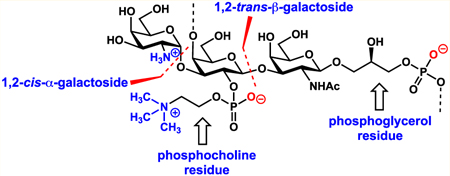
Abstract
Zwitterionic polysaccharides (ZPSs) activate T-cell-dependent immune responses by major histocompatibility complex class II presentation. Herein, we report the first synthesis of a Morganella morganii ZPS repeating unit as an enabling tool in the synthesis of novel ZPS materials. The repeating unit incorporates a 1,2-cis-α-glycosidic bond; the problematic 1,2-trans-galactosidic bond, Gal-β-(1 → 3)-GalNAc; and phosphoglycerol and phosphocholine residues which have not been previously observed together as functional groups on the same oligosaccharide. The successful third-generation approach leverages a first in class glycosylation of a phosphoglycerol-functionalized acceptor. To install the phosphocholine unit, a highly effective phosphocholine donor was synthesized.
Graphical abstract
INTRODUCTION
The capsule is a protective structure on the surface of a number of microbes. Its surface is coated with capsular polysaccharides (CPSs), incorporating residues that differ significantly in structure from those of the mammalian glycome.1,2 Due to their incongruity, CPSs are ligands for the human immune system.3,4 CPSs are antigens that work by activating B cells through cross-linking of cell surface receptors (Figure 1A). These B cells are partially activated and differentiate into plasma B cells that produce low-affinity IgM antibodies. In the absence of external mediators, only a T-cell-independent humoral response is induced.
Figure 1.
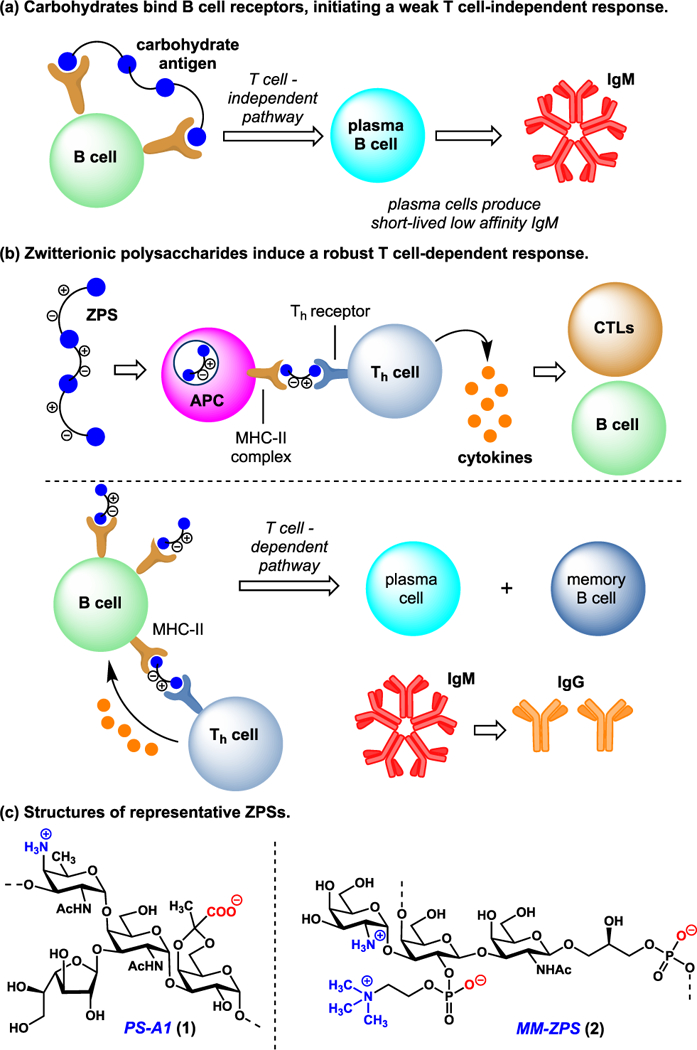
(a),(b) Immunological response to carbohydrate antigens. (c) Structures of PS-A1 (1) and MM-ZPS (2).
Unlike other carbohydrate antigens, zwitterionic polysaccharides (ZPSs) possess an alternating charge motif and elicit a T-cell-dependent response from the immune system (Figure 1B).5,6 Mechanistically, an antigen-presenting cell (APC) consumes and lyses the ZPS. Next, antigenic fragments are loaded onto major histocompatibility class II (MHC-II) receptors. The APC then presents the resulting antigen/MHC-II complex on the cell surface. Helper T cells (Th cells) bind to the antigen/MHC-II complex through the T-cell receptor (TCR), initiating cytokine release from the Th cell which activates cytotoxic T cells (CTLs). B cells are also activated. In a parallel pathway, activated Th cells bind to antigen-bound B cells to produce cytokines that fully activate B cells. This action results in immunoglobulin class switching and affinity maturation to produce high affinity IgG antibodies and memory B cells. Memory B cell formation is central to a long- lasting immune response.
Due to their immuno-stimulatory properties, ZPSs have been studied in a number of immunology settings. For example, ZPSs function as adjuvants when coadministered with poorly immunogenic antigens. In studies by the Wack group, ZPSs coadministered with the tetanus toxoid antigen were observed to increase antibody titers in vivo.7
The most common use of ZPSs is in cancer immunotherapy as substitutes for carrier proteins in vaccine development. ZPSs offer several advantages over carrier proteins such as keyhole limpet hemocyanin (KLH).8–13 Carrier protein–antigen conjugates are difficult to synthesize and characterize. They also elicit low levels of IgG antibodies in patients.14,15 It is believed this profile is due to the strong immune response elicited by carrier proteins themselves and their inadvertent suppression of immune responses to the antigen.16 The most well-characterized ZPS is PS-A1 (1), a Bacteroides fragilis CPS (Figure 1C).17 Foundational work by the Andreana lab has shown that, when administered to rodents, PS-A1 conjugates to tumor-associated carbohydrate antigens (TACAs) elicit high antibody titers.18–21 Moreover, the antibodies are highly selective for their respective antigen. The Seeberger and Andreana laboratories have independently completed total syntheses of PS-A1 to better characterize its immunostimulatory properties.22,23
Recently, we took note of the Morganella morganii ZPS (MM-ZPS, 2).24 In vitro binding assays revealed that 2 engages the MHCII in a manner competitive with PS-A1. Moreover, cellular studies suggest that 2 activates CD4+ T-cells. Antibody binding assays of hydrolyzed fragments and oligomers of the repeating unit revealed that the phosphocholine functional group was the dominant element of the epitope. Isolating MM-ZPS from the microbial cell surface for further evaluation is challenged by two problems. First, extraction and purification are complex and result in the isolation of small, impure batches. Second, the isolation process can alter the ZPS. Thus, there is an unmet need to synthesize structurally defined antigens. While it is general practice in the field to synthesize repeating units that are ready for chemical polymerization, our strategy differs in that we plan to use the repeating unit as ink to “print” unnatural ZPS nanomaterials.25 Indeed, three-dimensional (3D) printing is a frontier area of nanoscience due to its ability to produce 3D objects in advanced applications in areas ranging from tissue engineering to biomaterials. We hypothesize that nanoprinting can be used to synthesize ZPS materials, in the absence of traditional conjugation handles, through deposition of the ZPS onto polyelectrolyte (PE) complexes with nanometer precision in all dimensions. Described herein is the first step in that process, the total synthesis of the MM-ZPS trisaccharide repeating unit.
RESULTS AND DISCUSSION
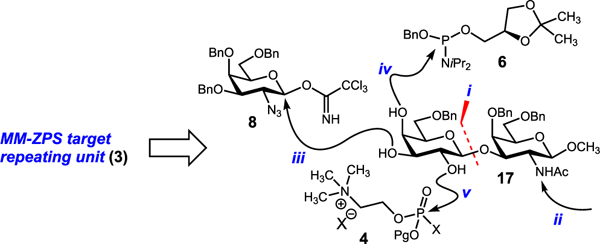
At the planning stage, we were drawn to repeating unit 3 which features a tetra-substituted galactose residue housing three of the repeating unit’s four charges (Figure 2). This molecule also contains two sites of phosphorylation, including a phosphocholine residue which is rarely observed on carbohydrates.26 Each residue is galactose derived, and one of the glycosidic bonds is a Gal-β-(l → 3)-GalNAc linkage. Regardless of the conditions employed, installing this 1,2-trans-galactosyl bond is regularly accompanied by orthoester formation (when participating groups are located at C2).27–32 Based on these considerations, our first-generation approach focused on stepwise functionalization of a central epoxygalactal residue 5. After forming the 1,2-cis-α-glycosidic bond (GalN3-α-(l → 3)-Gal) (step i), we planned to install the phosphoglycerol arm using phosphoramidite 6 (step ii). Installation of the 1,2-trans-β-glycosidic bond and second oxygen–phosphorus bond would hinge on ring opening of the epoxide (steps iii and iv, respectively).33,34
Figure 2.
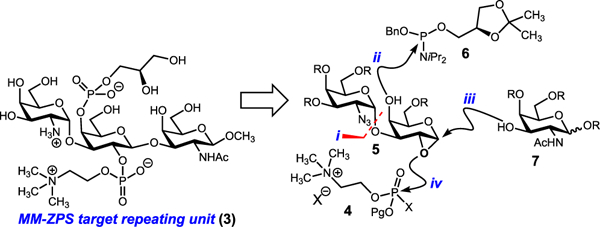
First-generation analysis.
The synthesis started from Schmidt trichloroacetimidate 8,35 prepared in seven steps from D-galactose (Scheme 1A). BF3∙Et2O-mediated glycosylation with known 6-O-TBS galactal acceptor36 9 proceeded selectively at C3 to provide α−10 in 65% yield as the major product. The high α-selectivity can be attributed to solvent participation in a β-fashion by diisopropyl ether.37 To avoid temporarily protecting the C3′ position, we installed the phosphoglycerol unit using phosphoramidite 6.38–40
Scheme 1.
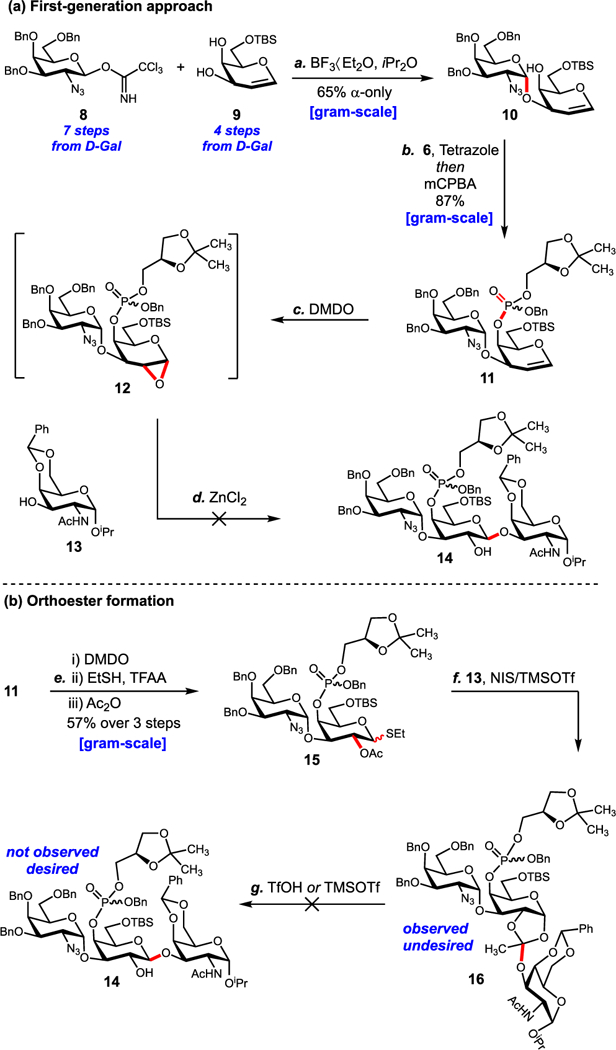
(a) First-Generation Approach and (b) Alternative Donor Leading to an Undesired Orthoester Producta aReagents and conditions: (a) BF3∙Et2O, 4:1 iPr2O/CH2Cl2, −40 °C → 25 °C, 1 h, 4 Å MS, 65% α-only; (b) 6 (1.5 equiv), 1H-tetrazole (1.5 equiv), CH3CN, 0 °C → 25 °C, 0.5 h then mCPBA (1.5 equiv), CH2Cl2, −78 °C → 0 °C, 2 h, 87%; (c) DMDO (1.2 equiv), CH2Cl2, −78 °C → 0 °C, 1 h, then 13 (1.2 equiv), ZnCl2 (2.5 equiv), −78 °C → 25 °C, >3 days, <5%; (d) DMDO (1.2 equiv), CH2Cl2, 0 °C, 20 min, then EtSH (28 equiv), TFAA (0.1 equiv), CH2Cl2, −78 °C → 0 °C, 1 h then Ac2O (10 equiv), Et3N (10 equiv), DMAP (0.1 equiv) CH2Cl2, 0 °C, 10 h, 57% over 3 steps; (e) 13 (2.5 equiv), NIS (1.2 equiv), TMSOTf (0.15 equiv), CH2Cl2, −78 °C → 25 °C, 2.5 h, <5%; (f) TMSOTf (0.15 equiv) or TfOH (0.15), CH2Cl2, −78 °C→ 25 °C, 2.5 h.
Subsequent oxidation of the intermediate phosphorus(III) species with mCPBA provided phosphate ester 11 in 87% yield as an inseparable mixture of phosphorus epimers. Next, epoxidation of the glycal with DMDO gave intermediate epoxide 12.34 Unfortunately, ring opening the epoxide was intractable. To circumvent this issue, glycal 11 was converted to thioglycoside donor 15 using a sequence of epoxidation, thiylation, and acylation of the resultant C2′ alcohol (Scheme 1B).41 Interestingly, glycosylation of 15 with acceptor 13 provided orthoester 16 instead of glycoside 14. Based on literature precedence, we hypothesized that the orthoacetate could be rearranged to the desired glycoside under Kochetkov conditions.42,43 However, exposing 16 to acidic media resulted in hydrolysis and decomposition over prolonged reaction times. Derivatives containing electron-deficient acyl-protecting groups at C2 were also examined as donors (see SI) as electron-withdrawing groups have been shown to slow competitive orthoester formation.37 Orthoester formation dominated regardless of the protecting group at C2.
As we terminated the first-generation approach, it was clear that the Gal-β-(l → 3)-GalNAc bond was more difficult to install than we anticipated. In fact, with few exceptions, a large number of routine glycosylation procedures favor orthoester formation over glycosidic bond formation.27–29,31,32,44–49 One obscure tactic used to generate this bond is to mask the C2 amine of the galactosamine acceptor as an azide.27,50 We incorporated this maneuver into a second-generation approach targeting 3 (Figure 3). The plan called for Gal-β-(l → 3)-GalNAc glycosidic bond formation early in the synthesis (step i). Following conversion of the azide to an acetamide (step ii), we would install the α-glycosidic bond (step iii). The final steps would involve installation of the phosphoglycerol (step iv) and phosphocholine (step v) arms. Installing the phosphoglycerol unit in the latter stages of the synthesis would minimize the number of subsequent steps requiring handling and characterization of phosphorus epimers.
Figure 3.
Second-generation analysis.
Donor 18 and acceptor 19 were synthesized from d-galactose in 651 and 12 steps, respectively (see SI). Glycosylation between donor 18 and 19 was followed by saponification to provide β-linked disaccharide 20 in 91% yield over two steps (Scheme 2). Subsequent acetonide protection of the C3′-C4′ alcohols and PMB protection of the C2′ alcohol (selected to allow for C4′ protecting group orthogonality in subsequent steps) gave fully protected disaccharide 21. Exchange of the C2 amine-protecting group at this stage proved critical as the C2 and C2″ amines are differentially functionalized in the final product. Thus, we planned to use a GalN3-based glycosyl donor to establish the trisaccharide core. LiAlH4 reduction of the C2 azide and protection of the resulting amine as its diacetyl imide afforded 22 in quantitative yield.
Scheme 2.
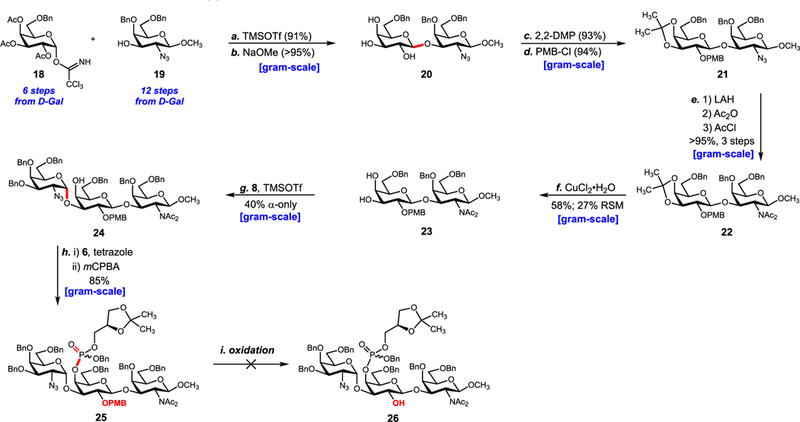
Second-Generation Approacha aReagents and conditions: (a) TMSOTf (0.15 equiv), CH2Cl2, 0 °C → 25 °C, 1 h, β-only, 91%; (b) NaOCH3, CH3OH, 25 °C, 2 h, > 95%; (c) 2,2-DMP, p-TsOH, CH2Cl2, 25 °C, 0.5 h, 93%; (d) PMBCl (1.2 equiv), NaH (2.0 equiv), DMF, 0 °C → 25 °C, 1 h, 94%; (e) LiAlH4, THF, 0 °C → 25 °C, 1 h then Ac2O, pyr., DMAP, 0 °C → 25 °C, 2 h then AcCl, DIPEA, 2:3 CH2Cl2/CH3CN, μwave 85 °C, 3 h, >95% over three steps; (f) CuCl2·H2O, CH3CN, 0 °C → 25 °C, 3 h, 58% with 27% RSM; (g) 8 (2.5 equiv), TMSOTf (0.15 equiv), CH2Cl2, −78 °C→ −40 °C, 2.5 h, α-only, 40%; (h) 6 (1.5 equiv), 1H-tetrazole (1.5 equiv), CH3CN, 0 °C → 25 °C, 0.5 h then mCPBA (1.5 equiv), CH2Cl2, −78 °C → 0 °C, 2 h, 85%.
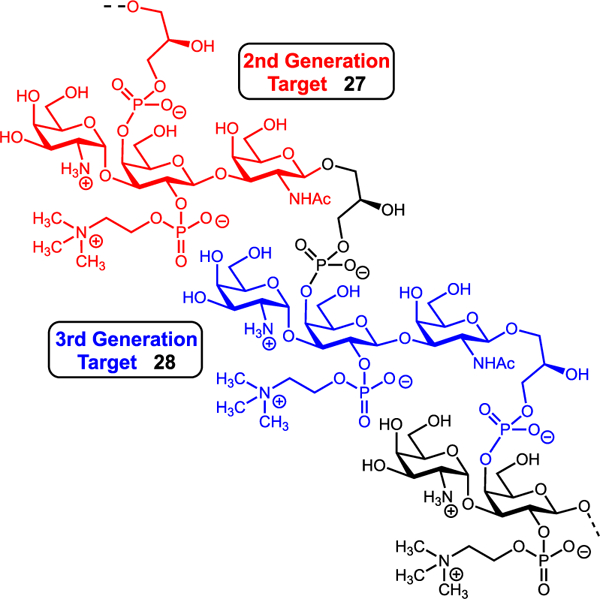
Next, deprotection of the C3′–C4′ acetonide with CuCl2∙H2O gave the C3′–C4′ diol acceptor 23 in modest yield along with recovered starting material which could be recycled. Conventional methods of acetonide removal (80% AcOH, p-TsOH/MeOH, aq. TFA, etc.) proved unsuccessful and resulted in hydrolysis of the glycosidic bond or conversion of the diacetyl imide to the acetamide. Leveraging the enhanced nucleophilicity of the equatorial C3′ alcohol over the axial C4′ alcohol, we examined glycosylation with imidate donor 8. While optimized conditions gave a complex mixture of every possible glycosylation product, the desired α-anomer 24 was the major product in 40% yield. Efforts to improve this reaction by esterifying the C4′ position proved futile as the modification curtailed the nucleophilicity of the C3′ alcohol. Having established the trisaccharide core, we next focused on phosphorylation. The C4′ phosphoglycerol residue was installed by coupling 24 with phosphoramidite 6 and subsequently oxidizing at phosphorus using mCPBA. 25 was isolated in 85% yield. At this stage, completion of the MM-ZPS 2 required installation of the C2′ phosphocholine residue and global deprotection. Unfortunately, oxidative removal of the C2′ PMB ether was unsuccessful. Both traditional (DDQ,52 CAN) and nontraditional (HCl/HFIP,53 TfOH,54,55 CBr4-TPP,56,57 silver(I),58 thermolysis,59 MgBr2,59 and homogeneous electron transfer60) conditions proved ineffective. Reassessing the route, we hypothesized the central galactose residue was too hindered to allow for installation of all substituents. Accordingly, we identified a new target repeating unit where the phosphoglycerol residue was shifted to the reducing end (Figure 4). This modification transfers a substituent away from the central residue and eliminates synthesis of a repeating unit with an unnatural O-alkyl capping unit at the reducing end.
Figure 4.
Target repeating unit frameshift.
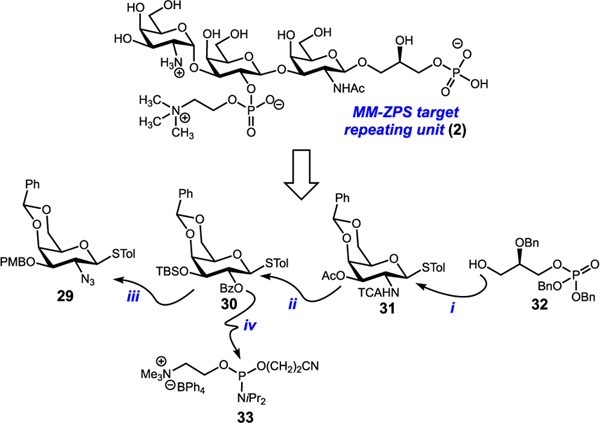
At the planning stage (Figure 5), it was clear that the synthesis of this new target (2) would maintain its novelty as the strategy would now feature an unprecedented glycosylation reaction, use of a phosphoglycerol-containing acceptor (step i). We opted to use thioglycoside donors to form each glycosidic bond due to their stability and scalability. The trisaccharide backbone would be constructed from its nonreducing end to its reducing end to minimize waste of the more valuable acceptor (steps ii and iii). The final challenge would be installation of the phosphocholine unit (step iv), which has thus far proved elusive. Thus, we anticipated developing a new reagent to install this motif.
Figure 5.
Third-generation analysis.
The third-generation approach commenced with an NIS-TMSOTf-promoted coupling of thioglycoside 31 to phosphoglycerol acceptor 32 (see SI). After glycosylation, NaOCH3-mediated removal of the C3 acetate gave β−34 in 93% yield over two steps (Scheme 3). Presumably, neighboring group participation from the C2 trichloroacetamide assisted in exclusive formation of the β-anomer. Subsequent NIS-TMSOTf-promoted glycosylation with thioglycoside 30 occurred in 91% yield to give 35.
Scheme 3.
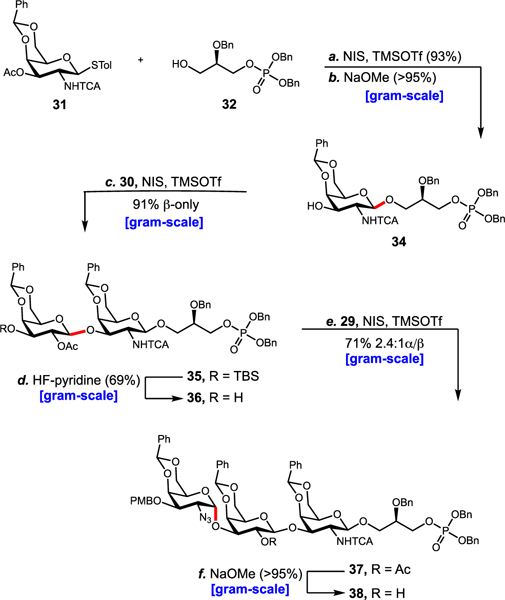
Third-Generation Approacha aReagents and conditions: (a) NIS (2.0 equiv), TMSOTf (0.15 equiv), CH2Cl2, 4 Å MS, 0 °C → 25 °C, 1.0 h, β-only, 93%; (b) NaOCH3, CH3OH, 25 °C, 2 h, >95%; (c) 30 (1.5 equiv), NIS (1.5 equiv), TMSOTf (0.10 equiv), CH2Cl2, 4 Å MS, −78 °C, 1.0 h, β-only, 91%; (d) HF-pyridine (10.0 equiv), pyridine, 25 °C, 3 h, 69%; (e) 29 (1.4 equiv), NIS (2.0 equiv), TMSOTf (0.15 equiv), 2.4:1 CH2Cl2/iPr2O, 4 Å MS, −60 °C → 0 °C, 5.0 h, 2.4:1 α/β, 71%; (f) NaOCH3, CH3OH, 25 °C, 2 h, >95%.
Again, the high β-selectivity can be attributed to neighboring group participation, this time from the C2′ acetate. Next, we moved forward with liberating the C3′ alcohol, which would serve as the acceptor in the final glycosylation event. To our surprise, we encountered difficulties while removing the silyl ether. The most productive conditions, HF-pyridine buffered by additional pyridine, provided acceptor 36 in 69% yield. A third NIS-TMSOTf-promoted glycosylation with thioglycoside 29 (see SI) gave trisaccharide 37 in 71% yield as a 2.4 to 1 mixture of α and β anomers. A number of α-selective additives (DMF, thioethers, and ethers) were evaluated in the reaction. Ultimately, 25 equiv of diisopropyl ether was the most α-selective additive. Finally, NaOCH3-mediated removal of the C2′ acetate provided trisaccharide 38 in 95% yield.
With the trisaccharide core in hand, we arrived at the most critical step of the synthesis, installation of the phosphocholine residue (Scheme 4). The initial approach to phosphorylation focused on using the well-established cyclic phospholanes 40 and 41. In theory, following phosphorylation, the 5-membered ring of 42 can be opened by trimethylamine to provide the phosphocholine unit. Unfortunately, this line of inquiry was met by two challenges. First, the C2′ alcohol of 38 proved to be both too sterically hindered and poorly nucleophilic to couple with either phospholane. Second, cyclic phospholanes are vulnerable to heat, moisture, and silica gel chromatography.
Scheme 4.
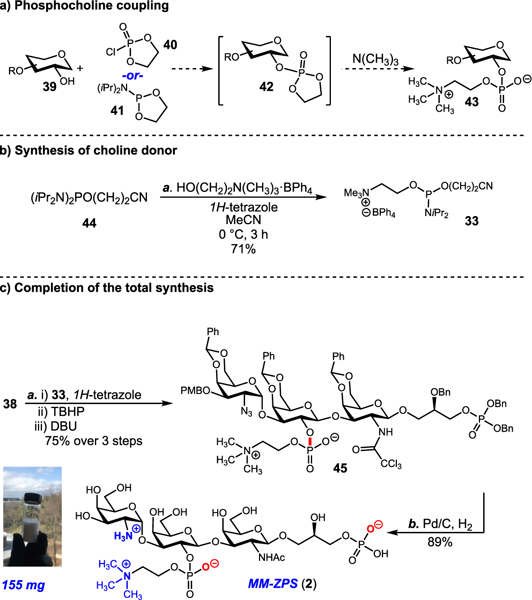
(a) Phosphocholine Coupling and (b) Completion of the Total Synthesisa aReagents and conditions: (a) 33 (1.2 equiv), 1H-tetrazole (1.2 equiv), CH3CN, 0.5 h, 25 °C then TBHP (1.2 equiv), CH3CN, 0 °C, 1 h then DBU (5.0 equiv), CH2Cl2, 25 °C, 18 h, 78% over three steps; (b) Pd/C (15% w/w), H2 (balloon), CH3OH, 25 °C, 88 h, 89%.
To introduce this residue, we synthesized a new phosphocholine donor, choline-2-cyanoethyl N,N-diisopropyl-phosporamidate tetraphenylborate 44, which functioned very well when using tetrazole as an activator. After formation of the oxygen–phosphorus bond (33 + 38), subsequent oxidation to the phosphate was achieved using tert-butyl hydroperoxide (TBHP). Finally, exposure to DBU led to removal of the cyanoethyl group, via β-elimination, to provide the desired phosphodiester 45 in 75% yield over the three steps.
With the complete core structure in hand, the final maneuver in the synthesis was a remarkable, exhaustive catalytic hydrogenation of 45. This reaction served to remove three benzylidene acetals, one PMB ether, and three benzyl ethers. The reductive environment also converted the C2 trichloroacetamide to the acetamide and the C2″ azide to an amine. Starting from 330 mg (0.2 mmol) of 45, purification using size exclusion chromatography (P2-Biogel) gave 155 mg of MM-ZPS 2 in 89% isolated yield. Charge-deconvoluted ESI FT ICR (electron spray ionization Fourier transformation ion cyclotron resonance) mass spectrometry revealed molecular mass spectral peaks in agreement with the desired calculated mass (calcd [M + H] for C28H55N3O23P2 = 864.2780, found 864.2772). 1H NMR coupling constants revealed the presence of one α glycosidic bond at C1″ (J = 5.33, 3.6 Hz) and two β glycosidic bonds at C1′ (J = 4.70, 7.9 Hz) and C1 (J = 4.46, 7.6 Hz). 2-D NMR experiments, including 1H–31P HSQC, enabled full assignment of the ZPS’s glycosidic bonds and phosphorus functionality. Additionally, the NMR data from the synthetic repeating unit 2 are in complete agreement with the data reported for the naturally occuring MM-ZPS polymer (Table S1).
CONCLUSION
In summary, we have completed the first total synthesis of the repeating unit of MM-ZPS 2. Key steps include: (1) early stage phosphoglycerol glycosylation occurring in high yield and excellent β-selectivity, (2) formation of the challenging Gal-β- (l → 3)-GalNAc bond without generating any undesired orthoacetate byproduct, and (3) installation of the phosphocholine group using a new choline donor. As studies with the naturally occurring MM-ZPS demonstrated that single repeating units do not elicit an immune response, current efforts are focused on using the synthetic repeating unit to synthesize non-natural ZPS materials. Studies regarding the synthesis and immunological properties of these materials are currently underway.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported, in part, by the National Institutes of Health under Grant No. 1R35GM133602. The Mass Spectrometry Research Center at Vanderbilt University is acknowledged for acquisition of high-resolution mass spectral data. Dr. Markus Voehler and Dr. Donald Stec are acknowledged for assistance with NMR experiments.
Footnotes
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/jacs.9b06830.
Experimental procedures, characterization data, and NMR spectra (PDF)
The authors declare no competing financial interest.
REFERENCES
- (1).Roberts IS The biochemistry and genetics of capsular polysaccharide production in bacteria. Annu. Rev. Microbiol. 1996, 50, 285–315. [DOI] [PubMed] [Google Scholar]
- (2).Reid CW; Fulton KM; Twine SM Never take candy from a stranger: the role of the bacterial glycome in host-pathogen interactions. Future Microbiol. 2010, 5 (2), 267–88. [DOI] [PubMed] [Google Scholar]
- (3).Cobb BA; Wang Q; Tzianabos AO; Kasper DL Polysaccharide processing and presentation by the MHCII pathway. Cell 2004, 117 (5), 677–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Avci FY; Kasper DL How bacterial carbohydrates influence the adaptive immune system. Annu. Rev. Immunol. 2010, 28, 107–30. [DOI] [PubMed] [Google Scholar]
- (5).Kalka-Moll WM; Tzianabos AO; Bryant PW; Niemeyer M; Ploegh HL; Kasper DL Zwitterionic polysaccharides stimulate T cells by MHC class II-dependent interactions. J. Immunol. 2002, 169 (11), 6149–53. [DOI] [PubMed] [Google Scholar]
- (6).Berti F; Adamo R Recent mechanistic insights on glycoconjugate vaccines and future perspectives. ACS Chem. Biol. 2013, 8 (8), 1653–63. [DOI] [PubMed] [Google Scholar]
- (7).Gallorini S; Berti F; Mancuso G; Cozzi R; Tortoli M; Volpini G; Telford JL; Beninati C; Maione D; Wack A Toll-like receptor 2 dependent immunogenicity of glycoconjugate vaccines containing chemically derived zwitterionic polysaccharides. Proc. Natl. Acad. Sci. U. S. A. 2009, 106 (41), 17481–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Danishefsky SJ; Shue YK; Chang MN; Wong CH Development of Globo-H cancer vaccine. Acc. Chem. Res. 2015, 48 (3), 643–52. [DOI] [PubMed] [Google Scholar]
- (9).Keding SJ; Danishefsky SJ Prospects for total synthesis: a vision for a totally synthetic vaccine targeting epithelial tumors. Proc. Natl. Acad. Sci. U. S. A. 2004, 101 (33), 11937–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Slovin SF; Ragupathi G; Musselli C; Olkiewicz K; Verbel D; Kuduk SD; Schwarz JB; Sames D; Danishefsky S; Livingston PO; Scher HI Fully synthetic carbohydrate-based vaccines in biochemically relapsed prostate cancer: clinical trial results with alpha-N-acetylgalactosamine-O-serine/threonine conjugate vaccine. J. Clin. Oncol. 2003, 21 (23), 4292–8. [DOI] [PubMed] [Google Scholar]
- (11).Ragupathi G; Koide F; Sathyan N; Kagan E; Spassova M; Bornmann W; Gregor P; Reis CA; Clausen H; Danishefsky SJ; Livingston PO A preclinical study comparing approaches for augmenting the immunogenicity of a heptavalent KLH-conjugate vaccine against epithelial cancers. Cancer Immunol. Immunother. 2003, 52 (10), 608–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Wang ZG; Williams LJ; Zhang XF; Zatorski A; Kudryashov V; Ragupathi G; Spassova M; Bornmann W; Slovin SF; Scher HI; Livingston PO; Lloyd KO; Danishefsky SJ Polyclonal antibodies from patients immunized with a globo H-keyhole limpet hemocyanin vaccine: isolation, quantification, and characterization of immune responses by using totally synthetic immobilized tumor antigens. Proc. Natl. Acad. Sci. U. S. A. 2000, 97(6), 2719–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Ragupathi G; Slovin SF; Adluri S; Sames D; Kim IJ; Kim HM; Spassova M; Bornmann WG; Lloyd KO; Scher HI; Livingston PO; Danishefsky SJ A Fully Synthetic Globo H Carbohydrate Vaccine Induces a Focused Humoral Response in Prostate Cancer Patients: A Proof of Principle. Angew. Chem., Int. Ed. 1999, 38 (4), 563–566. [DOI] [PubMed] [Google Scholar]
- (14).Gilewski T; Ragupathi G; Bhuta S; Williams LJ; Musselli C; Zhang XF; Bornmann WG; Spassova M; Bencsath KP; Panageas KS; Chin J; Hudis CA; Norton L; Houghton AN; Livingston PO; Danishefsky SJ; et al. Immunization of metastatic breast cancer patients with a fully synthetic globo H conjugate: a phase I trial. Proc. Natl. Acad. Sci. U. S. A. 2001, 98 (6), 3270–3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Kudryashov V; Glunz PW; Williams LJ; Hintermann S; Danishefsky SJ; Lloyd KO Toward optimized carbohydrate-based anticancer vaccines: epitope clustering, carrier structure, and adjuvant all influence antibody responses to Lewis(y) conjugates in mice. Proc. Natl. Acad. Sci. U. S. A. 2001, 98 (6), 3264–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Herzenberg LA; Tokuhisa T Epitope-specific regulation. I. Carrier-specific induction of suppression for IgG anti-hapten antibody responses. J. Exp. Med. 1982, 155 (6), 1730–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Surana NK; Kasper DL The yin yang of bacterial polysaccharides: lessons learned from B. fragilis PSA. Immunol Rev. 2012, 245 (1), 13–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).De Silva RA; Wang Q; Chidley T; Appulage DK; Andreana PR Immunological response from an entirely carbohydrate antigen: design of synthetic vaccines based on Tn-PS A1 conjugates. J. Am. Chem. Soc. 2009, 131 (28), 9622–3. [DOI] [PubMed] [Google Scholar]
- (19).De Silva RA; Appulage DK; Pietraszkiewicz H; Bobbitt KR; Media J; Shaw J; Valeriote FA; Andreana PR The entirely carbohydrate immunogen Tn-PS A1 induces a cancer cell selective immune response and cytokine IL-17. Cancer Immunol. Immunother. 2012, 61 (4), 581–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Nishat S; Andreana PR Entirely Carbohydrate-Based Vaccines: An Emerging Field for Specific and Selective Immune Responses. Vaccines (Basel, Switz.) 2016, 4 (2), 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Trabbic KR; Bourgault JP; Shi M; Clark M; Andreana PR Immunological evaluation of the entirely carbohydrate-based Thomsen-Friedenreich - PS B conjugate. Org. Biomol. Chem. 2016, 14 (13), 3350–5. [DOI] [PubMed] [Google Scholar]
- (22).Eradi P; Ghosh S; Andreana PR Total Synthesis of Zwitterionic Tetrasaccharide Repeating Unit from Bacteroides fragilis ATCC 25285/NCTC 9343 Capsular Polysaccharide PS A1 with Alternating Charges on Adjacent Monosaccharides. Org. Lett. 2018, 20 (15), 4526–4530. [DOI] [PubMed] [Google Scholar]
- (23).Pragani R; Seeberger PH Total synthesis of the Bacteroides fragilis zwitterionic polysaccharide A1 repeating unit. J. Am. Chem. Soc. 2011, 133 (1), 102–7. [DOI] [PubMed] [Google Scholar]
- (24).Young NM; Kreisman LS; Stupak J; MacLean LL; Cobb BA; Richards JC Structural characterization and MHCII-dependent immunological properties of the zwitterionic O-chain antigen of Morganella morganii. Glycobiology 2011, 21 (10), 1266–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Ventrici de Souza J; Liu Y; Wang S; Dorig P; Kuhl TL; Frommer J; Liu GY Three-Dimensional Nanoprinting via Direct Delivery. J. Phys. Chem. B 2018, 122 (2), 956–962. [DOI] [PubMed] [Google Scholar]
- (26).Vance DE; Vance JE Biochemistry of lipids, lipoproteins, and membranes, 4th ed.; Elsevier: Amsterdam; Boston, 2002; p xxiv, 607 p. [Google Scholar]
- (27).Li Q; Guo Z Pondering the Structural Factors that Affect 1,2-trans-Galactosylation: A Lesson Learnt from 3-O-beta-Galactosylation of Galactosamine. J. Carbohydr. Chem. 2017, 47 (5), 347–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Dziadek S; Brocke C; Kunz H Biomimetic synthesis of the tumor-associated (2,3)-sialyl-T antigen and its incorporation into glycopeptide antigens from the mucins MUC1 and MUC4. Chem. - Eur. J. 2004, 10 (17), 4150–62. [DOI] [PubMed] [Google Scholar]
- (29).Brocke C; Kunz H Synthetic glycopeptides of the tandem repeat sequence of the epithelial mucin MUC4 with tumour-associated carbohydrate antigens. Synlett 2003, No. 13, 2052–2056. [Google Scholar]
- (30).Yi W; Shao J; Zhu L; Li M; Singh M; Lu Y; Lin S; Li H; Ryu K; Shen J; Guo H; Yao Q; Bush CA; Wang PG Escherichia coli O86 O-antigen biosynthetic gene cluster and stepwise enzymatic synthesis of human blood group B antigen tetrasaccharide. J. Am. Chem. Soc. 2005, 127 (7), 2040–1. [DOI] [PubMed] [Google Scholar]
- (31).Cai H; Huang ZH; Shi L; Zou P; Zhao YF; Kunz H; Li YM Synthesis of Tn/T Antigen MUC1 Glycopeptide BSA Conjugates and Their Evaluation as Vaccines. Eur. J. Org. Chem. 2011, 2011 (20–21), 3685–3689. [Google Scholar]
- (32).Reipen T; Kunz H Synthesis of peptide and glycopeptide partial structures of the homophilic recognition domain of epithelial cadherin. Synthesis 2003, No. 16, 2487–2502. [Google Scholar]
- (33).Baertschi SW; Raney KD; Stone MP; Harris TM Preparation of the 8,9-epoxide of the mycotoxin aflatoxin B1: the ultimate carcinogenic species. J. Am. Chem. Soc. 1988, 110 (23), 7929–7931. [Google Scholar]
- (34).Danishefsky SJ; Bilodeau MT Glycals in organic synthesis: The evolution of comprehensive strategies for the assembly of oligosaccharides and glycoconjugates of biological consequence. Angew. Chem., Int. Ed. Engl. 1996, 35 (13–14), 1380–1419. [Google Scholar]
- (35).Kalikanda J; Li ZT Study of the Stereoselectivity of 2-Azido-2-deoxygalactosyl Donors: Remote Protecting Group Effects and Temperature Dependency. J. Org. Chem. 2011, 76 (13), 5207–5218. [DOI] [PubMed] [Google Scholar]
- (36).Kozikowski AP; Lee JM A Synthetic Approach to the Cis-Fused Marine Pyranopyrans, (3e)-Dactomelyne and (3z)-Dactomelyne - X-Ray Structure of a Rare Organomercurial. J. Org. Chem. 1990, 55 (3), 863–870. [Google Scholar]
- (37).Boons G-J; Hale K Organic synthesis with carbohydrates; Sheffield Academic Press; Blackwell Science: Sheffield, England Malden, MA U.S.A., 2000; p xi, 336 p. [Google Scholar]
- (38).Chen J; Feng L; Prestwich GD Asymmetric total synthesis of phosphatidylinositol 3-phosphate and 4-phosphate derivatives. J. Org. Chem. 1998, 63 (19), 6511–6522. [Google Scholar]
- (39).Guanti G; Banfi L; Basso A; Bondanza L; Guglieri G; Powles K; Riva R Optimized synthesis of phosphatidylserine. Amino Acids 2010, 39 (2), 367–373. [DOI] [PubMed] [Google Scholar]
- (40).Sureshan KM; Riley AM; Potter BVL Rapid and efficient routes to phosphatidylinositol 3,4,5-trisphosphates via myoinositol orthobenzoate. Tetrahedron Lett. 2007, 48 (11), 1923–1926. [Google Scholar]
- (41).Seeberger PH; Eckhardt M; Gutteridge CE; Danishefsky SJ Coupling of glycal derived thioethyl glycosyl donors with glycal acceptors. An advance in the scope of the glycal assembly. J. Am. Chem. Soc. 1997, 119 (42), 10064–10072. [Google Scholar]
- (42).Kochetkov NK; Khorlin AJ; Bochkov AF Tetrahedron 1967, 23 (2), 693–707. [Google Scholar]
- (43).Wang W; Kong F New synthetic methodology for regio- and stereoselective synthesis of oligosaccharides via sugar ortho ester intermediates. J. Org. Chem. 1998, 63 (17), 5744–5745. [DOI] [PubMed] [Google Scholar]
- (44).Xia J; Alderfer JL; Matta KL Chemical synthesis of a core 2 branched pentasaccharide containing a carboxylate group. Bioorg. Med. Chem. Lett. 2000, 10 (21), 2485–7. [DOI] [PubMed] [Google Scholar]
- (45).Liao WS; Locke RD; Matta KL Selectin ligands: 2,3,4-tri-O-acetyl-6-O-(2-naphthyl)methyl (NAP) alpha-D-galactopyranosyl imidate as a novel glycosyl donor for the efficient total synthesis of branched mucin core 2-structure containing the NeuAc alpha 2,3(SO3Na-6)Gal beta 1,3GalNAc alpha sequence. Chem. Commun. 2000, No. 5, 369–370. [Google Scholar]
- (46).Xia J; Alderfer JL; Piskorz CF; Matta KL Total synthesis of sialylated and sulfated oligosaccharide chains from respiratory mucins. Chem. - Eur. J. 2000, 6 (18), 3442–51. [DOI] [PubMed] [Google Scholar]
- (47).Xia J; Srikrishnan T; Alderfer JL; Jain RK; Piskorz CF; Matta KL Chemical synthesis of sulfated oligosaccharides with a beta-D-Gal-(1->3). Carbohydr. Res. 2000, 329 (3), 561–77. [DOI] [PubMed] [Google Scholar]
- (48).Dowlut M; Hall DG; Hindsgaul O Investigation of nonspecific effects of different dyes in the screening of labeled carbohydrates against immobilized proteins. J. Org. Chem. 2005, 70 (24), 9809–9813. [DOI] [PubMed] [Google Scholar]
- (49).Brocke C; Kunz H Synthetic tumor-associated glycopeptide antigens from the tandem repeat sequence of the epithelial mucin MUC4. Synthesis 2004, No. 4, 525–542. [Google Scholar]
- (50).Zhang SN; Li ZJ; Cai MS Synthesis of N-sugar-substituted phthalimides and their derivatives from sugar azides and phthalic anhydride. Carbohydr. Res. 2004, 339 (8), 1419–1420. [DOI] [PubMed] [Google Scholar]
- (51).Lu D; Hu Y; He X; Sollogoub M; Zhang Y Total synthesis of a sialyl Lewis(x) derivative for the diagnosis of cancer. Carbohydr. Res. 2014, 383, 89–96. [DOI] [PubMed] [Google Scholar]
- (52).Lloyd D; Bylsma M; Bright DK; Chen XZ; Bennett CS Mild Method for 2-Naphthylmethyl Ether Protecting Group Removal Using a Combination of 2,3-Dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) and beta-Pinene. J. Org. Chem. 2017, 82 (7), 3926–3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Volbeda AG; Kistemaker HA; Overkleeft HS; van der Marel GA; Filippov DV; Codee JDC Chemoselective Cleavage of p-Methoxybenzyl and 2-Naphthylmethyl Ethers Using a Catalytic Amount of HCl in Hexafluoro-2-propanol. J. Org. Chem. 2015, 80 (17), 8796–8806. [DOI] [PubMed] [Google Scholar]
- (54).Correa IR; Pilli RA Total synthesis and structural elucidation of (–)-delactonmycin. Angew. Chem. Int. Ed. 2003, 42 (26), 3017–3020. [DOI] [PubMed] [Google Scholar]
- (55).Jung ME; Koch P Mild, selective deprotection of PMB ethers with triflic acid/1,3-dimethoxybenzene. Tetrahedron Lett. 2011, 52 (46), 6051–6054. [Google Scholar]
- (56).Rival N; Grados AA; Schiavo L; Colobert F; Hanquet G Mild deprotection of PMB ethers using tert-butyl bromide. Tetrahedron Lett. 2015, 56 (49), 6823–6826. [Google Scholar]
- (57).Yadav JS; Mishra RK Single step transformation of PMB ethers to bromides using a CBr4-TPP reagent system. Tetrahedron Lett. 2002, 43 (31), 5419–5422. [Google Scholar]
- (58).Kern N; Dombray T; Blanc A; Weibel JM; Pale P Silver(I)-Catalyzed Deprotection of p-Methoxybenzyl Ethers: A Mild and Chemoselective Method. J. Org. Chem. 2012, 77 (20), 9227–9235. [DOI] [PubMed] [Google Scholar]
- (59).Yadav JS; Meshram HM; Reddy GS; Sumithra G Microwave thermolysis IV: Selective deprotection of MPM ethers using clay supported ammonium nitrate “clayan” in dry media. Tetrahedron Lett. 1998, 39 (19), 3043–3046. [Google Scholar]
- (60).Schmidt W; Steckhan E Indirect Electrochemical Processes 0.8. Selective Deprotection of Primary and Secondary Hydroxy Functions by Homogeneous Electron-Transfer. Angew. Chem. Int. Ed. Engl. 1979, 18 (10), 802–803. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


