Editorial
Fifteen years ago, the society that produces this journal was established to advance research on the developmental origins of health and disease (DOHaD). But, as we show here, extending our previous work1, DOHaD research has been more concerned with exposures in the fetal period than in any other window of development. This interest manifests as an abundance of studies on the potential effects of the health and lifestyle of mothers around the time of pregnancy on the health of their children. We argue that this focus reflects deeply-held assumptions, amongst researchers, clinicians, policy makers, the media and the public, that maternal pregnancy exposures are the most important, causal determinants of offspring health.1 We call for the DOHaD research community to recognise and challenge these assumptions.
Evidence of an imbalance
As shown in our previous article,1 nearly 20 times more papers have been published mentioning terms relating to DOHaD and “maternal”/”mother” compared to the same terms and “paternal”/”father”. In an attempt to further quantify the scale of the DOHaD literature imbalance towards studies of maternal pregnancy exposures, we extracted information about each original research article published in the Journal of the Developmental Origins of Health and Disease since it began almost a decade ago (Figure 1).
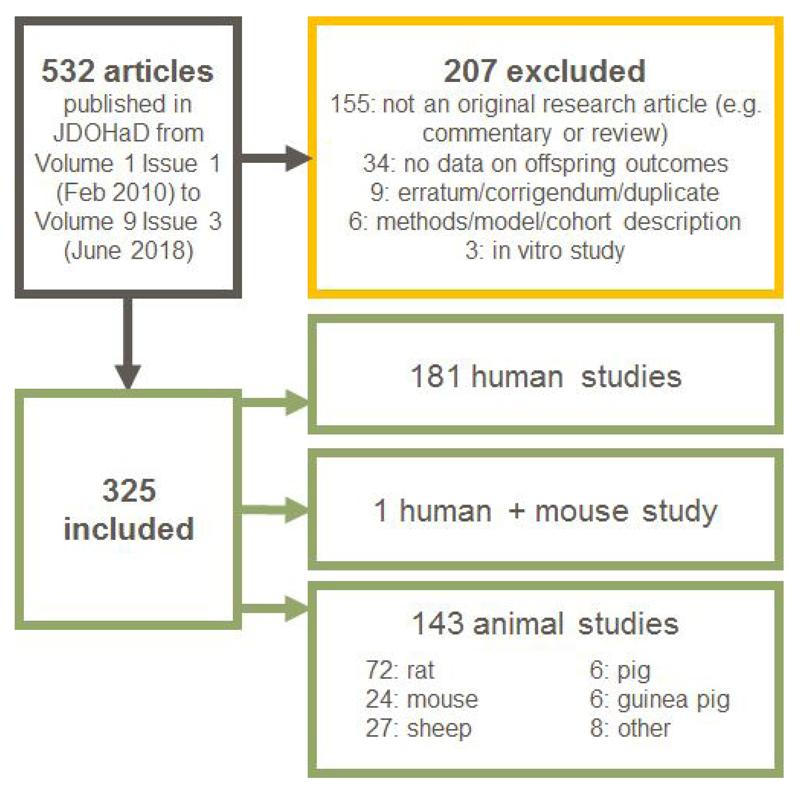
Figure 1.
Summary of identification of articles for review. We extracted information on 325 articles published in JDOHaD from 2010-2018. Data extraction was performed independently by at least two authors, with any differences reconciled through discussion with a third.
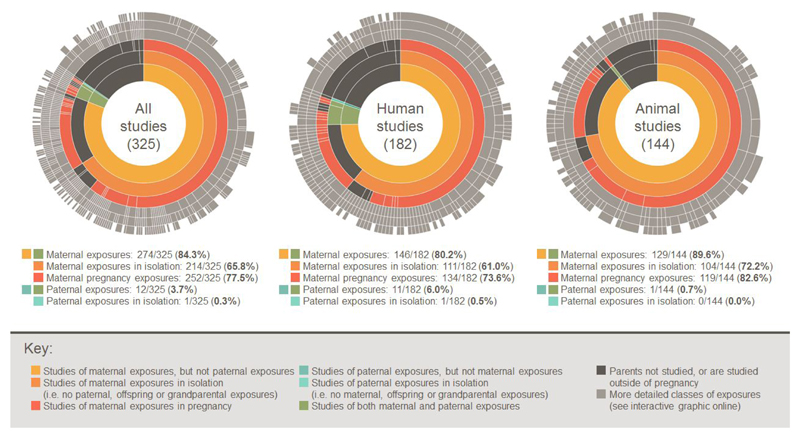
Of 325 eligible articles, 274 (84%) describe studies of maternal exposures, with 214 (66%) describing studies of maternal exposures in isolation (i.e. these studies did not consider paternal, offspring or grandparental exposures). Maternal exposures in pregnancy were studied in 252 articles (77%), with 167 articles (51%) reporting on maternal pregnancy exposures in isolation (Figure 2). In stark contrast, only 12 articles (4%) described studies of paternal exposures (in any period) and only one study (0.3%) considered paternal exposures in isolation.
Figure 2.
Sunburst charts showing the proportion of studies considering different classes of exposures. An interactive version of this graphic is available at https://gs8094.shinyapps.io/sunburst/.
Where studied as exposures, we categorised fetal or birth characteristics (fetal growth and intrauterine growth restriction, birth size/weight, molecules in cord blood, gestational age at delivery, and mode of delivery) as maternal pregnancy exposures, because these characteristics are often considered to be largely influenced by the intrauterine environment afforded by the mother during pregnancy. However, even if we reclassify these exposures as pertaining to offspring in all 52 relevant studies, there is still a striking imbalance towards studies of maternal pregnancy exposures (198 studies; 61%).
To allow readers to further explore all of the data that we extracted, we have produced an interactive version of the graphic presented in Figure 2 (available at https://gs8094.shinyapps.io/sunburst/). Using this web app, readers can view more specific information about exposures, subdivide into animal and human studies, and further explore the impact of classifying fetal/birth characteristics as pertaining to offspring or mothers during pregnancy. The web app also provides links to the data and code used in this analysis.
Why has DOHaD research traditionally focussed on maternal pregnancy exposures?
“Mothers are easier to study”
Many human birth cohort studies take advantage of maternal health services to recruit mothers as the primary participant, often regarding them as the “gatekeeper” to the recruitment of other family members.2 Conversely, there is often less opportunity to recruit fathers directly and retain them throughout the study period. For example, researchers in the Born in Bradford study tried a number of strategies, including going to sports grounds, places of worship and working men’s clubs, but recruitment rates were still low compared to mothers.3
Since these difficulties do not apply to animal studies, we might expect more animal studies of paternal exposures. However, we were surprised to find that our review of studies published in the Journal of DOHaD suggests this literature is similarly imbalanced: of 144 eligible animal studies, 129 (90%) considered maternal exposures, 119 (83%) in pregnancy, and 76 (53%) in pregnancy in isolation (Figure 2). This suggests that difficulties in studying fathers might make only a small contribution to DOHaD’s research focus on maternal exposures.
“The scientific rationale for studying maternal pregnancy exposures is stronger”
The proximal and intimate relationship between a mother and offspring around pregnancy, and the potential for efficient health promotion during the antenatal period, provide a strong rationale to study the influence of maternal pregnancy exposures on offspring health. However, with a few well-known exceptions (e.g. maternal smoking and birthweight4), the current evidence for a causal influence of most studied maternal pregnancy exposures on offspring outcomes is weak5–8. Additionally, the relative contribution of maternal pregnancy exposures is difficult to ascertain because other exposures (including paternal exposures and postnatal offspring non-familial exposures) have not been studied with the same intensity. We would argue that with over two decades of correlative research on maternal pregnancy exposures with relatively little evidence of robust causal effects, there is currently no strong scientific rationale for continuing to focus research efforts on maternal pregnancy exposures so intensively.
“It just makes more sense”
We argue that the main reason for the current imbalance in DOHaD research is that it reflects implicit, unquestioned, and deeply-held starting assumptions that maternal pregnancy exposures are the most important, causal drivers of offspring health.1 This explains why, despite the lack of robust causal findings, DOHaD research on maternal pregnancy exposures is: a) greater in intensity (and potentially quality), in both human and animal studies, than that on other exposures; b) more likely to be published (publication bias is a factor9); and c) more likely to be subsequently translated in the media, clinic and public health policy. In a looping effect,10 this wide public uptake of weak, but “common sense” claims about maternal pregnancy effects reinforces assumptions about the causal primacy of maternal pregnancy exposures, which in turn further drives the over-focus of the DOHaD research agenda on the fetal developmental period.1
The potential negative impact of imbalanced DOHaD research
The potential impact of these assumptions, and the resultant imbalanced DOHaD research, is far from benign. It increases the risk of missing more appropriate, more easily modifiable targets for intervention. Paternal or postnatal factors might mitigate or amplify the effect of any maternal or pregnancy exposure and could yield more effective and less expensive intervention targets than maternal pregnancy exposures. For example, higher rates of smoking cessation in pregnant women are consistently associated with their partners’ cessation11. Additionally, pregnancy interventions designed to maximise offspring health can have adverse effects. For example, it is current practice to weigh women throughout pregnancy in an attempt to limit maternal weight gain to prevent offspring obesity. However, there is limited evidence that gestational weight gain has a causal effect on offspring adiposity and associated adverse cardio-metabolic health, or that it can be modified safely in pregnancy.6,7 Conversely, the practice of continual monitoring of gestational weight gain and the pressure to conform to recommended levels that are not evidence-based may be associated with maternal anxiety.12
When DOHaD findings, despite a lack of causal evidence, are rushed into policy and clinical practice, concerns for the fetus are often placed above those of the mother. For example, the International Association of Diabetes and Pregnancy Study Group (IADPG) recently updated their recommendations for diagnosing gestational diabetes following DOHaD research highlighting the potential influence of gestational diabetes on the risk of greater offspring adiposity at birth and beyond.13 In a notable shift from previous decades, where the thresholds used to define gestational diabetes were directed towards reducing the future risk of maternal type 2 diabetes, the newly proposed IADPG thresholds are directed towards reducing birth size and future offspring overweight or obesity. The widespread use of these thresholds in clinical practice results in an increase in the number of women identified with gestational diabetes,14 but any benefit for future offspring risk of obesity beyond birth is unknown.
In the media, DOHaD findings are often reported using alarmist, inflammatory language, with pregnant mothers presented as individually responsible for a host of specific harms to future generations, ignoring the societal systems that influence health behaviours. This public discourse can have coercive and autonomy-limiting effects for women, as we have previously described.1,15 For example, a recent report by Amnesty International showed that fetal endangerment laws in the USA, designed to promote healthy pregnancies, discourage pregnant women who are dependent on drugs from seeking healthcare services for fear of criminal conviction.16
Tackling the imbalance
The DOHaD field needs to remain critical of assumptions around the causal primacy of maternal pregnancy effects. To do this, we recommend:
-
1)
Collaborating with social scientists to consider the role of cognitive assumptions and the social and ethical implications of DOHaD research throughout the research cycle;17
-
2)
Systematically reviewing and monitoring publication bias in the literature to further raise awareness of the current imbalance and help promote a cultural change;
-
3)
Collecting better quality data on other factors that influence offspring health, including social factors, postnatal life, and partner/paternal factors (which should be straightforward in animal studies, and for human studies will involve working collectively nationally and internationally to promote the importance of exploring the effect of fathers on their child’s health and wellbeing);
-
4)
Improving and contextualising the causal evidence base by scrutinising the influence of these factors alongside potential maternal pregnancy effects using causal inference techniques;
-
5)
Accurately communicating DOHaD research in a way that does not sensationalise or overstate the findings, in both the academic literature and translations in the media, clinic and policy.
We are encouraged to see progress in these areas. For example, the recently launched WRISK project, draws on women’s experiences to understand and improve the development and communication of risk messages in pregnancy.18 It is also encouraging to see the wider discussion of paternal exposures in the DOHaD literature19,20 (although we are concerned that referring to the “Paternal Origins of Health and Disease” or “POHaD” continues the unhelpful reductionist attitudes that have contributed to the current focus on maternal pregnancy exposures and would suggest using a more systems-based approach).
We hope that strategies such as these will help ensure that DOHaD research supports effective policies and clinical practice to maximise the health of all family members.
Acknowledgements
GCS, LS and DAL work in a unit that receives funds from the University of Bristol and UK Medical Research Council [MC_UU_00011/5 and MC_UU_00011/6]. DAL's contribution to this work is supported by grants from the US NIH [R01 DK1034] and the European Union's Seventh Framework Programme [FP/2007-2013) / ERC Grant Agreement (Grant number 66945; DevelopObese)]. DAL is a National Institute of Health Research Senior Investigator [NF-SI-0611-10196]. The views expressed in this paper are those of the authors and not necessarily any funders. The funders had no influence on the content of the paper.
Contributor Information
Gemma C Sharp, MRC Integrative Epidemiology Unit at the University of Bristol and Bristol Dental School, University of Bristol.
Laura Schellhas, Tobacco and Alcohol Research Group, School of Psychological Sciences, University of Bristol and MRC Integrative Epidemiology Unit at the University of Bristol.
Sarah S Richardson, Department of the History of Science and Studies of Women, Gender and Sexuality, Harvard University.
Deborah A Lawlor, MRC Integrative Epidemiology Unit at the University of Bristol, Population Health Sciences, Bristol Medical School, University of Bristol and Bristol NIHR Biomedical Research Centre.
References
- 1.Sharp GC, Lawlor DA, Richardson SS. It’s the mother!: How assumptions about the causal primacy of maternal effects influence research on the developmental origins of health and disease. Soc Sci Med. 2018 Sep;213:20–27. doi: 10.1016/j.socscimed.2018.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kiernan K. Fathers and Partners in National and International Birth Cohort Studies. 2014 [Google Scholar]
- 3.Wright J, Small N, Raynor P, et al. Int J Epidemiol. 4. Vol. 42. Oxford University Press; 2013. Aug 1, Cohort Profile: The Born in Bradford multi-ethnic family cohort study; pp. 978–991. [DOI] [PubMed] [Google Scholar]
- 4.Tyrrell J, Huikari V, Christie JT, et al. Hum Mol Genet. 24. Vol. 21. Oxford University Press; 2012. Dec 15, Genetic variation in the 15q25 nicotinic acetylcholine receptor gene cluster (CHRNA5–CHRNA3–CHRNB4) interacts with maternal self-reported smoking status during pregnancy to influence birth weight; pp. 5344–5358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mamluk L, Edwards HB, Savović J, et al. Low alcohol consumption and pregnancy and childhood outcomes: time to change guidelines indicating apparently ‘safe’ levels of alcohol during pregnancy? A systematic review and meta-analyses. BMJ Open. 2017 Aug 3;7(7):e015410. doi: 10.1136/bmjopen-2016-015410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lawlor DA. The Society for Social Medicine John Pemberton Lecture 2011. Developmental overnutrition--an old hypothesis with new importance? Int J Epidemiol. 2013 Feb 1;42(1):7–29. doi: 10.1093/ije/dys209. [DOI] [PubMed] [Google Scholar]
- 7.Lawlor DA, Relton C, Sattar N, Nelson SM. Nat Rev Endocrinol. 11. Vol. 8. Nature Publishing Group, a division of Macmillan Publishers Limited. All Rights Reserved; 2012. Nov 25, Maternal adiposity--a determinant of perinatal and offspring outcomes? pp. 679–88. [DOI] [PubMed] [Google Scholar]
- 8.Taylor AE, Carslake D, de Mola CL, et al. Maternal Smoking in Pregnancy and Offspring Depression: a cross cohort and negative control study. Sci Rep. 2017 Dec 3;7(1):12579. doi: 10.1038/s41598-017-11836-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stern JM, Simes RJ. Publication bias: evidence of delayed publication in a cohort study of clinical research projects. BMJ. 1997;315(7109) doi: 10.1136/bmj.315.7109.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lombrozo T. Using Science to Blame Mothers. 13.7 Cosm Cult NPR. 2014 [Internet]. Available from: https://www.npr.org/sections/13.7/2014/08/25/343121679/using-science-to-blame-mothers-check-your-values. [Google Scholar]
- 11.McBride CM, Baucom DH, Peterson BL, et al. Prenatal and Postpartum Smoking Abstinence. Am J Prev Med. 2004 Oct;27(3):232–238. doi: 10.1016/j.amepre.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 12.Farrar D, Duley L. Commentary: but why should women be weighed routinely during pregnancy? Int J Epidemiol. 2007 Dec 1;36(6):1283–4. doi: 10.1093/ije/dym210. [DOI] [PubMed] [Google Scholar]
- 13.International Association of Diabetes and Pregnancy Study Groups Consensus Panel. Metzger BE, Gabbe SG, et al. International Association of Diabetes and Pregnancy Study Groups Recommendations on the Diagnosis and Classification of Hyperglycemia in Pregnancy. Diabetes Care. 2010 Mar 1;33(3):676–682. doi: 10.2337/dc09-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farrar D, Fairley L, Santorelli G, et al. Association between hyperglycaemia and adverse perinatal outcomes in south Asian and white British women: analysis of data from the Born in Bradford cohort. Lancet Diabetes Endocrinol. 2015 Oct;3(10):795–804. doi: 10.1016/S2213-8587(15)00255-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richardson SS, Daniels CR, Gillman MW, et al. Society: Don’t blame the mothers. Nature. 2014 Aug 14;512(7513):131–2. doi: 10.1038/512131a. [DOI] [PubMed] [Google Scholar]
- 16.Amnesty International. Criminalizing pregnancy: policing pregnant women who use drugs in the USA. 2017 [Google Scholar]
- 17.Müller R, Hanson C, Hanson M, et al. The biosocial genome? EMBO Rep. 2017 Oct;18(10):1677–1682. doi: 10.15252/embr.201744953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trickey H. WRISKY business? Improving the communication of risk in pregnancy. [cited 2018 Nov 21];DECIPHer. 2018 [Internet]. Available from: http://decipher.uk.net/wrisky-business-improving-the-communication-of-risk-in-pregnancy/ [Google Scholar]
- 19.Soubry A. Epigenetics as a Driver of Developmental Origins of Health and Disease: Did We Forget the Fathers? Bioessays. 2017 Nov 23;:1700113. doi: 10.1002/bies.201700113. [DOI] [PubMed] [Google Scholar]
- 20.Braun JM, Messerlian C, Hauser R. Fathers Matter: Why It’s Time to Consider the Impact of Paternal Environmental Exposures on Children’s Health. Curr Epidemiol Reports. 2017 Mar 11;4(1):46–55. doi: 10.1007/s40471-017-0098-8. [DOI] [PMC free article] [PubMed] [Google Scholar]