Abstract
Introduction Bevacizumab offers a medical treatment that may slow the growth of vestibular schwannomas (VS) and possibly preserve hearing in patients with neurofibromatosis type 2 (NF2). This study aims to investigate the effect of long-term bevacizumab treatment on VS progression.
Methods Demographic, clinical, audiometric, and radiographic data were collected from the medical records of NF2 patients treated with bevacizumab at a tertiary medical center.
Results Eleven tumors from seven NF2 patients treated with bevacizumab were analyzed. The median age was 17 years (range: 12–47 years). Median bevacizumab treatment time was 33 months (range: 12–74 months). Of five patients with serviceable hearing pretreatment, one (20%) maintained serviceable hearing during bevacizumab therapy. Significantly slower growth rates for both tumor diameters and tumor volumes were identified during active bevacizumab treatment. Median tumor diameters and volumes during active bevacizumab treatment were 0 cm/year (range: –0.13–0.17 cm/year) and 0.1 cm 3 /year (range: –0.92–0.41), compared with 0.37 cm/year (range: 0–1.5 cm/year, p = 0.0011) and 1.38 cm 3 /year (range: 0.013–3.74), respectively, without bevacizumab treatment ( p = 0.0263). Reduced tumor progression was noted with bevacizumab treatment utilizing both linear greatest diameter (hazard ratio 0.16, p = 0.006) and segmentation volumes (hazard ratio 0.15, p = 0.023). Complications of bevacizumab treatment included fatigue (43%), nausea/vomiting (43%), hypertension (43%), epistaxis (29%), and proteinuria (29%). One subject had a cerebrovascular accident detected on screening magnetic resonance imaging without symptoms or neurological sequelae.
Discussion Bevacizumab may reduce tumor growth rate and the risk of progression based on both volumetric and linear measurements.
Keywords: vestibular schwannoma, neurofibromatosis type 2, bevacizumab, Avastin, acoustic neuroma
Introduction
Neurofibromatosis type 2 (NF2) is a tumor predisposition syndrome which affects ∼1 in 30,000 individuals worldwide. 1 NF2 develops as a result of mutation in the tumor suppressor gene which encodes the protein Merlin and is inherited in an autosomal-dominant manner, although 50% of patients suffer a de novo mutation. Patients with NF2 are at risk of the development of several nerve associated benign tumors, particularly bilateral vestibular schwannomas (VS), meningiomas, cranial and peripheral nerve schwannomas, ependymomas, posterior subcapsular opacities, and spinal cord tumors. 2 Hearing loss is the most common presenting symptom in patients with VS. 2 3 Because surgical resection and radiation can lead to worsening of hearing and nerve damage, the treatment algorithm for patients with NF2 often involves observation until hearing is no longer serviceable or the patient develops other intractable symptoms. Although there are microsurgical approaches that offer the potential for hearing preservation, such as the middle fossa or retrosigmoid approaches, hearing preservation rates may be lower in patients with NF2 as compared with those patients with sporadic VS. 4 5 6 Similarly, hearing preservation rates following radiation treatment for VS in NF2 patients may be worse than microsurgery. 7
Recently, bevacizumab has been shown to slow or even halt VS growth while improving hearing in some patients with NF2. 8 9 10 Bevacizumab is a monoclonal antibody that targets vascular endothelial growth factor, an important driver of angiogenesis, and was originally developed as a chemotherapeutic agent for colorectal cancer, renal cell cancer, and non-small cell lung cancer. 11 This study aims to investigate the effects of long-term bevacizumab treatment on VS growth rates and progression at a university-based NF2 program.
Methods
This study is a retrospective chart review of all NF2 patients treated with bevacizumab at the University of Texas Southwestern (UTSW) Medical Center Neurofibromatosis Type 2 Program from October 2010 through September 2017. This study was approved by the UTSW Institutional Review Board (122016–038). Patients were treated with bevacizumab at doses of 10 mg/kg every 2 weeks for 6 months before transitioning to a maintenance dose of 5 mg/kg every 3 weeks indefinitely or until side effects resulted in treatment cessation. Demographic, clinical, and audiometric data were collected from the electronic medical record. Primary endpoints were linear and volumetric tumor growth rates, as well as a categorical definition of tumor progression, defined by > 2 mm increase in a tumor's greatest axial dimension or >20% increase in tumor volume. Hearing data, specifically pure tone average (PTA) and word recognition score (WRS), were also collected. PTA was calculated based on a four-frequency average (0.5, 1, 2, and 3 kHz) at the following time points: (1) audiogram at presentation, (2) most recent audiogram prior to starting bevacizumab, (3) audiogram at the end of bevacizumab treatment, and (4) latest audiogram. For the purposes of hearing preservation, serviceable hearing was defined as class A or B hearing according to the 1995 AAOHNS guidelines. 12 Clinical notes were reviewed to determine if adverse effects occurred during bevacizumab treatment.
All magnetic resonance imaging (MRI) studies, which were obtained every 6 months to 1 year, were imported into Brainlab iPlan software (Munich, Germany). Utilizing T1-weighted post-contrast (T1c) images, measurements were conducted by two reviewers. Measurements included the greatest axial tumor diameter and tumor volumes, the latter of which were calculated using the automatic segmentation software package. This required manual tracing of several individual axial slices of the tumor. Growth rates were calculated by determining the difference in linear and volumetric measurements and dividing this by the intervening time period between imaging studies.
Continuous variables were summarized with medians, ranges, frequency counts, and percentages for non-normally distributed data. Linear and volumetric tumor growth rates were compared for patients on and off bevacizumab treatment, the latter of which was considered the longest period of surveillance prior to or following bevacizumab therapy, using the Wilcoxon Rank-Sum test. Cox regression models were then used to assess and compare tumor progression while on and off bevacizumab treatment using the exact marginal likelihood method with progression as the failure event. All statistics were performed with Stata/IC 14.1, 2016.
Results
Eleven tumors in seven subjects with NF2 were treated with bevacizumab. Demographic and treatment data are summarized in Table 1 . The median age of the subjects at the start of treatment was 17 years (range: 12–47 years). Four subjects were white (57.1%), two were Hispanic (28.6%), and one was African American (14.3%). All patients had lost serviceable hearing in one ear prior to the start of bevacizumab treatment. After a discussion of the risks, benefits, and alternatives to bevacizumab treatment, patients consented to treatment. The median duration of bevacizumab treatment was 33 months (range: 12–74 months). Complications of bevacizumab treatment ( Fig. 1 ) included fatigue (3/7, 43%), nausea/vomiting (3/7, 43%), hypertension (3/7, 43%), epistaxis (2/7, 29%), proteinuria (2/7, 29%), irregular menstrual cycle (2/7, 28%), mucositis (1/7, 14%), conjunctivitis (1/7, 14%), and diarrhea (1/7, 14%). All episodes of hypertension were controlled with antihypertensive medications, and no episodes required cessation of bevacizumab treatment. One subject had a cerebrovascular accident detected on screening MRI without symptoms or neurological sequelae while on bevacizumab therapy.
Table 1. Demographic and treatment characteristics of enrolled subjects.
| Total patients | 7 patients |
|---|---|
| Gender | 4 (57.1%) male, 3 (42.9%) female |
| Age | Median 17 years (range: 12–47) |
| Ethnicity | White 4 (57.1%) |
| African American 1 (14.3%) | |
| Hispanic 2 (28.6%) | |
| Bevacizumab induction dose | 10 mg/kg every 2 weeks |
| Bevacizumab maintenance dose | 5 mg/kg every 3 weeks |
| Bevacizumab treatment time | Median 33 months (range: 12–74) |
| Follow-up | Median 57 months (range: 15–147) |
| Hearing at start of bevacizumab treatment | 5 ears with serviceable hearing |
| Median PTA 40 dB (range: 7.5–47.5) | |
| Median WRS 100% (all 5 ears with 100%) | |
| Hearing at end of bevacizumab treatment | 1 ear with serviceable hearing |
| Median PTA 58.75 dB (range: 31.75–115) | |
| Median WRS 90% (range: 0–92). |
Abbreviations: PTA, pure tone average; WRS, word recognition score.
Fig. 1.
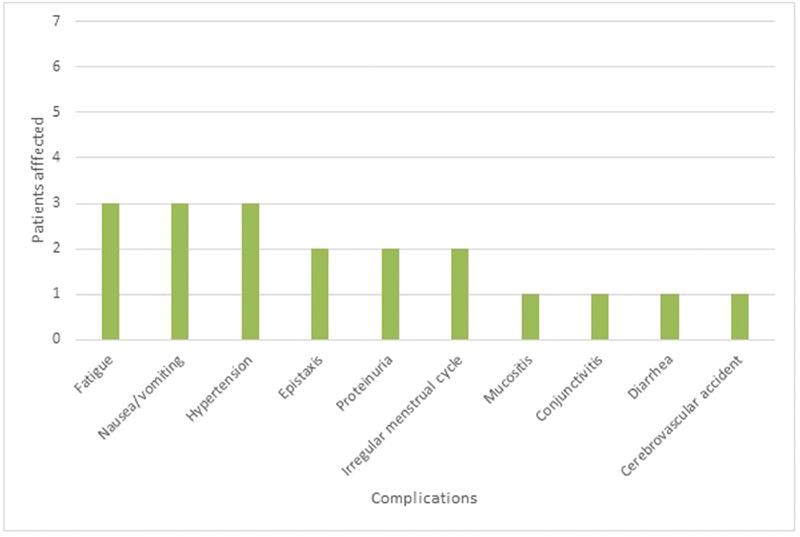
Complications associated with bevacizumab treatment.
Hearing data was available for six patients ( Table 1 ). Median audiometric follow-up off and during bevacizumab treatment was 25.7 months (range: 11.6–74.9 months) and 47.5 months (range: 14.1–77.7 months), respectively. Five patients (83.3%) had class A or B hearing in one ear at the start of bevacizumab treatment—class A:1 ear, class B: 4 ears, class C: 0 ears, class D: 7 ears. The median PTA for these 5 ears at the start of bevacizumab treatment was 40 dB (range: 7.5–47.5) and all had WRS of 100%. During bevacizumab treatment, hearing did not improve in any patient, and one (20%) preserved class A or B hearing at the conclusion of bevacizumab treatment—class A: 0 ears, class, B: 1 ear, class C: 2 ears, class D: 9 ears. Post-treatment median PTA was 58.75 dB (range: 31.75–115) and median WRS was 90% (range: 0–92).
Table 2 outlines the effect of bevacizumab on tumor growth rates. Fig. 2 displays an example MRI of a VS prior to bevacizumab (A) and at the end of bevacizumab therapy (B), demonstrating a reduction in tumor volume to 49.5% of the initial tumor volume at the conclusion of bevacizumab therapy. The median greatest axial linear dimension and tumor volume at presentation was 2.1 cm (range: 0.7–3.8 cm) and 2.89 cm 3 (range: 0.04–15.75 cm 3 ), respectively, whereas the median greatest axial linear dimension and tumor volume at the start of treatment was 2.6 cm (range: 1.4–4.1 cm 3 ) and 3.23 cm 3 (range: 0.30–18.48 cm 3 ), respectively. Linear axial tumor growth rate during bevacizumab treatment (median 0 cm/year, range: –0.13–0.17 cm/year) was significantly slower ( p = 0.0011) when compared with the linear growth rate off bevacizumab (the longer period of observation between prior to initiation of treatment or following the cessation of treatment) (median 0.37 cm/year, range: 0–1.5 cm/year). Tumor volume growth rate during bevacizumab treatment (median 0.1 cm 3 /year, range: -0.92–0.41 cm 3 /year) was also significantly slower ( p = 0.0263) when compared with the tumor volumetric growth rate off bevacizumab (median 1.38 cm 3 /year, range: 0.013–3.74 cm 3 /year).
Table 2. Comparison of tumor growth for tumors on and off bevacizumab treatment.
| Characteristic | On bevacizumab | Off bevacizumab | Wilcoxon Rank-Sum ( p -value) |
|---|---|---|---|
| Growth in tumor diameter | Median 0 cm/year (range: −0.13–0.17) | Median 0.37 cm/year (range: 0–1.5) | p = 0.0011 |
| Growth in tumor volume | Median 0.1 cm 3 /year (range: −0.92–0.41) | Median 1.38 cm 3 /year (range: 0.013–3.74) | p = 0.0263 |
Fig. 2.
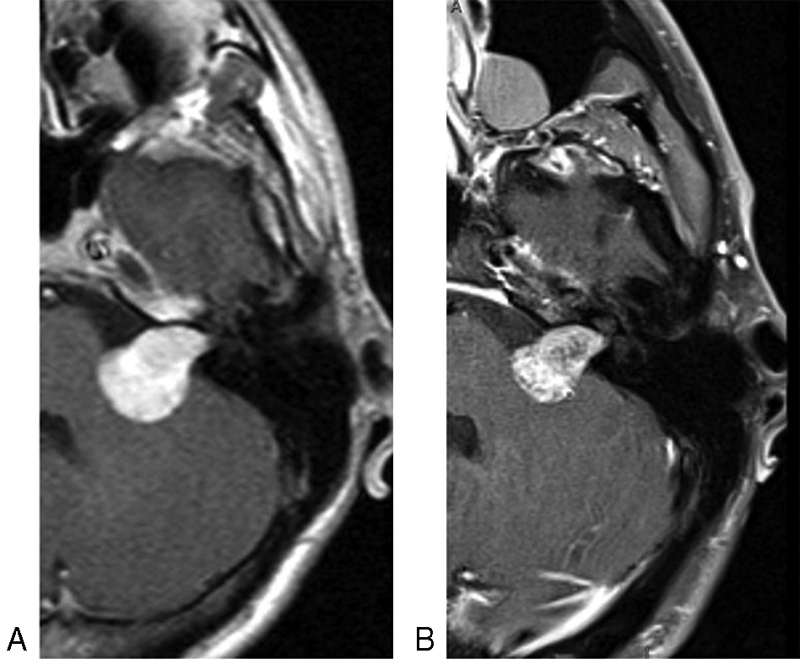
Axial T1-weighted postgadolinium magnetic resonance imaging demonstrating left vestibular schwannoma extending into the cerebellopontine angle prior to bevacizumab therapy ( A ) and at the end of bevacizumab therapy ( B ). Tumor volume at the end of bevacizumab was 49.5% of the starting volume.
Axial linear tumor diameter freedom from growth while on bevacizumab at 1, 2, and 3 years were 90, 79, and 53%, respectively, compared with 60, 36, and 0% for tumors off bevacizumab. Axial linear growth ( Fig. 3 ) during bevacizumab treatment was reduced (hazard ratio 0.16, p = 0.006) compared with tumor progression off bevacizumab. Volumetric tumor freedom from growth while on bevacizumab at 1, 2, and 3 years were 100, 88, and 70%, respectively, compared with 85, 64, and 0% for tumors of bevacizumab. Volumetric tumor progression ( Fig. 4 ) during bevacizumab treatment was reduced (hazard ratio 0.15, p = 0.023) compared with progression off bevacizumab.
Fig. 3.
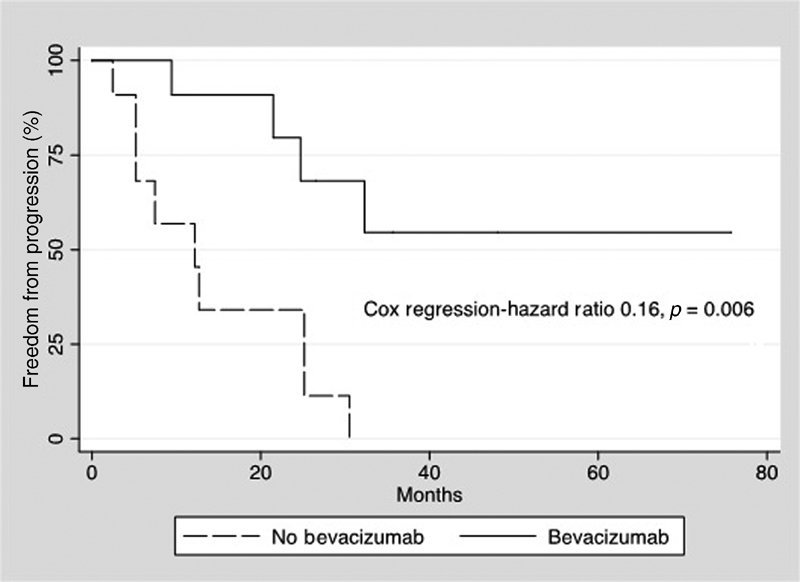
Kaplan–Meier plot demonstrates freedom from linear progression for tumors while off bevacizumab (dashed line) and during bevacizumab treatment (solid line). Cox regression demonstrated a significant reduction in linear progression with bevacizumab treatment.
Fig. 4.
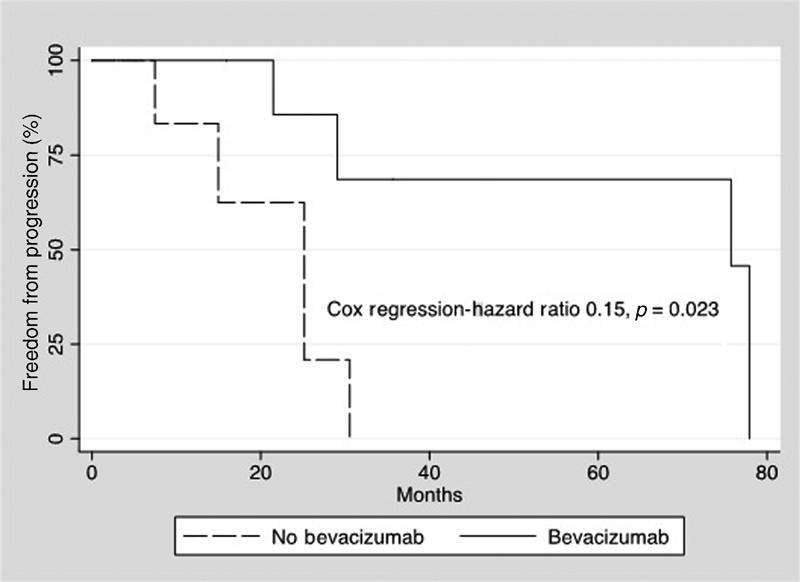
Kaplan–Meier plot demonstrates freedom from volumetric progression for tumors while off bevacizumab (dashed line) and during bevacizumab treatment (solid line). Cox regression demonstrated a significant reduction in volumetric progression with bevacizumab treatment.
Discussion
Treatment of VS in NF2 is challenging. Tumor control rates with stereotactic radiosurgery in NF2 have been reported to be 85% over 5 years in a recent review, 13 compared with 97.1% for sporadic VS, 14 though there was no statistically significant difference between tumor control in a recent matched cohort study. 15 Chung et al 7 performed a systemic review comparing surgery and radiation in NF2 patients. Radiation had a control rate of 75.1% compared with 91.9% with surgery, and radiation had significantly worse hearing preservation (40.1 vs 52.0%) but significantly improved facial nerve preservation compared with surgery (92.3 vs 75.7%). 7
Nevertheless, NF2 patients may have worse rates of hearing preservation following surgery when compared with patients with sporadic VS. For middle cranial fossa approaches, Doyle and Shelton reported a 38% hearing preservation rate for NF2 patients in 1993, 5 compared with a hearing preservation rate in 68% of patients with sporadic VS. 4 However, Slattery et al later showed a hearing preservation rate of 65% in NF2 patients following a gross-total resection via middle cranial fossa approach, though 57.4% of patients with a minimum follow-up of 5 years had recurrent tumor in the surgical field. 6 With regard to the retrosigmoid approach, Chen et al reported a hearing preservation rate of 41.6% with a gross tumor resection rate of 84.6% for NF2 patients, 16 compared with hearing preservation rate of 72% for small sporadically arising VS by Yamakami et al. 17
Given the bilateral nature of VS in NF2 and the possibility of worsening hearing with either surgery or radiation, conservative management of these tumors has gained favor. With regard to hearing for stable tumors in NF2 patients, a recent systematic review by Lloyd et al suggested that the best management option would be observation with surveillance MRI until the tumors are confirmed to be growing. 18 Medical treatment with bevacizumab offers another possible treatment modality for growing tumors in hopes of preserving serviceable hearing. Bevacizumab (literature summarized in Table 3 ) was first reported to show efficacy in patients with NF2 with bilateral VS in 2009, in which 9/10 patients treated for a median of 12 months had a reduction in tumor size. 8 In a series of 31 patients with a median treatment time of 14 months, Plotkin et al demonstrated tumor stability, defined as <20% volume growth, in 88% by 1 year and 67% by 2 years of bevacizumab treatment. 9 Alanin et al reported on a series of 12 patients treated for a median length of 22 months and showed that 83% had a reduction in tumor volume while 3/13 (23%) had improvement of 10 to 22% in WRS. 10 In their recent systematic review, Lloyd et al reported on a total of 60 tumors treated with bevacizumab and found that 66.9% of patients had a reduction in tumor volume and 48.3% had hearing improvement, with a median improvement in WRS by 10%. 18 Lloyd et al suggested that treatment of growing tumors with bevacizumab offers an improved probability of hearing preservation over surgery or radiation. 18
Table 3. Summary of other bevacizumab studies.
| Article | Sample size | Dose | Median treatment time | Response |
|---|---|---|---|---|
| Plotkin et al 8 | 10 patients | 5 mg/kg every 2 weeks | 12 months | 90% with reduction in tumor size |
| Plotkin et al 9 | 31 patients | 5 mg/kg every 2 weeks | 14 months | Freedom from progression in 88% by 1 year and 67% by 2 years |
| Alanin et al 10 | 12 patients | First 6 months: 10 mg/kg every 2 weeks Maintenance: 15 mg/kg every 3 weeks |
22 months | Reduction in tumor volume in 88% |
| Current study | 7 patients | First 6 months: 10 mg/kg every 2 weeks Maintenance: 5 mg/kg every 3 weeks |
33 months | Freedom from linear and volumetric progression, respectively: 1 year: 90%, 100% 2 years: 79%, 88% 3 years: 53%, 70% |
In the present series, we report longer term use of bevacizumab than previously published studies (median length of treatment of 33 months). Moreover, we demonstrate significantly reduced tumor growth with treatment. Freedom from both linear and volumetric progression (1 year: 90%, 100%; 2 years: 79%, 88%; 3 years: 53%, 70%) were similar to those reported by Plotkin et al Both median linear growth and volumetric growth were significantly reduced, and time to growth was significantly prolonged by both linear and volumetric measurements. In our series, 20% of patients preserved serviceable hearing at the conclusion of bevacizumab treatment, though this only applied to five ears that started with serviceable hearing (class A or B hearing), and no ears demonstrated improvement with bevacizumab treatment. Given our small sample size and small number of ears with serviceable hearing, robust statistical analysis was impossible, and drawing conclusions regarding hearing improvement or preservation in this series is problematic.
Bevacizumab is generally well tolerated but has been associated with adverse effects, including thrombosis, bleeding, hypertension, and proteinuria. 11 Plotkin et al reported side effects of hypertension, epistaxis, and proteinuria in their series treating NF2 patients. 9 More concerning, Alanin et al reported one NF2 patient treated with bevacizumab who died following a cerebral hemorrhage. 10 In our study, toxicity was overall manageable with similar complications reported in the literature, namely hypertension, proteinuria, epistaxis, fatigue, mucositis. One patient had a cerebrovascular accident, though there were no residual neurologic deficits.
This study is limited in several aspects. The sample size is small. While the median treatment time is longer than other studies, there is one patient with a treatment time of only 1 year. Additionally, this study is retrospective in nature. Furthermore, due to the sample size, multivariate analysis was not possible to further analyze factors affecting the response to treatment. Additionally, this study is limited in that only six patients had audiometric data, limiting our ability to report on hearing outcomes. Nevertheless, this study demonstrates using both linear and volumetric measurements that bevacizumab may have efficacy in slowing tumor progression in VS in NF2.
Conclusion
This study demonstrates that long-term bevacizumab use is effective at reducing tumor progression for VS in patients with NF2. Toxicity was limited and treatment was well tolerated, although there was one case of cerebrovascular accident. Patients and providers should be aware of the risk of serious adverse events with bevacizumab therapy.
Acknowledgments
None.
Funding Statement
Funding None.
Conflicts of Interests None.
IRB
This project was approved and in compliance with University of Texas Southwestern Medical Center IRB (122016–038).
Authorship Participation
Study design: Killeen, Tolisano, Hunter, Kutz
Data acquisition: Killeen, Hunter
Data Analysis: Killeen, Tolisano, Hunter, Kutz
Manuscript drafting: Killeen, Tolisano, Hunter, Kutz
Critical Revisions: Killeen, Klesse, Tolisano, Hunter, Kutz
References
- 1.Evans J A, Vitez M, Czeizel A. Patterns of acrorenal malformation associations. Am J Med Genet. 1992;44(04):413–419. doi: 10.1002/ajmg.1320440405. [DOI] [PubMed] [Google Scholar]
- 2.Slattery W H. Neurofibromatosis type 2. Otolaryngol Clin North Am. 2015;48(03):443–460. doi: 10.1016/j.otc.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 3.Strasnick B, Glasscock M E, III, Haynes D, McMenomey S O, Minor L B. The natural history of untreated acoustic neuromas. Laryngoscope. 1994;104(09):1115–1119. doi: 10.1288/00005537-199409000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Slattery W H, III, Brackmann D E, Hitselberger W. Middle fossa approach for hearing preservation with acoustic neuromas. Am J Otol. 1997;18(05):596–601. [PubMed] [Google Scholar]
- 5.Doyle K J, Shelton C. Hearing preservation in bilateral acoustic neuroma surgery. Am J Otol. 1993;14(06):562–565. [PubMed] [Google Scholar]
- 6.Slattery W H, III, Brackmann D E, Hitselberger W. Hearing preservation in neurofibromatosis type 2. Am J Otol. 1998;19(05):638–643. [PubMed] [Google Scholar]
- 7.Chung L K, Nguyen T P, Sheppard J P et al. A systematic review of radiosurgery versus surgery for neurofibromatosis type 2 vestibular schwannomas. World Neurosurg. 2018;109:47–58. doi: 10.1016/j.wneu.2017.08.159. [DOI] [PubMed] [Google Scholar]
- 8.Plotkin S R, Stemmer-Rachamimov A O, Barker F G, II et al. Hearing improvement after bevacizumab in patients with neurofibromatosis type 2. N Engl J Med. 2009;361(04):358–367. doi: 10.1056/NEJMoa0902579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Plotkin S R, Merker V L, Halpin C et al. Bevacizumab for progressive vestibular schwannoma in neurofibromatosis type 2: a retrospective review of 31 patients. Otol Neurotol. 2012;33(06):1046–1052. doi: 10.1097/MAO.0b013e31825e73f5. [DOI] [PubMed] [Google Scholar]
- 10.Alanin M C, Klausen C, Caye-Thomasen P et al. The effect of bevacizumab on vestibular schwannoma tumour size and hearing in patients with neurofibromatosis type 2. Eur Arch Otorhinolaryngol. 2015;272(12):3627–3633. doi: 10.1007/s00405-014-3398-3. [DOI] [PubMed] [Google Scholar]
- 11.Bergsland E, Dickler M N. Maximizing the potential of bevacizumab in cancer treatment. Oncologist. 2004;9 01:36–42. doi: 10.1634/theoncologist.9-suppl_1-36. [DOI] [PubMed] [Google Scholar]
- 12.Monsell E, Balkany T, Gates G, Goldenberg R A, Meyerhoff W L, House J W. Committee on hearing and equilibrium guidelines for the evaluation of hearing preservation in acoustic neuroma (vestibular schwannoma). American Academy of Otolaryngology-Head and Neck Surgery Foundation, INC. Otolaryngol Head Neck Surg. 1995;113(03):179–180. doi: 10.1016/S0194-5998(95)70101-X. [DOI] [PubMed] [Google Scholar]
- 13.Mallory G W, Pollock B E, Foote R L, Carlson M L, Driscoll C L, Link M J.Stereotactic radiosurgery for neurofibromatosis 2-associated vestibular schwannomas: toward dose optimization for tumor control and functional outcomes Neurosurgery 20147403292–300., discussion 300–301 [DOI] [PubMed] [Google Scholar]
- 14.Boari N, Bailo M, Gagliardi Fet al. Gamma Knife radiosurgery for vestibular schwannoma: clinical results at long-term follow-up in a series of 379 patients J Neurosurg 2014121(Suppl):123–142. [DOI] [PubMed] [Google Scholar]
- 15.Kruyt I J, Verheul J B, Hanssens P EJ, Kunst H PM. Gamma Knife radiosurgery for treatment of growing vestibular schwannomas in patients with neurofibromatosis type 2: a matched cohort study with sporadic vestibular schwannomas. J Neurosurg. 2018;128(01):49–59. doi: 10.3171/2016.9.JNS161463. [DOI] [PubMed] [Google Scholar]
- 16.Chen L-H, Zhang H-T, Xu R-X, Zhang L, Li W-D, Sun K. Microsurgery for patients diagnosed with neurofibromatosis type 2 complicated by vestibular schwannomas: clinical experience and strategy for treatments. Medicine (Baltimore) 2018;97(17):e0270. doi: 10.1097/MD.0000000000010270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamakami I, Ito S, Higuchi Y. Retrosigmoid removal of small acoustic neuroma: curative tumor removal with preservation of function. J Neurosurg. 2014;121(03):554–563. doi: 10.3171/2014.6.JNS132471. [DOI] [PubMed] [Google Scholar]
- 18.Lloyd S KW, King A T, Rutherford S A et al. Hearing optimisation in neurofibromatosis type 2: a systematic review. Clin Otolaryngol. 2017;42(06):1329–1337. doi: 10.1111/coa.12882. [DOI] [PubMed] [Google Scholar]


