Abstract
Background
Previous studies had demonstrated hemostatic abnormalities in patients with heart failure (HF) and several studies have shown that abnormal coagulation indices, represented by elevated D-dimer, had prognostic significance in patients with compatible or acute decompensated HF. However, the impact of D-dimer on the outcome in patients with end-stage HF remains unclear.
Methods
A total of 244 consecutive patients with end-stage HF due to idiopathic dilated cardiomyopathy (DCM) were prospectively enrolled from February 2011 to September 2014. D-dimer levels were measured and its prognostic value was assessed. Primary endpoint was all-cause mortality during the follow-up period. Secondary endpoints were stroke, bleeding, occurrence of sustained ventricular tachycardia or ventricular fibrillation, and major adverse cardiovascular events (MACE).
Results
D-dimer was significantly elevated in the non-survivors (median: 0.8 vs. 1.1 mg/L, P < 0.001). Traditional markers including B-type natriuretic peptide, troponin I, left ventricular ejection fraction, and left ventricular end-diastolic dimension provided limited prognostic value; but the addition of D-dimer refined the risk stratification. The optimal cut-off value of D-dimer to predict all-cause mortality was 0.84 mg/L by receiver operator characteristic analysis. Elevated D-dimer level was independently associated with increased risk of long-term all-cause mortality (HR = 2.315, 95% CI: 1.570–3.414, P < 0.001) and MACE (HR = 1.256, 95% CI: 1.058–1.490, P = 0.009), and the predictive value was independent of age, sex, atrial fibrillation and anticoagulation status.
Conclusions
Elevated D-dimer level was independently associated with poor long-term outcome in patients with end-stage HF secondary to idiopathic DCM, and the predictive value was superior to that of traditional prognostic markers.
Keywords: D-dimer, End-stage heart failure, Idiopathic dilated cardiomyopathy, Long-term outcome
1. Introduction
Although mortality from cardiovascular disease as a whole has declined, the prevalence of heart failure (HF) is still increasing[1] and the long-term prognosis associated with HF is poor.[2],[3] It is very troublesome to manage HF if it progresses to an advanced stage, and the annual mortality for end-stage HF with medical therapy alone remains as high as 75%.[4] It is the responsibility of clinicians to give a reasonable evaluation for patients with end-stage HF to determine the optimal management strategies and assess the prognosis. Previous studies had shown some biomarkers such as B-type natriuretic peptide (BNP),[5] and troponin I (TnI),[6] and parameters that represent cardiac structure and function such as left ventricular ejection fraction (LVEF)[7] and left ventricular end-diastolic dimension (LVEDD),[8] can provide important prognostic information. However, not only is end-stage HF associated with impaired cardiac structure and/or function, but some significant pathophysiological processes are involved, such as hemodynamics disorders,[9] mobility limitation,[10] and inflammation activation,[11] among which coagulation dysfunction secondary to HF stands out and has prognostic significance.[12]–[14] D-dimer is the degradation product of cross-linked fibrin and is elevated in patients with HF. Several studies have demonstrated that elevated D-dimer was associated with poor outcome in patients with systolic or acute decompensated HF;[15],[16] however, the prognostic value of D-dimer level in patients with end-stage HF is not well understood. In this study, we aimed to investigate the impact of D-dimer level on long-term outcome in patients with end-stage HF due to idiopathic dilated cardiomyopathy (DCM), a common etiology of HF in China[17] and to compare the prognostic and predictive value of D-dimer with that of traditional prognostic markers.
2. Methods
2.1. Study population
This is a prospective observational study. A total of 244 consecutive patients with idiopathic DCM and end-stage HF were recruited between February 2011 and September 2014. Idiopathic DCM was defined by the presence of left ventricular dilatation and left ventricular systolic dysfunction confirmed by echocardiography in the absence of abnormal loading conditions (hypertension, valve disease) or coronary artery disease sufficient to cause global systolic impairment according to the recommendations for classifying cardiomyopathies.[18] End-stage HF was defined as New York Heart Association class III-IV, despite optimal medical and device therapy, intolerance or withdrawal of evidence-based HF medications such as beta blockers, angiotensin converting enzyme inhibitors (ACEIs) due to hypotension, diuretic-refractory volume overload, or need for intravenous inotrope. Patients with previous diagnosis of malignancy, deep vein thromboembolism, suspected and/or confirmed pulmonary thromboembolism, recent surgery or recent trauma (within the last month) that may cause D-dimer elevation were excluded in the study. Protocols were approved by the appropriate institutional review boards of the first affiliated hospital of Chongqing medical university and complied with the declaration of Helsinki. All subjects were provided with written informed consent.
2.2. Study measurement
Plasma D-dimer was measured by an immunoturbidometric technique with the Innovance D-dimer assay (Siemens, Marburg, Germany) on the Sysmex CA-7000 coagulation analyzer. Using the lyophilized reference plasma, the intra-assay and inter-assay variability (coefficient of variability) were 6%–9% and 2%–6%, respectively. The cutoff value was 0.5 mg/L fibrinogen equivalent units (FEU) and the measurable range was 0.19–35 mg/L FEU. Briefly, 5 mL of venous blood was collected into tubes containing sodium citrate. The tubes were then centrifuged for 10 min (1700 × g) and the plasma was separated for assays according to standard protocols provided by the supplier. BNP levels were measured by a fluorescence immunoassay kit (Triage, Biosite, SanDiego, CA) with a measurable range of 5 to 5000 pg/mL, and the interassay and intraassay coefficients of variability were 4.0%–5.8% and 2.8%–4.5%, respectively. Plasma TnI levels were measured using fluorescence immunoassay kit (Alere™ Triage®Cardio2) with a measurable range of 0.05 to 30 pg/mL, and the interassay and intraassay coefficients of variability were 7.2%–9.4% and 5.1%–8.2%, respectively. LVEDD values were obtained by M-mode echocardiography and LVEF was evaluated by Simpson's rule.
2.3. Study endpoints
Patients were followed up every three to six months after enrollment through telephone communication. Primary endpoint was all-cause mortality during the follow-up period. Secondary endpoints were stroke, bleeding, occurrence of sustained ventricular tachycardia (VT) or ventricular fibrillation (VF), and major adverse cardiovascular events (MACE) (combined all-cause mortality, stroke, bleeding, and occurrence of sustained VT/VF).
The definition of every event was as follows. All-cause mortality was defined as death from any cause during the follow-up period. Stroke was defined as focal neurological deficits lasting at least 24 h and confirmed by computed tomographic scans or magnetic resonance imaging. Bleeding included major bleeding (critical location, bleeding leading to surgical intervention, overt bleeding resulting in a drop in hemoglobin concentration of ≥ 20 g/L or leading to transfusion of two or more units of blood, or fatal bleeding) and minor bleeding (not meeting the criteria for major bleeding). Sustained VT was defined by the presence of a series of consecutive ectopic ventricular beats at a rate of more than 100 beats/min lasting more than 30 s or accompanied by hemodynamic instability requiring direct-current. VF was defined as irregular electrocardiogram waves of inconsistent shape and unidentifiable QRS complexes accompanied by hemodynamic compromise requiring cardioversion.
2.4. Statistical analysis
The baseline characteristics of the patients are presented as the mean ± SD or interquartile range for continuous variables and were compared by Student's t test if the data were normally distributed; otherwise, the Wilcoxon signed rank test was used. Categorical variables are presented as percentages and were compared by Pearson's chi-square test. Survival curves were constructed using the Kaplan-Meier method and were compared by log rank tests. The association between D-dimer level and other variables was analyzed by Spearman's correlation test. Receiver operator characteristic (ROC) analysis was performed to determine the optimal cut-off point of D-dimer to predict the all-cause mortality and the areas under the curve (AUC) were calculated as measures of the accuracy of the tests. Multivariate Cox proportional hazard regression models were performed to identify the association between D-dimer and the follow-up outcomes and the model was corrected by multivariable adjustment. The adjusted hazard ratios (HRs) with their respective 95% confidence intervals (CIs) were calculated. All statistical tests were two-tailed, and P < 0.05 was considered to be statistically significant. All statistical analyses were carried out using the SPSS statistical software, version 20.0 (SPSS Inc., Chicago, Illinois, USA).
3. Results
The median follow-up period was 20 months (4 to 26 months). Of 244 consecutive patients with idiopathic DCM, 16 patients were lost to follow-up and 10 patients had incomplete data. Therefore, data from the 218 remaining patients were analyzed.
Table 1 summarizes the baseline characteristics between survivors and non-survivors. The mean age, sex distribution and co-morbidities were comparable between the two groups (all P > 0.05). On admission, the non-survivors presented with relatively lower systolic blood pressure (SBP), diastolic blood pressure and LVEF, but had higher D-dimer and creatinine levels compared to the survivors (all P < 0.05). The admission heart rate, BNP, TnI, LVEDD and mural thrombus of ventrium were comparable between survivors and non-survivors (all P > 0.05). During hospitalization, beta blockers and aldosterone receptor antagonists were less likely to be administered to the non-survivors (all P < 0.05) while the use of diuretics, ACEIs or angiotensin receptors blockers (ARBs), inotropes/vasoconstrictors, ultrafiltration and device implantation were comparable between the two groups (all P > 0.05).
Table 1. Comparison of baseline characteristics and treatment between survivors and non-survivors.
| Variables | Survivors (n = 95) | Non-survivors (n = 123) | P-value |
| Epidemiologics | |||
| Age, yrs | 53.8 ± 11.7 | 55.7 ± 11.2 | 0.216 |
| Male | 70 (73.7%) | 88 (71.5%) | 0.761 |
| Body mass index, kg/m2 | 20.9 ± 3.9 | 20.6 ± 4.1 | 0.640 |
| Co-morbidities | |||
| Coronary atherosclerosis | 7 (7.4%) | 11 (8.9%) | 0.806 |
| Hypertension | 15 (15.8%) | 12 (9.8%) | 0.215 |
| Diabetes mellitus | 43 (45.5%) | 60 (48.8%) | 0.682 |
| Atrial fibrillation | 62 (65.3%) | 91 (74.0%) | 0.181 |
| Previous or current smoking | 60 (63.2%) | 64 (52.0%) | 0.129 |
| Previous or current alcohol use | 38 (40.0%) | 47 (38.2%) | 0.889 |
| Admission vital signs | |||
| SBP, mmHg | 100.4 ± 16.9 | 92.4 ± 11.1 | < 0.001 |
| DBP, mmHg | 63.3 ± 8.8 | 66.4 ± 8.4 | 0.010 |
| Heart rate, bmp | 86.0 ± 18.0 | 90.0 ± 17.0 | 0.087 |
| Lab examination | |||
| BNP, pg/mL | 1206.0 (823.0–2020.0)* | 915.0 (346.0–2416.0)* | 0.352 |
| Troponin I, ug/L | 0.05 (0.04–0.11)* | 0.06 (0.04–1.55)* | 0.084 |
| D-dimer, mg/L | 0.8 (0.5–1.2)* | 1.1 (0.8–2.0)* | < 0.001 |
| Serum creatinine, umol/L | 93.0 (76.6–125.0)* | 105.8 (81.1–162.5)* | 0.023 |
| LVEDD, mm | 73.1 ± 9.5 | 74.4 ± 9.8 | 0.353 |
| LVEF | 35.4% ± 3.9% | 33.6% ± 5.1% | 0.004 |
| Mural thrombus of ventrium | 16 (16.8%) | 23 (18.7%) | 0.859 |
| Drug and device therapy | |||
| Diuretics | 93 (97.9%) | 119 (96.7%) | 0.699 |
| Beta blockers | 37 (38.9%) | 23 (18.7%) | 0.001 |
| ACEIs (or ARBs) | 42 (44.2%) | 44 (35.8%) | 0.212 |
| Aldosterone receptor antagonist | 87 (91.6%) | 101 (82.1%) | 0.049 |
| Digoxin | 66 (69.5%) | 99 (80.5%) | 0.079 |
| Warfarin | 16 (16.8%) | 24 (19.5%) | 0.725 |
| Aspirin | 32 (33.7%) | 66 (53.7%) | 0.004 |
| Clopidogrel | 29 (30.5%) | 59 (48.0%) | 0.012 |
| Amiodarone | 9 (9.5%) | 26 (21.1%) | 0.025 |
| Inotropes/vasoconstrictors | 53 (55.8%) | 69 (56.1%) | 1.000 |
| Ultrafiltration | 20 (21.1%) | 33 (26.8%) | 0.344 |
| VVI or DDD implantation | 10 (10.5%) | 5 (4.1%) | 0.103 |
| CRT (D) implantation | 11 (11.6%) | 11 (8.9%) | 0.651 |
Data are presented as means ± SD or n (%). *Presented as median (25th–75th). ACEIs: angiotensin-converting enzyme inhibitors; ARBs: angiotensin receptors blockers; BNP: brain natriuretic peptide; CRT: cardiac resynchronization therapy; DBP: diastolic blood pressure; LVEDD: left ventricular end diastolic diameter; LVEF: left ventricular ejection fraction; SBP: systolic blood pressure.
Due to the significant difference in the D-dimer level between survivors and non-survivors, the biomarker, D-dimer, was deeply investigated. Table 2 shows the correlations between D-dimer level and other clinical variables. The D-dimer level was independent of common variables such as age, BNP, TnI, LVEDD, LVEF, creatinine, and atrial fibrillation (AF), indicating that this coagulational parameter may reflect disease severity independent of traditional markers.
Table 2. Spearman correlation coefficients between D-dimer and other clinical variables.
| Variables | D-dimer | P-value |
| Age | –0.068 | 0.320 |
| Atrial fibrillation | –0.086 | 0.208 |
| BNP | –0.038 | 0.576 |
| LVEDD | 0.093 | 0.170 |
| LVEF | –0.019 | 0.778 |
| Troponin I | 0.131 | 0.053 |
| Serum creatinine | 0.096 | 0.157 |
BNP: brain natriuretic peptide; LVEDD: left ventricular end diastolic diameter; LVEF: left ventricular ejection fraction.
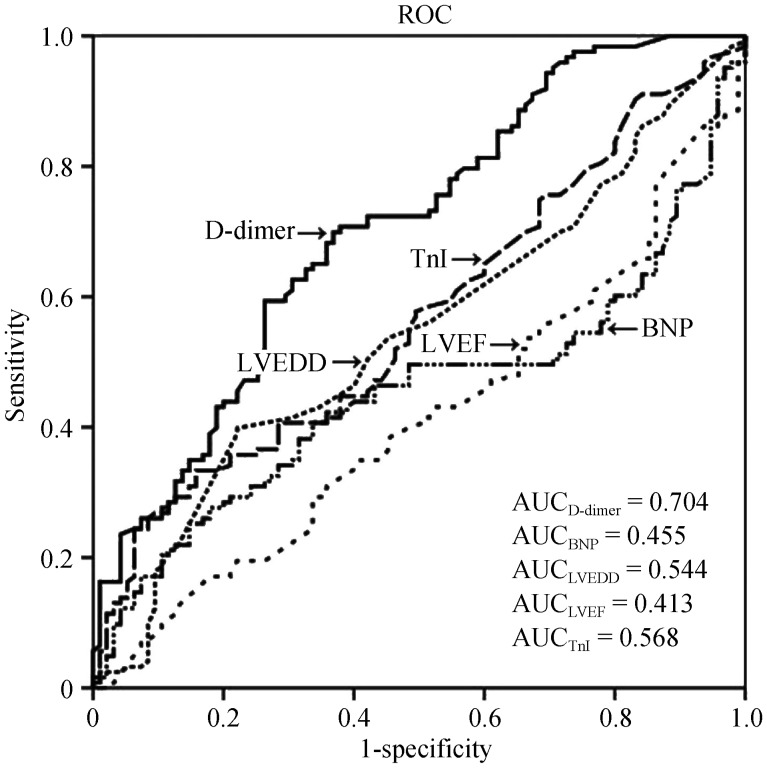
The long-term all-cause mortality stratified by D-dimer and the traditional markers was analyzed (Figure 1). When divided into quartiles, the traditional markers including BNP, TnI, LVEF, and LVEDD provided risk stratification to some extent; however, combining with the D-dimer improved the prognostic stratification. The general trend was that as D-dimer, BNP, LVEDD, and TnI increased or LVEF decreased, the mortality increased. Figure 2 shows the comparison of the predictive ability of D-dimer and other traditional markers for all-cause mortality by ROC analysis. The AUC of D-dimer, BNP, LVEDD, LVEF, and TnI for predicting all-cause mortality were 0.704, 0.455, 0.544, 0.413, and 0.568, respectively; indicating that D-dimer had a superior predictive value compared with the traditional markers. The best cut-off value of D-dimer to predict all-cause mortality by ROC analysis was 0.84 mg/L (sensitivity: 69.9%, specificity: 63.2%).
Figure 1. All-cause mortality stratified by combining D-dimer with BNP (A), TnI (B), LVEF (C), and LVEDD (D).
BNP: B-type natriuretic peptide; LVEDD: left ventricular end-diastolic dimension; LVEF: left ventricular ejection fraction; TnI: troponin I.
Figure 2. ROC analysis of D-dimer, BNP, TnI, LVEF, and LVEDD for prediction of all-cause mortality.
AUC: areas under the curve; BNP: B-type natriuretic peptide; LVEDD: left ventricular end-diastolic dimension; LVEF: left ventricular ejection fraction; ROC: receiver operating characteristic; TnI: troponin I.
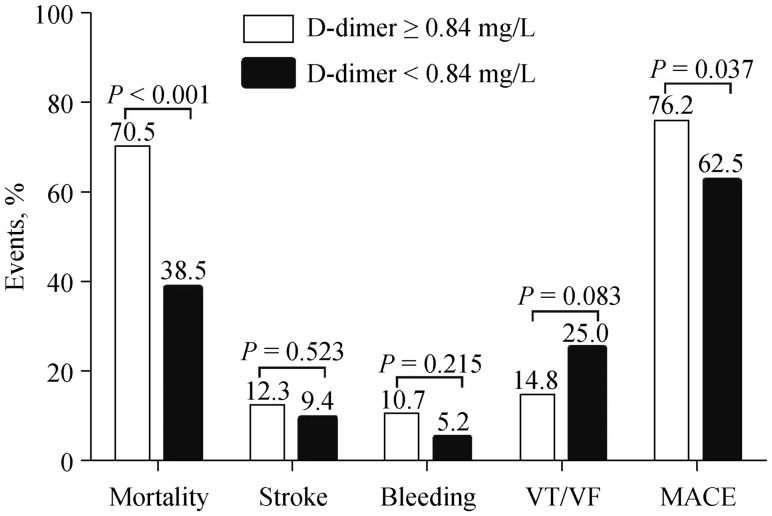
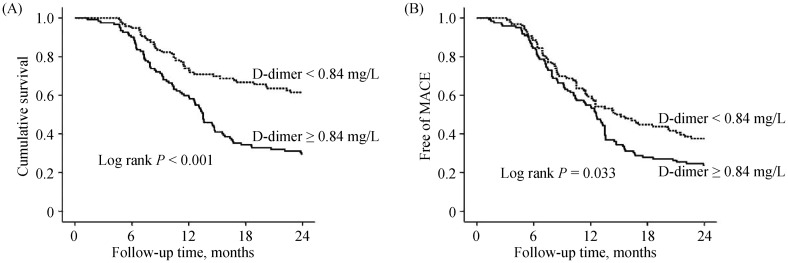
Table 3 compares clinical features between patients with D-dimer ≥ 0.84 mg/L and < 0.84 mg/L. Patients with D-dimer ≥ 0.84 mg/L were more likely to present with lower admission SBP, higher heart rate and serum creatinine; but had lower LVEF and were more likely to receive inotropes/vasoconstrictors or ultrafiltration therapy (all P < 0.05). Figure 3 shows the long-term outcomes. Compared with patients with D-dimer < 0.84 mg/L, patients with D-dimer ≥ 0.84 mg/L had higher all-cause mortality (70.5% vs. 38.5%, P < 0.001) and MACE rate (76.2% vs. 62.5%, P = 0.037), but the incidences of stroke, bleeding, and VT/VF were comparable (all P > 0.05). Figure 4 shows the Kaplan-Meier curves survival and MACE rate stratified by admission D-dimer level. It was found that patients with D-dimer ≥ 0.84 mg/L had significantly higher cumulative mortality (Figure 4A) and MACE rate (Figure 4B) than patients with D-dimer < 0.84 mg/L (log rank P < 0.001 and P = 0.033, respectively).
Table 3. Comparison of baseline characteristics and treatment between patients with D-dimer ≥ 0.84 and < 0.84 mg/L.
| Variables | D-dimer ≥ 0.84 mg/L (n = 95) | D-dimer < 0.84 mg/L (n = 123) | P-value |
| Age, yrs | 54.9 ± 11.0 | 55.0 ± 12.0 | 0.946 |
| Male | 88 (72.1%) | 70 (72.9%) | 1.000 |
| Hypertension | 14 (11.5%) | 13 (13.5%) | 0.682 |
| Atrial fibrillation | 89 (73.0%) | 64 (66.7%) | 0.371 |
| SBP, mmHg | 93.6 ± 13.6 | 98.5 ± 15.3 | 0.015 |
| Heart rate, beats/min | 92.0 ± 19.0 | 85.0 ± 16.0 | 0.005 |
| BNP, pg/mL | 1166.0 (360.5–2345.3)* | 1208.0 (627.5–1986.3)* | 0.866 |
| Troponin I, ug/L | 0.06 (0.04–0.52)* | 0.06 (0.04–0.11)* | 0.391 |
| Serum creatinine, umol/L | 105.9 (82.3–155.4)* | 94.0 (76.1–124.5)* | 0.035 |
| LVEDD, mm | 74.1 ± 9.9 | 73.5 ± 9.4 | 0.665 |
| LVEF | 33.2% ± 5.0% | 34.6% ± 4.4% | 0.029 |
| Diuretics | 118 (96.7%) | 94 (97.9%) | 0.697 |
| Beta blockers | 32 (26.2%) | 28 (29.2%) | 0.649 |
| ACEIs (or ARBs) | 42 (34.4%) | 44 (45.8%) | 0.095 |
| Aldosterone receptor antagonist | 100 (82.0%) | 88 (91.7%) | 0.048 |
| Warfarin | 19 (15.6%) | 21 (21.9%) | 0.290 |
| Aspirin | 68 (55.7%) | 30 (31.2%) | < 0.001 |
| Clopidogrel | 50 (41.0%) | 38 (39.6%) | 0.890 |
| Inotropes/vasoconstrictors | 77 (63.1%) | 45 (46.9%) | 0.020 |
| Ultrafiltration | 37 (30.3%) | 16 (16.7%) | 0.026 |
Data are presented as means ± SD or n (%). *Presented as median (25th–75th). ACEIs: angiotensin-converting enzyme inhibitors; ARBs: angiotensin receptors blockers; BNP: brain natriuretic peptide; LVEDD: left ventricular end diastolic diameter; LVEF: left ventricular ejection fraction; SBP: systolic blood pressure.
Figure 3. Comparison of long-term outcomes in patients with D-dimer ≥ 0.84 and < 0.84 mg/L.
MACE: major adverse cardiovascular event; VF: ventricular fibrillation; VT: ventricular tachycardia.
Figure 4. Survival (A) and MACE (B) curves in patients with D-dimer ≥ 0.84 and < 0.84 mg/L.
MACE: major adverse cardiovascular event.
The independent factors associated with long-term outcome were analyzed by the multivariate Cox model (Table 4). After adjusting for age, sex, body mass index, AF, SBP, heart rate, TnI, LVEDD, LVEF, creatinine, and main medications, compared with D-dimer < 0.84 mg/L, D-dimer ≥ 0.84 mg/L was associated with 2.3-fold increased risk of all-cause mortality (HR = 2.315, 95% CI: 1.570–3.414, P < 0.001). Admission D-dimer level was positively associated with long-term MACE (HR = 1.256, 95% CI: 1.058–1.490, P = 0.009). Other factors independently associated with all-cause mortality were admission SBP, LVEF, and use of beta blockers. Other factors associated with MACE included admission SBP, LVEF, use of ACEIs/ARBs, and occurrence of VT/VF (Table 4).
Table 4. Risk factors for all-cause mortality and MACE by multivariate Cox analysis.
| All-cause mortality |
MACE |
||||
| Variables | HR (95% CI) | P-value | Variables | HR (95% CI) | P-value |
| D-dimer ≥ 0.84 mg/L (vs. < 0.84 mg/L) | 2.315 (1.570–3.414) | < 0.001 | D-dimer | 1.256 (1.058–1.490) | 0.009 |
| SBP | 0.984 (0.972–0.996) | 0.010 | SBP | 0.984 (0.973–0.995) | 0.003 |
| LVEF | 0.942 (0.909–0.976) | 0.001 | LVEF | 0.943 (0.912–0.976) | 0.001 |
| Beta blockers use | 0.515 (0.327–0.812) | 0.004 | ACEIs/ARBs use | 0.627 (0.447–0.879) | 0.007 |
| VT/VF occurrence | 3.309 (2.276–4.811) | < 0.001 | |||
ACEIs: angiotensin-converting enzyme inhibitors; ARBs: angiotensin receptors blockers; LVEF: left ventricular ejection fraction; MACE: major adverse cardiovascular event; SBP: systolic blood pressure; VF: ventricular fibrillation; VT: ventricular tachycardia.
Finally, as the D-dimer level is influenced by multiple factors, such as age, sex, AF and anticoagulation status, the D-dimer level was compared in multiple subgroups. However, D-dimer level was comparable between patients aged ≥ 60 and < 60 years (median: 0.87 vs. 0.90 mg/L, P = 0.519), males and females (median: 0.89 vs. 0.92 mg/L, P = 0.818), AF and non-AF patients (median: 0.97 vs. 0.84 mg/L, P = 0.207), and anticoagulation and non-anticoagulation patients (median: 0.80 vs. 0.96 mg/L, P = 0.105). Moreover, the interactions between D-dimer and the abovementioned clinical factors were not significant (data not shown), indicating the homogeneous effect of D-dimer on patients with different clinical backgrounds.
4. Discussion
The major findings from the present study are as follows. Firstly, patients with poor outcome had significantly elevated admission D-dimer level. Secondly, compared with the traditional markers, admission D-dimer had a better predictive value for long-term mortality in patients with end-stage HF. Last but not least, elevated D-dimer independently predicted adverse long-term outcome regardless of the clinical status that affected the D-dimer level. Our present study demonstrated D-dimer as a superior prognostic biomarker in patients with end-stage HF.
Previous studies had shown that HF is associated with a pro-thrombotic state and disturbed blood coagulation system regardless of the HF type (systolic or diastolic HF).[13],[19] Hypercoagulability in HF could have multiple causes, such as blood stasis, dilatation of cardiac chambers, reduced myocardial contractility and cardiac output, inflammation reaction, neuro-hormonal activation, impaired endothelial function, and arrhythmias such as AF.[20] The hypercoagulability status predisposes the patients to embolic events. Previous studies had shown that approximately 11% of patients with DCM have one or more embolic events during the course of their illness,[21] and mural thrombus of ventrium was associated with high risk of stroke.[22] In our present study, 39 patients (17.9%) were found to have mural thrombus of the ventrium; however, the incidence between survivors and non-survivors was comparable and the D-dimer level was also comparable between those with and without mural thrombus of the ventrium (data not shown). Consistent with previous studies,[21],[22] indicating that mural thrombus of the ventrium was mainly associated with embolic events, but not with mortality.
D-dimer originates from the formation and degradation of cross-linked fibrin and reflects the activation of coagulation and fibrinolysis. Previous studies had shown that elevated D-dimer level not only predicted the development of incident systolic HF,[23] but also predicted adverse outcome in patients with HF. Minami, et al.[15] found that a high admission D-dimer level (> 3.85 µg/mL) was associated with adverse in-hospital and poor medium-term prognosis in patients with acute decompensated HF. Zorlu, et al.[16] analyzed the data of 174 patients with systolic HF and found that elevated D-dimer (> 1.43 µg/mL) independently predicted increased cardiovascular mortality. Moreover, even in patients with symptoms compatible to HF, increased D-dimer (> 0.25 mg/L) was found to confer a 3.3 times increased risk for cardiovascular death.[24] However, HF involved in these studies were symptom compatible or acute decompensated and dynamics stable, studies concerning the impact of D-dimer on the outcome in patients with end-stage HF are lacking. Due to the complex pathphysiological process involved in end-stage HF, hemostatic abnormalities may represent the integrated effect of insufficient systemic organ perfusion, organ congestion and cardiac structural, functional, and electrophysiological abnormalities. Therefore, D-dimer, as a marker of hemostatic abnormalities, may correlate with disease severity. In our present study, the D-dimer level was significantly elevated in the non-survivors, consistent with the previous reports,[15],[16] indicating its potential prognostic value in patients with end-stage HF. It is worth mentioning that, inconsistent with findings from previous studies,[16],[24] our study found that the D-dimer value had no significant correlation with several variables determined by previous studies, such as the left atrial size, age, LVEF etc.; which indicated that the D-dimer level in patients with end-stage HF might be a marker reflecting the severity of HF independent of traditional demographic, cardiac, and other morbidity characteristics.
Currently, many markers have been used to evaluate the prognosis in patients with HF such as BNP, TnI, LVEDD, LVEF, etc.,[5]–[8] however, our study revealed that these classic markers were not linearly associated with the outcome. Adding D-dimer to these traditional markers offered an incremental prognostic value. We further demonstrated that D-dimer, compared with the traditional markers, had better predictive value for prognosis by ROC analysis, which suggested that D-dimer might have superior prognostic value for patients with end-stage HF. The reason why D-dimer provided incremental prognostic value in addition to traditional markers may be that end-stage HF is characterized by increasing inability to meet the metabolic demands of end organs and results in end-organ dysfunction, such as hepatic and renal insufficiency and hemostatic abnormalities, which in turn increases the mortality associated with HF. Compared with traditional markers that only reflect the global cardiac structure and function, D-dimer may reflect the function status of non-cardiac organs such as the liver, kidney, and hemostasis system.
Because the D-dimer level is affected by multiple factors, we analyzed the D-dimer level in subgroups and found that the D-dimer level was comparable regardless of age, sex, AF, or anticoagulation status. Moreover, the interaction of D-dimer with the abovementioned subgroups for predicting long-term mortality was not statistically significant, which indicated that D-dimer offers similar predictive value in different subgroups of patients.
Cox regression demonstrated that D-dimer was an independent factor associated with long-term outcome in patients with end-stage HF. Our study confirms and extends previous research, indicating that elevated D-dimer level independently predicted poor prognosis regardless of the HF type (compensated HF, acute decompensated HF, systolic HF, or end-stage HF). Our present study also identified several factors associated with long-term outcome that have been extensively confirmed, such as admission SBP,[25] LVEF, [7] and use of beta blockers.[26] However, inconsistent with previous findings, BNP as a continuous variable was not an independent factor associated with long-term outcome. Our previous study found a “U-like” shape between the BNP level and mortality in patients with end-stage HF, the so called BNP paradox, which may be a reflection of an impaired neurohormonal response in end-stage HF.[27] Moreover, ACEIs/ARBs were not independent factors for long-term mortality in our study and we inferred that the benefit from ACEIs/ARBs may be limited by the duration and dosage of ACEIs/ARBs treatment, because patients with end-stage HF usually have lower blood pressure and the target of ACEIs/ARBs treatment cannot be easily achieved.
The precise mechanisms of elevated D-dimer level as an independent risk factor for adverse long-term outcome in patients with end-stage HF are not well understood. There are some possible explanations. Firstly, patients with significantly elevated D-dimer level presented with relatively lower admission SBP and LVEF, but had higher HR and creatinine levels, and were more likely to receive inotropes/vasoconstrictors and ultrafiltration therapy, which were important signs of disease severity and indicated poor outcome.[27] Secondly, hemostatic abnormalities, represented by elevated D-dimer, may reflect the integrated effect of cardiac and non-cardiac functional status, which is the disease severity. Thirdly, our present study showed that patients with significantly elevated D-dimer had a relatively higher incidence of stroke and bleeding. It is known that either thrombotic or hemorrhagic events are associated with increased risk of poor outcome.[28] Furthermore, previous studies had shown that D-dimer can promote inflammatory reactions by inducing the synthesis and release of some inflammatory cytokines such as interleukin-1β and interleukin-6,[29] resulting in increased mortality. Last but not least, patients with end-stage HF are usually confronted with mobility limitation, which increases the risk of thrombosis and subsequent thrombotic events, all of which are reflected by elevated D-dimer level. However, these supposed interpretations need to be confirmed by further studies.
The present study has some implications for clinical practice, especially in the treatment of HF in China. Firstly, our current study suggests that D-dimer level might be an important biomarker for risk stratification and prognosis evaluation in patients with end-stage HF and that the prognostic value of D-dimer might be superior to traditional prognostic markers. Secondly, owing to socioeconomic status and healthcare policy limitations, the implantation of cardiac resynchronization therapy or implantable cardioverter defibrillator is still very limited in China compared with developed countries.[30],[31] Meanwhile, although heart transplantation is now considered the gold standard therapy for patients with end-stage HF, routine treatment in China is still challenging due to the shortage of donor organs and technical and economic limitations.[32] Therefore, medical treatment is still the main strategy for most HF patients in China and D-dimer level may provide additional prognostic information beyond the traditional risk factors.
4.1. Limitations
There are some limitations in our study. Firstly, the D-dimer value was obtained on admission instead of before discharge and some patients may have received anticoagulation therapy, which may potentially confound the association between D-dimer and outcomes. Secondly, this is a single-center observational study, and our results may not be extended to patients in other regions due to the heterogeneity in clinical characteristics, co-morbidities, and treatment compliance in individuals with DCM. Thirdly, the present study focused on DCM as the cause of incident HF, and whether our conclusions can be generalized to other causes of HF, such as ischemic cardiomyopathy or valvular heart disease, needs further study. In addition, we recorded the medication use during hospitalization only and follow-up data were unavailable. Last but not least, due to the observational study design, the effect of unmeasured confounders may have impacted the results, although we performed statistical adjustments. Therefore, more studies are required to confirm our results.
4.2. Conclusions
Elevated D-dimer level was independently associated with poor long-term outcome in patients with end-stage HF secondary to idiopathic DCM, and the predictive value was superior to that of traditional prognostic markers.
Acknowledgments
All authors had no conflicts of interest to disclose. Many thanks to the staff in our team who participated in the patient follow up.
References
- 1.Sanderson JE, Tse TF. Heart failure: a global disease requiring a global response. Heart. 2003;89:585–586. doi: 10.1136/heart.89.6.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khand A, Gemmel I, Clark AL, et al. Is the prognosis of heart failure improving? J Am Coll Cardiol. 2000;36:2284–2286. doi: 10.1016/s0735-1097(00)00995-5. [DOI] [PubMed] [Google Scholar]
- 3.Ammar KA, Jacobsen SJ, Mahoney DW, et al. Prevalence and prognostic significance of heart failure stages: application of the American College of Cardiology/American Heart Association heart failure staging criteria in the community. Circulation. 2007;115:1563–1570. doi: 10.1161/CIRCULATIONAHA.106.666818. [DOI] [PubMed] [Google Scholar]
- 4.Rose EA, Gelijns AC, Moskowitz AJ, et al. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med. 2001;345:1435–1443. doi: 10.1056/NEJMoa012175. [DOI] [PubMed] [Google Scholar]
- 5.van Veldhuisen DJ, Linssen GC, Jaarsma T, et al. B-type natriuretic peptide and prognosis in heart failure patients with preserved and reduced ejection fraction. J Am Coll Cardiol. 2013;61:1498–1506. doi: 10.1016/j.jacc.2012.12.044. [DOI] [PubMed] [Google Scholar]
- 6.Aimo A, Januzzi JL, Jr, Vergaro G, et al. Prognostic value of high-sensitivity troponin T in chronic heart failure: an individual patient data meta-analysis. Circulation. 2018;137:286–297. doi: 10.1161/CIRCULATIONAHA.117.031560. [DOI] [PubMed] [Google Scholar]
- 7.Curtis JP, Sokol SI, Wang Y, et al. The association of left ventricular ejection fraction, mortality, and cause of death in stable outpatients with heart failure. J Am Coll Cardiol. 2003;42:736–742. doi: 10.1016/s0735-1097(03)00789-7. [DOI] [PubMed] [Google Scholar]
- 8.Kajimoto K, Minami Y, Otsubo S, et al. Sex differences in left ventricular cavity dilation and outcomes in acute heart failure patients with left ventricular systolic dysfunction. Can J Cardiol. 2018;34:477–484. doi: 10.1016/j.cjca.2018.01.019. [DOI] [PubMed] [Google Scholar]
- 9.Tanai E, Frantz S. Pathophysiology of heart failure. Compr Physiol. 2015;6:187–214. doi: 10.1002/cphy.c140055. [DOI] [PubMed] [Google Scholar]
- 10.Albabtain M, Brenner MJ, Nicklas JM, et al. Hyponatremia, cognitive function, and mobility in an outpatient heart failure population. Med Sci Monit. 2016;22:4978–4985. doi: 10.12659/MSM.898538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y, Bauersachs J, Langer HF. Immune mechanisms in heart failure. Eur J Heart Fail. 2017;19:1379–1389. doi: 10.1002/ejhf.942. [DOI] [PubMed] [Google Scholar]
- 12.Marcucci R, Gori AM, Giannotti F, et al. Markers of hypercoagulability and inflammation predict mortality in patients with heart failure. J Thromb Haemost. 2006;4:1017–1022. doi: 10.1111/j.1538-7836.2006.01916.x. [DOI] [PubMed] [Google Scholar]
- 13.Jug B, Vene N, Salobir BG, et al. Procoagulant state in heart failure with preserved left ventricular ejection fraction. Int Heart J. 2009;50:591–600. doi: 10.1536/ihj.50.591. [DOI] [PubMed] [Google Scholar]
- 14.Itani R, Minami Y, Haruki S, et al. Prognostic impact of disseminated intravascular coagulation score in acute heart failure patients referred to a cardiac intensive care unit: a retrospective cohort study. Heart Vessels. 2017;32:872–879. doi: 10.1007/s00380-017-0946-y. [DOI] [PubMed] [Google Scholar]
- 15.Minami Y, Haruki S, Jujo K, et al. Elevated D-dimer levels predict an adverse outcome in hospitalized patients with acute decompensated heart failure. Int J Cardiol. 2016;204:42–44. doi: 10.1016/j.ijcard.2015.11.156. [DOI] [PubMed] [Google Scholar]
- 16.Zorlu A, Yilmaz MB, Yucel H, et al. Increased D-dimer levels predict cardiovascular mortality in patients with systolic heart failure. J Thromb Thrombolysis. 2012;33:322–328. doi: 10.1007/s11239-011-0635-0. [DOI] [PubMed] [Google Scholar]
- 17.Jiang H, Ge J. Epidemiology and clinical management of cardiomyopathies and heart failure in China. Heart. 2009;95:1727–1731. doi: 10.1136/hrt.2008.150177. [DOI] [PubMed] [Google Scholar]
- 18.Elliott P, Andersson B, Arbustini E, et al. Classification of the cardiomyopathies: a position statement from the European Society of Cardiology Working Group on myocardial and pericardial diseases. Eur Heart J. 2008;29:270–276. doi: 10.1093/eurheartj/ehm342. [DOI] [PubMed] [Google Scholar]
- 19.Lip GY, Pearce LA, Chin BS, et al. Effects of congestive heart failure on plasma von Willebrand factor and soluble P-selectin concentrations in patients with non-valvar atrial fibrillation. Heart. 2005;91:759–763. doi: 10.1136/hrt.2004.036160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jafri SM. Hypercoagulability in heart failure. Semin Thromb Hemost. 1997;23:543–545. doi: 10.1055/s-2007-996133. [DOI] [PubMed] [Google Scholar]
- 21.Gottdiener JS, Gay JA, VanVoorhees L, et al. Frequency and embolic potential of left ventricular thrombus in dilated cardiomyopathy: assessment by 2-dimensional echocardiography. Am J Cardiol. 1983;52:1281–1285. doi: 10.1016/0002-9149(83)90588-x. [DOI] [PubMed] [Google Scholar]
- 22.Crawford TC, Smith WT, 4th, Velazquez EJ, et al. Prognostic usefulness of left ventricular thrombus by echocardiography in dilated cardiomyopathy in predicting stroke, transient ischemic attack, and death. Am J Cardiol. 2004;93:500–503. doi: 10.1016/j.amjcard.2003.10.056. [DOI] [PubMed] [Google Scholar]
- 23.de Boer RA, Nayor M, deFilippi CR, et al. Association of cardiovascular biomarkers with incident heart failure with preserved and reduced ejection fraction. JAMA Cardiol. 2018;3:215–224. doi: 10.1001/jamacardio.2017.4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alehagen U, Dahlström U, Lindahl TL. Elevated D-dimer level is an independent risk factor for cardiovascular death in out-patients with symptoms compatible with heart failure. Thromb Haemost. 2004;92:1250–1258. doi: 10.1160/TH04-05-0278. [DOI] [PubMed] [Google Scholar]
- 25.Tremblay-Gravel M, Khairy P, Roy D, et al. Systolic blood pressure and mortality in patients with atrial fibrillation and heart failure: insights from the AFFIRM and AF-CHF studies. Eur J Heart Fail. 2014;16:1168–1174. doi: 10.1002/ejhf.168. [DOI] [PubMed] [Google Scholar]
- 26.Chatterjee S, Biondi-Zoccai G, Abbate A, et al. Benefits of β blockers in patients with heart failure and reduced ejection fraction: network meta-analysis. BMJ. 2013;346:f55–f55. doi: 10.1136/bmj.f55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang B, Shen J, Li L, et al. Effect of B-type natriuretic peptide level on long-term outcome in patients with end-stage heart failure. Am J Cardiol. 2016;118:383–388. doi: 10.1016/j.amjcard.2016.05.018. [DOI] [PubMed] [Google Scholar]
- 28.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 29.Robson SC, Shephard EG, Kirsch RE. Fibrin degradation product D-dimer induces the synthesis and release of biologically active IL-1 beta, IL-6 and plasminogen activator inhibitors from monocytes in vitro. Br J Haematol. 1994;86:322–326. doi: 10.1111/j.1365-2141.1994.tb04733.x. [DOI] [PubMed] [Google Scholar]
- 30.Hua W. Cardiac resynchronization therapy for chronic heart failure in China: guideline and practice. Chin Med J (Engl) 2010;123:2293–2294. [PubMed] [Google Scholar]
- 31.Chia YMF, Teng TK, Tan ESJ, et al. Disparity between indications for and utilization of implantable cardioverter defibrillators in Asian patients with heart failure. Circ Cardiovasc Qual Outcomes. 2017;10:e003651–e003651. doi: 10.1161/CIRCOUTCOMES.116.003651. [DOI] [PubMed] [Google Scholar]
- 32.Hu XJ, Dong NG, Liu JP, et al. Status on heart transplantation in China. Chin Med J (Engl) 2015;128:3238–3242. doi: 10.4103/0366-6999.170238. [DOI] [PMC free article] [PubMed] [Google Scholar]