MA, for the use of the Specialized Histopathology Core, which provided histology and immunohistochemistry service for the OPSC-CR cohort. We thank Paul Goodfellow for helpful comments and suggestions on an earlier draft of the manuscript.
Supplementary Material
References
- 1. Blomberg M, Nielsen A, Munk C, Kjaer SK.. Trends in head and neck cancer incidence in Denmark, 1978-2007: focus on human papillomavirus associated sites. Int J Cancer. 2011;1293:733–741. [DOI] [PubMed] [Google Scholar]
- 2. Jemal A, Simard EP, Dorell C.. Annual report to the nation on the status of cancer, 1975-2009, featuring the burden and trends in human papillomavirus (HPV)-associated cancers and HPV vaccination coverage levels. J Natl Cancer Inst. 2013;1053:175–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hwang TZ, Hsiao JR, Tsai CR, Chang JS.. Incidence trends of human papillomavirus-related head and neck cancer in Taiwan, 1995-2009. Int J Cancer. 2015;1372:395–408. [DOI] [PubMed] [Google Scholar]
- 4. Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;3631:24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nichols AC, Faquin WC, Westra WH, et al. HPV-16 infection predicts treatment outcome in oropharyngeal squamous cell carcinoma. Otolaryngol Head Neck Surg. 2009;1402:228–234. [DOI] [PubMed] [Google Scholar]
- 6. Nguyen NP, Sallah S, Karlsson U, Antoine JE.. Combined chemotherapy and radiation therapy for head and neck malignancies: quality of life issues. Cancer. 2002;944:1131–1141. [DOI] [PubMed] [Google Scholar]
- 7. Shim GJ, Gherman D, Kim HJ, et al. Differential expression of oestrogen receptors in human secondary lymphoid tissues. J Pathol. 2006;2083:408–414. [DOI] [PubMed] [Google Scholar]
- 8. Arbeit JM, Howley PM, Hanahan D.. Chronic estrogen-induced cervical and vaginal squamous carcinogenesis in human papillomavirus type 16 transgenic mice. Proc Natl Acad Sci USA. 1996;937:2930–2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chung SH, Wiedmeyer K, Shai A, Korach KS, Lambert PF.. Requirement for estrogen receptor alpha in a mouse model for human papillomavirus-associated cervical cancer. Cancer Res. 2008;6823:9928–9934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Spurgeon ME, den Boon JA, Horswill M, et al. Human papillomavirus oncogenes reprogram the cervical cancer microenvironment independently of and synergistically with estrogen. Proc Natl Acad Sci USA. 2017;11443:E9076–E9085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. den Boon JA, Pyeon D, Wang SS, et al. Molecular transitions from papillomavirus infection to cervical precancer and cancer: role of stromal estrogen receptor signaling. Proc Natl Acad Sci USA. 2015;11225:E3255–E3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nair HB, Luthra R, Kirma N, et al. Induction of aromatase expression in cervical carcinomas: effects of endogenous estrogen on cervical cancer cell proliferation. Cancer Res. 2005;6523:11164–11173. [DOI] [PubMed] [Google Scholar]
- 13. Mroz EA, Tward AD, Hammon RJ, Ren Y, Rocco JW.. Intra-tumor genetic heterogeneity and mortality in head and neck cancer: analysis of data from the Cancer Genome Atlas. PLoS Med. 2015;122:e1001786.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. The Cancer Genome Atlas Network. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 2015;5177536:576–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lewis JS Jr, Beadle B, Bishop JA, et al. Human papillomavirus testing in head and neck carcinomas: guideline from the College of American Pathologists. Arch Pathol Lab Med. 2018;142(5):559–597. [DOI] [PubMed] [Google Scholar]
- 16. Allred DC, Clark GM, Elledge R, et al. Association of p53 protein expression with tumor cell proliferation rate and clinical outcome in node-negative breast cancer. J Natl Cancer Inst. 1993;853:200–206. [DOI] [PubMed] [Google Scholar]
- 17. Allred DC, Harvey JM, Berardo M, Clark GM.. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998;112:155–168. [PubMed] [Google Scholar]
- 18. Harvey JM, Clark GM, Osborne CK, Allred DC.. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol. 1999;175:1474–1481. [DOI] [PubMed] [Google Scholar]
- 19. Hammond ME, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;2816:2784–2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Barbie DA, Tamayo P, Boehm JS, et al. Systematic RNA interference reveals that oncogenic KRAS-driven cancers require TBK1. Nature. 2009;4627269:108–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hanzelmann S, Castelo R, Guinney J.. GSVA: gene set variation analysis for microarray and RNA-seq data. BMC Bioinformatics. 2013;14:7.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schaefer CF, Anthony K, Krupa S, et al. PID: the Pathway Interaction Database. Nucleic Acids Res. 2009;37(Database issue):D674–D679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Huang Z, Zhu W, Szekeres G, Xia H.. Development of new rabbit monoclonal antibody to estrogen receptor: immunohistochemical assessment on formalin-fixed, paraffin-embedded tissue sections. Appl Immunohistochem Mol Morphol. 2005;131:91–95. [DOI] [PubMed] [Google Scholar]
- 24. Amini A, Jasem J, Jones BL, et al. Predictors of overall survival in human papillomavirus-associated oropharyngeal cancer using the National Cancer Data Base. Oral Oncol. 2016;56:1–7. [DOI] [PubMed] [Google Scholar]
- 25. Fakhry C, Westra WH, Wang SJ, et al. The prognostic role of sex, race, and human papillomavirus in oropharyngeal and nonoropharyngeal head and neck squamous cell cancer. Cancer. 2017;1239:1566–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gillison ML, Koch WM, Capone RB, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;929:709–720. [DOI] [PubMed] [Google Scholar]
- 27. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG), Davies C, Godwin J, Gray R, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;3789793:771–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ishida H, Wada K, Masuda T, et al. Critical role of estrogen receptor on anoikis and invasion of squamous cell carcinoma. Cancer Sci. 2007;985:636–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ku TK, Crowe DL.. Coactivator-mediated estrogen response in human squamous cell carcinoma lines. J Endocrinol. 2007;1931:147–155. [DOI] [PubMed] [Google Scholar]
- 30. Egloff AM, Rothstein ME, Seethala R, Siegfried JM, Grandis JR, Stabile LP.. Cross-talk between estrogen receptor and epidermal growth factor receptor in head and neck squamous cell carcinoma. Clin Cancer Res. 2009;1521:6529–6540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chung SH, Franceschi S, Lambert PF.. Estrogen and ERalpha: culprits in cervical cancer? Trends Endocrinol Metab. 2010;218:504–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Deligeoroglou E, Michailidis E, Creatsas G.. Oral contraceptives and reproductive system cancer. Ann N Y Acad Sci. 2003;997:199–208. [DOI] [PubMed] [Google Scholar]
- 33. Ramachandran B. Functional association of oestrogen receptors with HPV infection in cervical carcinogenesis. Endocr Relat Cancer. 2017;244:R99–R108. [DOI] [PubMed] [Google Scholar]
- 34. de Villiers EM. Relationship between steroid hormone contraceptives and HPV, cervical intraepithelial neoplasia and cervical carcinoma. Int J Cancer. 2003;1036:705–708. [DOI] [PubMed] [Google Scholar]
- 35. Chi AC, Day TA, Neville BW.. Oral cavity and oropharyngeal squamous cell carcinoma–an update. CA Cancer J Clin. 2015;655:401–421. [DOI] [PubMed] [Google Scholar]
- 36. Mirghani H, Amen F, Moreau F, Lacau St Guily J.. Do high-risk human papillomaviruses cause oral cavity squamous cell carcinoma? Oral Oncol. 2015;513:229–236. [DOI] [PubMed] [Google Scholar]
- 37. Lyford-Pike S, Peng S, Young GD, et al. Evidence for a role of the PD-1: PD-L1 pathway in immune resistance of HPV-associated head and neck squamous cell carcinoma. Cancer Res. 2013;736:1733–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chung SH. Targeting female hormone receptors as cervical cancer therapy. Trends Endocrinol Metab. 2015;268:399–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet. 2015;38610001:1341–1352. [DOI] [PubMed] [Google Scholar]
- 40. Li L, Wang Q, Lv X, et al. Expression and localization of estrogen receptor in human breast cancer and its clinical significance. Cell Biochem Biophys. 2015;711:63–68. [DOI] [PubMed] [Google Scholar]
- 41. Platet N, Cathiard AM, Gleizes M, Garcia M.. Estrogens and their receptors in breast cancer progression: a dual role in cancer proliferation and invasion. Crit Rev Oncol Hematol. 2004;511:55–67. [DOI] [PubMed] [Google Scholar]
- 42. Schuller DE, Abou-Issa H, Parrish R.. Estrogen and progesterone receptors in head and neck cancer. Arch Otolaryngol. 1984;11011:725–727. [DOI] [PubMed] [Google Scholar]
- 43. Lukits J, Remenar E, Raso E, Ladanyi A, Kasler M, Timar J.. Molecular identification, expression and prognostic role of estrogen- and progesterone receptors in head and neck cancer. Int J Oncol. 2007;301:155–160. [DOI] [PubMed] [Google Scholar]
- 44. Scambia G, Panici PB, Battaglia F, et al. Receptors for epidermal growth factor and steroid hormones in primary laryngeal tumors. Cancer. 1991;675:1347–1351. [DOI] [PubMed] [Google Scholar]
- 45. Virolainen E, Tuohimaa P, Aitasalo K, Kytta J, Vanharanta-Hiltunen R.. Steroid hormone receptors in laryngeal carcinoma. Otolaryngol Head Neck Surg. 1986;944:512–517. [DOI] [PubMed] [Google Scholar]
- 46. Oğretmenoğlu O, Ayas K.. Laryngeal carcinoma and estrogen receptor analysis in patients after long-term follow-up. Eur Arch Otorhinolaryngol. 1998;2559:457–461. [DOI] [PubMed] [Google Scholar]
- 47. Chang Y-L, Hsu Y-K, Wu T-F, et al. Regulation of estrogen receptor alpha function in oral squamous cell carcinoma cells by FAK signaling. Endocr Relat Cancer. 2014;214:555–565. [DOI] [PubMed] [Google Scholar]
- 48. Grimm M, Biegner T, Teriete P, et al. Estrogen and progesterone hormone receptor expression in oral cavity cancer. Med Oral Patol Oral Cir Bucal. 2016;215:e554–e558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Thomas C, Gustafsson JA.. The different roles of ER subtypes in cancer biology and therapy. Nat Rev Cancer. 2011;118:597–608. [DOI] [PubMed] [Google Scholar]
- 50. Mirghani H, Amen F, Blanchard P, et al. Treatment de-escalation in HPV-positive oropharyngeal carcinoma: ongoing trials, critical issues and perspectives. Int J Cancer. 2015;1367:1494–1503. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.